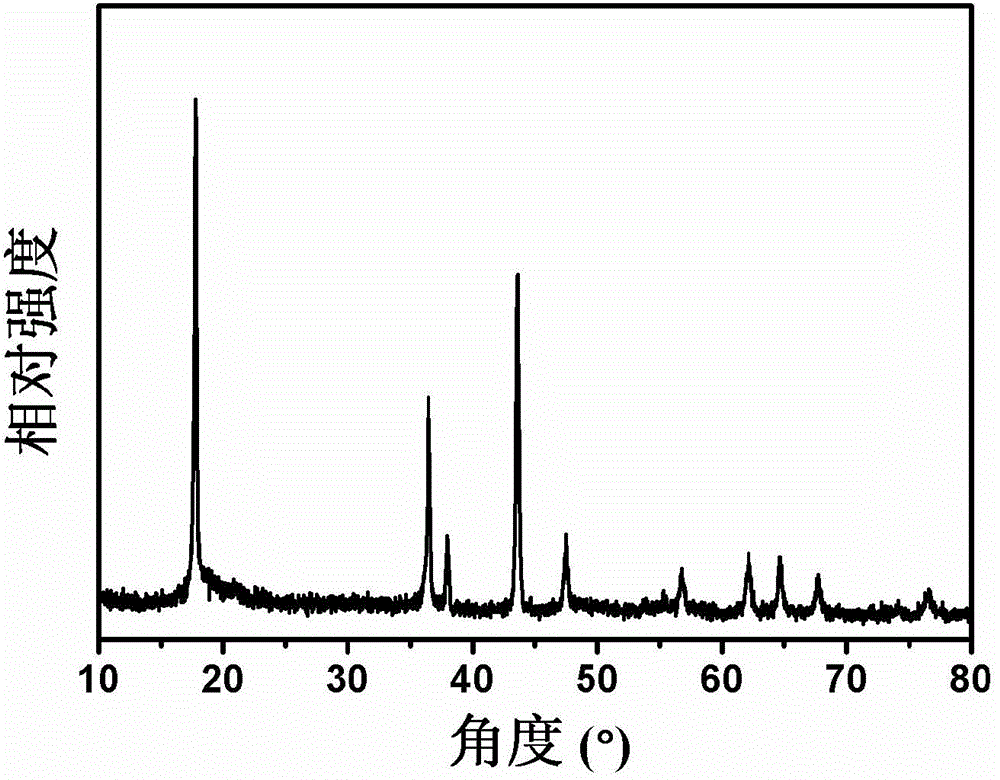
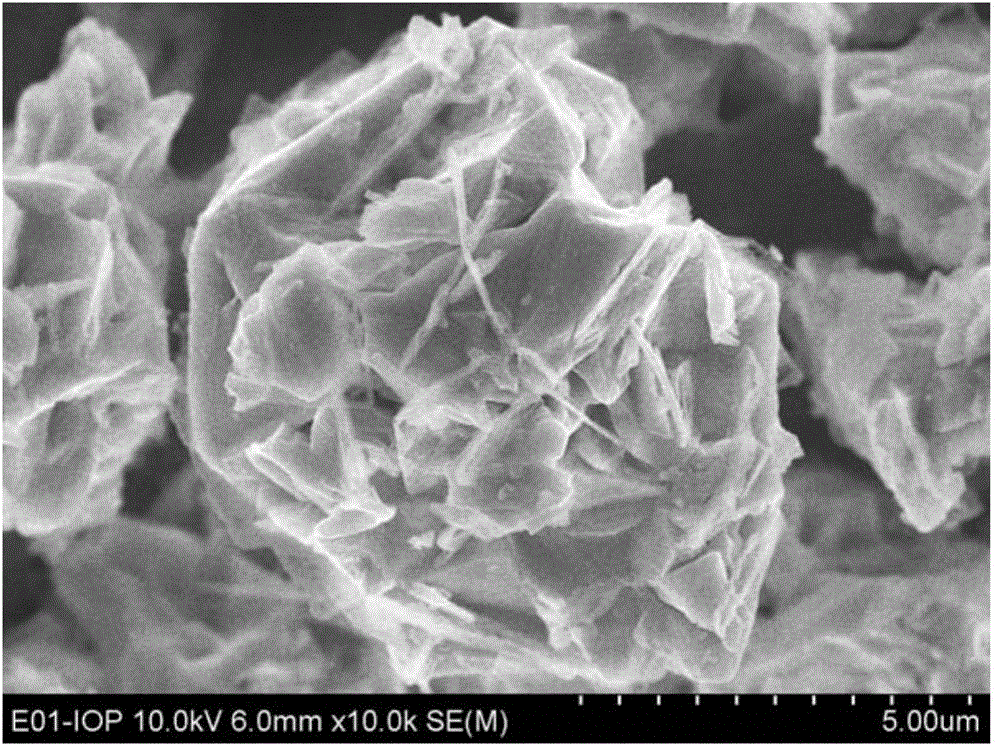
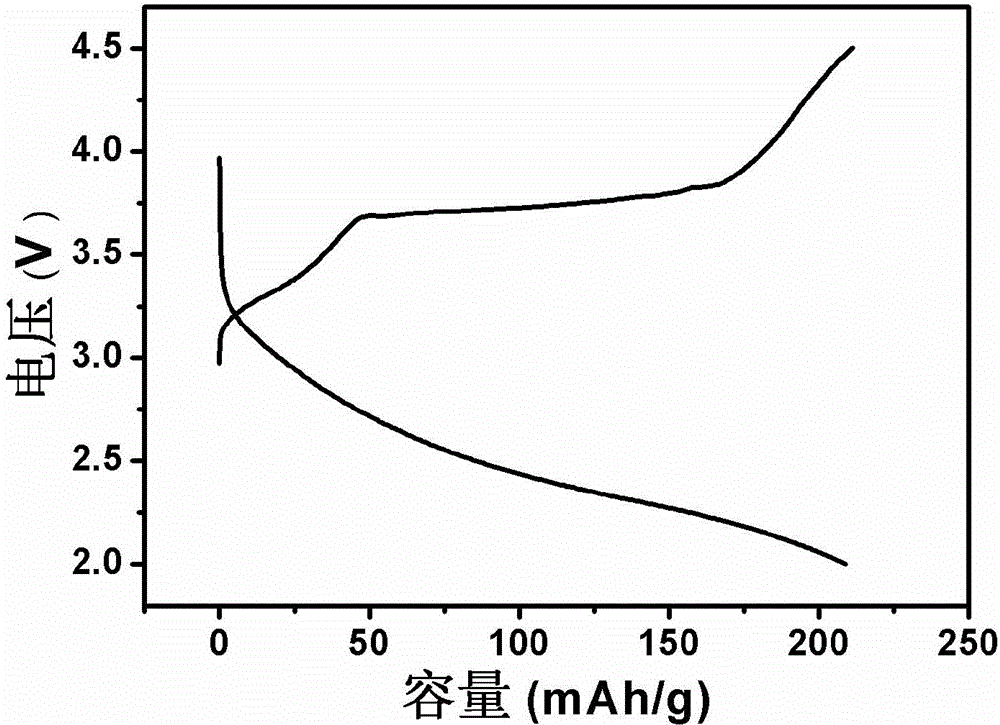
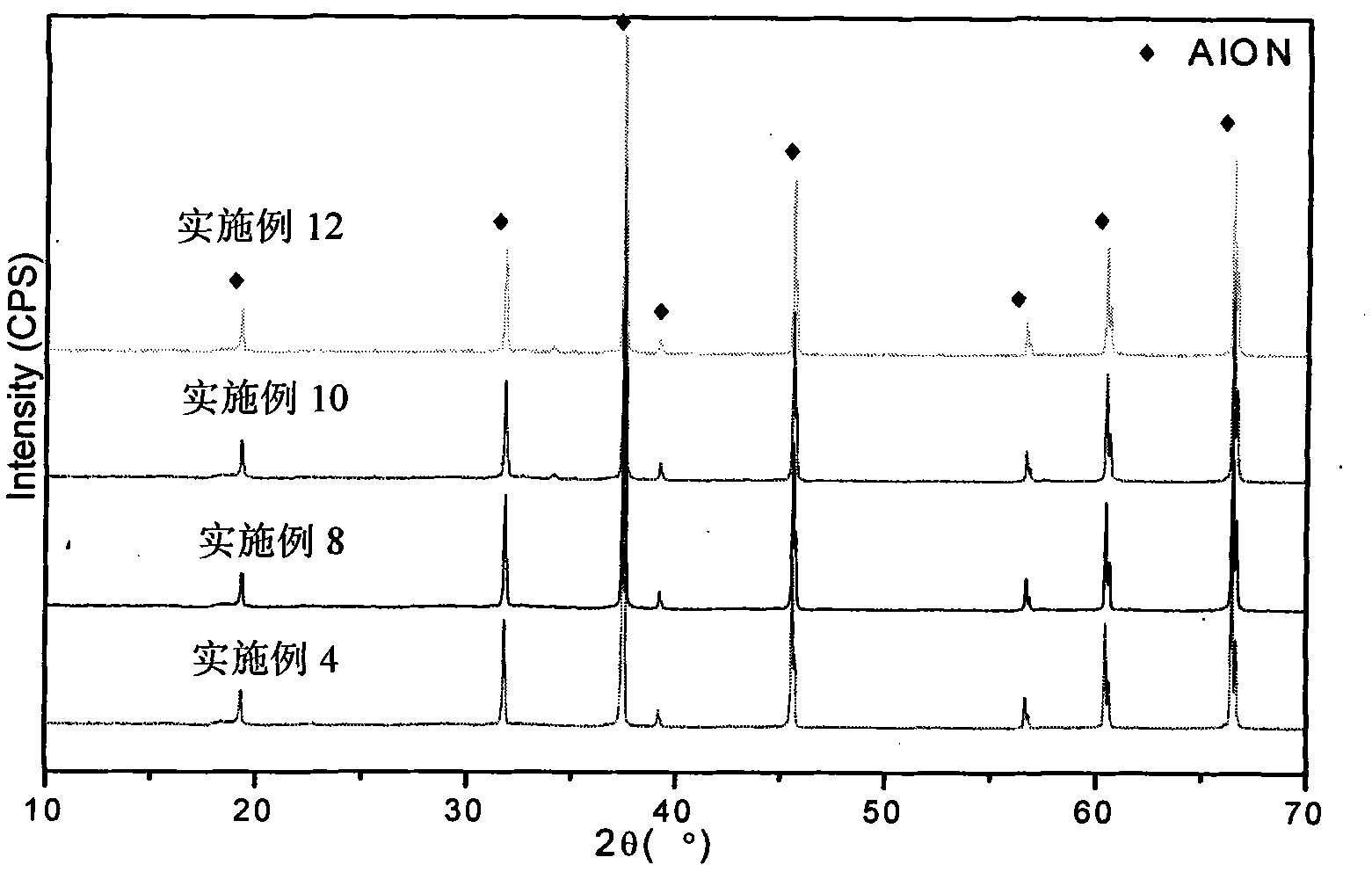
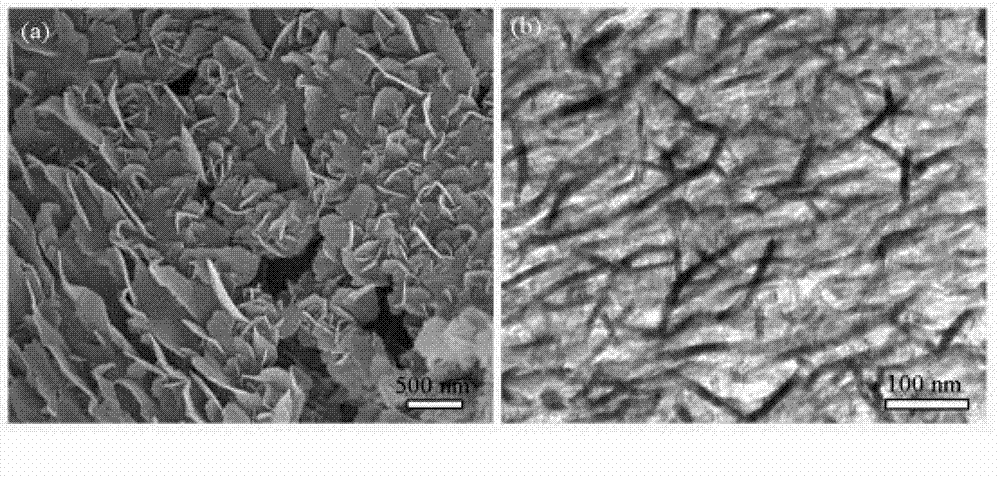
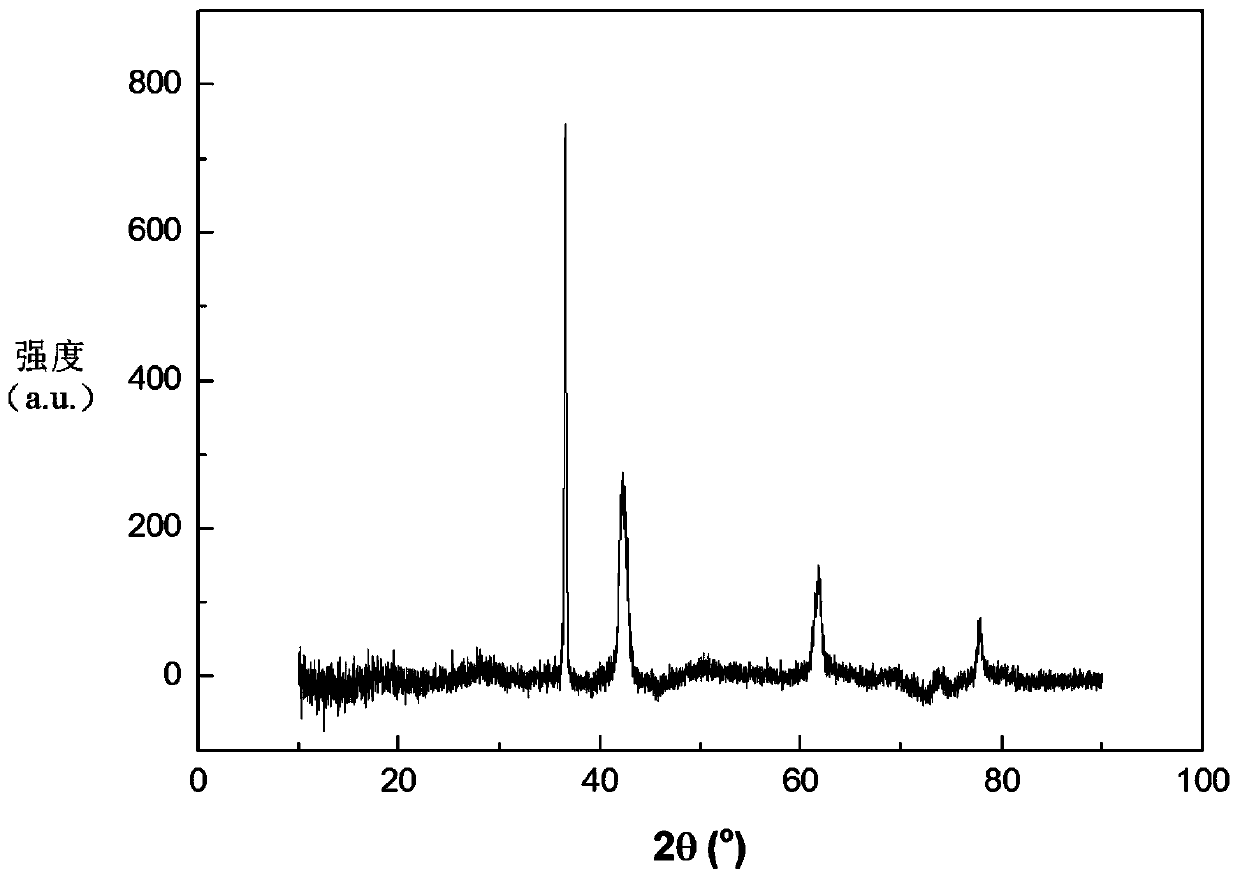
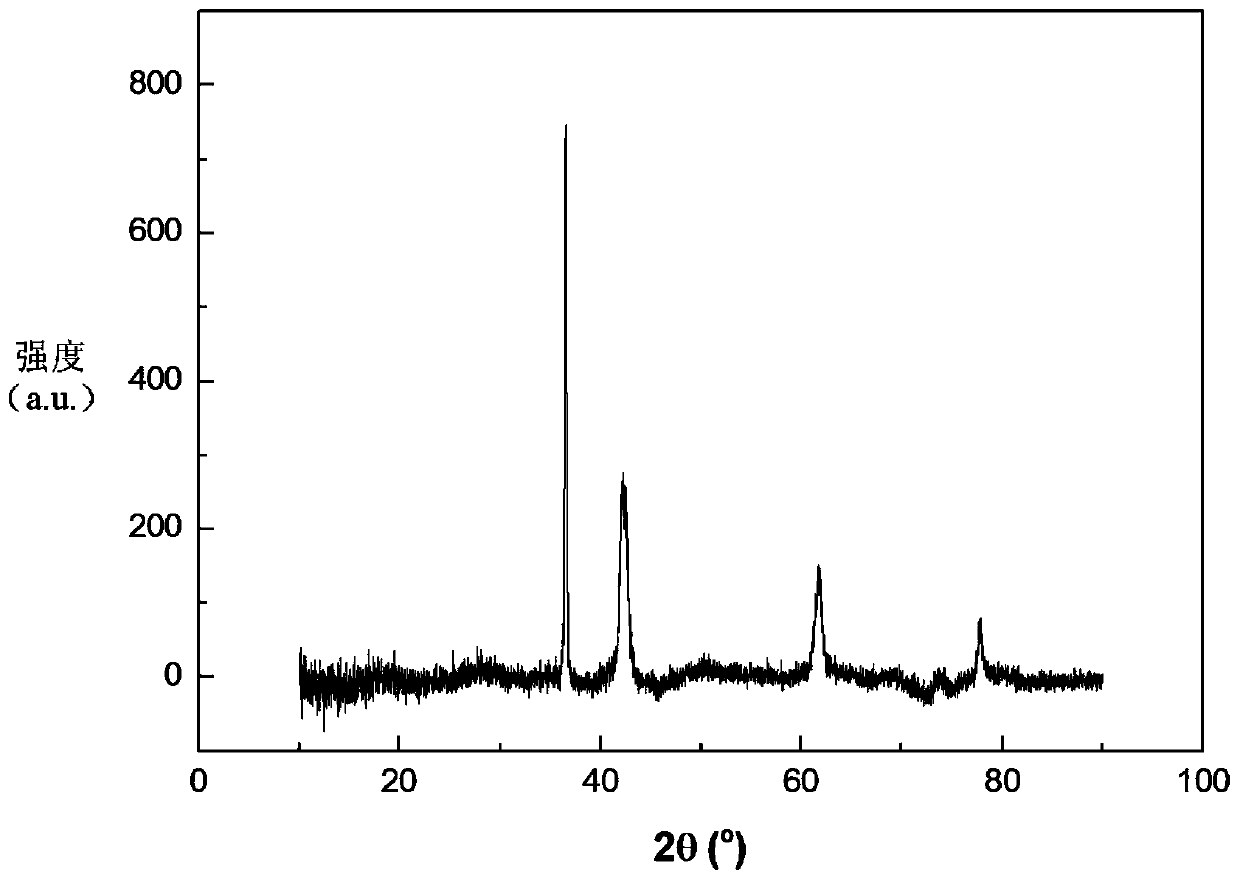
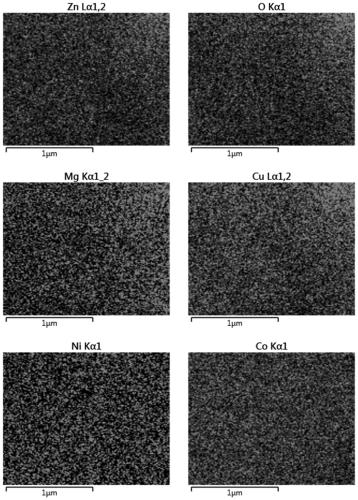
Patents
Literature
1551results about How to "Synthesis temperature is low" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bismuth layered perovskite-like structure oxide up-conversion luminescent piezoelectric material and preparation method thereof
InactiveCN102276248AHigh emission intensityThe synthesis process is simpleChemistryThree dimensional display
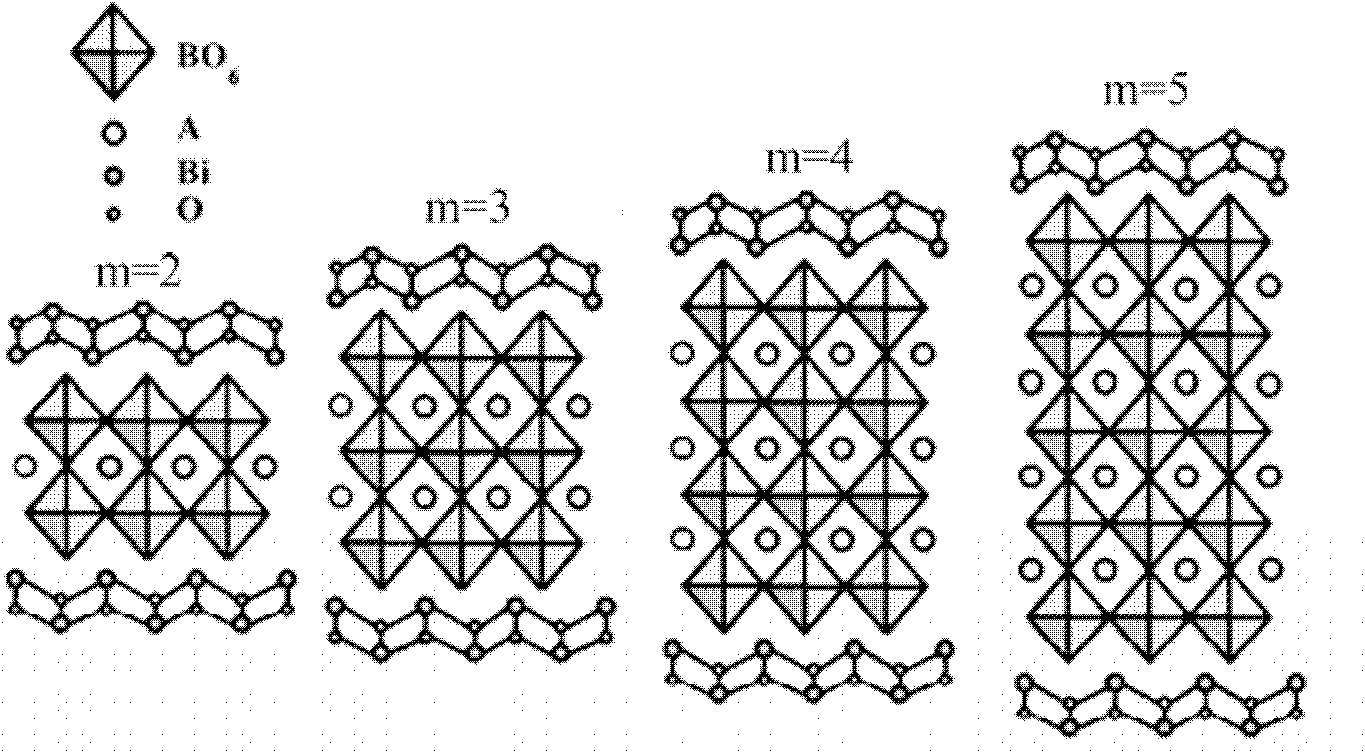
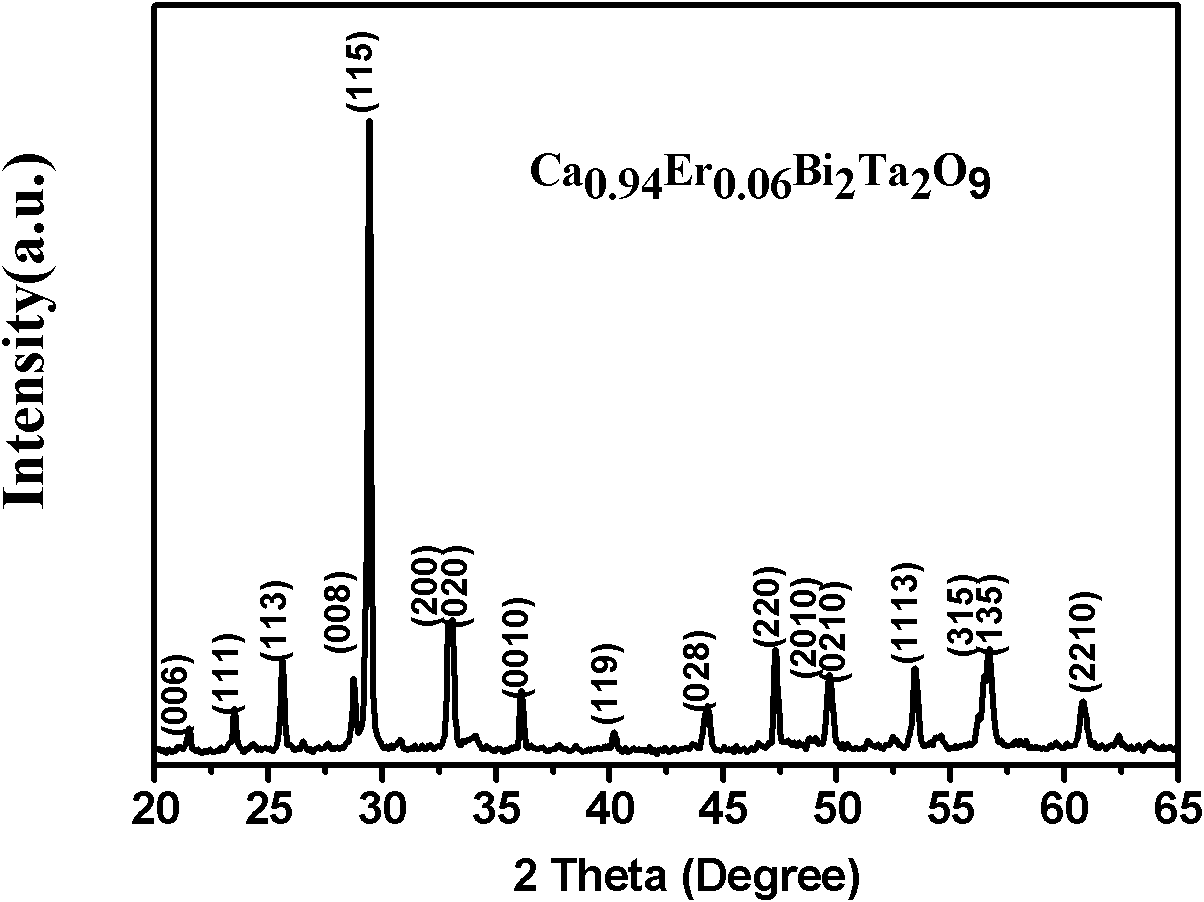
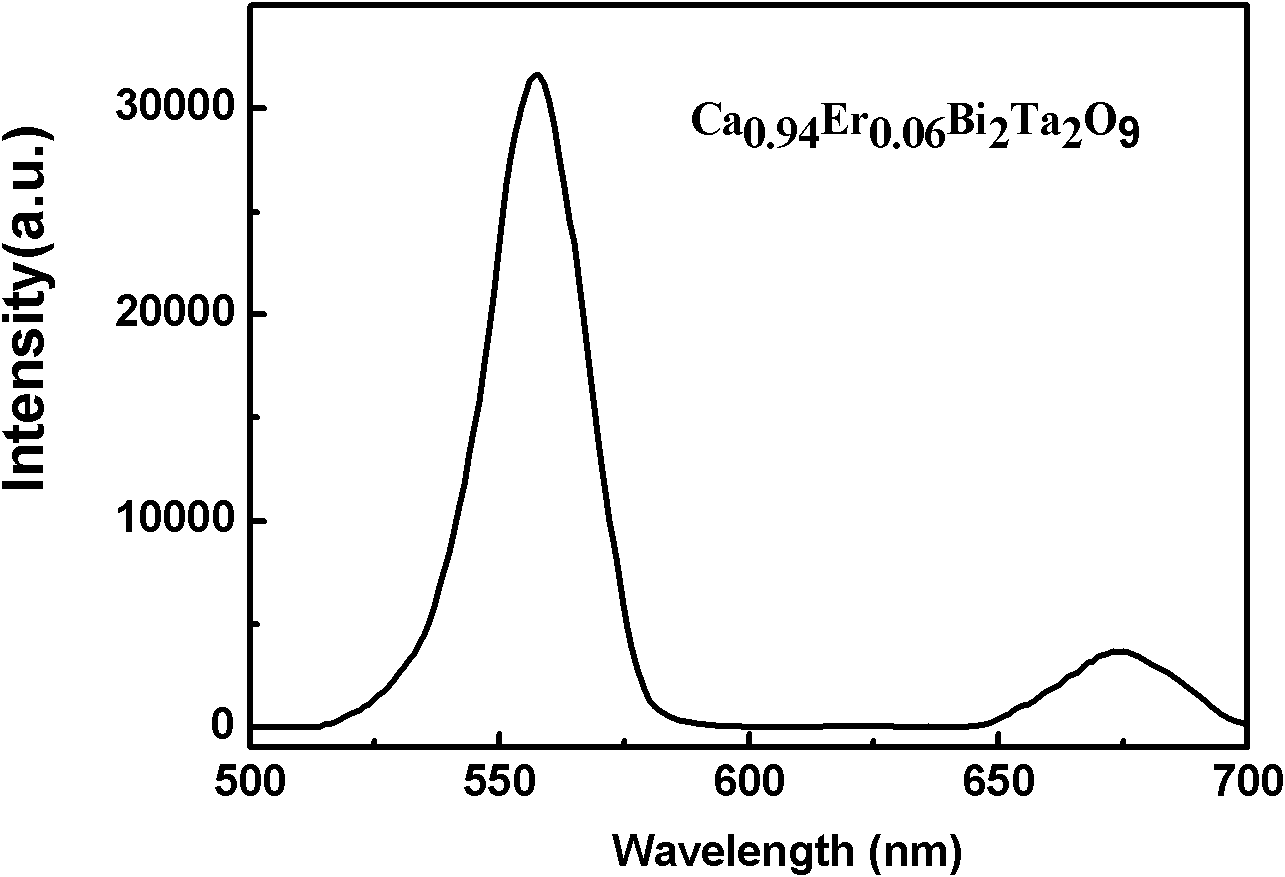
The invention relates to an infrared-excited oxide up-conversion luminescence piezoelectric material of a bismuth lamellar perovskite-like structure and a preparation method thereof. The up-conversion luminescence piezoelectric material has a chemical general formula of Am-1-x-RxYbyBi2BmO3M<+3>, wherein R is selected from Er<3+>, Ho<3+> and Tm<3+>, A is selected from Bi<3>, Ca<2+>, Sr<2+>, Ba<2+>, Pb<2+>, Na<+>, K<+>, La<3+> and Y<3+>, B is selected from Ti<4+>, Zr<4+>, Nb<5+>, Ta<5+>, W<6+> and Mo<6+>, m is a positive integer not smaller than 2 and not more than 8, x is not smaller than 0.000001 and not more than 0.3, and y is not smaller than 3.0 and not more than 0.6. The up-conversion luminescence piezoelectric material is prepared by adopting a solid-phase reaction method, has the characteristics of good thermal stability, good chemical stability, easiness for synthesis, high luminous