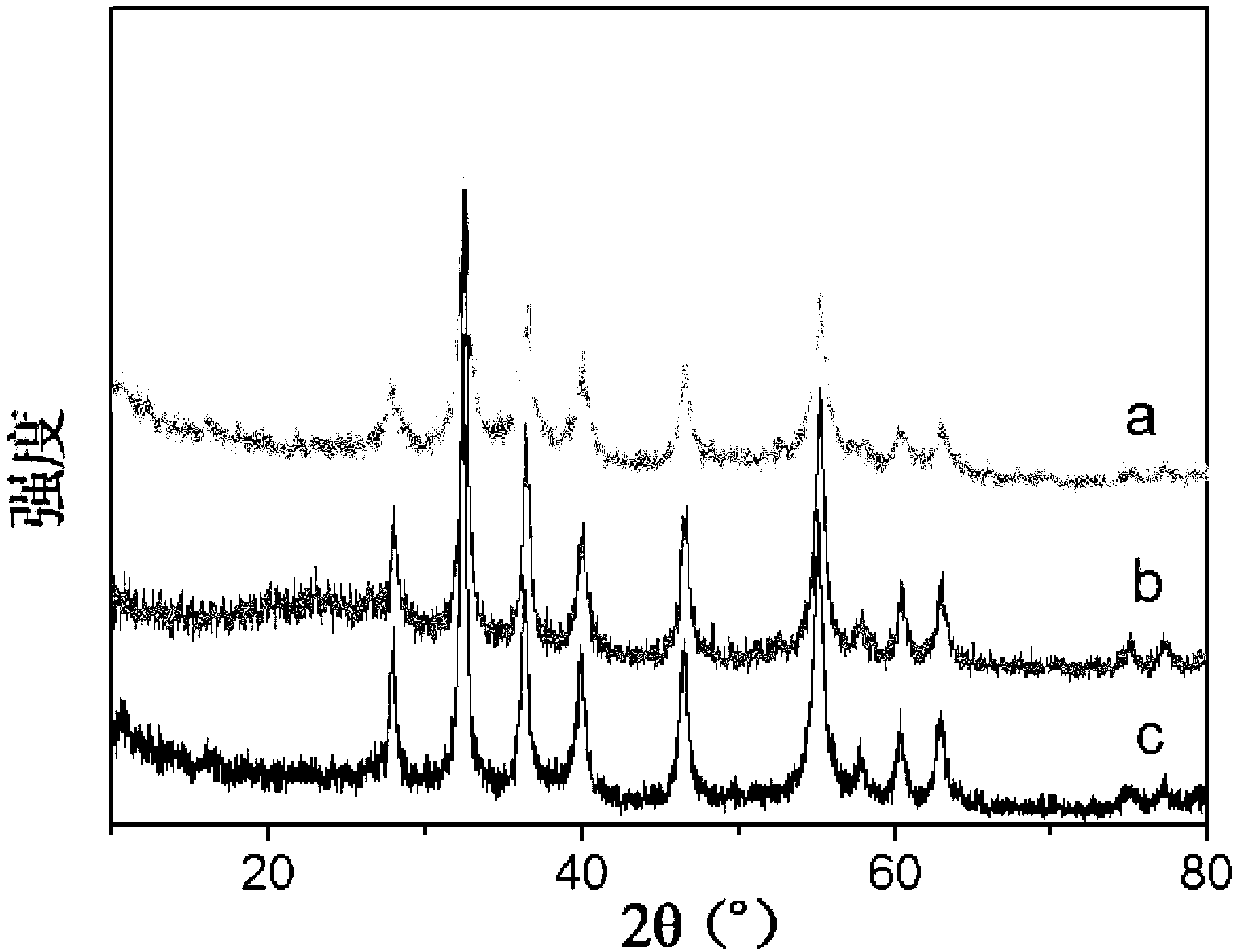
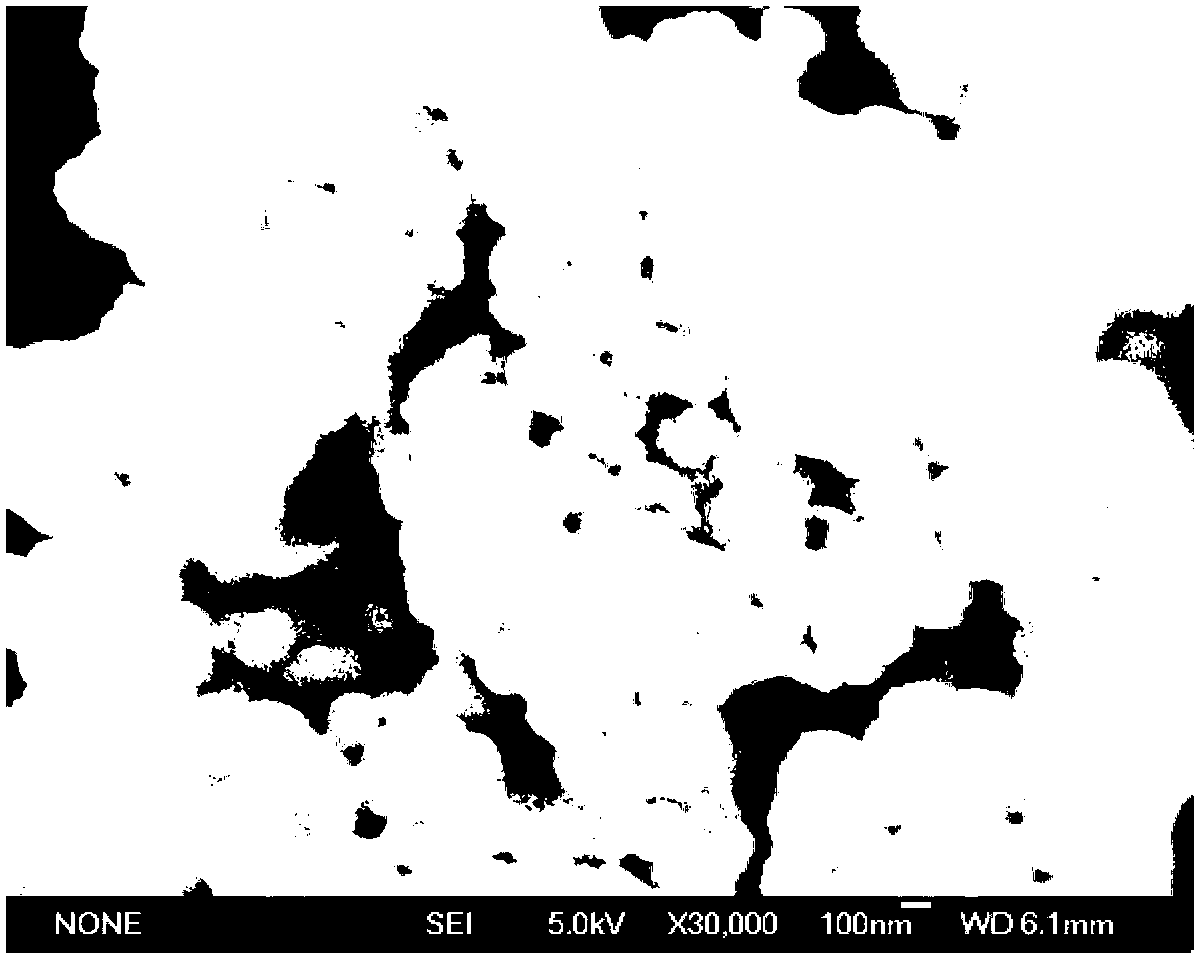
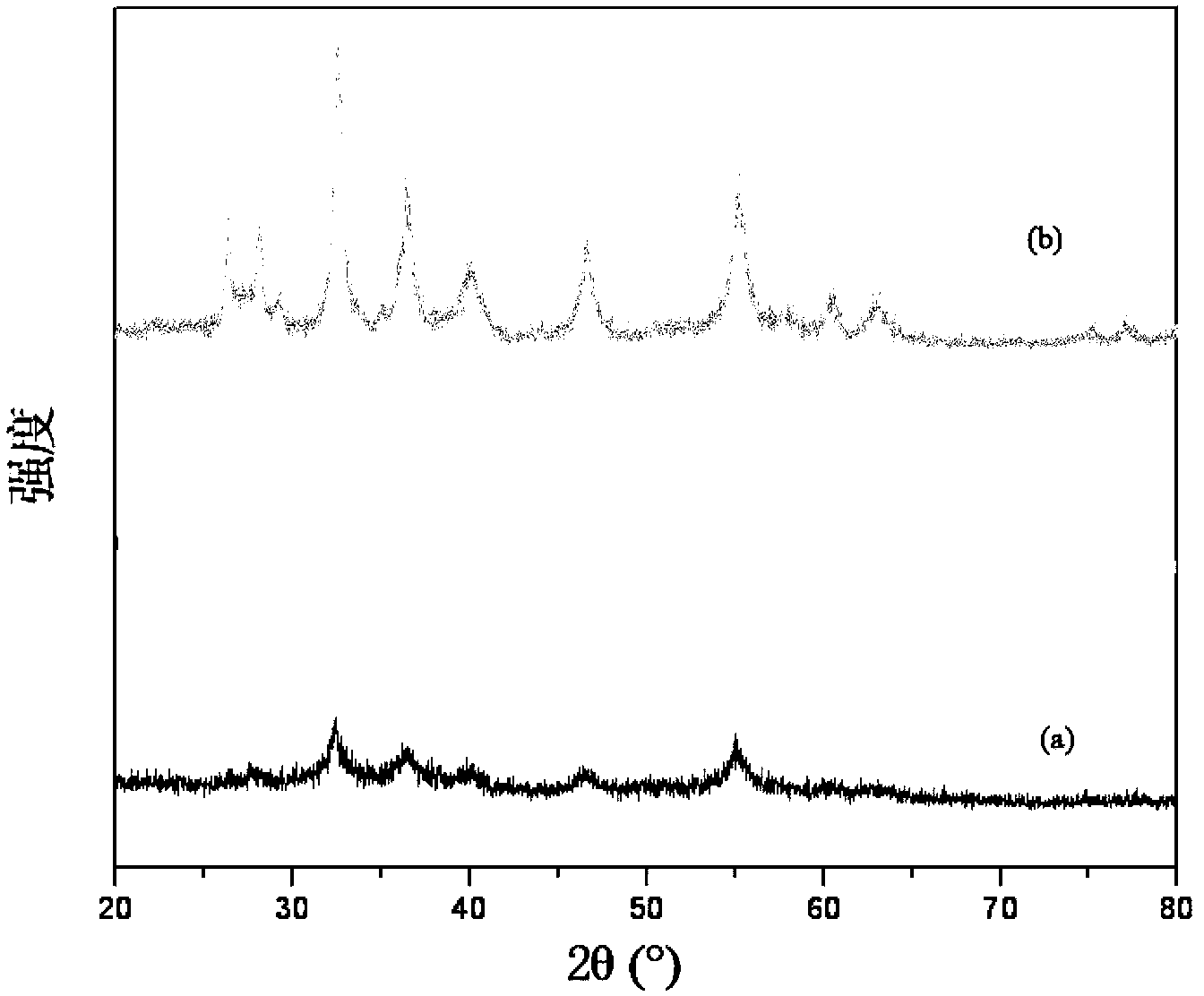
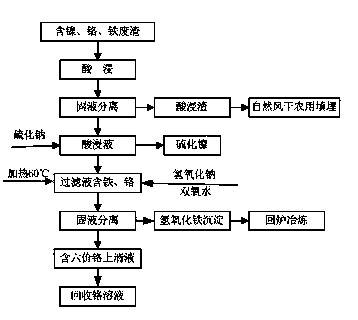
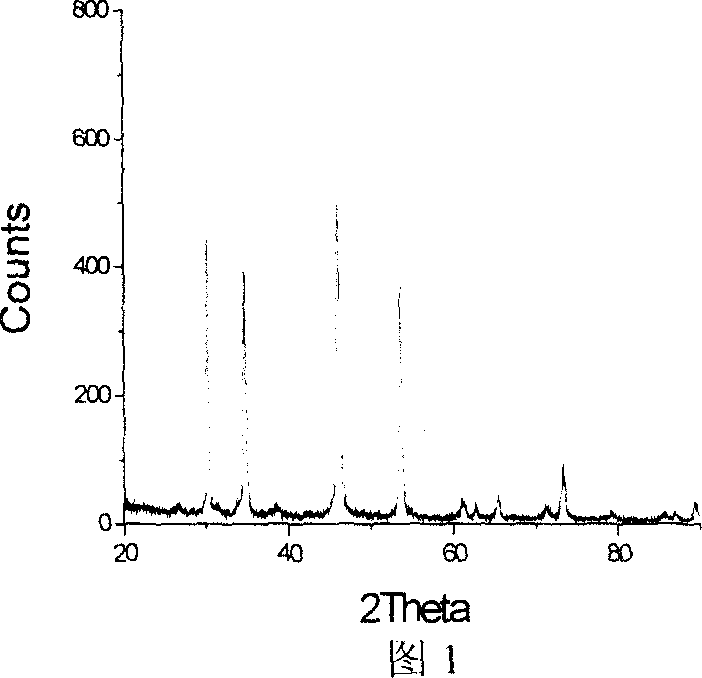
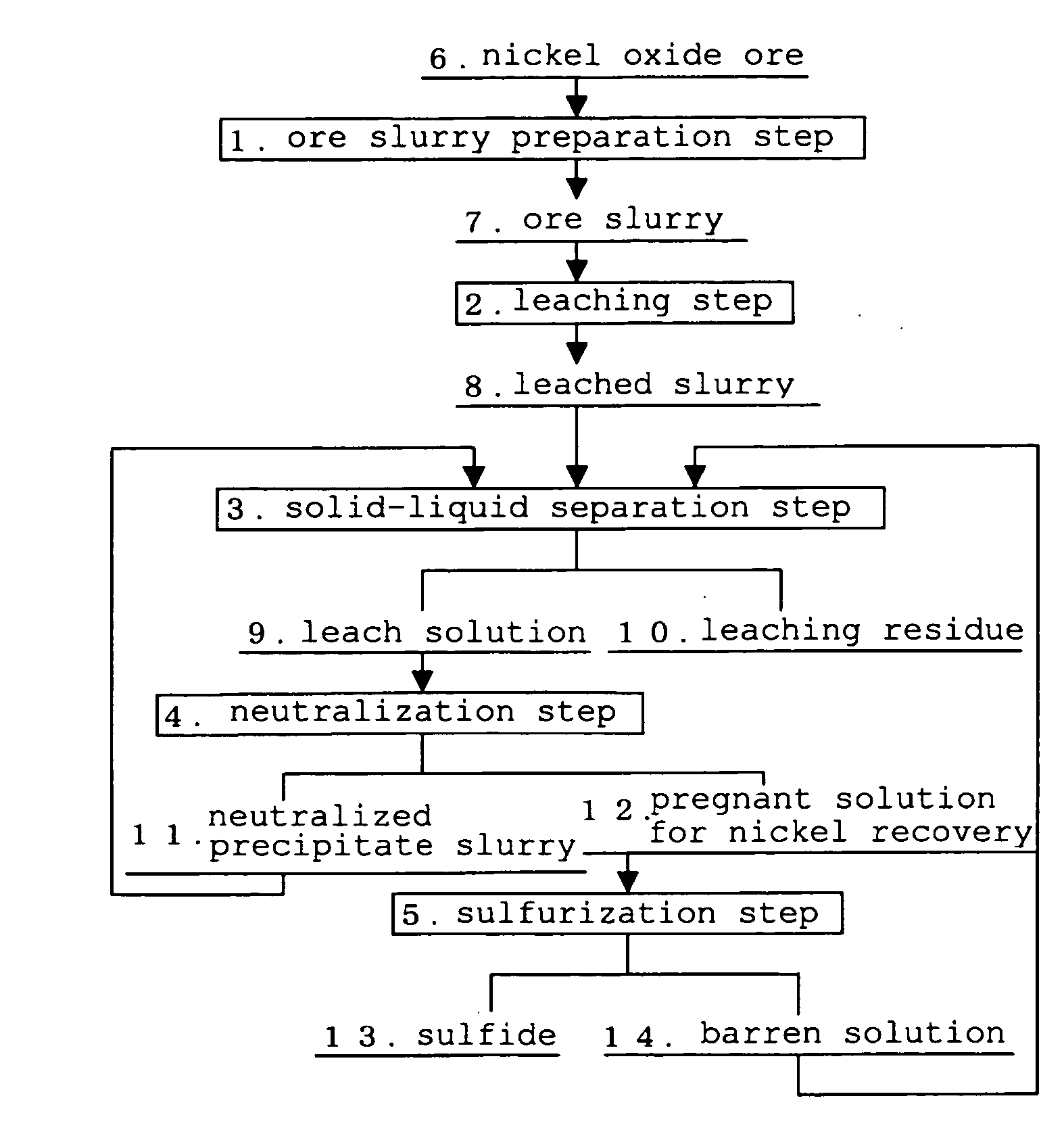
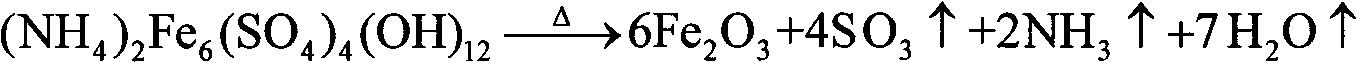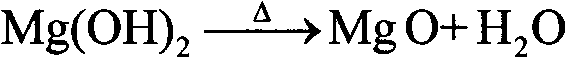Patents
Literature
284results about "Nickel sulfides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
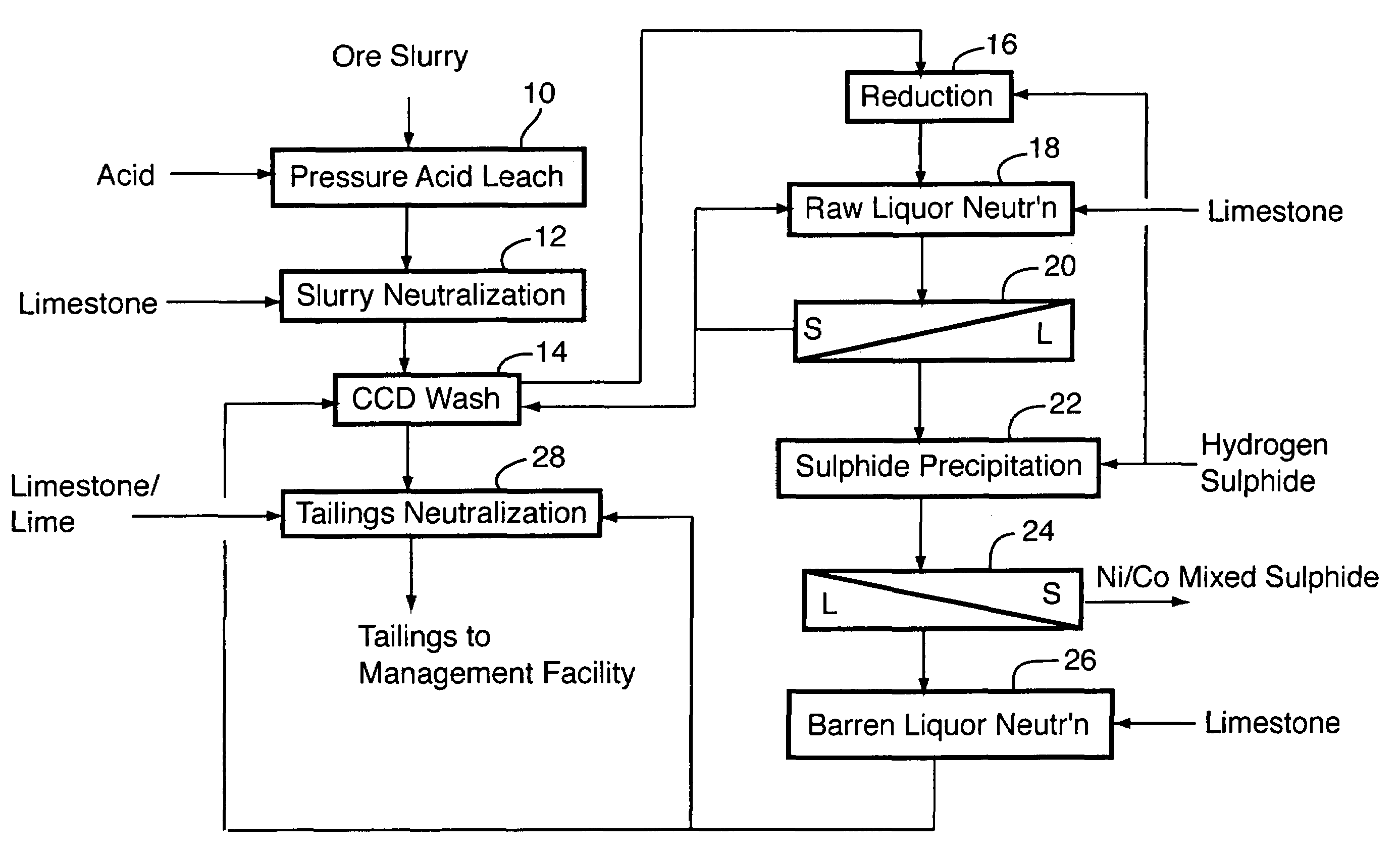
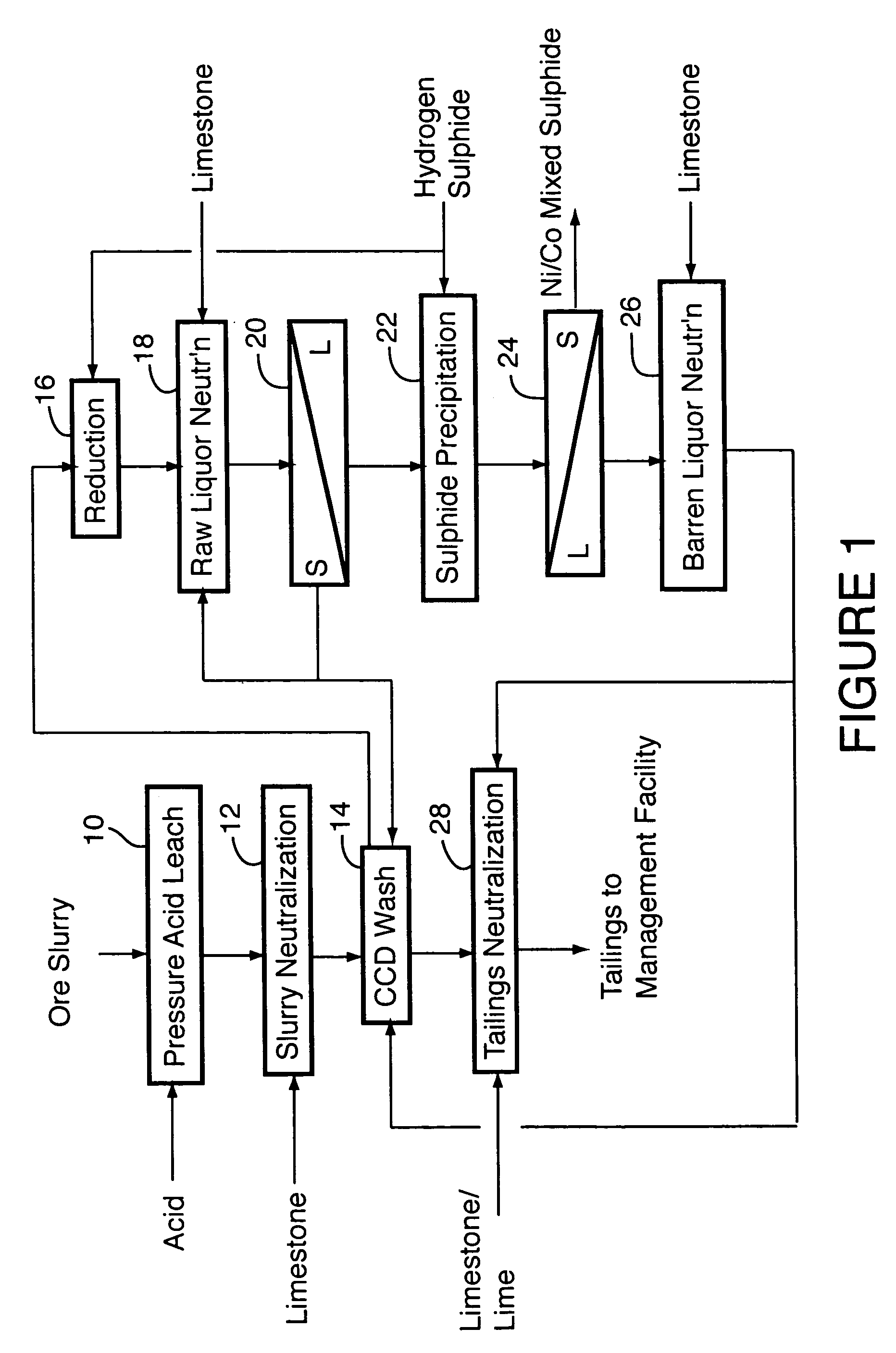
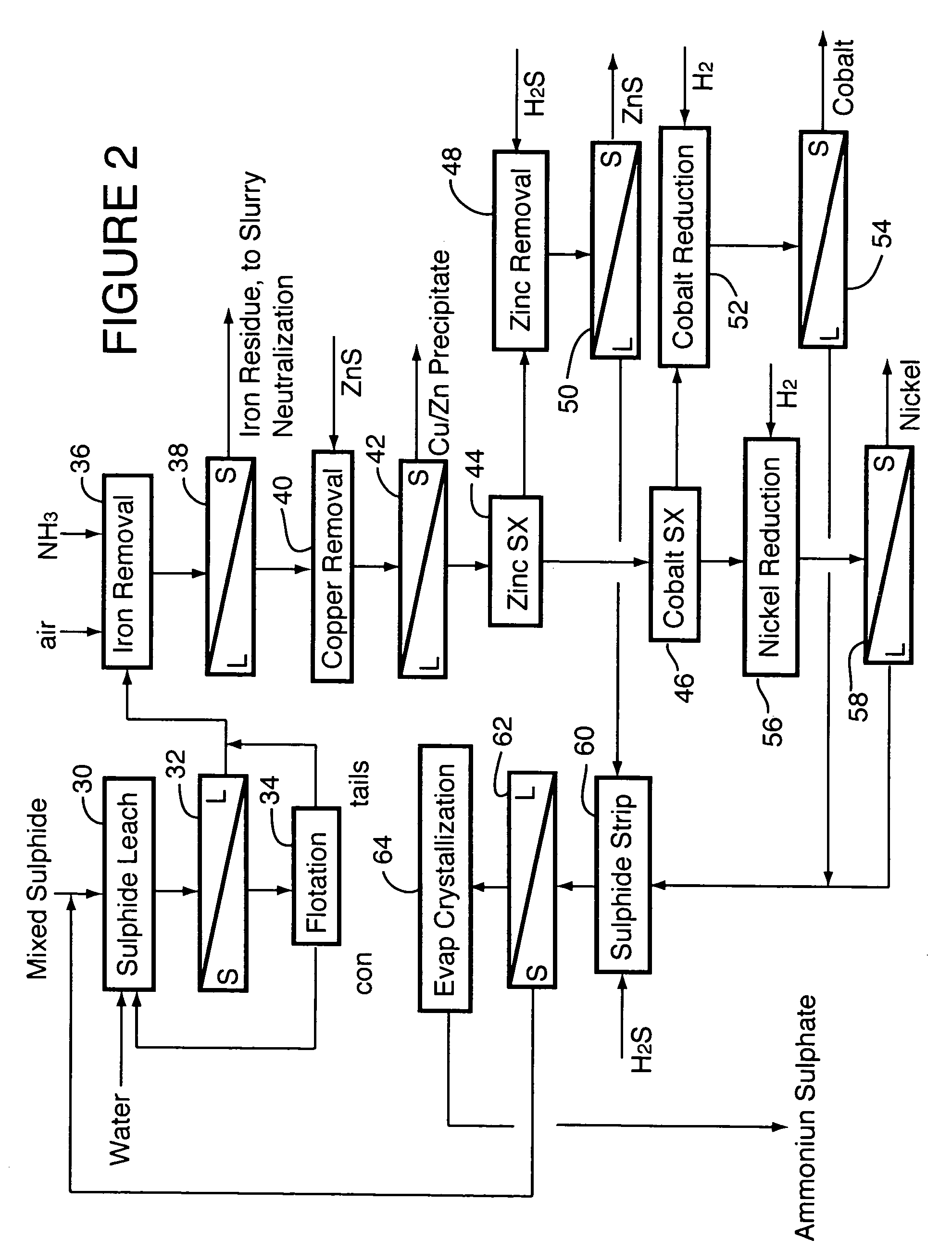
Process for recovery of nickel and cobalt from laterite ore
ActiveUS20060228279A1Efficient separation and recoveryHigh purityCobalt sulfidesSolvent extractionFree solutionSlurry
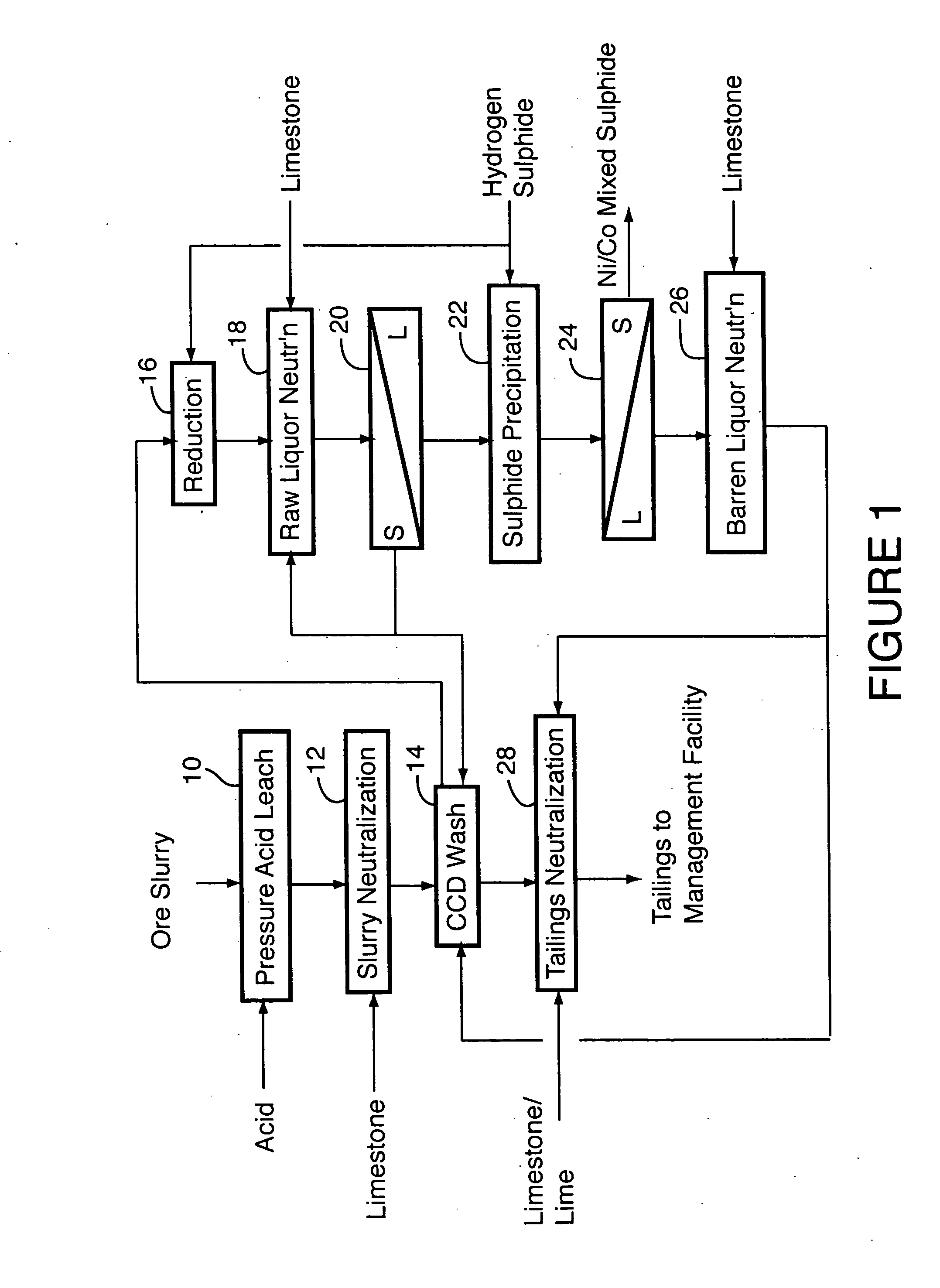
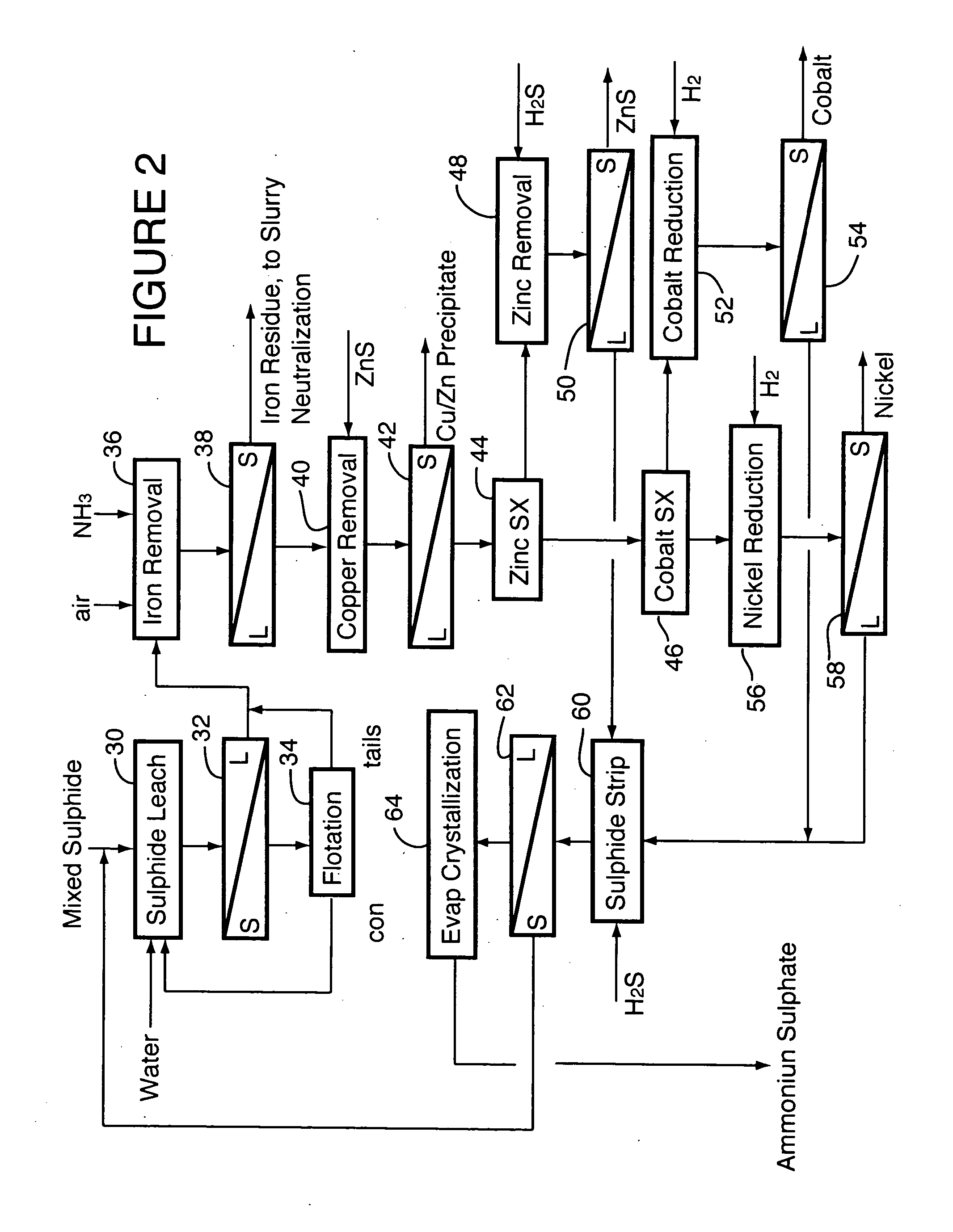
A process for recovering nickel and cobalt values from nickel- and cobalt-containing laterite ores as an enriched mixed nickel and cobalt sulphide intermediate and for producing nickel and cobalt metal from the nickel and cobalt sulphide intermediate. The laterite ore is leached as a slurry in a pressure acid leach containing an excess of aqueous sulphuric acid at high pressure and temperature, excess free acid in the leach slurry is partially neutralized to a range of 5 to 10 g / L residual free H2SO4 and washed to yield a nickel- and cobalt-containing product liquor, the product liquor is subjected to a reductant to reduce any Cr(VI) in solution to Cr(III), the reduced product liquor is neutralized to precipitate ferric iron and silicon at a pH of about 3.5 to 4.0, and the neutralized and reduced product liquor is contacted with hydrogen sulphide gas to precipitate nickel and cobalt sulphides. The precipitated nickel and cobalt sulphides can be leached in a water slurry in a pressure oxidation leach, the leach solution subjected to iron hydrolysis and precipitation, the iron-free solution contacted with zinc sulphide to precipitate copper, the iron- and copper-free solution subjected to zinc and cobalt extraction by solvent extraction to produce a nickel raffinate, the nickel raffinate contacted with hydrogen gas to produce nickel powder and the cobalt strip solution from the solvent extraction step contacted with hydrogen gas to produce cobalt powder.
