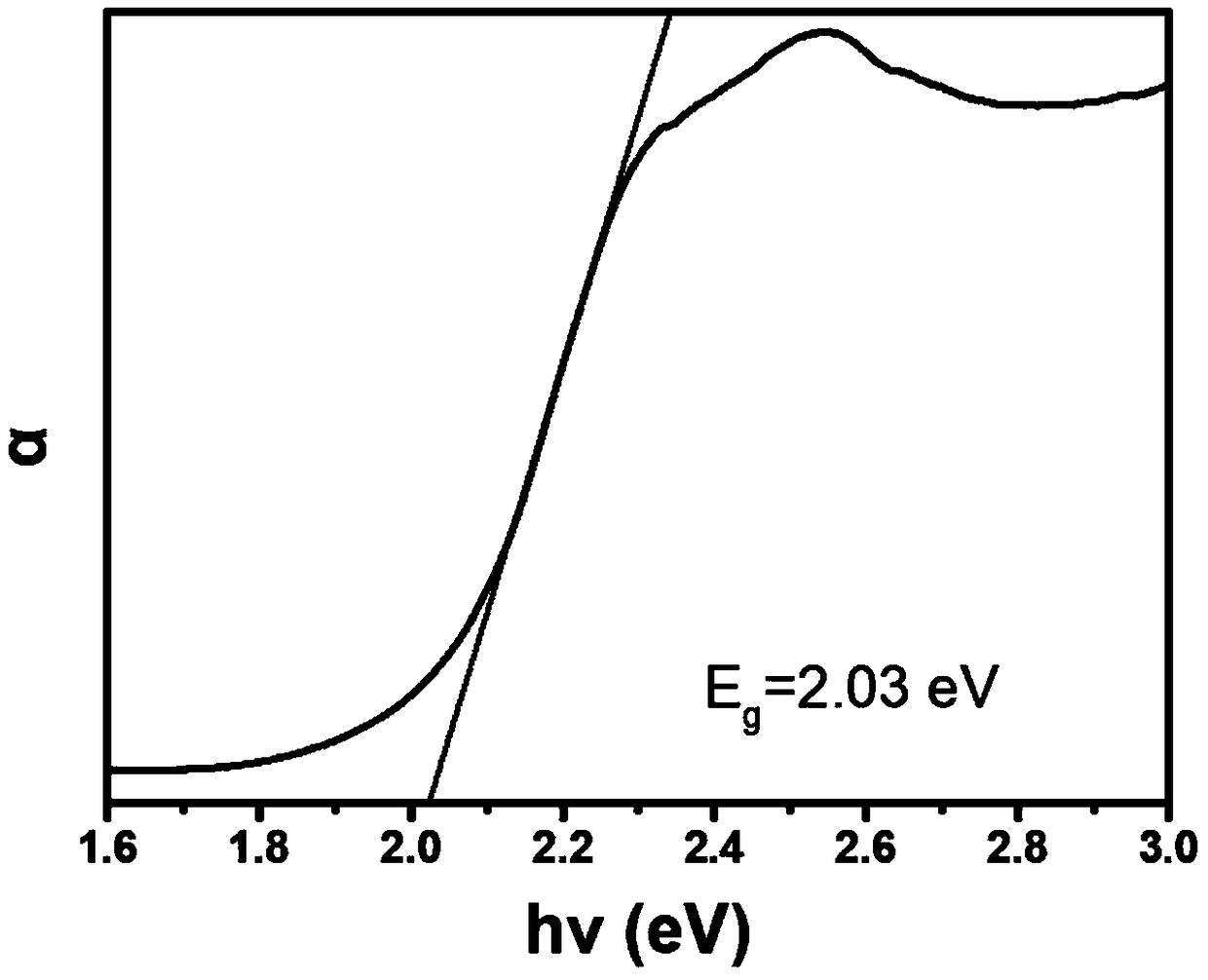
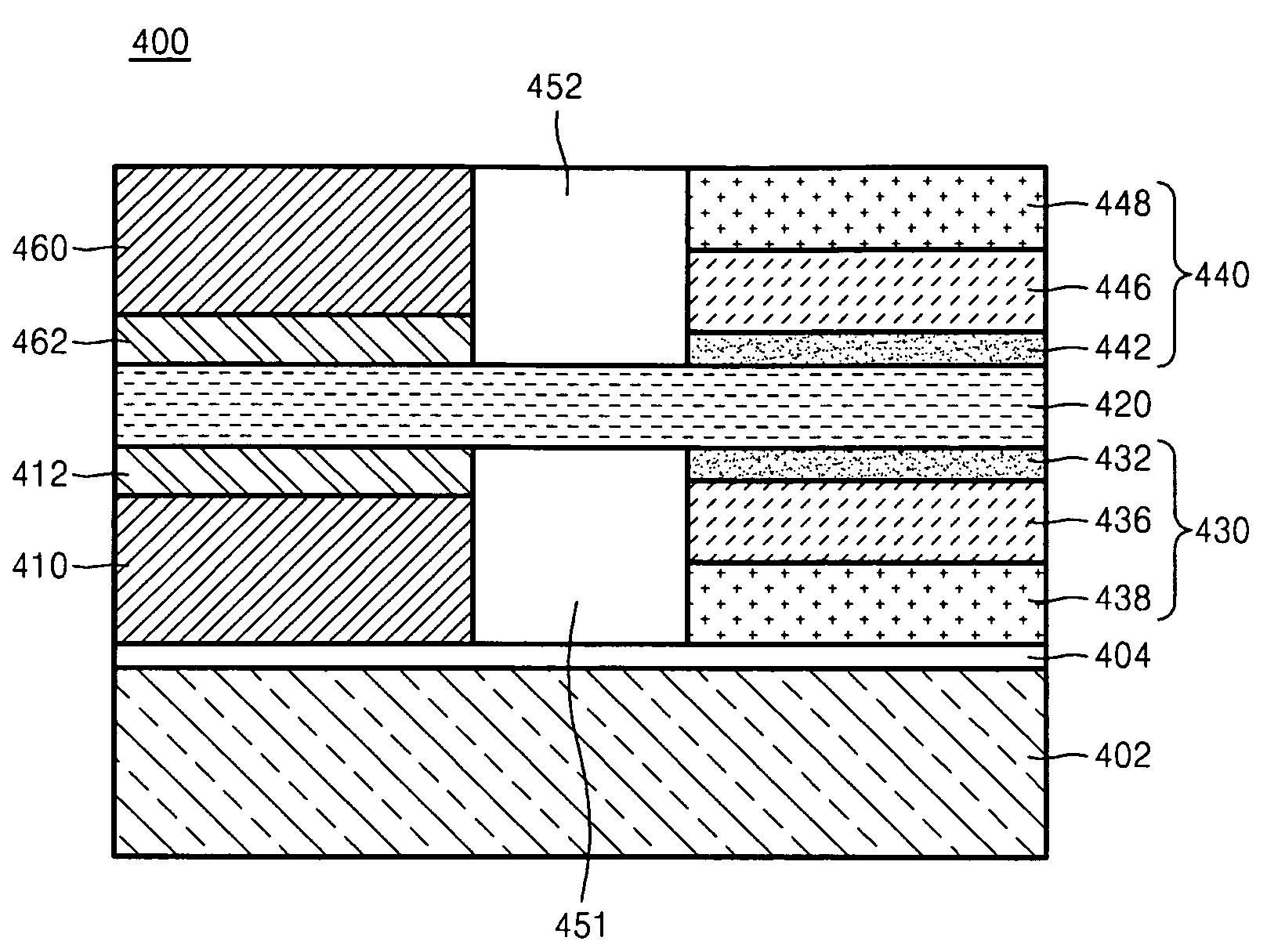
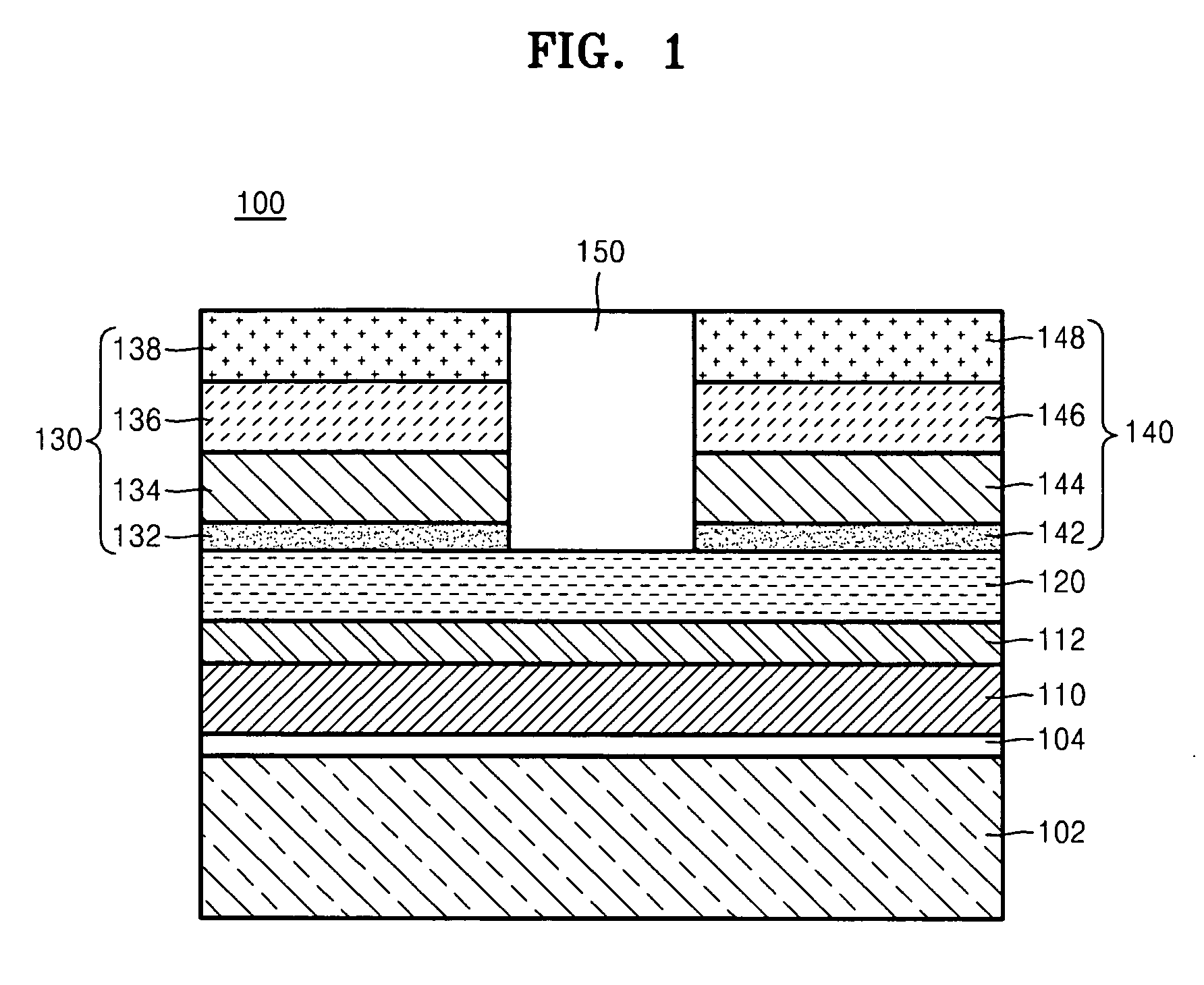
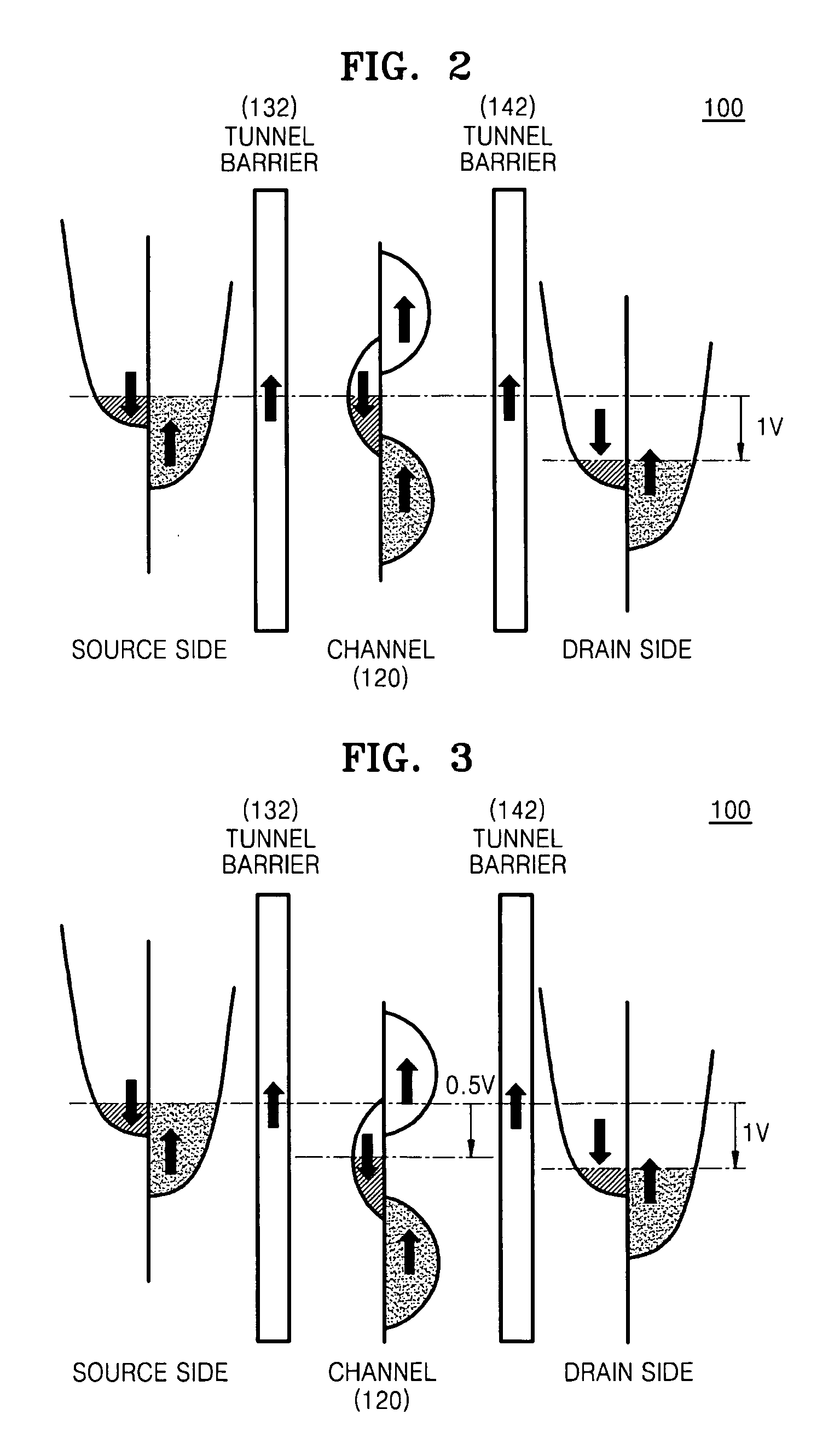
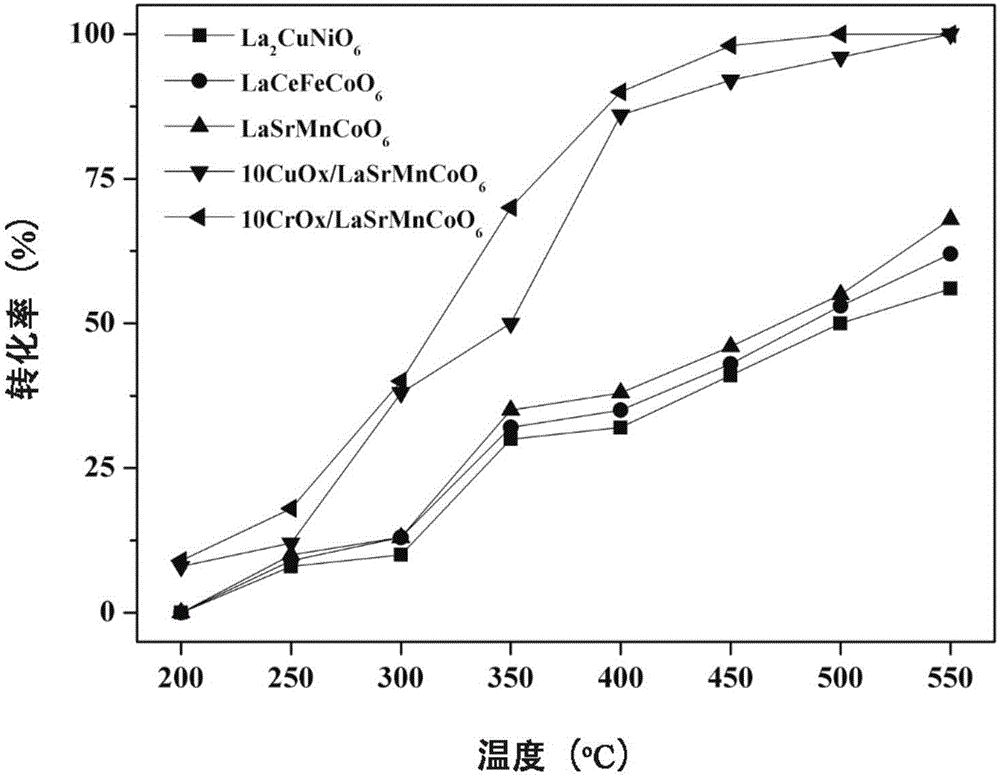
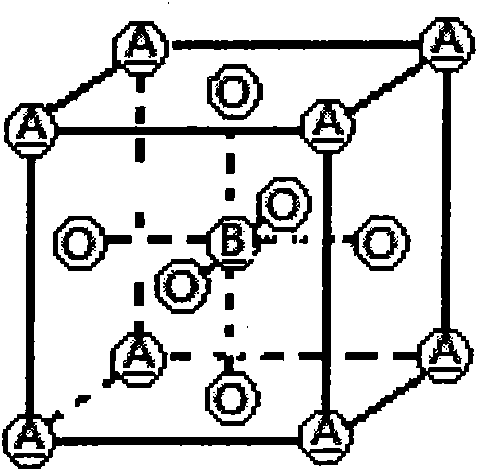
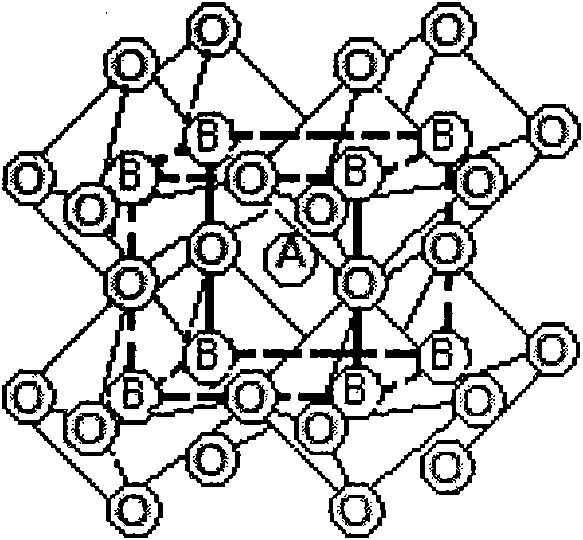
Patents
Literature
146 results about "Double perovskite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Perovskite-based fuel cell electrode and membrane
InactiveUS7504172B2Lower cost of capitalReduce energy costsCell electrodesFinal product manufactureSyngasFuel cells
The present invention provides a material suitable for use in a solid oxide fuel cell, wherein the material is of an, optionally doped, double perovskite oxide material having the general formula (I): (LnaXb)e(Z1cZ2d)fOg (I) wherein Ln is selected from Y, La and a Lanthanide series element, or a combination of these and X also represents an element occupying the A site of a perovskite oxide and is selected from Sr, Ca and Ba, and Z1 and Z2 represent different elements occupying the B site of a perovskite oxide and are selected from Cr, Mn, Mg and Fe, and wherein a has a value from 0 to 1, preferably 0.7 to 1.0, b has a value of from 1 to 0, preferably 0.3 to 0, and each of c and d has a value of from 0.25 to 0.75, provided that a+b has a value of 1, and c+d, has a value of 1, and wherein e has a value of from 0.8 to 1, wherein f has a value of from 0.8 to 1, and g has a value of from 2.5 to 3.2. Also provided are SOFCs having an electrode or functional layer of a material or containing a material of the invention, as well as mixed ionic / electronic conducting membranes suitable for use in a syngas reactor or oxygen separator, comprising a layer of a double perovskite material of the invention, and a method of oxidising a fuel in an SOFC having an anode of a double perovskite material of the invention.