intensity and adjustable color, and can be widely applied to various aspects such as three-dimensional display, infrared detection, counterfeiting prevention, solar cells and photoelectric integration, micro-electro-mechanical systems, photoelectric sensing and the like.
Owner:TONGJI UNIV
Synthetic method of visible light catalyst Bi2MoO6
InactiveCN101254463AEvenly dispersedSmall particle sizeMetal/metal-oxides/metal-hydroxide catalystsMolybdateSynthesis methods
A synthetic method of a novel visible light photocatalyst Bi2MoO6 (bismuth molybdate) is provided. The method includes the following steps: weighing Bi(NO3)3*5H2O and (NH4)6Mo7O24*4H2O solids at the theoretical ratio by mass of 14:1, dispersing the weighed solids into an appropriate amount of deionized water, stirring to obtain white curdy precipitates, placing the precipitates in a magnetic stirrer and stirring at normal temperature for 30 minutes, subjecting to ultrasonic treatment with a ultrasonic generator for 30 minutes to allow intensively mixing, allowing reactions of the treated white precipitates at 150-200 DEG C under sealed conditions, filtering the reaction product to collect flavescent precipitates, washing, drying, and grinding to obtain Bi2MoO6 (bismuth molybdate) photocatalyst. The photocatalyst has good photodegradation effect on target pollutants; and when the temperature of hydrothermal synthesis is 160 DEG C, the removal rate of target pollutant 4BS after photodegradation for 90 minutes reaches 99.5%. The inventive synthetic method has the advantages of simple process and low requirement for equipment, and is suitable for the synthesis of highly-active visible light photocatalyst Bi2MoO6 (bismuth molybdate).
