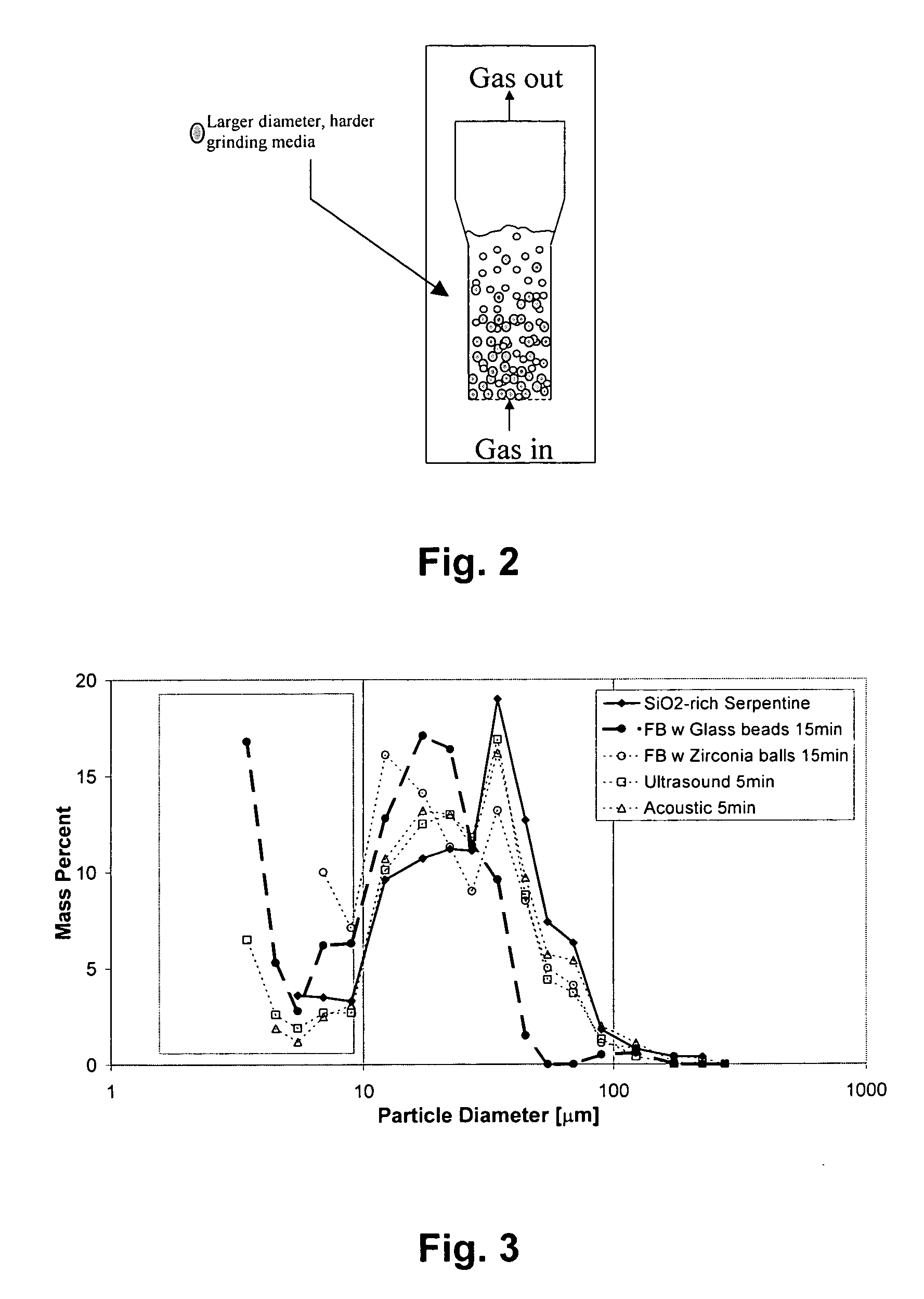
Patents
Literature
1281results about "By chemical separation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
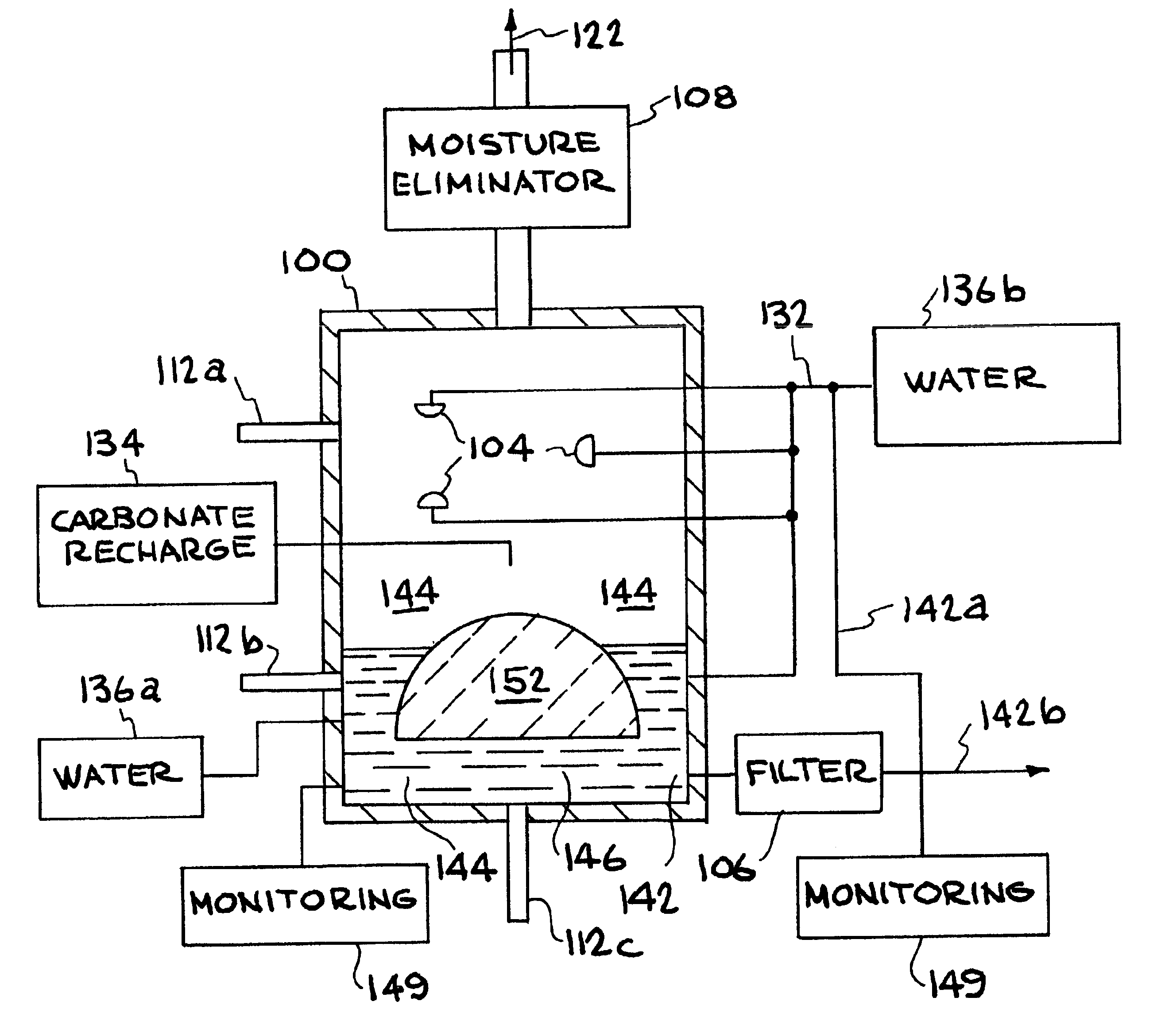
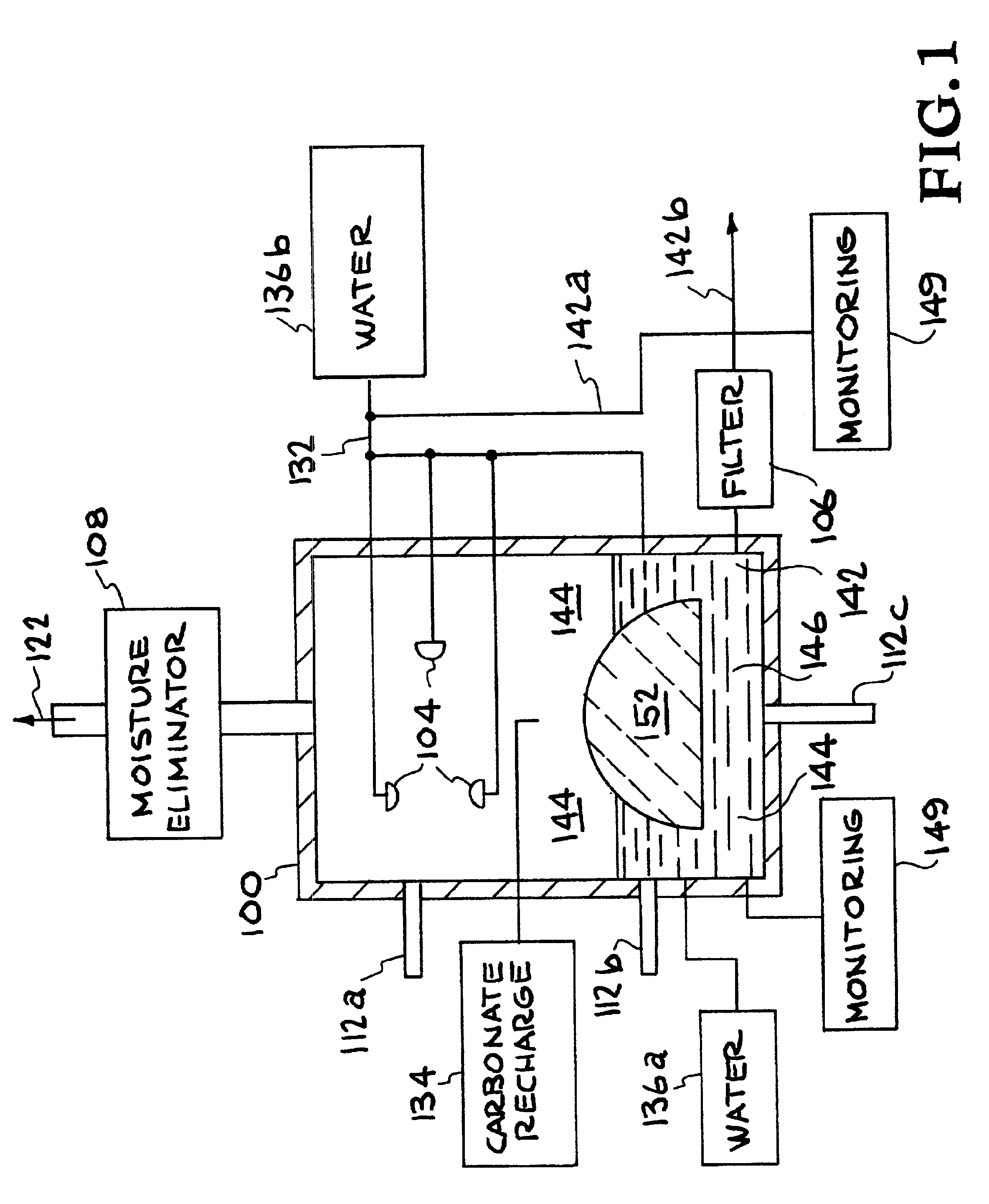
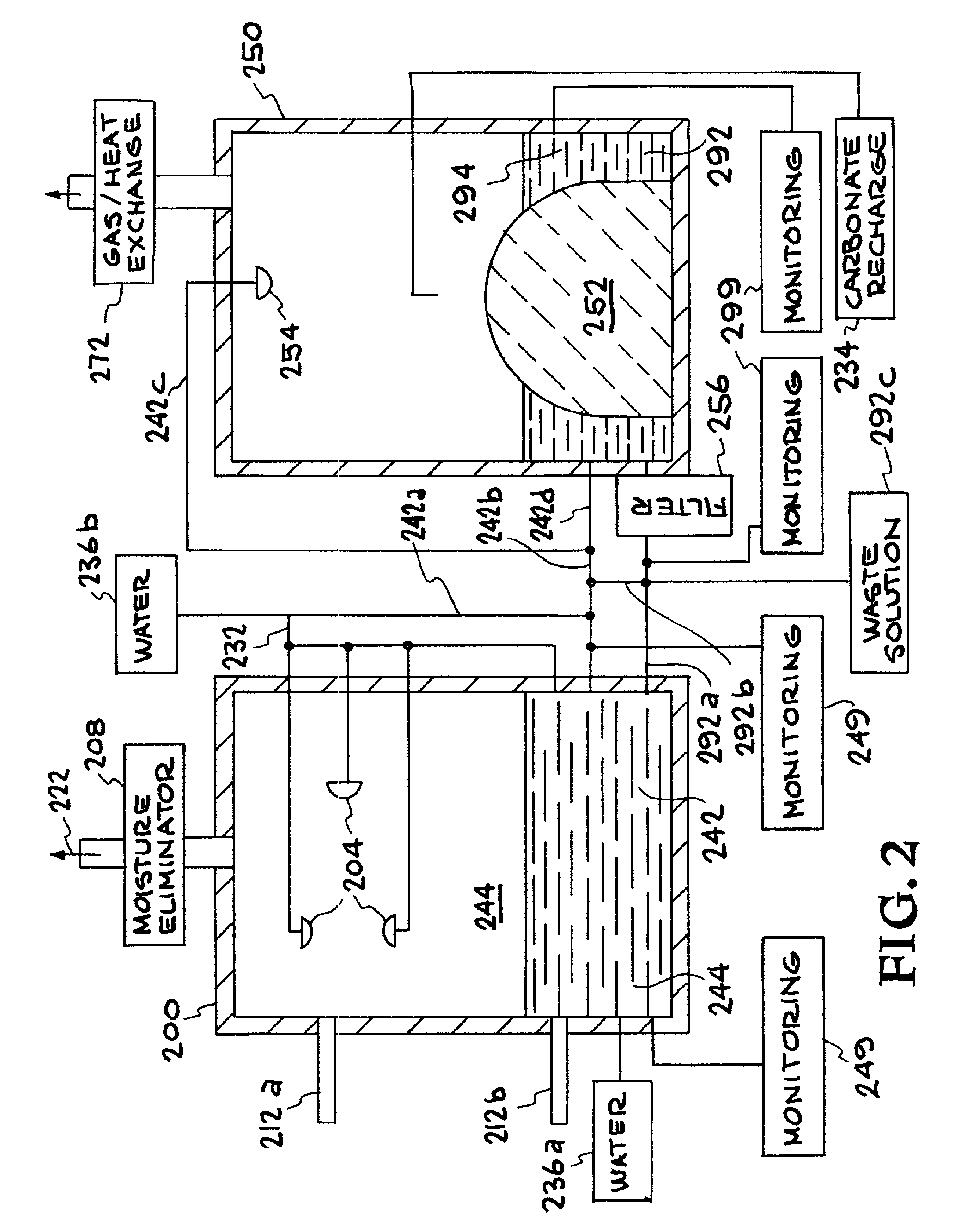
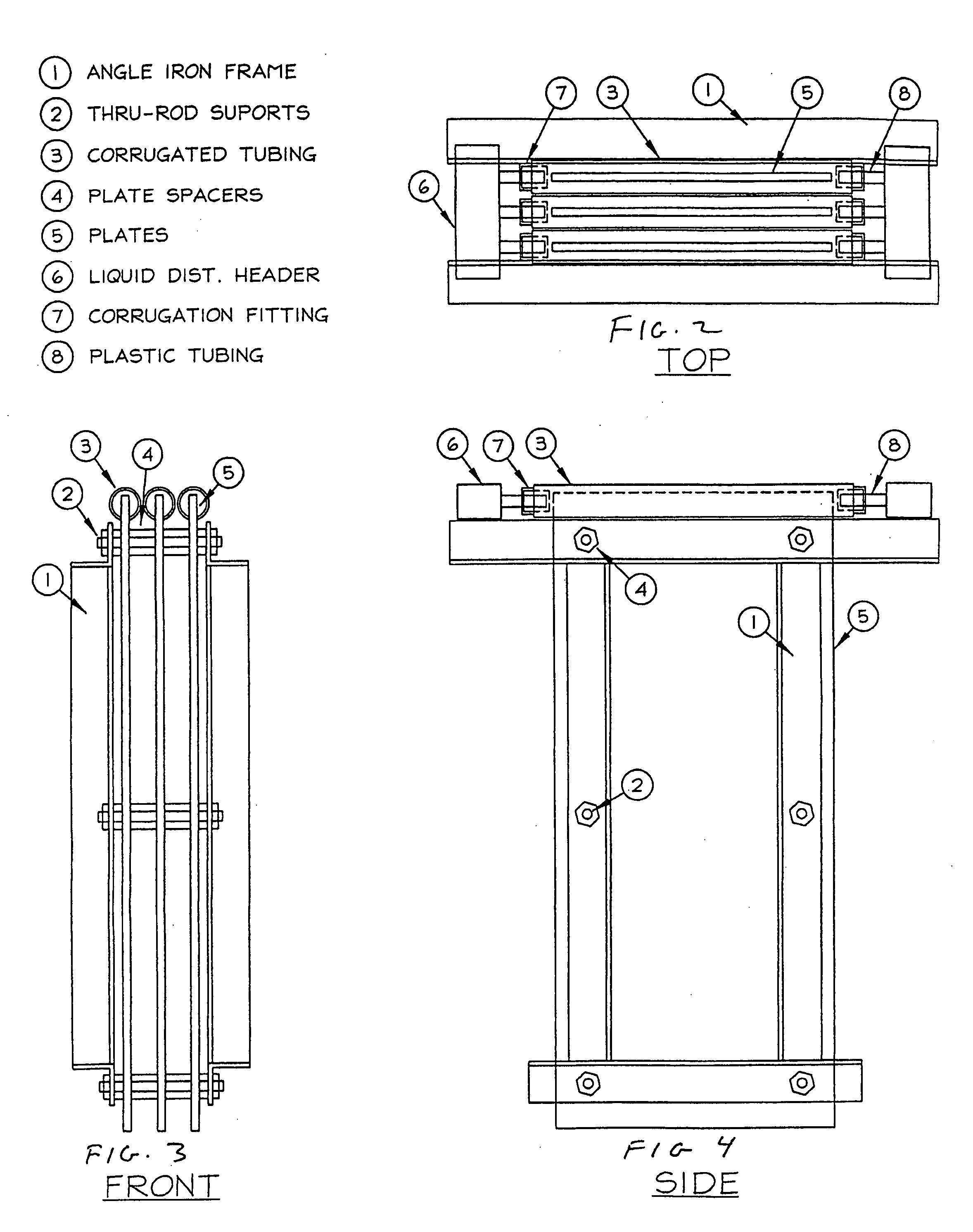
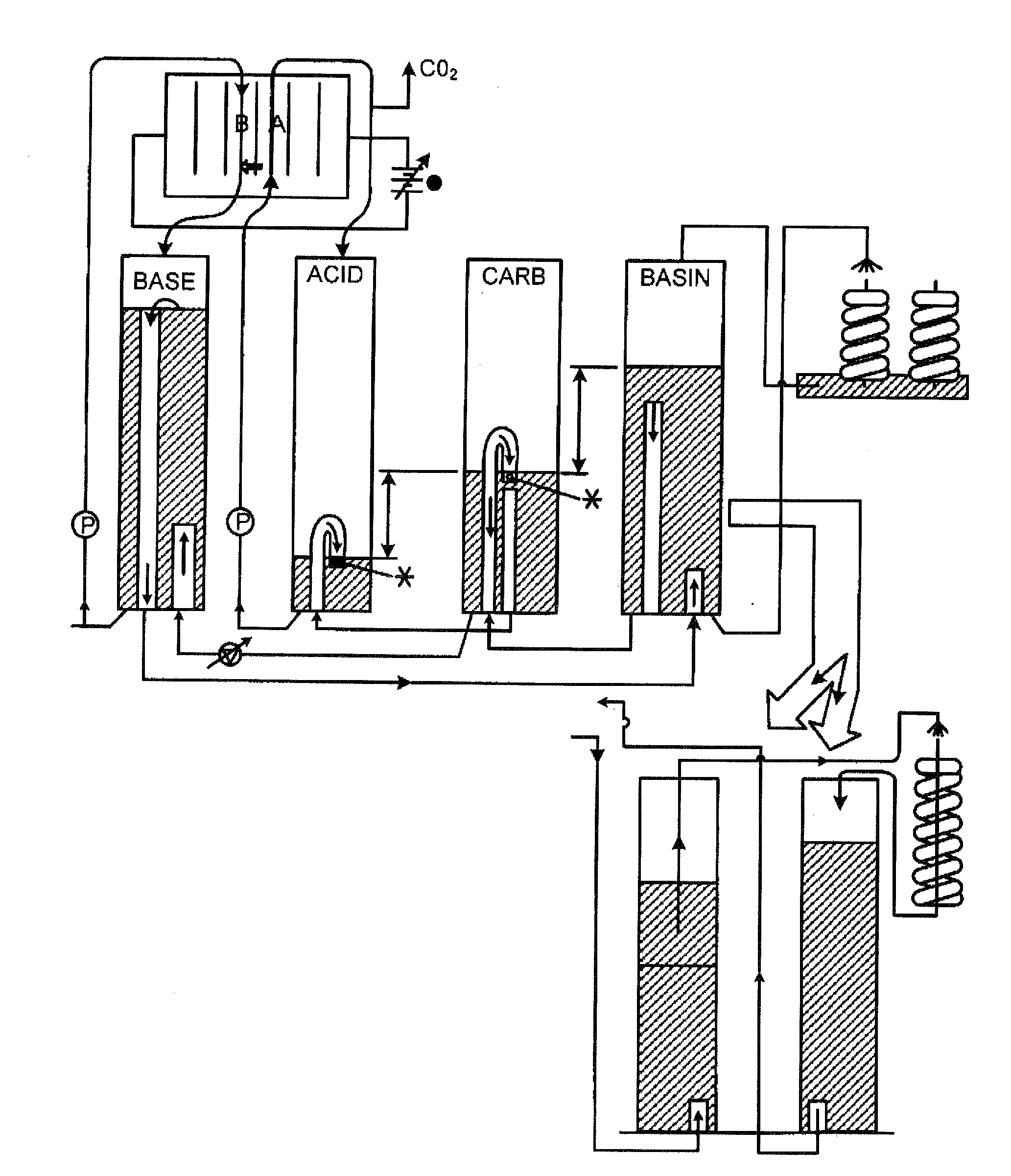
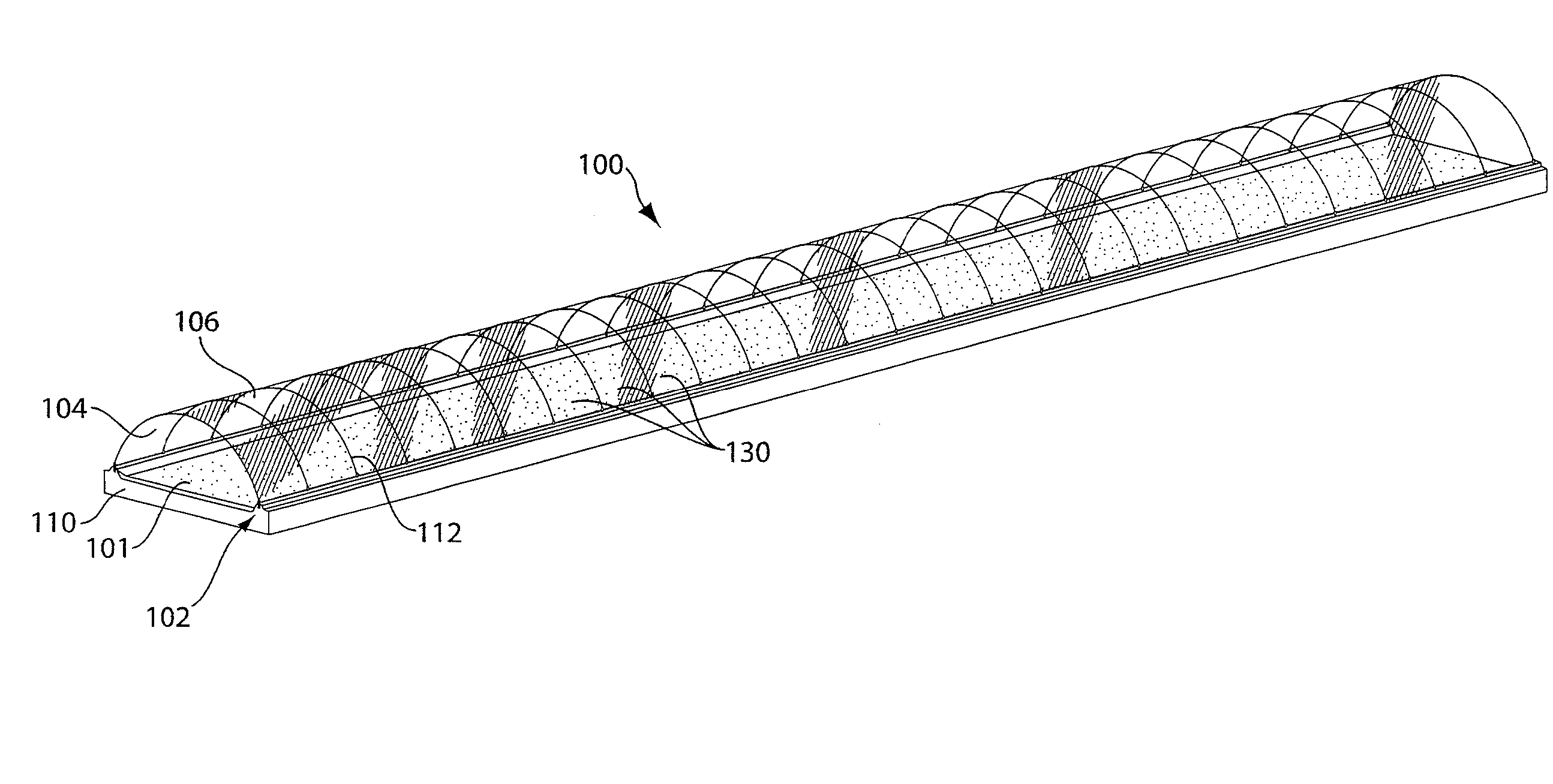
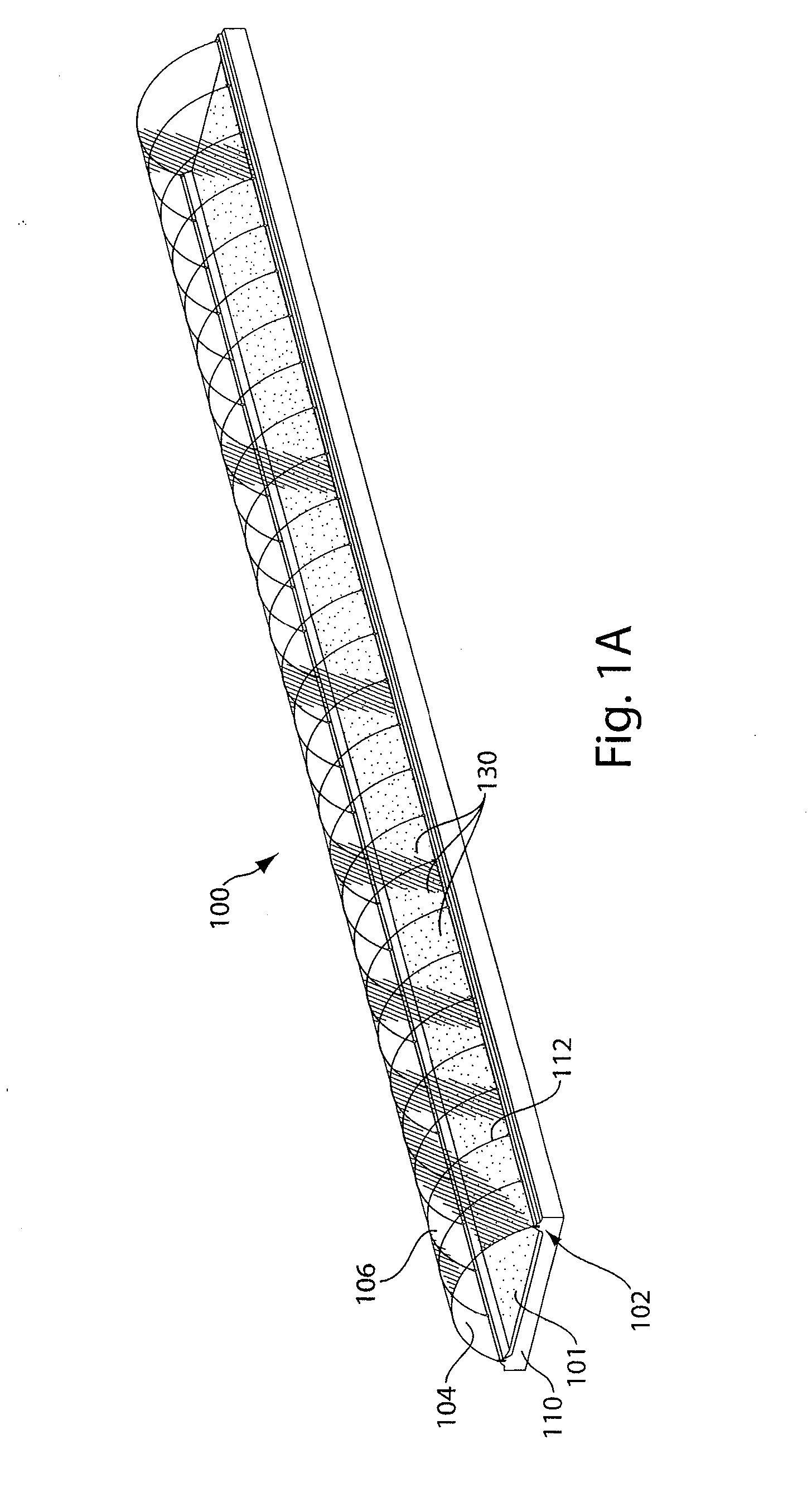
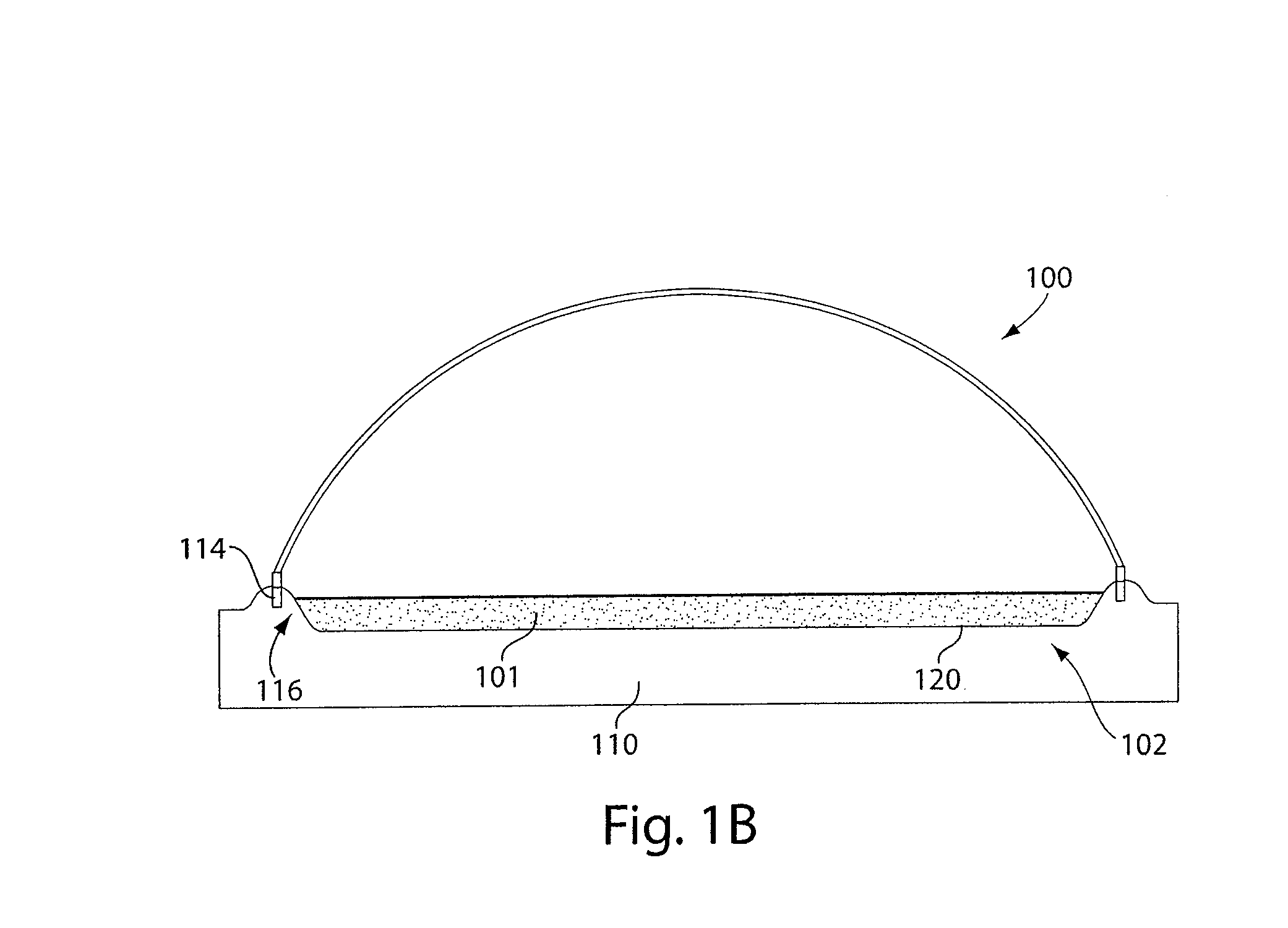
Method for extracting and sequestering carbon dioxide
InactiveUS6890497B2Reduce CO burdenWithout significant expenditureCalcium/strontium/barium carbonatesCombination devicesDicarbonateAlkaline earth metal
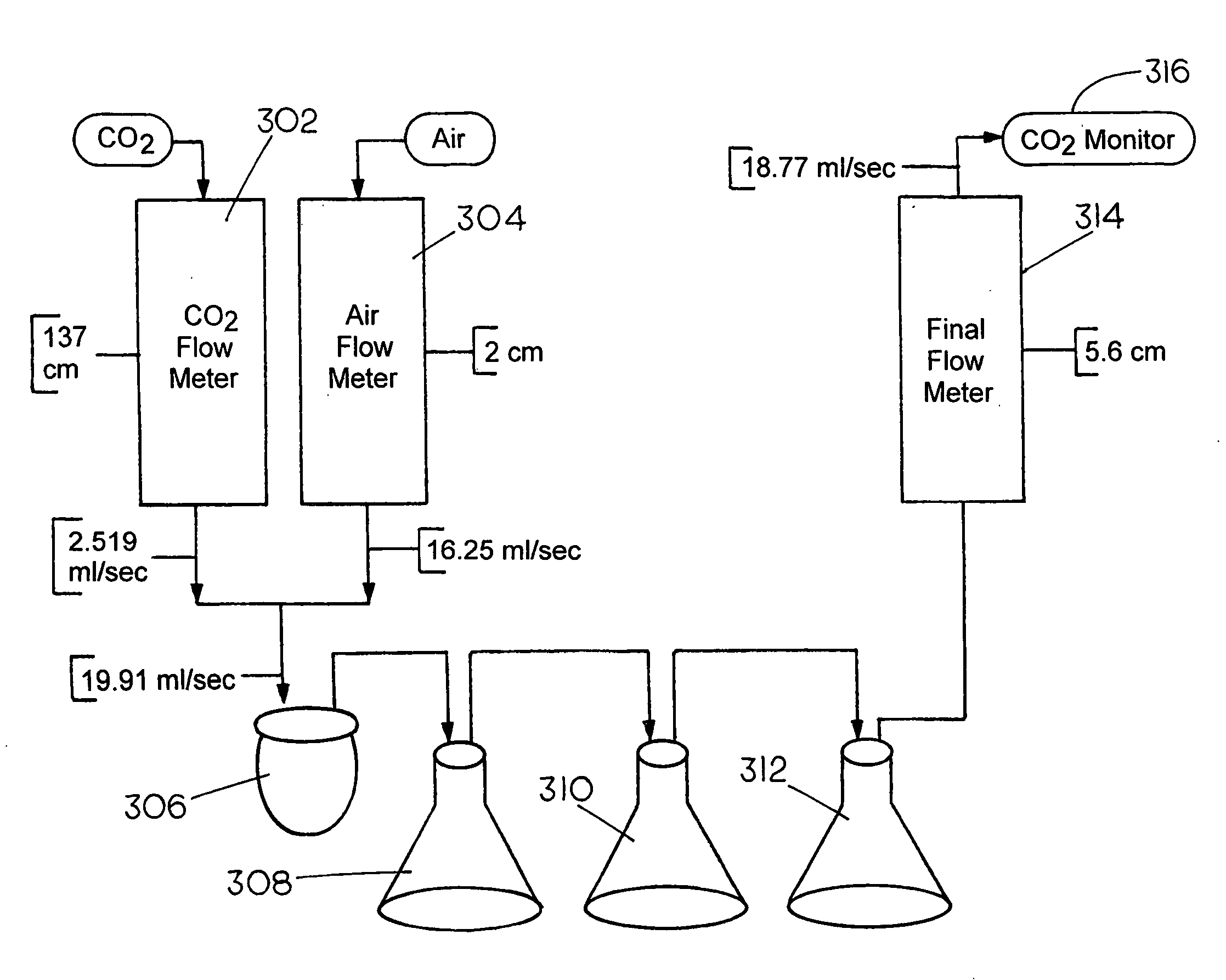
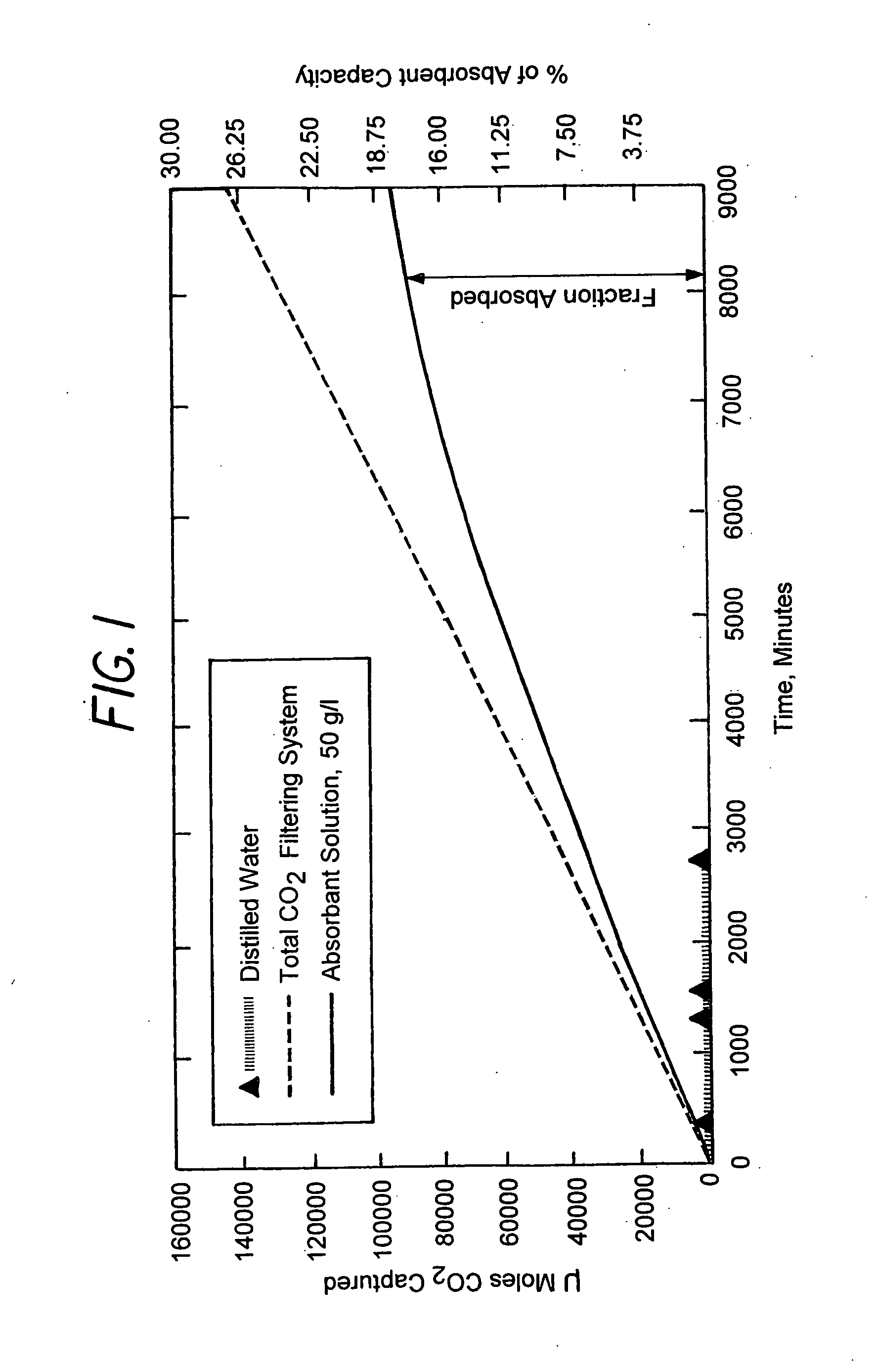
A method and apparatus to extract and sequester carbon dioxide (CO2) from a stream or volume of gas wherein said method and apparatus hydrates CO2, and reacts the resulting carbonic acid with carbonate. Suitable carbonates include, but are not limited to, carbonates of alkali metals and alkaline earth metals, preferably carbonates of calcium and magnesium. Waste products are metal cations and bicarbonate in solution or dehydrated metal salts, which when disposed of in a large body of water provide an effective way of sequestering CO2 from a gaseous environment.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Methods of sequestering co2
Methods of sequestering carbon dioxide (CO2) are provided. Aspects of the methods include precipitating a storage stable carbon dioxide sequestering product from an alkaline-earth-metal-containing water and then disposing of the product, e.g., by placing the product in a disposal location or using the product as a component of a manufactured composition. Also provided are systems for practicing methods of the invention.
Owner:ARELAC INC
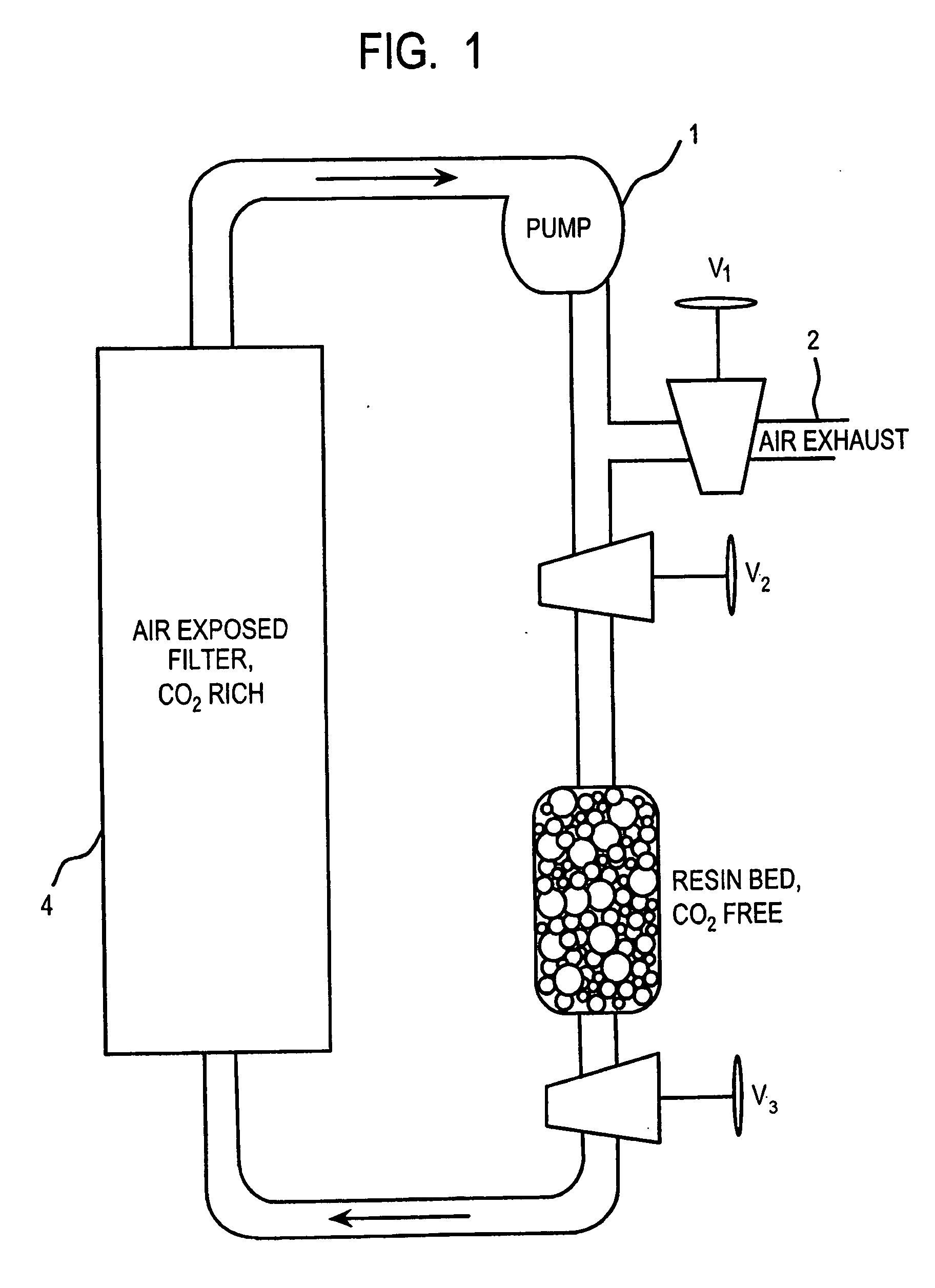
Removal of carbon dioxide from air
InactiveUS20060051274A1Simple processReduce energy consumptionSemi-permeable membranesHydrogen sulfidesSolventCarbon dioxide binding