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
Ultraviolet light detector of lead-free double perovskite single crystal and manufactruing method thereof
ActiveCN109830550ASolve the problems caused by biological toxicityImprove stabilityFinal product manufactureSolid-state devicesUltraviolet lightsSilver colloid
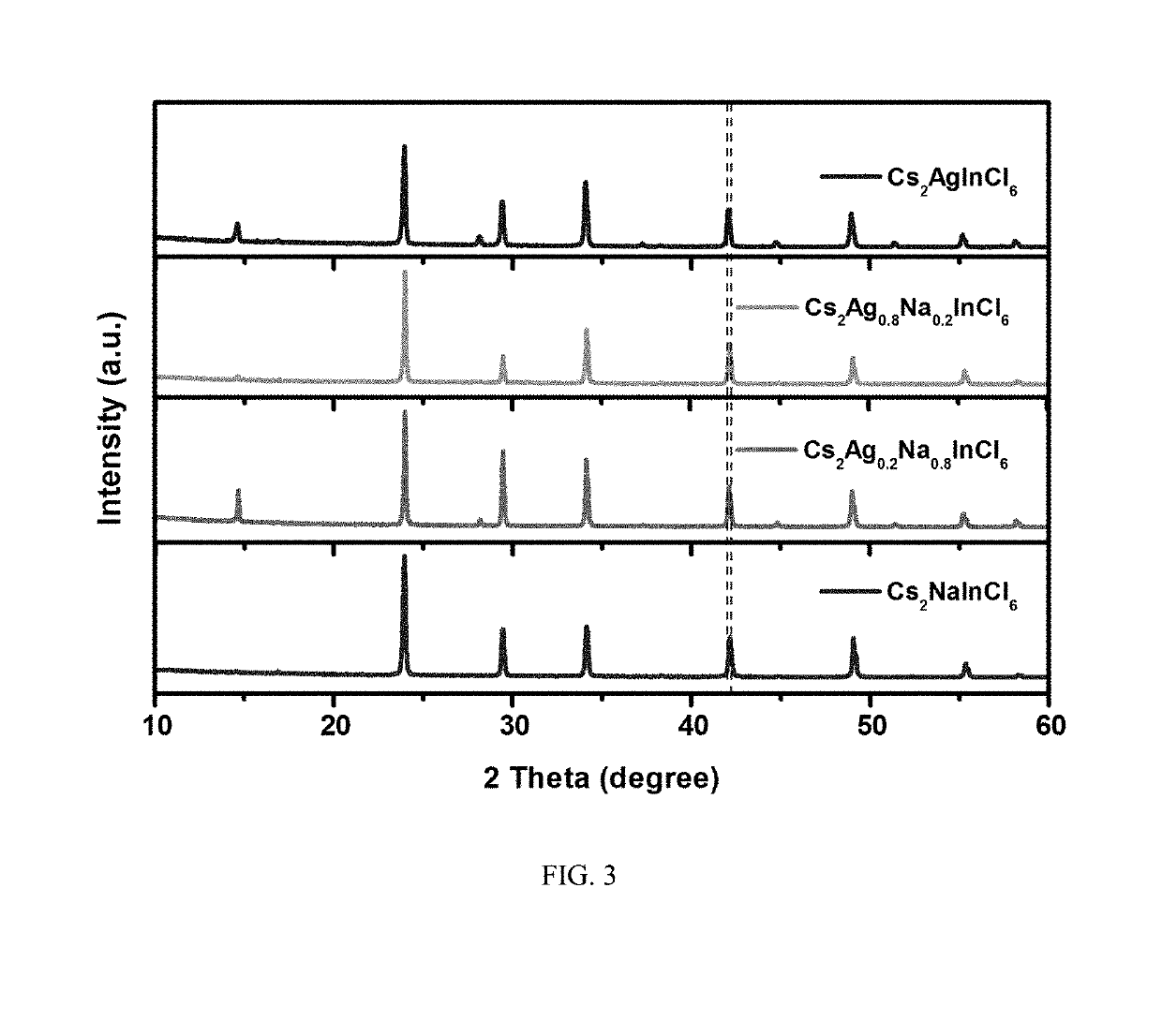
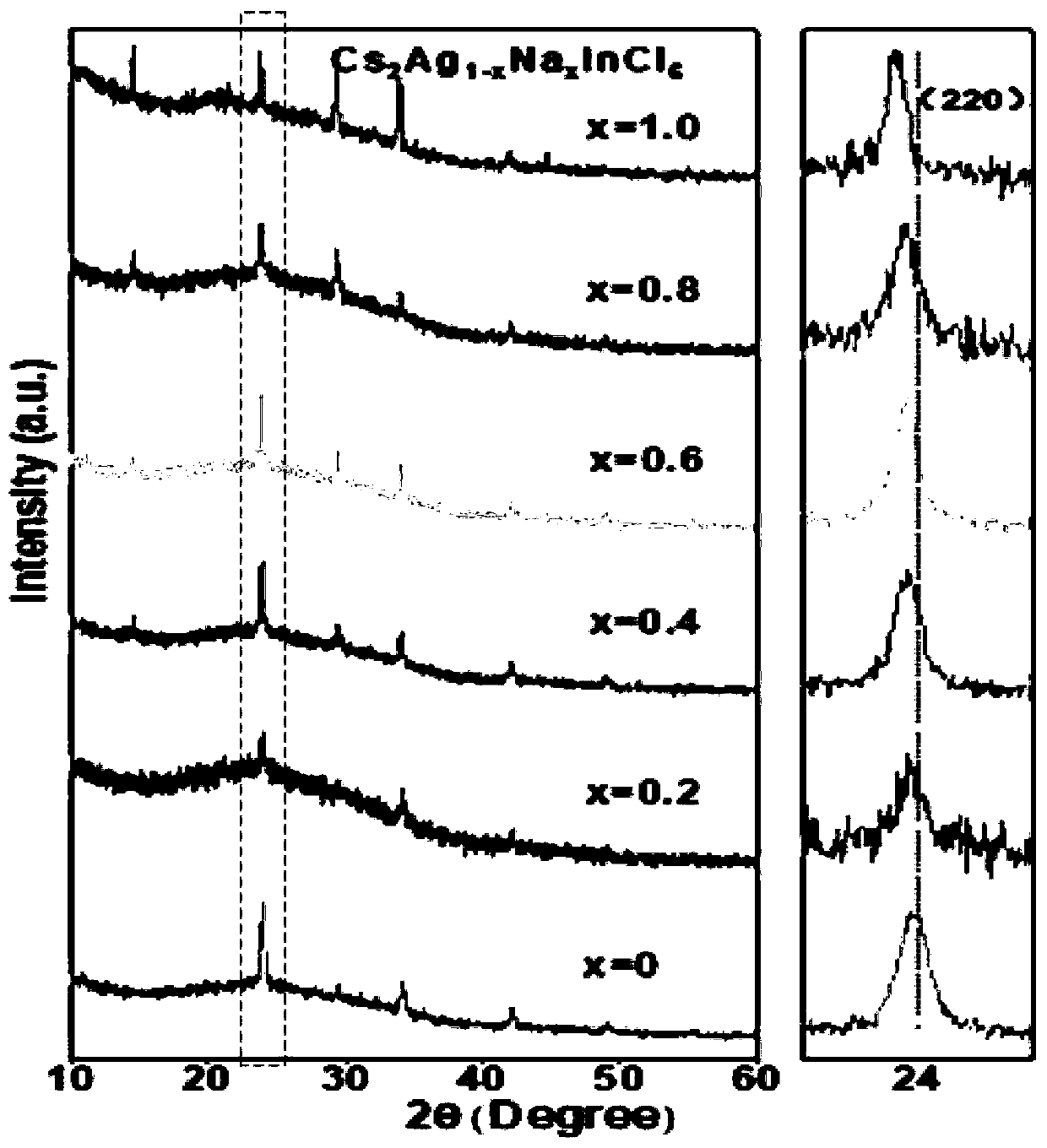
The invention discloses an ultraviolet light detector of a lead-free double perovskite single crystal. The detector successively comprises a substrate, the lead-free double perovskite single crystal,an electrode, a silver colloid and a conductive gold wire from bottom to top. The lead-free double perovskite single crystal is formed by A, BI, BIII, and X. A molecular structural formula is A2BIBIIIX6, wherein the A is methylamine (MA) or Cs, the BI is Ag or Na, the BIII is Bi, Sb or In, and the X is Cl or I. A lead-free double perovskite single crystal material is selected from any one of Cs2AgInCl6, Cs2NaInCl6, MA2AgBiI6, and MA2AgSbI6. The type of double perovskite material solves a problem caused by biotoxicity of lead in traditional perovskite and has excellent photoelectric performance. Compared with a traditional perovskite material, the perovskite material of the invention has a high and rapid ultraviolet response. Good performance can be maintained in an air and humidity environment and good stability is achieved.
Owner:JINAN UNIVERSITY
Double-perovskite structured red fluorescent powder as well as preparation method and application thereof
ActiveCN102250616AChemically stableImprove luminosityGas discharge lamp usageLuminescent compositionsGreen-lightRed fluorescence
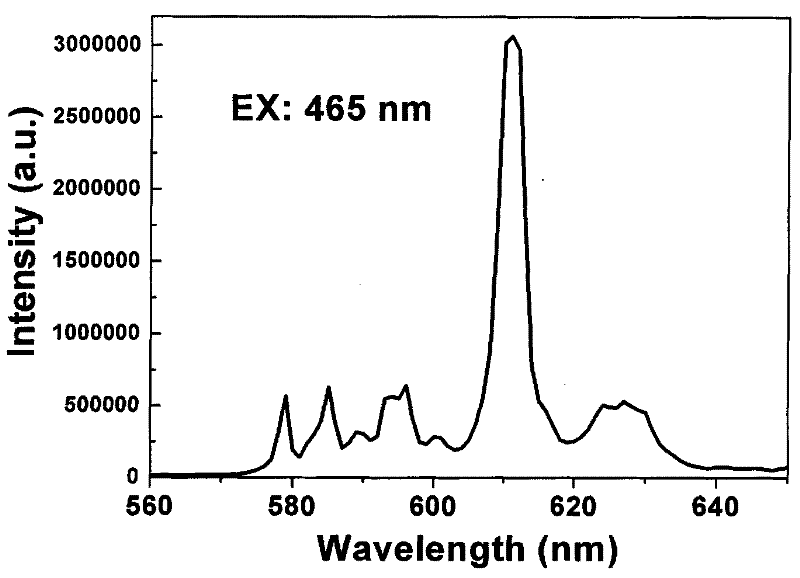
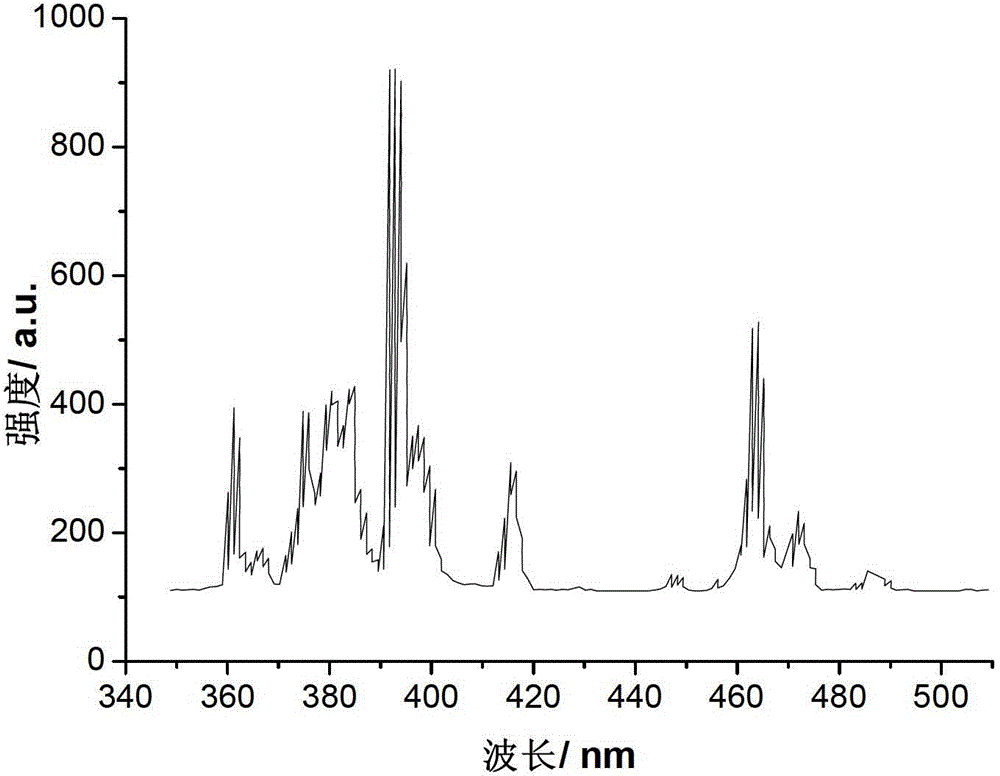
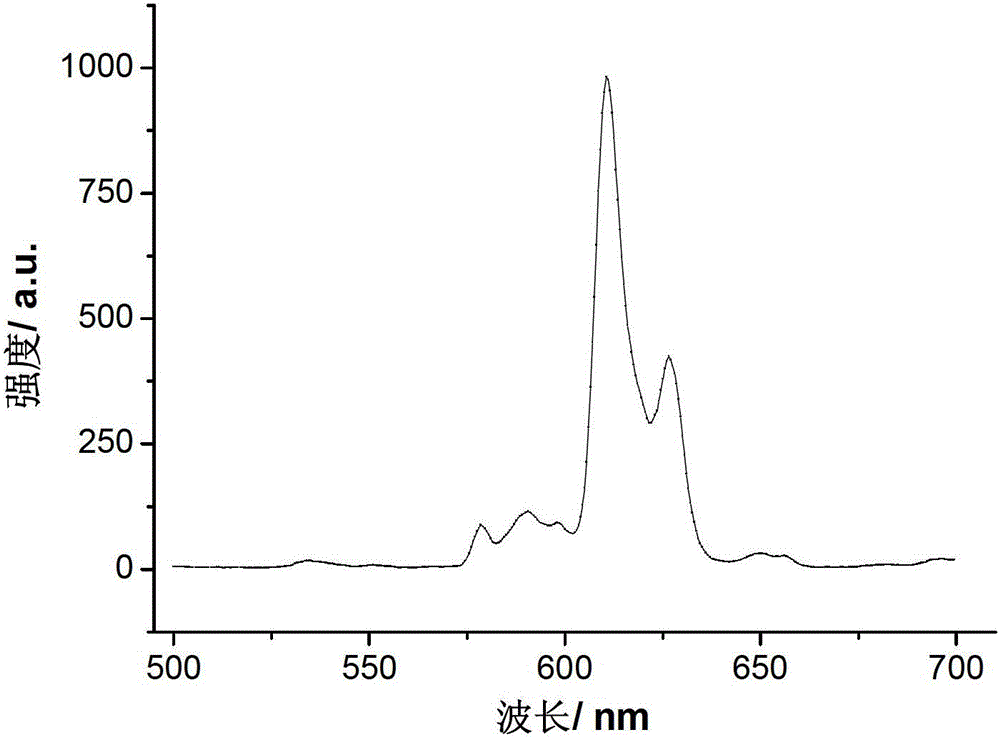
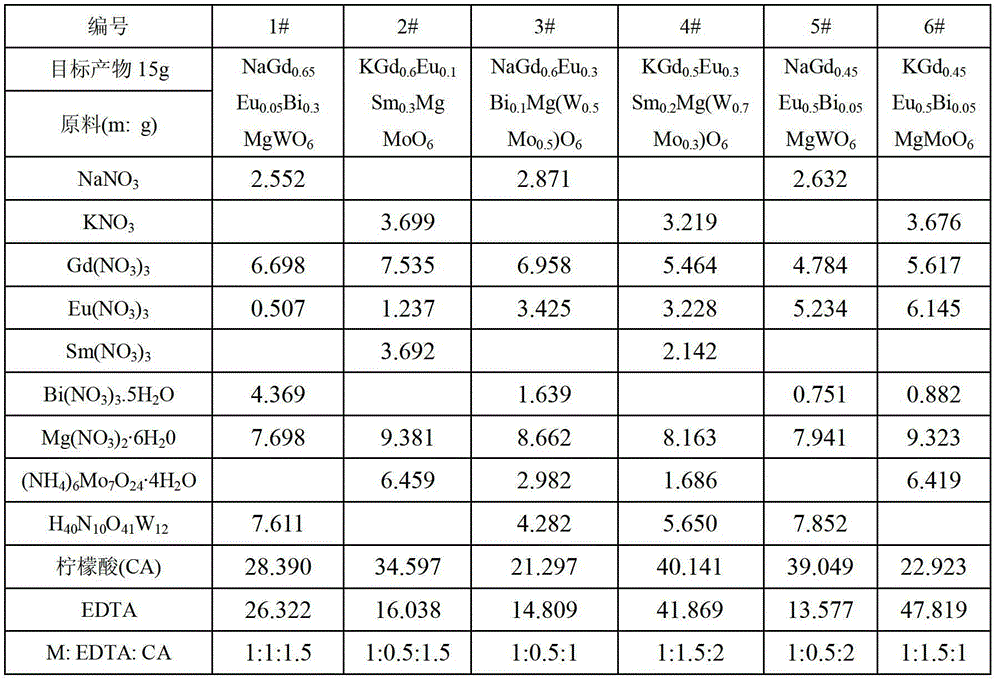
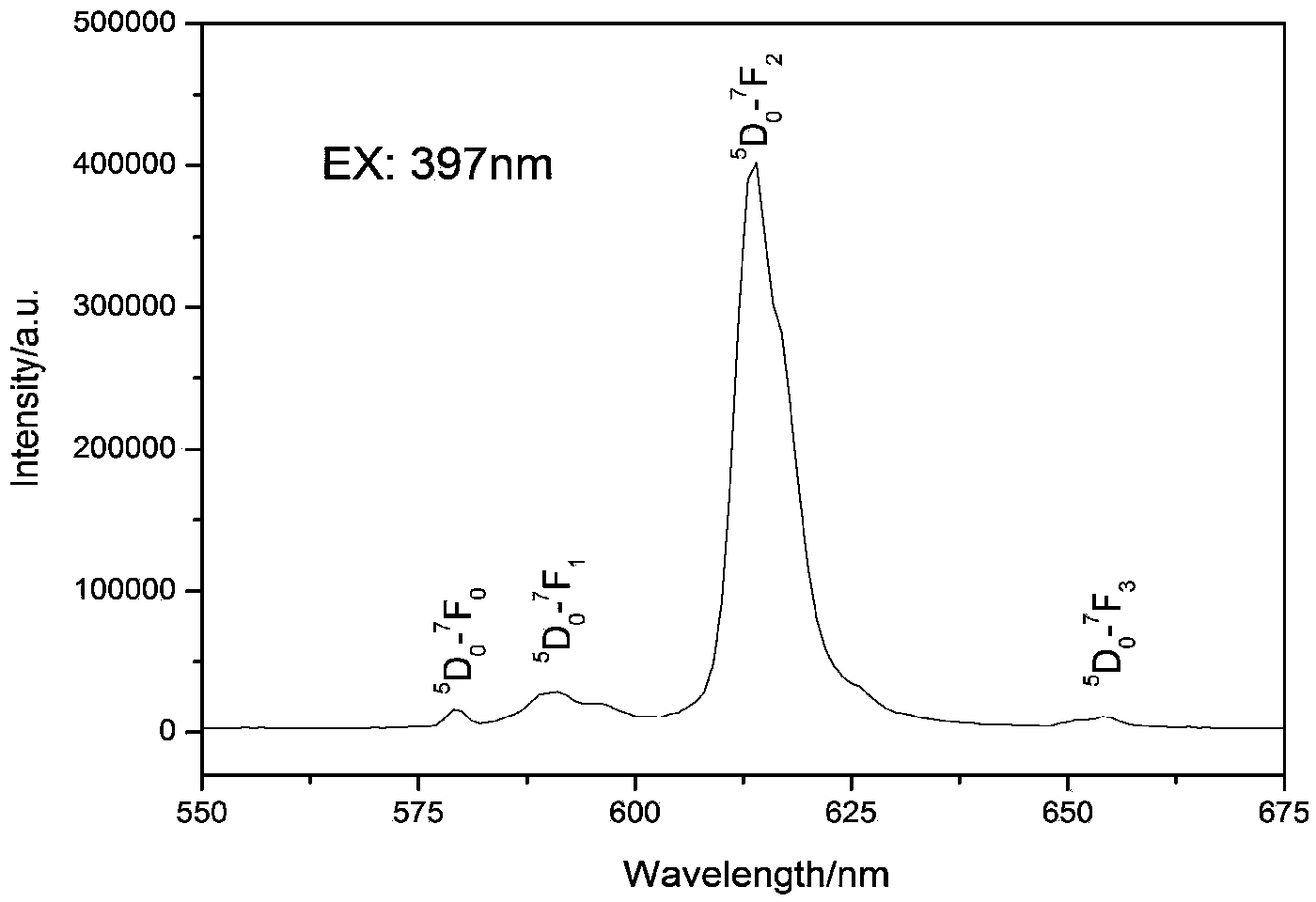
The invention discloses double-perovskite structured red fluorescent powder as well as a preparation method and application thereof, belonging to the field of fluorescent materials. The chemical formula of the fluorescent powder can be shown as Ln2-xAMO6:xEu and meets the following conditions: Ln is one, two or three of La, Gd and Y; A is one, two or three of Li, Na and K; M is one, two or three of Sb, Nb and Ta; and Eu is a luminescent center and is doped in the Ln position and the doping value x is 0.01-1.0. The fluorescent powder disclosed by the invention can emit fluorescence of 570-640 nm under the excitation of a green light chip (528-533 nm), a blue light chip (460-470 nm) or a near-ultraviolet light chip (390-399 nm), and red fluorescence is of 600-620 nm. The fluorescent powder can be used for white LED (light-emitting diode) and related display and lighting devices, has a simple preparation process, stable chemical properties and excellent luminescent performance, and is ideal fluorescent powder used for white LED.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI +1
