Method for synthesizing high-purity nickel disulfide
A technology of nickel disulfide and synthesis method, applied in the direction of nickel sulfide, etc., to achieve the effects of simple process technology, mild preparation conditions, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
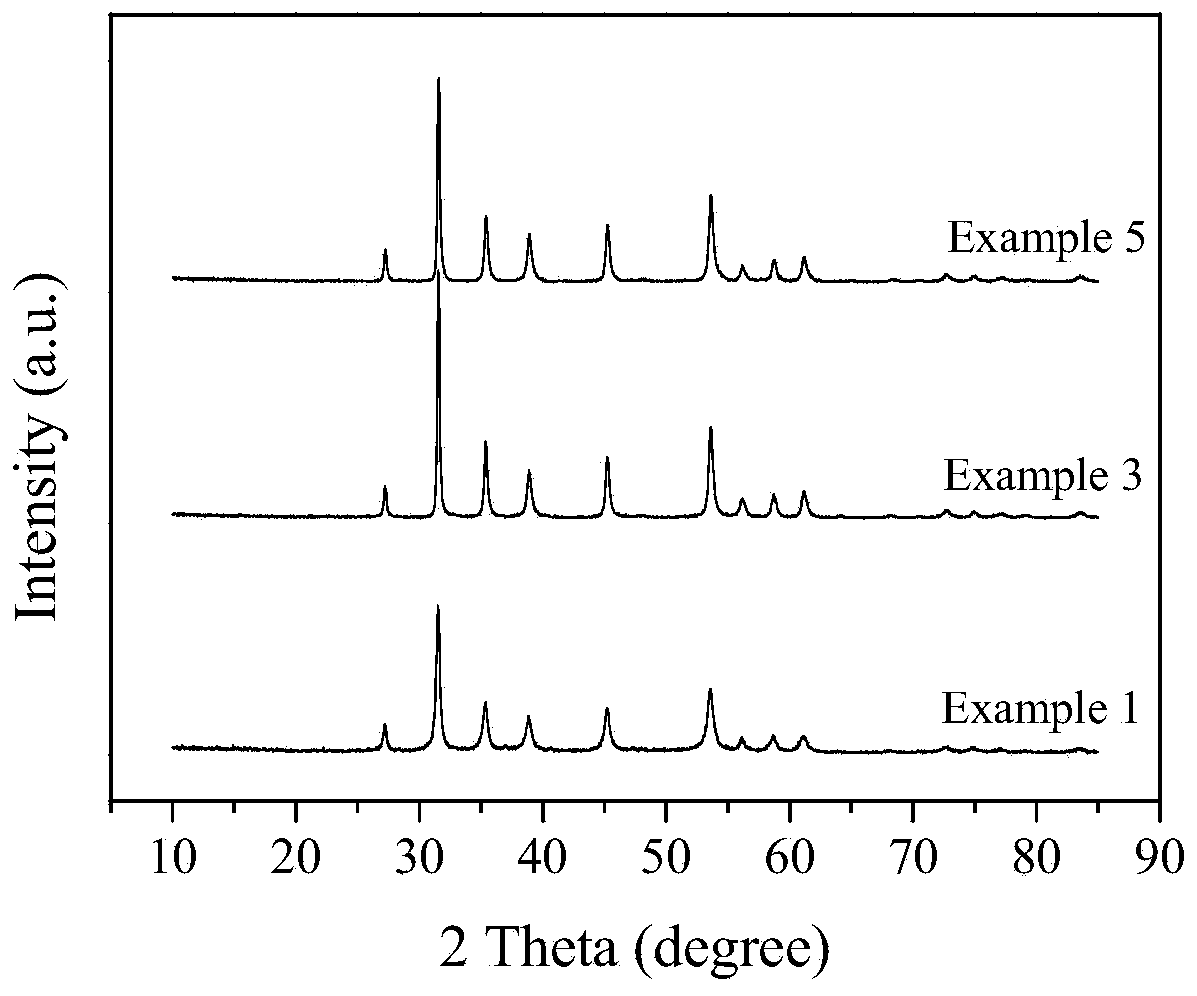
Embodiment 1
[0019] Dissolve 6mmol of nickel nitrate in 250mL of deionized water, add nitric acid to adjust the pH value of the solution to 0.7, then add 35mmol of thiourea, fully dissolve, move to a 300mL closed hydrothermal reaction kettle, heat up to 200°C, react for 24 hours, and the reaction is over Finally, the solution was taken out, filtered, washed with water, washed with alcohol, and vacuum dried to obtain 0.70 g of nickel disulfide with a purity greater than 99%. Calculated based on the reacted metallic nickel, the yield of nickel disulfide was 94.9%.
Embodiment 2
[0021] Dissolve 6mmol of nickel sulfate in 250mL of deionized water, add sulfuric acid to adjust the pH value of the solution to 0.6, then add 35mmol of sodium sulfide, after fully dissolved, move to a 300mL closed hydrothermal reaction kettle, heat up to 220°C, react for 12 hours, and the reaction ends Finally, the solution was taken out, filtered, washed with water, washed with alcohol, and vacuum dried to obtain 0.63 g of nickel disulfide with a purity greater than 99%. Calculated based on the reacted metallic nickel, the yield of nickel disulfide was 86.2%.
Embodiment 3
[0023] Dissolve 6mmol of nickel acetate in 250mL of deionized water, add acetic acid to adjust the pH value of the solution to 0.5, then add 35mmol of thioacetamide, fully dissolve, move to a 300mL closed hydrothermal reaction kettle, heat up to 200°C, and react for 36 hours. After the reaction, the solution was taken out, filtered, washed with water, washed with alcohol, and vacuum-dried to obtain 0.66 g of nickel disulfide with a purity greater than 99%. Calculated based on the reacted metallic nickel, the yield of nickel disulfide was 89.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com