Owner:SHERRITT INTERNATIONAL
Flotation Reagents and Flotation Processes Utilizing Same
ActiveUS20100021370A1Improve froth structureReduce froth viscosityCell electrodesFlotationSulfideEngineering
Methods of enhancing recovery of value sulfide or precious minerals from an ore containing Mg-silicate, slime forming minerals, and / or clay by crushing the ore, grinding the ore, and subjecting the ground ore to a flotation process, in conjunction with the addition of at least one monovalent ion modifier enhancing agent and / or froth phase modifier agent to the ore, are provided herein.
Owner:CYTEC TECH CORP
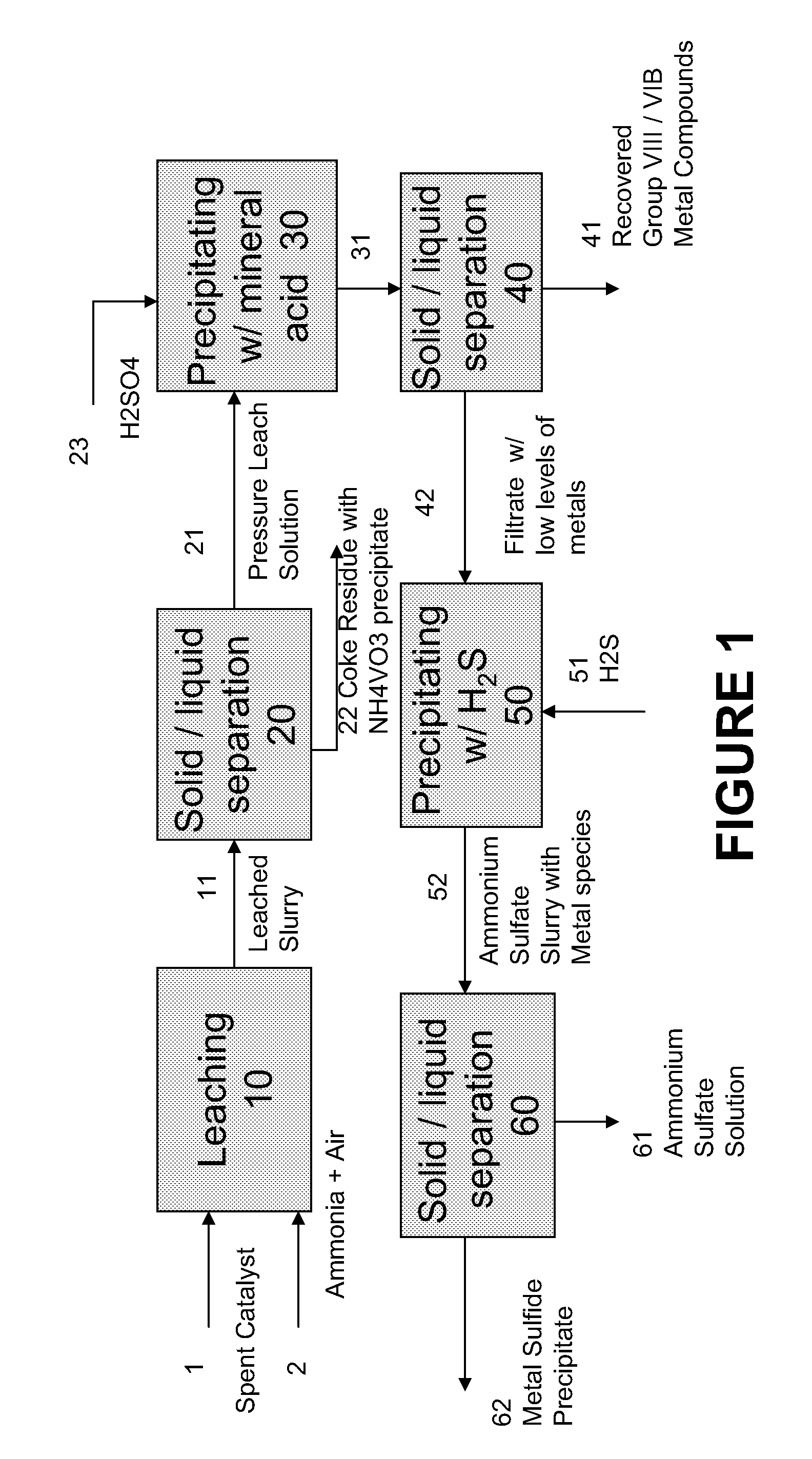
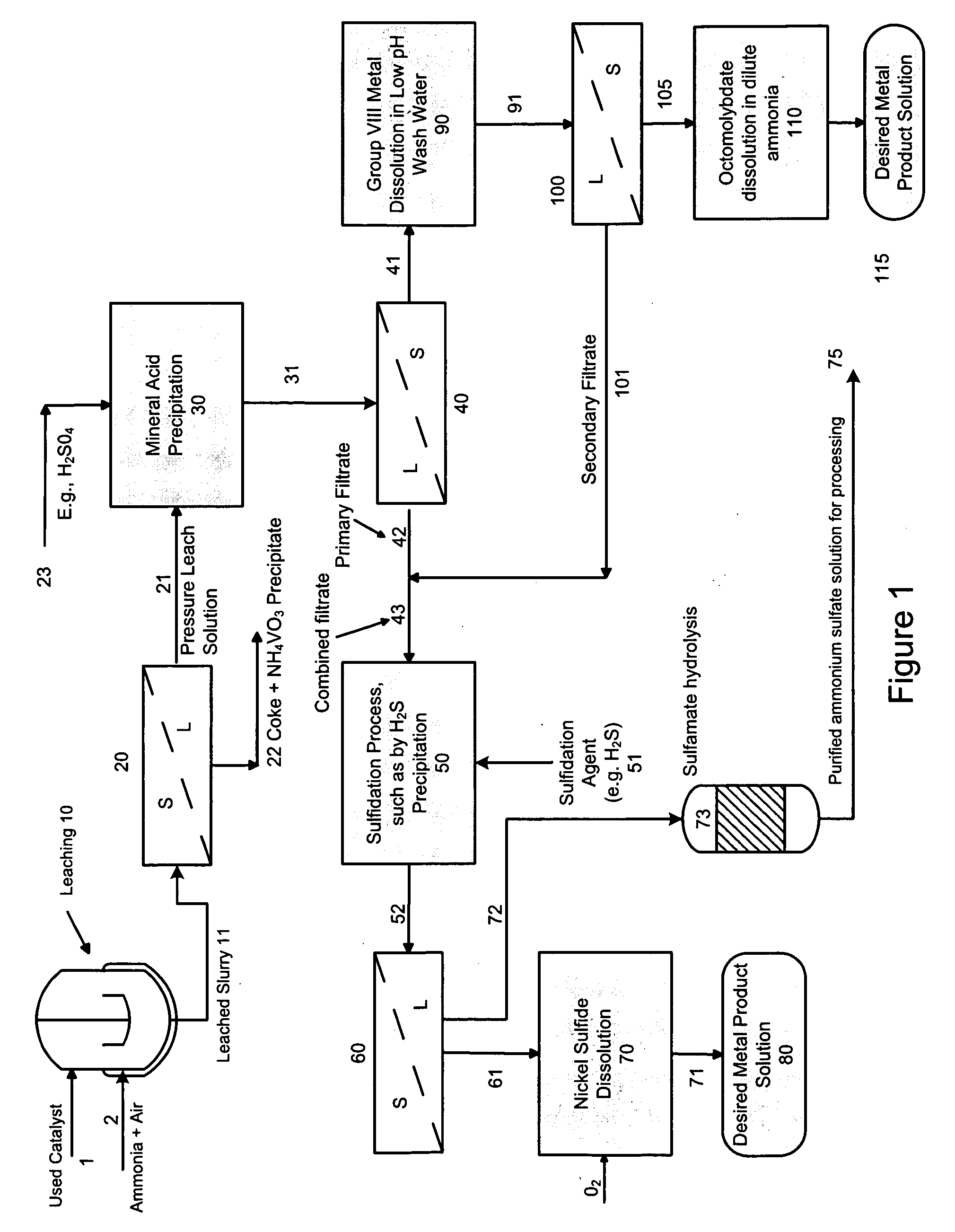
Process for separating and recovering base metals from used hydroprocessing catalyst
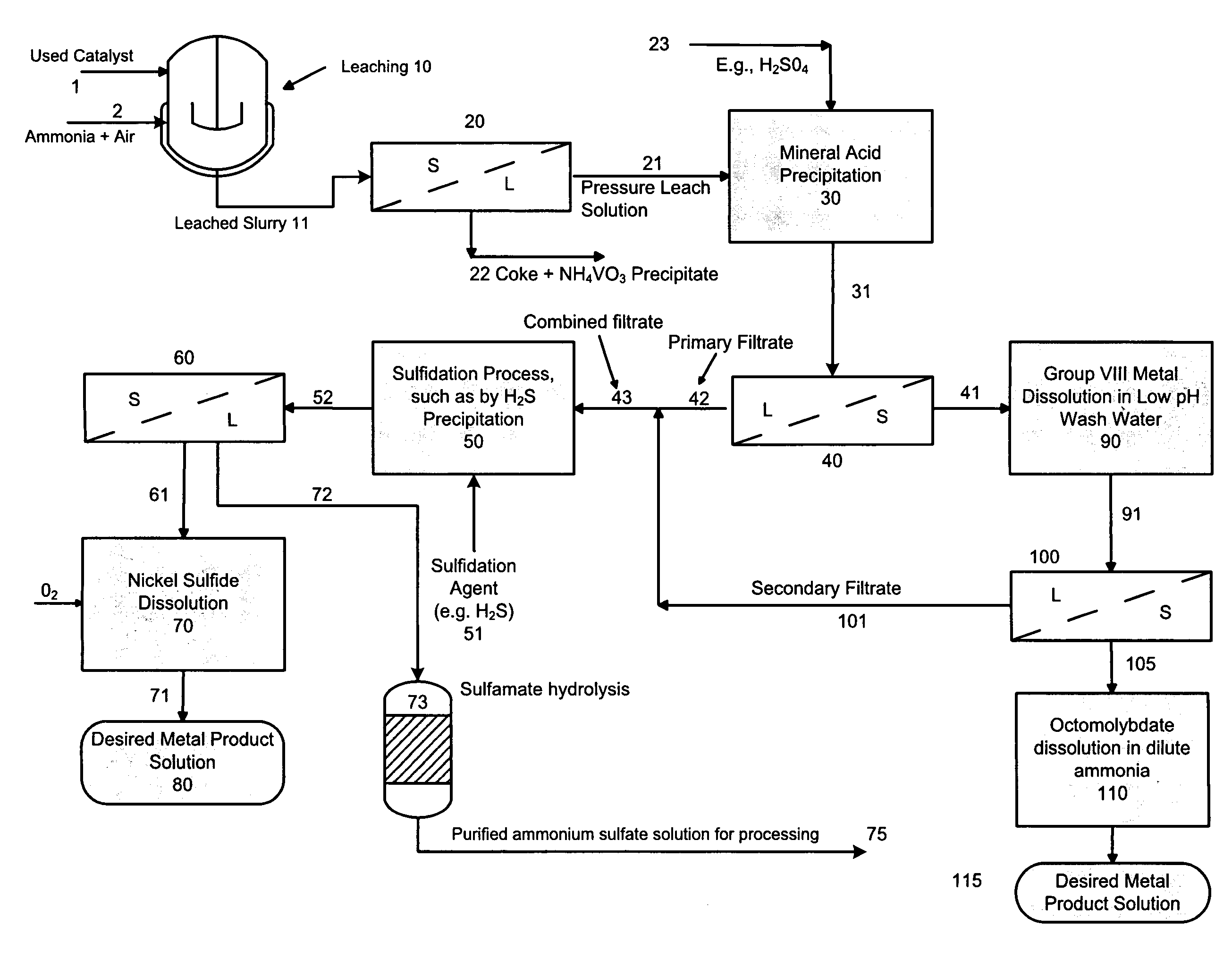
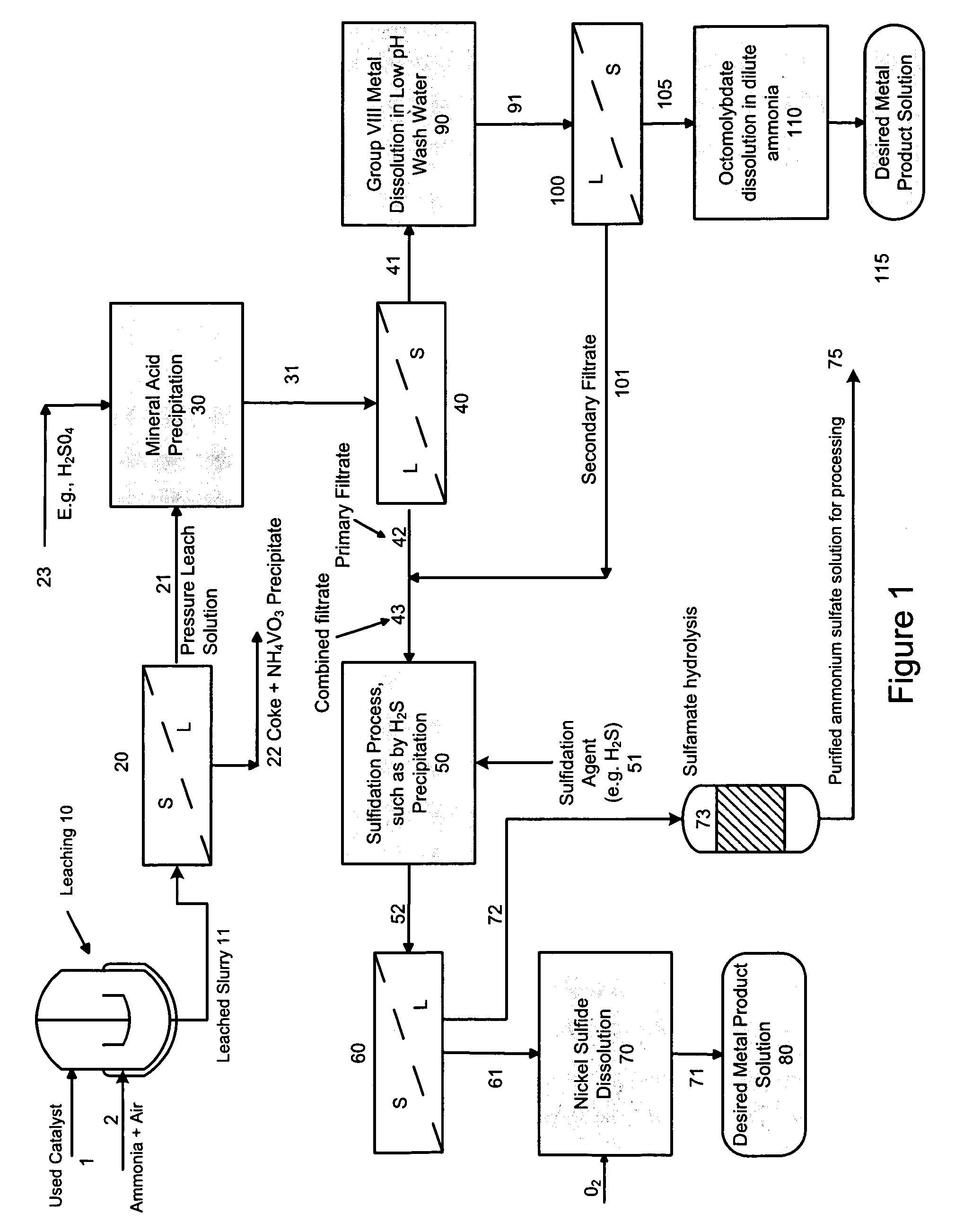
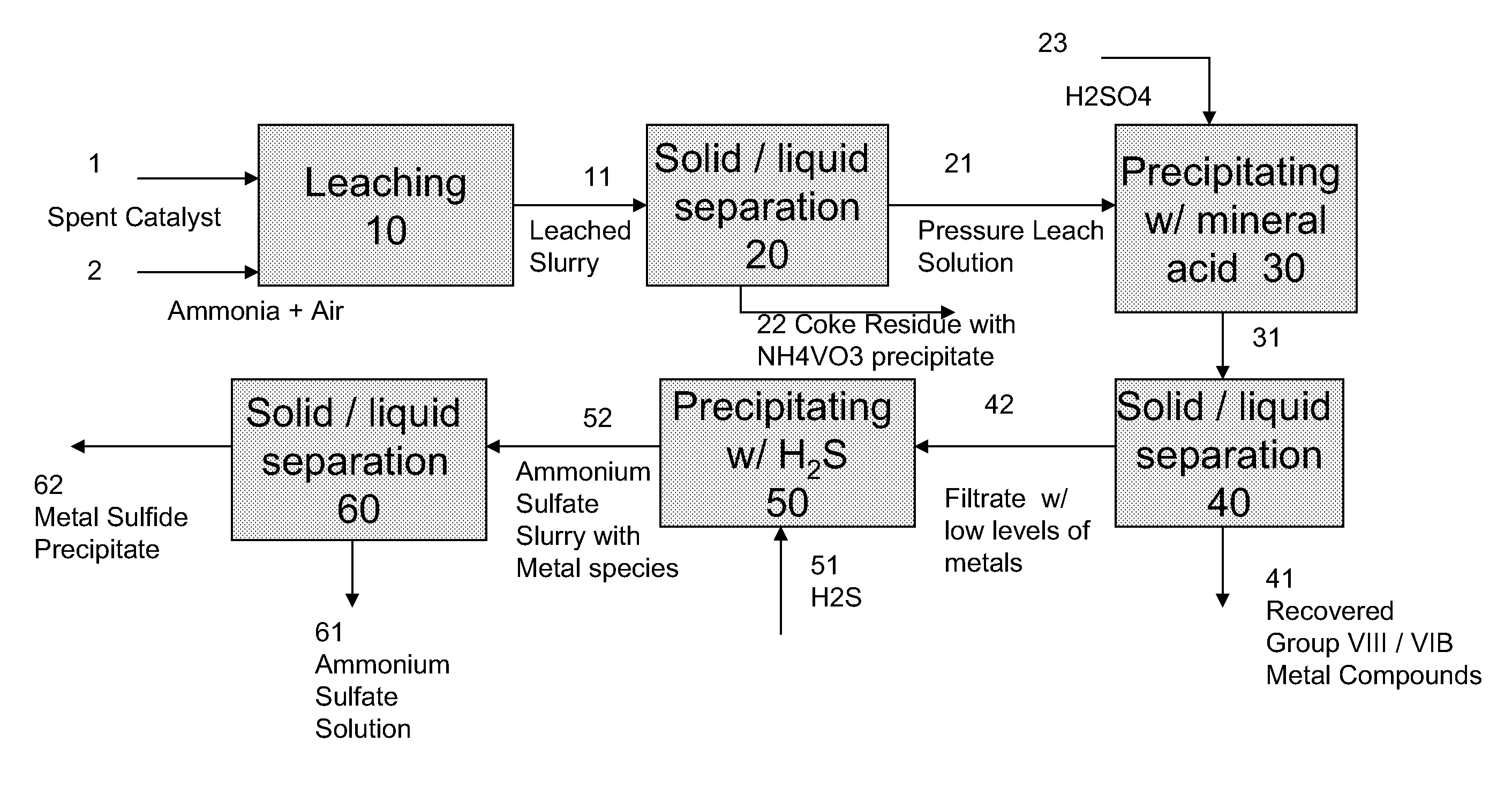
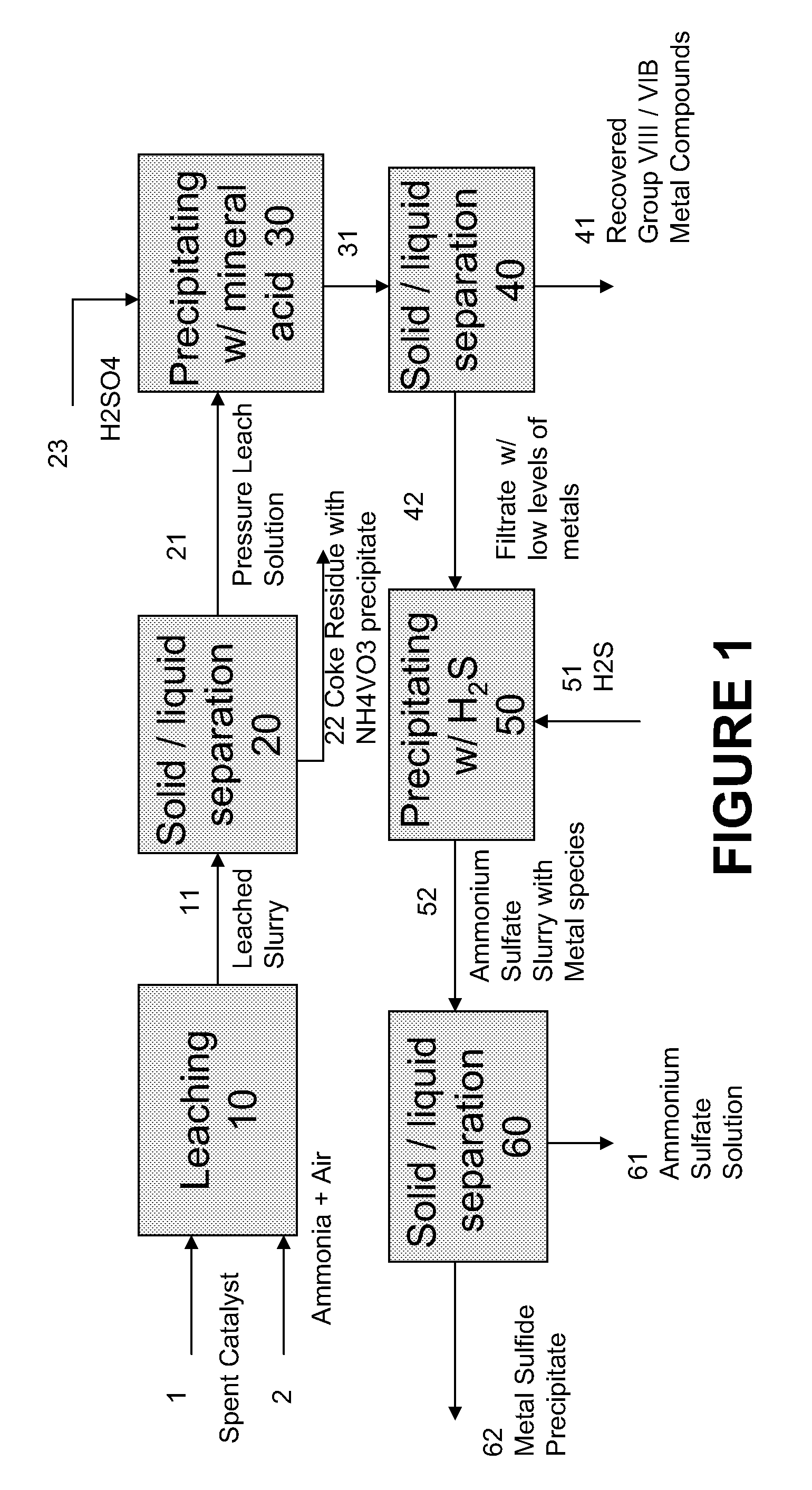
A method is disclosed for separating and recovering base metals from a used hydroprocessing catalyst originating from Group VIB and Group VIII metals and containing at least a Group VB metal. In one embodiment, the method comprises the steps of: contacting the used catalyst with an ammonia leaching solution under conditions sufficient to dissolve the group VIB metal and the Group VIII metal into the leaching solution; forming a leached slurry containing at least a group VIB metal complex and at least a group VIII metal complex, ammonium sulfate and a solid residue containing at least a Group VB metal complex and coke; separating and removing from the leached slurry the solid residue and coke; precipitating from the remaining solution at least a portion of the Group VIB metal complex and at least a portion of the Group VIII metal complex by controlling the pH to form a liquid material substantially free of Group VB, Group VIB and Group VIII metals and another solid material comprising substantially Group VIB and Group VIII metal complexes. Said solid material is further processed by dissolution, means of separation, further means of precipitation and oxidative dissolution to produce, separately, a Group VIB metal product solution, a Group VIII metal product solution and a purified ammonium sulfate product solution.
Owner:CHEVROU USA INC
Process for recovering base metals from spent hydroprocessing catalyst
A method for recovering metals from a spent dispersed catalyst originating from a Group VIB metal sulfide catalyst containing at least a Group VB and Group VIII metal for hydrocarbon oil hydroprocessing is disclosed. In one embodiment, the method comprises the steps of: contacting the spent dispersed catalyst with a leaching solution containing ammonia and air to dissolve the group VIB metal and the Group VIII metal into the leaching solution at sufficient temperature and pressure; forming a slurry containing at least a group VIB metal complex and at least a group VIII metal complex, ammonium sulfate and solid residue containing at least a Group VB metal complex and coke; separating and removing the solid residue containing ammonium metavanadate and coke from the pressure leach solution (PLS); precipitating from the PLS at least a portion of the Group VIB metal and at least a portion of the Group VIII metal by controlling the pH at a pre-selected pH to selectively precipitate as metal complexes the Group VIB and Group VIII metals.
Owner:CHEVROU USA INC
Method for preparing hollow ball of sulfide and oxide of nickel
ActiveCN103058289ASimplified protocolEasy to operateNanotechnologyNickel oxides/hydroxidesCysteine thiolateTe element