Method for preparing dual-perovskite type methyl hydride combustion catalyst containing titanium
InactiveCN101293200ASimple structureHigh catalytic activityGaseous fuelsMetal/metal-oxides/metal-hydroxide catalystsEvaporationCoordination complex
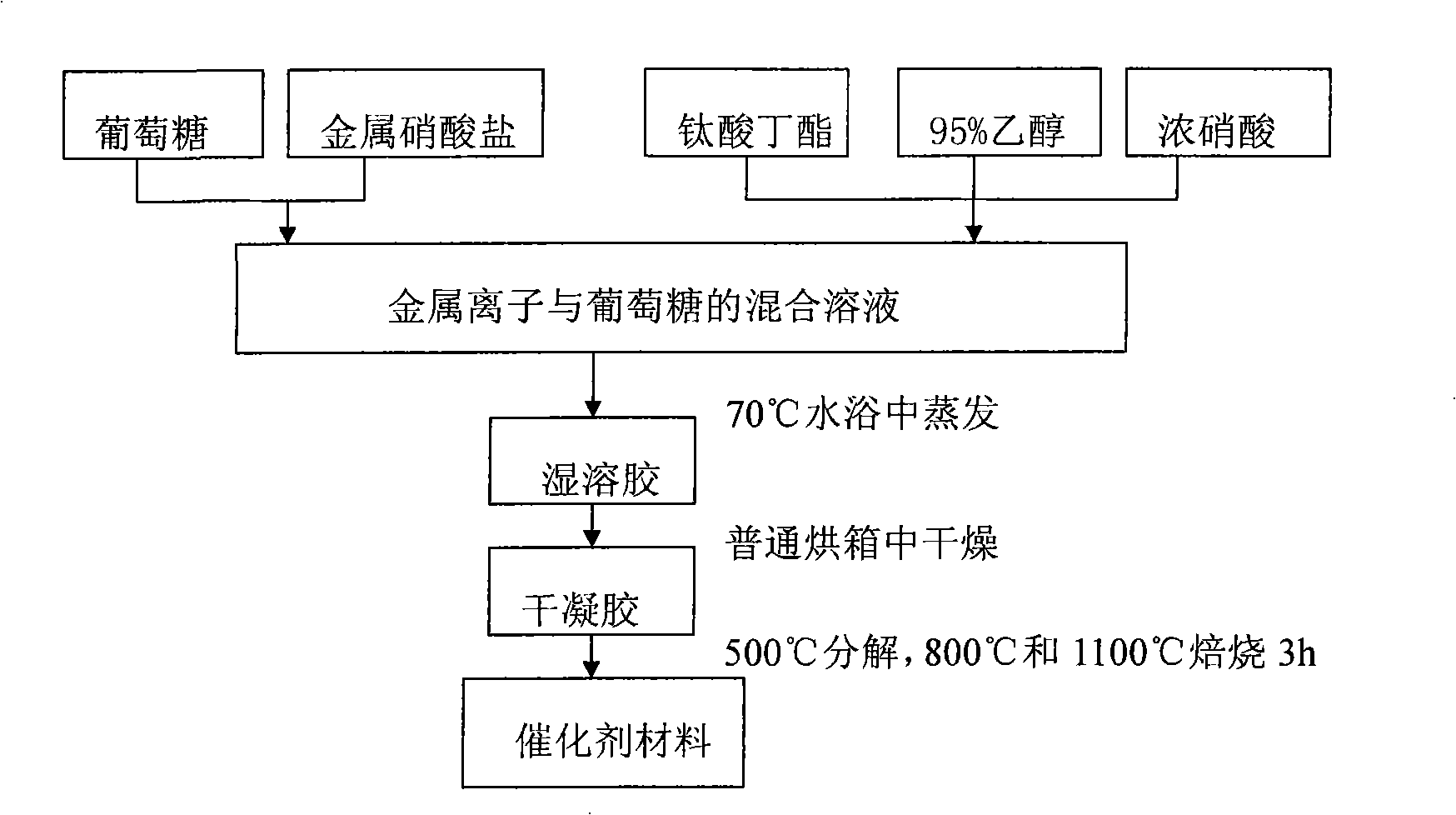
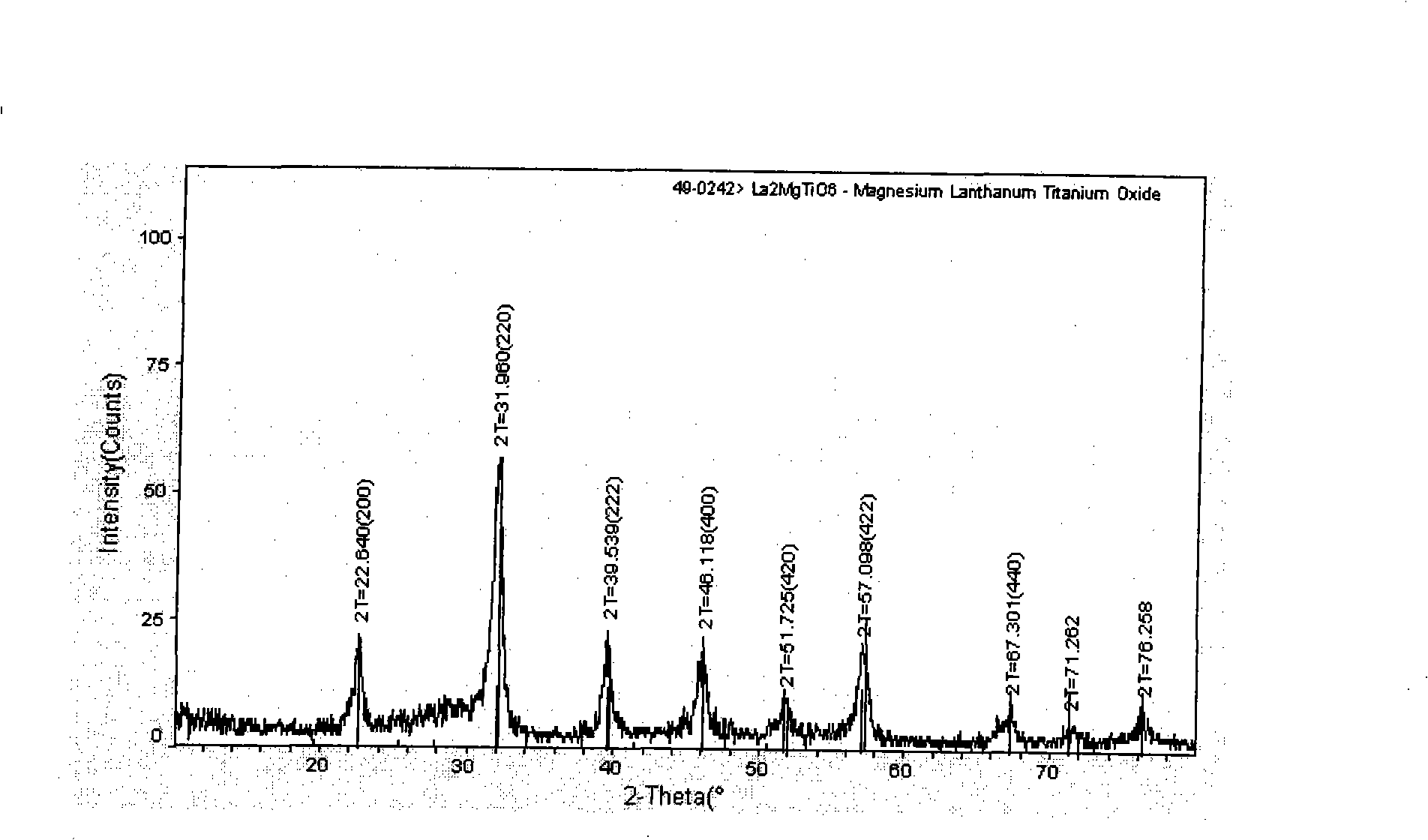
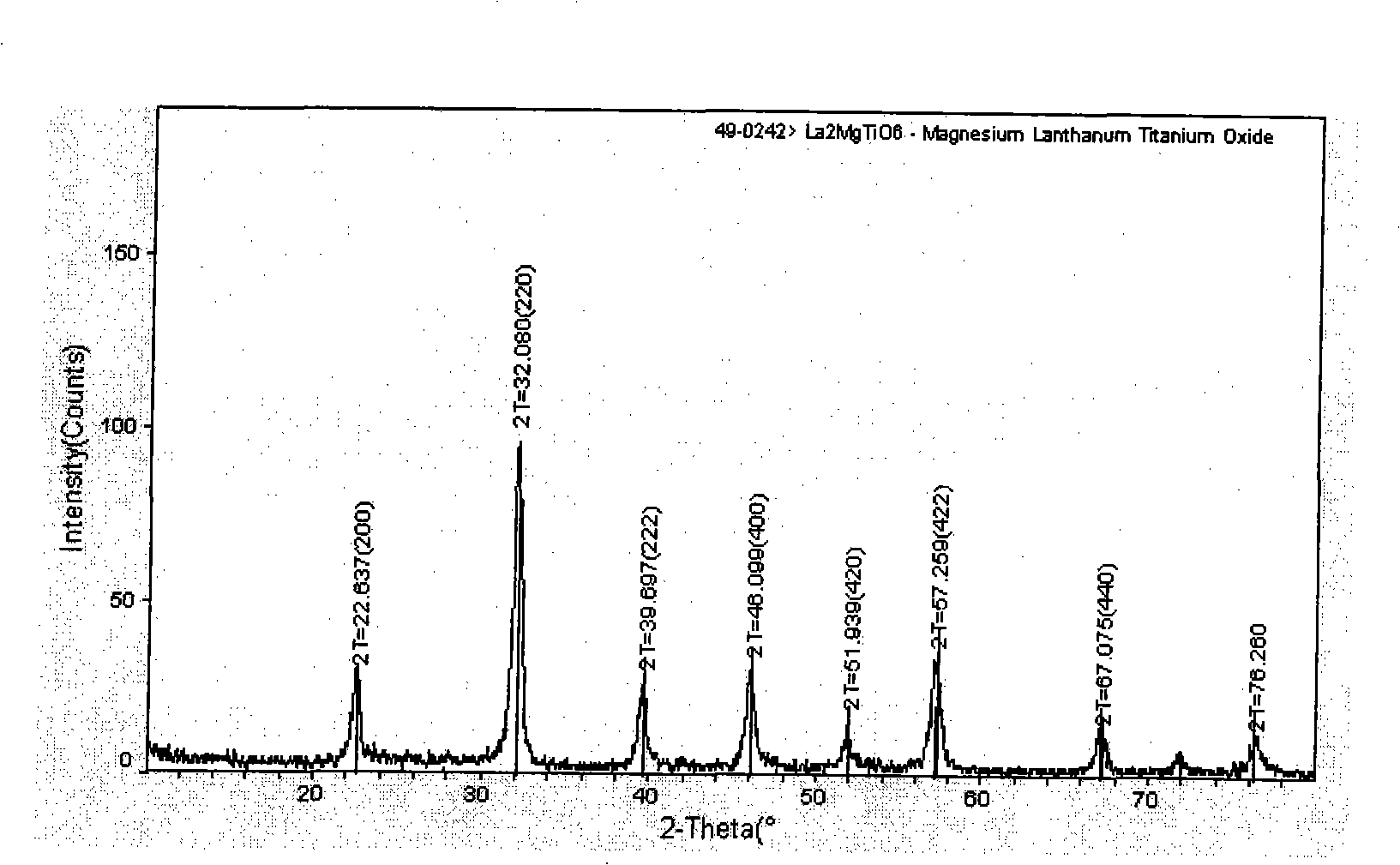
The present invention relates to a preparation method of titanium-containing double perovskite type methane combustion catalyst. The process is characterized in that a certain amount of butyl titanate is added into ethanol to produce white sediment; concentrated nitric acid is added until the sediment is totally dissolved and transparent solution is obtained; lanthanum nitrate and magnesium nitrate are weighed and dissolved in the deionized water and a certain amount of dextrose is added, thus completely forming composition solution; then gel is formed by constant-temperature stirring and evaporation; the gel obtained is dried and sintered at the temperature of 500 DEG C, 800 DEG C and 1,100 DEG C in the air for three hours to obtain the catalyst La2MgTiO6. The present invention is characterized in that the Sol-Gel Preparation Method with the dextrose as the complexing agent is adopted and the single-phase double layer perovskite type composite oxide catalyst is prepared at lower temperature. The preparation method is simple and low in cost. The catalyst used in methane catalytic combustion reaction has the characteristics of low initiation temperature and low whole transformation temperature.
Owner:INNER MONGOLIA UNIVERSITY
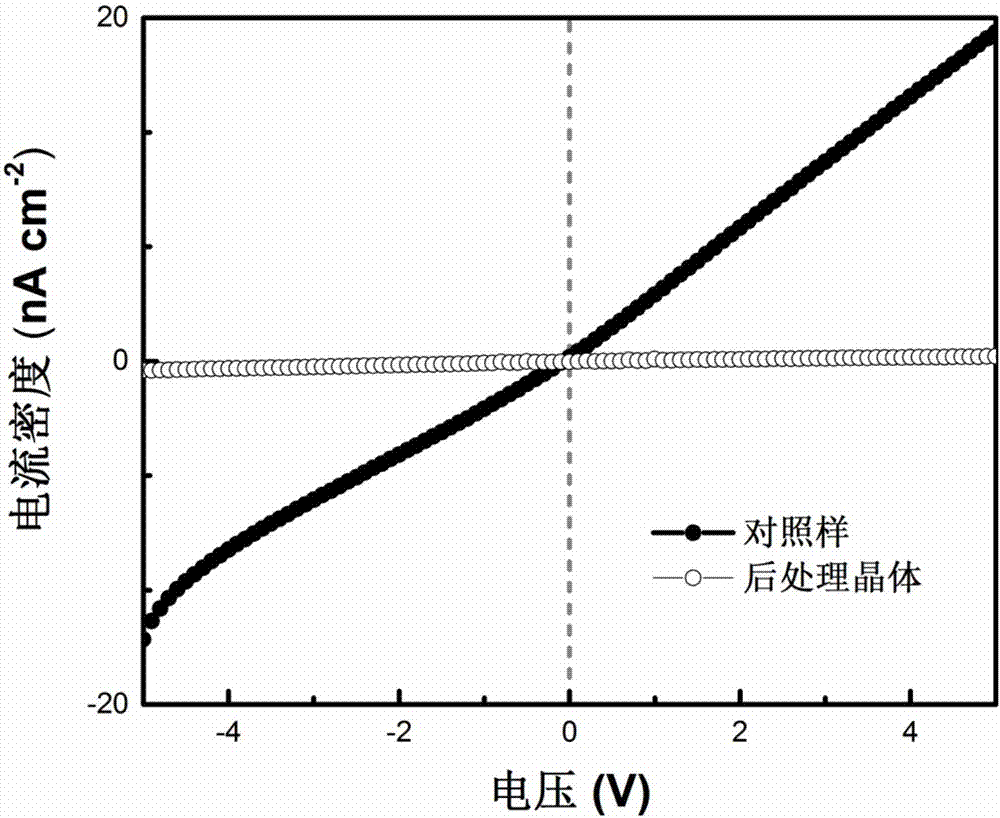
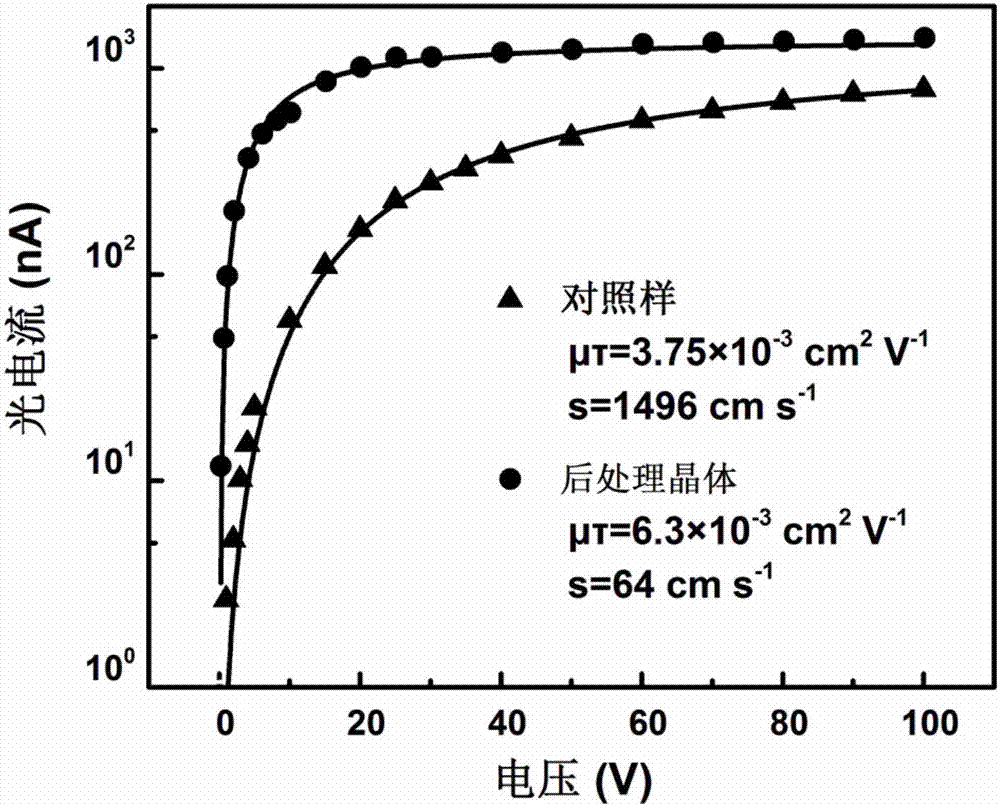
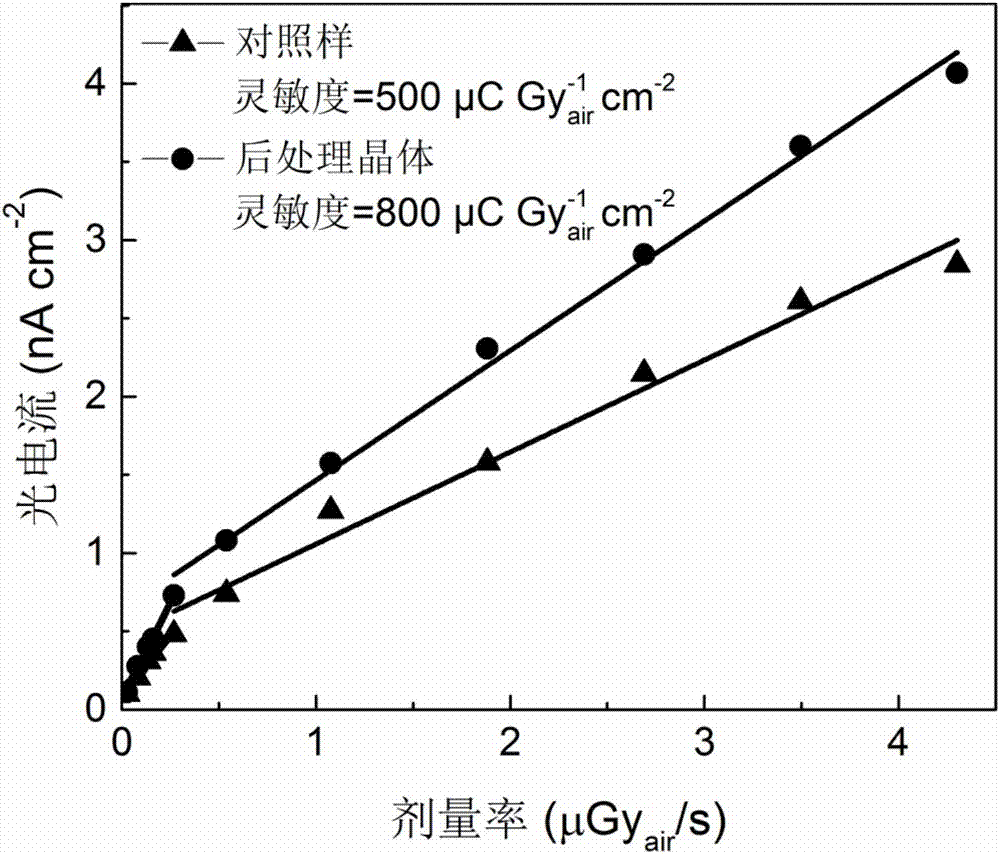
Post-processing method and application of double perovskite crystals
ActiveCN107248538ARaise the μτ productReduce leakage currentFinal product manufactureSemiconductor devicesCharge carrier mobilitySolvent
The invention discloses a post-processing method and application of double perovskite crystals. The post-processing method of double perovskite crystals comprises the following steps: performing annealing treatment on Cs2AgBiX6 double perovskite crystals; then cooling; and performing surface passivation treatment on the cooled crystals by means of a solvent to improve the mobility of the double perovskite crystals and reduce the surface recombination rate. The invention improves the technological process adopted in the key post-processing and the specific condition parameters adopted in each process step, therefore, the problems of the double perovskite Cs2AgBiX6 crystals in the prior art that the probability of occurrence of Ag and Bi dislocation is high, the internal defects of the crystals are more, the product of the carrier mobility multiply by the carrier lifetime ([mu] [tau]) of the crystals is not high are effectively solved; moreover, the crystals obtained by means of the post-processing method disclosed by the invention are especially suitable for using in radiation detectors.