Owner:NANJING UNIV
Preparation method of carbon coated vanadium sodium phosphate positive electrode material
InactiveCN105336924AEasy diffusion distanceImprove electrochemical performanceNon-aqueous electrolyte accumulatorsCell electrodesSodium phosphatesElectrical battery
A preparation method of a carbon coated vanadium sodium phosphate positive electrode material comprises the steps: with glucose as a reducing agent and a carbon source and water as a dispersant, carrying out ball milling of NH4VO3, NaH2PO4.2H2O and glucose in water, carrying out spray drying, calcining, and thus obtaining the carbon coated vanadium sodium phosphate positive electrode material. The method has the advantages of low synthesis temperature, simple steps, easily obtained raw materials, and advantageous industrialization; the obtained carbon coated vanadium sodium phosphate positive electrode material has a structure with uniform primary particles, has the particle size of 100-200 nm, and has the characteristics of short sodium ion diffusion distance, fast transmission speed, high specific surface area, high electrical conductivity and fast ion transmission and the like. The obtained carbon coated vanadium sodium phosphate positive electrode material is assembled into a battery; in a voltage scope of 2.0-3.75 V and under 1 C multiplying power, the highest first charge and discharge capacity per gram can reach 93.5 mAh*g<-1>, the capacity retention rate can be up to 97.7% after cycling for 50 circles with the 1C multiplying power, and excellent electrochemical performance is showed.
Owner:CENT SOUTH UNIV
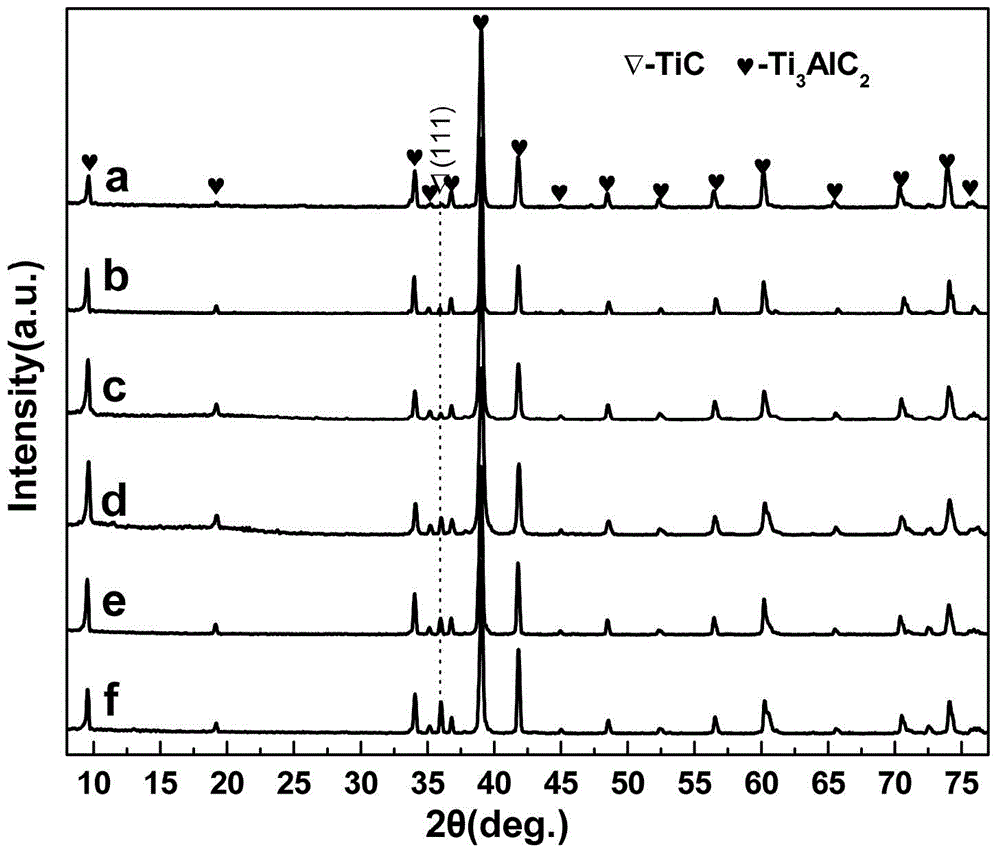
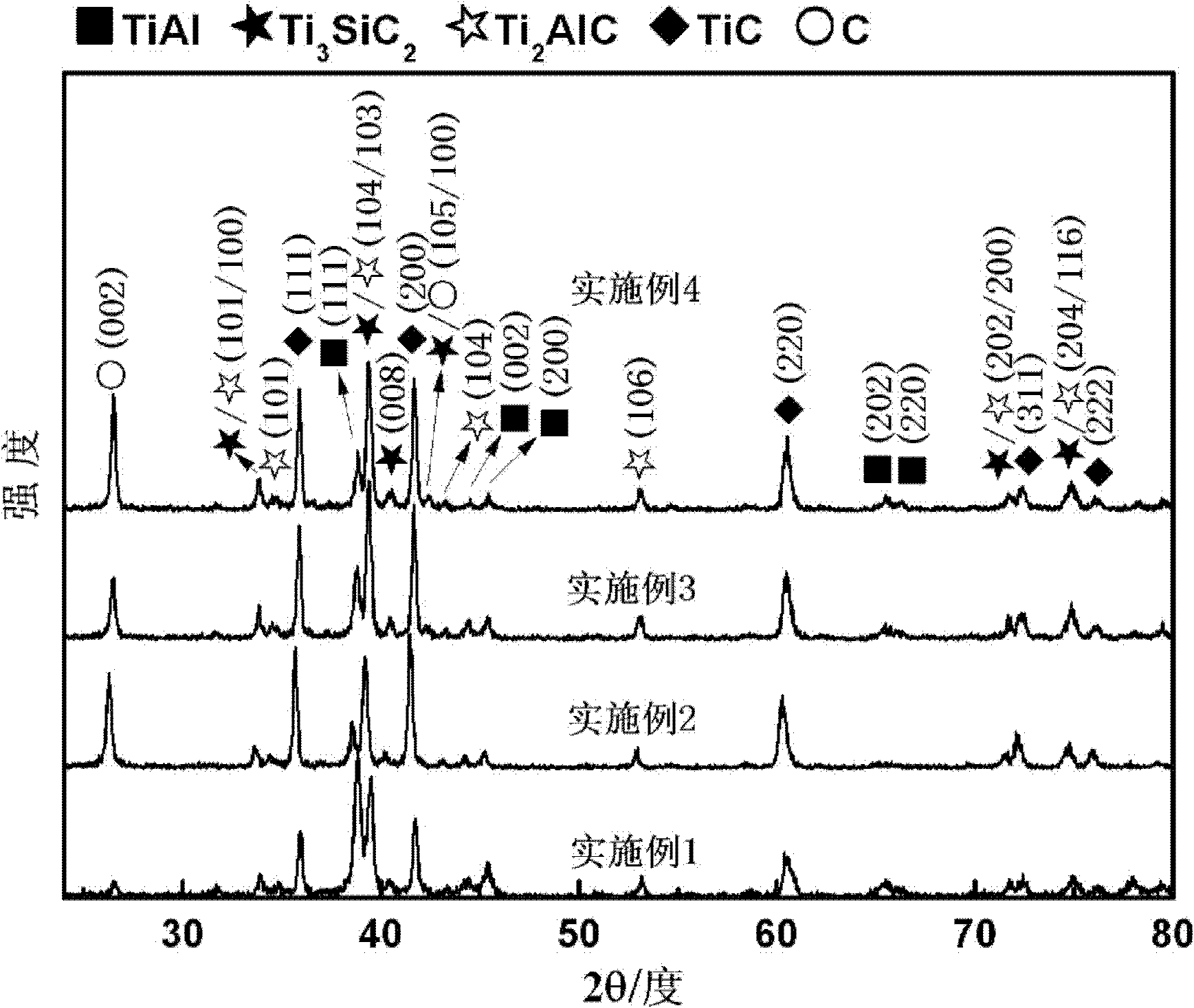
Preparation method of Ti3AlC2 ceramic powder
The invention discloses a preparation method of a Ti3AlC2 ceramic powder, and belongs to the technical field of material science. The preparation method includes the following steps: taking Ti, Al and TiC respectively according to a molar ratio of Ti:Al:TiC:Sn:Si equal to 1:(1-1.3): 2:(0.05-0.2):(0.05-0.15), then adding a Sn powder and a Si powder and mixing well to obtain a mixture; 2) adding ethanol, conducting fully ball milling to obtain a uniform powder and drying; and 3) sintering the dried mixture in vacuum and cooling to obtain the Ti3AlC2 ceramic powder. The simple process provided by the invention is simple; addition of a grinding aids improves mixing uniformity of the mixture; addition of synthetic additives improves the purity of the product and reduces the impurities; and using TiC powder as a C source not only reduces the synthesis temperature but also improves the purity of the product.
Owner:SHAANXI UNIV OF SCI & TECH
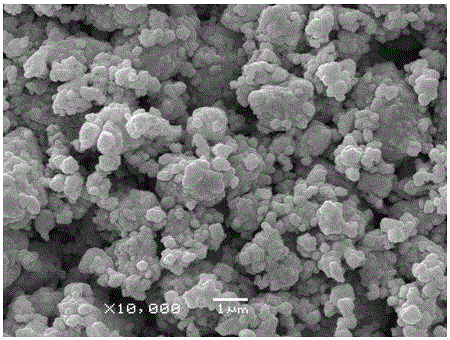
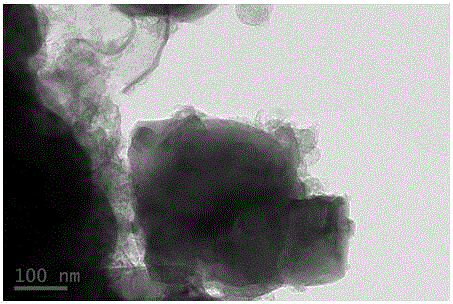
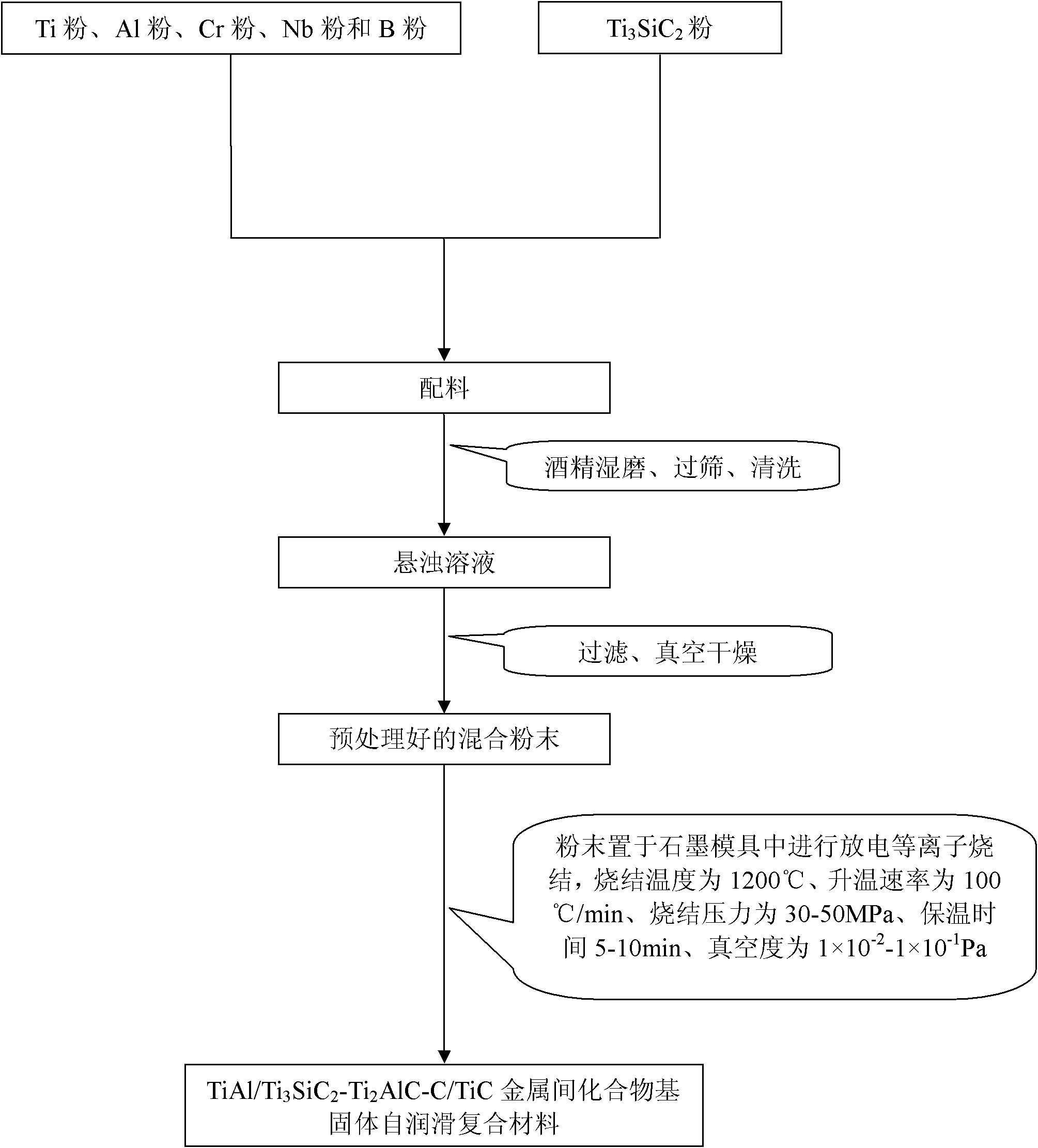
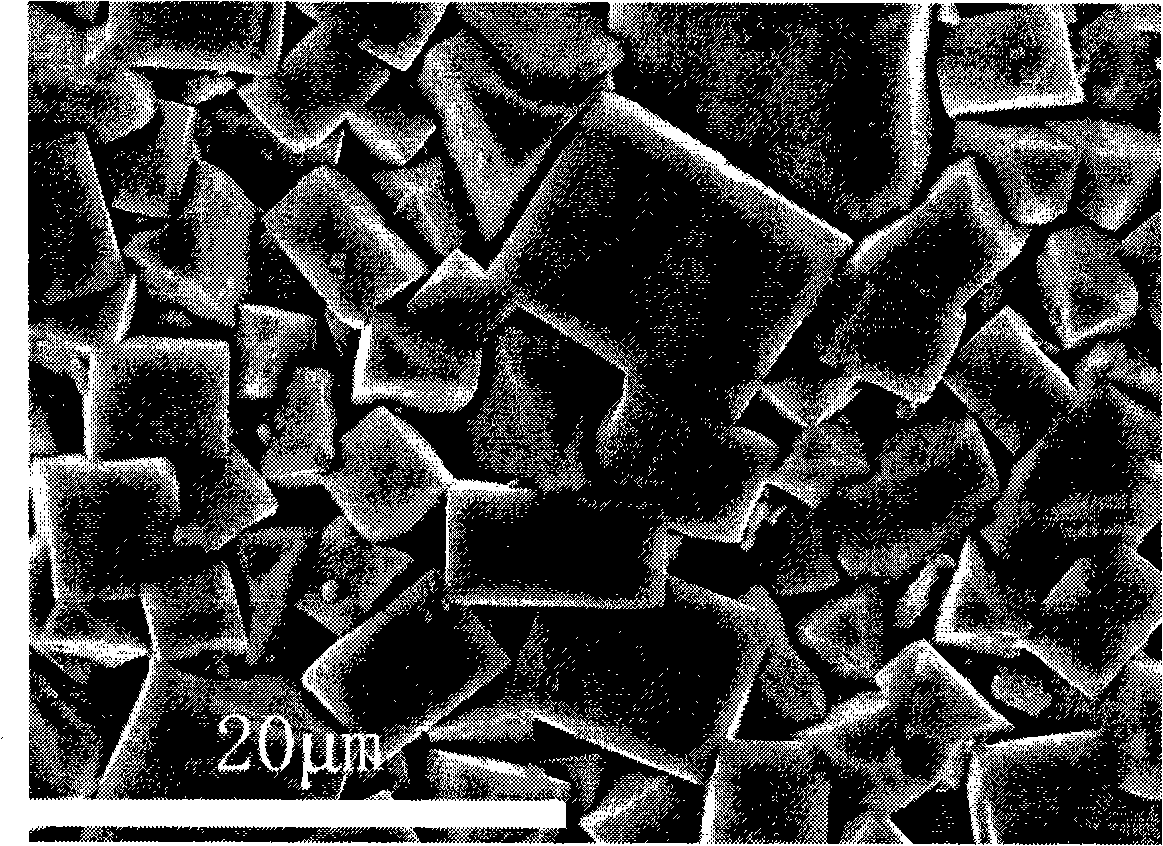
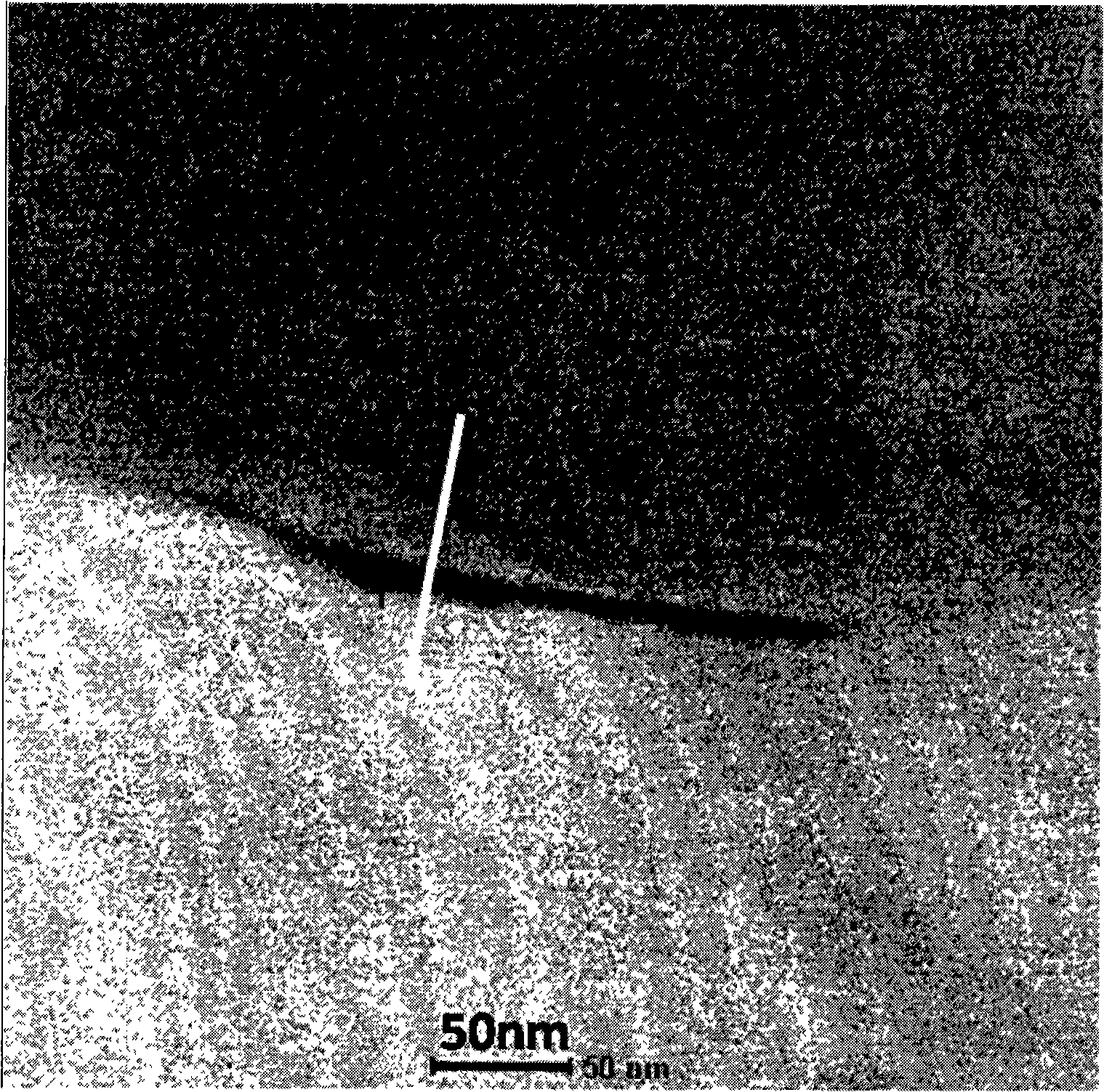
TiAl intermetallic compound-based solid seif-lubricating composite material and preparation method thereof
The invention relates to a TiAl intermetallic compound-based solid seif-lubricating composite material which comprises Ti3SiC2, Ti2AlC and C which are used as the ternary composite lubricating phase and TiC which is used as the reinforced phase, and a preparation method thereof. The TiAl intermetallic compound-based solid seif-lubricating composite material is characterized in that the material is prepared from Ti powder, Al powder, Cr powder, Nb powder, B powder and Ti3SiC2 powder, wherein the molar ratio of Ti, Al, Cr, Nb and B is 48:47:2:2:1 and the dosage of the Ti3SiC2 powder is 5-20wt.% of the total weight of the Ti powder, Al powder, Cr powder, Nb powder and B powder. The TiAl / Ti3SiC2-Ti2AlC-C / TiC intermetallic compound-based solid seif-lubricating composite material synthesized by the method is novel in component design (the intermetallic compound matrix, the composite lubricating phase and the reinforced phase), high in density, good in tribological properties, stable in technological parameters, fast and simple in preparation process and easy in operation and the method is suitable to be used to prepare the high performance TiAl intermetallic compound-based solid seif-lubricating composite material.