The present invention is directed to methods for removing carbon dioxide from air, which comprises exposing solvent covered surfaces to air streams where the airflow is kept laminar, or close to the laminar regime. The invention also provides for an apparatus, which is a laminar scrubber, comprising solvent covered surfaces situated such that they can be exposed to air stream. In another aspect, the invention provides a method and apparatus for separating carbon dioxide (CO2) bound in a solvent. The invention is particularly useful in processing hydroxide solvents containing CO2 captured from air.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +2
Carbon Dioxide Capture and Mitigation of Carbon Dioxide Emissions
InactiveUS20080031801A1Pressure dropCalcium/strontium/barium carbonatesLiquefactionSorbentSolid surface
The present invention describes methods and systems for extracting, capturing, reducing, storing, sequestering, or disposing of carbon dioxide (CO2), particularly from the air. The CO2 extraction methods and systems involve the use of chemical processes, mineral sequestration, and solid and liquid sorbents. Methods are also described for extracting and / or capturing CO2 via condensation on solid surfaces at low temperature.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
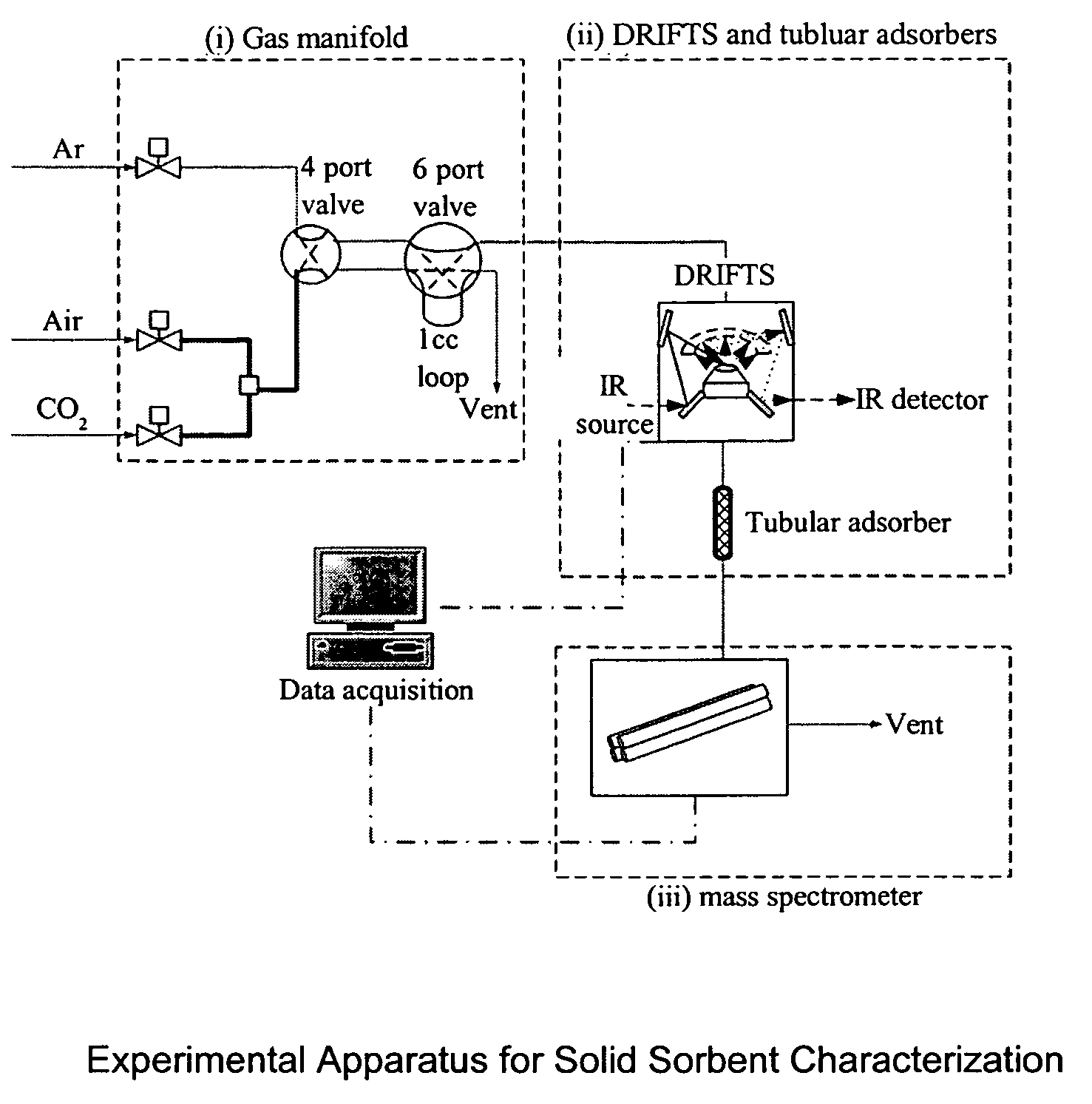
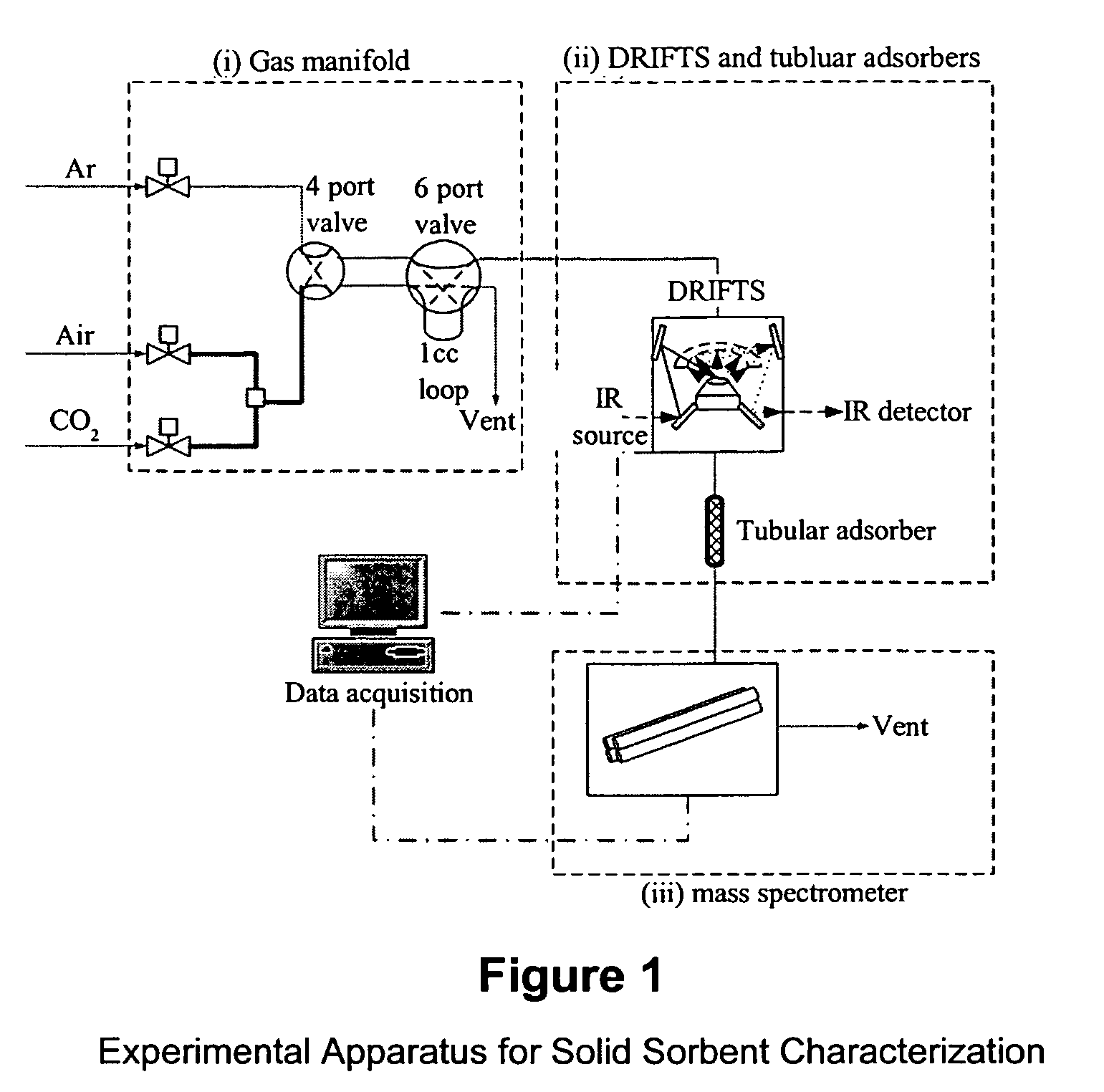
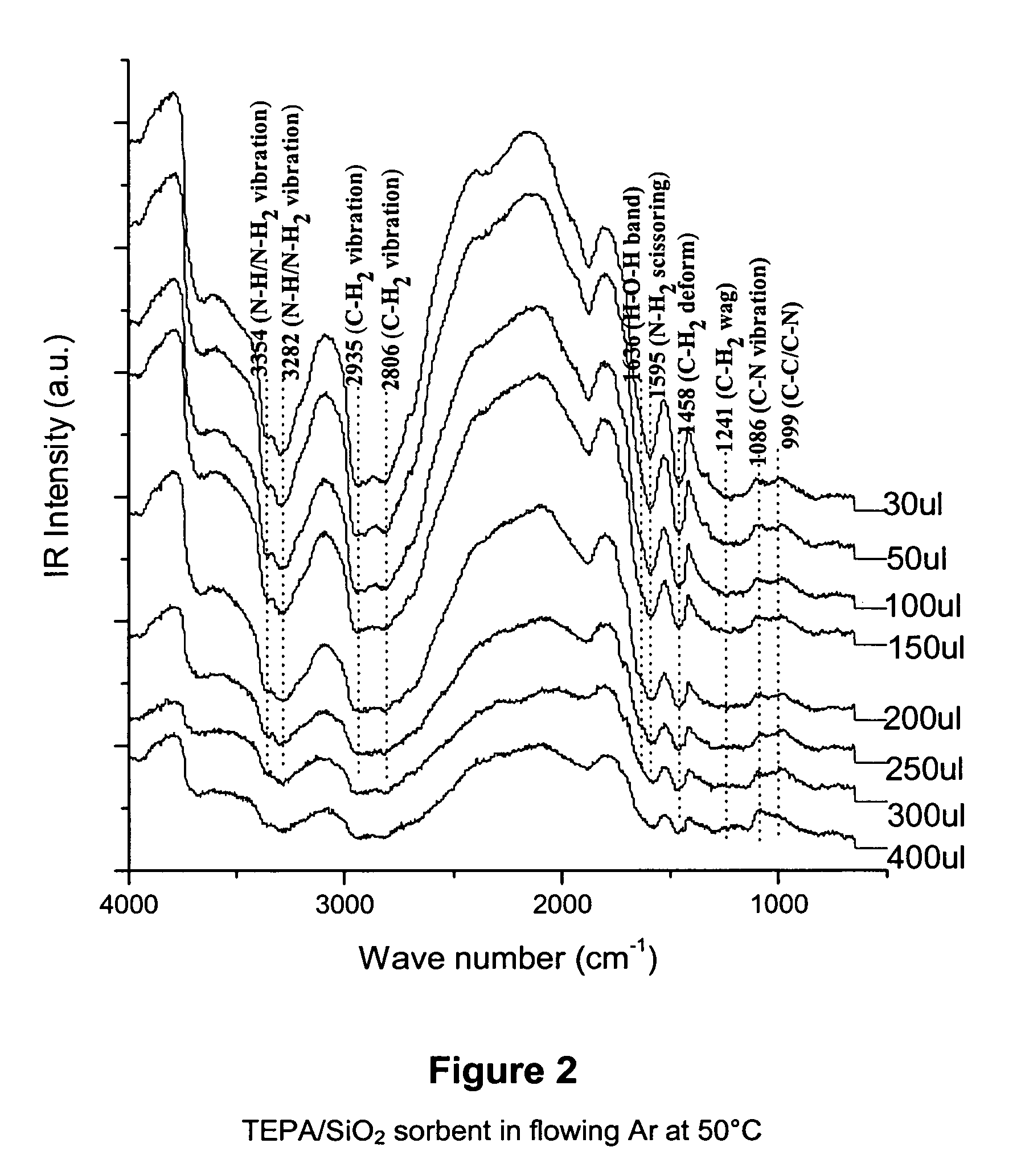
Solid sorbents for removal of carbon dioxide from gas streams at low temperatures
InactiveUS6908497B1Promote regenerationRegeneration process is inexpensiveGas treatmentOther chemical processesGas solidSorbent
New low-cost CO2 sorbents are provided that can be used in large-scale gas-solid processes. A new method is provided for making these sorbents that involves treating substrates with an amine and / or an ether so that the amine and / or ether comprise at least 50 wt. percent of the sorbent. The sorbent acts by capturing compounds contained in gaseous fluids via chemisorption and / or physisorption between the unit layers of the substrate's lattice where the polar amine liquids and solids and / or polar ether liquids and solids are located. The method eliminates the need for high surface area supports and polymeric materials for the preparation of CO2 capture systems, and provides sorbents with absorption capabilities that are independent of the sorbents' surface areas. The sorbents can be regenerated by heating at temperatures in excess of 35° C.