Owner:HUAZHONG UNIV OF SCI & TECH
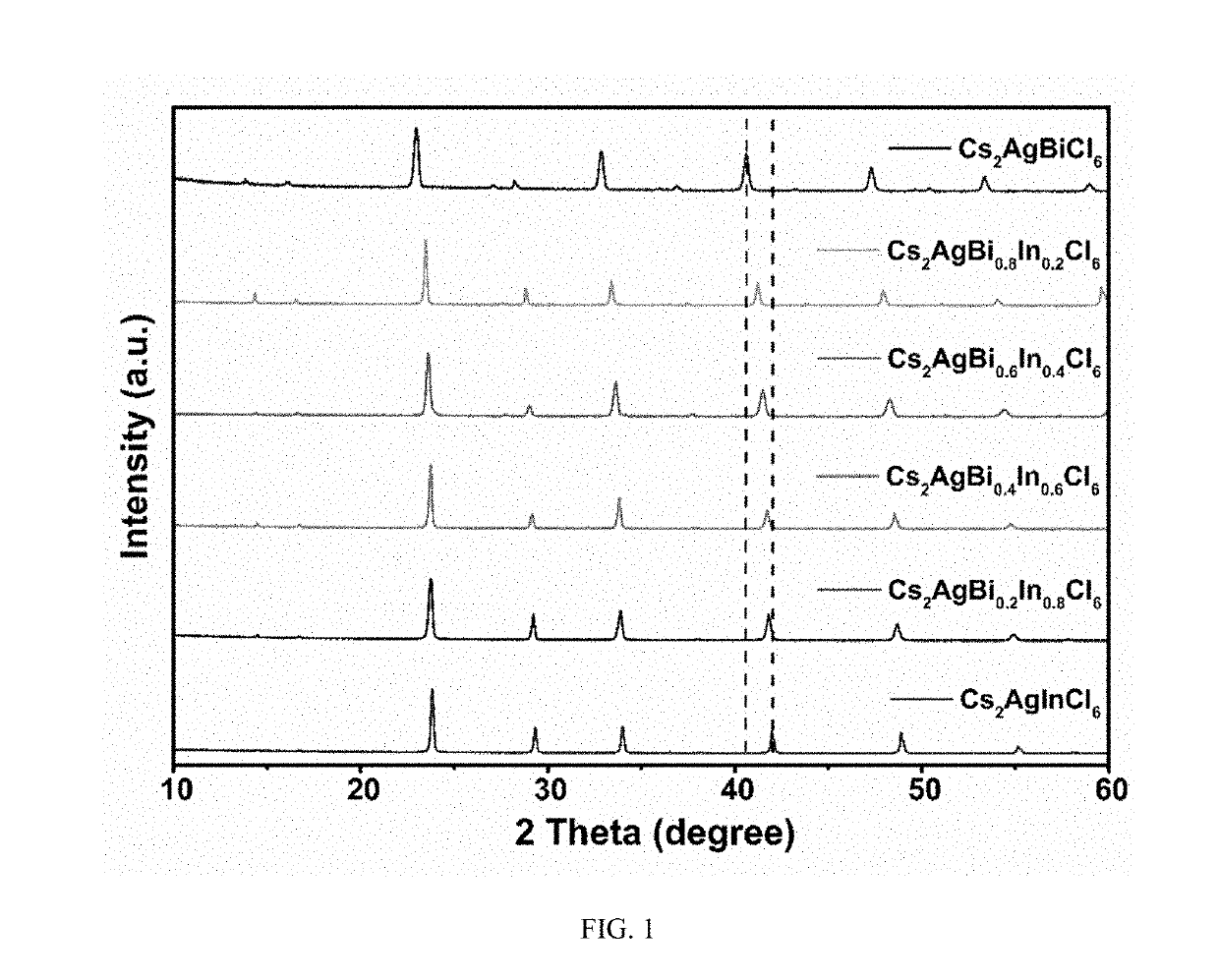
Multi-element perovskite material as well as preparation and luminescent application thereof
ActiveUS20190330074A1High fluorescence yieldImprove luminous performancePolycrystalline material growthFrom normal temperature solutionsPhotoluminescenceSingle crystal
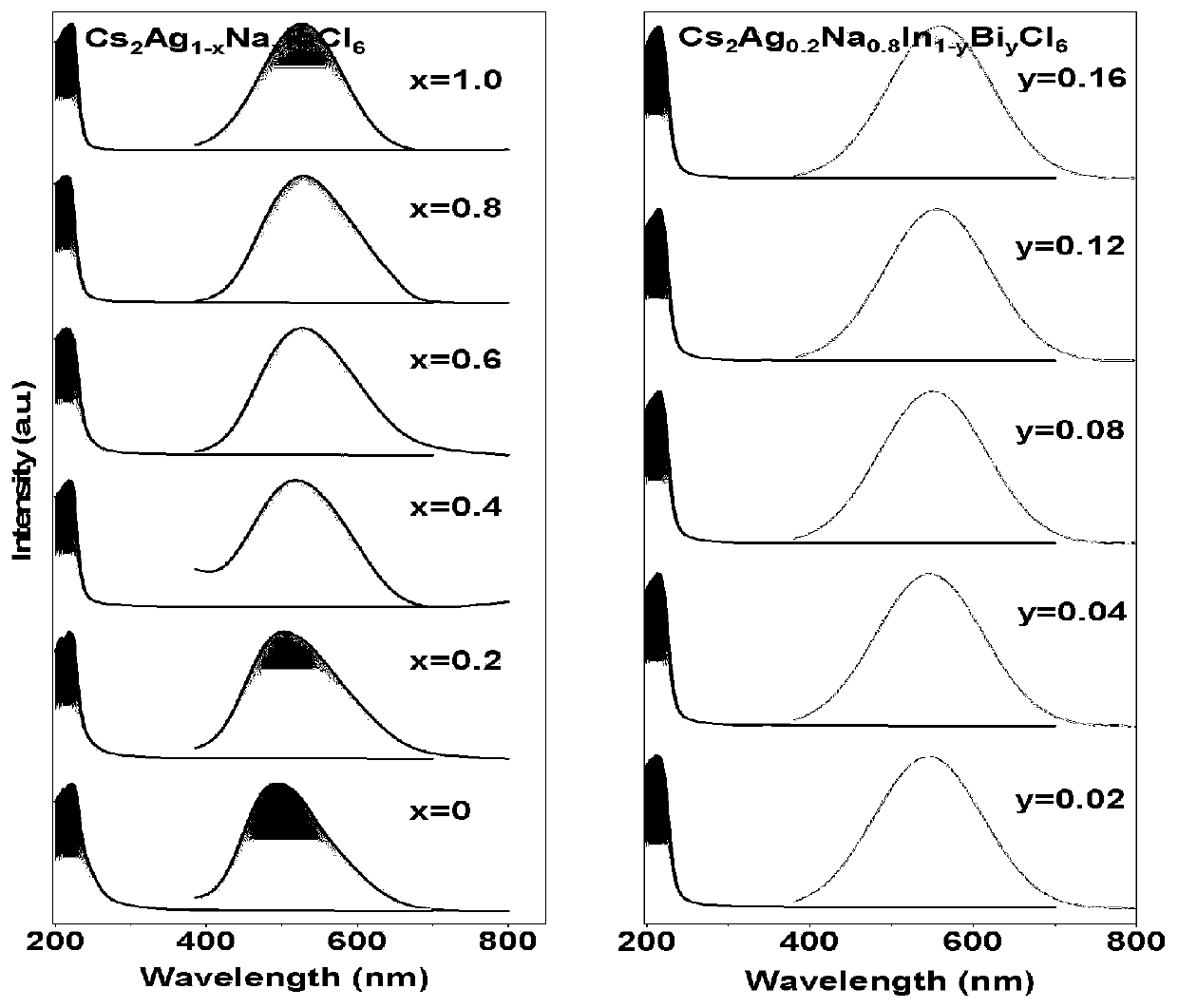
The present invention discloses a multi-element perovskite material, and a single crystal, powder and a film thereof, as well as the applications thereof in photoluminescence and electroluminescence, in which the multi-element perovskite material is a multi-element fully-inorganic salt of non-lead metal halide and has a perovskite structure; and the chemical formula of the multi-element perovskite material is Cs2NaxAg1-xInyBi1-yCl6, wherein 0≤x≤1, 0≤y≤1. Meanwhile, based on the very strong self-trapped excitors states of the double perovskite, the present invention proposes a high-efficiency single-phase broadband phosphor and an electroluminescent device.
Owner:HUAZHONG UNIV OF SCI & TECH
Double perovskite type inorganic nano fiber and preparation method thereof
InactiveCN104313729AStable one-dimensional structureLarge specific surface areaInorganic material artificial filamentsFiberNickel salt
The invention relates to double perovskite type inorganic nano fiber and a preparation method thereof and belongs to the field of inorganic nano fiber materials. The preparation method comprises the following steps: mixing polyvinylpyrrolidone with a solvent to obtain a spinning precursor solution, and performing electrostatic spinning, preoxidation and carbonization to the spinning precursor solution to obtain the double perovskite type inorganic nano fiber; the spinning precursor solution comprises the following components in mass percent: 10% to 20% of inorganic salt, 10% to 30% of polyvinylpyrrolidone and the balance of solvent, wherein the inorganic salt is the mixture of lanthanum salt, cobalt salt and metal salt III, the molar ratio of the lanthanum salt, the cobalt salt and the metal salt III is 2:1:1, and the metal salt III is nickel salt, ferric salt or manganese salt. The invention has the advantages that the preparation method is simple and easy to implement, the raw material consumption is low, the product purity is high, and the obtained double perovskite type inorganic nano fiber has a stable one-dimensional structure, is relatively high in draw ratio and uniform in diameter.
Owner:DALIAN JIAOTONG UNIVERSITY
Deep-ultraviolet detector based on lead-free double-perovskite film, and preparation method
ActiveCN108400244ASimple preparation processOvercome instabilityFinal product manufactureSolid-state devicesElectronic transmissionInstability
The invention belongs to the technical field of the semiconductor photoelectric detection, and specifically relates to a deep-ultraviolet detector based on a lead-free double-perovskite film, and a preparation. The structure is composed of a double-side polished Al2O3 substrate, n-type bandwidth gap electronic transmission layer, a Cs2AgBiBr6 light absorption layer and a contact electrode. On theone hand, the instability and the lead positioning and like adverse factors of the traditional perovskite structure are overcome; on the other hand, the fast separation and transmission of the photo-generated carrier can be realized by means of the energy band matching between the bandwidth gap electronic transmission layer and the Cs2AgBiBr6 light absorption layer, and the light detection range of the device can be extended to the deep ultraviolet region from the visible light. The preparation method of the device disclosed by the invention is simple and practical, and environment-friendly; the prepared photoelectric detector has high detection rate and response speed, and has very important application value.
Owner:ZHENGZHOU UNIV
Method for preparing double perovskite magnetic material Sr2MWO6(M=Fe or Mn)
InactiveCN1885445AUniform sizeLow reaction temperatureInorganic material magnetismAmmonium paratungstateSolid reaction
The provided preparation method for a Sr2MWO6 (M = Fe or Mn) material comprises: using the Sr(NO3)2, Fe(NO3)3, Mn(NO3)4 and ammonium paratungstate as material, citric acid as complexant, and ethylene glycol as dispersant; reacting for 20-40h at 950-1150Deg to obtain the biperovskite-structure product. This invention uses sol-gel technique with low temperature and short time, removes the SrWO4 in solid phase reaction, and fit to large-scale production.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Lead-free hybrid two-dimensional double perovskite material and preparation method thereof
InactiveCN109369725AGood chemical stabilitySuitable and tunable optical absorption bandgapOrganic chemistry methodsBismuth organic compoundsPhoto stabilityOpto electronic
Owner:XI AN JIAOTONG UNIV
Buffer layers for rebco films for use in superconducting devices
InactiveUS20120264612A1Superconductors/hyperconductorsSuperconductor detailsAlkaline earth metalRare earth
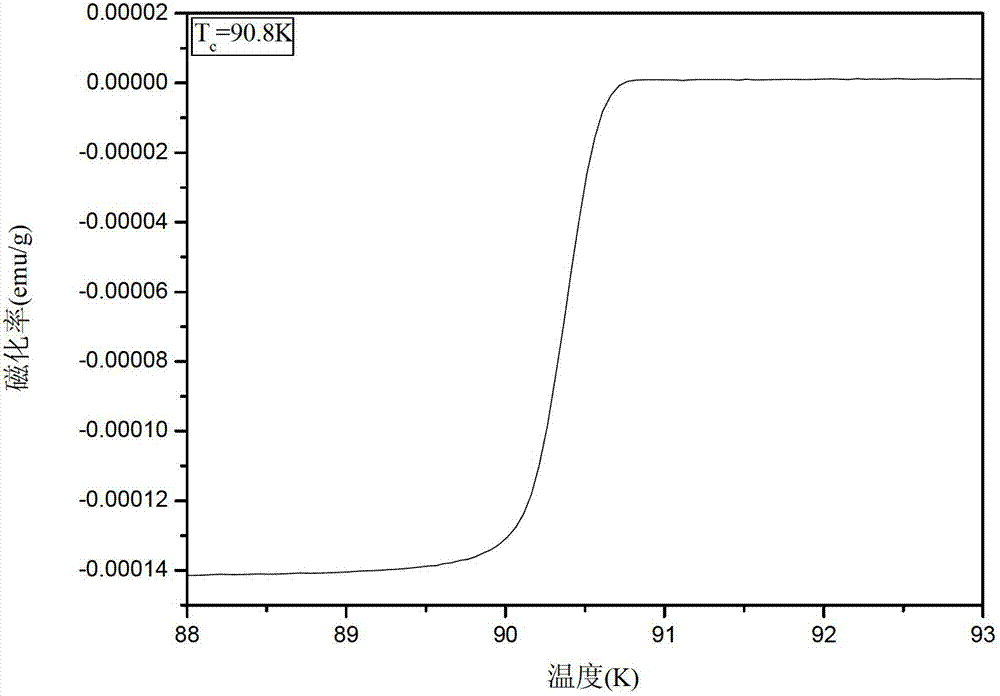
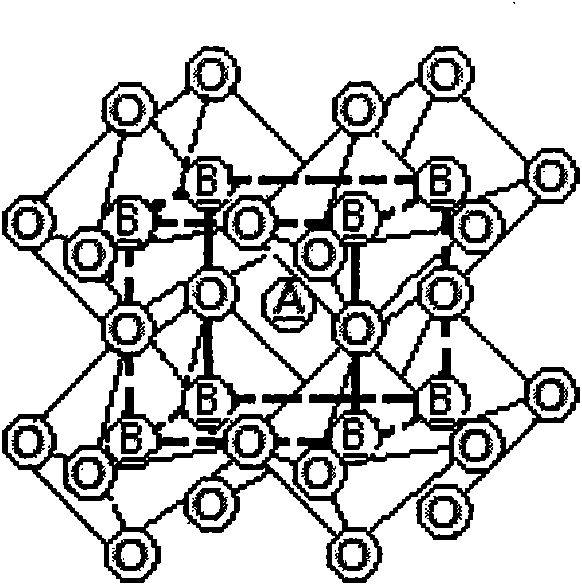
A superconducting article includes a substrate having a biaxially textured surface. A biaxially textured buffer layer, which can be a cap layer, is supported by the substrate. The buffer layer includes a double perovskite of the formula A2B′B″O6, where A is rare earth or alkaline earth metal and B′ and B″ are different transition metal cations. A biaxially textured superconductor layer is deposited so as to be supported by the buffer layer. A method of making a superconducting article is also disclosed.
Owner:UNIV OF TENNESSEE RES FOUND +1
Spin field effect transistor using half metal and method of manufacturing the same
ActiveUS20090121267A1Increase charge mobilityReduce the amount requiredNanomagnetismSemiconductor/solid-state device manufacturingHalf-metalAlloy
A spin field effect transistor may include at least one gate electrode, a channel layer, a first stack and a second stack separate from each other on a substrate, wherein the channel layer is formed of a half metal. The half metal may be at least one material selected from the group consisting of chrome oxide (CrO2), magnetite (Fe3O4), a double perovskite structure material, a Heusler alloy, NiMnSb, La(1-x)AxMnO3 (A=Ca, Ba, Sr, x˜0.3), and GaN doped with Cu, and the double perovskite structure material is expressed as a chemical composition of A2BB′O6, and a material corresponding to A is Ca, Sr, or Ba, a material corresponding to B is a 3d orbital transition metal, and a material corresponding to B′ is a 4d orbital transition metal. The 3d orbital transition metal may be Fe or Co, and the 4d orbital transition metal is Mo or Re.