Owner:WUHAN UNIV OF TECH
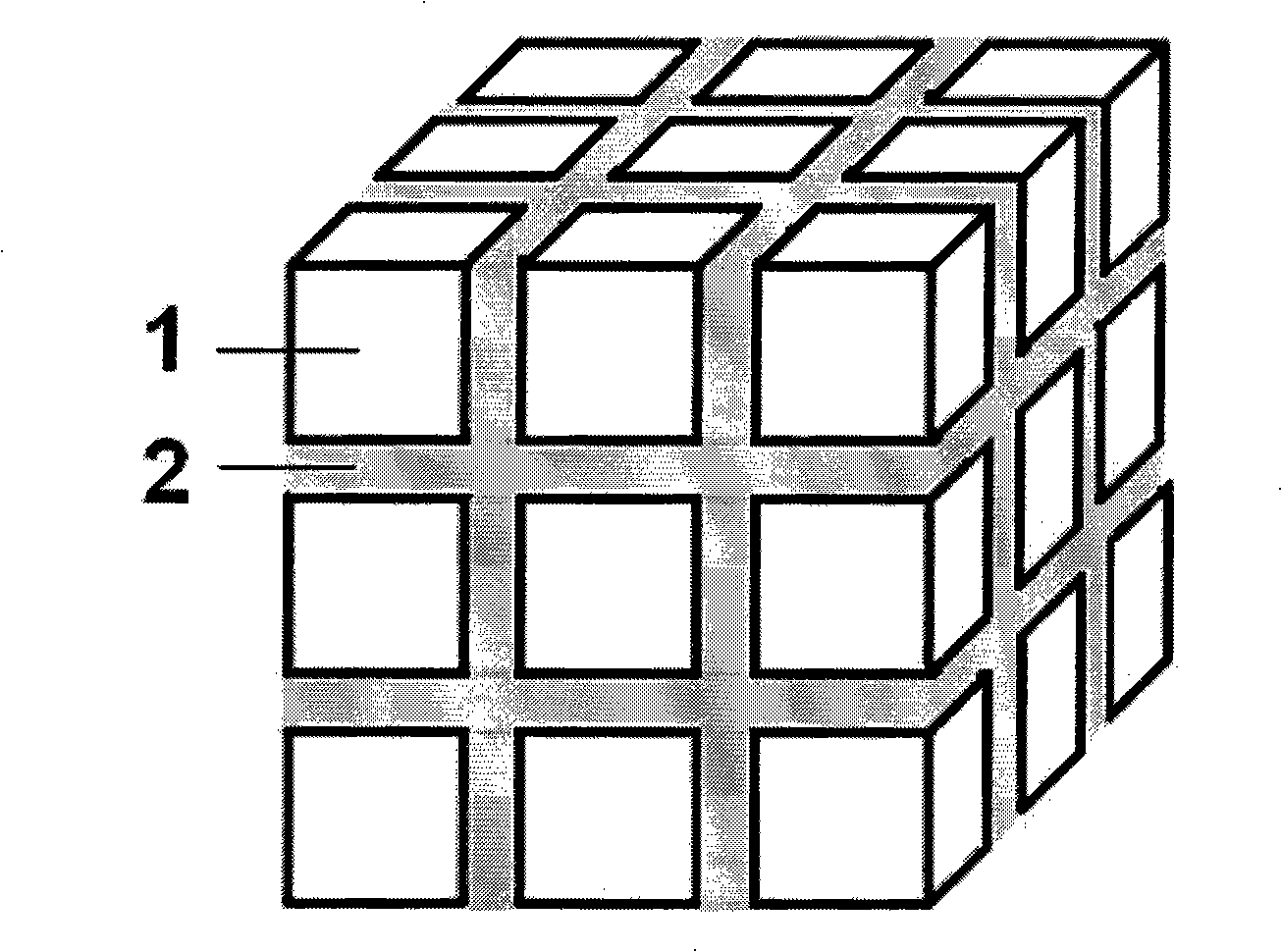
Lithium lanthanum titanium oxygen LLTO composite solid-state electrolyte material and synthesizing method thereof
ActiveCN101325094AGood lifting effectSimple processSolid electrolyte cellsSecondary cellsSolid state electrolyteComposite ceramic
The invention provides a La-Li-Ti-O (LLTO) composite solid electrolyte material containing an amorphous silicon oxidate grain boundary layer and the synthetic method thereof, and belongs to the field of the lithium ion battery. The material is characterized in that: composite ceramics of an amorphous nano-silicon oxidate layer 2 are contained in the position of the grain boundary between materialcrystal grains, and the induction of the amorphous nano-silicon oxidate layer 2 is realized by adopting the wet chemical process, in the wet chemical process, inexpensive organic silicide is adopted as the additive to be added to the LLTO solid electrolyte material, and when the silicone content is 1 to 10 percent, the LLTO composite solid electrolyte material containing the amorphous silicon oxidate grain boundary layer can be synthesized through agglomeration. The electrical conductivity of the grain boundary is obviously improved, thereby improving the total electrical conductivity of the material. The composite solid electrolyte material has the advantages that the preparation process is simple, the operation is easy, the experimental period is greatly shortened, and the synthesis temperature is reduced, the energy consumption and the production cost are saved.
Owner:TSINGHUA UNIV +1
Lithium molybdate serving as secondary battery electrode material
InactiveCN104577088AImprove cycle performanceImprove securityCell electrodesSecondary cellsMetallurgyLithium molybdate
The invention discloses lithium molybdate serving as a secondary battery electrode material. In an execution mode, a chemical formula of the electrode material is Li (2-x)MoyMzO(3-u), wherein x is larger than or equal to minus 2 and smaller than or equal to 2, y is larger than 0 and smaller than or equal to 5, z is larger than or equal to 0 and smaller than or equal to 9, u is larger than or equal to minus 9 and smaller than or equal to 3, and M comprises one element selected from C, N, F, Na, Mg, Al, Si, P, S, Cl, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Cd, In, Sn, Sb, Te, I, Cs, Ba, Ta, W, Re, Os, Ir, Pt, Au, Hg, Pb, Bi, Po, At, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu or combination of the elements. The electrode material is characterized by having very high specific capacity, excellent cycle performance, rate capability and safety.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
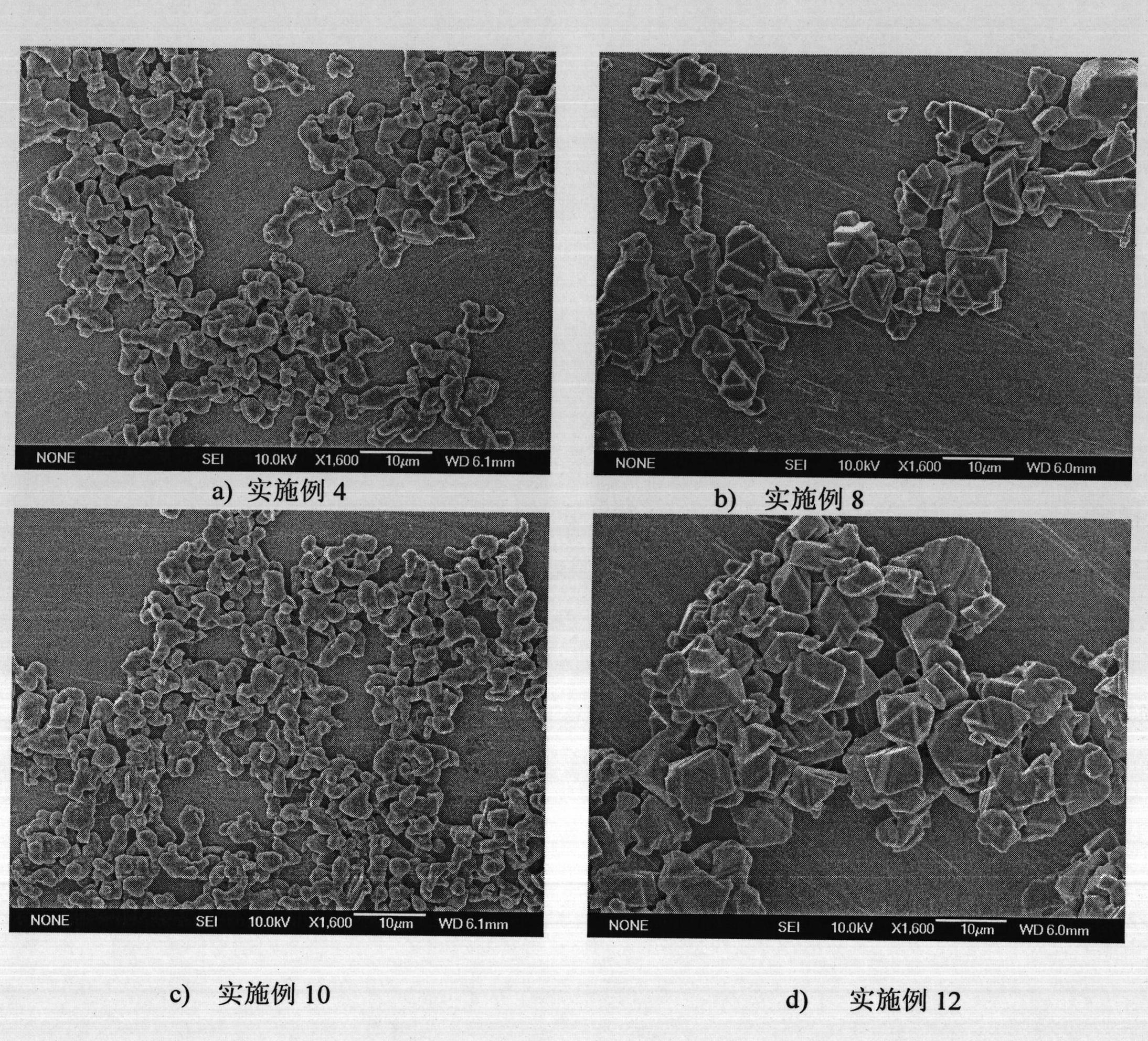
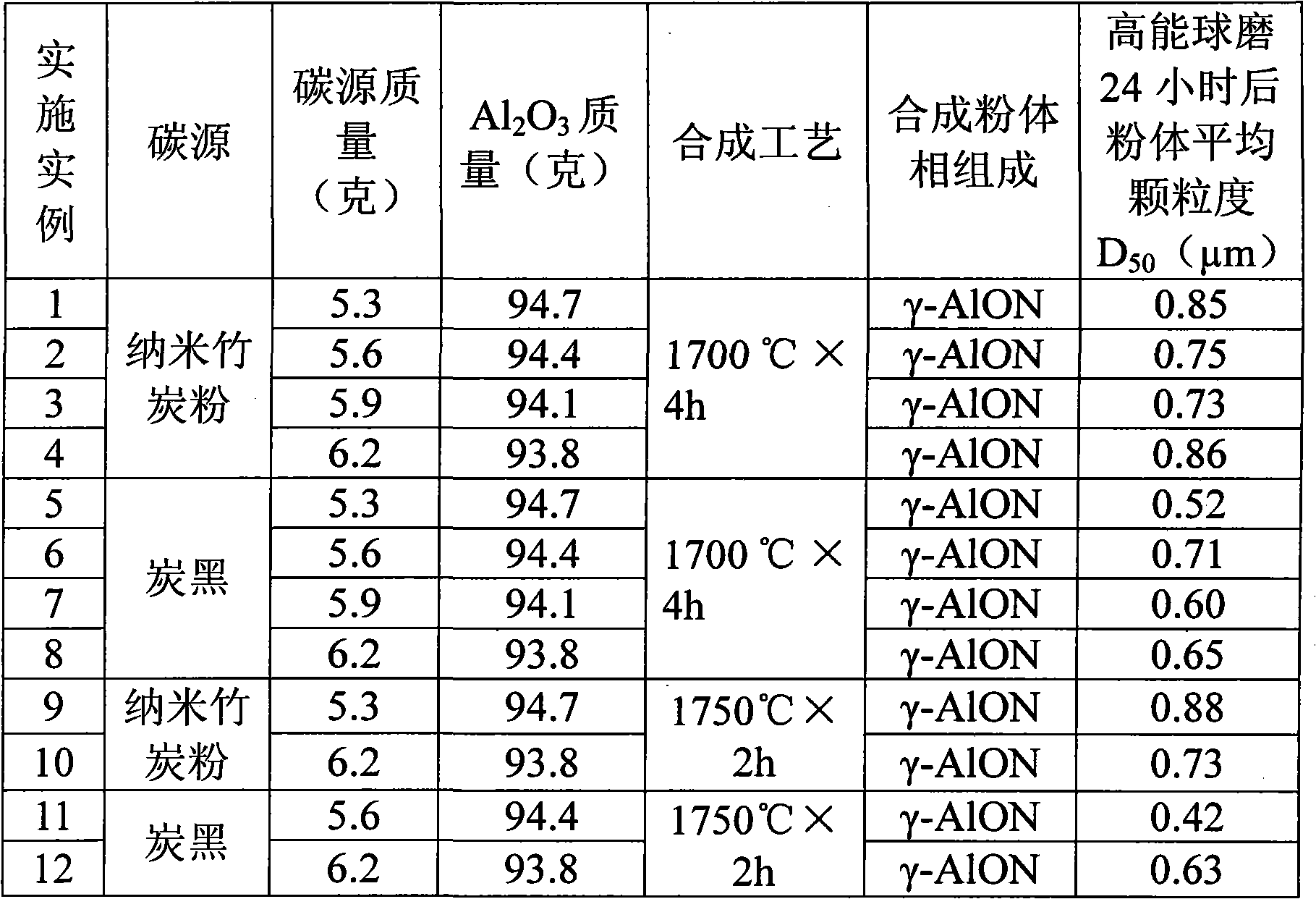
Preparation method of superfine and high-purity gamma-ALON transparent ceramics powder
The invention relates to a preparation method of superfine and high-purity gamma-ALON transparent ceramics powder, belonging to the filed of transparent ceramics material preparation. In the invention, the gamma-ALON transparent ceramics powder is prepared by combining a high energy ball mill with a carbothermic reduction nitriding method. The method of the invention is characterized by comprising the following steps: taking the gamma-Al2O3 powder with high specific surface area and carbon source, i.e. carbon black or nanometer powdered carbon as raw materials, mixing evenly by a wet process high energy ball mill and then drying; placing into a combined crucible of alumina and graphite to carry out the carbothermic reduction nitriding reaction; decarburizing by low-temperature process; and finally obtaining superfine and high-purity gamma-ALON transparent ceramics powder after being crushed by the high energy ball mill. The invention can synthesize pure phase alumina powder under the lower resultant temperature, has simple and feasible process, saves cost, and is suitable for industry production.