The invention discloses a method for preparing a hollow ball of sulfide and oxide of nickel. The method comprises the following steps: (1), adopting ultrasonic or mechanical stirring to disperse nickel nitrate, L-cysteine and urea uniformly in deionized water; (2), transferring the mixed liquid into a reaction kettle, and reacting for 8 to 48 hours at a temperature of 140 DEG C to obtain a nickel disulphide hollow ball; (3), calcinating nickel disulphide at different temperatures to obtain hollow balls of nickel monosulfide and nickel oxide respectively; and (4), under hydrothermal condition, reacting nickel disulphide with selenium source and tellurium source respectively for 4 to 48 hours at a temperature of 140 DEG C to obtain hollow balls of nickel diselenide and nickel telluride. According to the method, raw materials are cheap, easy to obtain, and convenient to compound; the required devices are simple; no pollution is caused in the production process; and the large scale production can be realized rapidly.
Owner:CENT SOUTH UNIV
Metal-organic framework compound derived metal sulfide nanosheet and preparation method thereof
ActiveCN109835937AStrong structural adjustabilityWide choice of chemical compositionNanotechnologyCobalt compoundsChemical compositionMetal-organic framework
The invention discloses a metal-organic framework compound derived metal sulfide nanosheet and a preparation method thereof. The derived metal sulfide has a pleated nanosheet or porous nanosheet structure, and the chemical composition can abbreviated as MSx, wherein M represents one or more of Co, Zn Ni, Fe, Cu, Mn and common transition metal elements, S represents the sulfur element, and x is ina range of 0.5-2. The preparation method comprises the following steps: reacting a metal salt with an organic ligand to obtain a two-dimensional metal organic framework, and then carrying out low-temperature liquid phase sulfuration treatment on the metal organic framework to obtain metal sulfide nanosheets having different two-dimensional structures and compositions. Different sulfide nanosheetscan be prepared through adopting the two-dimensional metal organic framework as a precursor and selecting different sulfurizing reagents and solvents for effective control by the method. The method issimple, and easy to implement, and can be used to prepare nanostructures which are difficult to realize by other methods, and the metal-organic framework compound derived metal sulfide nanosheet hasgreat application prospects in the fields of electrochemical energy storage, catalysis and adsorption.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hydrothermal preparation method for NiS2 with controllable shape
InactiveCN102633309AEasy to operateMild reaction conditionsNanotechnologyNickel sulfidesNickel saltReaction temperature
The invention discloses a hydrothermal preparation method for NiS2 with a controllable shape, and the method mainly comprises the following steps of: mixing nickel salt, a sulphur source and a complexing agent in a certain molar ratio, transferring the mixture into a high-pressure reactor, adding distilled water, and stirring to adequately dissolve soluble solids; adjusting the pH value of the reaction system, sealing the reactor, and reacting at a certain temperature; and washing the obtained product by use of distilled water and ethanol respectively, centrifugally separating, and drying. The hydrothermal preparation method for NiS2 with a controllable shape disclosed by the invention has the advantages of being simple in operation by using a hydrothermal method, moderate in reaction condition, narrow in particle size distribution, high in product purity, easy in industrial production, and the like. The particle shape and size of NiS2 can be controlled by adjusting the synthesis conditions such as hydrothermal reaction temperature, reaction time, complexing agent type and pH value, and the prepared NiS2 can be approximately-cube-shaped, spherical, sheet-shaped and the like. Compared with a solid-state reaction method, an ultrasonic spray pyrolysis method, a gamma-ray irradiation method and an organic solvent hot method, the NiS2 prepared by the hydrothermal method has the advantages of being low in reaction temperature and equipment demand, less in toxicity on a human body, high in safety factor, low in production cost, capable of meeting the requirements of energy conservation and emission reduction, and the like.
