Double-perovskite tungsten molybdate red fluorescent powder for white light LED and preparation method of double-perovskite tungsten molybdate red fluorescent powder
A technology of red phosphor and double perovskite, applied in chemical instruments and methods, luminescent materials, sustainable buildings, etc., can solve problems such as difficulty in obtaining red light emission, increased process cost, and low luminous efficiency, and achieve increased lumens Efficiency, increase luminous intensity, increase the effect of doping concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
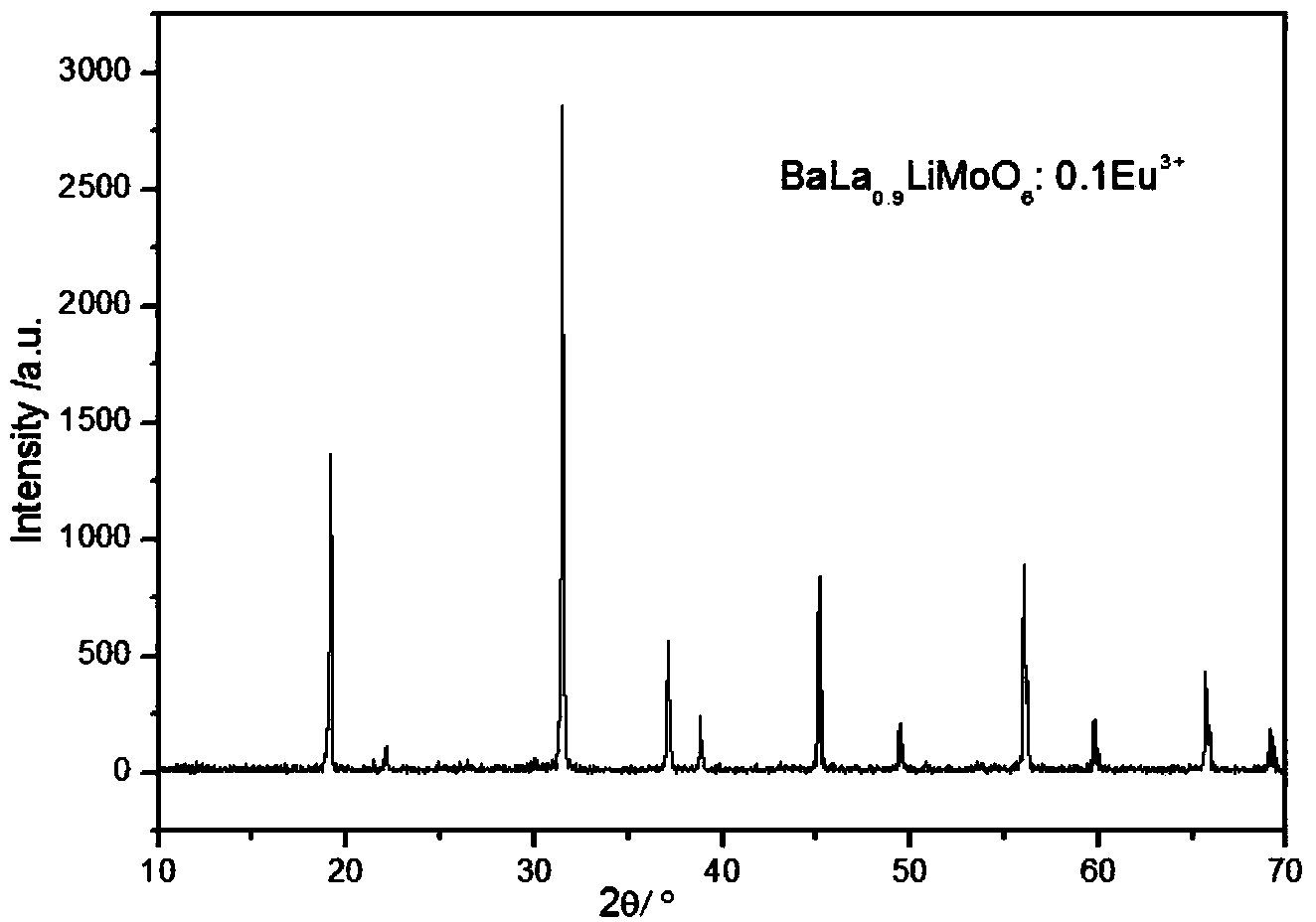
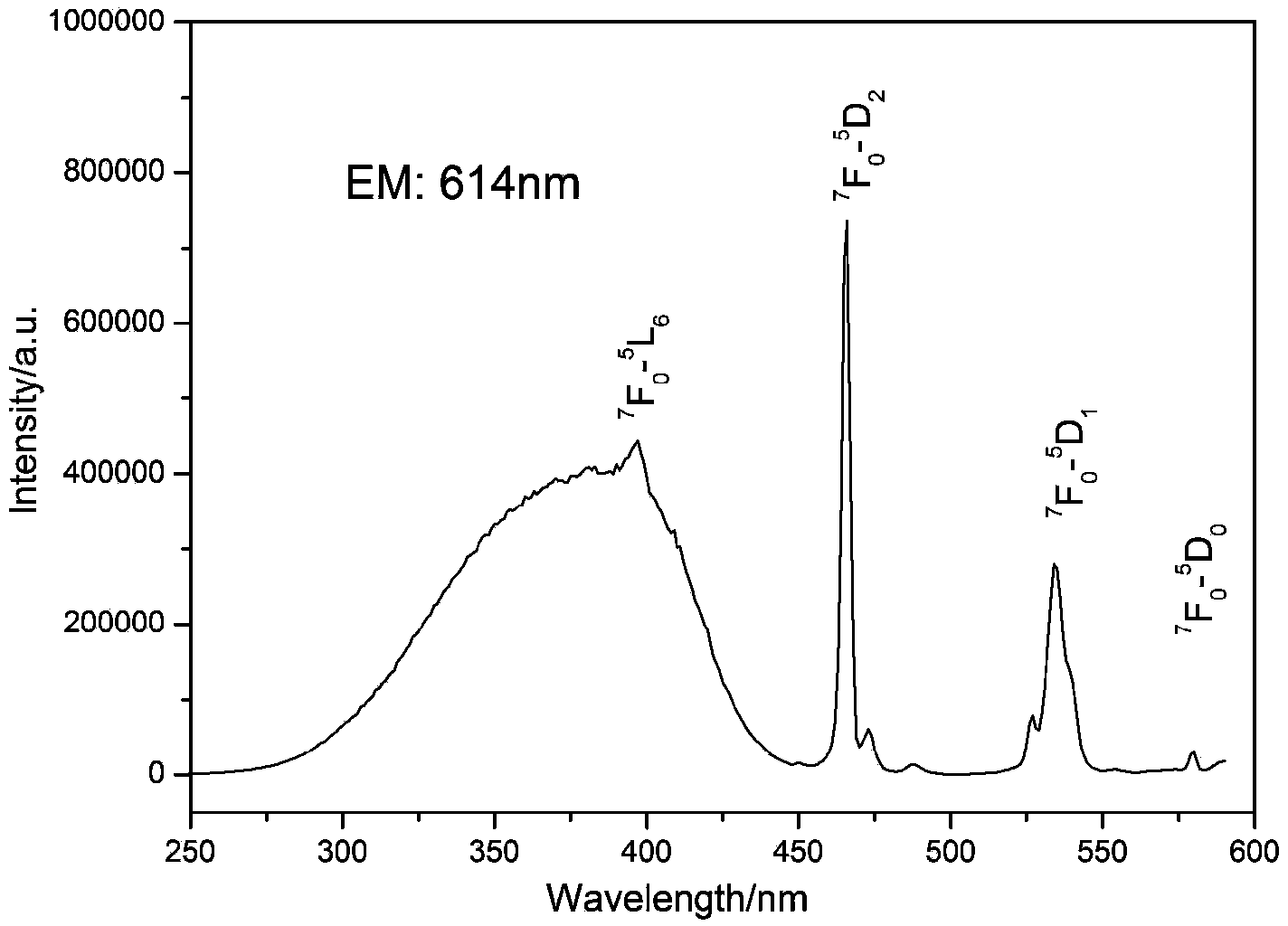
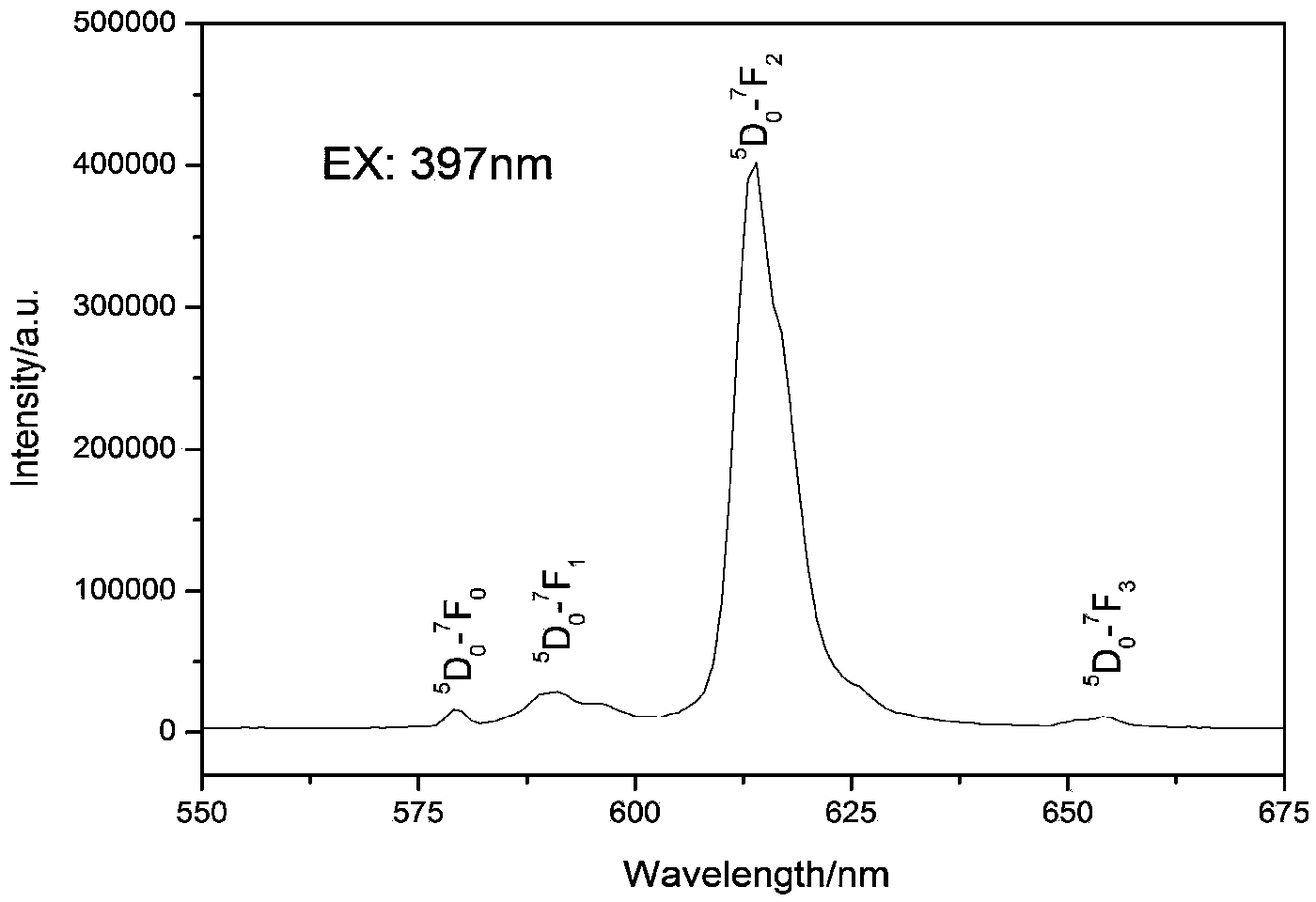
[0033] 1), with BaCO 3 , La 2 o 3 , Li 2 CO 3 , Eu 2 o 3 , (NH 4 ) 6 Mo 7 o 24 4H 2 O is the raw material, according to BaLa 0.9 LiMoO 6 :0.1Eu 3+ Stoichiometric ratio for weighing;