Owner:SHANGHAI FRP RES INST
Solvent-thermal method for preparing single-phase bismuth titanate Bi2Ti2O7
InactiveCN102351242AExcellent solar photocatalytic activityHigh purityTitanium compoundsMetal/metal-oxides/metal-hydroxide catalystsSolar photocatalysisPhoto catalytic
The invention discloses a solvent-thermal method for preparing single-phase bismuth titanate Bi2Ti2O7. Bismuth nitrate and butyl titanate are taken as raw materials, and the method is characterized by comprising the following steps of: dissolving the bismuth nitrate in alcohol serving as a solvent, and adding the butyl titanate and ether, wherein the using amount of the bismuth nitrate is lower than the stoichiometric ratio; uniformly mixing, and reacting at the temperature of between 110 and 230DEG C in an enclosed reaction kettle; and cooling, filtering and drying a solid, and calcining at the temperature of between 450 and 550DEG C to obtain the single-phase Bi2Ti2O7. The prepared bismuth titanate compound has a single-phase structure, is spherical nanoparticles, and has excellent solar photo-catalytic activity. The method has the advantages of low synthesis temperature, high powder purity and the like, the preparation process is simple, the raw materials are readily available, thecost is low, and the equipment is simple, and easy to operate.
Owner:NANJING NORMAL UNIVERSITY
Lithium titanate/titanium black anode material and preparation method thereof
ActiveCN102496704AImproved magnification performanceImprove cycle performanceCell electrodesTitanium compoundsElectrical batteryTitanium oxide
The invention relates to a lithium titanate / titanium black anode material and a preparation method thereof belonging to the technical field of lithium ion batteries. The lithium titanate / titanium black anode material has a chemical formula of (1-0.8x)Li4Ti5O12-xTi4O7, wherein x is more than 0,03 and is not more than 0.30. The lithium titanate / titanium black anode material can be synthesized by adopting two methods, the first method is as follows: lithium titanate is carbonized in vacuum, a compact titanium black high-conductive membrane is formed on the surface of the lithium titanate through controlling temperature, reaction time and pressure to have higher multiplying power performance; and the second method is as follows: Ti4O7 is directly added in the lithium titanate, and the lithium titanate / titanium black anode material is synthesized under an inert atmosphere at high temperature. The lithium titanate / titanium black anode material has a simple manufacture process and is beneficial to industrialized production. Compared with the current marketized anode material, the lithium titanate / titanium black anode material has higher electrical conductivity and corrosion resistance and higher large-current discharge cycling performance; and multiplying power charge and discharge performances and cycling performance of a lithium ion power battery can be further improved.
Owner:北京盟固利新材料科技有限公司
Preparation method of nickel/vanadium layered double hydroxide nano-sheet array water oxidation catalyst
InactiveCN107497444ASynthesis temperature is lowLow costCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsElectrolysisVacuum drying
The invention provides a preparation method of a nickel / vanadium layered double hydroxide nano-sheet array water oxidation catalyst. The preparation method includes the steps of: 1) soaking foam nickel in a pure acetone solution for ultrasonic washing, soaking foam nickel in hydrochloric acid for ultrasonic washing, alternately washing the foam nickel with ethanol and deionized water, and finally vacuum-drying the foam nickel to obtain treated foam nickel; 2) simultaneously adding NiCl2.6H2O, VCl3 and Co(NH2)2 to deionized water to obtain a clear solution A; and 3) placing the foam nickel in a reaction lining, pouring the solution A into the reaction lining, sealing the reaction lining, and arranging and fixing the reaction lining in an outer kettle, placing the kettle in a homogeneous-phase reactor to perform hydrothermal reaction, and naturally cooling the reaction product to room temperature; 4) moving the cooled foam nickel product out, alternately washing the foam nickel product with water and alcohol, collecting the product, and vacuum-drying the product to obtain NiV-LDH nano-sheet array. The produce has homogeneous chemical composition, high purity and uniform appearance, and has excellent electrochemical performance when being used as an electrode material for electrolyzing water.
Owner:SHAANXI UNIV OF SCI & TECH
Manufacture method of lithium lanthanum titanium oxide
ActiveCN1970455AImprove electrical performanceThe experiment process is simpleCell electrodesLithium compoundsAlcoholLithium-ion battery
The invention discloses a making method of Li-La-Ti oxide in the lithium ion battery domain, which comprises the following steps: adopting lithium nitrate, lanthanum nitrate and butyl titanate as raw material with molecular formula as Li3xLa2 / 3-xTiO3 (0<x<0.16); setting the molar rate of Li, La and Ti at 1: 1: 2; adopting alcohol or ethylene glycol monomethyl ether as solvent; or adjusting pH value under 1 through water and acetate; drying solution; sintering under 800-900 deg.c for 2h; obtaining pure LLTO. the invention shortens experimental period and synthesizing temperature with grain size about 200nm, which possesses excellent compact and electric property.
Owner:TSINGHUA UNIV
A ball hydroxide oxidated Ni-Co-Mn and its making method
InactiveCN101127398AHigh tap densityImprove electrochemical activityElectrode manufacturing processesCobalt compoundsReaction timingOxidizing agent
The utility model discloses a spherical hydroxyl nickel oxide cobalt manganese and the fabrication method, relating to the anode material of a lithium battery, and aiming to provide a spherical hydroxyl nickel oxide cobalt manganese and the fabrication method. The fabricated anode material of the lithium battery has the advantages of big tap density, good electrochemical activity, big specific volumetric capacity, low synthesis temperature and short reaction time. The key points of the technical proposal of the utility model are that: the general formula of the spherical hydroxyl nickel oxide cobalt manganese is Ni1-x-yCoxMnyOOH; the spherical hydroxyl nickel oxide cobalt manganese and the fabrication method comprise the following steps: (1)a mixed salt solution of nickel, cobalt and manganese is confected according to the ratio that Ni:Co:Mn=1:(1 / 5-2 / 5):(1 / 5-2 / 5); (2) an alkaline solution of two to ten mol / L is confected; (3) the mixed salt solution of nickel, cobalt and manganese, the alkaline solution and the ammonia are conducted to an reaction kettle; (4) the intermediate products are added to the reaction kettle according to the solid-liquid ratio of 1:2 to 10, and reacted with the oxidant to produce the utility model. The utility model is used as the anode material of lithium batteries.
Owner:HENAN NORMAL UNIV +1
Hexagonal crystal system Y-type ferrite electromagnetic material and preparation method thereof
InactiveCN102674823AFacilitated DiffusionFully contactedInorganic material magnetismCrystal systemHexagonal crystal system
The invention provides a hexagonal crystal system Y-type ferrite electromagnetic material and a preparation method thereof. The material is a Ba2Co2-xZnxFeyO22 (x is more than or equal to 0 and less than or equal to 2, and y is more than or equal to 10 and less than or equal to 14) ferrite material with hexagonal flaky morphology, and is obtained by uniformly mixing reactants and a reaction medium and calcining, wherein the reactants at least comprise a barium source, an iron source and a cobalt source; the reaction medium is one kind of chlorate or a mixture of two kinds of chlorate; the molar ratio of various elements in the reactants is that the ratio of Ba to Fe is 2:(10-14), and the ratio of Ba to Co is 2:(0-2); and the ratio of the mass of the reaction medium to the total mass of the reactants is (1-4):1. The invention has the advantages that the morphology of ferrite powder particles can be well controlled; inorganic molten salt is taken as the reaction medium, and the reactants are quickly dispersed and fully contacted in the molten salt by utilizing the dissolution of the reactants in the molten salt so as to reduce reaction temperature and improve reaction rate; the molten salt runs through generated barium ferrite particles in the reaction process, so the mutual agglomeration among the particles can be stopped; and the process is simple, the product is high in purity, the resultant temperature is low, the mechanical ball milling is not required, the doping is avoided, and the particle size distribution is narrow.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
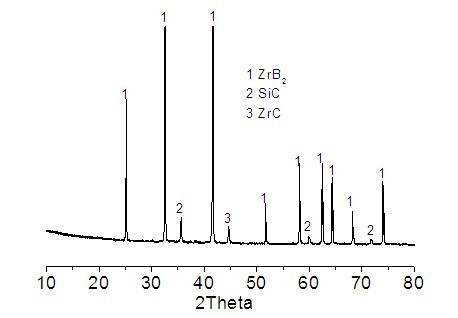
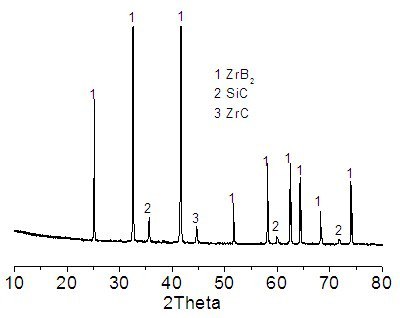
ZrB2-SiC composite powder and preparation method thereof
InactiveCN102320850AFix issues with restricted usageSynthesis temperature is lowUltra-high-temperature ceramicsRefractory
The invention discloses ZrB2-SiC composite powder and a preparation method thereof. According to the invention, zircon sand, a boron raw material and a carbon raw material are adopted as main materials. The main materials are mixed, grinded, and are heated under a temperature of 1350 to 1480 DEG C, such that the powder is obtained. The powder comprises components of, by weight: 37 to 63% of ZrSiO4, 16 to 32% of B2O3, and 20 to 28% of C, wherein the boron raw material is calculated according to the amount of B2O3, and the carbon raw material is calculated according to the amount of C element. According to the invention, natural zircon sand with a relatively low price is used as a raw material for producing high-grade ZrB2-SiC. The advantages of ZrB2 and SiC are combined. Mutual complement of advantages can be realized when ZrB2 and SiC are used in the field of high-temperature materials. ZrB2-SiC provides relatively high thermal conductivity, excellent thermal shock resistance and corrosion resistance. When ZrB2-SiC is introduced into the composite powder, high-temperature mechanical properties, oxidative stabilities and corrosion resistances of ultra-high temperature ceramics and refractory materials can be improved.
Owner:ZHENGZHOU UNIV
Metallic carbide/carbon composite coating on surface of carbon material and preparation method thereof
InactiveCN101570443ADelay the time of high temperature anti-oxidationSynthesis temperature is lowCarbon fibresCarbon compositesNitrogen gas
The invention relates to a metallic carbide / carbon composite coating prepared on the surface of a carbon material and a method thereof. The method comprises the following steps: placing the carbon material into a chemical vapor deposition furnace, introducing propylene and nitrogen of which flow rates are 100 to 800sccm and 100 to 2,000sccm respectively into the furnace, simultaneously heating the carbon material to between 700 and 1,300 DEG C at a rate of 1 to 20 DEG C per minute, and preserving the heat for 40 to 120 hours to obtain the carbon material of which surface is deposited with a pyrolytic carbon coating; placing the carbon material into a crucible, covering a mixture of 1 to 1.9 times of assistant and 0.01 to 1.2 times of transition metal powder in terms of the carbon material on the carbon material, heating the carbon material to between 600 and 1,200 DEG C at a rate of 1 to 30 DEG C per minute under the atmosphere of the nitrogen, preserving the heat for 1 to 20 hours, cooling the mixture and then taking out a product, washing the product by water and drying the product to obtain the composite coating. The method can prepare various transition metallic carbide / carbon composite coatings on the surface of the carbon material, the thickness and form of the coating are controllable, the combination of the coating and the carbon material basal body is good, and the properties of high-temperature oxidation resistance and ablation resistance of a base material are improved.
