New method for two-step process preparation of nickel sulfide
A nickel sulfide, step method technology, applied in nickel sulfide and other directions, can solve problems such as the wide application of unfavorable nickel sulfide micro-nano materials, environmental pollution, etc., and achieve stable and reliable product performance, no environmental pollution, and good consistency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve 2.5 mmol nickel chloride in 35 mL deionized water, add 10 mmol urea, then transfer to an autoclave, heat at 120 °C for 16 h, cool naturally, filter, wash the product, and dry it at 60 °C The nickel hydroxide precursor can be obtained in 12 h. Weigh 0.3 g of nickel hydroxide precursor, ultrasonically disperse (100 W power, 15 minutes) into 50 mL of ethylene glycol, then add 10 mL of aqueous solution containing 5 g of sodium sulfide nonahydrate, and dissolve the above mixed solution Transfer to a 100 mL round bottom flask, place in an oil bath, condense and reflux at 160 °C for 12 h, cool naturally, wash the obtained black precipitate with water and absolute ethanol (three times each), and then Nickel sulfide can be obtained by drying at 60 °C for 12 h.
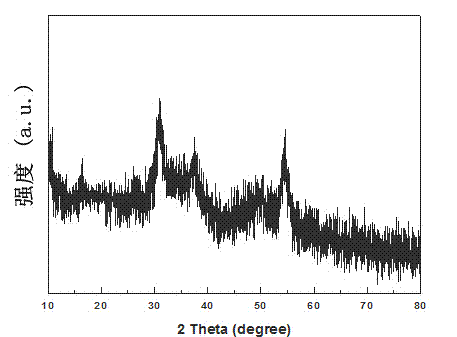
[0032] Such as figure 1 As shown, the obtained precursor is Ni(OH)2 ((JCPDS Card.14-117).
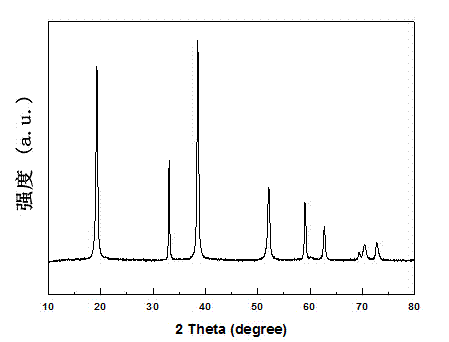
[0033] Such as figure 2 As shown, the composition of the obtained nickel sulfide is pure phase Ni3S4 ((JCPDS Card....
Embodiment 2
[0037] Dissolve 2.5 mmol of nickel chloride in 30 mL of deionized water, add 2 mL of ammonia water, then transfer to an autoclave, heat at 160 °C for 8 h, cool naturally, wash the product, and dry at 60 °C for 12 h A nickel hydroxide precursor can be obtained. Weigh 0.3 g of nickel hydroxide precursor, ultrasonically disperse (100 W power, 16 minutes) into 50 mL of ethylene glycol, then add 10 mL of aqueous solution containing 5 g of sodium sulfide nonahydrate, and dissolve the above mixed solution Transfer to a 100 mL round-bottomed flask, place in an oil bath, condense and reflux at 160 °C for 24 h, after natural cooling, wash the obtained black precipitate with water and absolute ethanol (washing three times each), and in Nickel sulfide can be obtained by drying at 60 °C for 12 h.
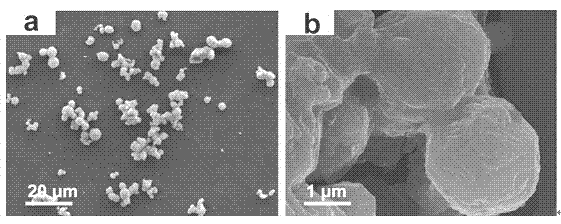
[0038] Such as Figure 5 As shown, the prepared nickel sulfide is composed of microspheres with a diameter of about 3 μm, and the microspheres have a flower-like structure.
Embodiment 3
[0040] Dissolve 2.5 mmol of nickel chloride in 25 mL of ethylene glycol, add 10 mL of deionized water to 2 mL of ammonia water, mix the two solutions slowly, transfer to an autoclave, heat at 160 °C for 16 h, and cool naturally , the product was washed and dried at 60 °C for 12 h to obtain the nickel hydroxide precursor. Weigh 0.3 g of nickel hydroxide precursor, ultrasonically disperse (100 W power, 16 minutes) into 50 mL of ethylene glycol, then add 10 mL of aqueous solution containing 5 g of sodium sulfide nonahydrate, and dissolve the above mixed solution Transfer to a 100 mL round-bottomed flask, place in an oil bath, condense and reflux at 160 °C for 16 h, and after natural cooling, wash the obtained black precipitate with water and absolute ethanol (three times each), and in Nickel sulfide can be obtained by drying at 60 °C for 12 h.
[0041] Such as Figure 6 As shown, the prepared nickel sulfide is composed of hexagonal sheets with uniform shape and side length of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com