Owner:SHENYANG LIGONG UNIV
Composite nanostructure based on three-dimensional porous transition metal carbide Ti3C2MXene and general preparation method thereof
InactiveCN110589786AFix production issuesSolve application problemsTitanium carbideNickel oxides/hydroxidesChemical reactionMetal-organic framework
The invention discloses a composite nanostructure based on a three-dimensional porous transition metal carbide Ti3C2MXene and a general preparation method thereof, and belongs to the field of nanomaterials. The three-dimensional composite structure is composed of a three-dimensional porous Mxene-supported inorganic nanostructure, and has a honeycomb hierarchical porous structure. A precursor of atwo-dimensional transition metal carbide and a metal-organic framework compound is subjected to high-temperature pyrolysis or a chemical reaction in an inert or reactive atmosphere to prepare the composite nanostructure with a controllable size. According to the composite nanostructure, stacking of MXene itself is inhibited, an active surface area, porosity, and ion permeability of MXene are increased, and thereby a surface interface of MXene is efficiently used. At the same time, introduction of the metal-organic framework compound realizes uniform and stable compounding of the three-dimensional porous MXene and an inorganic nanomaterial, the fundamental difficult problem that plagues exerting and application of inorganic nanomaterial performance is solved, and the composite nanostructurehas wide application prospects in the fields such as catalysis, energy, photo-electricity, space technology, and military industry.
Owner:DALIAN UNIV OF TECH
Process for Recovering Base Metals from Spent Hydroprocessing Catalyst
A method for recovering metals from a spent dispersed catalyst originating from a Group VIB metal sulfide catalyst containing at least a Group VB and Group VIII metal for hydrocarbon oil hydroprocessing is disclosed. In one embodiment, the method comprises the steps of: contacting the spent dispersed catalyst with a leaching solution containing ammonia and air to dissolve the group VIB metal and the Group VIII metal into the leaching solution at sufficient temperature and pressure; forming a slurry containing at least a group VIB metal complex and at least a group VIII metal complex, ammonium sulfate and solid residue containing at least a Group VB metal complex and coke; separating and removing the solid residue containing ammonium metavanadate and coke from the pressure leach solution (PLS); precipitating from the PLS at least a portion of the Group VIB metal and at least a portion of the Group VIII metal by controlling the pH at a pre-selected pH to selectively precipitate as metal complexes the Group VIB and Group VIII metals.
Owner:CHEVROU USA INC
New method for two-step process preparation of nickel sulfide
InactiveCN104261490ASimple production processReaction conditions are easy to controlNickel sulfidesSODIUM SULFIDE NONAHYDRATEHigh pressure
The invention relates to a new method for two-step process preparation of nickel sulfide. The new method comprises the following steps: transferring a water and urea mixed solution of nickel chloride or a water, ammonia water and ethylene glycol mixed solution of nickel chloride into a high-pressure kettle, performing heating reaction at the temperature of 120-160 DEG C for 8-16h, filtering and washing a generated nickel hydroxide precipitate, and drying to prepare a nickel hydroxide precursor; and then dispersing into ethylene glycol, performing ultrasonic dispersion, adding sodium sulfide nonahydrate, transferring into a reactor, performing reflux reaction at the temperature of 160 DEG C for 12-24h, filtering, washing, and performing vacuum drying for 10-12h to prepare nickel sulfide. The new method provided by the invention has the advantages of simple production process, easiness in control of reaction conditions, no need of using a template agent and a surfactant, environmental friendliness and good consistency of the obtained product, is conductive to production of nickel sulfide, and has important practical significance.
Owner:XUZHOU NORMAL UNIVERSITY
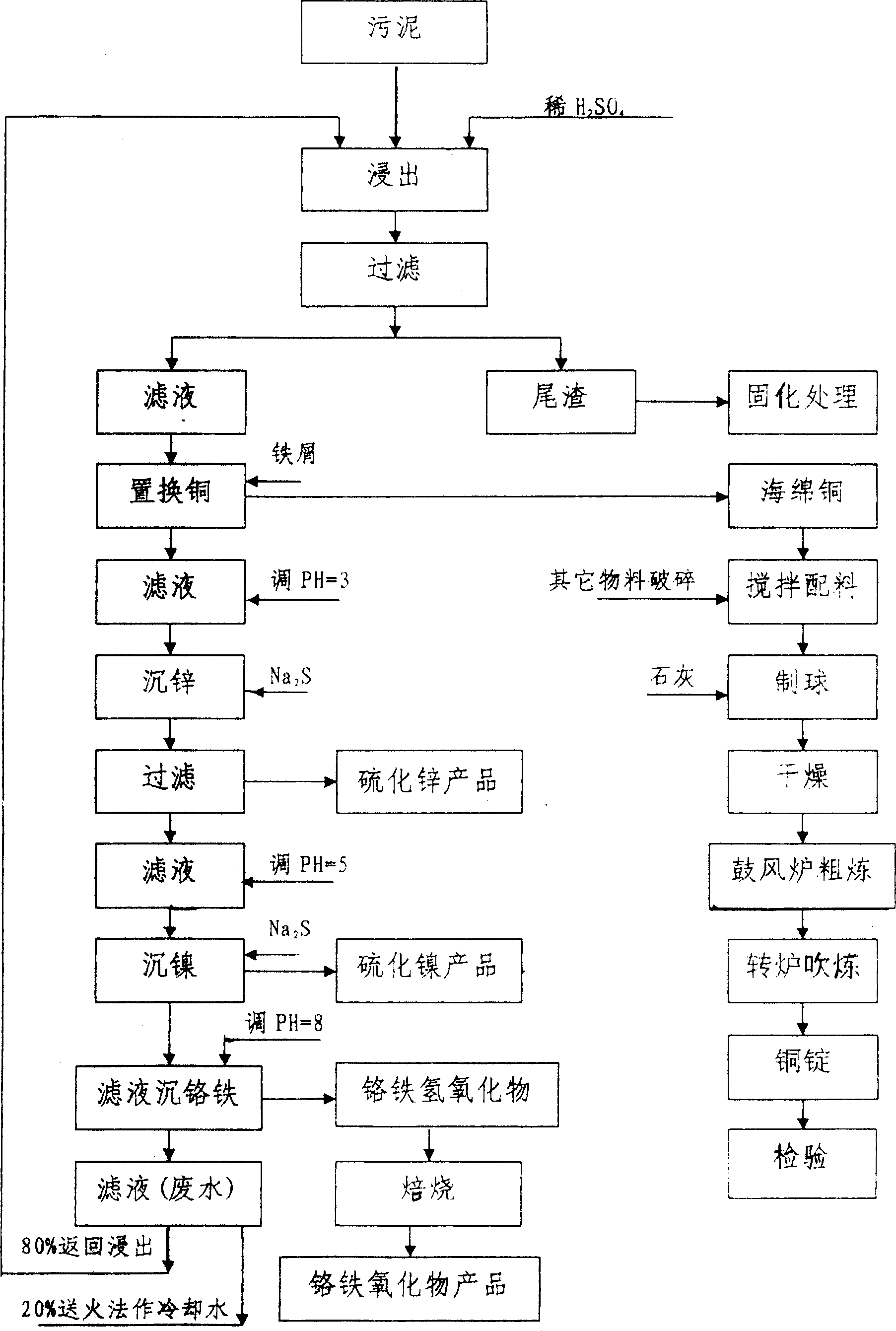
Circuit board and electric plating sludge resource recovery process
InactiveCN101058436AConforms to the requirements of raw materials for the manufacture of ferrochrome alloysRealize the purpose of comprehensive recovery of metal resourcesSludge treatmentZinc sulfidesSludgeSlag
The invention discloses a circuit board and resouring recycling technique of plated sludge, which is characterized by the following: leaching the sludge through dilute sulfuric acid; controlling Na2S and pH value to sediment zinc and nickel; recycling chromium iron through liquid alkaline sediment; using iron chips to replace and recycle copper; solidifying the leached tail slag as building material with reliable technique; separating and extracting zinc and nickel effectively; improving the utility of zinc and nickel resource; reducing the discharge of waste; realizing environmental protection.
Owner:上饶市华丰铜业有限公司
Recovery of nickel, cobalt, iron, silica, zinc and copper from laterite ore by sulfuric acid leaching
ActiveUS7387767B2Efficient separation and recoveryHigh purityCobalt sulfidesSolvent extractionFree solutionSlurry
A process for recovering nickel and cobalt values from nickel- and cobalt-containing laterite ores as an enriched mixed nickel and cobalt sulphide intermediate and for producing nickel and cobalt metal from the nickel and cobalt sulphide intermediate. The laterite ore is leached as a slurry in a pressure acid leach containing an excess of aqueous sulphuric acid at high pressure and temperature, excess free acid in the leach slurry is partially neutralized to a range of 5 to 10 g / L residual free H2SO4 and washed to yield a nickel- and cobalt-containing product liquor, the product liquor is subjected to a reductant to reduce any Cr(VI) in solution to Cr(III), the reduced product liquor is neutralized to precipitate ferric iron and silicon at a pH of about 3.5 to 4.0, and the neutralized and reduced product liquor is contacted with hydrogen sulphide gas to precipitate nickel and cobalt sulphides. The precipitated nickel and cobalt sulphides can be leached in a water slurry in a pressure oxidation leach, the leach solution subjected to iron hydrolysis and precipitation, the iron-free solution contacted with zinc sulphide to precipitate copper, the iron- and copper-free solution subjected to zinc and cobalt extraction by solvent extraction to produce a nickel raffinate, the nickel raffinate contacted with hydrogen gas to produce nickel powder and the cobalt strip solution from the solvent extraction step contacted with hydrogen gas to produce cobalt powder.
Owner:SHERRITT INC
Nickel doped copper sulfide nano material as well as preparation method and application thereof
ActiveCN104548094AEnhanced magnetic resonance imagingFunctionalEnergy modified materialsNMR/MRI constrast preparationsTherapeutic effectCopper sulfide
The invention provides a nickel doped copper sulfide nano material. The chemical general formula of the nickel doped copper sulfide nano material is Cu1-xNixS, wherein CuS refers to a substrate, Ni<2+> refers to doping ions, and the value range of x is that x is larger than 0 and is smaller than or equal to 0.8; the nickel doped copper sulfide nano material provided by the invention is a multifunctional nano material and simultaneously has a magnetic resonance imaging function and a photothermal therapy effect, so that the nickel doped copper sulfide nano material can be used as a magnetic resonance imaging probe and a photothermal therapy reagent and is a nano material integrating detection and therapy. The invention further provides a preparation method and an application of the nickel doped copper sulfide nano material.
Owner:SHENZHEN INST OF ADVANCED TECH
Universal method for preparing nano metal sulfides and complexes thereof in one step
ActiveCN108675267AReduce manufacturing costLow priceMaterial nanotechnologySulfide/polysulfide preparationOxalateMetallic sulfide
The invention relates to the field of nano-materials and particularly relates to a universal method for preparing nano metal sulfides and complexes thereof in one step. According to the universal method, nano metal sulfides are subjected to hydrothermal synthesis by adopting elemental sulfur as a sulfur source, adopting oxalic acid as a reducer and adopting a metal oxide or metal salt as a metal source. According to the method, sulfur powder is directly adopted as the sulfur source without using organic sulfur as the sulfur source, so that the cost is lower, and the safety is higher; meanwhile, the oxalic acid with stable properties is adopted as the reducer, water serves as a solvent, and preparation can be carried out in relatively moderate environments, so that the safety of a preparation process is further improved, and the production cost is further reduced.
Owner:SOUTHWEST UNIVERSITY
Process for separating and recovering base metals from used hydroprocessing catalyst
A method is disclosed for separating and recovering base metals from a used hydroprocessing catalyst originating from Group VIB and Group VIII metals and containing at least a Group VB metal. In one embodiment, the method comprises the steps of: contacting the used catalyst with an ammonia leaching solution under conditions sufficient to dissolve the group VIB metal and the Group VIII metal into the leaching solution; forming a leached slurry containing at least a group VIB metal complex and at least a group VIII metal complex, ammonium sulfate and a solid residue containing at least a Group VB metal complex and coke; separating and removing from the leached slurry the solid residue and coke; precipitating from the remaining solution at least a portion of the Group VIB metal complex and at least a portion of the Group VIII metal complex by controlling the pH to form a liquid material substantially free of Group VB, Group VIB and Group VIII metals and another solid material comprising substantially Group VIB and Group VIII metal complexes. Said solid material is further processed by dissolution, means of separation, further means of precipitation and oxidative dissolution to produce, separately, a Group VIB metal product solution, a Group VIII metal product solution and a purified ammonium sulfate product solution.
Owner:CHEVROU USA INC
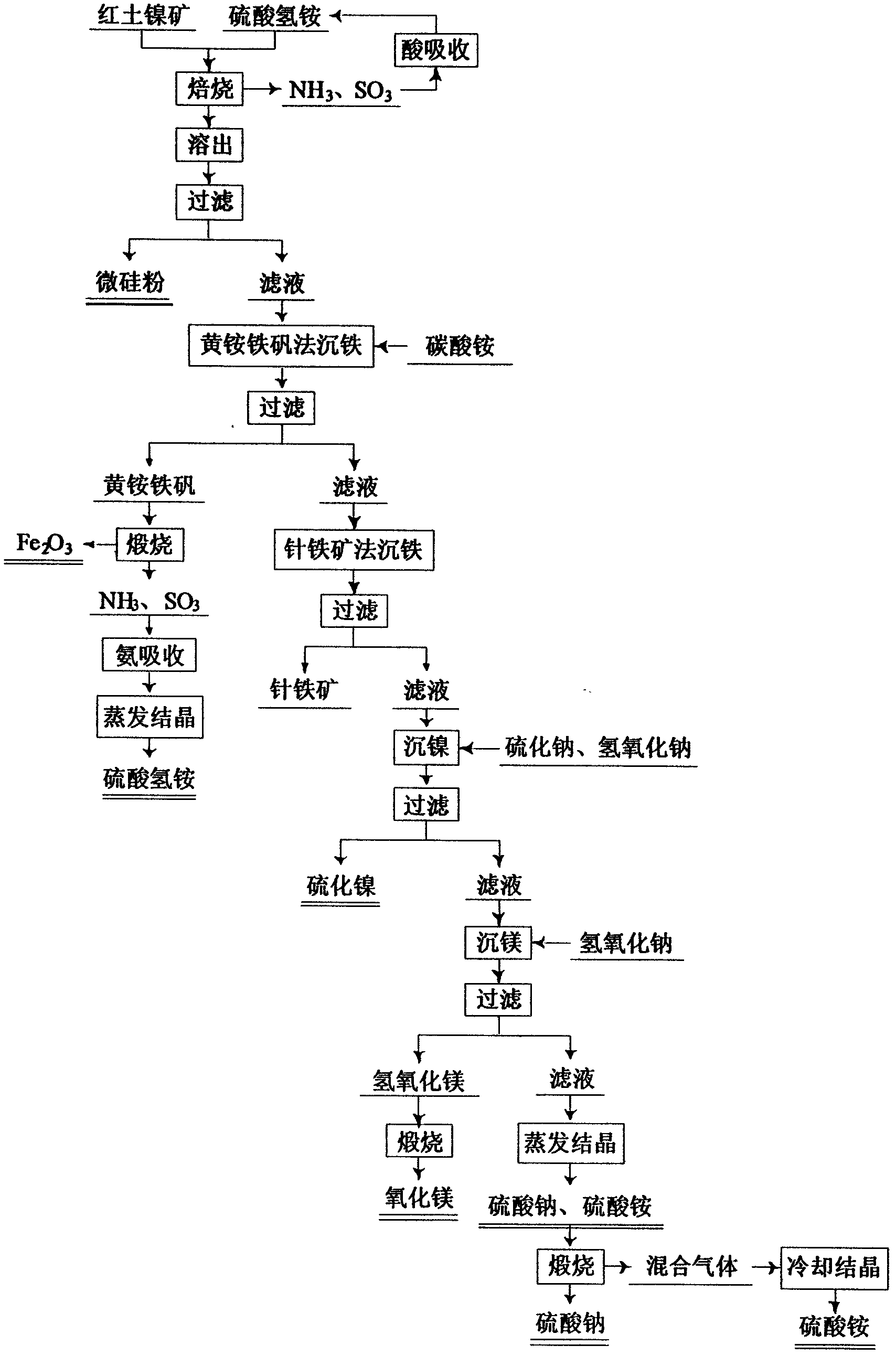
Method for comprehensive utilization of laterite nickel ore
A method for comprehensive utilization of laterite nickel ore comprises the following steps: (1) pulverizing laterite nickel ore, grinding, mixing with ammonium bisulfate, and roasting; (2) dissolving and filtering the roasted clinker to obtain a filtrate, depositing iron by an ihleite method, and depositing iron by a goethite method; (3) performing nickel deposition of the filtrate obtained after iron deposition by sodium sulfide, preparing a nickel sulfide product; (4) performing magnesium deposition of the filtrate obtained after nickel deposition by sodium hydroxide to obtain magnesium hydroxide; (5) washing, drying and calcining magnesium hydroxide to prepare a magnesium oxide product; (6) using the roasted clinker dissolved slag as a microsilica fume product directly, wherein the main component of the slag is silica; (7) calcining ammonium jarosite to obtain an iron oxide product.