Owner:AEROSPACE RES INST OF MATERIAL & PROCESSING TECH +1
Method for preparing ball-flower-shaped gamma-bismuth trioxide powder
InactiveCN102491417ADiffraction peak is weakHigh purityNanotechnologyBismuth compoundsDispersityFiltration
The invention discloses a method for preparing ball-flower-shaped gamma-bismuth trioxide powder, which comprises the following steps: 1 adding Bi(NO3)3 5H2O into glycol, and performing stirring to enable the Bi(NO3)3 5H2O to be dissolved, 2 sequentially adding NaOH solution, water and polyethylene glycol into prepared bismuth salt solution during the stirring to obtain reaction solution, adding the prepared reaction solution into a hydrothermal reaction kettle containing a polytetrafluoroethylene substrate, and performing hydrothermal reaction for producing Bi2O3 in an airtight state, and 3 cooling an obtained material to the room temperature and then performing air pump filtration, washing and drying to obtain a gamma-Bi2O3 powder product. The prepared gamma-Bi2O3 has uniform morphology and good dispersity. The microstructure is in a ball-like shape, and further, the ball-like shape is a ball-flower shape with diameter of 1-25 micrometers and large specific surface area. The thickness of nanometer sheets for forming micro-balls is smaller than 100 micrometers, visible light response is good, and photo-catalytic activity is high.
Owner:临沂润泰新型建材有限公司
Sol-solvent-thermal method for synthesizing nanocrystalline oxide powder
InactiveCN101348240AUniform particle sizeNarrow distributionOxide/hydroxide preparationIron oxides/hydroxidesInorganic saltsLiquid medium
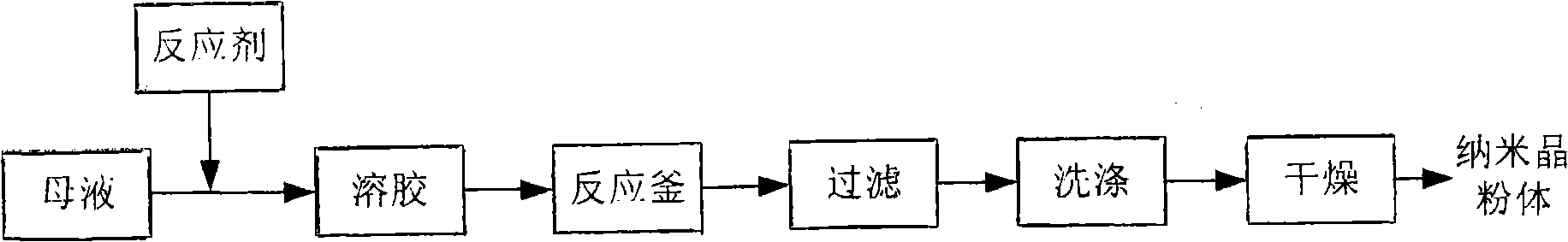
The invention provides a sol-solvent thermal method for synthesizing a nanocrystalline oxide powder. The method comprises the following: (1) a step of preparation of inorganic salt mother liquids, during which the inorganic salt taken as a material is dissolved in an organic liquid medium or water to prepare a mother liquid of the inorganic salt with definite concentration; (2) a step of preparation of a precipitation reactant liquid, during which alkali is dissolved in an organic liquid medium or water, proper quantity of surfactants are added to prepare the precipitation reactant liquid with definite concentration; (3) a step of preparation of sols, during which, at a certain temperature, while stirring, the prepared reactant liquid is slowly added in the prepared mother liquid of the inorganic salt, the reactant liquid and the mother liquid of the inorganic salt are kept reacting for a period of time after adding the reactant liquids in the mother liquid of the inorganic salt, and the transparent sols are obtained; and (4) a step of solvent thermal reaction of the sols, during which the prepared sols are put in a reaction kettle and reacted for a period of time under a certain temperature and pressure, the products obtained are subjected to filtering, washing and drying, and the nanocrystalline oxide powder is obtained.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of transition metal carbide material
InactiveCN1751990ASynthesis temperature is lowImprove conversion rateTungsten/molybdenum carbideFiberCrucible
A process for preparing the carbide of transition metal includes such steps as putting carbon in crucible, covering it by assistant and transition metal, heating to 600-1300 deg.C at 0.1-30 deg.C / min under protection of Ar gas or in vacuum, holding the temp for 0.1-200 hr, cooling, boiling the crucible in water, taking the carbide, water washing and drying.
Owner:WUHAN UNIV OF SCI & TECH
Preparation method of MIL-100(Fe) packaged phosphotungstic heteropolyacid catalyst
ActiveCN103191786ALess prone to elution and lossEvenly dispersedOrganic-compounds/hydrides/coordination-complexes catalystsWater basedPtru catalyst
The invention discloses a preparation method of an MIL-100(Fe) packaged phosphotungstic heteropolyacid catalyst. The preparation method comprises the steps of: adding raw materials for synthesizing phosphotungstic heteropolyacid into a certain amount of deionized water based on certain proportion; subsequently adding a certain amount of an iron source, mixing and stirring, further adding a certain amount of an organic ligand, mixing, stirring, adding a certain amount of an acid solution, and mixing and stirring; mixing, stirring and reacting for 5-20 hours under normal pressure at 80-95 DEG C; and filtering the obtained solid, washing for 10-20 hours at 60-80 DEG C by using absolute ethyl alcohol, subsequently treating for 10-15 hours at 60-80 DEG C by using a 30-60mmol / L ammonium fluoride solution, sufficiently washing by using deionized water, and finally drying for 5-10 hours at 100-200 DEG C so as to obtain the MIL-100(Fe) packaged phosphotungstic heteropolyacid catalyst. The method is temperate in synthesis condition, low in synthesis energy consumption and high in catalyst synthesis yield.
Owner:日照经济技术开发区客商服务有限公司
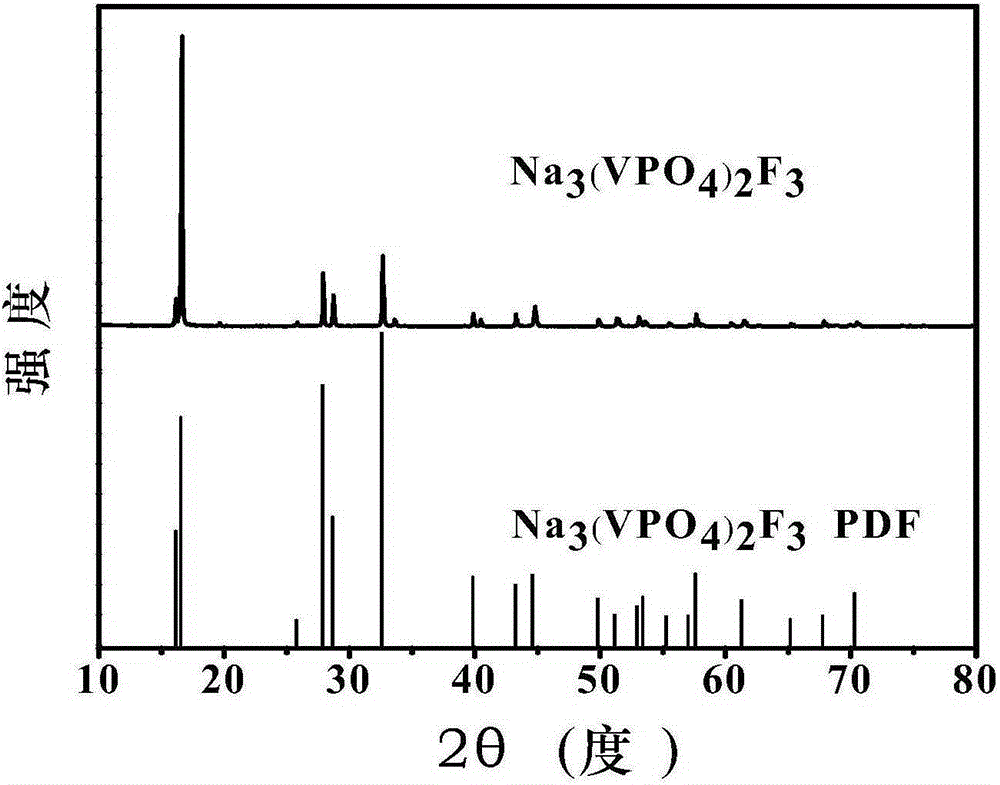
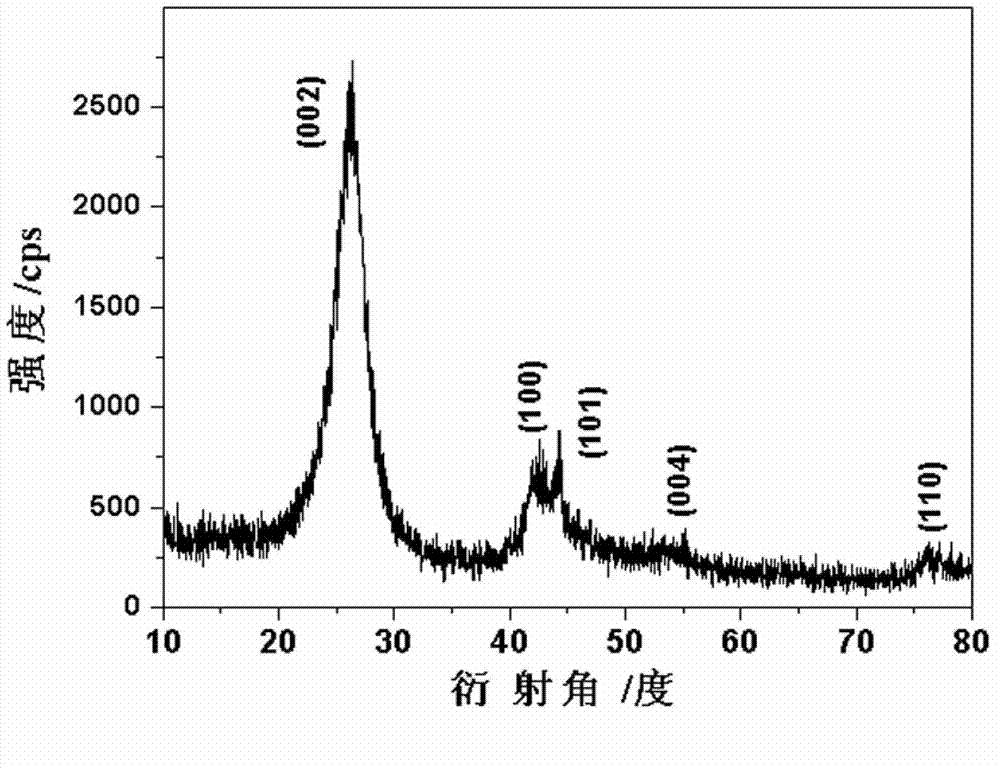
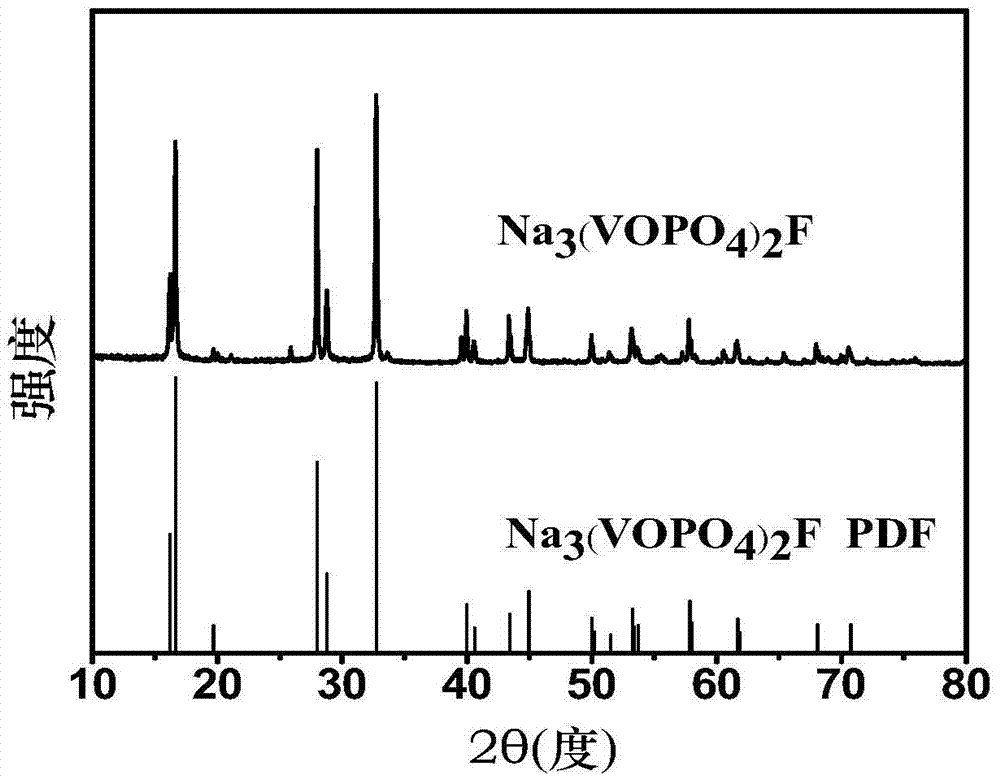
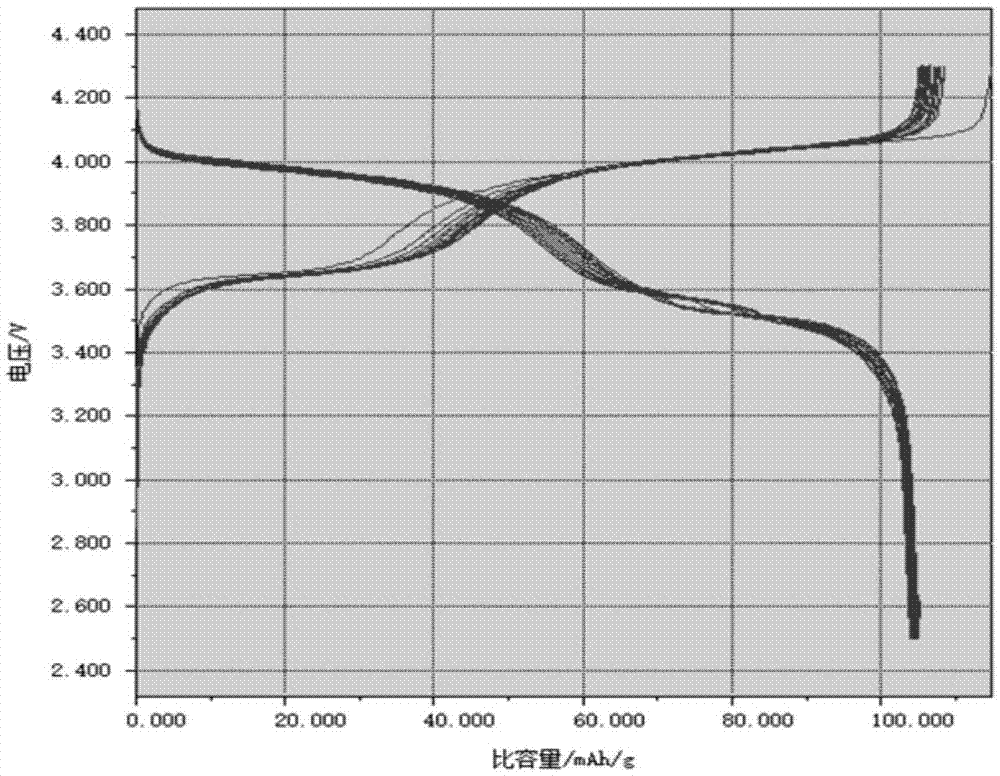
Sodium vanadium fluorophosphate as well as low-temperature environment-friendly preparation method and use thereof
InactiveCN106495124AImprove electrochemical performanceSynthesis temperature is lowCell electrodesPhosphorus compoundsAqueous solutionSpecific discharge
The invention belongs to the technical field of electrode materials and relates to sodium vanadium fluorophosphate as well as a low-temperature environment-friendly preparation method and use thereof. The preparation method comprises the steps of preparing a mixed water solution of a sodium source, a vanadium source, a phosphorus source and a fluorine source, reacting by virtue of the mixed water solution at 20-180 DEG C to obtain sodium vanadium fluorophosphate, wherein the vanadium source is a trivalent vanadium source and / or a tetravalent vanadium source; the chemical constitution of sodium vanadium fluorophosphate is Na3(VOxPO4)2F3-2x, and x is more than or equal to 0 and less than or equal to 1. According to the low-temperature environment-friendly preparation method, the mixed water solution of the sodium source, thephosphorus source, the fluorine source and the trivalent vanadium source and / or a tetravalent vanadium source can generate spontaneous reaction at 20-35 DEG C, the reaction can be accelerated at a temperature high than 35 DEG C and lower than 180 DEG C, and well-crystallized sodium vanadium fluorophosphate can be obtained. Sodium vanadium fluorophosphate can be used as an anode to be assembled into a battery, the specific discharge capacity is not lower than 100mAh / g, and the cycling stability is good.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Hexagonal boron nitride two-dimensional ultrathin nanometer sheet as well as preparation method and application thereof
InactiveCN103043634ALow reaction temperatureIncrease productionMaterial nanotechnologyNitrogen compoundsHexagonal boron nitrideHeat stability
The invention discloses a hexagonal boron nitride two-dimensional ultrathin nanometer sheet as well as a preparation method and application thereof and belongs to the technical field of nanometer materials. According to the invention, metal boride (such as calcium boride, lanthanum boride, magnesium boride, titanium boride and the like) is adopted as a boron resource; ammonium salt (such as ammonium chloride, ammonium bromide, ammonium nitrate and the like) is adopted as a nitrogen source; and the hexagonal boron nitride two-dimensional ultrathin nanometer sheet with the thickness of 0.5-4.0nm is obtained through the reaction under a mild condition (at the temperature of 500-600 DEG C). The invention aims to realize the macro-quantity preparation of the hexagonal boron nitride two-dimensional ultrathin nanometer sheet at the mild temperature by adopting cheaper raw materials; the preparation method has the advantages of saving the energy, simplifying the experimental step and greatly reducing the product cost; and due to the high heat conductivity, heat stability and chemical stability, the hexagonal boron nitride two-dimensional ultrathin nanometer sheet can be applied to the fields of heat dissipation materials, polymer filling materials, catalyst carriers and the like.