Owner:ENERGY U S DEPARMENT OF
Methods and devices for the production of Hydrocarbons from Carbon and Hydrogen sources
InactiveUS20080283411A1Reduce the environmentEasy to controlPhotography auxillary processesInternal combustion piston enginesElectrolysisAtmospheric air
Devices and methods are described for converting a carbon source and a hydrogen source into hydrocarbons, such as alcohols, for alternative energy sources. The influents may comprise carbon dioxide gas and hydrogen gas or water, obtainable from the atmosphere for through methods described herein, such as plasma generation or electrolysis. One method to produce hydrocarbons comprises the use of an electrolytic device, comprising an anode, a cathode and an electrolyte. Another method comprises the use of ultrasonic energy to drive the reaction. The devices and methods and related devices and methods are useful, for example, to provide a fossil fuel alternative energy source, store renewable energy, sequester carbon dioxide from the atmosphere, counteract global warming, and store carbon dioxide in a liquid fuel.
Owner:PRINCIPLE ENERGY SOLUTIONS
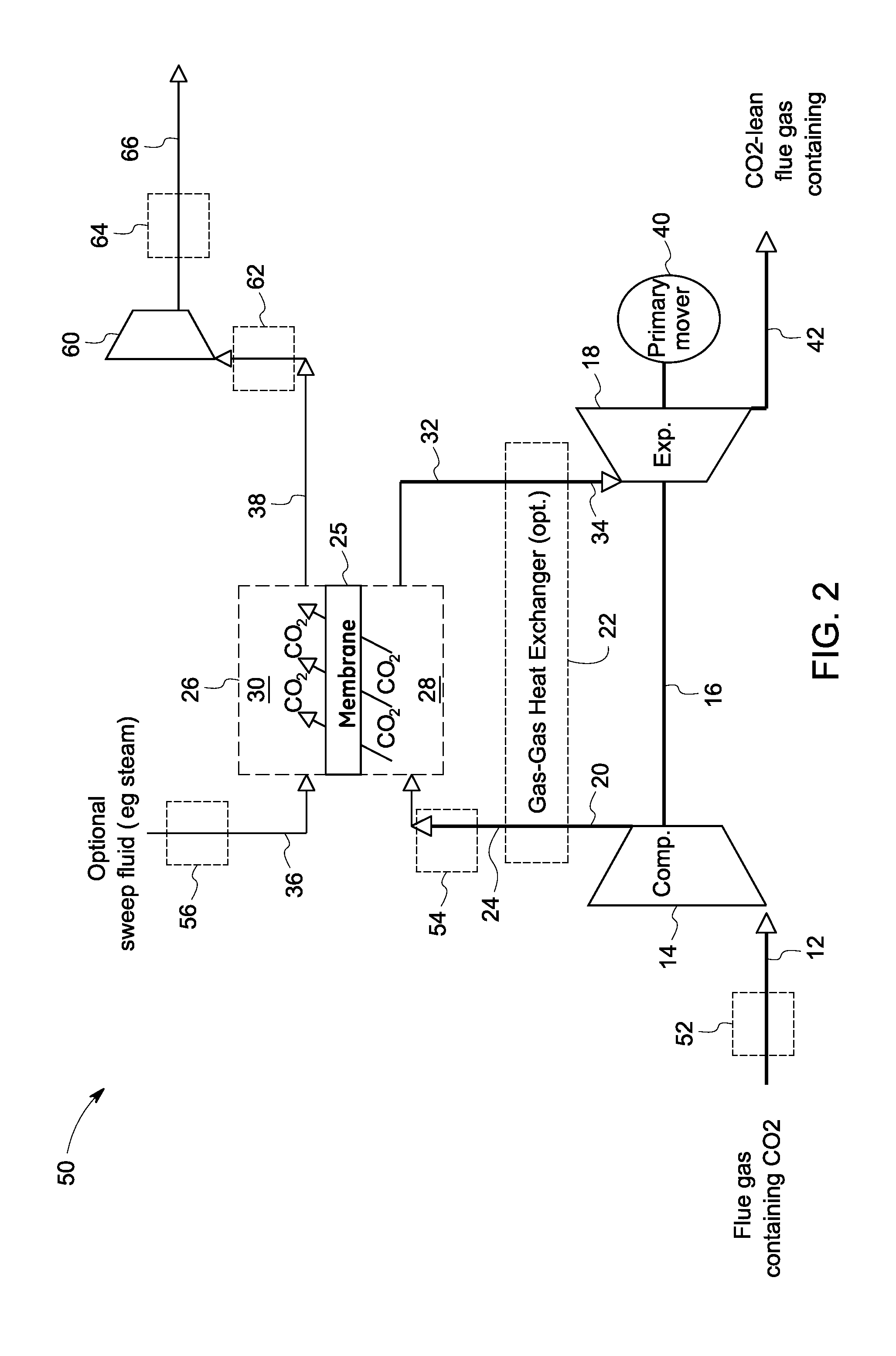
Carbon dioxide capture systems and methods
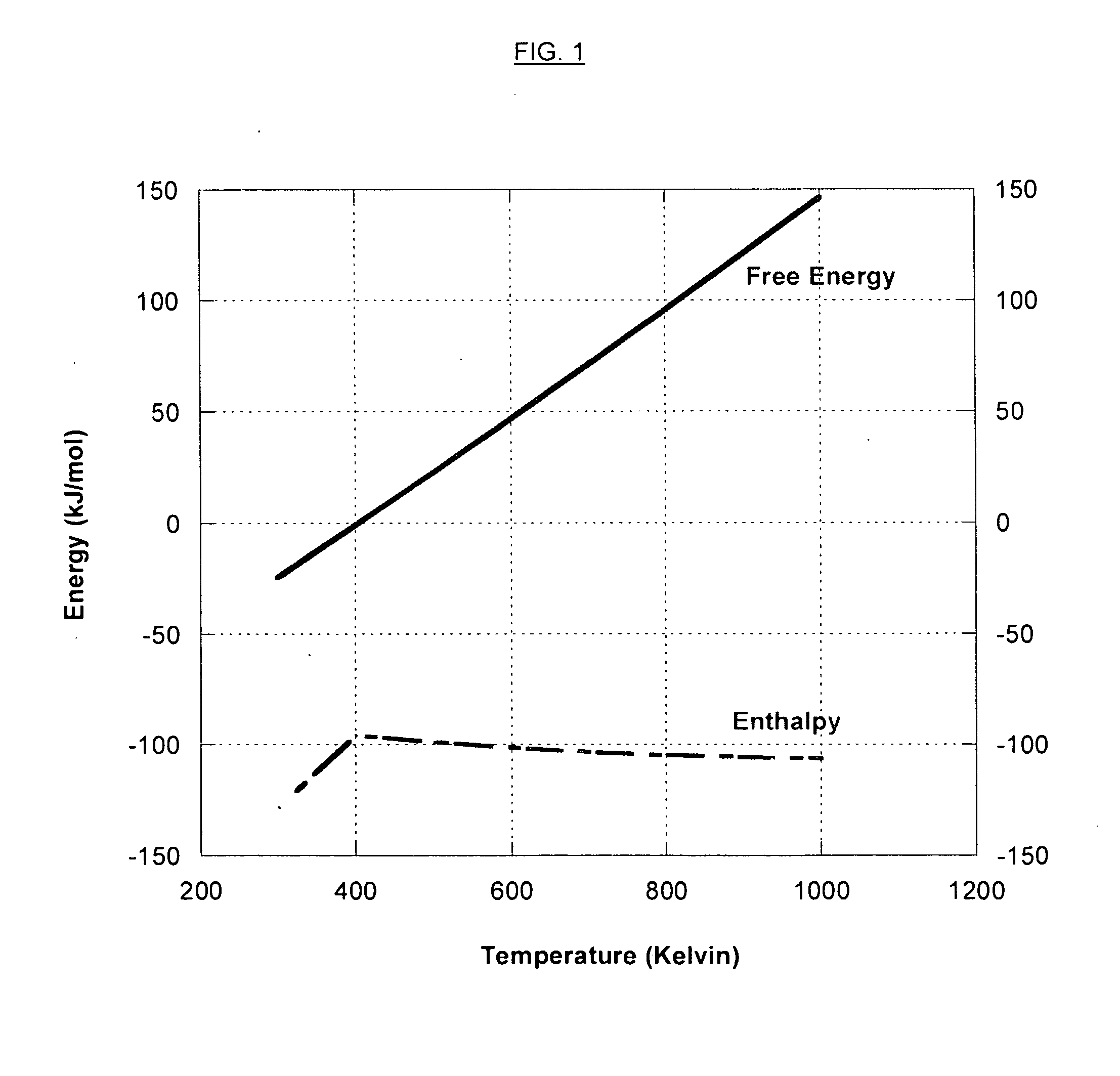
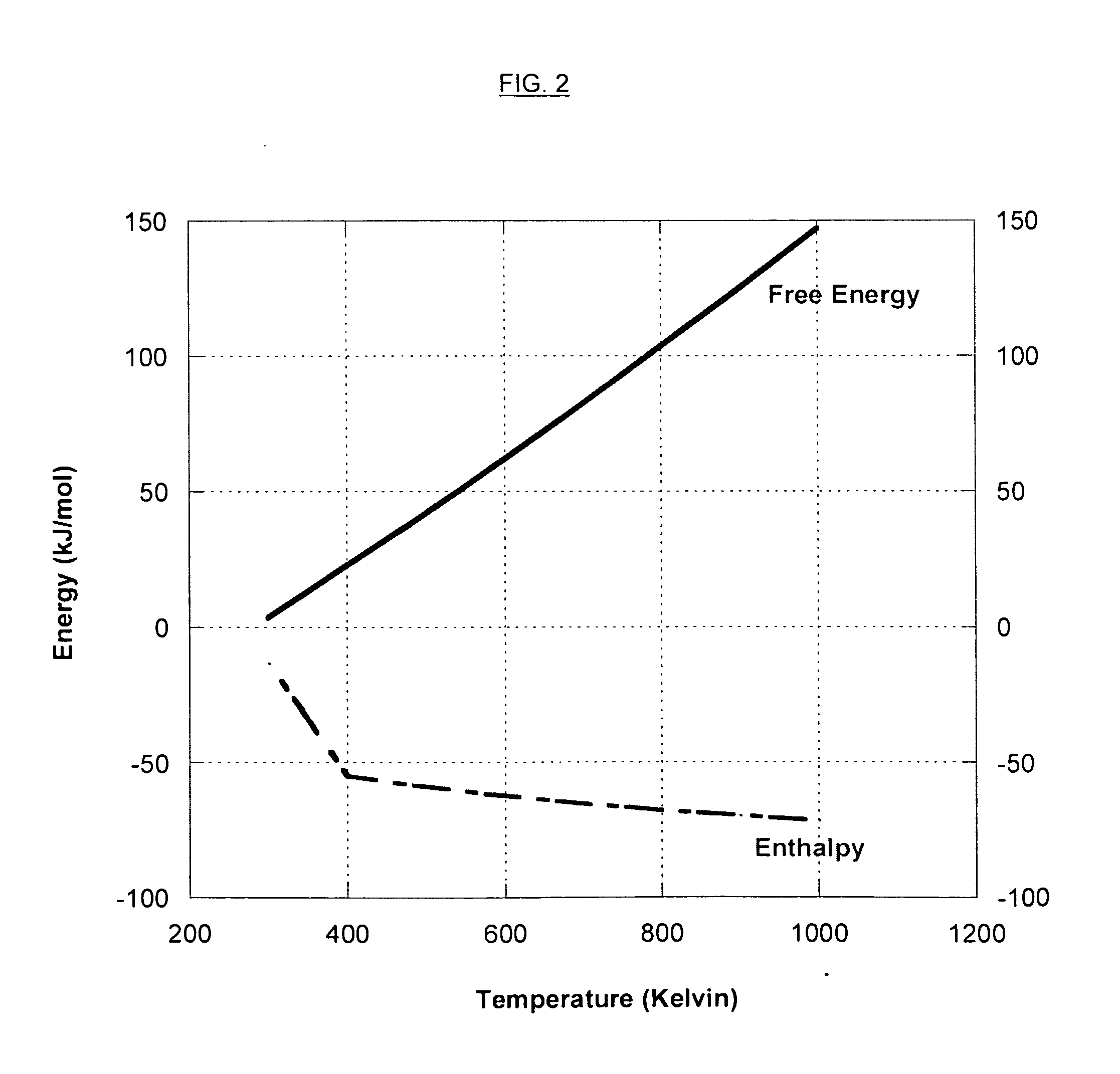
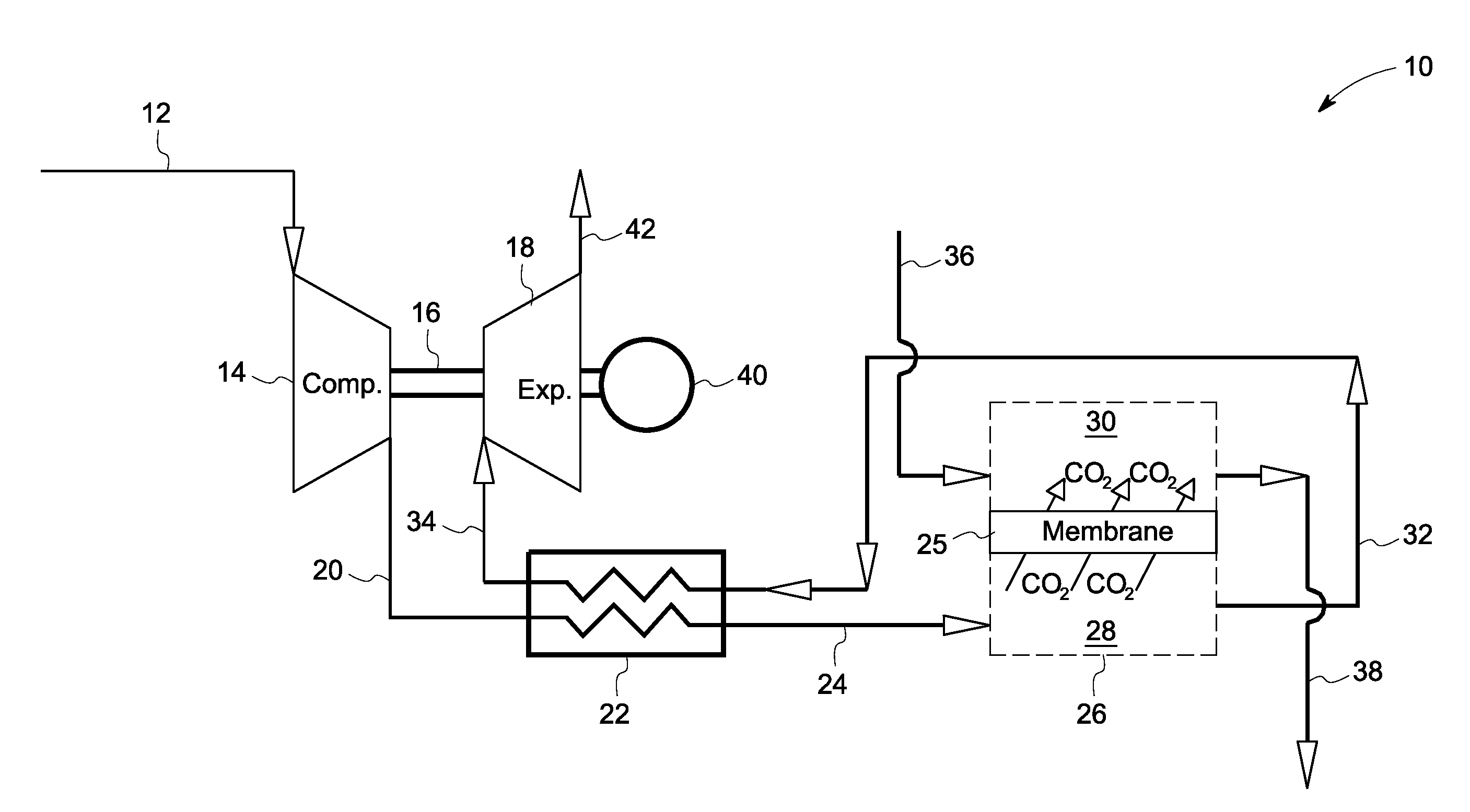
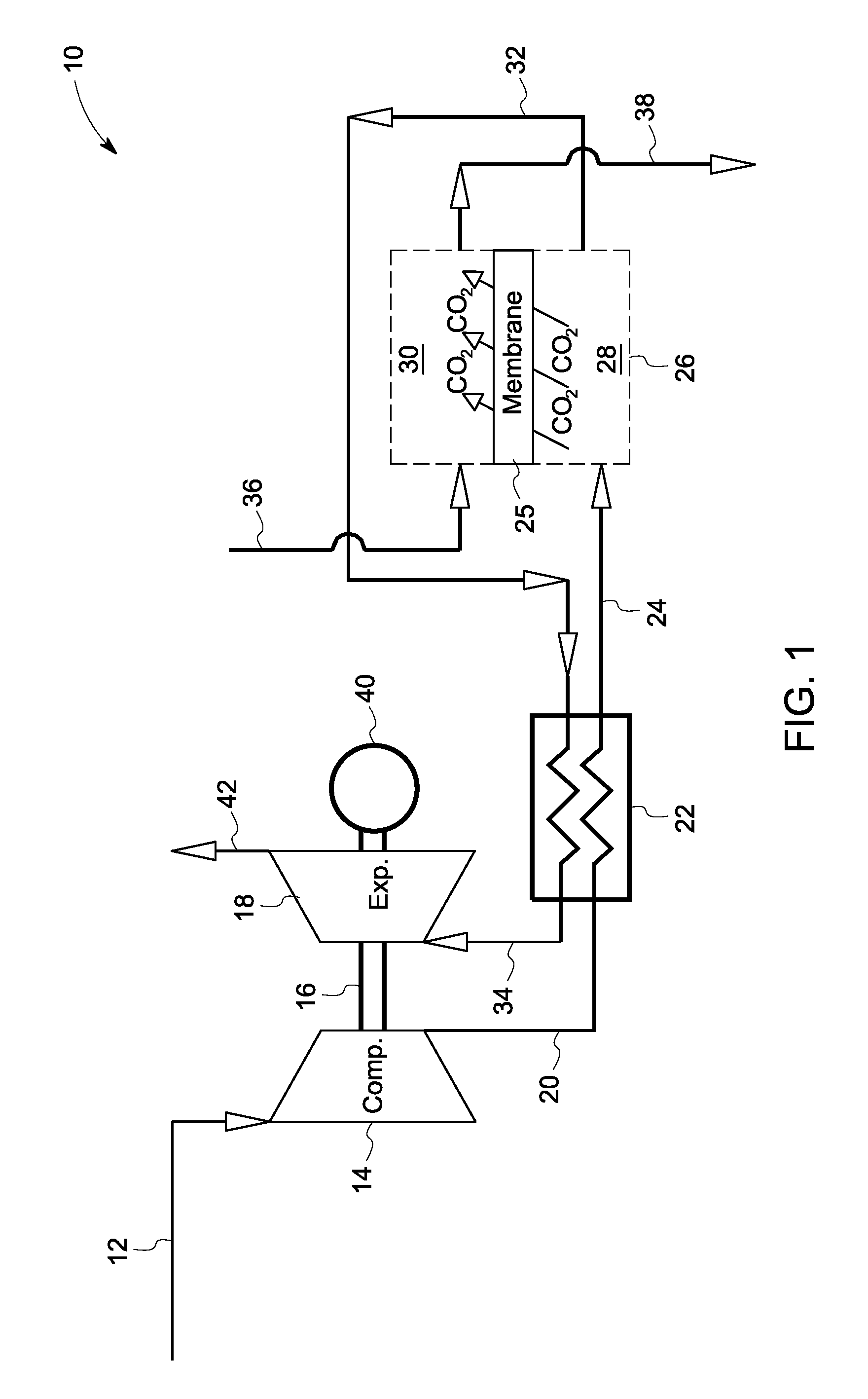
InactiveUS20080127632A1More cost-effectivelyEasy and cost-effectiveGas treatmentExhaust apparatusCarbon dioxide transportProcess engineering
A carbon dioxide separation system includes a compressor for receiving an exhaust gas comprising CO2 and generate a compressed exhaust gas and a separator configured to receive the compressed exhaust gas and generate a CO2 lean stream. The separator includes a first flow path for receiving the compressed exhaust gas, a second flow path for directing a sweep fluid therethrough, and a material with selective permeability of carbon dioxide for separating the first and the second flow paths and for promoting carbon dioxide transport therebetween. The system further includes an expander coupled to the compressor for receiving and expanding the CO2 lean stream to generate power and an expanded CO2 lean stream.
Owner:GENERAL ELECTRIC CO
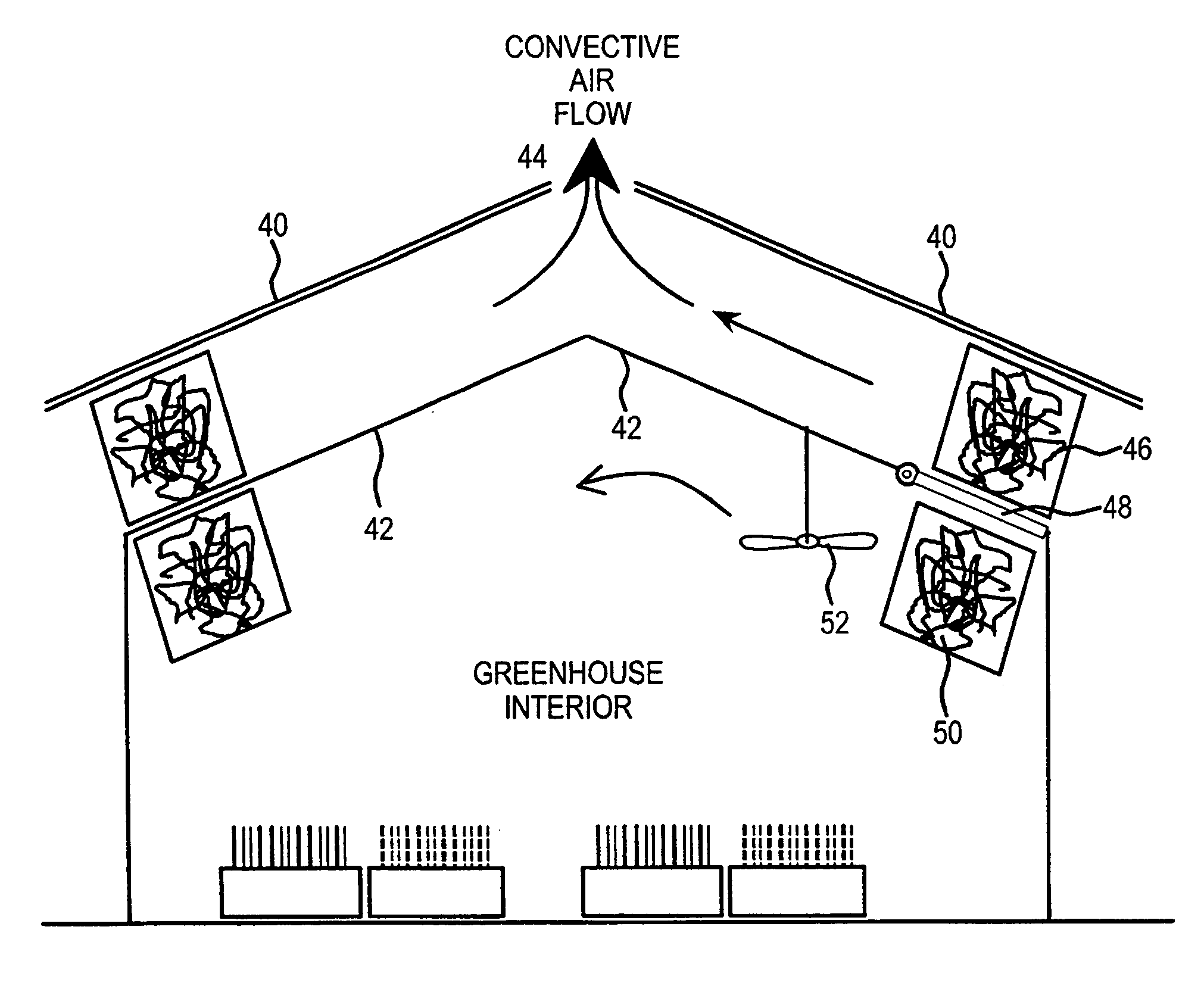
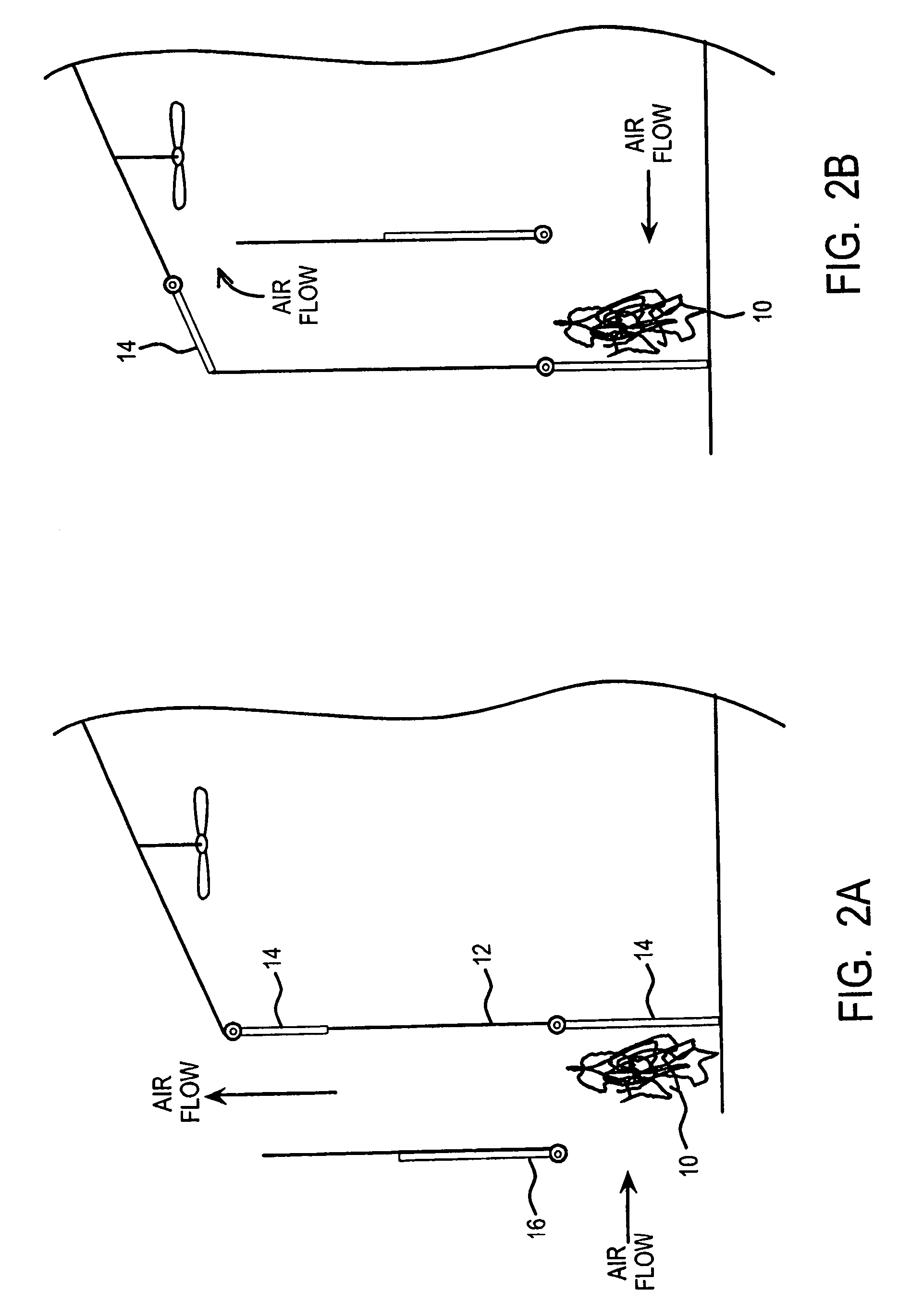
Method and apparatus for extracting carbon dioxide from air
A method and apparatus for extracting CO2 from air comprising an anion exchange material formed in a matrix exposed to a flow of the air, and for delivering that extracted CO2 to controlled environments. The present invention contemplates the extraction of CO2 from air using conventional extraction methods or by using one of the extraction methods disclosed; e.g., humidity swing or electro dialysis. The present invention also provides delivery of the CO2 to greenhouses where increased levels of CO2 will improve conditions for growth. Alternatively, the CO2 is fed to an algae culture.