Owner:NORTHEASTERN UNIV
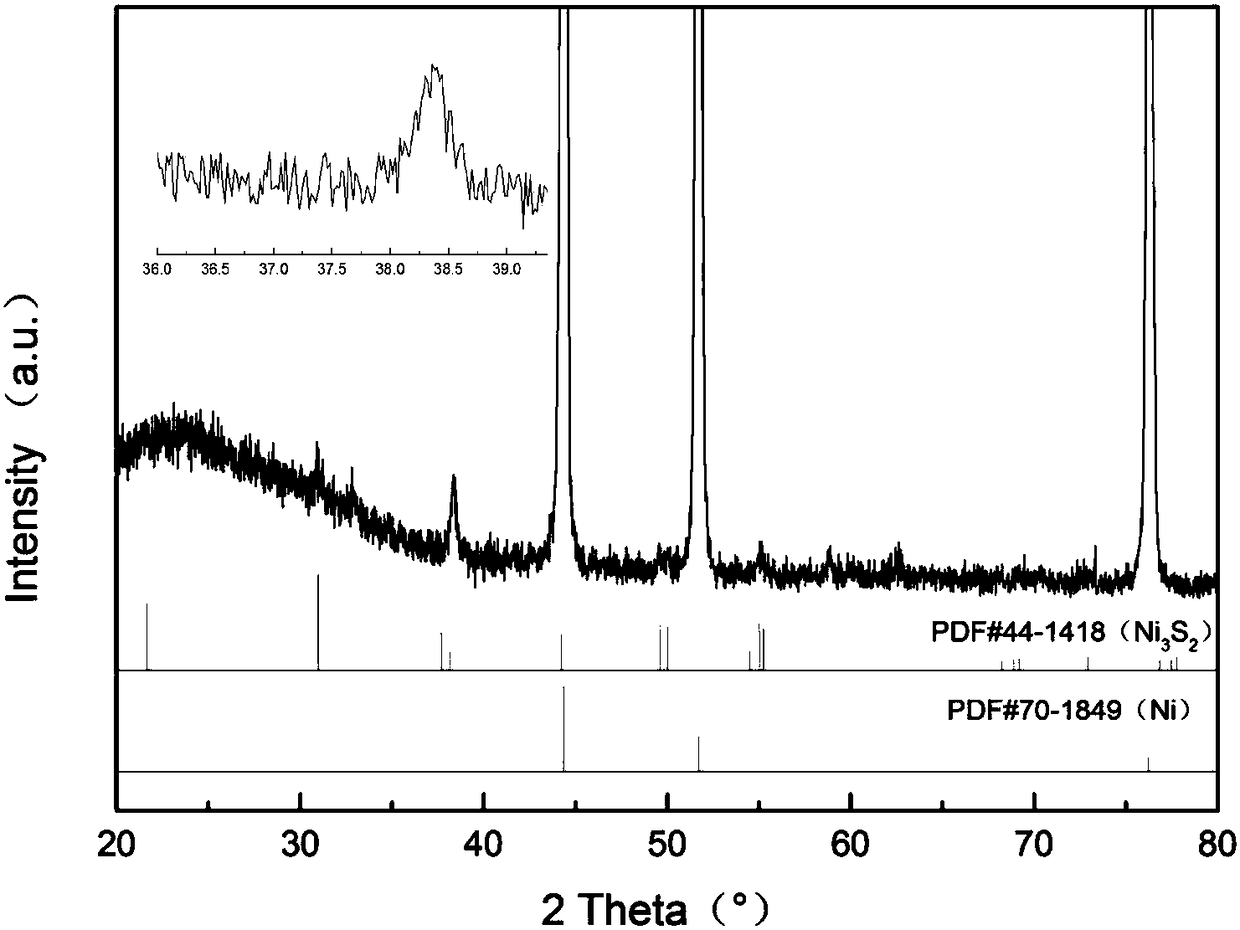
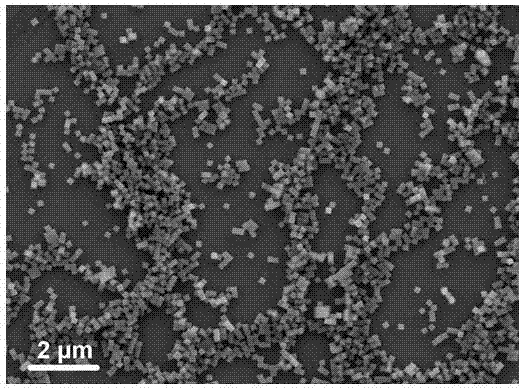
Synthesis method of self-assembled Ni3S2 nano-sheet
ActiveCN108423717ASimple processShort preparation cycleMaterial nanotechnologyPhysical/chemical process catalystsSynthesis methodsThiourea
The invention provides a synthesis method of a self-assembled Ni3S2 nano-sheet. The method comprises steps as follows: firstly, foamed nickel is ultrasonically cleaned in acetone and an HCl solution in sequence, then washed with deionized water and absolute ethyl alcohol respectively and air-dried at the room temperature; secondly, air-dried foamed nickel is put in a high-temperature and high-pressure reactor provided with a polytetrafluoroethylene lining, a thiourea solution and a polyvinylpyrrolidone morphological regulating agent are added to the reactor, the reactor is sealed and put in adrying oven, after the reaction is finished, a mixture is naturally cooled to the room temperature, and foamed nickel is taken out and repeatedly washed with water and absolute ethyl alcohol; finally,washed foamed nickel is put in a vacuum drying oven and dried at the room temperature, and a self-assembled Ni3S2 nano-sheet array self-supporting electrode is obtained. The solvothermal method has the characteristics of simple process, short preparation cycle and easily controllable reaction conditions, and reaction progress and morphology size can be controlled by adopting different temperatures, so that different special structural morphologies are obtained.
Owner:吉林省春泽露科技有限公司
Separation method for zinc sulfide
InactiveUS20100034716A1Improve filtering effectAvoid cloggingSolvent extractionCell electrodesPregnant leach solutionFiltration
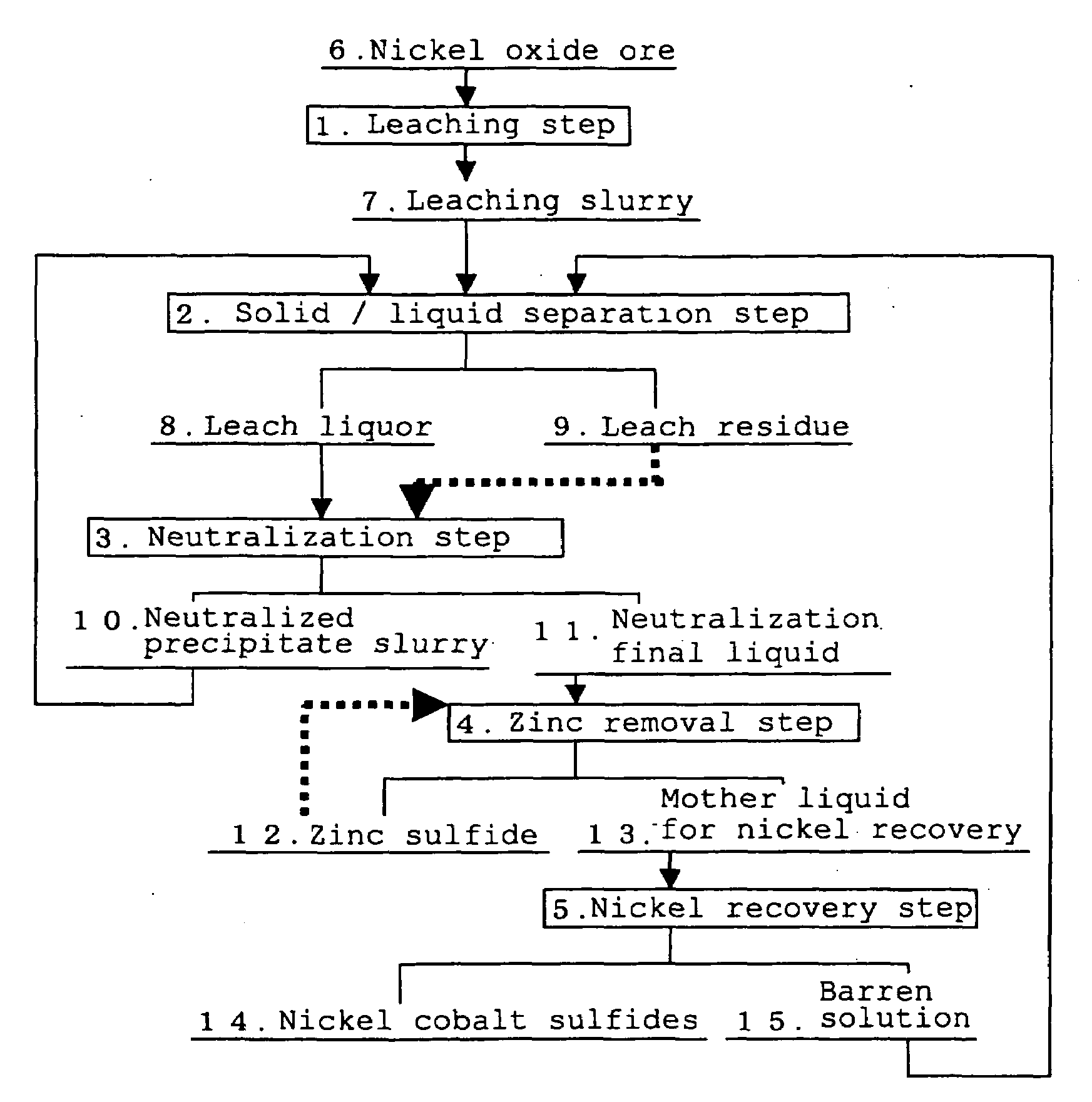
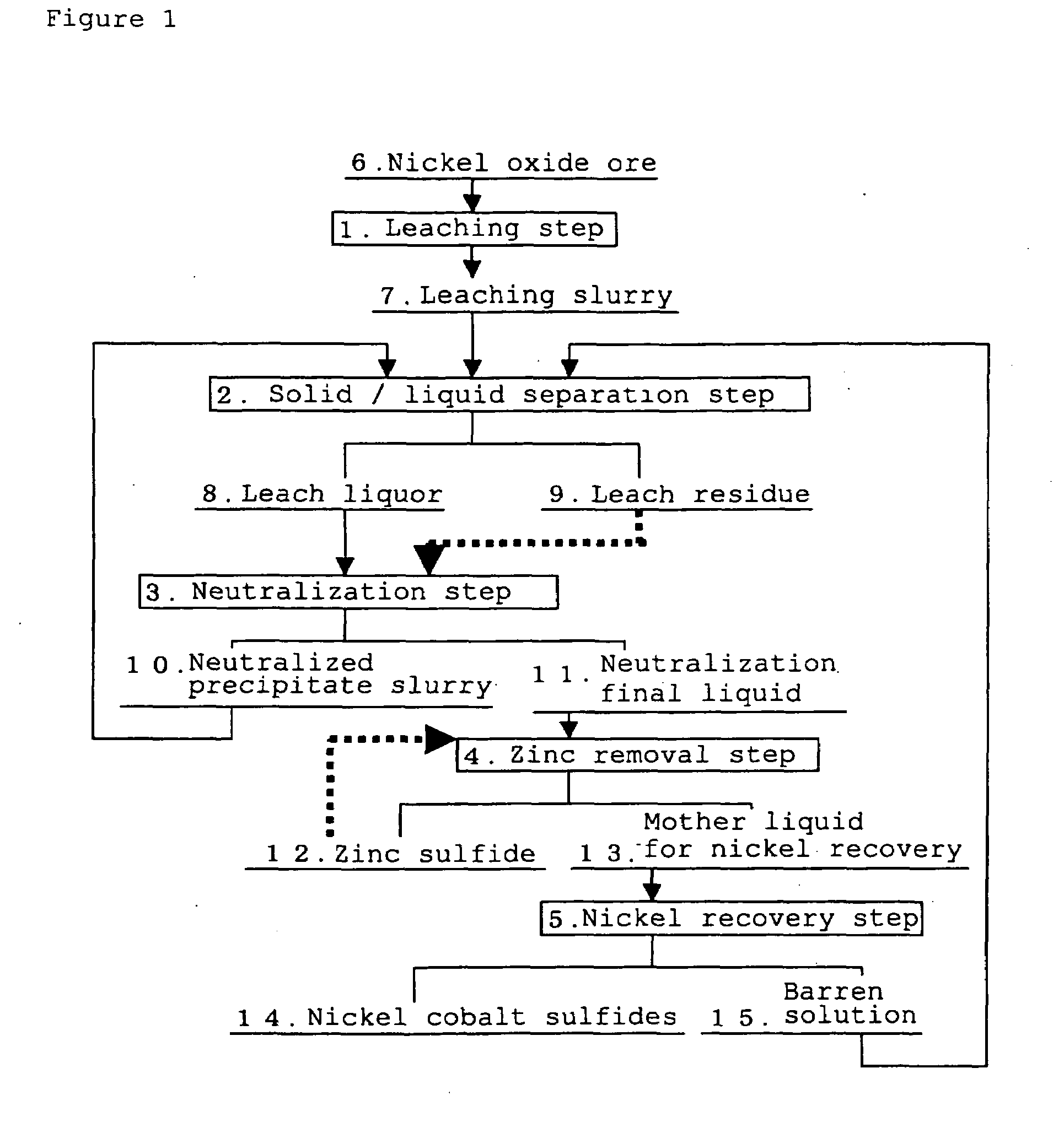
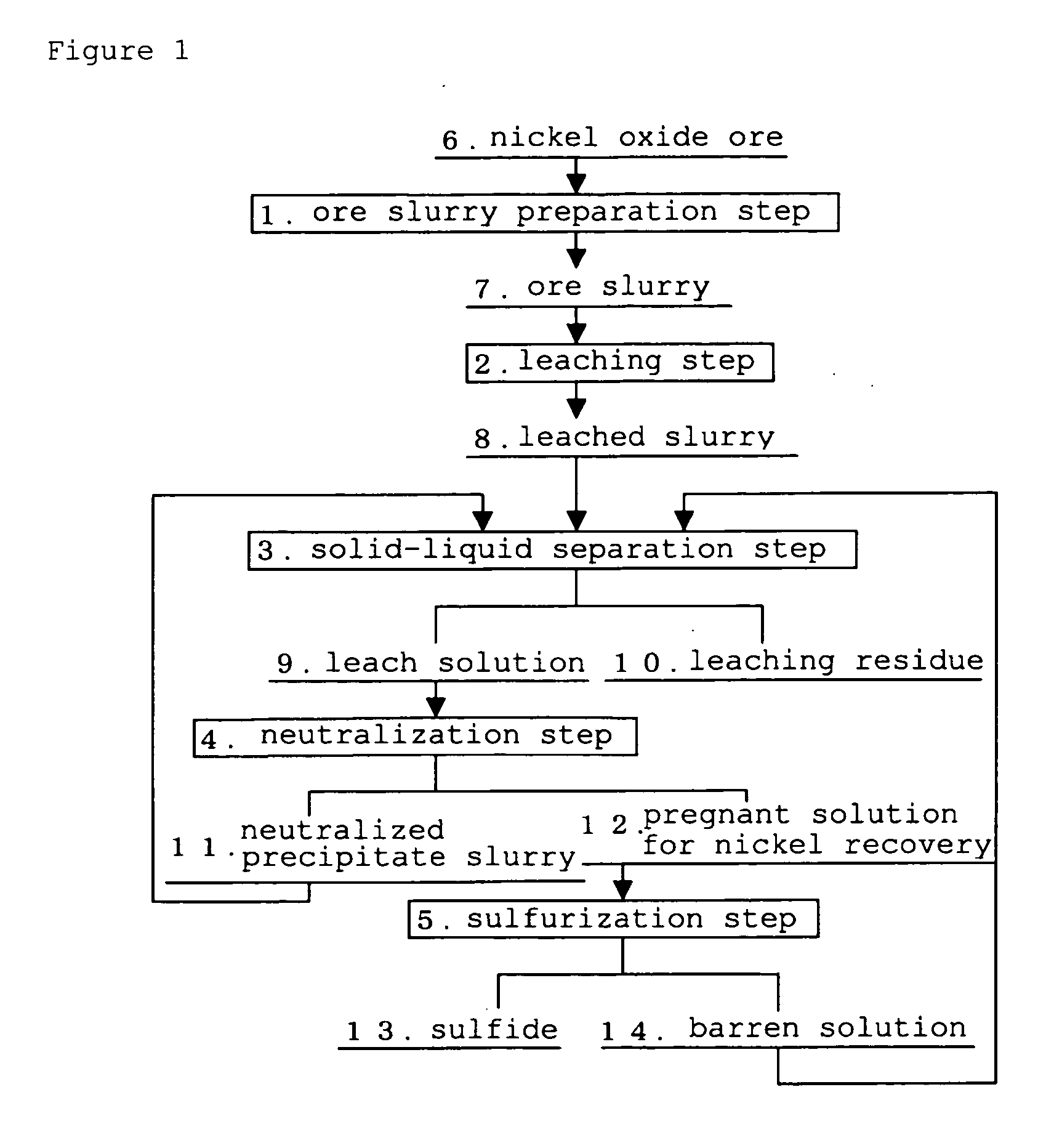
The separation method for zinc sulfide, in the hydrometallurgical process by a High Pressure Acid Leach for nickel oxide ore comprising leaching and solid / liquid separation step, neutralization step, zinc removal step, and nickel recovery step, which can inhibit clogging of a filter cloth and reduce a frequency of washing operation and replacement operation of a filter cloth by improving filtration performance of zinc sulfide, and inhibit decrease of nickel recovery ratio, in the zinc removal step in which zinc sulfide is formed by adding a sulfurizing agent to the neutralization final liquid containing zinc as well as nickel and cobalt and zinc sulfide is separated to obtain a mother liquid for nickel recovery containing nickel and cobalt.The separation method for zinc sulfide of the present invention is characterized in that in the above-described neutralization step, the leach residue is added to the leach liquor, and pH of the neutralization final liquid is adjusted so as to fall to the range from 3.0 to 3.5, and in the zinc removal step, the suspended solid comprising the neutralized precipitate and the leaching reside are kept remained in said neutralization final liquid so that turbidity thereof falls in the range from 100 to 400 NTU.