Owner:SOUTH CHINA AGRI UNIV
Preparation method of high-entropy oxide ceramic
The invention discloses a preparation method of high-entropy oxide ceramic. The method comprises the following steps that 1, according to metal atom mole ratio being 1:1:1:1 or 1:1:1:1:1, four or fiveor less kinds of metal oxide powder are weighed; the oxide powder includes MgO, ZnO, NiO, CuO, CaO, CoO, ZrO2, CeO2, Al2O3, Gd2O3, La2O3, Er2O3, Y2O3, Fe2O3, Co3O4 and CaCO3; 2, powder weighed in step 1 is subjected to ball milling, drying and granulation; 3, granulated powder is pressed and molded to form a green body; 4, the green body obtained in step 3 is subjected to thermal treatment; 5, the green body obtained after thermal treatment in step 4 is heated to the preset temperature, an electric field of the preset electric field intensity is applied to a sample, after flash-burning appears, a power source is converted from the constant voltage state to the constant current state, the temperature is kept constant for 1-60 min under the preset current density, and then quenching is performed to obtain the needed high-entropy oxide ceramic. The flash-burning is adopted for preparing the high-entropy oxide ceramic, the sintering temperature is remarkably lowered, and the sintering time is remarkably shortened.
Owner:SOUTHWEST JIAOTONG UNIV
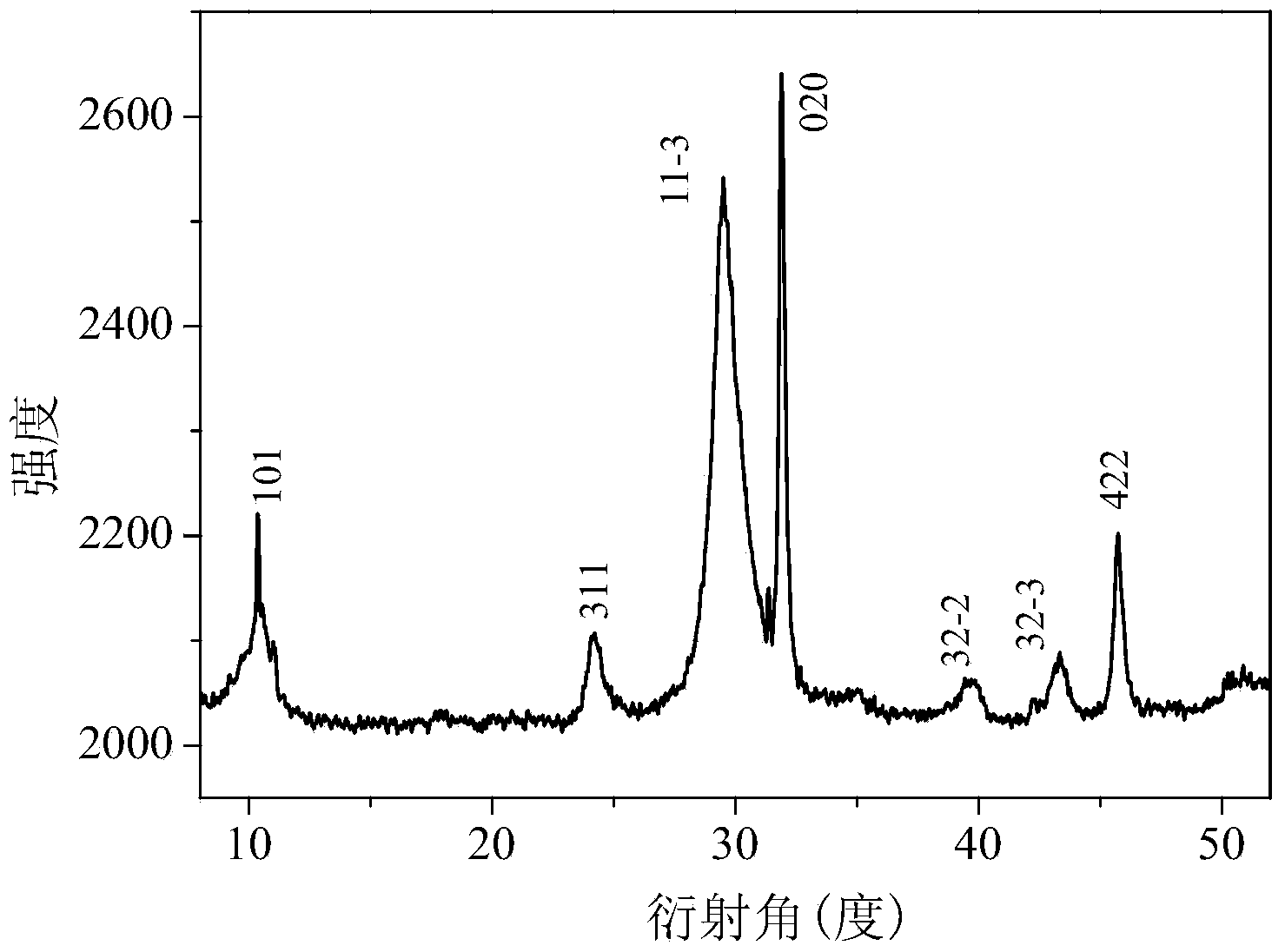
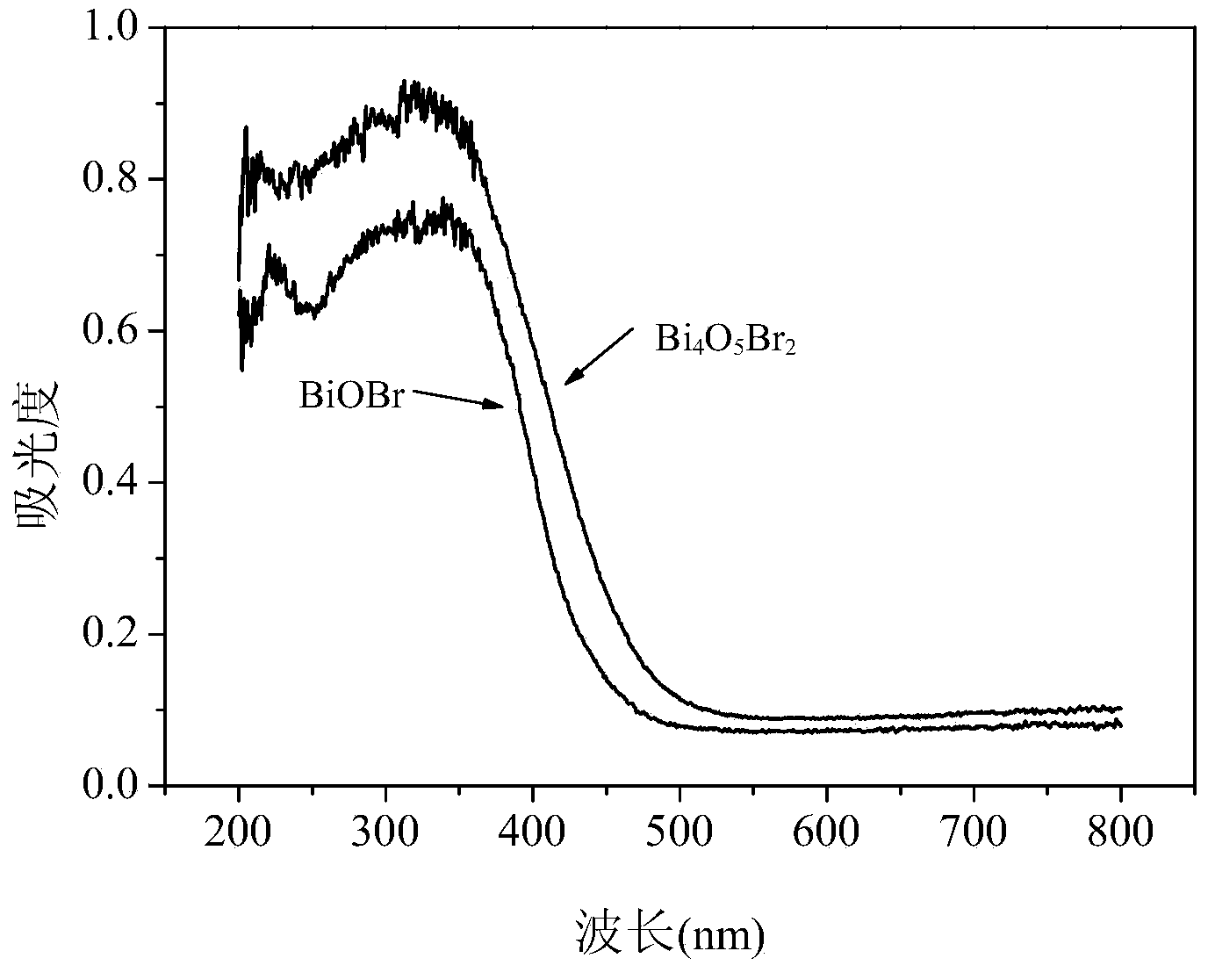
Visible-light-induced photocatalyst Bi4O5Br2 and preparation method thereof
InactiveCN104226339AImprove photocatalytic performanceEasy to recyclePhysical/chemical process catalystsWater/sewage treatment by irradiationSolar photocatalysisMethyl orange
The invention discloses a visible-light-induced photocatalyst Bi4O5Br2 and a preparation method thereof. According to the visible-light-induced photocatalyst Bi4O5Br2 and the preparation method thereof, an improved low-temperature hydrothermal method is adopted, composition of Bi, O and Br in BiOX is controlled by controlling amount of a bismuth source and a bromine source, and a novel layered-cake-shaped visible-light-induced photocatalyst Bi4O5Br2 is prepared successfully. The preparation method is simple in production process, easy to operate, low in synthesis temperature, high in reaction yield, environment-friendly and low in cost and meets the requirement of actual production, the reaction yield is 92%, and raw materials are easy to obtain. The visible-light-induced photocatalyst has good visible-light catalytic activity, can completely degrade various organic pollutants such as rhodamine b, methyl orange and methylene blue in short time under the visible light irradiation, is small in light corrosion and good in reusability, can be applied to industrial production and particularly has a better application value in organic pollutant degradation through solar photocatalysis, and the market potential is large.
Owner:YULIN NORMAL UNIVERSITY
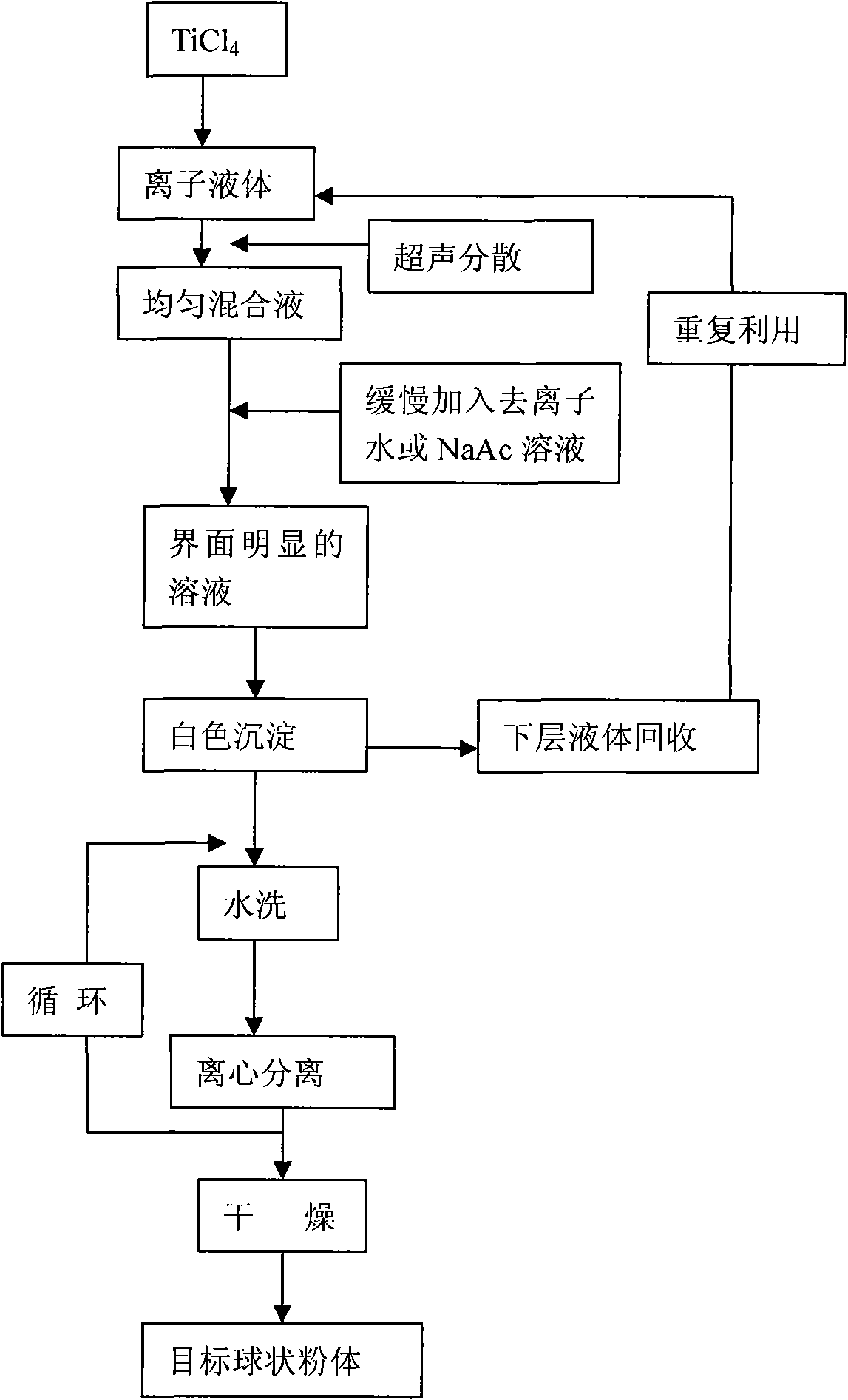
Method for preparing titanium dioxide hollow spherical powder
InactiveCN101580275AAdjust diameter sizeAdjust the distributionTitanium dioxideLiquid layerCentrifugation
The invention discloses a method for preparing titanium dioxide hollow spherical powder, belonging to the technical field of material powder preparation. The method comprises the following steps: firstly, TiCl4 is added into ion liquid with a proper amount; the mixture is stirred uniformly with ultrasonic; deionized water is slowly added, wherein water is in the upper layer; after a period of time, water gradually enters an ion liquid layer to hydrolyze the TiCl4 to obtain a deposit ; because the obtained solution has lamination phenomenon, liquid at the upper layer is separated according to colors, and the ion liquid can be repeatedly utilized; and the deposit is washed by the deionized water and ethanol, separated by centrifugation, dried and baked to obtain the titanium dioxide hollow spherical powder with favorable dispersivity. The method has the advantages of simple and easily controlled process parameters, uniform and adjustable granularity, resource saving, little pollution, and the like.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Sodium vanadium fluorophosphate, preparation method and uses thereof
ActiveCN107154493AFast reaction kineticsLow costCell electrodesSecondary cellsSodium-ion batterySolvent
The present invention provides a sodium vanadium fluorophosphate, a preparation method and uses thereof, wherein the molecular formula of the sodium vanadium fluorophosphate is Na3(VOxPO4)2F3-2x, x is more than or equal to 0 and is less than or equal to 1, the morphology is a spherical wool ball, loose hollow ball or nanoparticle aggregate, and the size is from nanometer to micron. The preparation method comprises: (1) dissolving a vanadium source in water to obtain a vanadium source solution; (2) adding a phosphorus source, a fluorine source and a sodium source to the vanadium source solution to obtain a reaction mixture; and (3) post-treating the obtained reaction mixture to obtain the sodium vanadium fluorophosphate. According to the present invention, the water is directly used as the solvent so as to provide the advantage of no pollution; the sodium vanadium fluorophosphates can be directly synthesized at the room temperature of 10-35 DEG C, such that the temperature is low, the reaction kinetics is rapid, and the large-scale preparation of the sodium vanadium fluorophosphates can be achieved; the morphology and the crystallinity of the sodium vanadium fluorophosphate can be adjusted; the pentavalent vanadium industrial product can be used as the vanadium source so as to substantially reduce the cost of the vanadium source; and the prepared material has good electrochemical properties, and is suitable for the sodium ion battery positive electrode material.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
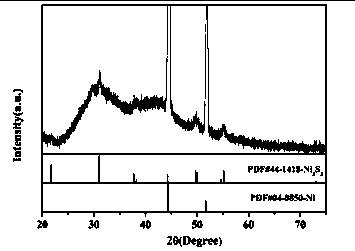
Synthesis method of vanadium-modified Ni3S2 electrocatalyst automatically assembled from rodlike shape into ball-flower shape
ActiveCN108325539ASynthesis temperature is lowShort synthesis timePhysical/chemical process catalystsElectrodesMicrowaveSulfur
The invention discloses a synthesis method of vanadium-modified Ni3S2 electrocatalyst automatically assembled from a rodlike shape into a ball-flower shape. The synthesis method comprises the following steps that clean foamed nickel is dipped into turbid liquid with the vanadium source concentration being 5-30 mM and the mole ratio of a vanadium source to a sulfur source being (1) to (0.5-12), a microwave solvent thermal reaction is conducted, and after the sufficient reaction is completed, the vanadium-modified Ni3S2 electrocatalyst material automatically assembled from the rodlike shape intothe ball-flower shape is obtained. The method is easy to operate, the reaction condition is mild, the consumed time is short, the prepared vanadium modified Ni3S2 product automatically assembled fromthe rodlike shape into the ball-flower shape is high in purity, and uniform in shape and size. In order to achieve the above purpose, the following technical scheme is adopted.