Owner:CARBON SINK
Removal of CO2, N2, or H2S from gas mixtures by swing adsorption with low mesoporosity adsorbent contactors
The present invention relates to the separation of one or more of CO2, N2, and H2S gas components from a gas mixture containing at least a second gas using a swing adsorption process unit. The adsorbent contactors of the swing adsorption process unit are engineered structured adsorbent contactors having a plurality of flow channels wherein 20 volume percent or less of the open pore volume of the contactors is in the mesopore and macropore range.
Owner:EXXON RES & ENG CO
Process for sequestering carbon dioxide and sulfur dioxide
A process for sequestering carbon dioxide, which includes reacting a silicate based material with an acid to form a suspension, and combining the suspension with carbon dioxide to create active carbonation of the silicate-based material, and thereafter producing a metal salt, silica and regenerating the acid in the liquid phase of the suspension.
Owner:PENN STATE RES FOUND
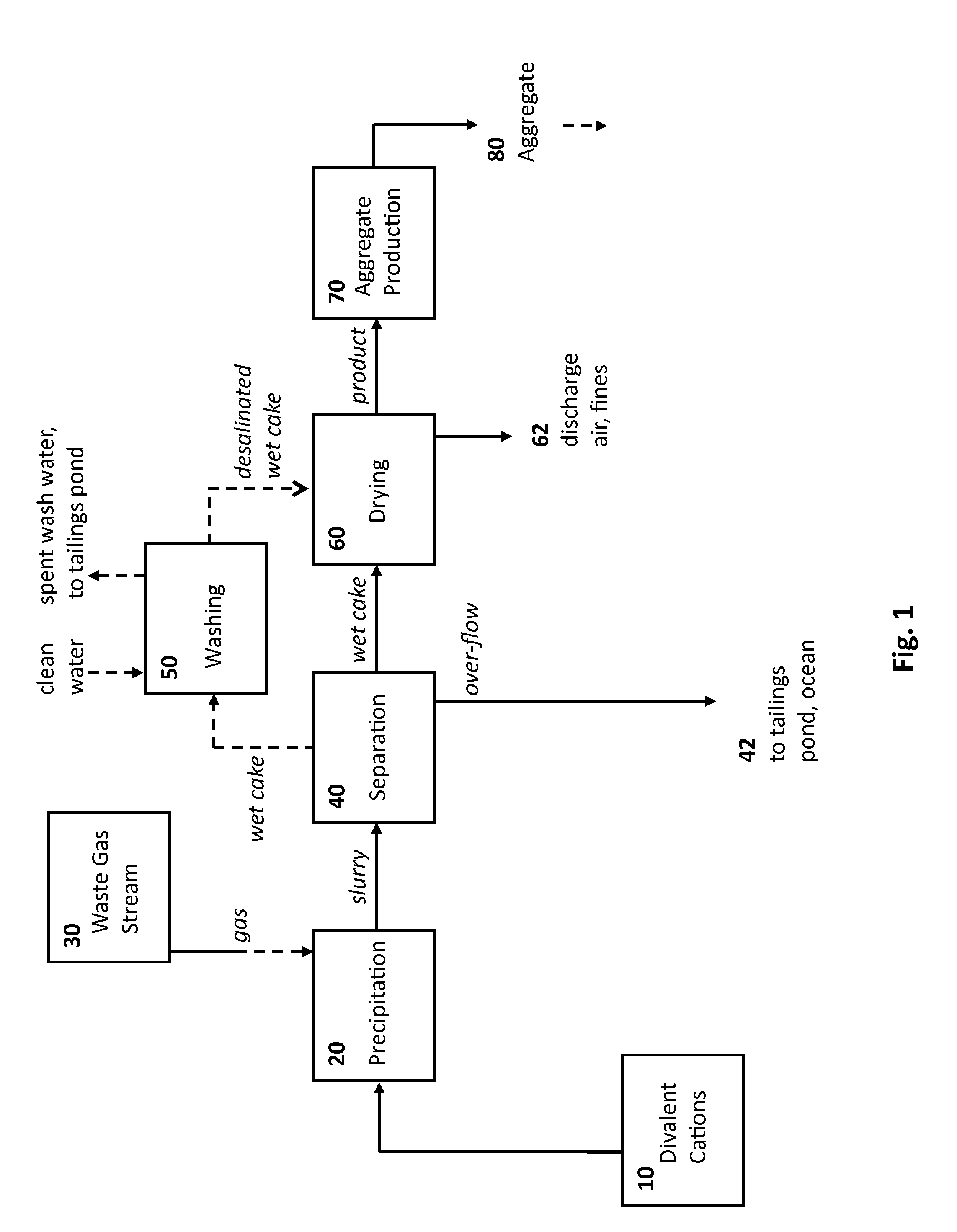
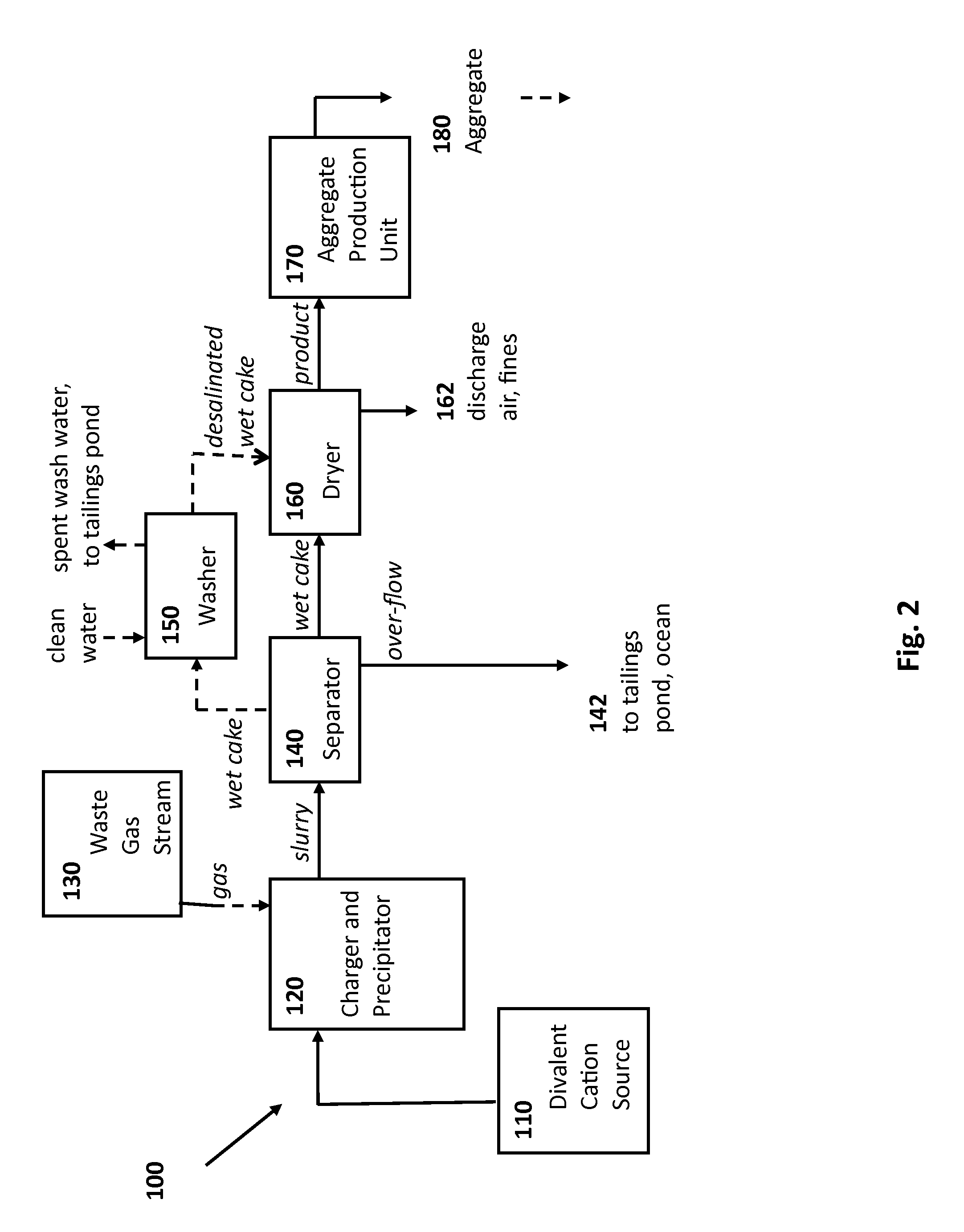
Rocks and aggregate, and methods of making and using the same
ActiveUS20100024686A1Calcium/strontium/barium carbonatesPigmenting treatmentCarbonateToxic industrial waste
Compositions comprising synthetic rock, e.g., aggregate, and methods of producing and using them are provided. The rock, e.g., aggregate, contains CO2 and / or other components of an industrial waste stream. The CO2 may be in the form of divalent cation carbonates, e.g., magnesium and calcium carbonates. Aspects of the invention include contacting a CO2 containing gaseous stream with a water to dissolve CO2, and placing the water under precipitation conditions sufficient to produce a carbonate containing precipitate product, e.g., a divalent cation carbonate.
Owner:ARELAC INC
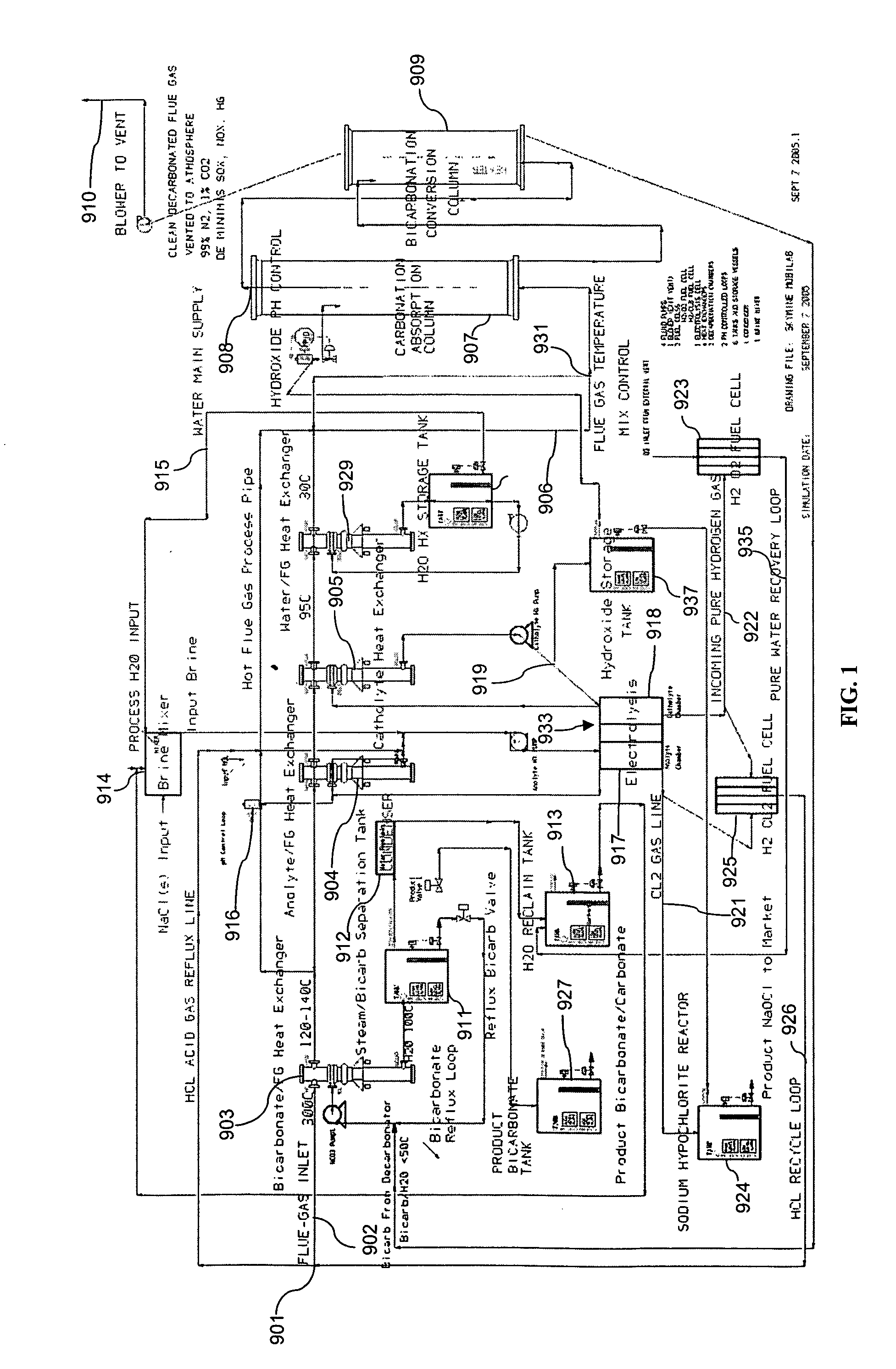
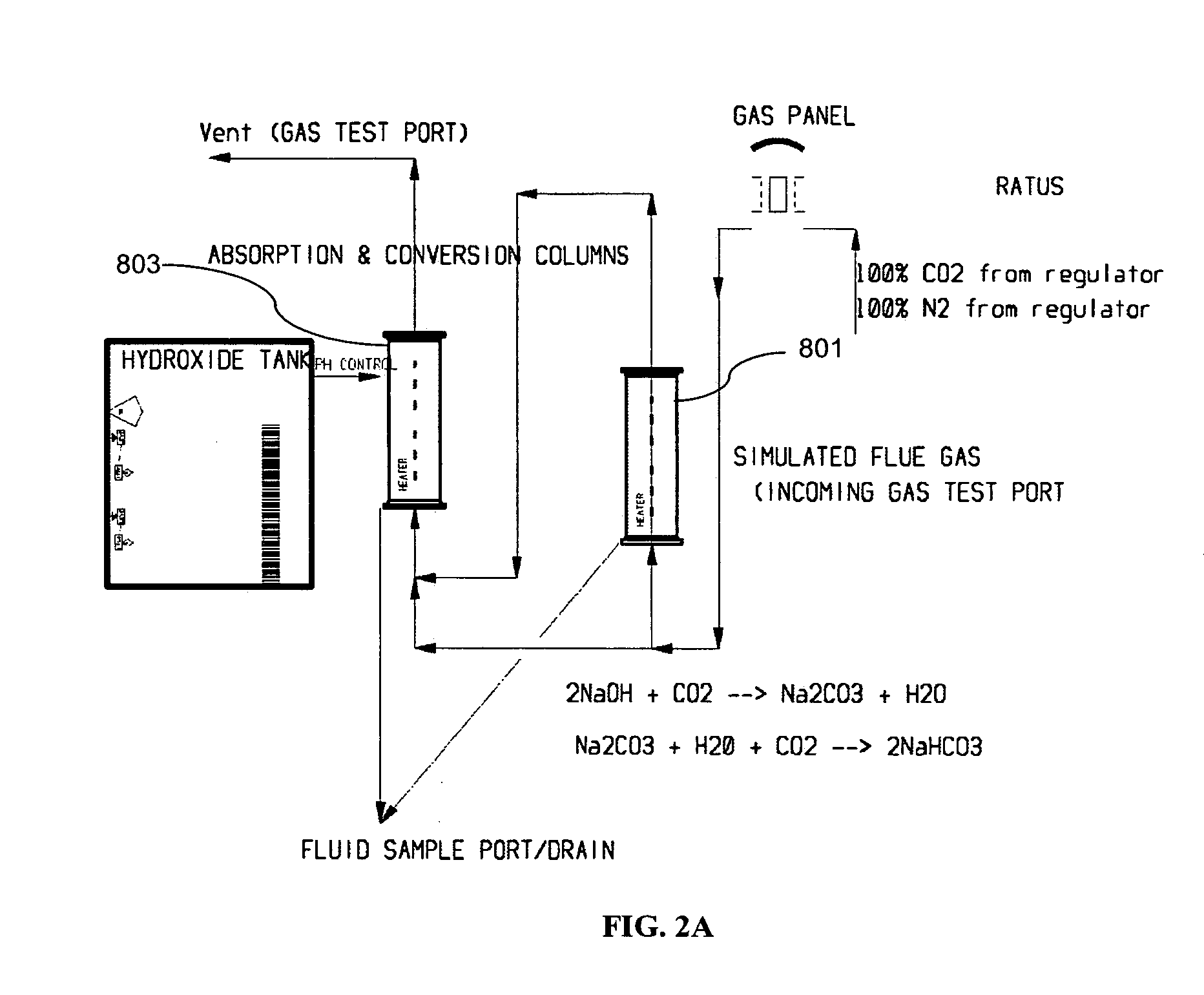
Removing carbon dioxide from waste streams through co-generation of carbonate and/or bicarbonate minerals
ActiveUS20060185985A1Improve ecologic efficiency of processEcologic efficiencyCalcium/strontium/barium carbonatesElectrolysis componentsElectrolysisWaste stream
Apparatuses and methods for removing carbon dioxide and other pollutants from a gas stream are provided. The methods include obtaining hydroxide in an aqueous mixture, and mixing the hydroxide with the gas stream to produce carbonate and / or bicarbonate. Some of the apparatuses of the present invention comprise an electrolysis chamber for providing hydroxide and mixing equipment for mixing the hydroxide with a gas stream including carbon dioxide to form an admixture including carbonate and / or bicarbonate.
Owner:CARBONFREE CHEM HLDG LLC
Reforming system for combined cycle plant with partial CO2 capture
A combined cycle system includes, a pre-steam-methane-reformer operating at a temperature of less than about 800 degrees Celsius to reform a mixed fuel stream to generate a first reformate stream, a water-gas-shift reactor to convert carbon monoxide in the first reformate stream to carbon dioxide and form a second reformate stream, a carbon dioxide removal unit for removing carbon dioxide from the second reformate stream and form a carbon dioxide stream and a third reformate stream; wherein less than about 50 percent of the carbon contained in the mixed fuel stream is recovered as carbon dioxide by the removal unit, a gas turbine unit for generating power and an exhaust stream, and a steam generator unit configured to receive the exhaust stream, wherein the heat of the exhaust stream is transferred to a water stream to generate the steam for the mixed fuel stream and for a steam turbine.
Owner:GENERAL ELECTRIC CO
Systems and methods for carbon capture and sequestration and compositions derived therefrom
ActiveUS20090143211A1Promote formationCalcium/strontium/barium carbonatesNitrogen compoundsInterior spaceProduct formation
A method of sequestering a greenhouse gas is described, which comprises: (i) providing a solution carrying a first reagent that is capable of reacting with a greenhouse gas; (ii) contacting the solution with a greenhouse gas under conditions that promote a reaction between the at least first reagent and the greenhouse gas to produce at least a first reactant; (iii) providing a porous matrix having interstitial spaces and comprising at least a second reactant; (iv) allowing a solution carrying the at least first reactant to infiltrate at least a substantial portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (v) allowing the at least first product to form and fill at least a portion of the interior spaces of the porous matrix, thereby sequestering a greenhouse gas.
Owner:RUTGERS THE STATE UNIV
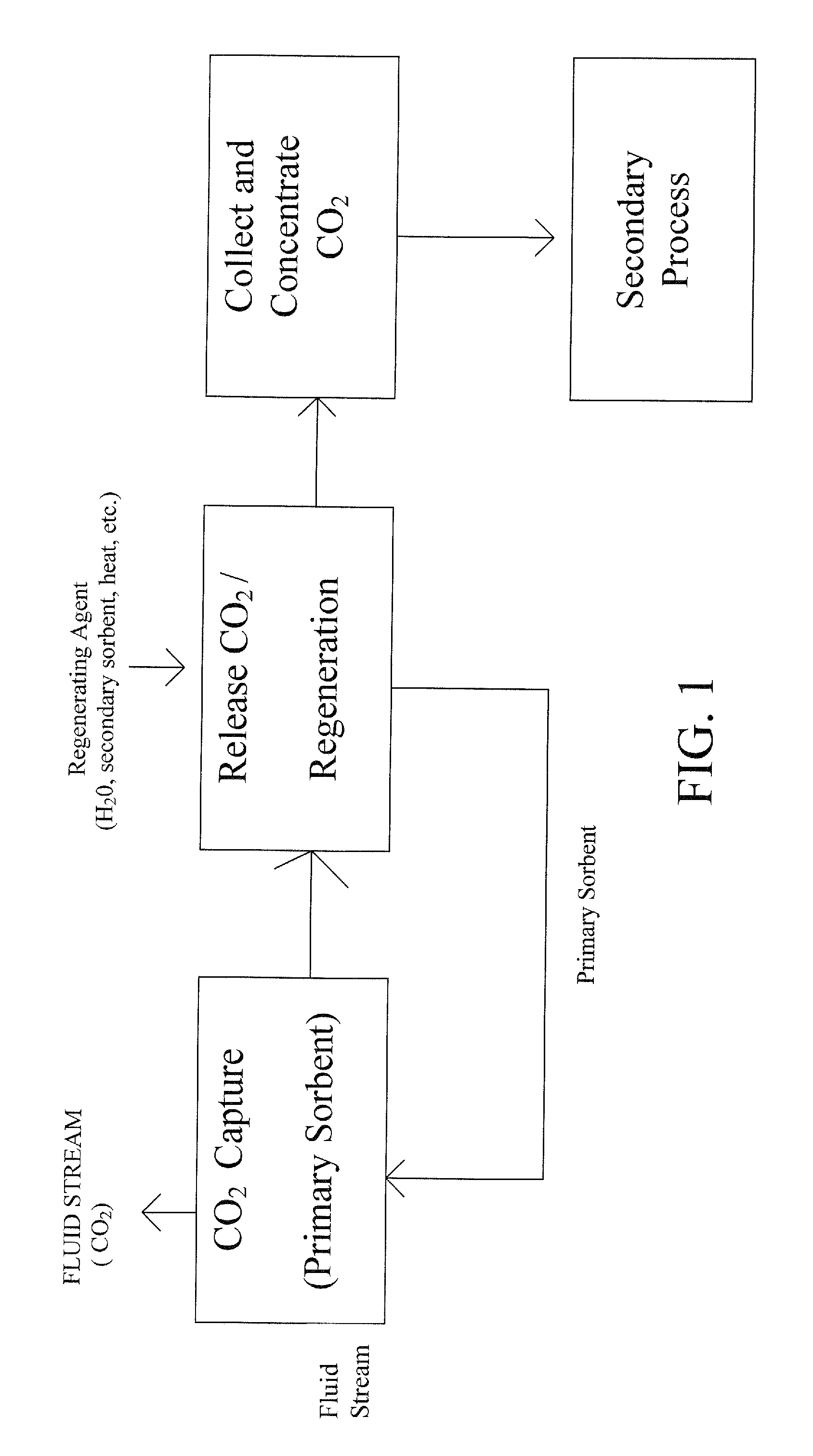
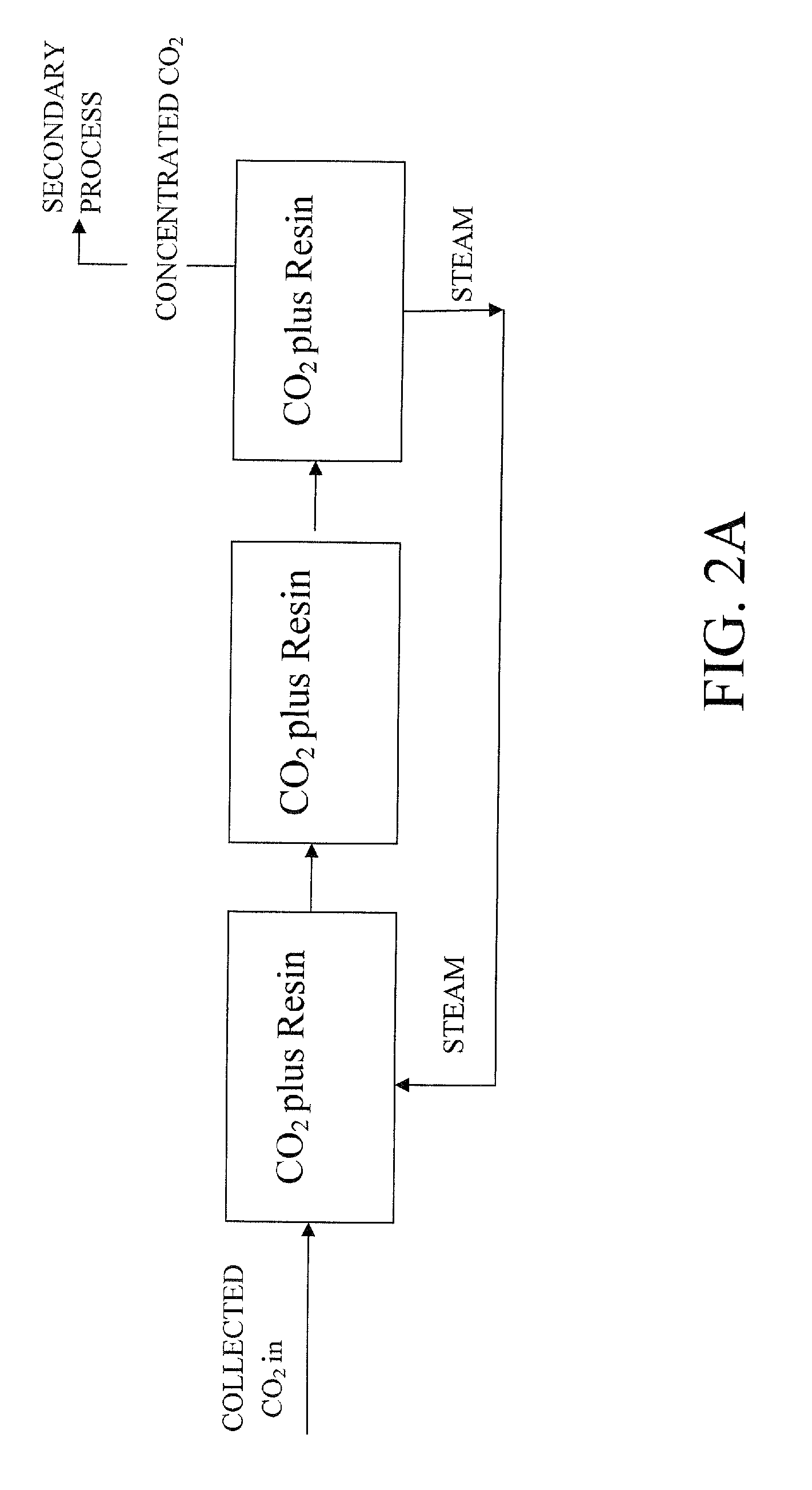
Extraction and sequestration of carbon dioxide
The present disclosure provides a method and apparatus for extracting carbon dioxide (CO2) from a fluid stream and for delivering that extracted CO2 to controlled environments for utilization by a secondary process. Various extraction and delivery methods are disclosed specific to certain secondary uses, included the attraction of CO2-sensitive insects, the ripening and preservation of produce, and the neutralization of brine.