Owner:SAMSUNG ELECTRONICS CO LTD
Double-perovskite composite metal oxide catalyst and preparation method and application thereof
ActiveCN106475105AThe synthesis method is simpleLow priceGas treatmentHeterogenous catalyst chemical elementsReaction temperatureTemperature resistance
The invention discloses a double-perovskite composite metal oxide catalyst and a preparation method and application thereof and belongs to the technical field of atmospheric pollution treatment. The condensed structural formula of the double-perovskite composite metal oxide catalyst is A2B2O6, wherein A is La, Sr or Ce, and B is Mn, Fe, Co, Cu or Ni. The double-perovskite composite metal oxide catalyst has the advantages that the catalyst is excellent in high temperature resistance, water resistance and chlorine resistance and applicable to effective degradation of CVOCs under an industrial high-temperature environment; the catalyst can achieve complete oxidization of industrial typical CVOCs (1,2-dichloroethane) under reaction temperature of 500-550 DEG C, reaction air speed of 20000-30000h<-1> and oxygen concentration of 10-20%; meanwhile, by regulating the loading capacity of transition metal oxide, the oxidizing efficiency of the catalyst can be increased effectively, and the selectivity of the reaction product CO2 can be increased greatly. The preparation method of the catalyst is simple, cheap in raw material and promising in industrial application prospect.
Owner:XI AN JIAOTONG UNIV
Double perovskite
ActiveUS20180290897A1Reduce usageSignificant environmental benefitsLight-sensitive devicesSolid-state devicesSemiconductor materialsDouble perovskites
The present invention relates to a semiconductor device comprising a semiconducting material, wherein the semiconducting material comprises a compound comprising: (i) one or more first monocations [A]; (ii) one or more second monocations [BI]; (iii) one or more trications [BIII]; and (iv) one or more halide anions [X]. The invention also relates to a process for producing a semiconductor device comprising said semiconducting material. Also described is a compound comprising: (i) one or more first monocations [A]; (ii) one or more second monocations [BI] selected from Cu+, Ag+ and Au+; (iii) one or more trications [BIII]; and (iv) one or more halide anions [X].
Owner:OXFORD UNIV INNOVATION LTD
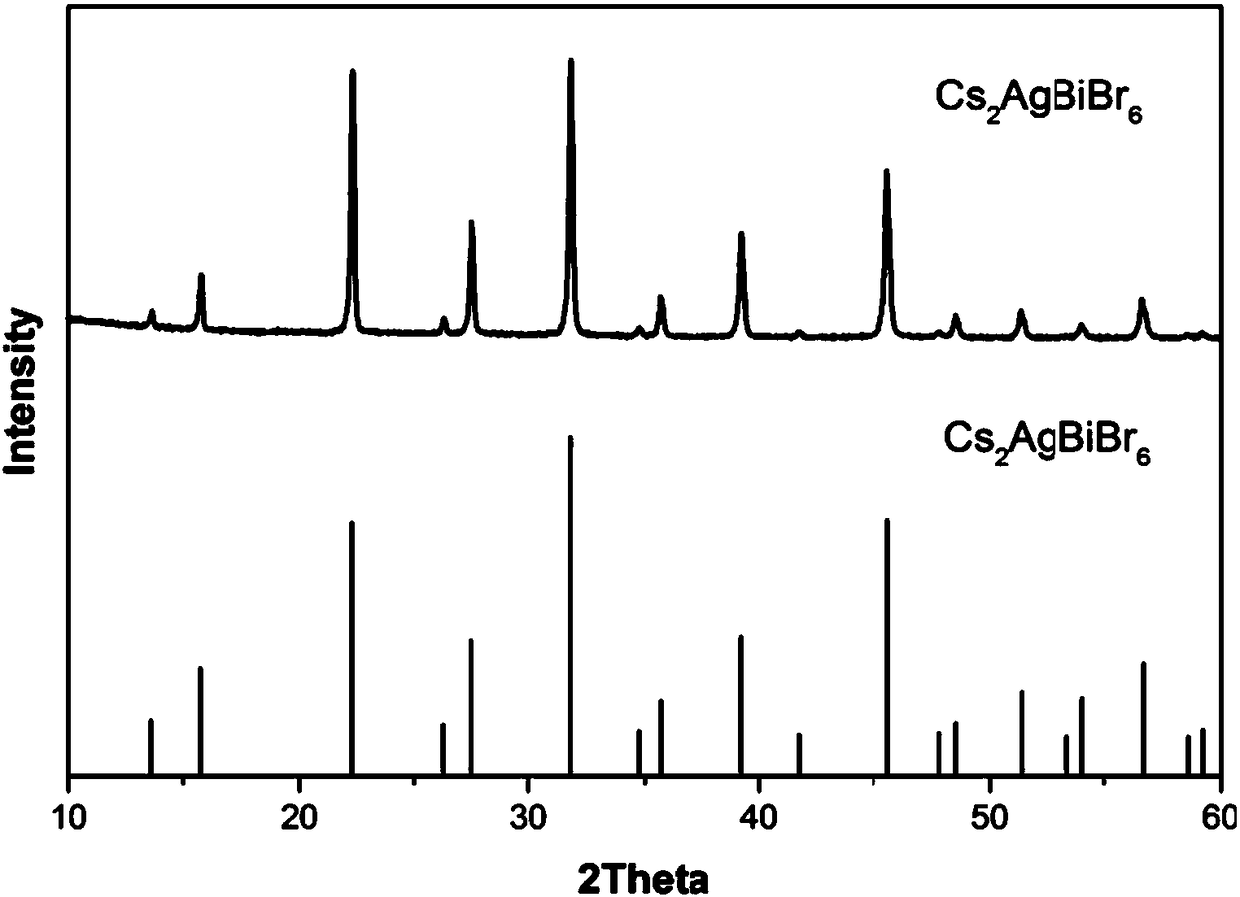
Cs2AgBiBr6 double perovskite and preparation method thereof
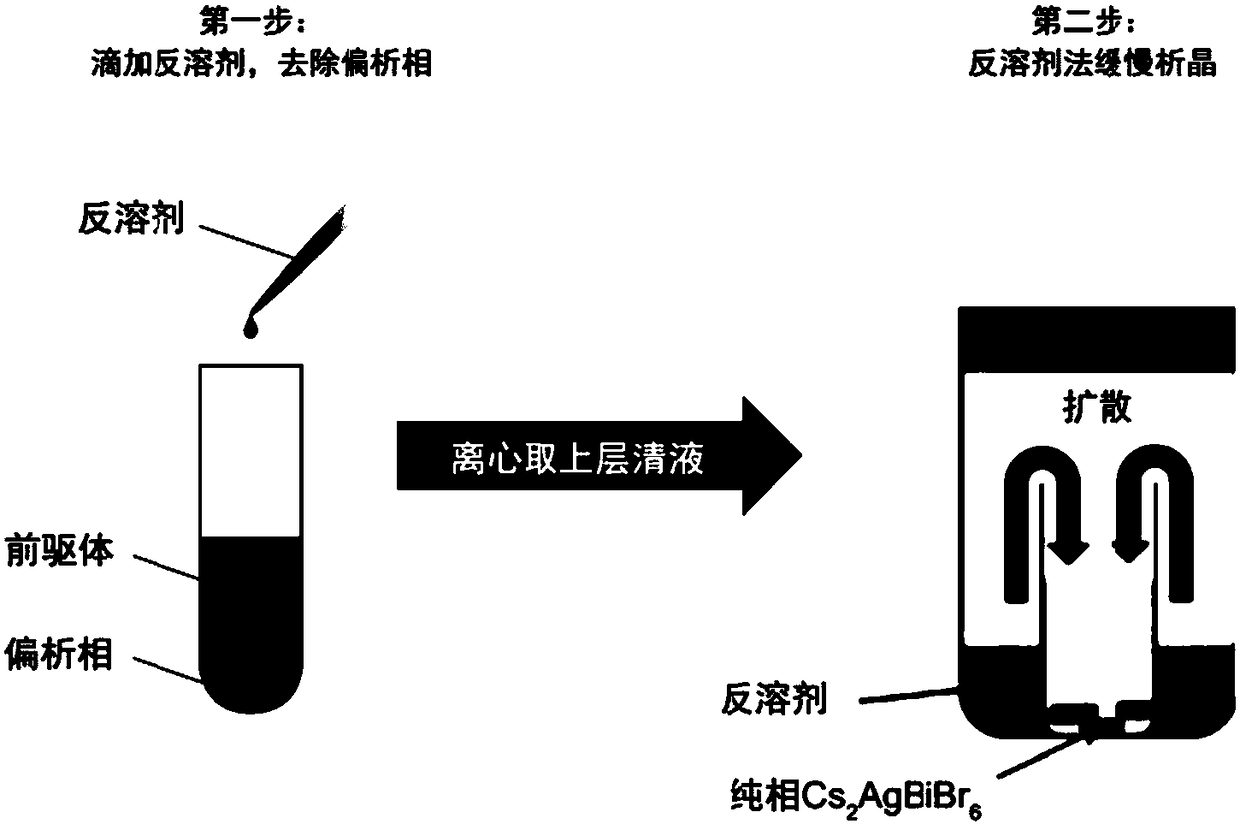
ActiveCN108559503AAvoid component segregationImprove solubilityLuminescent compositionsSolubilityOrganic solvent
The invention discloses Cs2AgBiBr6 double perovskite and a preparation method thereof. The preparation method is characterized in that a two-step crystallization process is introduced to avoid the component segregation phenomenon of Cs2AgBiBr6 perovskite in an organic solvent. The preparation method mainly comprises the following steps: titrating a precursor solution by using an anti-solvent, removing early crystallization by-products and taking clear liquid subjected to centrifugal separation as a crystal growth solution for the Cs2AgBiBr6 perovskite; then slowly synthesizing a Cs2AgBiBr6 perovskite material thorough the anti-solvent crystallization process. The preparation method of the Cs2AgBiBr6 double perovskite, disclosed by the invention, can effectively avoid the segregation phenomenon caused by different solubilities of components. Compared with acid as a solvent, the organic solvent has the advantages of greater solubility, lower requirements on sealing and corrosion resistance of containers; the synthesis efficiency can be significantly improved.
Owner:HUAZHONG UNIV OF SCI & TECH
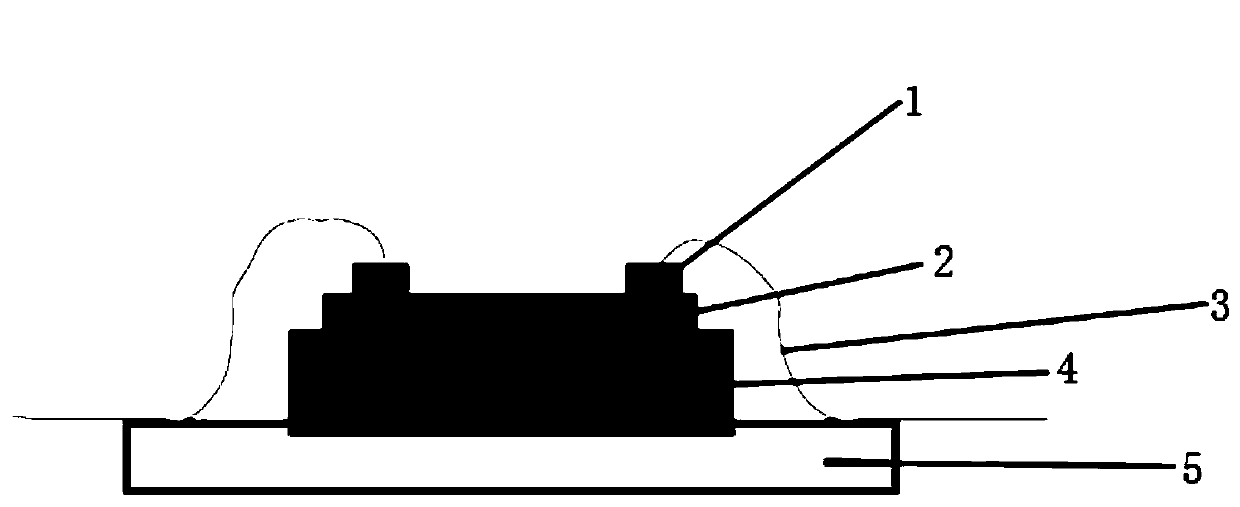
Double perovskite single crystal photodetector and preparation method thereof
InactiveCN109786486AEasy to detectIncrease the carrier concentrationFinal product manufactureSemiconductor devicesPhotovoltaic detectorsPhotodetector
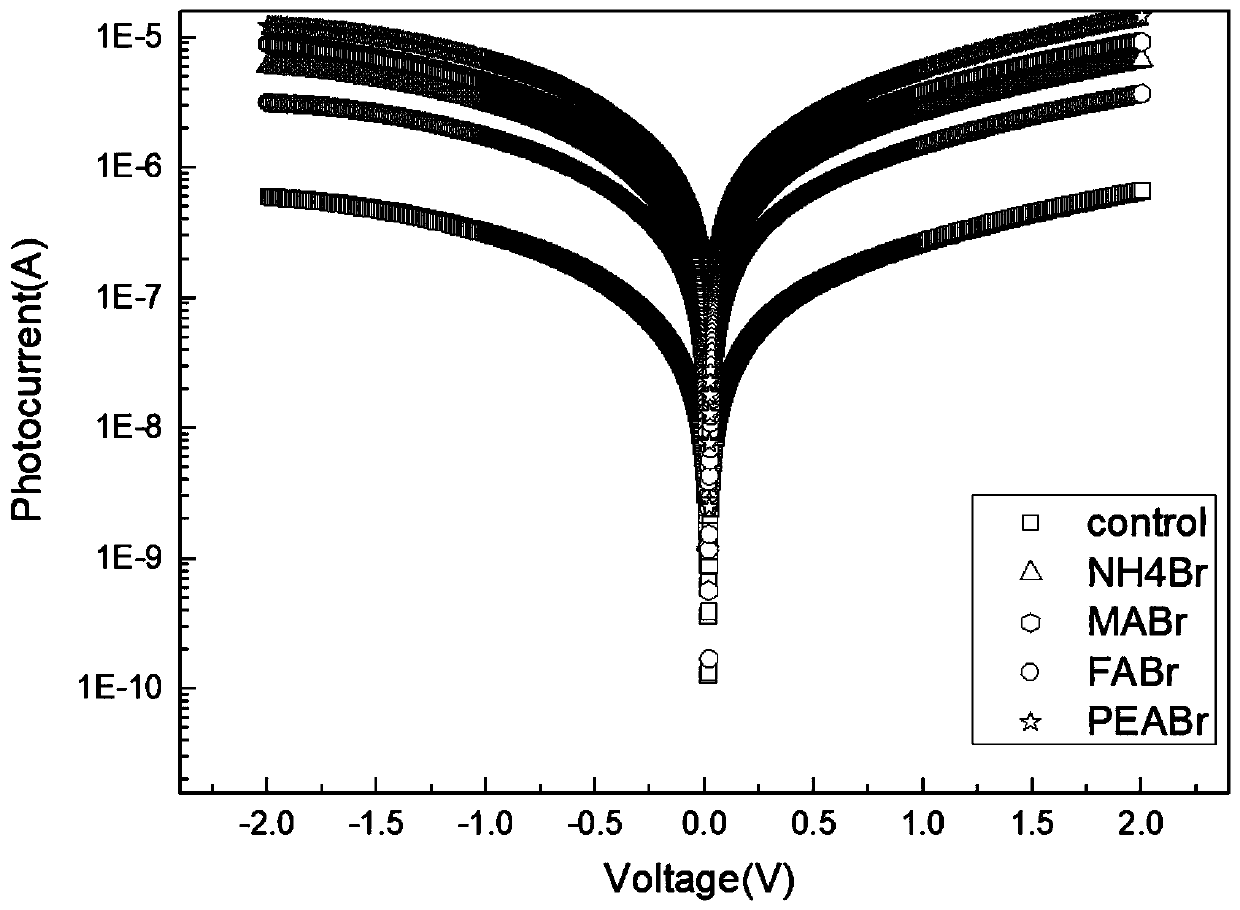
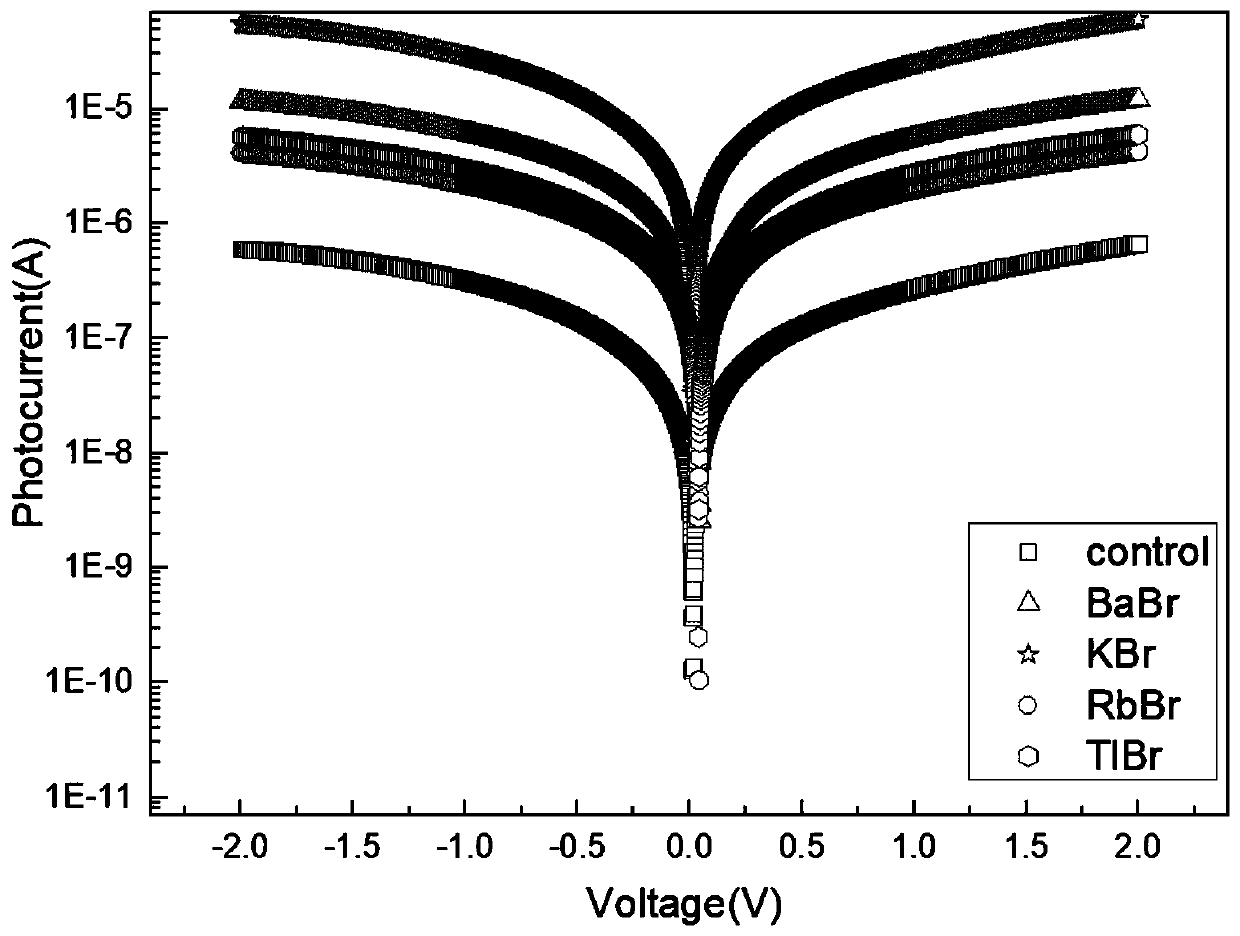
The invention discloses a double perovskite single crystal photodetector and a preparation method thereof. The double perovskite single crystal photodetector comprises a substrate, wherein the substrate is provided with a double perovskite single crystal, electrodes and a silver glue in sequence, the two electrodes are respectively connected to a conductive gold wire, the double perovskite singlecrystal is in a double perovskite structure formed by adding different cations in a solution of the perovskite growth single crystal, and each of the added cations is one of methylamine ion (MA+), formamidine ion (FA+), phenethylamine ion (PEA+), NH4+, butylamine ion (Ba+), K+, Rb+ and Tl+. By doping different cations in the double perovskite single crystal photodetector, the carrier concentrationof the device is increased under illumination conditions, which leads to an increase in photocurrent and enhances the detection performance of the photodetector.