Owner:SUMITOMO METAL MINING CO LTD
Preparation method of transition metal oxide/sulfide nano composite material
InactiveCN106966443ARealization reservationUniform sizeCobalt sulfidesNickel oxides/hydroxidesVulcanizationPotassium ferricyanide
The invention discloses a preparation method of a transition metal oxide / sulfide nano composite material. The preparation method includes: adopting a room-temperature standing method to combine cobalt and nickel transition metal ions with potassium ferricyanide organic ligand to prepare a Prussian blue derivative nanocube; adding sodium sulfide into a water solution for vulcanization reaction to obtain a Prussian blue derivative nanocube with a vulcanized shell; calcining to obtain a MOF-based transition metal oxide / sulfide nanocube composite material with a core-shell structure. The transition metal oxide / sulfide nanocube composite material prepared by the method breaks through limitation that most structures of conventional MOF-based composite materials are simple spherical hollow structures, and structure of the composite material is a nanocube with the core-shell structure, so that size is uniform; the composite material has wide applicability in preparation methods of MOF-based transition metal compounds and has great application prospect in the field of supercapacitors and lithium ion batteries.
Owner:FUZHOU UNIV
Two-dimensional material nanometer roll as well as preparation method and application thereof
InactiveCN108217608ASimple and flexible operationLower synthesis costMaterial nanotechnologyTantalum compoundsField-effect transistorNanomaterials
The invention relates to the field of a nanometer material and preparation thereof, and discloses a two-dimensional material nanometer roll as well as a preparation method and application thereof, wherein the two-dimensional material nanometer roll is obtained by soaking and / or dripping and coating a solution onto the surface of a two-dimensional material thin film and thus automatically curling the two-dimensional material thin film, wherein the two-dimensional material nanometer roll is in a hollow rod shape; the length is 50nm to 1cm; the outer diameter is 5 to 500nm; the inner hollow layerdiameter is 2 to 100 nm; the layer space is 0.3 to 10 nm. The migration rate of a prepared field effect transistor based on the two-dimensional material nanometer roll is 8 to 4000 cm / (V.sec). By using the method, the two-dimensional material nanometer roll with high quality can be prepared at high yield. In addition, the operation of the method is simple and flexible; the synthesis cost is low;the reaction time is short.
Owner:INST OF CHEM CHINESE ACAD OF SCI
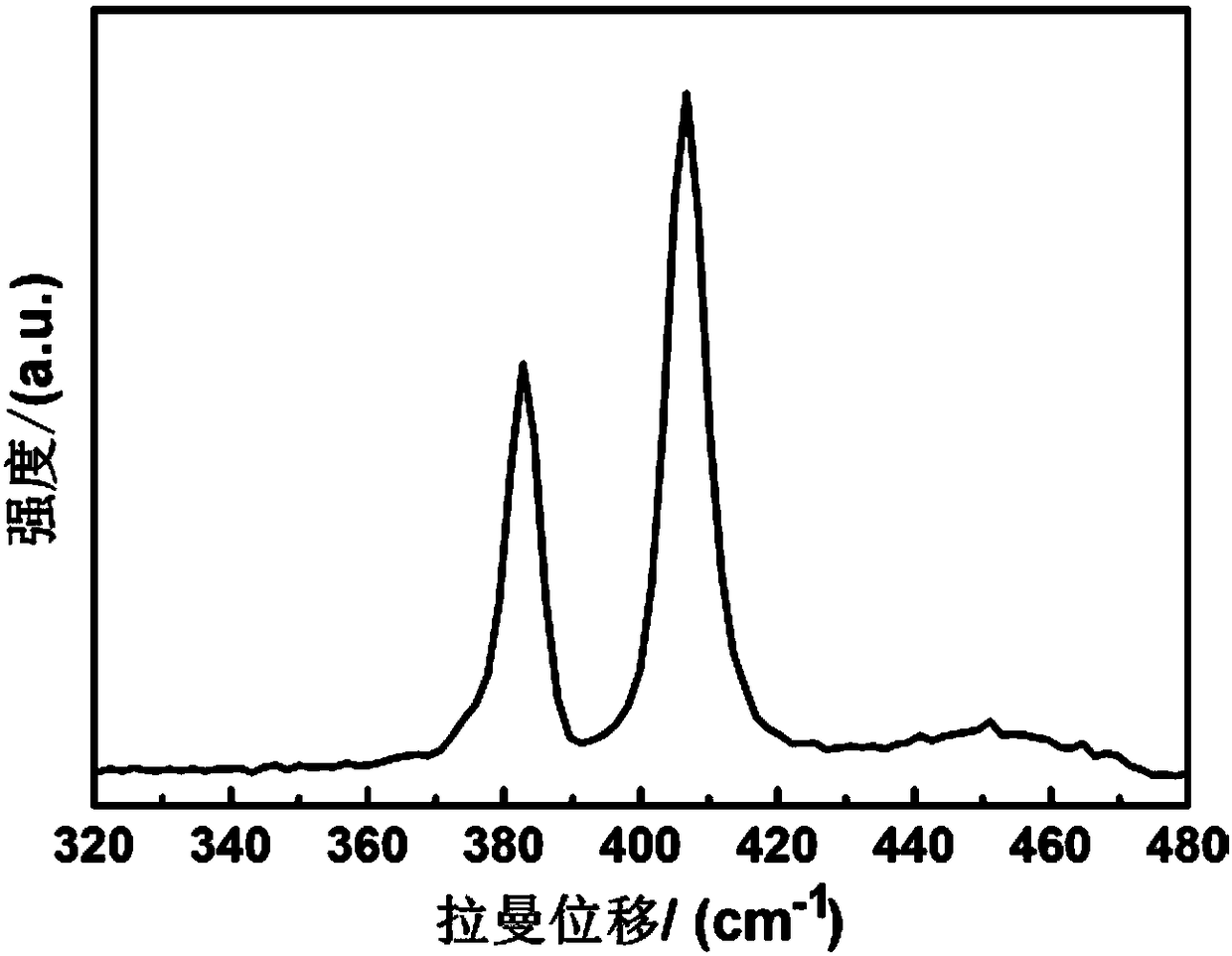
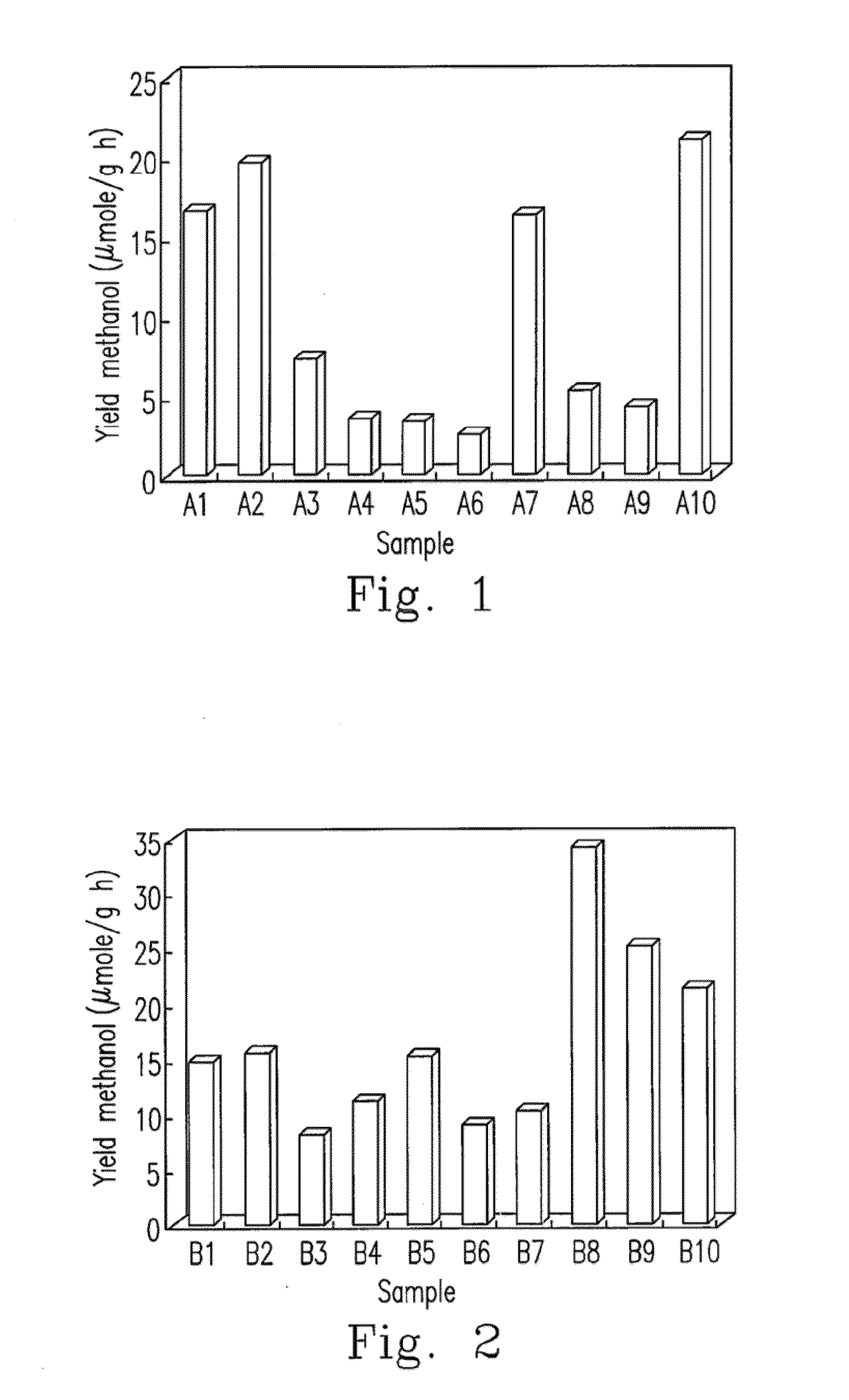
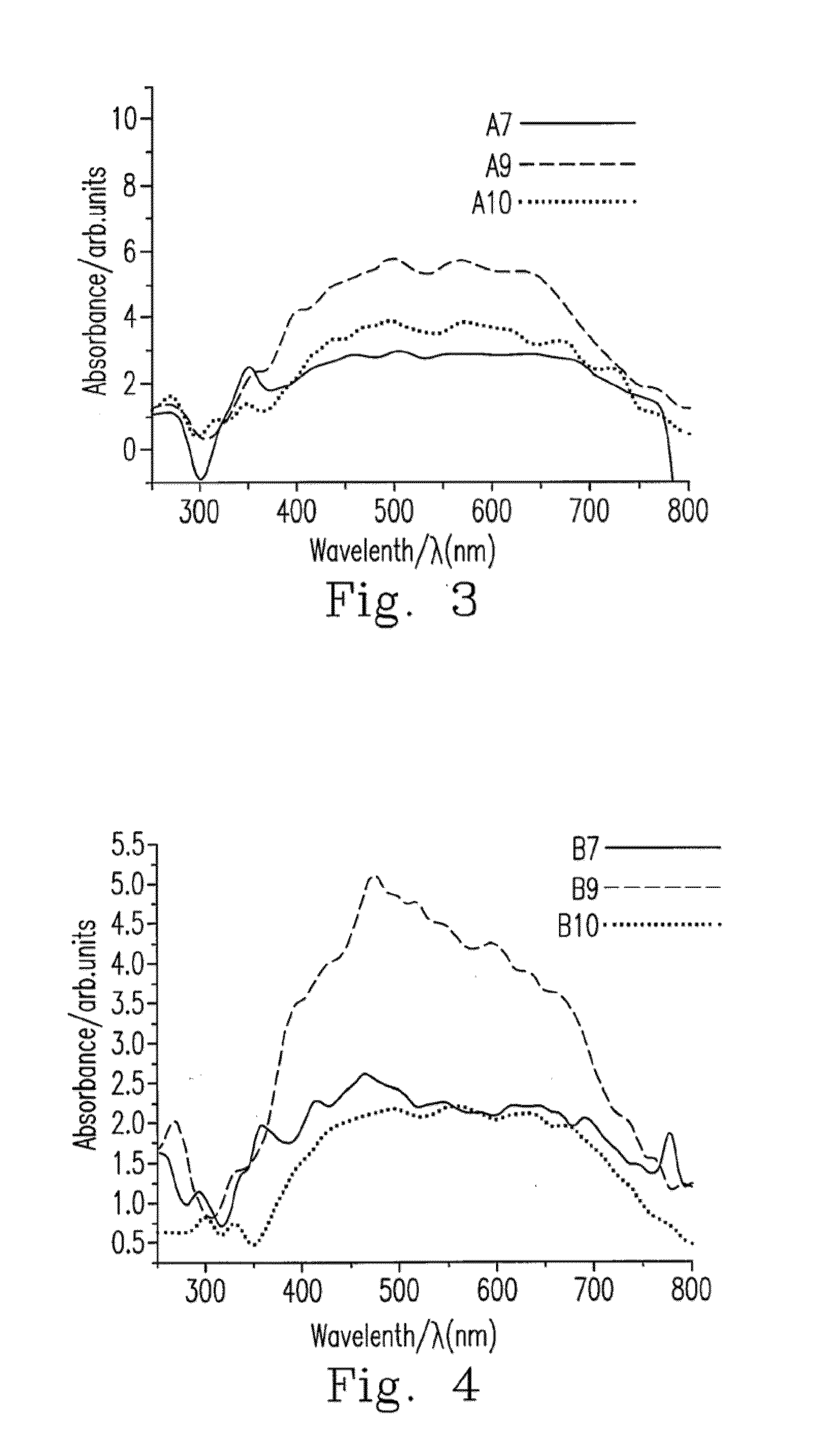
Metallic sulfide photocatalyst for carbon dioxide reduction and the preparation for the same
InactiveUS20130252798A1Readily apparentCell electrodesHydroxy compound preparationOxidation stateMetallic sulfide
Disclosed are the metallic sulfide photocatalyst and its preparation method. The photocatalyst includes at least one soluble metallic salt and a sulfide with the oxidation state of S atom ≦+4. The photocatalyst is afforded by reacting the sulfide with the at least one soluble metallic salt dissolved in the complexing agent. Additionally, the photocatalyst further is customized with co-catalyst such as RuCl to form Ru-carried metallic sulfide photocatalyst. The metallic sulfide photocatalyst and Ru-carried metallic sulfide photocatalyst are capable of effectively reducing CO2 to CH3OH under the visible light illumination.
Owner:NATIONAL TSING HUA UNIVERSITY
Flotation reagents and flotation processes utilizing same
ActiveUS8720694B2Promote recoveryImprovement recovery and gradeFlotationProcess efficiency improvementSulfideEngineering
Methods of enhancing recovery of value sulfide or precious minerals from an ore containing Mg-silicate, slime forming minerals, and / or clay by crushing the ore, grinding the ore, and subjecting the ground ore to a flotation process, in conjunction with the addition of at least one monovalent ion modifier enhancing agent and / or froth phase modifier agent to the ore, are provided herein.