[0034] 2), the weighed BaCO 3 and Li 2 CO 3 Pour into beaker A and dissolve completely with dilute nitric acid to obtain solution A; then put La in beaker B 2 o 3 and Eu 2 o 3 Dissolve with concentrated nitric acid, heat to drive off excess nitric acid, pour the solution in beaker B into beaker A and mix well;
[0035] 3), in beaker C, dissolve citric acid completely with deionized water to obtain citric acid solution, the amount of substance of citric acid and Ba 2+ , La 3+ 、Eu 3+ , Li + The ratio of the sum of the amounts of ionic substances, that is, the molar ratio, is 1:1. Add the configured citric acid solution into beaker A, and magnetically stir and mix evenly at room temperature to obtain solution C;
[0036] 4) Dissolve completely in deionized water in beaker D ...
Embodiment 2
[0043] 1), with SrCO 3 , Sc 2 o 3 , Li 2 CO 3 , Eu 2 o 3 , (NH 4 ) 6 Mo 7 o 24 4H 2 O as raw material, according to SrLa 0.7 LiMoO 6 :0.3Eu 3+ Stoichiometric ratio for weighing;
[0044] 2), the weighed SrCO 3 and Li 2 CO 3 Pour into beaker A and dissolve completely with dilute nitric acid to obtain solution A. And use beaker B to put Sc 2 o 3 and Eu 2 o 3 Dissolve in concentrated nitric acid, heat to drive off excess nitric acid, then add to beaker A and mix;
[0045] 3), completely dissolve carpamine with deionized water in beaker C, the amount of the substance of carpamine and Sr 2+ 、Sc 3+ 、Eu 3+ , Li + The ratio of the sum of the amounts of the ions is 1:1, and the prepared carbamide solution is added to the beaker A, and magnetically stirred and mixed at room temperature to obtain a solution C;
[0046] 4) Dissolve completely in deionized water in beaker D (NH 4 ) 6 Mo 7 o 24 4H 2 0, then proceed to obtain mixed solution in the beaker A, adj...
Embodiment 3
[0051] 1), with BaCO 3 , Gd 2 o 3 , Li 2 CO 3 , Eu 2 o 3 , (NH 4 ) 10 W 12 o 41 as raw material, according to BaGd 0.95 LiWO 6 :0.05Eu 3+ Stoichiometric ratio for weighing;
[0052] 2), the weighed BaCO 3 and Li 2 CO 3 Pour into beaker A and dissolve completely with dilute nitric acid to obtain solution A. And use beaker B to Gd 2 o 3 and Eu 2 o 3 Dissolve in concentrated nitric acid, heat to drive off excess nitric acid, then add to beaker A and mix;
[0053]3), fully dissolve citric acid and EDTA with deionized water in beaker C, the ratio of the amount of both citric acid and EDTA is 1:1, the sum of the amount of citric acid and EDTA is equal to Ba 2+ 、Gd 3+ 、Eu 3+ , Li + The ratio of the sum of the amount of ion substances is 1:2, and the configured citric acid solution is added to the beaker A, and magnetically stirred and mixed at room temperature to obtain a mixed solution;
[0054] 4) Dissolve completely in deionized water in beaker D (NH 4 ) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com