Owner:JINAN UNIVERSITY
Double-perovskite structure catalyst material for cathode of lithium air battery and preparation method of catalyst material
InactiveCN103545537AImprove energy conversion efficiencyEasy to prepareFuel and primary cellsCell electrodesAir atmosphereLithium-ion battery
The invention relates to a double-perovskite structure catalyst material for a cathode of a lithium air battery and a preparation method of the catalyst material. The molecular formula of the catalyst material is AxA'(2-x)CryMo(2-y)O6, wherein A is one of Sr, Ca or Ba; A' is one of Sr, Ca, Ba, La, Ce, Pr, Nd, Sm, Eu or Gd; x is greater than 1 and less than 2; y is greater than 0 and less than 2. The preparation method comprises the following steps: obtaining xerogel by a sol-gel method; performing one-step or multi-step presintering on the obtained xerogel in an air atmosphere to obtain solid powder; and tableting the obtained solid powder, and roasting in a reducing atmosphere to obtain the double-perovskite structure material. Compared with the prior art, the catalyst disclosed by the invention is a single pure phase with stable physicochemical property; the preparation method is simple with good repeatability. The catalyst for a cathode of the lithium air battery has the advantages that the specific capacity of the battery is increased and the energy conversion efficiency of the battery is effectively improved. Moreover, the lithium air battery assembled by use of the material has a good cycle life.
Owner:SHANGHAI JIAO TONG UNIV
Chemical looping combustion double perovskite type oxide oxygen carrier and preparation method and application thereof
InactiveCN102441397AHigh activityImprove thermal stabilityHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsRare earthEvaporation
The invention discloses a chemical looping combustion double perovskite type oxide oxygen carrier and a preparation method and application thereof. The oxygen carrier is a composite metal oxide with a double perovskite structure; and the general formula of the composite metal oxide is A2B'B''O6, wherein A is rare earth metal lanthanum, B' is transition metal nickel, and B'' is transition metal iron. In the application of the oxygen carrier in a chemical looping combustion technology, the temperature of the oxygen carrier in an air reactor is 500-1,000 DEG C; and the temperature of the oxygen carrier in a combustion reactor is 500-1,000 DEG C. The preparation method of the oxygen carrier comprises the following steps of: taking iron nitrate, nickel nitrate and lanthanum nitrate as precursors; taking citric acid as a complexing agent; preparing a solution from the precursors and the complexing agent, and evenly mixing and stirring; carrying out water evaporation while the solution changes into viscous gel from transparent colloidal sol; then, drying; and finally, roasting, wherein the roasted sample is the composite metal oxide with the double perovskite structure. The oxygen carrier disclosed by the invention has high oxygen-carrying rate, high activity and good stability.
Owner:CHINA PETROLEUM & CHEM CORP +1
Double-doped double perovskite red phosphor and preparation method of double-doped double perovskite red phosphor
InactiveCN103146385ABroaden the absorption widthHigh luminous intensityLuminescent compositionsTungstateRare earth
Owner:NANJING UNIV OF TECH
Application of double perovskite nanocrystalline material in preparation of inorganic white light LED
ActiveCN110484246AAdjust luminosityChange contentNanoopticsEnergy efficient lightingHalogenUltraviolet
The invention belongs to the technical field of luminescent materials, and more specifically relates to an application of a double perovskite nanocrystalline material in preparation of an inorganic white light LED, the chemical general formula of the lead-free double perovskite nanocrystalline material is Cs<2-x>A<x>Ag<1-y>B<y>In<1-z>C<z>D<6>, and A and B are independently positive monovalent cations; C is a positive trivalent cation; D is a halogen anion; 0 < = x < = 1; 0 < = y < = 1.0; 0 < = z < = 1.0. According to the invention, the composition of each lattice element in the double perovskite is partially substituted; by controlling and replacing the corresponding molar fractions x, y and z, the correspondingly obtained ultraviolet-excited double-perovskite single-matrix white-light nanocrystalline is wide in emission spectrum range and simple to prepare, the related color temperature can be flexibly regulated and controlled along with components, and the ultraviolet-excited double-perovskite single-matrix white-light nanocrystalline is particularly suitable for electroluminescent LED chips.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Double-perovskite tungsten molybdate red fluorescent powder for white light LED and preparation method of double-perovskite tungsten molybdate red fluorescent powder
ActiveCN104371719AEfficient excitationHigh luminous intensityEnergy efficient lightingLuminescent compositionsAlkaline earth metalMolybdate
The invention relates to double-perovskite tungsten molybdate red fluorescent powder for a white light LED and a preparation method of the double-perovskite tungsten molybdate red fluorescent powder. The chemical expression of the red fluorescent powder is AA<II>1-xLi<+>B<II>O6:xEu<3+>, wherein Eu<3+> is an active ion doped to an A<II> site, and the doping amount x is more than or equal to 0.01 and less than or equal to 0.6; A is any one of or a combination of more of divalent alkaline earth metal ions Ca<2+>, Sr<2+> and Ba<2+>; A<II> is any one of or a combination of more of trivalent rare-earth ions La<3+>, Gd<3+>, Y<3+> and Sc<3+>; B<II> is any one of or a combination of W<6+> and Mo<6+>; and the red fluorescent powder is prepared by adopting a low-temperature combustion method. The double-perovskite tungsten molybdate red fluorescent powder disclosed by the invention can be efficiently excited by near ultraviolet and blue light chips, the luminous efficiency is improved, the preparation process is simple, and the synthesis temperature is low, so that the double-perovskite tungsten molybdate red fluorescent powder is ideal red fluorescent powder suitable for the white light LED.
Owner:ANHUI UNIV OF SCI & TECH
Double perovskite type metal oxide catalyst and preparation method thereof
ActiveCN105107520ALarge specific surface areaHigh catalytic activityMetal/metal-oxides/metal-hydroxide catalystsAlkaline earth metalCatalytic combustion
Relating to the technical field of catalysts, the invention discloses a double perovskite type metal oxide catalyst and a preparation method thereof to solve the problems of low catalytic activity and poor high-temperature stability o existing LaMnO3. The general formula of the double perovskite type metal oxide catalyst is: La1-xMxD1-yMnyO3, wherein x=0-0.6, y=0.7-1, M is alkali metal ion or alkaline earth metal ion, and D is +1, +2 or +3 valence metal ion. According to the double perovskite type metal oxide catalyst provided by the invention, the M ion and D ion are introduced to adjust the composition and structure of the perovskite metal oxide and increase the specific surface area, thereby enhancing the catalytic activity and high temperature stability of the catalyst. The catalyst provided by the invention is mainly applied to catalytic combustion of methane.
Owner:ENN SCI & TECH DEV
Double-perovskite type intermediate temperature solid oxide fuel cell anode material and preparation method
InactiveCN104900887ALow average coefficient of thermal expansionLow costCell electrodesNitrateThermal expansion
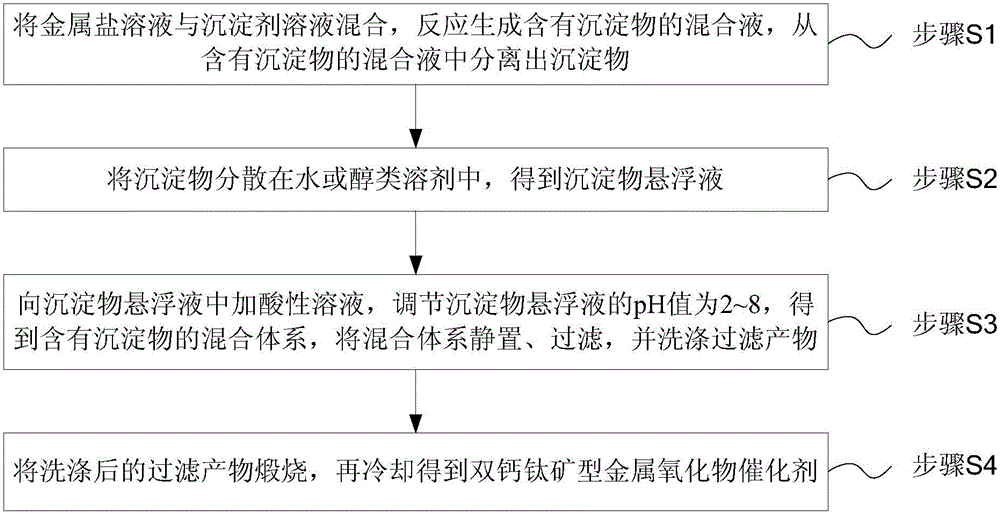
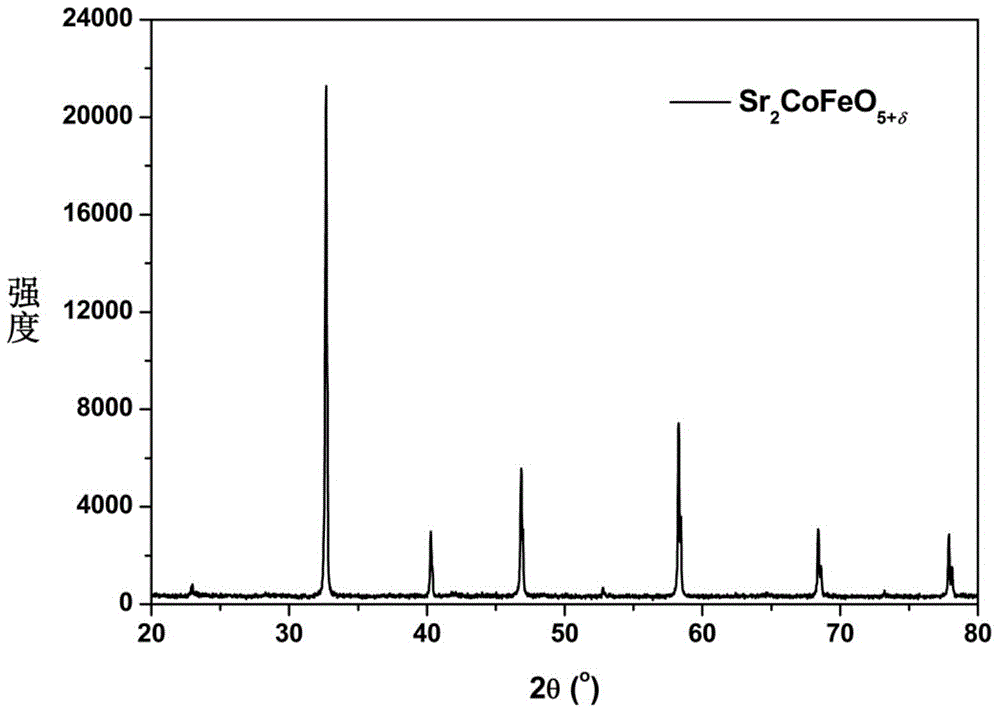
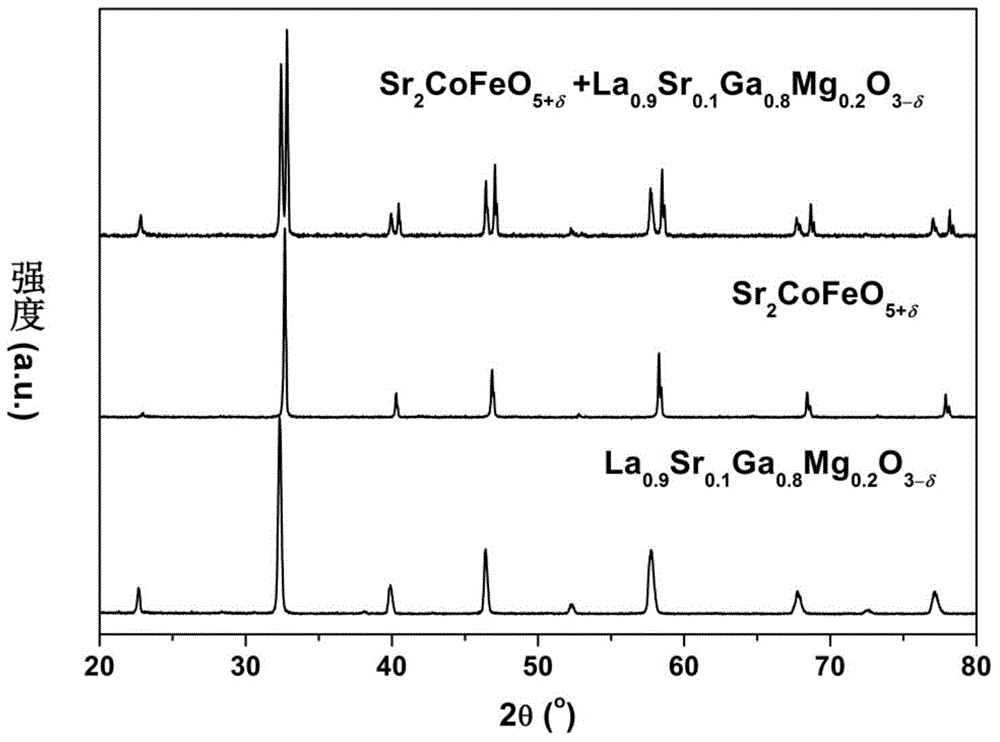
The invention discloses a double-perovskite type intermediate temperature solid oxide fuel cell anode material and a preparation method and belongs to the technical field of solid oxide fuel cells. The molecular formula of the anode material is A2Co<1-x>MxFeO<5+delta>, wherein A is Ca, Sr or Ba element, M is Mo, Nb, Ti, Ni, Cu or Al element, and x is greater than 0 and smaller than 1. The preparation method comprises the following steps: required solonetz nitrate and the like as well as a complexing agent are uniformly mixed in an aqueous solution to obtain gel, the gel is oven-dried, the oven-dried gel is calcined at 500-700 DEG C and 800-950 DEG C respectively, and the calcined powder is sintered at 1100-1300 DEG C for 10-20 hours to obtain the corresponding one-phase double-perovskite type anode material. The anode material has the characteristics of low cost, excellent conductivity, low coefficient of thermal expansion and chemistry compatibility with electrolyte material and is the intermediate temperature solid oxide fuel cell anode material having excellent application prospect.
Owner:JILIN UNIV
High-conductivity double perovskite aluminum-doped Sr2AlxMg1-xMoO6-Delta anode material and preparation method thereof
InactiveCN101867048AImprove conductivityIncreased concentration of free electronsCell electrodesAir atmosphereFuel cells
The invention relates to a high-conductivity double perovskite aluminum-doped Sr2AlxMg1-xMoO6- Delta anode material and a preparation method thereof, which belong to the field of the fuel cell. B site of double perovskite (A2BB'O6) solid oxide fuel cell anode material Sr2MgMoO6 is doped with aluminum to form a mixed conductor of double perovskite structure. Then binder of defined quantity is added to the B site doped Sr2AlxMgxMg1-xMoO6(x=0.01 to 0.1) powder to be compressed to sample strips under a given pressure after being uniformly mixed, the sample strips are sintered at high temperature under the air atmosphere and then are reduced under the low-oxygen condition, the measurement of the conductivity is performed after the reduction, the conductivity is 276 percent higher than that before the doping, thus favoring the improvement of the working property of the electrode. At the same time, the doped Sr2AlxMg1-xMoO6 has higher carbon sedimentation resistance and higher sulfur poisoning resistance than that of the traditional anode material Ni / YSZ.