Owner:KILIMANJARO ENERGY
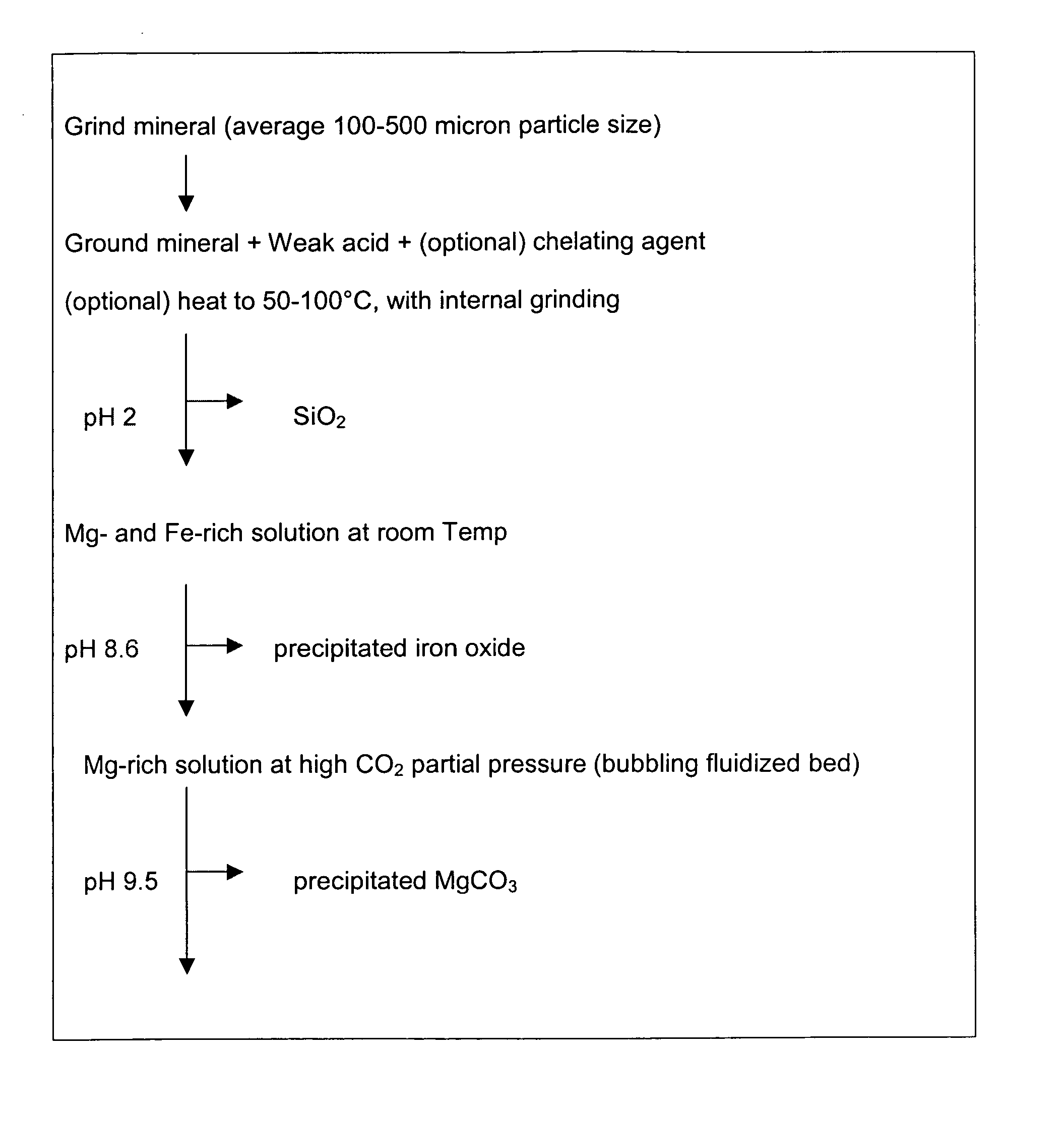
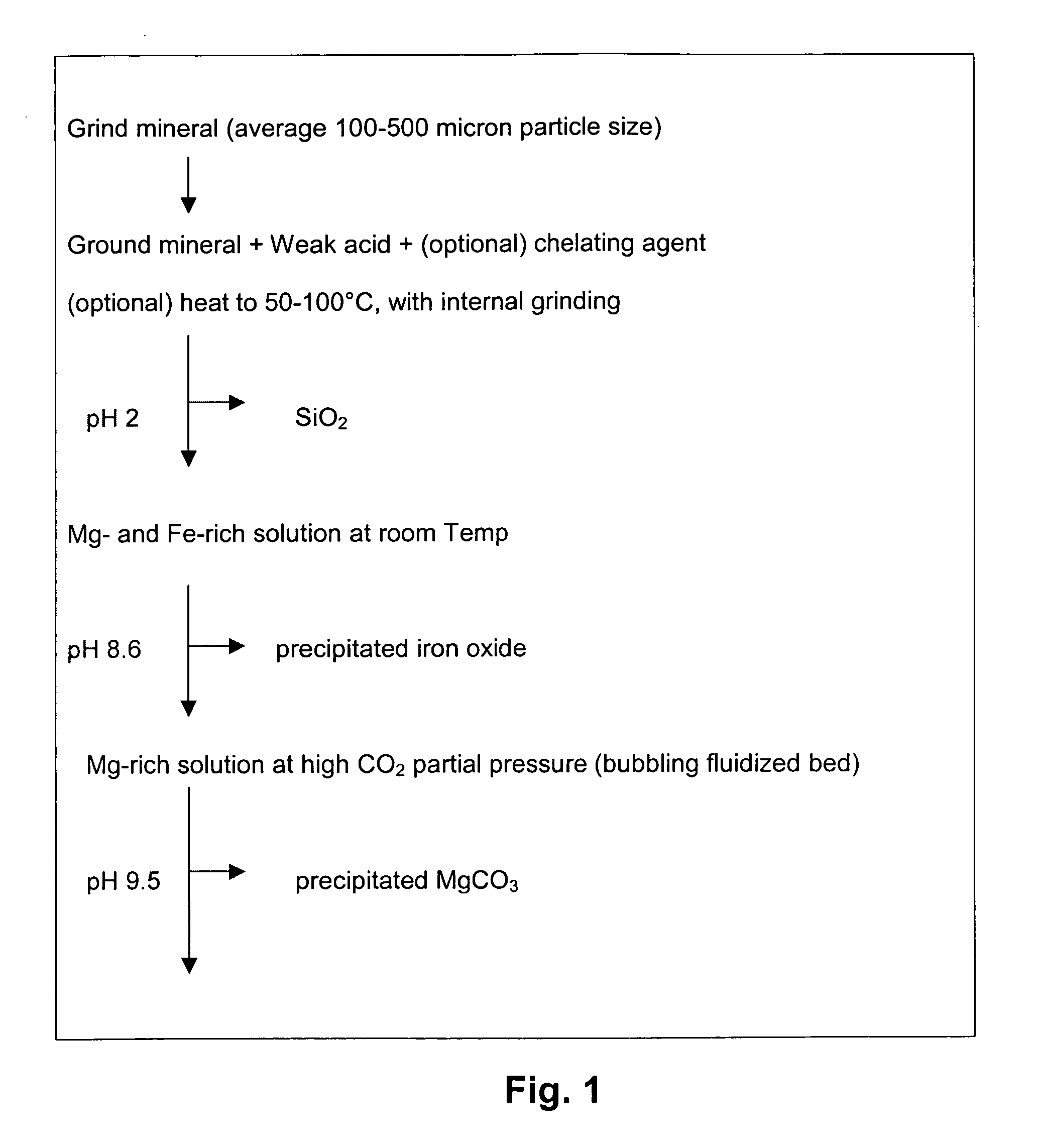
Carbon dioxide sequestration using alkaline earth metal-bearing minerals
ActiveUS20050180910A1High dissolution rateEfficient removalCalcium/strontium/barium carbonatesProductsParticulatesAlkaline earth metal
A method for mineral sequestration of pollutant gases resulting from the combustion of carbon-based fuels such as carbon and sulfur dioxides is provided and includes, providing a particulate magnesium-containing mineral and exposing the magnesium-containing mineral to a weak acid to dissolve magnesium from the mineral and form a magnesium-containing solution. The surface of the particulate magnesium-containing mineral is physically activated to expose and dissolve additional magnesium into the solution. Pollutant gases such as carbon dioxide are mixed with the magnesium-containing solution. When the pH of the magnesium-containing solution is increased, solid magnesium carbonate is formed.
Owner:THE OHIO STATES UNIV
Power plants that utilize gas turbines for power generation and processes for lowering co2 emissions
ActiveUS20080104958A1Reduce carbon dioxide emissionsEmission reductionGas treatmentInternal combustion piston enginesPower stationWorking fluid
Power plants and process for lowering CO2 emissions generally includes extracting a portion of the recirculated CO2-rich flue gas mid-way through the compression pathway of a gas turbine and removing the CO2 in a separation unit. The remaining portion of the CO2 rich flue gas (i.e., the portion of the recirculated flue gas that was not fed to the separation unit) is mixed with fresh air coming from an additional compressor-expander and then fed back to the compression pathway. As a result, flue gas recirculation increases the CO2 concentration within the working fluid, leading to an additional increase in CO2 partial pressure. As the concentration and partial pressure of CO2 is increased, a lower energy penalty is observed to remove the CO2. Moreover, a reduced volume is fed to the CO2 separation unit during operation. Consequently, the size of the separation equipment can be reduced as well as the energy required for the separation process.
Owner:GENERAL ELECTRIC CO +1
Photobioreactor systems and methods for treating CO2-enriched gas and producing biomass
InactiveUS20080178739A1Facilitate evaporative coolingSupport growthBioreactor/fermenter combinationsBiological substance pretreatmentsLiquid mediumStream flow
