Synthesis method of self-assembled Ni3S2 nano-sheet
A synthesis method and self-assembly technology, applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of catalytic activity, catalytic stability, and use range gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) First, the 1cm×2cm nickel foam was ultrasonically cleaned at 70W in acetone and 3mol / L HCl solution for 8 minutes, then washed with deionized water and absolute ethanol, and dried at room temperature;
[0019] 2) Next, put the dried nickel foam into a 50mL high-temperature and high-pressure reactor with a polytetrafluoroethylene liner, then add 5mL of thiourea solution with a concentration of 1.445mmol / L, and add 0.275-0.363g of polyethylene Pyrrolidone (PVP) morphology control agent, the reactor is sealed, placed in an oven at 130 ° C for 10 h;
[0020] 3) After the reaction is finished, after naturally cooling to room temperature, take out the nickel foam, and rinse repeatedly with water and absolute ethanol respectively;
[0021] 4) Finally, put the rinsed nickel foam into a vacuum oven to dry at room temperature to obtain self-assembled Ni 3 S 2 Nanosheet array self-supporting electrodes.
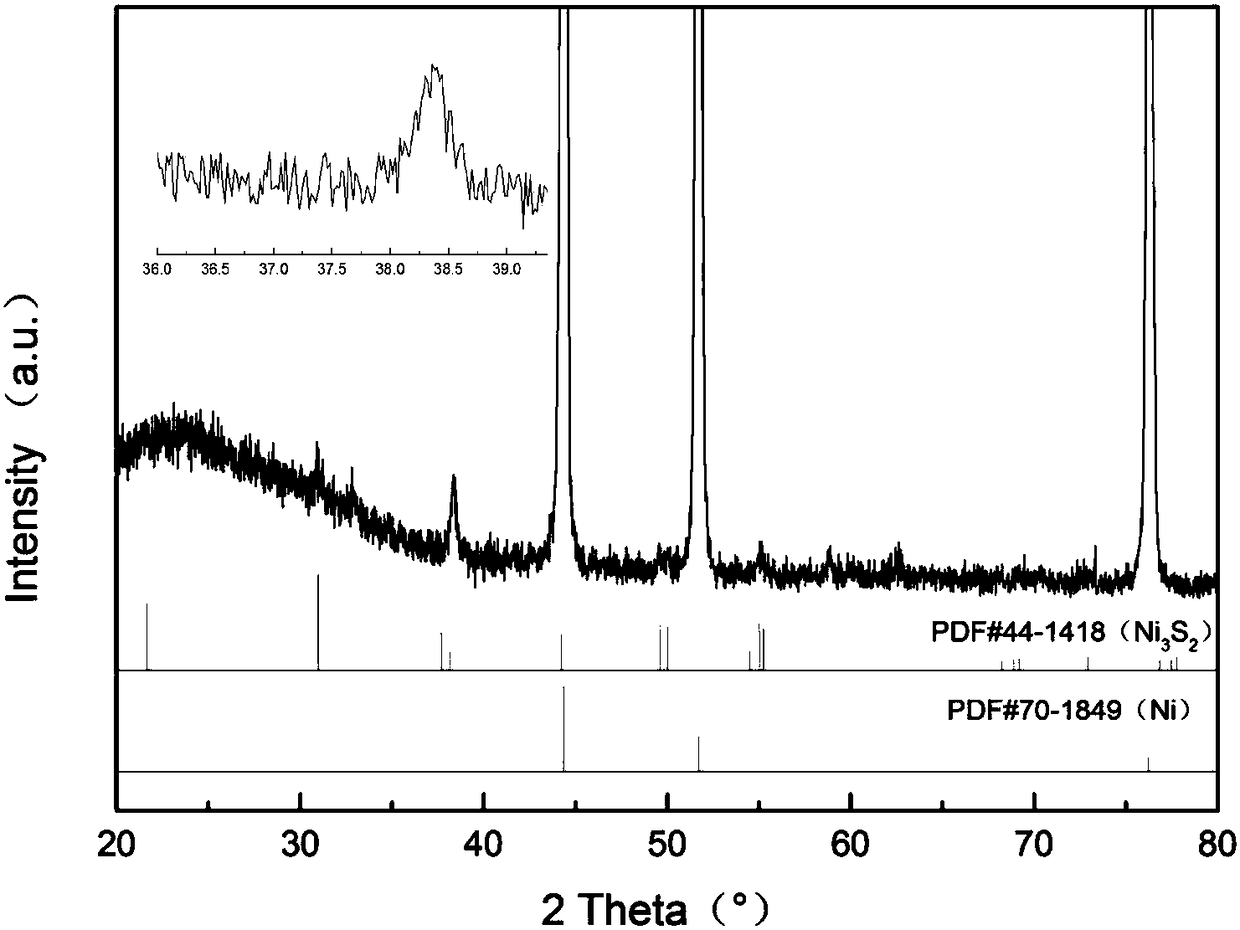
[0022] Depend on figure 1 It can be seen that the sample prepared in ...
Embodiment 2
[0024] 1) First, the 1cm×2cm nickel foam was ultrasonically cleaned at 80W in acetone and 3mol / L HCl solution for 5 minutes, then washed with deionized water and absolute ethanol, and dried at room temperature;
[0025] 2) Next, put the dried nickel foam into a 50mL high-temperature and high-pressure reactor with a polytetrafluoroethylene lining, then add 8mL of 1.445mmol / L thiourea solution, and add 0.2-0.264g of polyethylene Pyrrolidone (PVP) morphology control agent, the reactor is sealed, placed in an oven at 140 ° C for 9 hours;
[0026] 3) After the reaction is finished, after naturally cooling to room temperature, take out the nickel foam, and rinse repeatedly with water and absolute ethanol respectively;
[0027] 4) Finally, put the rinsed nickel foam into a vacuum oven to dry at room temperature to obtain self-assembled Ni 3 S 2 Nanosheet array self-supporting electrodes.
[0028] figure 2 It can be seen that a self-assembled Ni prepared in this example 3 S 2 N...
Embodiment 3
[0030] 1) First, the 1cm×2cm nickel foam was ultrasonically cleaned at 80W in acetone and 3mol / L HCl solution for 10min, then washed with deionized water and absolute ethanol, and dried at room temperature;
[0031] 2) Next, put the dried nickel foam into a 50mL high-temperature and high-pressure reactor with a polytetrafluoroethylene liner, then add 10mL of 1.445mmol / L thiourea solution, and add 0.15-0.198g of polyethylene Pyrrolidone (PVP) morphology control agent, the reaction kettle is sealed, placed in an oven at 150 ° C for 8 hours;
[0032] 3) After the reaction is finished, after naturally cooling to room temperature, take out the nickel foam, and rinse repeatedly with water and absolute ethanol respectively;
[0033] 4) Finally, put the rinsed nickel foam into a vacuum oven to dry at room temperature to obtain self-assembled Ni 3 S 2 Nanosheet array self-supporting electrodes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com