Owner:UNIV OF SCI & TECH BEIJING
Rare earth catalyst used in preparation of syngas through biomass and coal in supercritical water in co-gasification mode
InactiveCN102658163ASimple preparation processLow costHydrogenMetal/metal-oxides/metal-hydroxide catalystsSyngasManganese
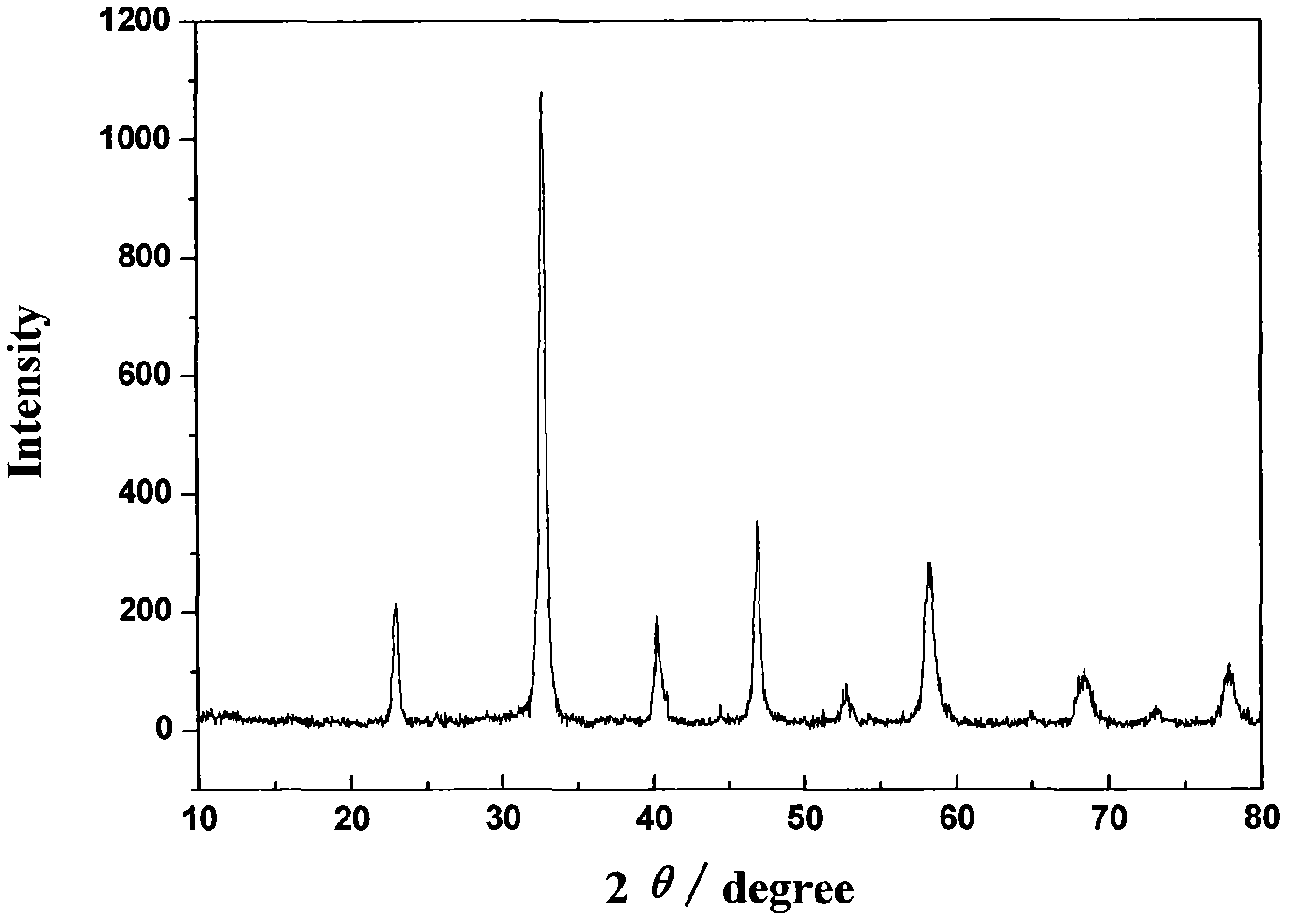
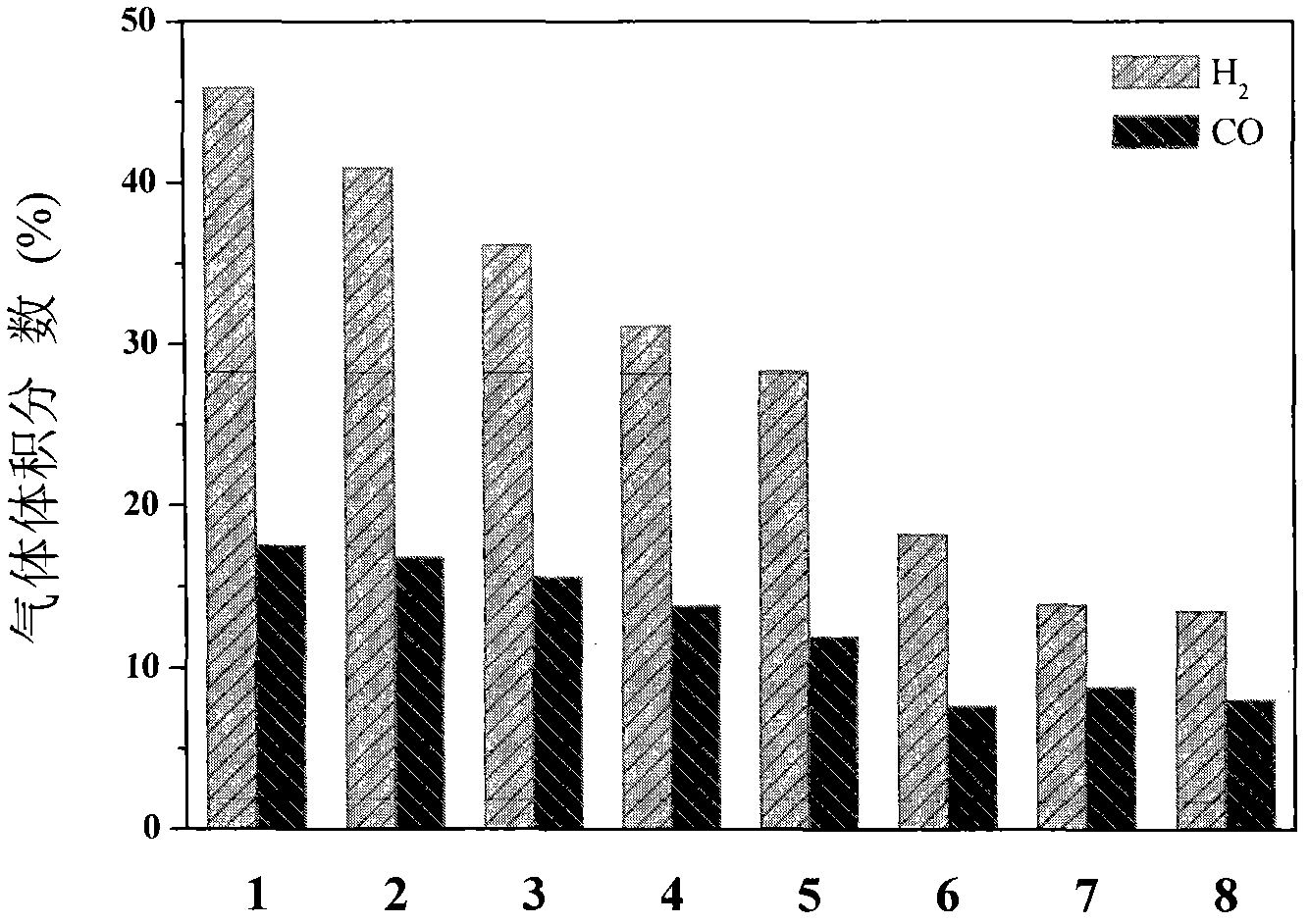
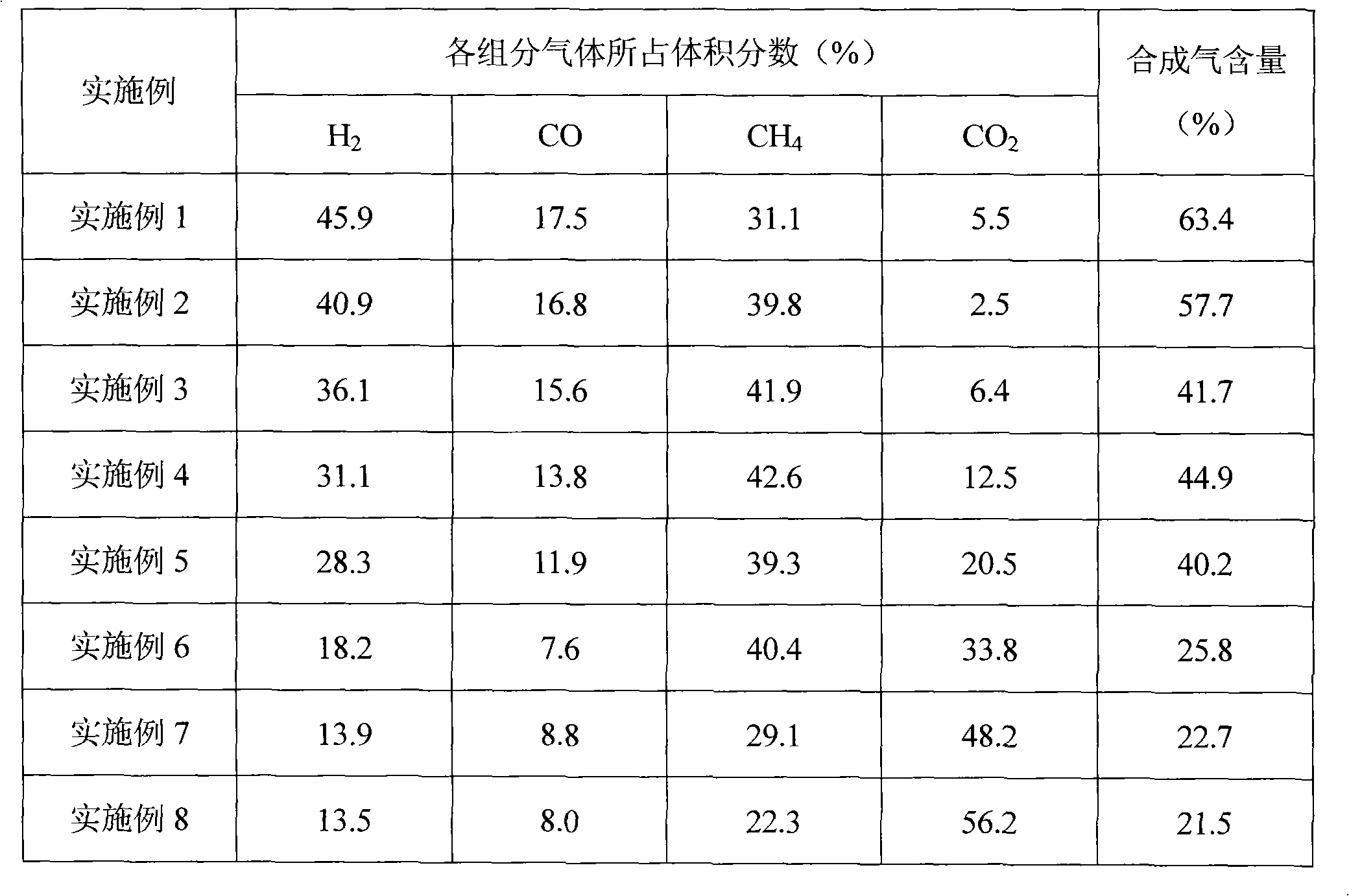
The utility model discloses a rare earth catalyst used in preparation of syngas through biomass and coal in supercritical water in co-gasification mode, in particular to a rare earth catalyst used for catalyzing gasified biomass to coal to prepare the syngas in the supercritical water. The rare earth catalyst selected in the method is rare earth double-perovskite type composite oxide containing lanthanum, cobalt and manganese. The catalyst is used for catalyzing the supercritical water co-gasification reaction of the biomass and the coal to obtain the syngas with the content reaching 63.4%, so that content of carbon monoxide in gas products increases, and content of carbon dioxide is remarkably reduced.
Owner:INNER MONGOLIA UNIVERSITY
Amorphous non-precious metal hydroxide modified perovskite composite catalyst used for oxygen evolution reaction and preparation method thereof
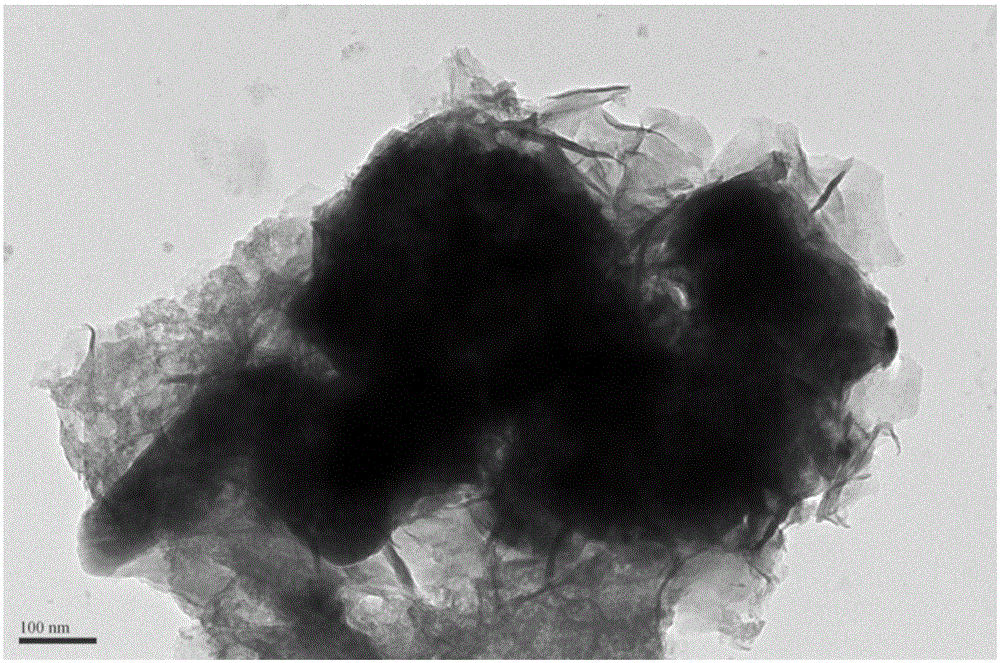
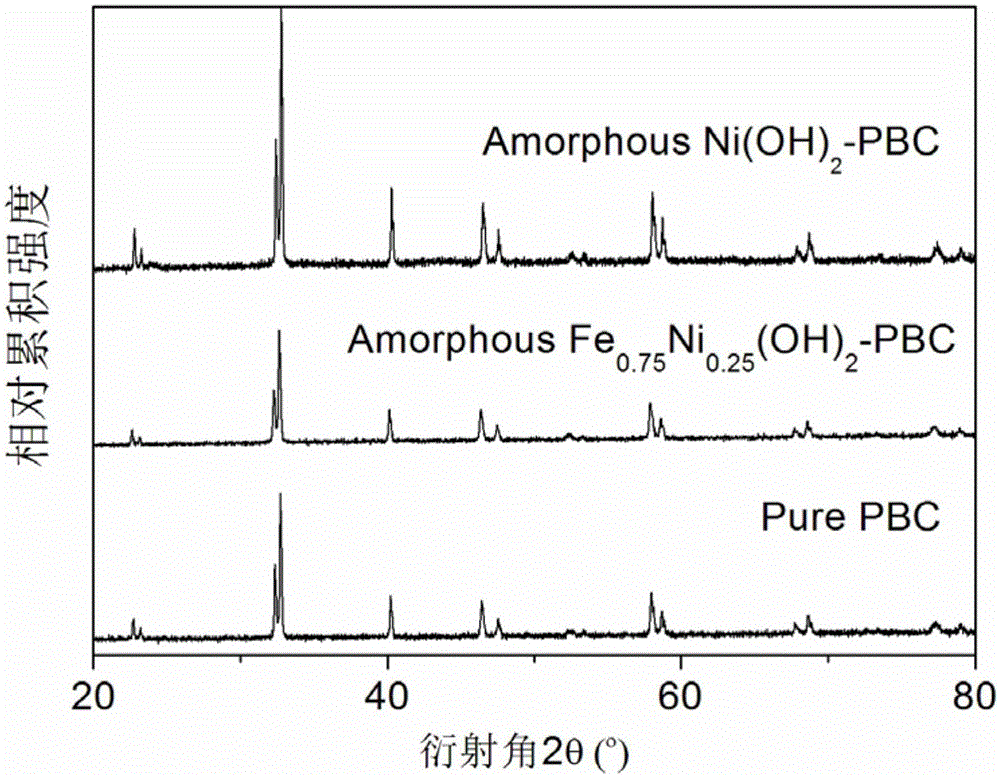
ActiveCN105056961AImprove electrochemical activityMaintain catalytic activityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsChemical compositionAmorphous silicon
The invention discloses an amorphous non-precious metal hydroxide modified perovskite composite catalyst used for oxygen evolution reaction and a preparation method thereof. The composite catalyst is composed of a main body material and a modification material, wherein the main body material is perovskite oxide and the modification material is amorphous non-precious metal hydroxide. The chemical composition of the amorphous non-precious metal hydroxide modification material is MxA1-x(OH)y, wherein M and A are selected from transition metal elements, x is no less than 0 and no more than 1, and y is no less than 2 and no more than 3. The main body material A-position ordered double perovskite oxide and has a molecular formula of Ln0.5Ba0.5CoO3-delta. The hydroxide modification material and the perovskite main body in the composite catalyst produce cooperative effect; the catalytic activity of the composite catalyst is greatly improved compared with catalytic activity of a main body catalyst; and after long-time electrochemical performance test, the composite catalyst can maintain catalytic activity and morphological stability.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
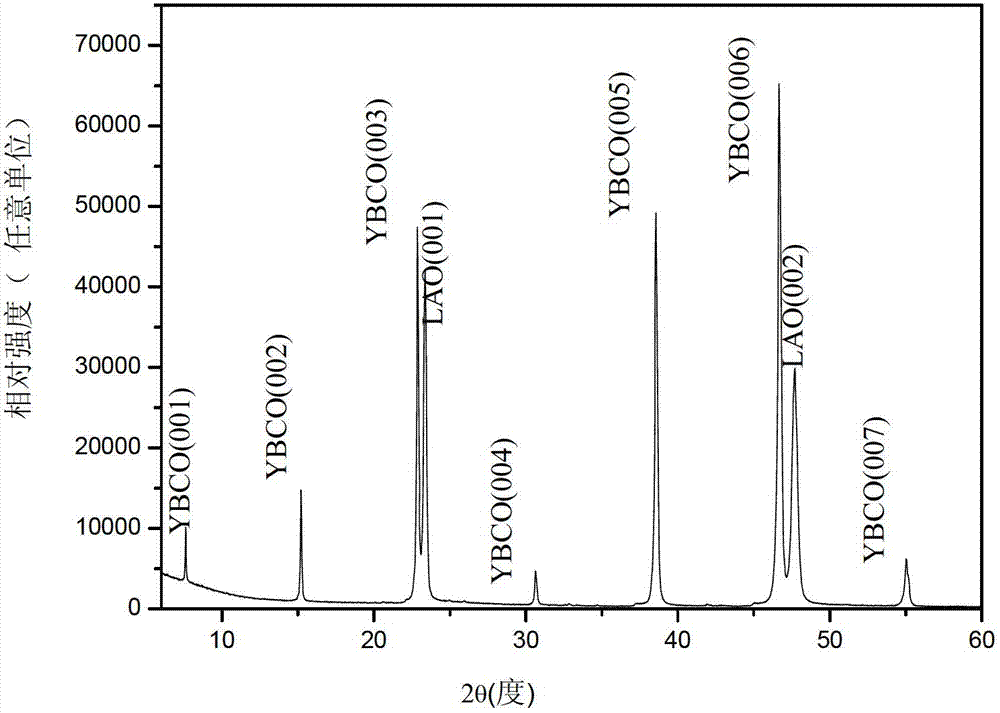
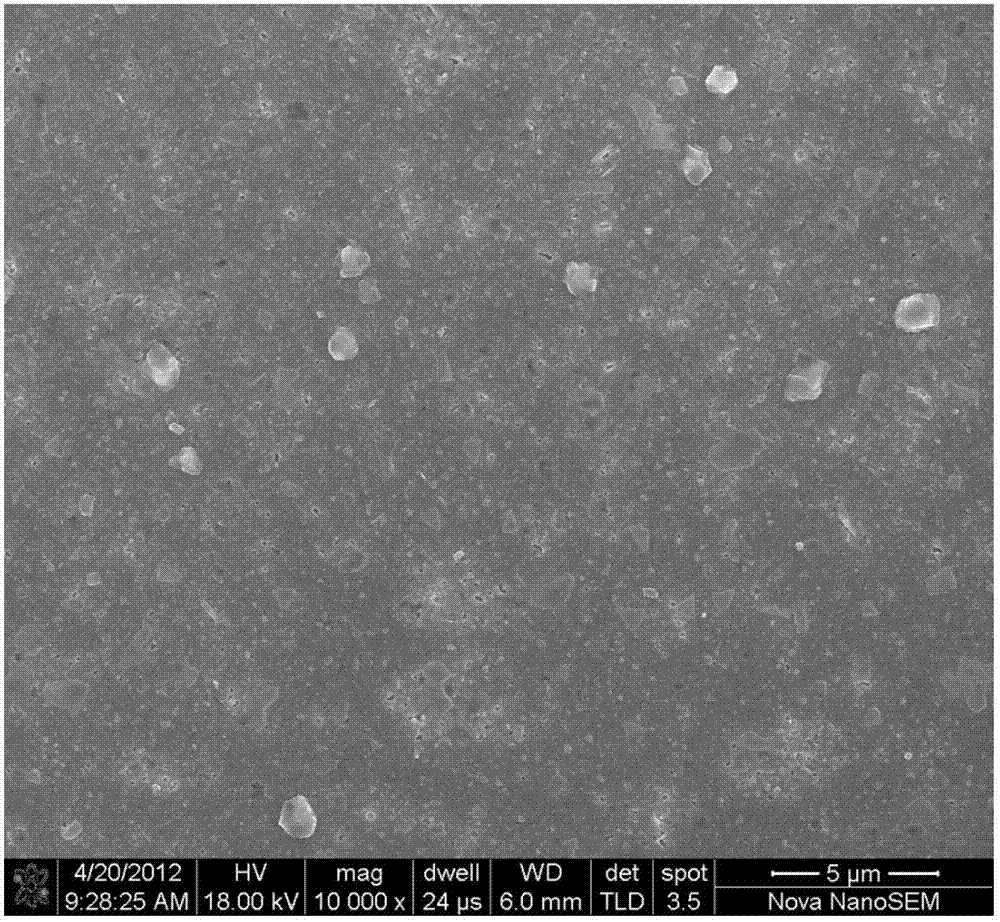
Nb-doped YBCO (Yttrium Barium Copper Oxide) super-conducting film and preparation method
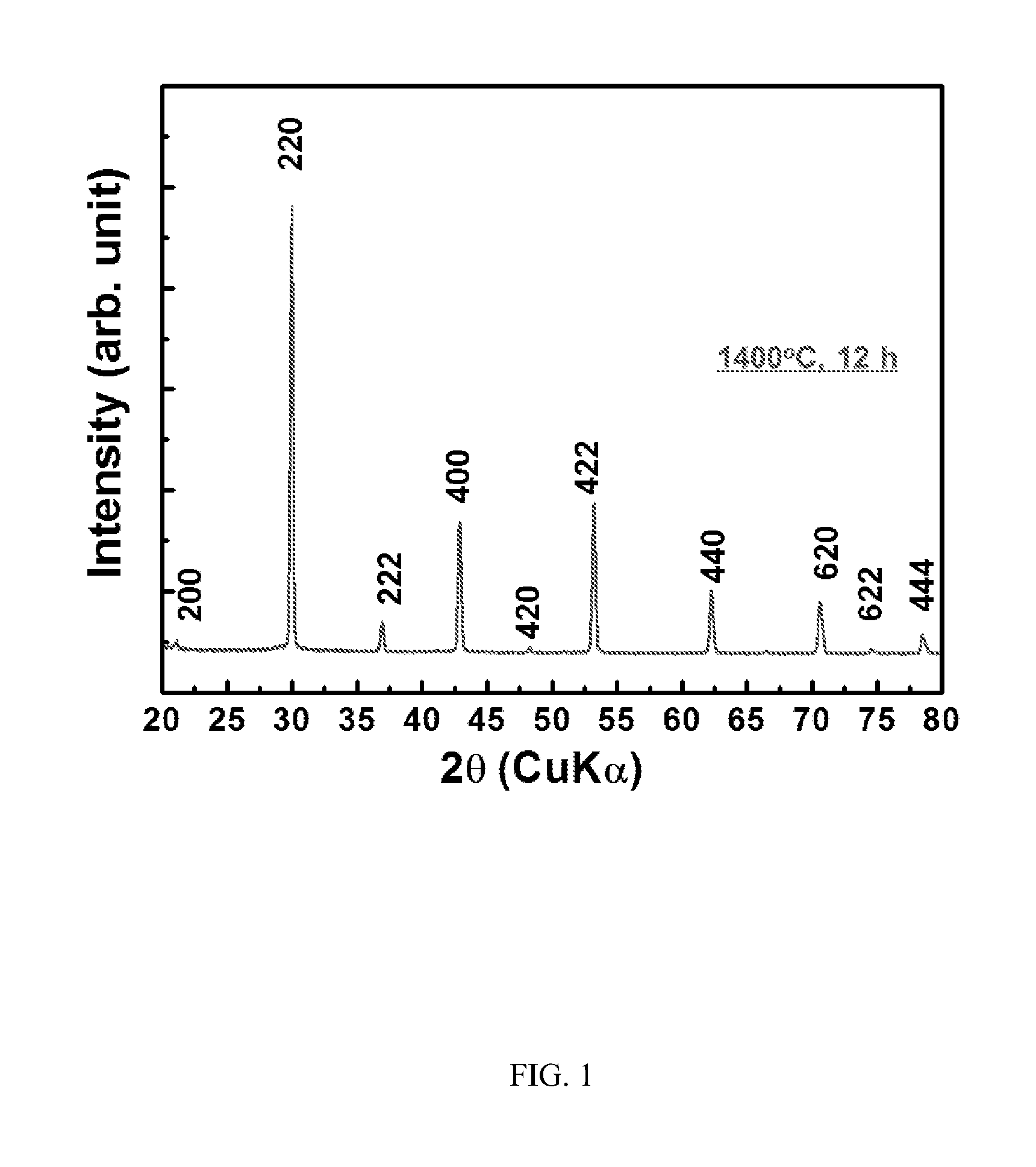
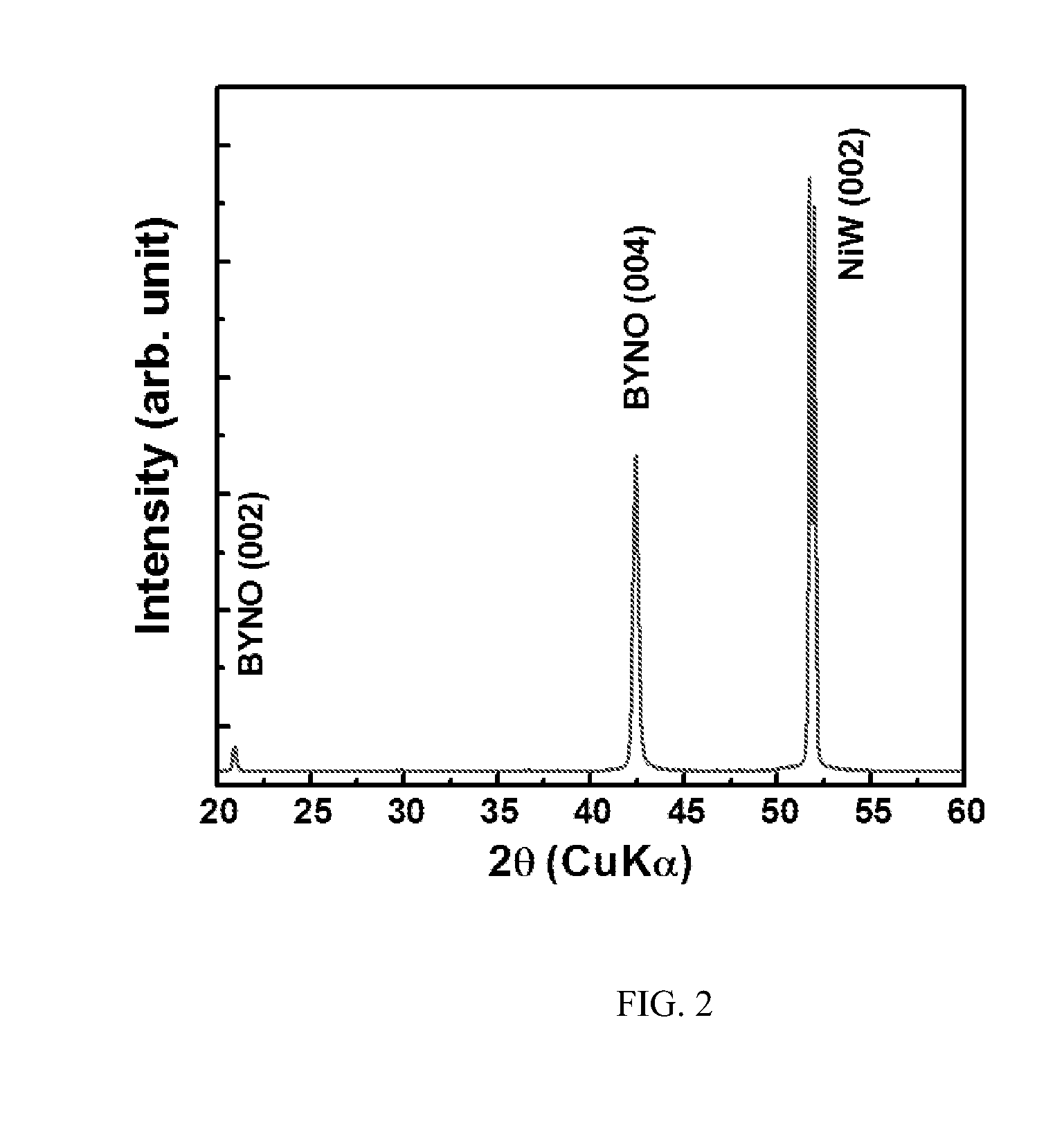
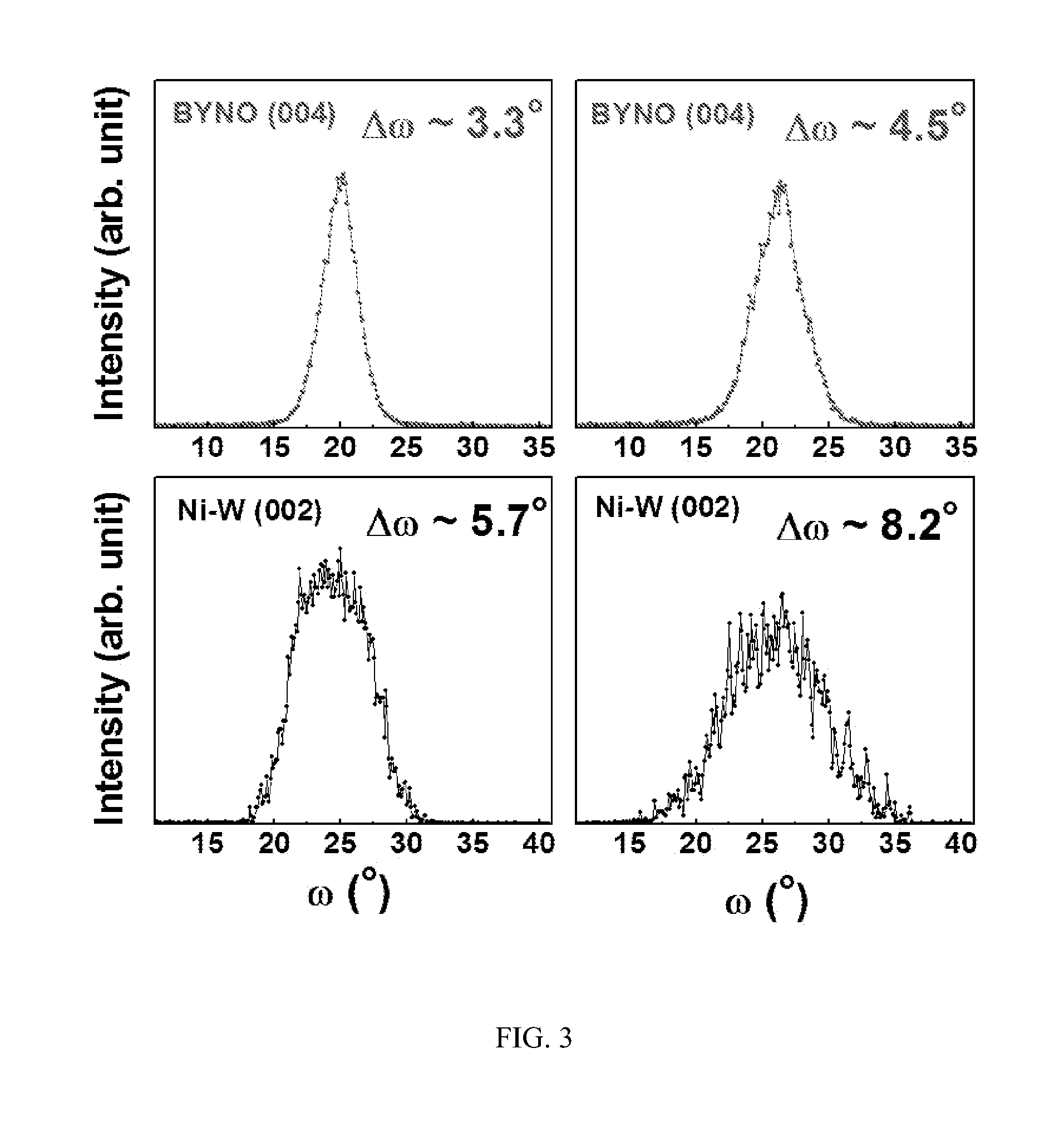
ActiveCN102875141ALow costRandom control of doping ratioSuperconductor elements usageNb dopedYttrium barium copper oxide
The invention provides an Nb-doped YBCO (Yttrium Barium Copper Oxide) super-conducting film and a preparation method. The super-conducting film is prepared by mixing a nano-particle BYNO (Ba2YNbO6) with a double perovskite structure into a YBCO film, wherein an Nb material is 1 to 10% of total quantity of a material of a metal cation. The preparation method comprises the following steps of: preparing a YBCO precursor solution; preparing an Nb precursor solution; preparing an Nb-doped YBCO precursor solution; coating same on a gel film; roasting at a low temperature; and sintering at a high temperature. The preparation method provided by the invention is simple and easy to carry out; a mixture ratio of doped materials can be randomly controlled; and furthermore, raw materials are cheap and easy to obtain, vacuum equipment is not needed, and low cost is ensured; a prepared film has higher critical transition temperature, critical current density and an excellent biaxial texture.
