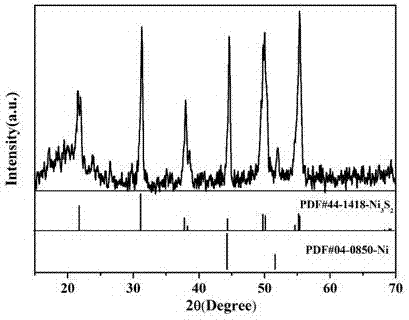
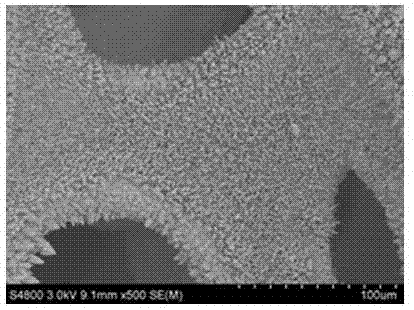
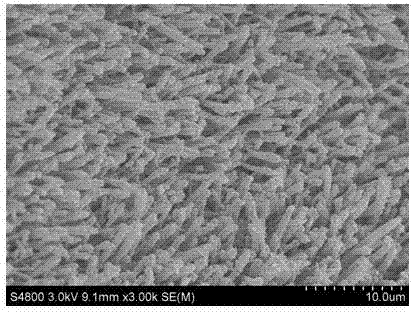
Synthetic method of Ni3S2 microrod array
A synthesis method and micro-rod technology, applied in nanotechnology, nanotechnology, electrode shape/type, etc., can solve the problems of harsh reaction conditions, large hydrogen evolution overpotential, complicated operation steps, etc., and achieve low synthesis temperature and uniform chemical composition. , the effect of cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Soak nickel foam (1×5) cm in acetone solution for 5 minutes, then immerse nickel foam in 2 mol / L hydrochloric acid for 5 minutes, and finally use ethanol and deionized water alternately Rinse 3 times and vacuum dry at 20 °C for 5 h to obtain the treated nickel foam;
[0031] (2) Weigh sodium dodecahydrate vanadate and sodium sulfide, take sodium dodecahydrate vanadate as 0.2 mmol, control the molar ratio of vanadium source and sulfur source to 1:1, add it into 20 ml deionized water at the same time, Stir magnetically at room temperature for 3 min to obtain a homogeneous solution A;
[0032] (3) Pour the stirred solution A into a hydrothermal kettle with a polytetrafluoroethylene liner, put the nickel foam treated in step (1) into the polytetrafluoroethylene liner, seal it, and place In a homogeneous reactor, the reaction was carried out at 70°C for a reaction time of 6 h;
[0033] (4) After the reaction, the reactor was cooled at room temperature, and the product ...
Embodiment 2
[0035] (1) Sonicate (1×5) cm nickel foam in acetone solution for 10 minutes, then immerse foam nickel in 3 mol / L hydrochloric acid for 15 minutes, and finally use ethanol and deionized water alternately Rinse 4 times and vacuum dry at 20 °C for 10 h to obtain the treated nickel foam;
[0036] (2) Take sodium vanadate and sodium diethylthiocarbamate, the sodium vanadate is 0.4 mmol, the molar ratio of vanadium source and sulfur source is 1:4, add it to 20 ml ethanol at the same time, and control it under magnetic stirring at room temperature 15 min to obtain a homogeneous solution A;
[0037] (3) Pour the stirred solution A into a hydrothermal kettle with a polytetrafluoroethylene liner, put the nickel foam treated in step (1) into the polytetrafluoroethylene liner, seal it, and place In a homogeneous reactor, the reaction was carried out at 90 °C for a reaction time of 10 h;
[0038](4) After the reaction, the reactor was cooled at room temperature, and the product was alter...
Embodiment 3
[0040] (1) Sonicate (1×5) cm nickel foam in acetone solution for 10 minutes, then immerse foam nickel in 3 mol / L hydrochloric acid for 15 minutes, and finally use ethanol and deionized water alternately Rinse 4 times and vacuum dry at 25 °C for 10 h to obtain the treated nickel foam;
[0041] (2) Take ammonium metavanadate and sulfur element, the ammonium metavanadate is 0.8mmol, control the molar ratio of vanadium source and sulfur source to 1:6, add it to 20 ml of methanol solvent at the same time, stir magnetically at room temperature for 15 min to get a homogeneous solution A;
[0042] (3) Pour the stirred solution A into a hydrothermal kettle with a polytetrafluoroethylene liner, put the nickel foam treated in step (1) into the polytetrafluoroethylene liner, seal it, and place In a homogeneous reactor, the reaction was carried out at 120 °C for a reaction time of 24 h;
[0043] (4) After the reaction, the reactor was cooled at room temperature, and the product was alter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com