Certain embodiments and aspects of the present invention relate to a photobioreactor including covered photobioreactor units through which a liquid medium stream and a gas stream flow. The liquid medium comprises at least one species of phototrophic organism therein. Certain methods of using the photobioreactor system as part of fuel generation system and / or a gas-treatment process or system at least partially remove certain undesirable pollutants from a gas stream. In certain embodiments, a portion of the liquid medium is diverted from a photobioreactor unit and reintroduced upstream of the diversion position. In certain embodiments, the disclosed photobioreactor system, methods of using such systems, and / or gas treatment apparatus and methods provided herein can be used as part of an integrated combustion method and system, wherein photosynthetic organisms used within the photobioreactor are harvested from the photobioreactor, processed, and used as a fuel source for a combustion system such as an electric power plant.
Owner:THE TRON GRP
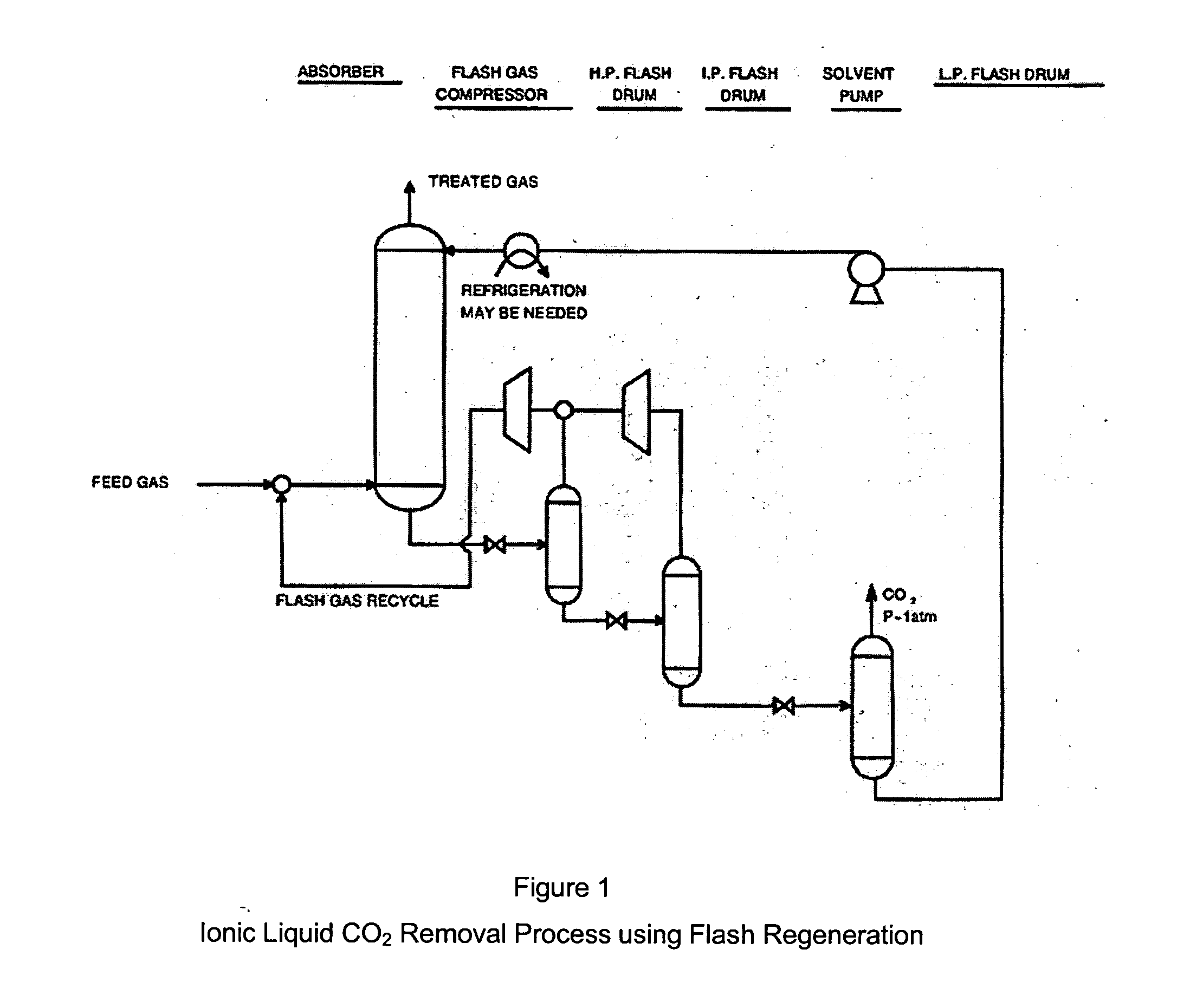
CO2 removal from gas using ionic liquid absorbents
InactiveUS20050129598A1High CO capacityLow hydrocarbon solubilityHydrogen sulfidesDispersed particle separationCo2 removalSorbent
A process and method for separating CO2 from a gaseous stream such as natural gas. An ionic liquid comprising an anion having a carboxylate function is used as an adsorbent to selectively complex the CO2 yielding a gaseous stream with a greatly reduced CO2 content. The ionic liquid can then be readily be regenerated and recycled.
Owner:CHEVROU USA INC
Separation of carbon dioxide (CO2) from gas mixtures
ActiveUS7618606B2Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentCo2 removalSorbent
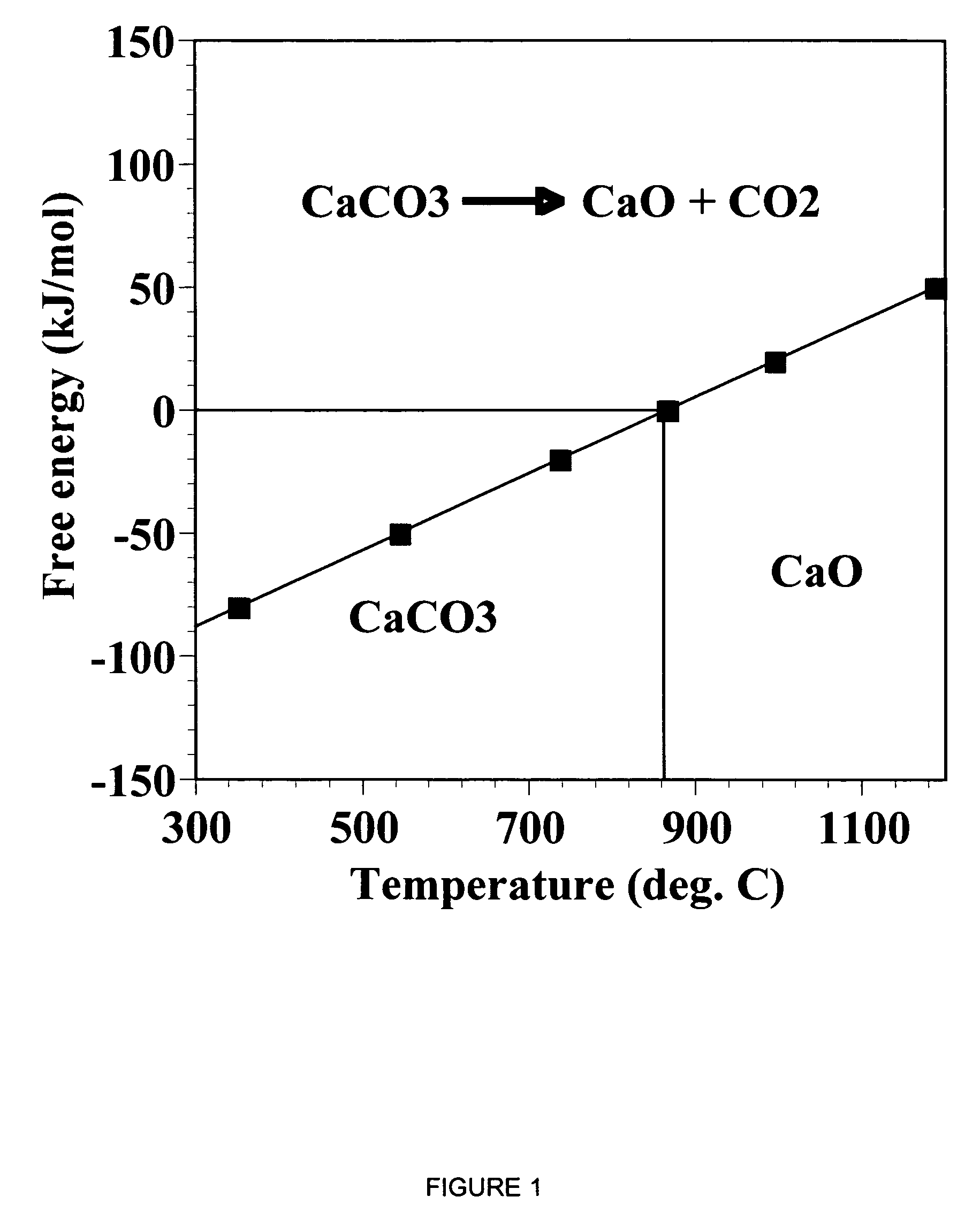
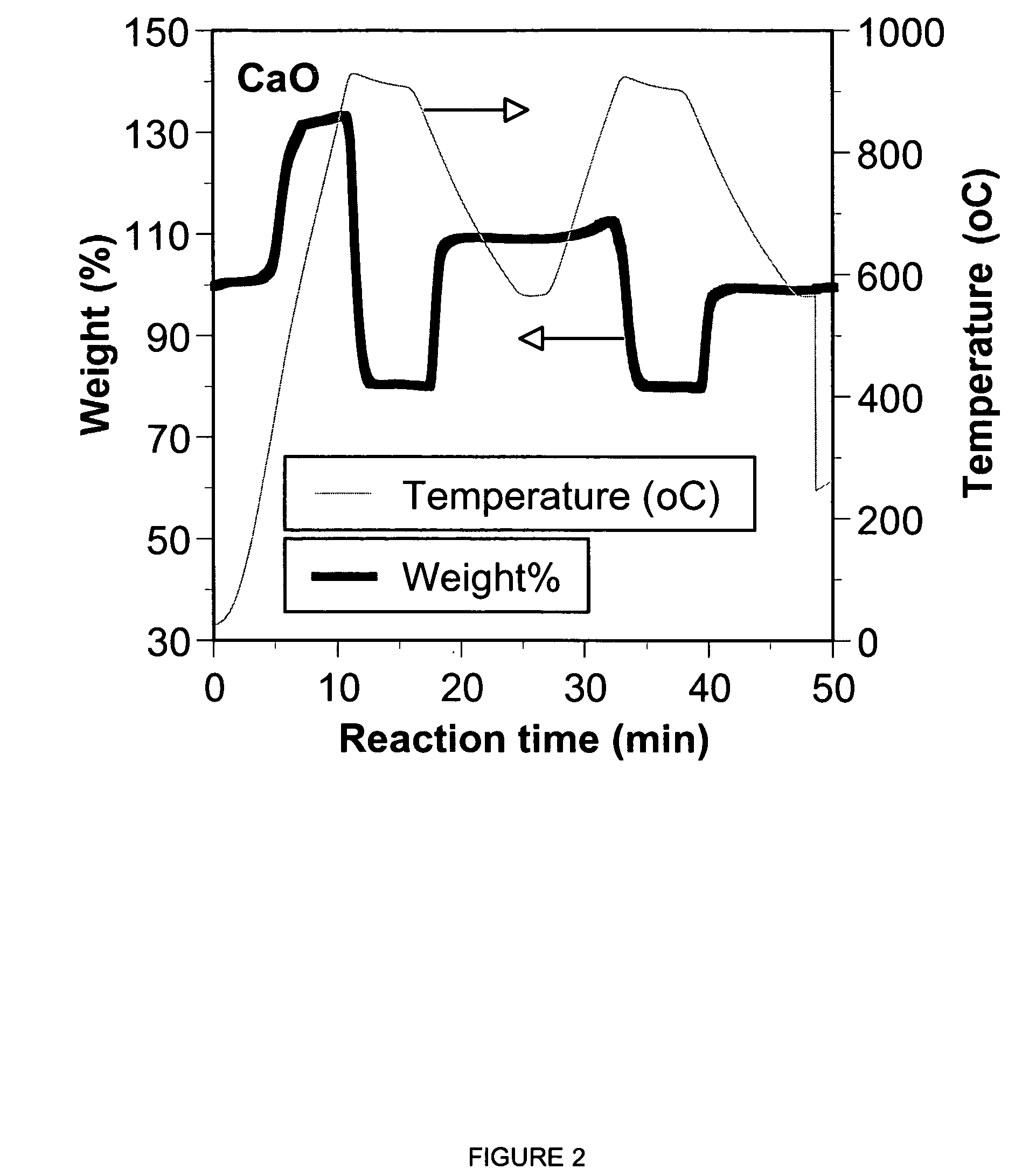
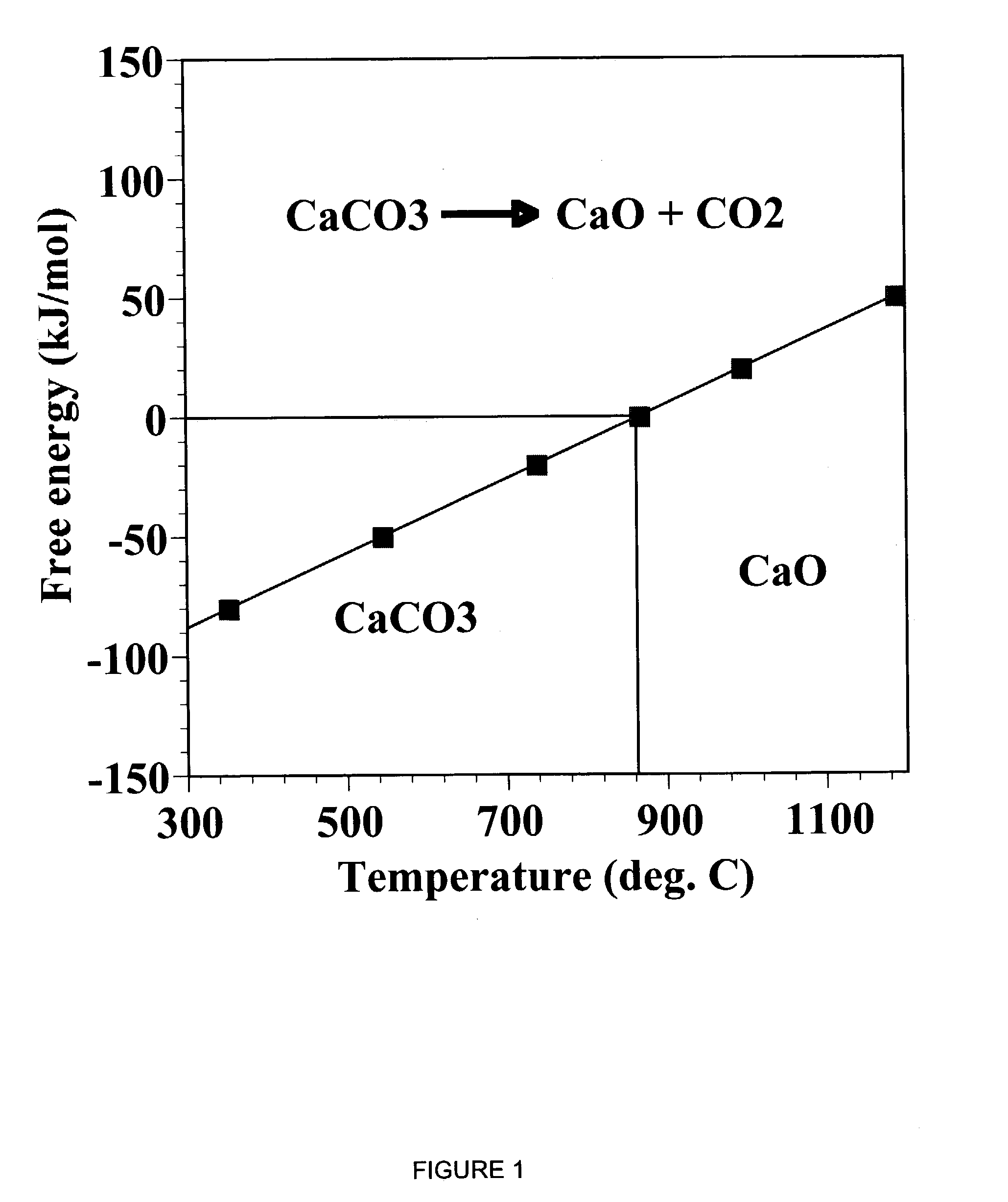
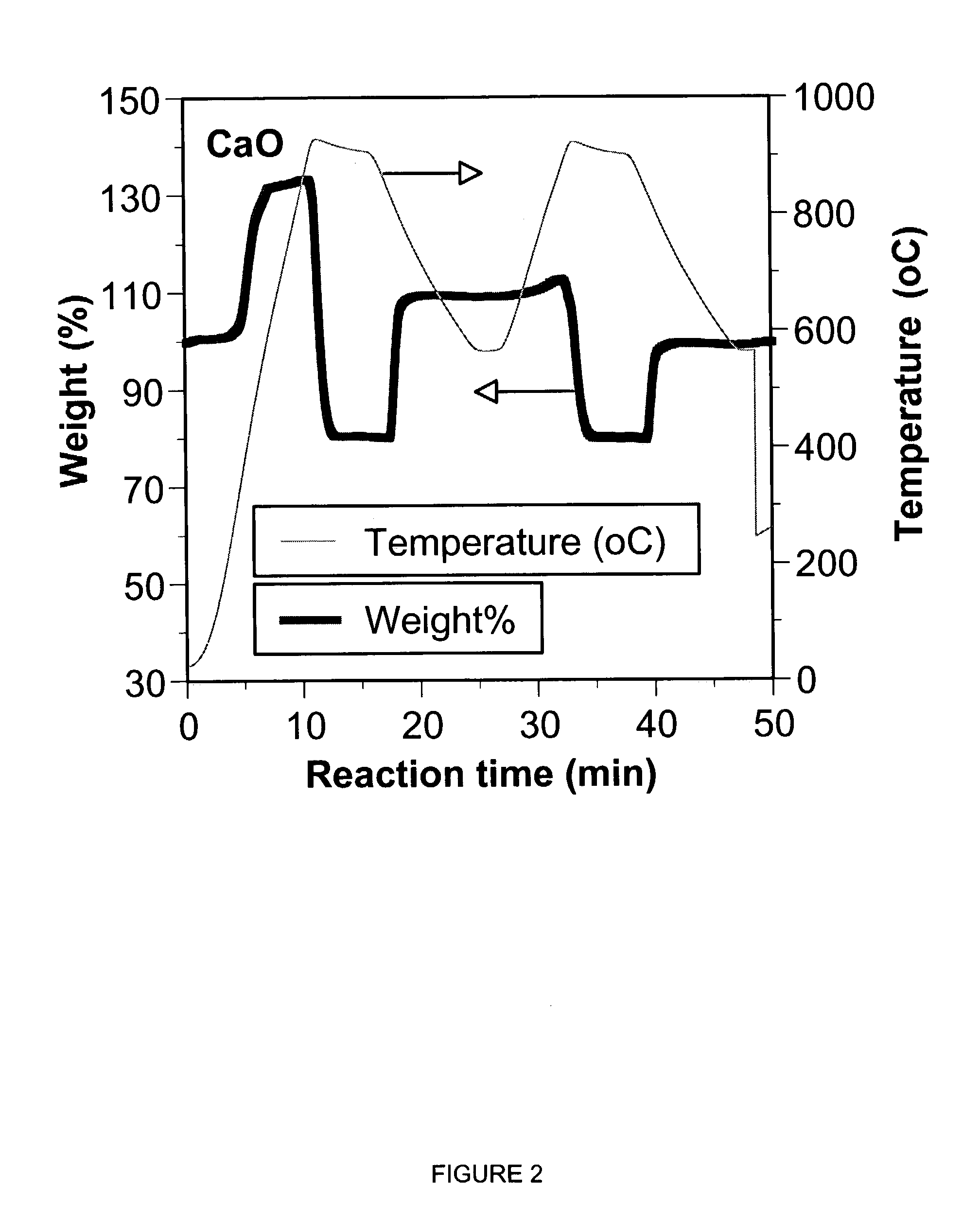
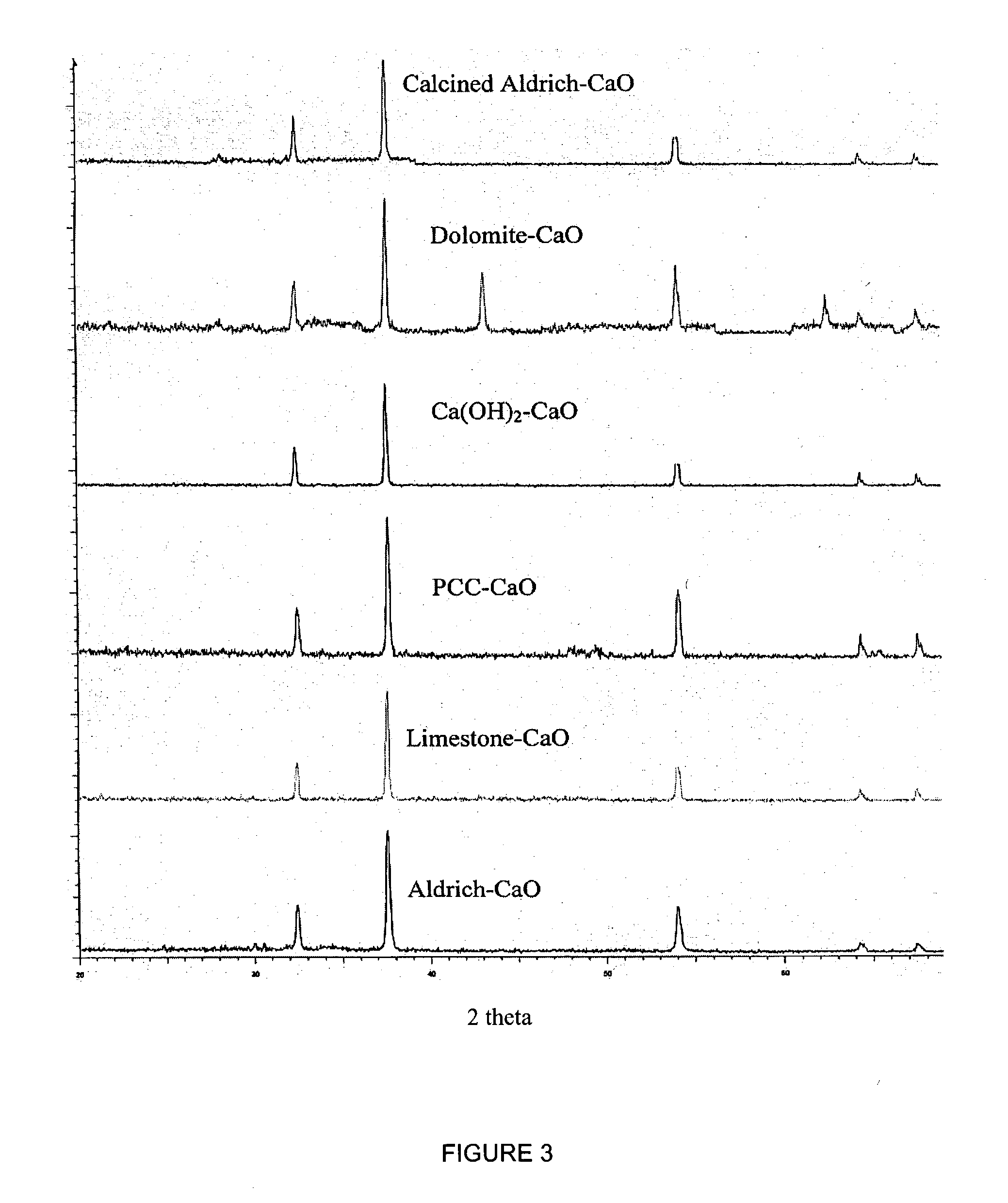
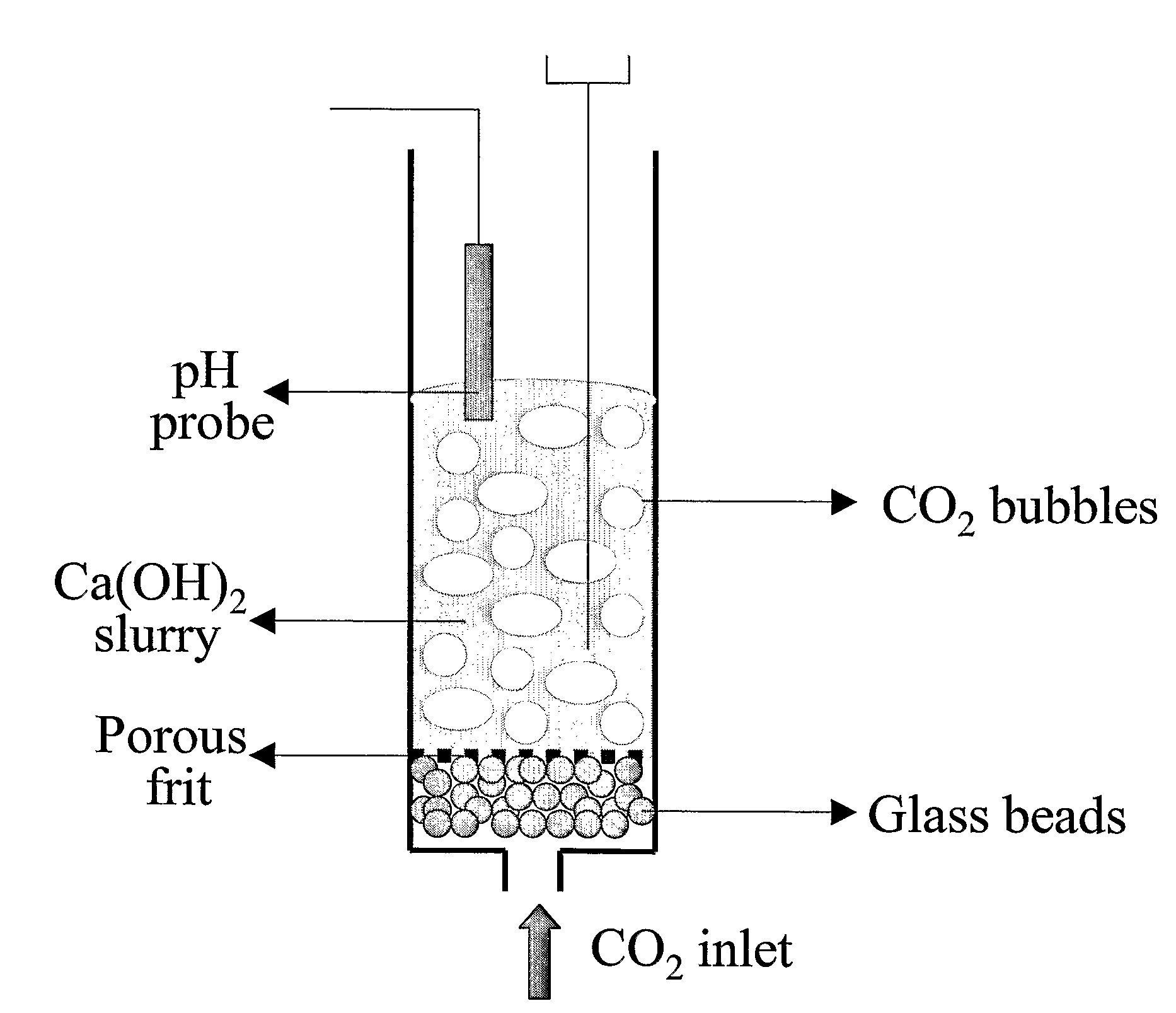
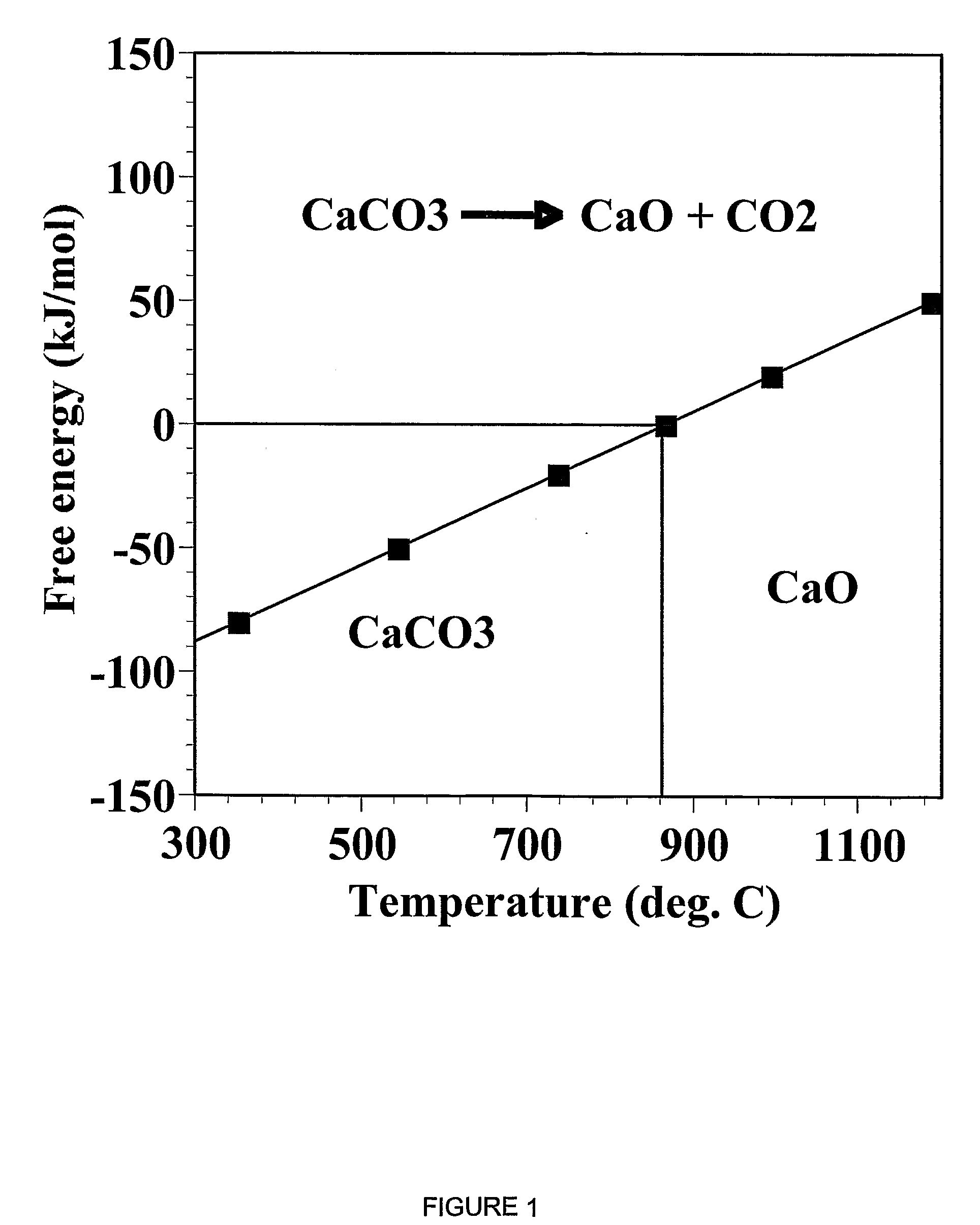
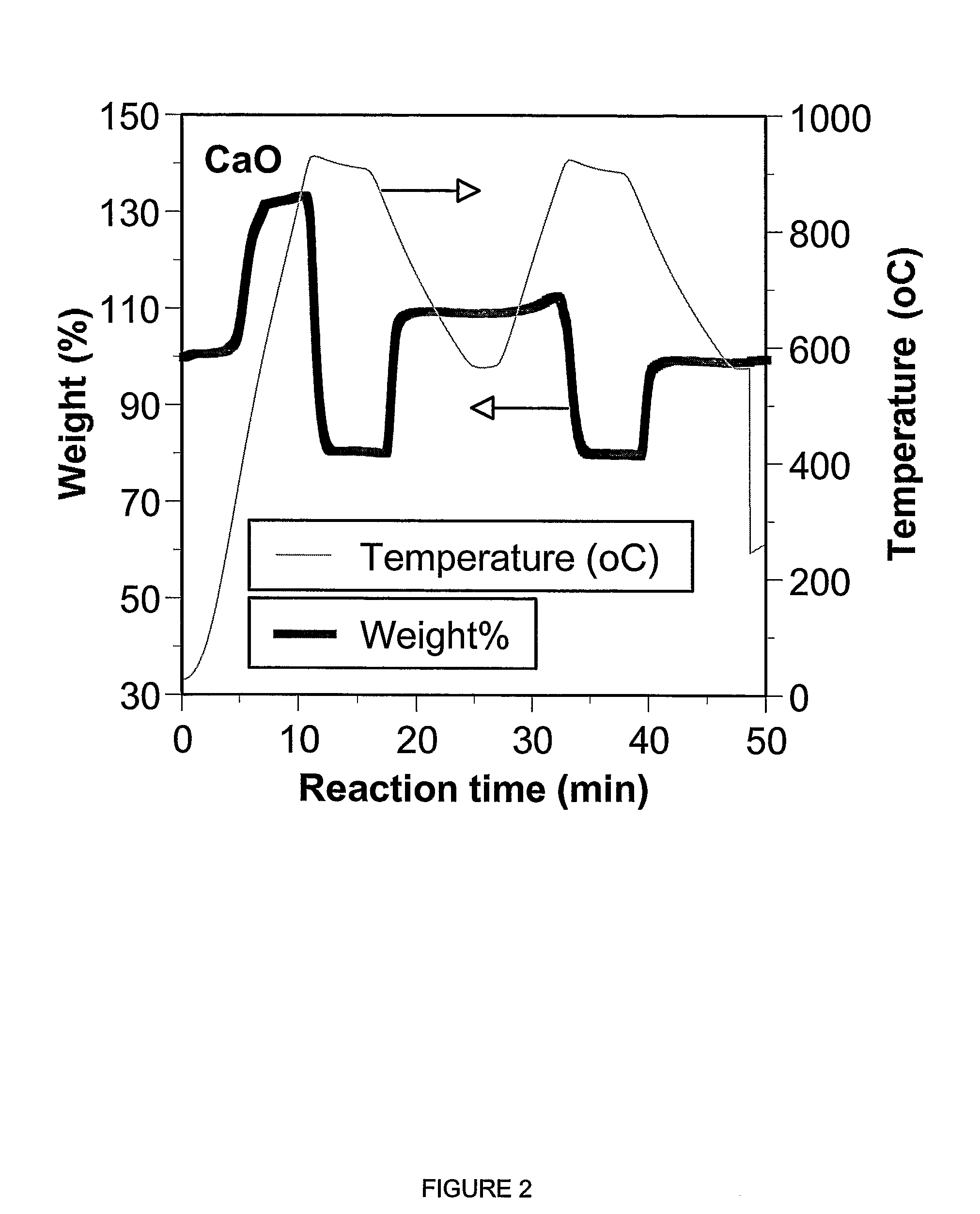
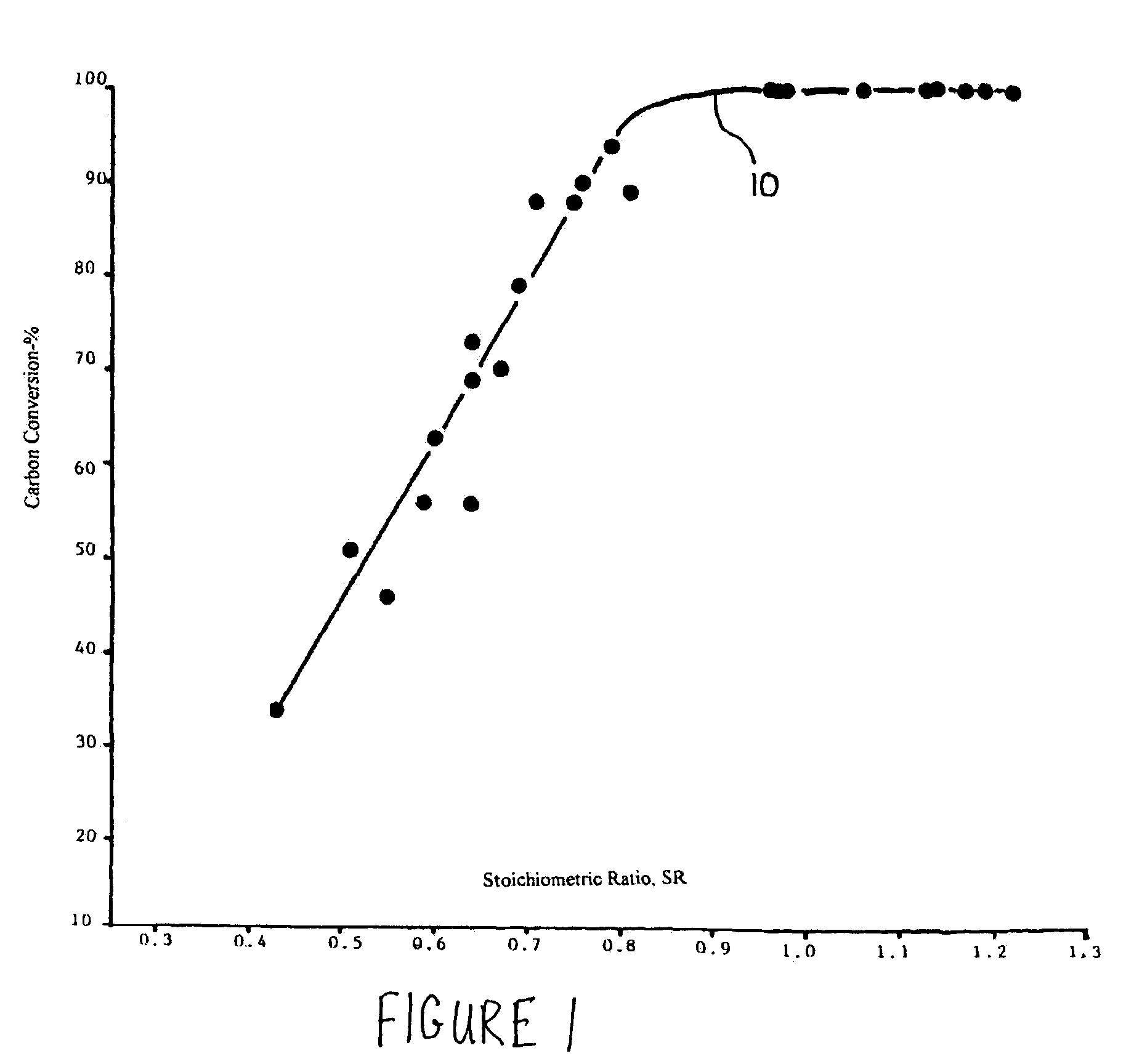