Owner:深创超导(深圳)科技有限公司
Near ultraviolet excitation double perovskite fluorescent powder for white light LED and preparation method thereof
InactiveCN103555327AAchieve outputStrong absorption capacityGas discharge lamp usageLuminescent compositionsRare-earth elementLuminous intensity
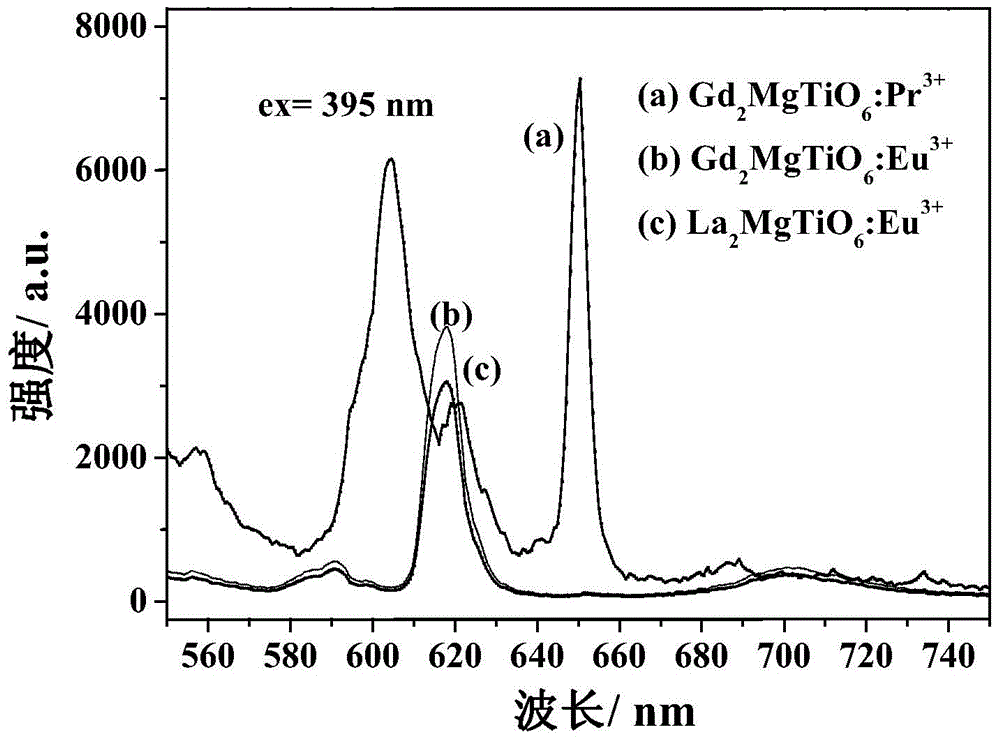
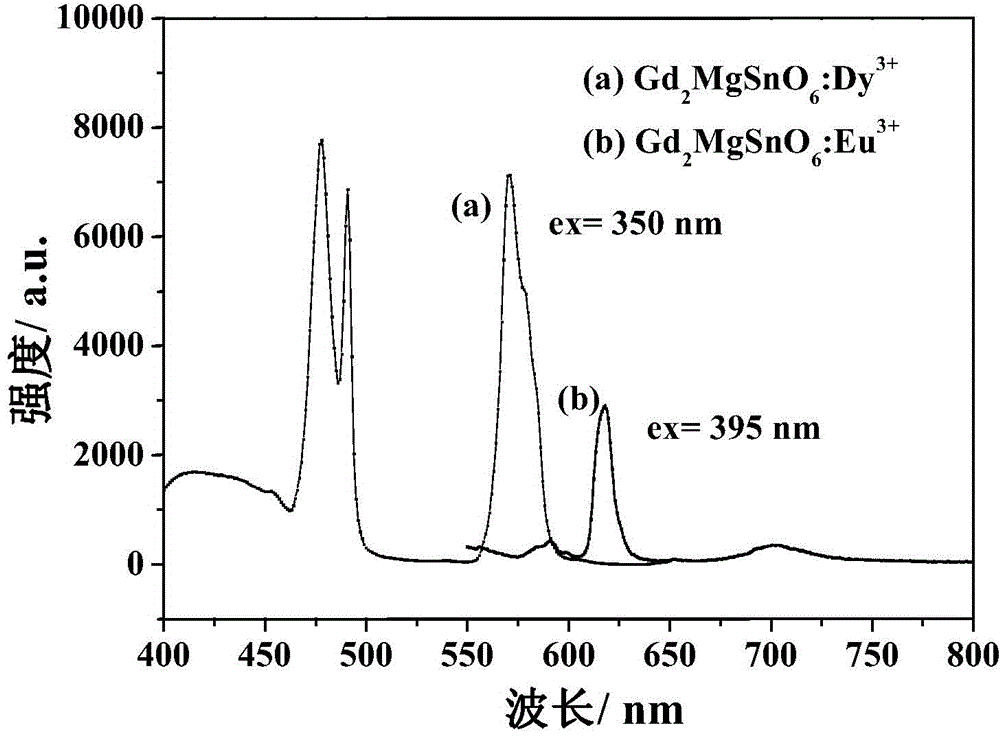
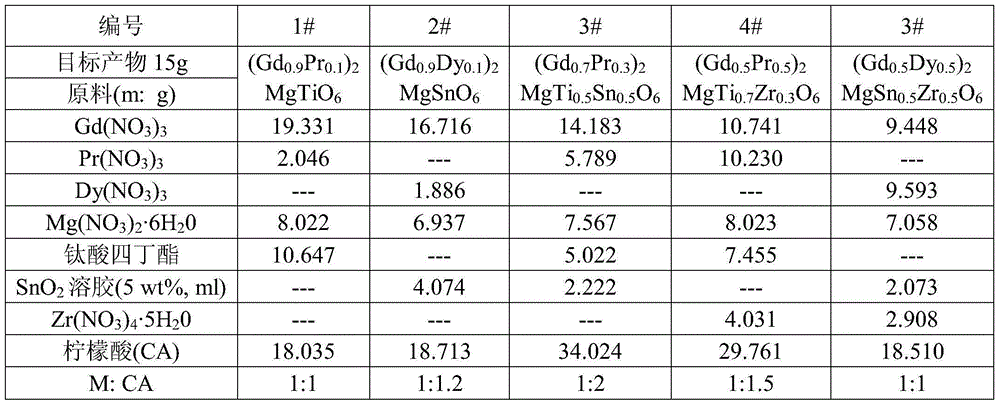
The invention relates to a near ultraviolet excitation double perovskite fluorescent powder for a white light LED and a preparation method thereof. The fluorescent powder is characterized by having a structural formula of (Gd1-xRE x)2MgMO6; M represents one or two selected from Ti, Sn and Zr, but M does not represents Zr individually and account for no more than 50% by molar ratio; and RE is one selected from rare earth elements Pr and Dy, wherein x satisfies the following relation: 0.1<=x<=0.5. The fluorescent powder is prepared by a sol-gel method using citric acid as a complexing agent. The fluorescent material provided by the invention emits Pr<3+> or Dy<3+> characteristic red light or near white light, and the two ions have strong absorption of excitation energy in the fluorescent powder, high luminous intensity, good color purity and good color rendering. The system gains greatly increased red light emitting intensity and achieves near white light output.
Owner:NANJING UNIV OF TECH
Double perovskite particle detector and preparation method thereof
InactiveCN110927769AImprove photoelectric performanceHigh stability and non-toxicX/gamma/cosmic radiation measurmentIndiumEthyl group
The invention discloses a double perovskite particle detector and a preparation method thereof. The double perovskite particle detector comprises, from top to bottom in sequence, a double perovskite single crystal and a photoelectric amplification device. The double perovskite single crystal is composed of A, B, C and X, the molecular structural formula of the double perovskite single crystal is A2BCX6, wherein A is methylamine (MA) or phenylethylamine (PEA) or cesium (Cs), B is silver (Ag) or potassium (K), C is bismuth (Bi), antimony (Sb) or indium (In), and X is halogen element chlorine (Cl), bromine (Br) or iodine (I). The double perovskite single crystal material is selected from any one of Cs2AgBiBr6, MA2AgBiBr6, Cs2KInCl6 and MA2AgSbI6. The double perovskite material can effectivelyavoid harm of heavy metal lead to human bodies and the environment, has excellent photoelectric properties, can effectively avoid formation of deep energy level defects, improves the crystal quality,and greatly improves the optical properties; compared with the traditional perovskite material, the perovskite material has better stability and is not easy to decompose and deteriorate in the air.
Owner:NANCHANG UNIV
Preparation method of double-perovskite ferroelectrics (FET)-antiferromagnetism (AFM) compound molecule with oxygen bridge
The invention discloses a preparation method of a double-perovskite ferroelectric (FET)-antiferromagnetic (AFM) compound molecule with an oxygen bridge. In the invention, a method for compounding an FET molecule with an AFM molecule with different physical properties is described. A chemically-synthesized AFF (Antiferromagnetism-ferroelectrics) material can form an ABO3 double-perovskite compound structure with an oxygen bridge Bafm-O-Bfet. A method for compounding BaTiO3 serving as an FET typical material with NdBaMnO3 serving as an AFM typical material is taken as an example in the invention.
Owner:纵坚平
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com