Owner:SHAANXI UNIV OF SCI & TECH
Method for preparing high radioactive waste curing treatment base material
InactiveCN1767077AInexpensive curing processPromote engineering applicationRadioactive decontaminationMetallurgyReaction temperature
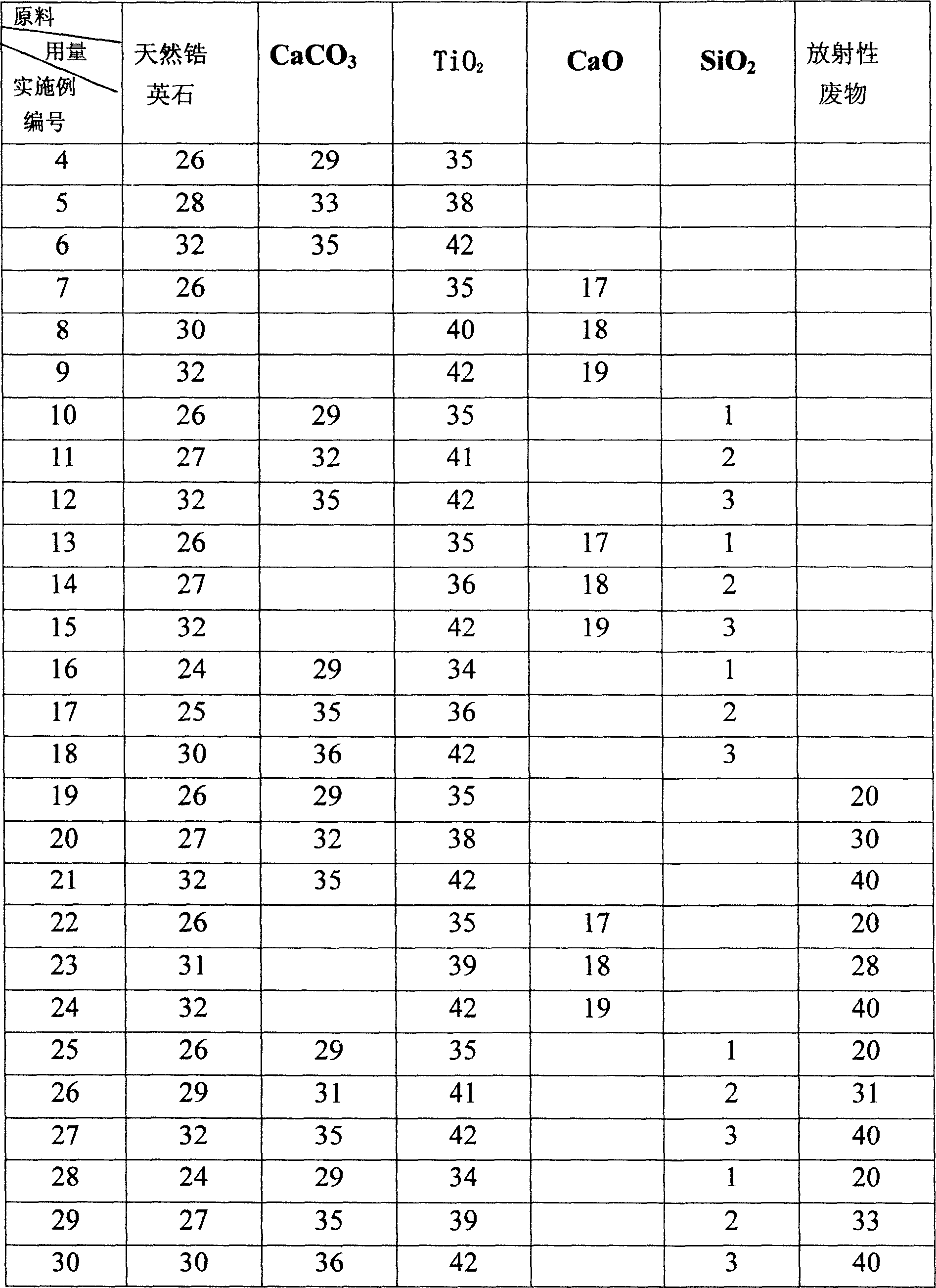
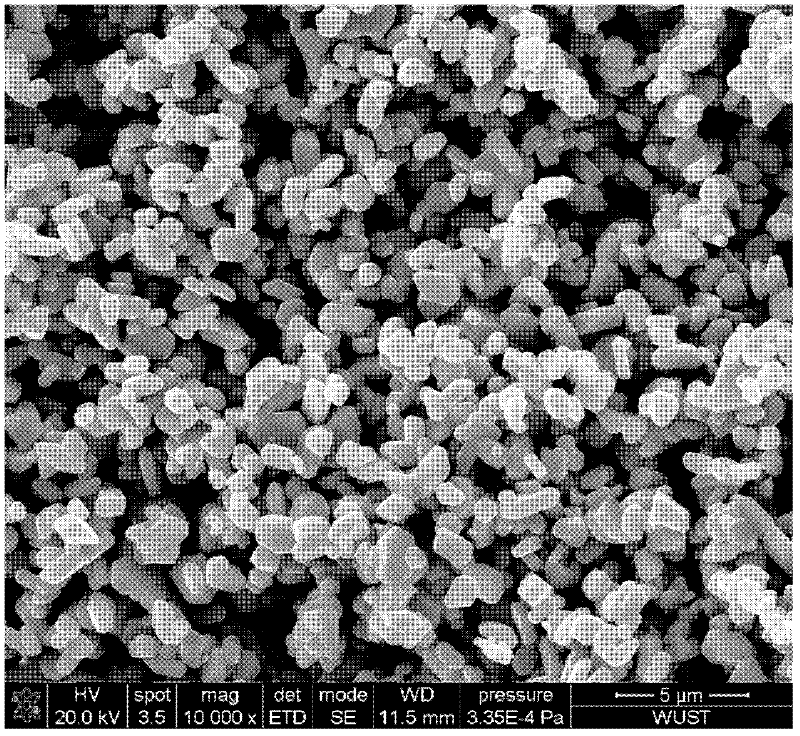
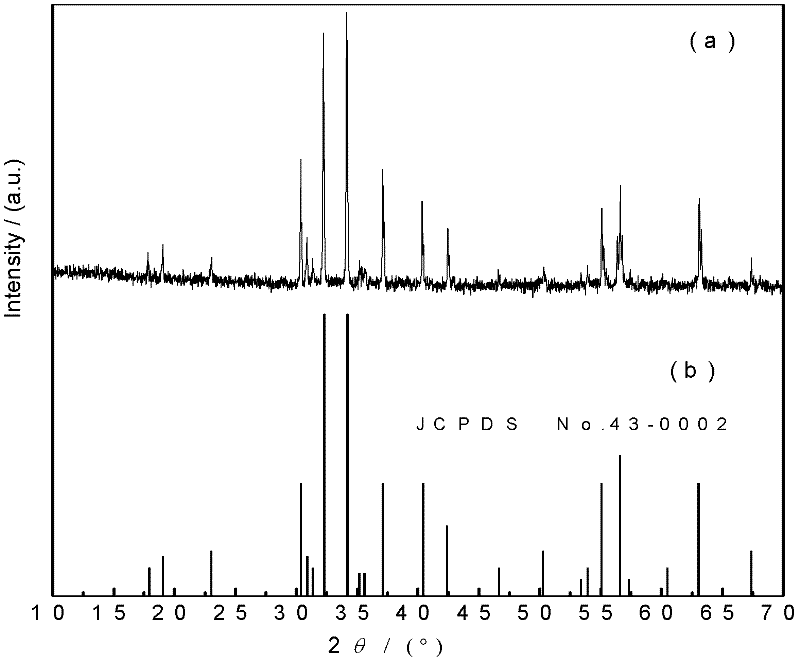
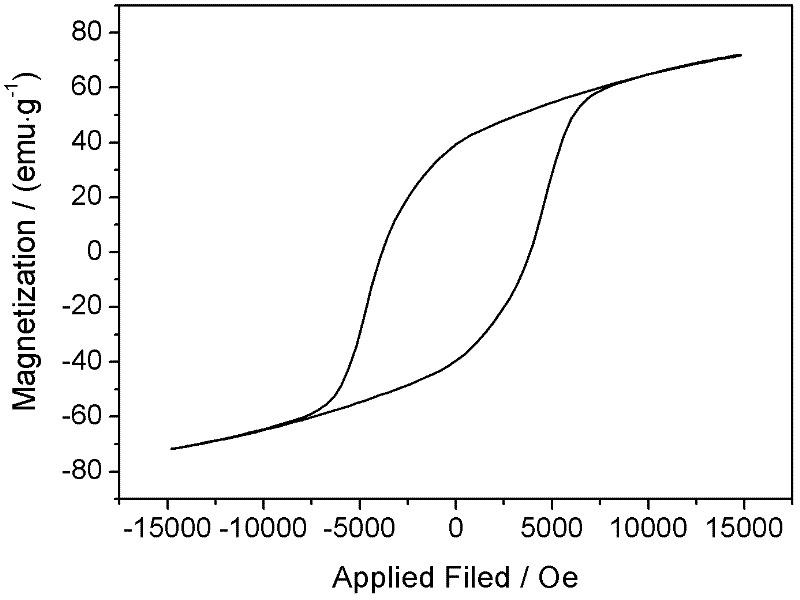
The invention relates to a method for preparing high radioactivity waste solidification treating base material which comprises the following steps: preparing the material: using nature hyacinth, CaCO3 and TiO2 as raw material and extracting them by weight ration: nature hyacinth: CaCO3:TiO2 =26-32:29-35:35-42; correct grinding: loading each raw material into the correct grinding device, adding the grinding ball to do correct grinding 30-60 minutes; burning: burning at the temperature 1150 deg. to 1350 deg. 20-60 minutes to obtain the product.
Owner:SOUTHWEAT UNIV OF SCI & TECH
A method for synthesizing high-performance barium ferrite with molten salt as flux and reaction medium
The invention relates to a preparation method of barium ferrite material. A method for synthesizing high-performance barium ferrite with molten salt as flux and reaction medium is characterized in that it comprises the following steps: 1) ferric oxide and barium carbonate are pressed by Fe3+: Ba2+ in a mol ratio of 10 to 12: 1. Weighing and mixing to obtain the reactant; 2) weighing NaCl and KCl according to the molar ratio of NaCl and KCl to be 1:1 and mixing to obtain the mixed salt; 3) according to the total mass of the mixed salt / total mass of the reactant=1~6, Mix the reactant with the mixed salt, ball mill and dry it, put it into a corundum crucible with a cover and place it at 750°C-1100°C for calcination for 1-3h; 4) After the reaction, cool down to room temperature naturally, wash and dry to obtain barium iron Oxygen. The method has the characteristics of simple process and low synthesis temperature, and the obtained barium ferrite has excellent performance.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
Synthetic method of Ni3S2 microrod array
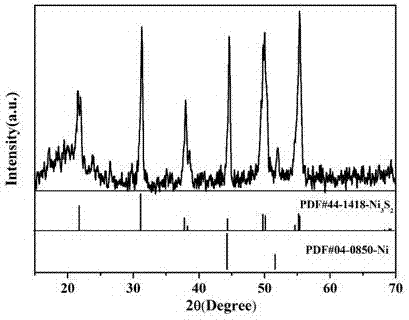
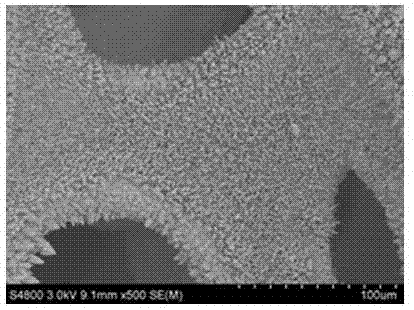
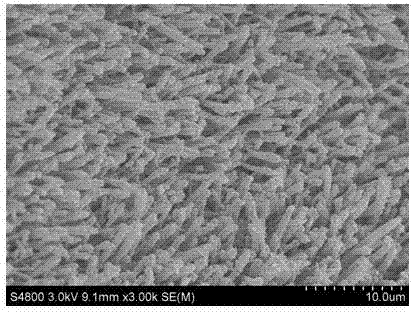
InactiveCN107324408ASynthesis temperature is lowRaw materials are cheap and easy to getMaterial nanotechnologyElectrode shape/formsSolventPolytetrafluoroethylene
The invention discloses a synthetic method of Ni3S2 microrod array. The synthetic method comprises following steps: 1, nickel foam to be treated is subjected to full ultrasonic cleaning and drying; 2, a vanadium source and a sulfur are dissolved in an appropriate amount of a solvent at a molar ratio of 1: (1-11), and full stirring is carried out so as to obtain a solution A, wherein the concentration of the vanadium source in the solution A is controlled to be 10 to 40mM; 3, the solution A is delivered into a hydrothermal reactor with a polytetrafluoroethylene lining, the nickel foam obtained via pretreatment in step 1 is immersed in the solution A, the hydrothermal reactor is sealed, and solvothermal reaction is carried out at 70 to 200 DEG C for 6 to 30h; and 4, after reaction, the hydrothermal reactor is cooled at room temperature, and an obtained product is washed fully and dried so as to obtain the Ni3S2 microrod array. Operation of the synthetic method is simple; reaction conditions are mild; reaction period is short; the purity of prepared Ni3S2 is high; the morphology is uniform; and excellent electrocatalytic hydrogen evolution performance is achieved.
Owner:SHAANXI UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com