A reaction-based process has been developed for the selective removal of carbon dioxide from a multicomponent gas mixture. The proposed process effects the separation of CO2 from a mixture of gases by its reaction with metal oxides. The Calcium based Reaction Separation for CO2 process consists of contacting a CO2 laden gas with calcium oxide in a reactor such that CaO captures the CO2 by the formation of calcium carbonate. Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent. The “regenerated” CaO is then recycled for the further capture of more CO2. This process also identifies the application of a mesoporous CaCO3 structure, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
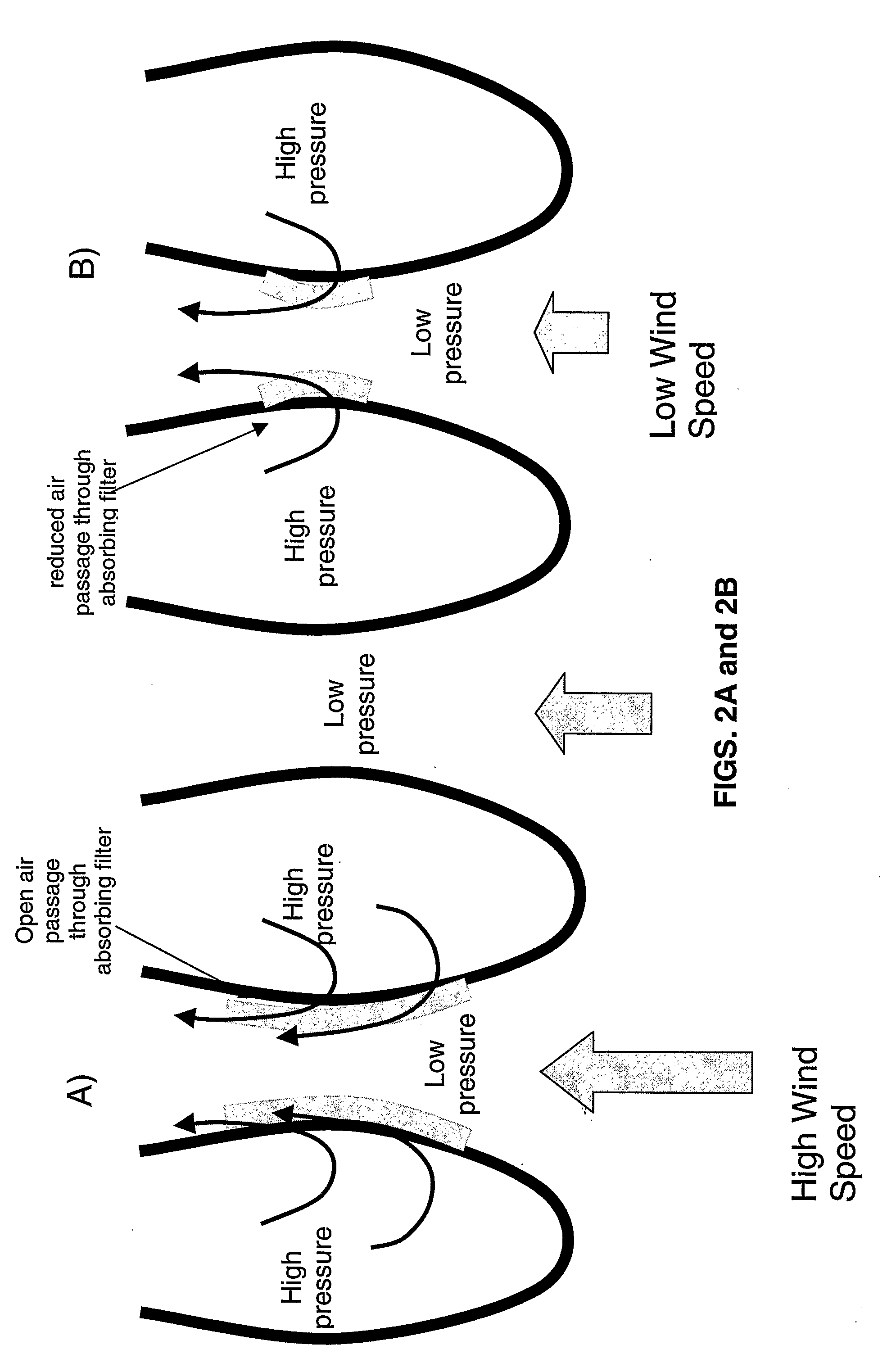
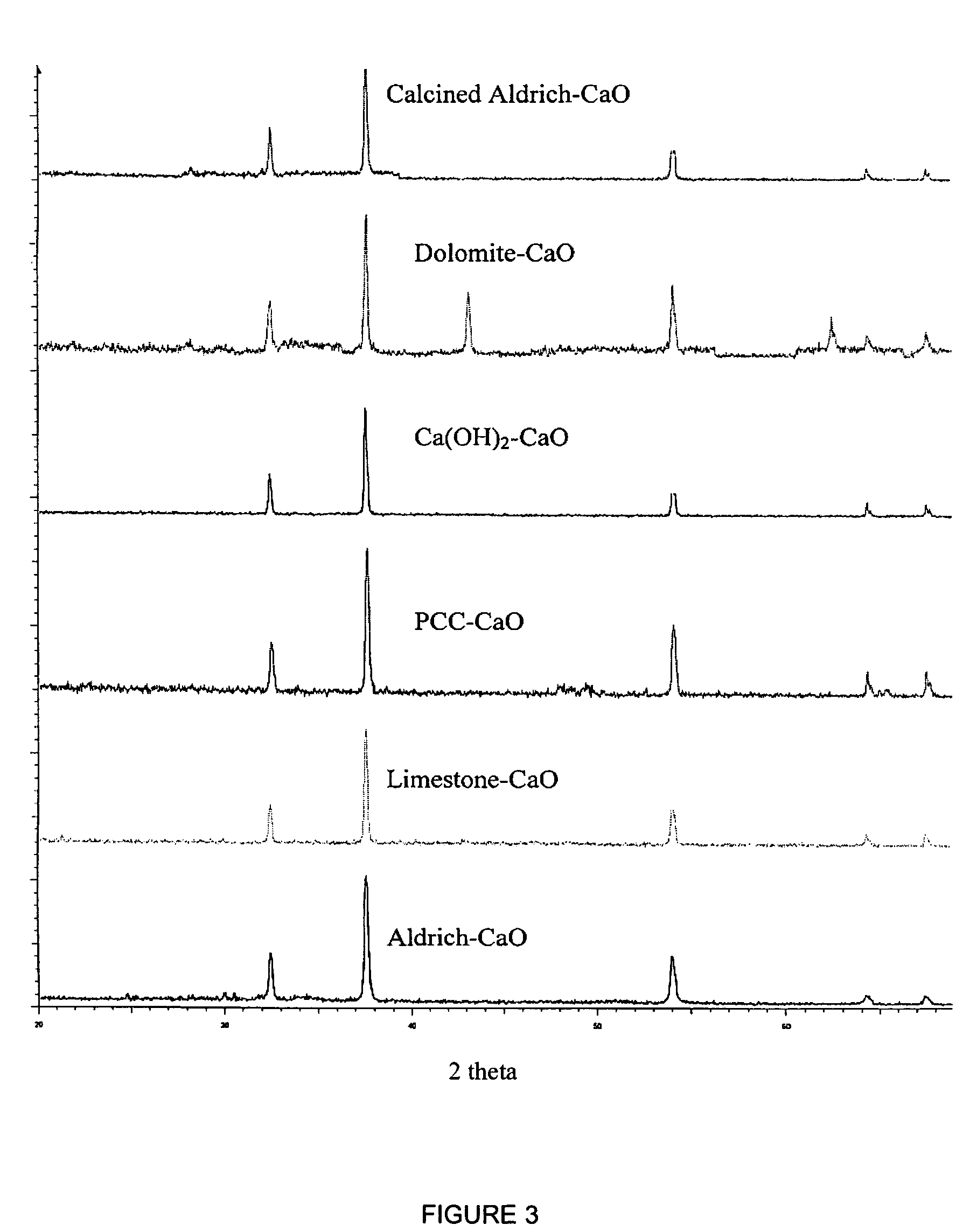
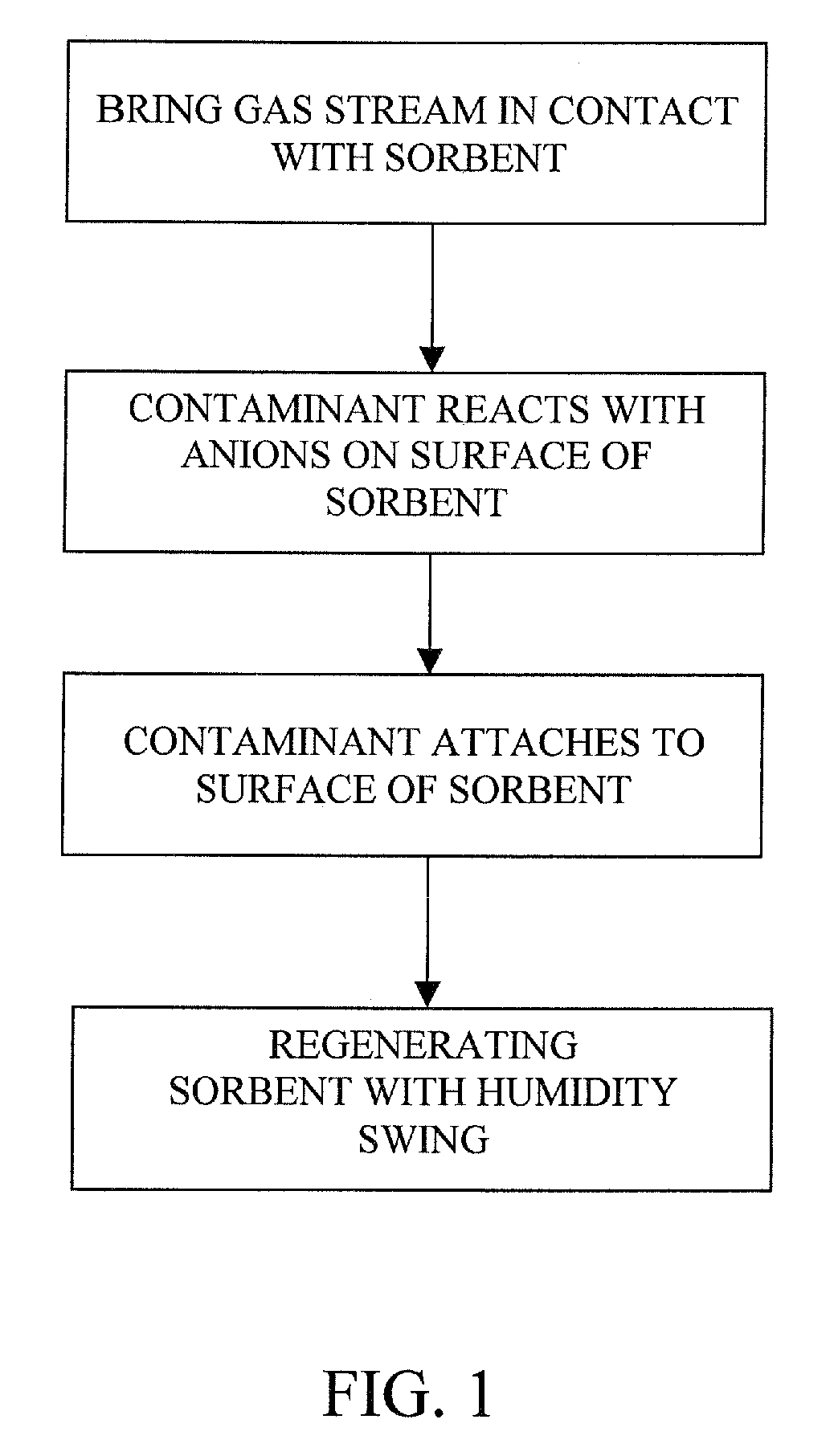
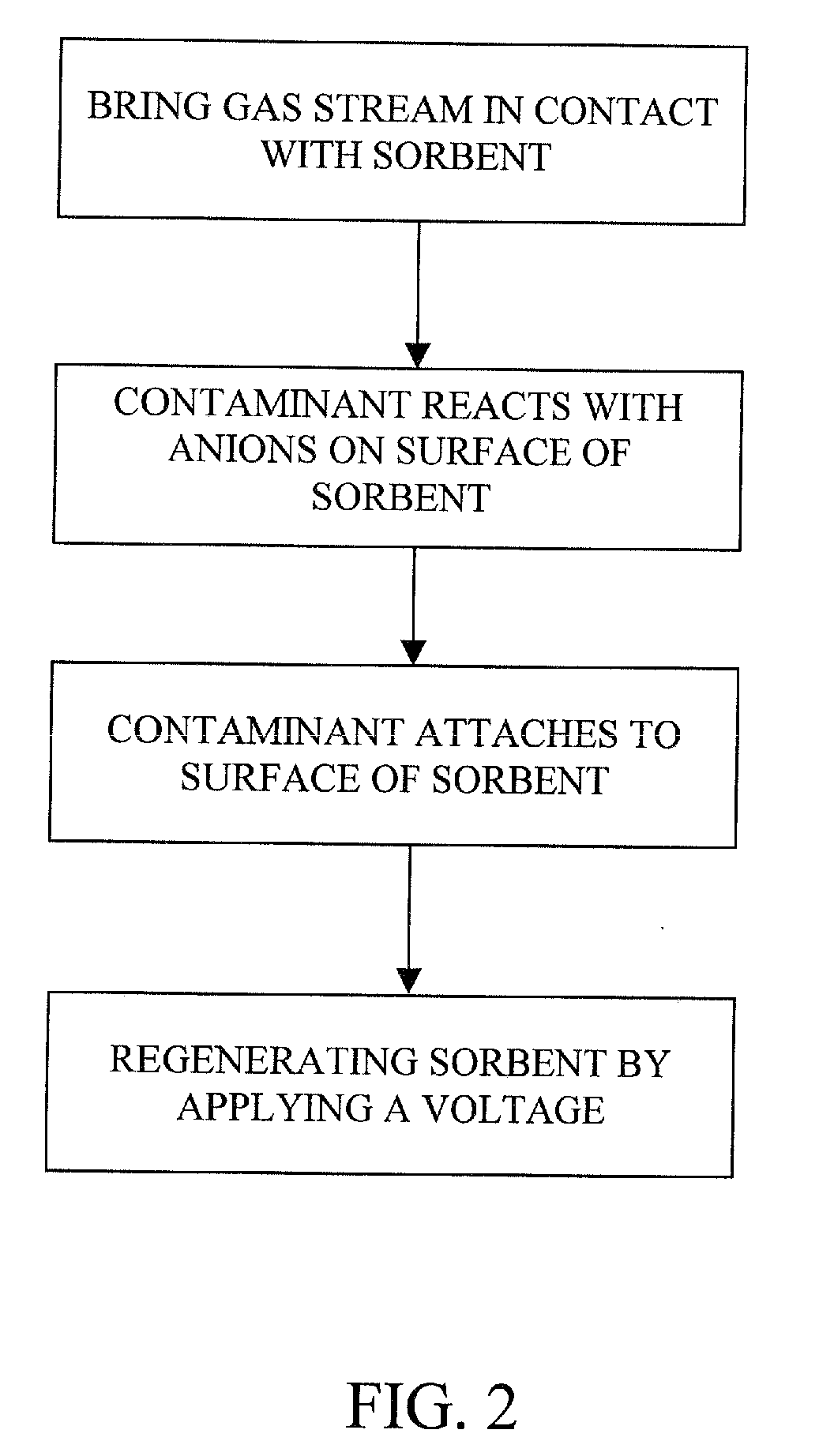
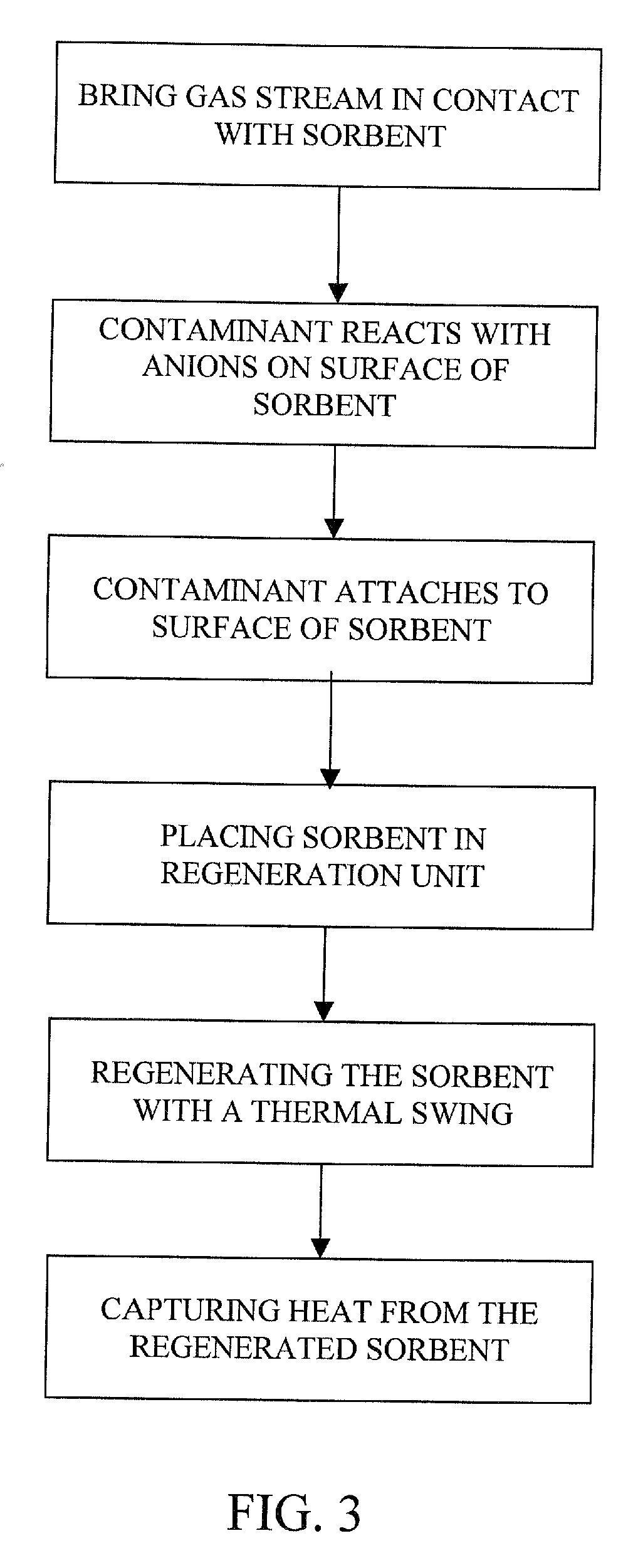
Removal of carbon dioxide from air
The present invention provides a method and apparatus for removing a contaminant, such as carbon dioxide, from a gas stream, such as ambient air. The contaminant is removed from the gas stream by a sorbent which may be regenerated using a humidity swing, a thermal swing, or a combination thereof. The sorbent may comprise a substrate having embedded positive ions and individually mobile negative ions wherein the positive ions are sufficiently spaced to prevent interactions between the negative ions. Where a thermal swing is used, heat may be conserved by employing a heat exchanger to transfer heat from the regenerated sorbent to an amount of sorbent that is loaded with the contaminant prior to regeneration.
Owner:CARBON SINK
Capture and Sequestration of Carbon Dioxide in Flue Gases
ActiveUS20090202410A1Calcium/strontium/barium carbonatesPigmenting treatmentAlkaline earth metalOxidation state
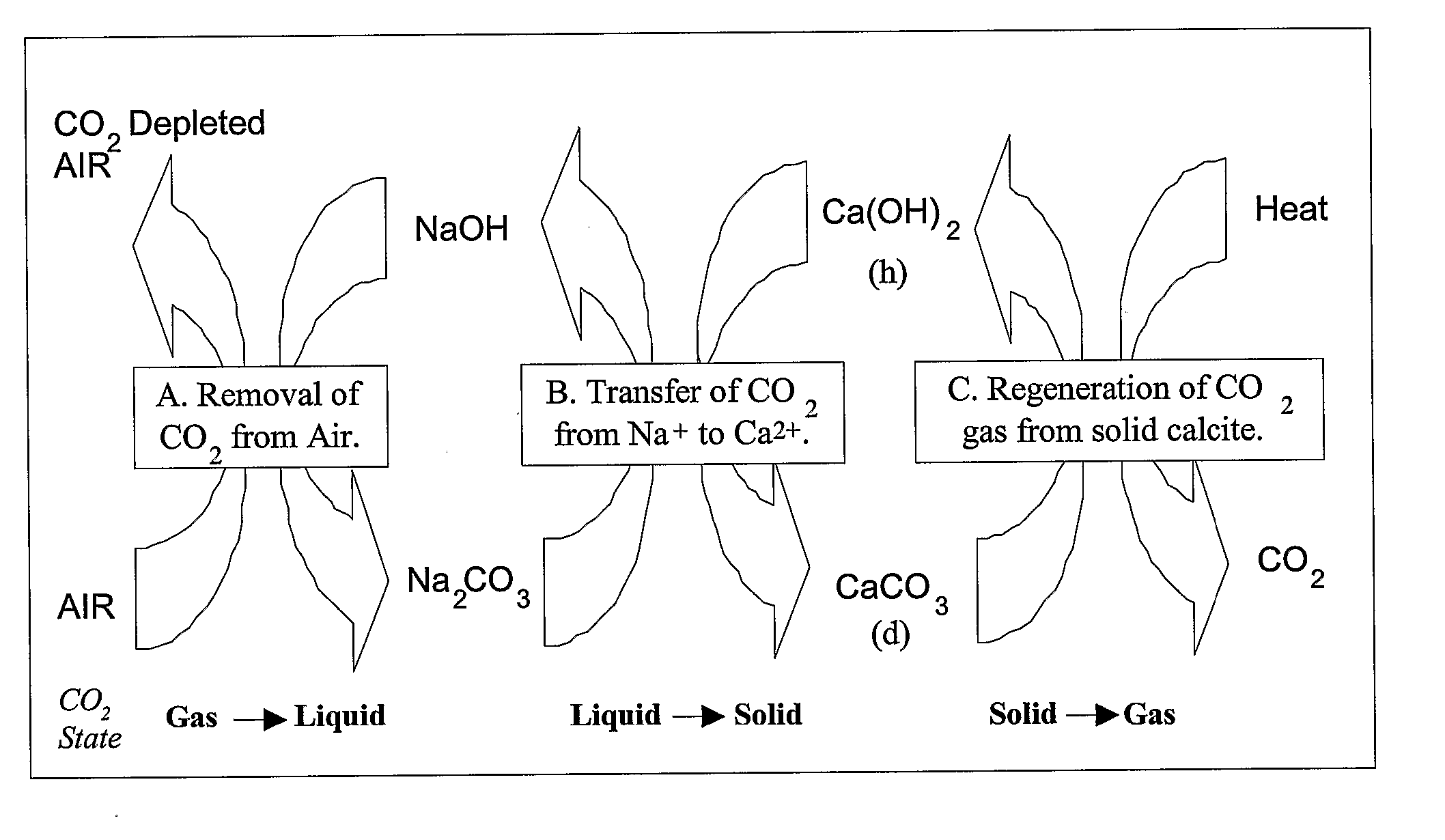
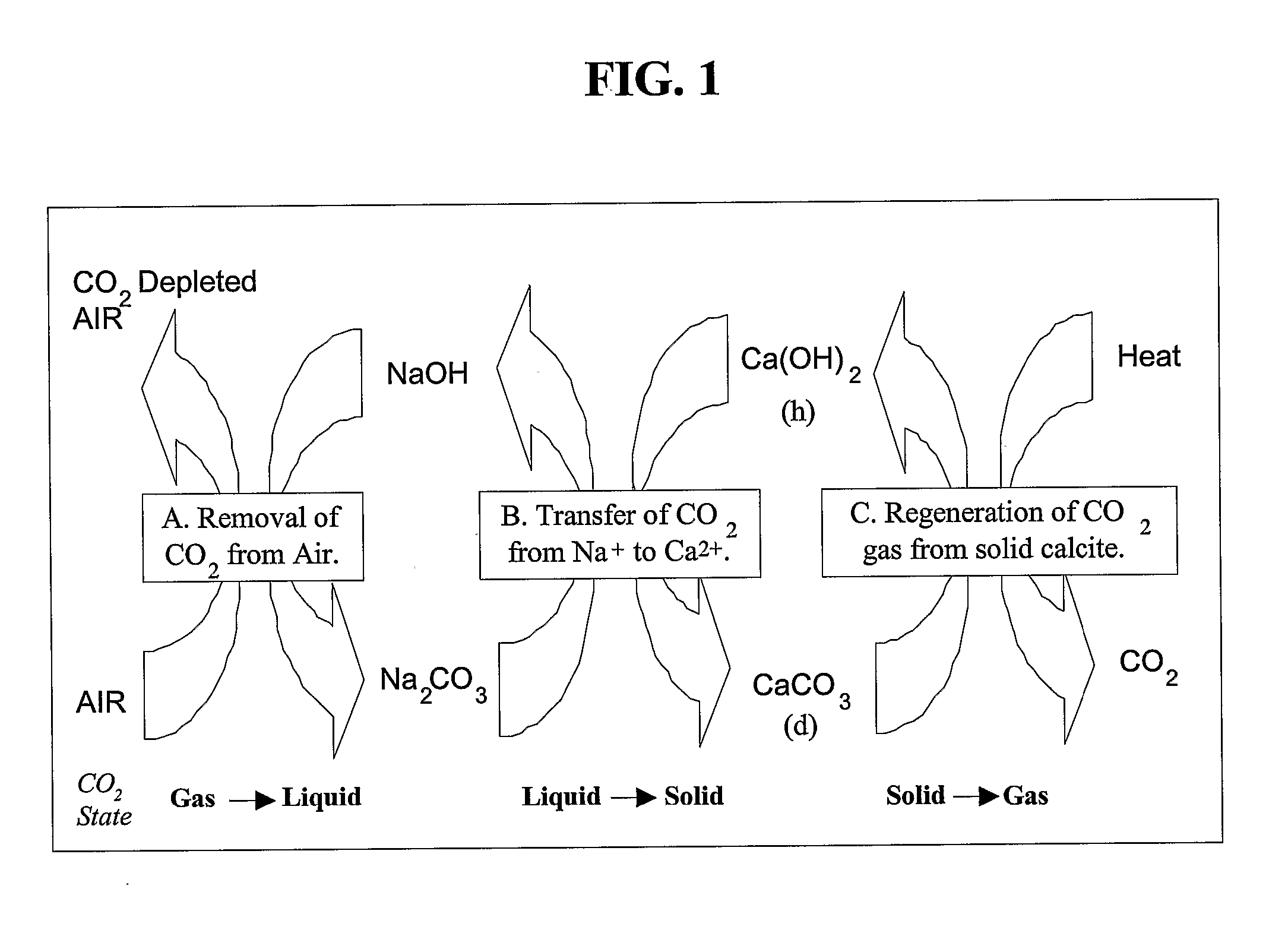
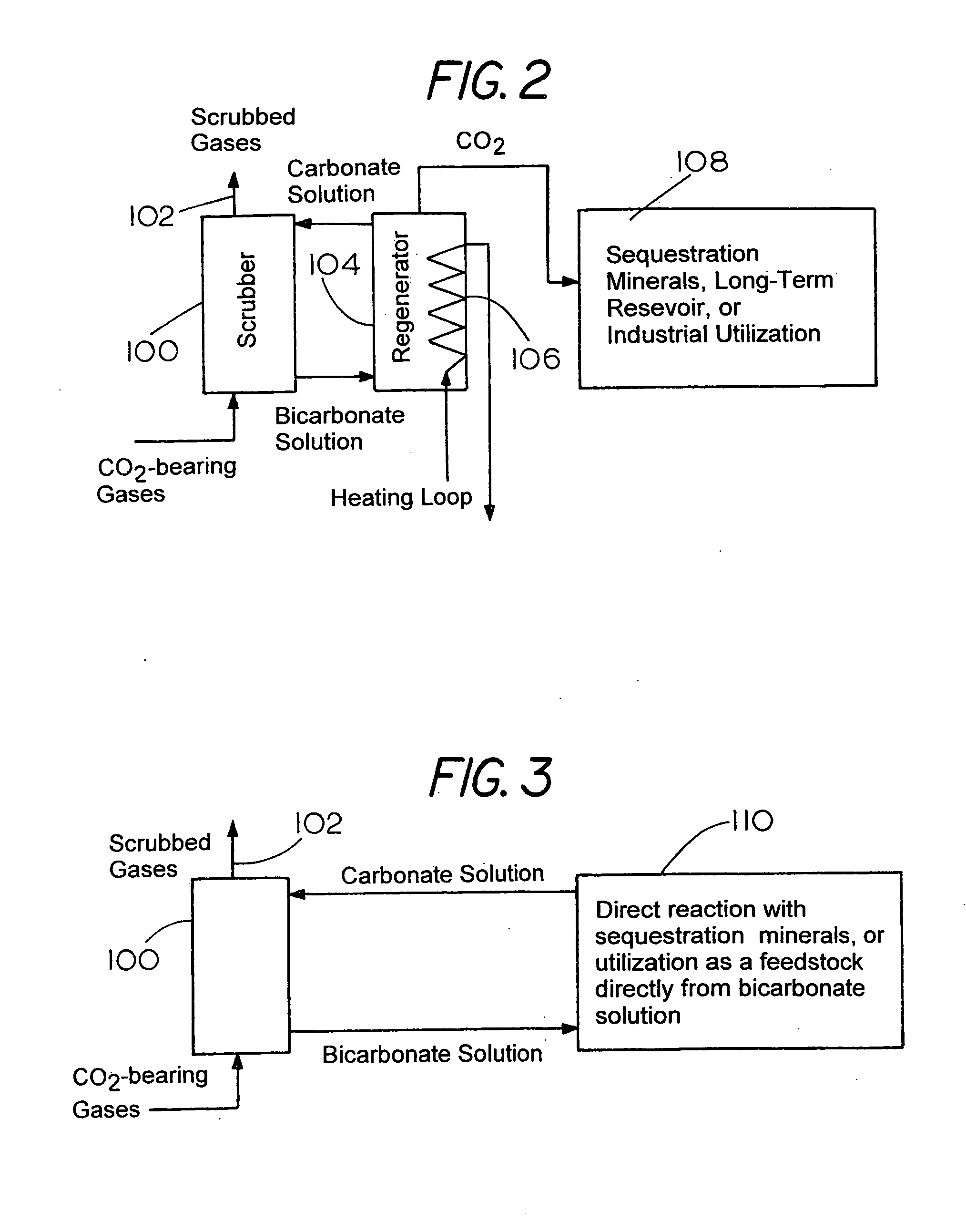
There is provided a process for the capture and sequestration of carbon dioxide that would otherwise enter the atmosphere and contribute to global warming and other problems. CO2 capture is accomplished by reacting carbon dioxide in flue gas with an alkali metal carbonate, or a metal oxide, particularly containing an alkaline earth metal or iron, to form a carbonate salt. A preferred carbonate for CO2 capture is a dilute aqueous solution of additive-free (Na2CO3). Other carbonates include (K2CO3) or other metal ion that can produce both a carbonate and a bicarbonate salt. Examples of suitable metal oxides include several alkaline earths including CaO and MgO. The captured CO2 is preferably sequestered using any available mineral or industrial waste that contains calcium magnesium or iron in non-carbonate forms, or iron in the Fe+2 oxidation state.