Owner:CYTEC TECH CORP
Comprehensive utilization method of serpentine and device used by method
Owner:NANYANG ORIENTAL APPL CHEM RES INST +1
Synthetic method of transition metal sulfide
InactiveCN103073073AAvoid pollutionReduce the temperatureCadmium sulfidesCobalt compoundsThioureaMetallic sulfide
The invention relates to a synthetic method of transition metal sulfide, and belongs to the technical field of inorganic synthetic methods. The method comprises the steps that nitrate of transition metal (cobalt, nickel or cadmium) and excessive thiourea are mixed, react for 4-24h at 180-240 DEG C, and are cooled to a room temperature, washed and filtered, and sulfide of the transition metal (the cobalt, nickel or cadmium) is obtained. In a reaction system of the synthetic method, thiourea serves as a reactant and a fluxing agent, so that the problems that other substances are required to be added as a fluxing agent in the traditional fluxing agent method, foreign ions are introduced to cause pollution to a synthetic crystal, and transition metal sulfide cannot be synthesized by the traditional fluxing agent method are overcome; transition metal sulfide can be synthesized under a low-temperature fuse salt condition; adopted raw materials are cheap and easy to obtain; and the synthetic method is simple in synthetic technology, short in reaction period, small in energy consumption, environment-friendly, and low in equipment requirement, and is suitable for large-scale industrial production.
Owner:JILIN UNIV
Processing method for recovering nickel, chromium and iron from stainless steel factory waste residue
ActiveCN104098148AHigh recovery rateEasy to separateIron oxides/hydroxidesChromates/bichromatesFerric hydroxideNickel sulfide
The invention discloses a processing method for recovering nickel, chromium and iron from stainless steel factory waste residue. The method is as below: adding mixed acid into the stainless steel factory waste residue, leaching out nickel, chromium and iron in the waste residue, and filtering to separate a pickle liquor and leaching residue; adding sodium sulfide to the pickle liquor under normal temperature, and after reaction conducting solid-liquid separation to obtain nickel sulfide and a filtrate; heating the filtrate, and adding a sodium hydroxide solution and a hydrogen peroxide solution; converting trivalent chromium into hexavalent chromium to stay in the supernatant by an alkali leaching oxidation method, and converting trivalent iron into a ferric hydroxide precipitate, conducting solid-liquid separation to obtain a sodium chromate solution; and recovering the chromium, smelting down the ferric hydroxide precipitate for smelting utilization, and subjecting the acid leaching residue to natural air drying for agricultural landfill. The whole process of the invention does not produce contaminants, and realizes complete recovery and utilization of resources; and the process is simple, easy to operate and low in cost, can effectively solve the problem of waste residue pollution in the production of stainless steel, and has high economic benefit and environmental benefit.
Owner:江苏森力威冶金设备有限公司
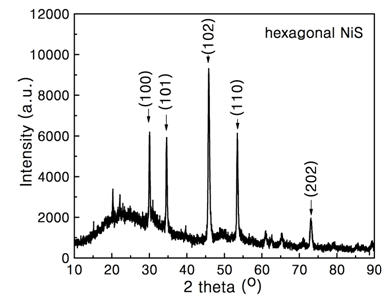
Prepn process of nanometer nickel sulfide rod
InactiveCN1974415ASimple operation processShort reaction timeNickel sulfidesHigh volume manufacturingNickel sulfide
The present invention relates to preparation process of nanometer nickel sulfide rod, and belongs to the field of nanometer material preparing technology. The improved preparation process includes dissolving nickel alkyl xanthate with C8-C22 straight chain or branched chain in ionic liquid, maintaining at 140-210 deg.c for 5 min to 3 hr, and separation to obtain the product. The ionic liquid consists of cation and anion, the cation is N, N'-dialkyl imidazole ion, and the cation is BF4-, PF6-, CF3COO-, C3F7COO-, CF3SO3-, C4F9SO3-, (CF3SO2)2N-, (C2F5SO2)2N-, (CF3SO2)3C-, SbF6- or AsF6- ion. The product of the process has relatively identical shape, and the process has the advantages of fast speed and environment friendship and is suitable for mass production.
Owner:HENAN UNIVERSITY
Hydrometallurgical process for nickel oxide ore
InactiveUS20100028227A1Preventing inevitable operation shutdownOperation efficiency is highSolvent extractionProcess efficiency improvementSlurryHydrometallurgy
The hydrometallurgical Process for a nickel oxide ore, which is capable of preventing inevitable operation shutdown of a leaching step and maintaining high operation efficiency as a whole process, in a trouble of the steps other than the leaching step, in a hydrometallurgical Process for a nickel oxide ore using a High Pressure Acid Leach.It is characterized in that, the hydrometallurgical Process for a nickel oxide ore using a High Pressure Acid Leach equipment equipped with the following means (a) to (c), in a trouble of the steps other than the above leaching step, the leached slurry discharged from the means (c), which is used in the above High Pressure Acid Leach equipment, is subjected to self-circulation inside the High Pressure Acid Leach equipment, by transferring to the means (a), which is used in the above High Pressure Acid Leach equipment, as well as by shutdown of receiving the ore slurry and the addition of sulfuric acid, in the above leaching step:means (a) to preliminarily increase temperature and pressure of the ore slurry;means (b) to form the leached slurry, by the addition of sulfuric acid to the ore slurry with preliminarily increased temperature and pressure, and leaching under blow of high-pressure steam and high-pressure air;means (c) to eliminate a pressurized state of the leached slurry formed.
Owner:SUMITOMO METAL MINING CO LTD
Preparation method of block NiS2 and application of block NiS2 to sodium-ion battery
InactiveCN105883940AIncrease contact areaLower electrode potentialMaterial nanotechnologyCell electrodesNickel saltAlcohol
The invention discloses a preparation method of block NiS2 and an application of the block NiS2 to a sodium-ion battery. According to the method, a 2-methylimidazole alcohol solution is added to a nickel salt alcohol solution, the mixture is evenly mixed, left to stand, subjected to centrifugal separation and dried, and nickel precursors are obtained; the nickel precursors and sulfur powder are ground and mixed, the mixture is placed in a vacuum environment for heat treatment, and a block NiS2 material comprising high-purity NiS2 nano spherical small particles is obtained. The material is used as an anode material to be applied to the sodium-ion battery and shows excellent electrochemical properties such as the high specific capacity, the long cycle life and the like; the preparation method of the block NiS2 material is simple, the cost is low, and industrial production requirements are met.
Owner:CENT SOUTH UNIV
Method for synthesizing nickel sulfide nanorods through solid-liquid phase reaction and prepared nanorods
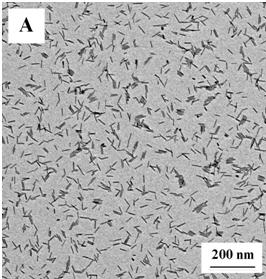
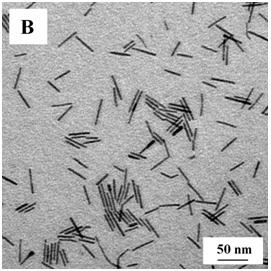
InactiveCN102198960ASmall sizeSimple reaction systemNanotechnologyNickel sulfidesChemical reactionDiameter ratio
The invention discloses a method for synthesizing nickel sulfide nanorods through solid-liquid phase reaction and the prepared nickel sulfide nanorods. The method comprises the following steps of: heating a mixture of an acetylacetone nickel solid, alkyl sulfhydryl and octadecene to the temperature of between 250 and 340 DEG C in a dry reactor, keeping the temperature for 15 to 45 minutes, and separating to obtain target products, namely the nickel sulfide nanorods. The nickel sulfide nanorods have regular nanorod-like structures, the average diameter of 4 to 5nm, the length of 25 to 70nm and the length-diameter ratio of 4 to 12. The nickel sulfide nanorods with smaller sizes, uniform appearances and hexagonal phase structures are synthesized through the solid-liquid phase chemical reaction at a low temperature under normal pressure, the process is simple, reaction time is short, and the method is suitable for mass production.
Owner:NANJING NORMAL UNIVERSITY
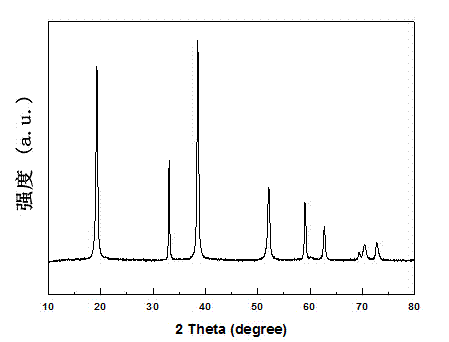
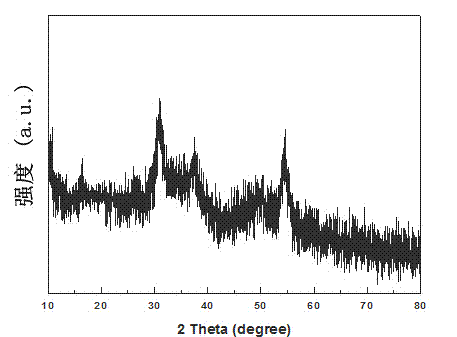
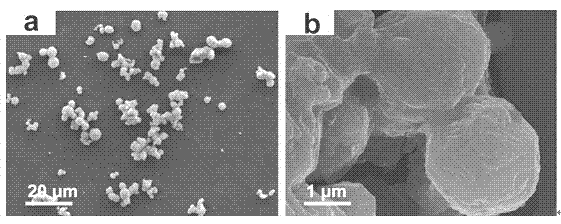
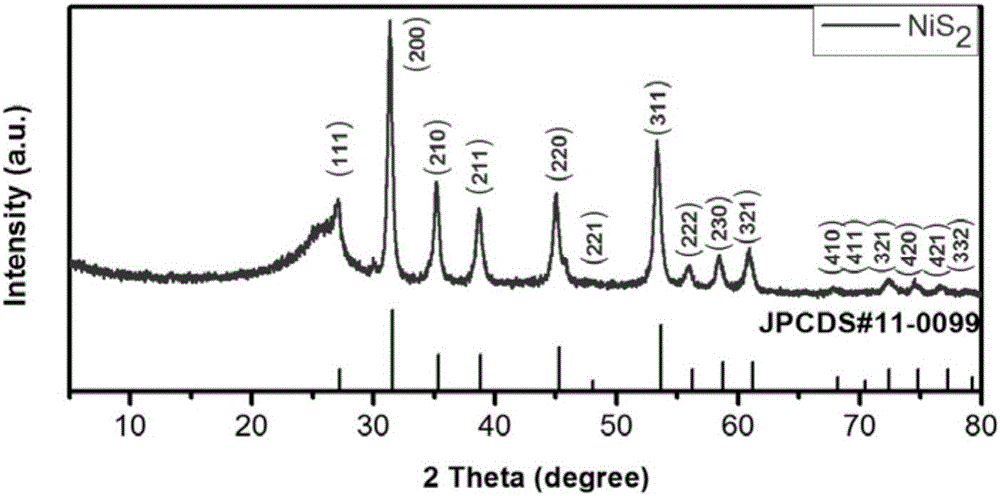
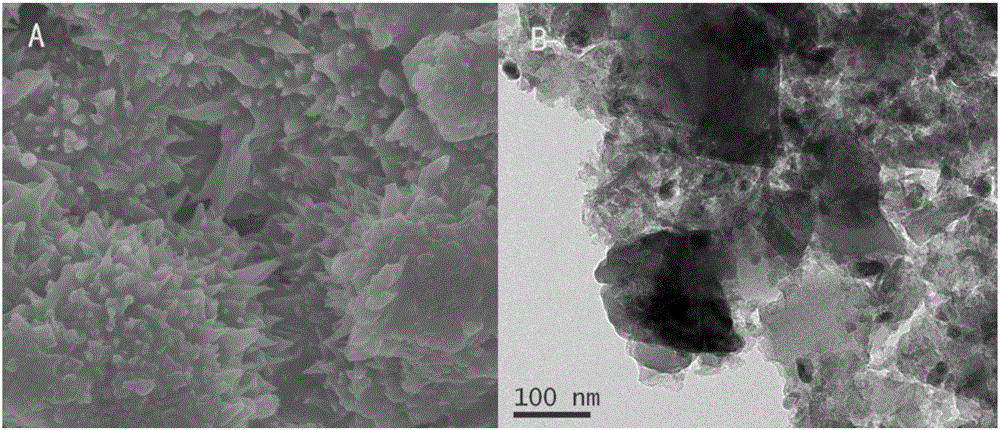
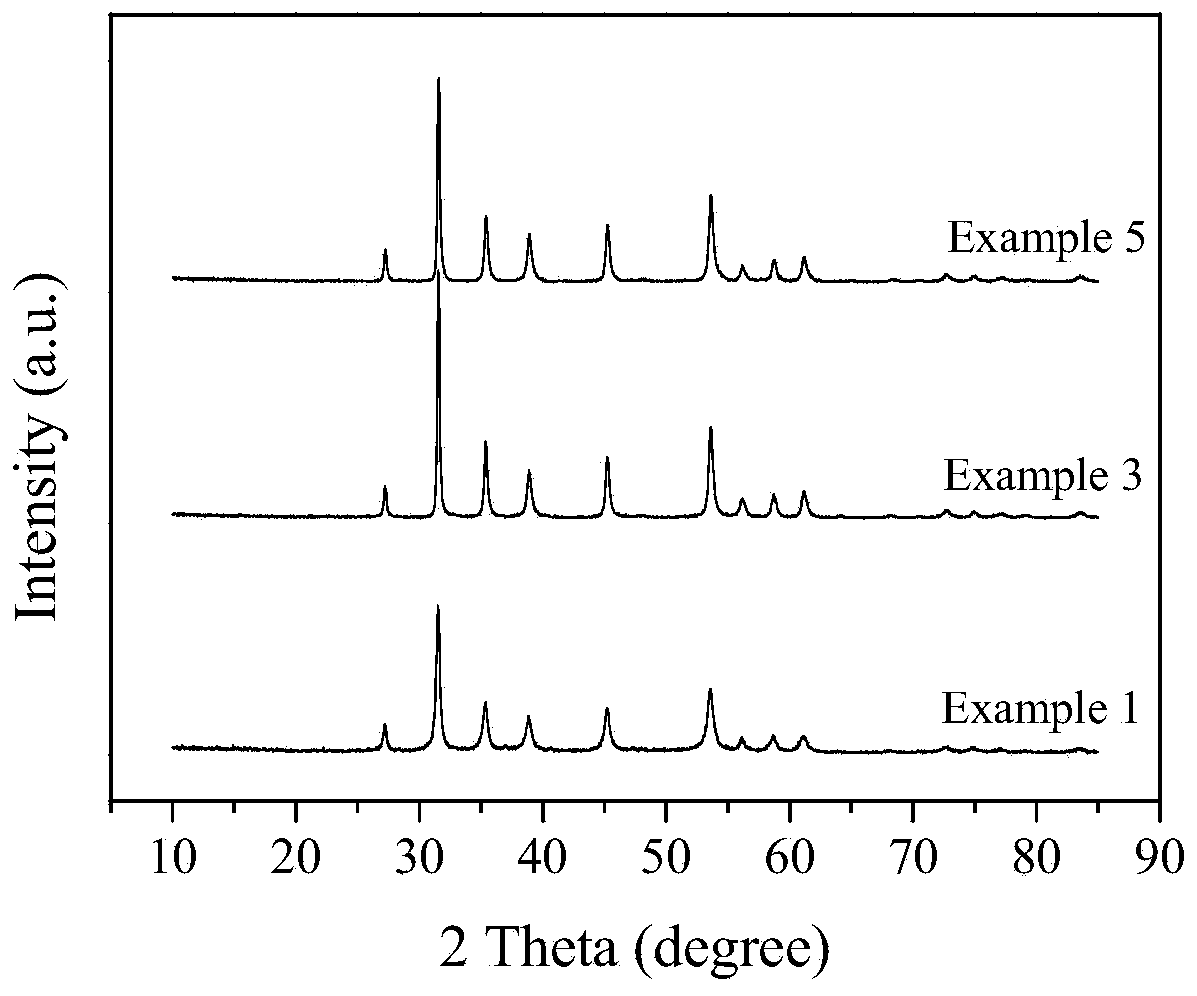
Method for synthesizing high-purity nickel disulfide
The invention discloses a method for synthesizing high-purity nickel disulfide. The method comprises the following steps: dissolving soluble nickel salt into deionized water, dropwise adding acid to adjust the pH value of the solution to be 0-3, further adding a water-soluble sulfur-containing compound with the molar ratio of molar ratio to nickel to be 4-12, stirring to completely dissolve, pouring the mixture into a sealed hydrothermal reaction kettle, reacting for 6-72 hours at 180-300 DEG C, washing with water and alcohol, and performing vacuum drying, thereby obtaining the high-purity nickel disulfide. The method adopts a one-step hydrothermal method to prepare the high-purity nickel disulfide, is cheap in raw material, simple in process, gentle in preparation condition, free of protecting gas, high in product purity, is a high-efficiency and environment-friendly synthesis method, and can be used for synthesizing high-purity cobalt disulfide, iron disulfide and the like.
Owner:XIANGTAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com