Owner:MICHIGAN TECHNOLOGICAL UNIVERSITY
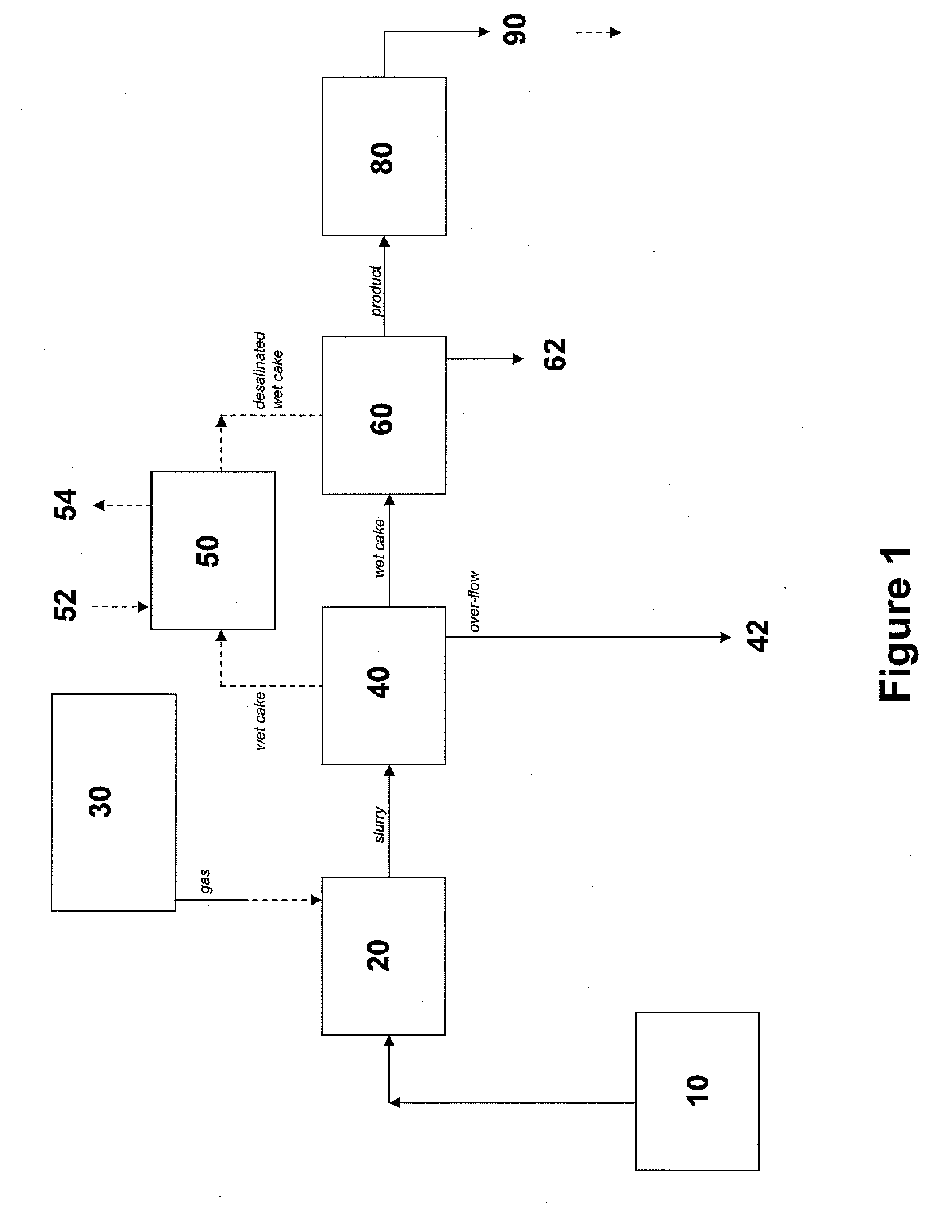
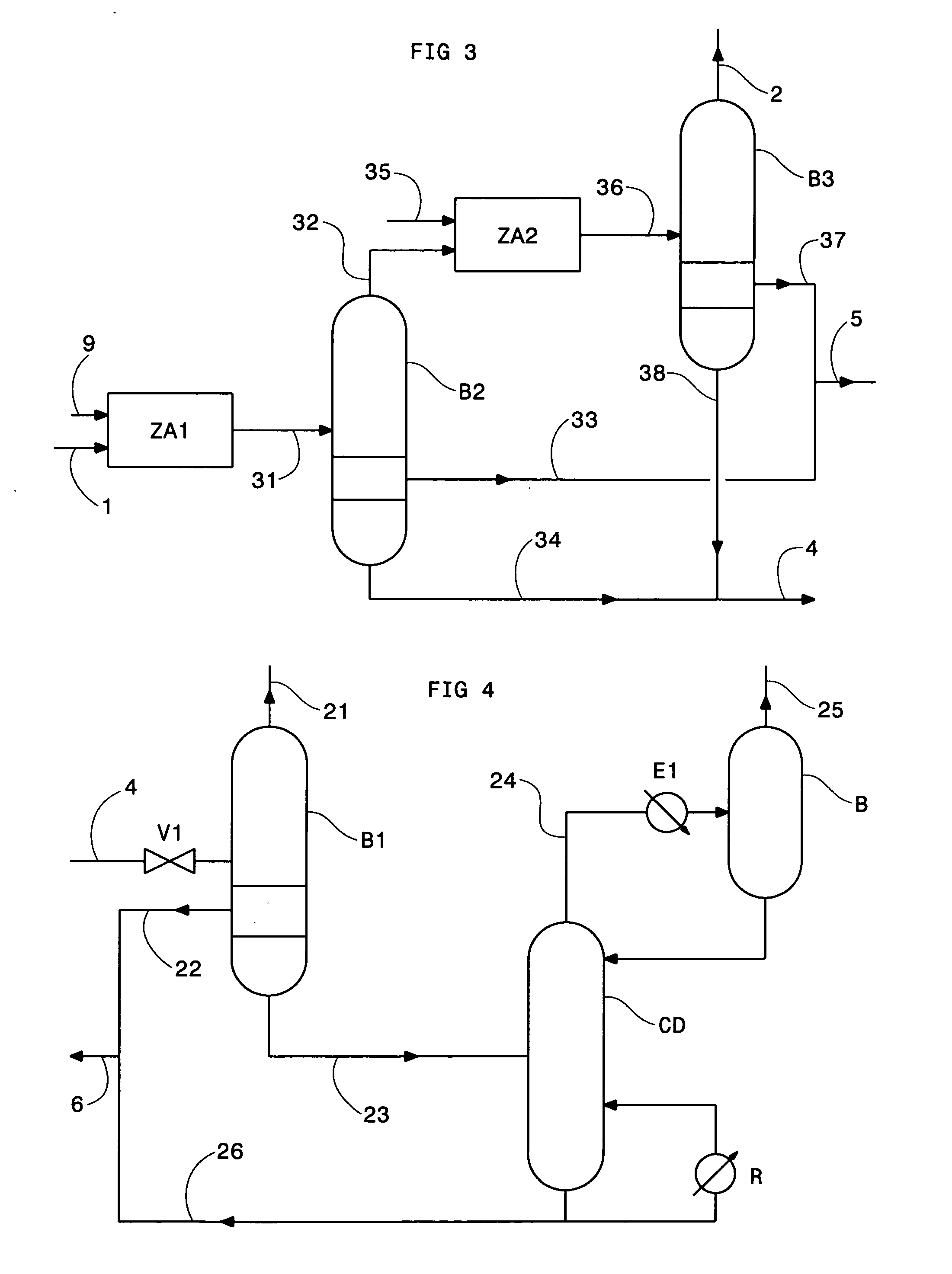
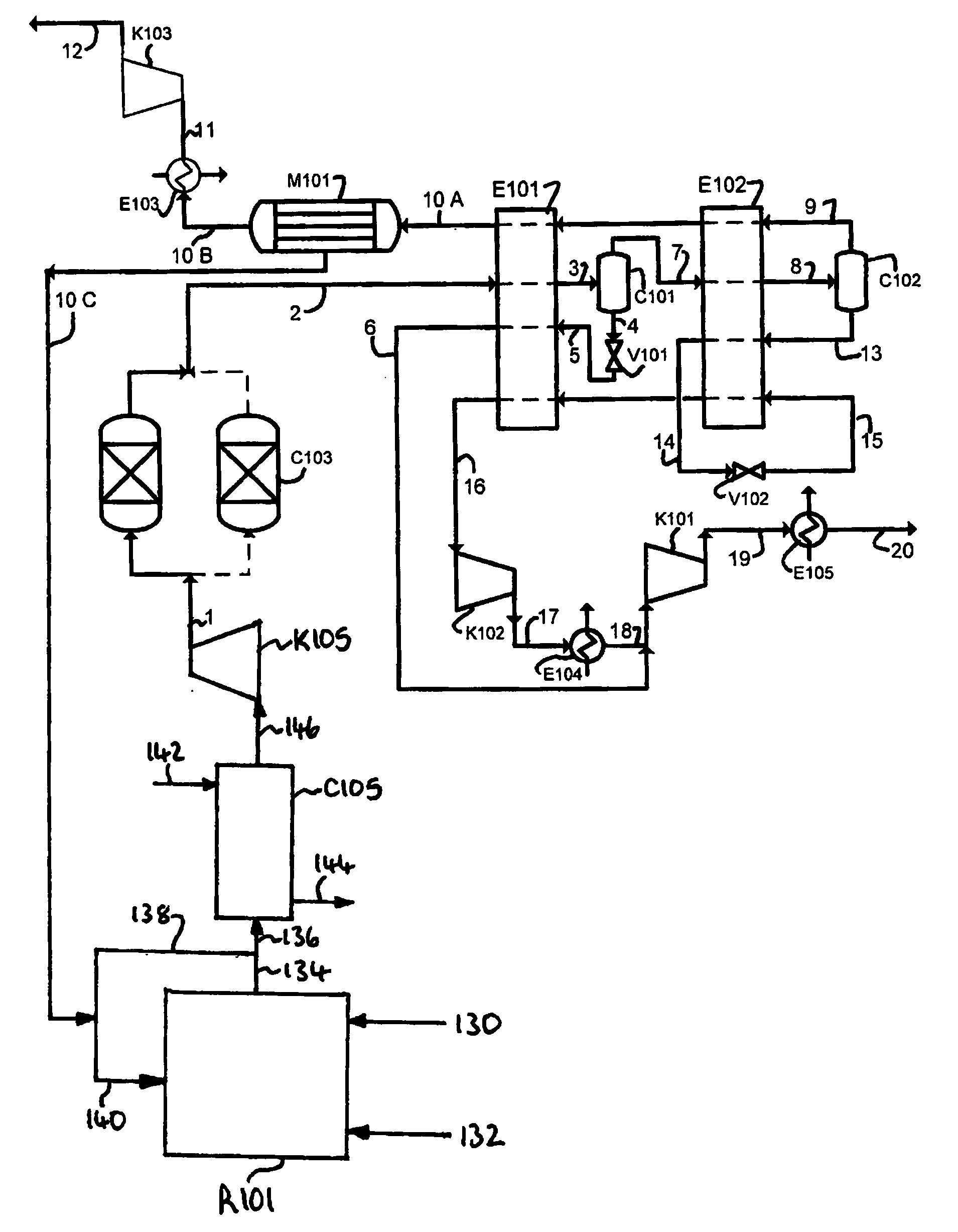
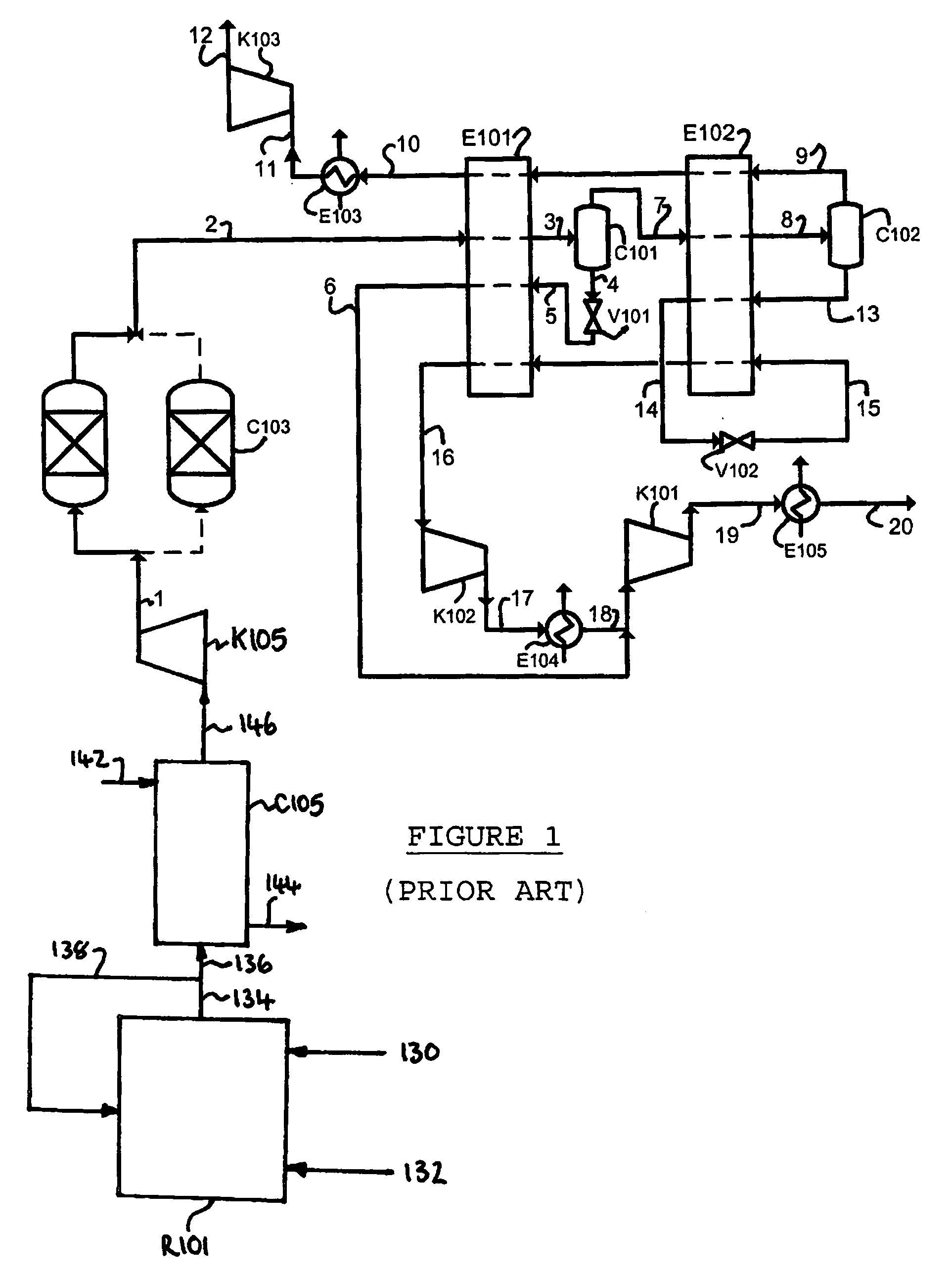
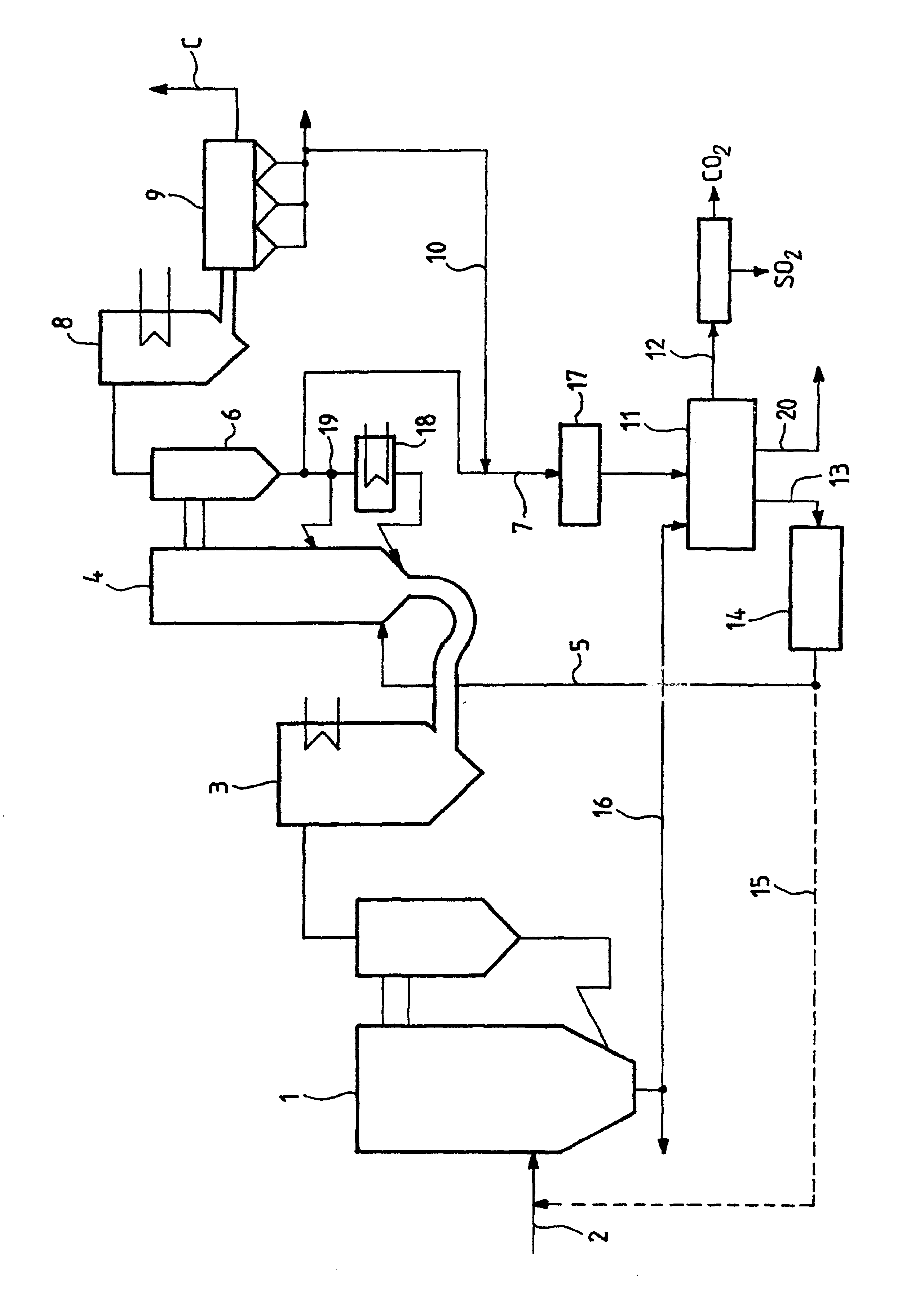
Method of deacidizing a gas with a fractional regeneration absorbent solution
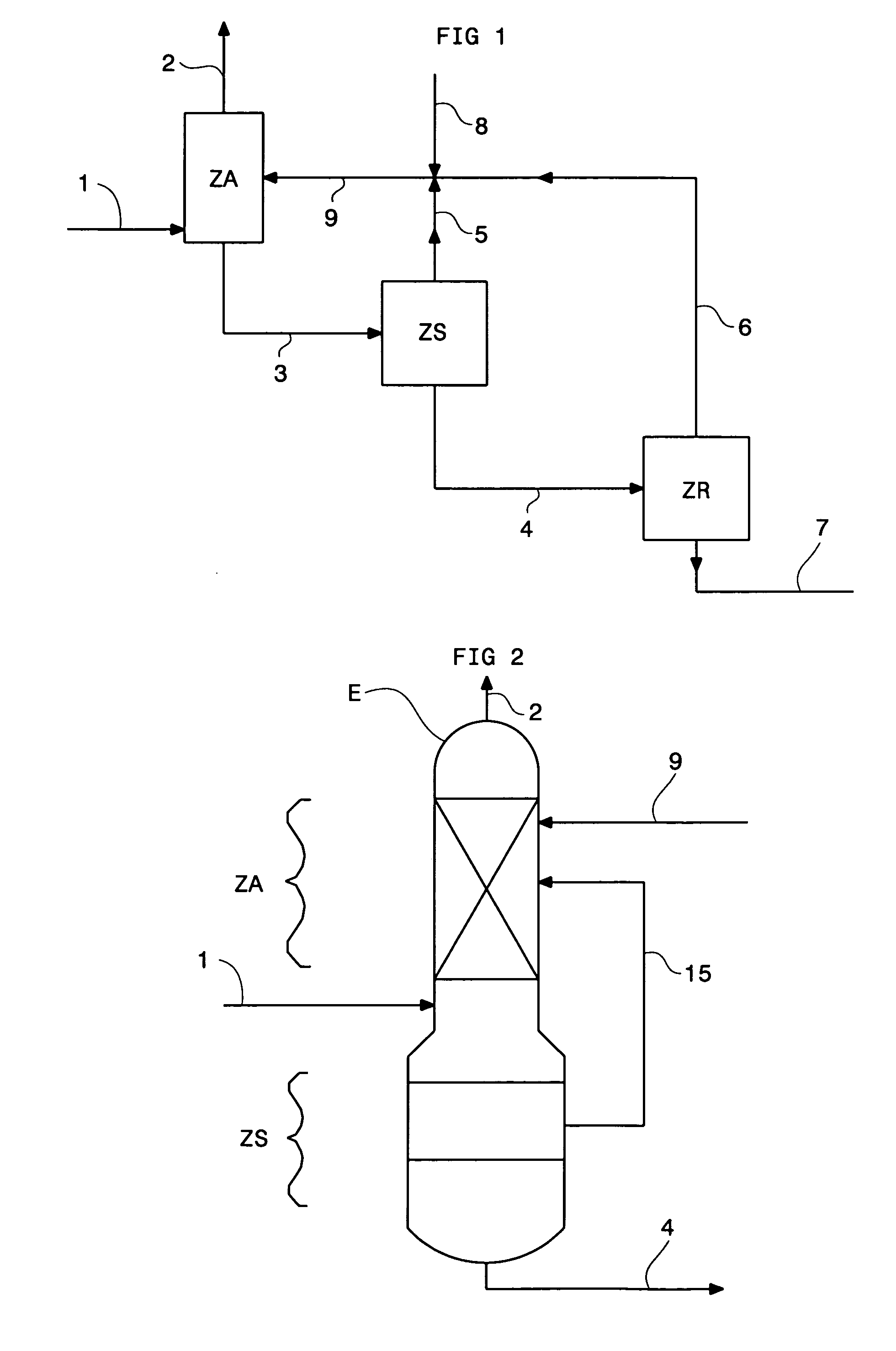
The gaseous effluent flowing in through line 1 is contacted in absorption zone ZA with the liquid absorbent solution flowing in through line 9. The gaseous effluent depleted in acid compounds is discharged through line 2. The absorbent solution laden with acid compounds is discharged through line 3. The absorbent solution once laden with acid compounds comprises two phases: a first phase poor in acid compounds and a second phase rich in acid compounds. The two phases are separated in zone ZS. The first phase is recycled through lines 5 and 9 to absorption zone ZA. The second phase is fed through line 4 into regeneration zone ZR. In zone ZR, the acid compounds are separated from the absorbent solution. The acid compounds are discharged through line 7. The regenerated absorbent solution is recycled through lines 6 and 9 to zone ZA.
Owner:INST FR DU PETROLE
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Separation of Carbon Dioxide (Co2) From Gas Mixtures By Calcium Based Reaction Separation (Cars-Co2) Process
InactiveUS20080233029A1Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentSorbentTransformation ratio
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS—CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCOa). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS—CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Amine absorber for carbon dioxide capture and processes for making and using the same
InactiveUS20100263534A1Lower potentialIncreasing amineGas treatmentMolecular sieve catalystsSulfurAbsorbent material
Owner:THE UNIVERSITY OF AKRON
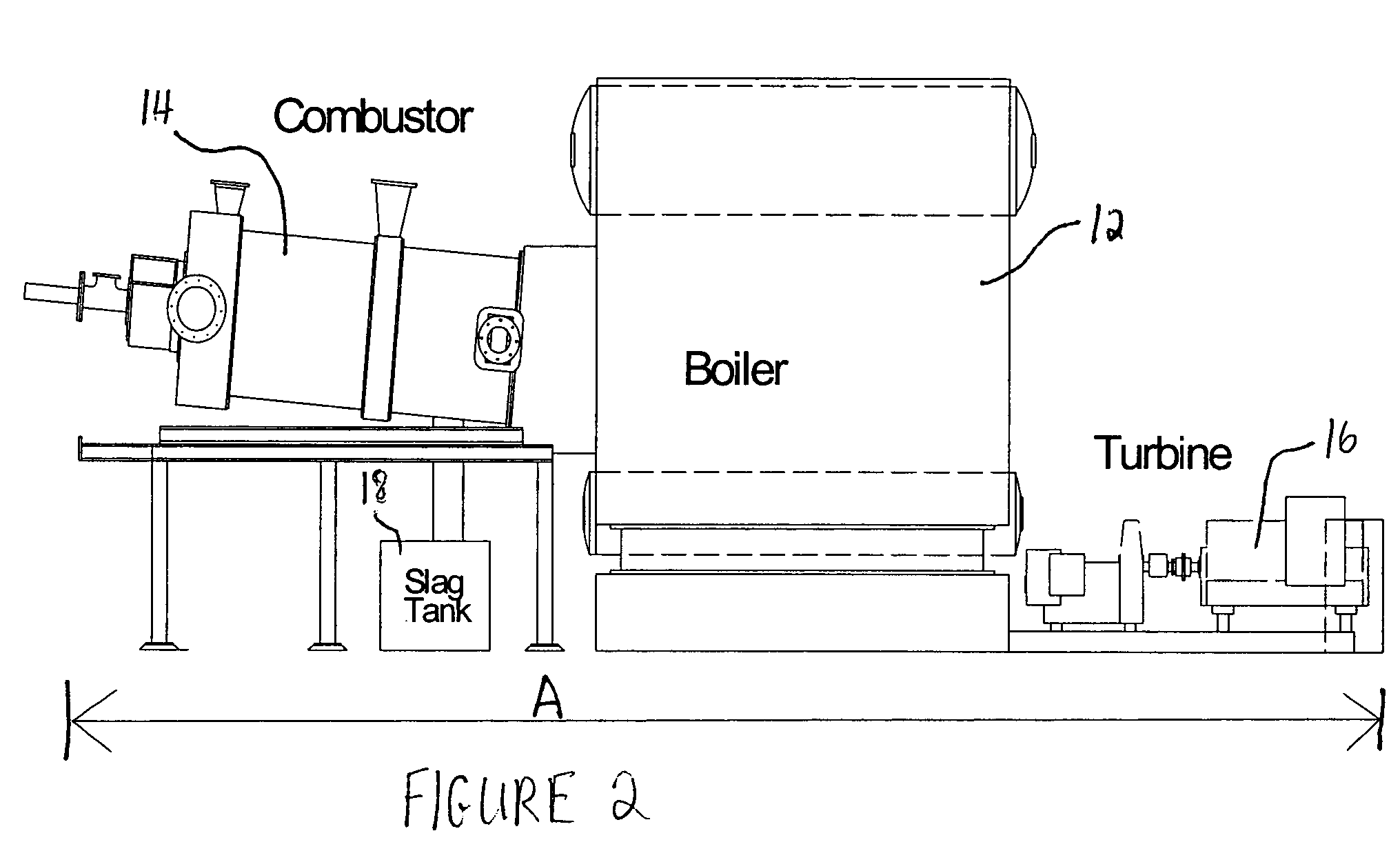
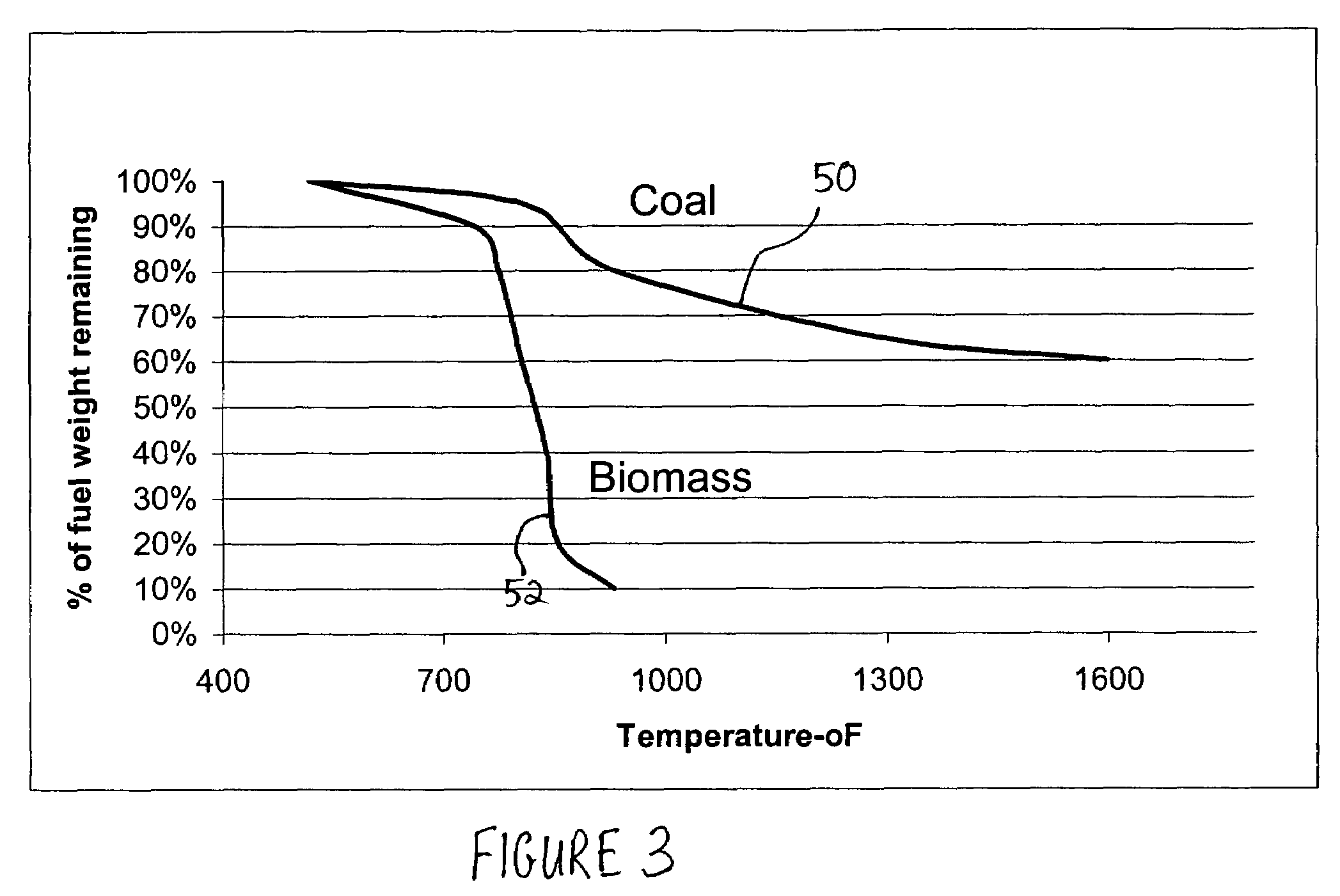
Production of hydrogen and removal and sequestration of carbon dioxide from coal-fired furnaces and boilers
InactiveUS7282189B2Increase ratingsValue maximizationOrganic chemistryNitrogen compoundsHydrogenProcess engineering
Methods for reducing and eliminating carbon dioxide from the emissions of solid fuel fired power plants, particularly coal fired power plants, and to sequester the carbon dioxide, typically by using existing equipment. In some embodiments, the methods involve pyrolyzing the solid fuel to remove volatile matter and using the volatile matter to produce hydrogen. Additionally, the methods may involve burning the solid fuel or pyrolized solid fuel at very fuel rich stoichiometric conditions. Sequestration may include the production of a carbon dioxide-containing solution and the pumping of the solution into the ground, particularly in areas high in limestone.
Owner:ZAUDERER BERT
Purification of carbon dioxide
Carbon dioxide is separated from a feed gas, preferably derived from flue gas from an oxyfuel combustion process, in a membrane separation system to produce separated carbon dioxide gas which is fed to the oxyfuel combustion process to improve the performance of the process.
Owner:AIR PROD & CHEM INC
Method of simultaneously reducing CO2 and SO2 emissions in a combustion installation
InactiveUS6737031B2Reduce carbon dioxide emissionsFluidized bed combustionGas treatmentCombustionFlue gas
The method of simultaneously reducing carbon dioxide (CO2) emissions and sulfur dioxide (SO2) emissions produced by the combustion of carbon-containing matter in a hearth consists in injecting into the hearth a calcium-based agent, a fraction of which absorbs SO2 after decarbonization, and then, after the flue gases have been subjected to intermediate cooling, in causing them to transit via a first reactor and in putting them in contact therein with the other fraction of the absorbant that has not reacted with SO2 so as to capture CO2 from the flue gases by carbonization, then, in a separator, in extracting the solids contained in the flue gases output from the first reactor so as to subject them to heat treatment in a second reactor in order to extract CO2 therefrom by decarbonization and in order to recycle the resulting regenerated CO2 absorbant to the first reactor.
Owner:GENERAL ELECTRIC TECH GMBH
Nano-structure supported solid regenerative polyamine and polyamine polyol absorbents for the separation of carbon dioxide from gas mixtures including the air
ActiveUS20080293976A1Increased CO absorption capacityIncreased COMaterial nanotechnologyElectrolysis componentsPolyolSorbent
The invention relates to regenerative, supported amine sorbents that includes an amine or an amine / polyol composition deposited on a nano-structured support such as nanosilica. The sorbent provides structural integrity, as well as high selectivity and increased capacity for efficiently capturing carbon dioxide from gas mixtures, including the air. The sorbent is regenerative, and can be used through multiple operations of absorption-desorption cycles.
Owner:UNIV OF SOUTHERN CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com