Sodium vanadium fluorophosphate, preparation method and uses thereof
A technology for vanadium fluorophosphate sodium salt and vanadium phosphate sodium salt is applied in the field of rapid preparation at room temperature, vanadium fluorophosphate sodium salt Na32F3-2x, and can solve the problems of long time consumption, high requirements for raw material solubility, long ball milling time and the like, To achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] A preparation method of vanadium sodium salt of fluorophosphate, said method comprising the steps of:
[0077] (1) Dissolving 3.260g of vanadyl sulfate in 40mL of deionized water to obtain a vanadium source solution;
[0078] (2) Add 4.716g sodium dihydrogen phosphate dihydrate and 1.285g sodium fluoride (V:P:F:Na about 1:3:3:6) to the vanadium source solution, stir to dissolve, and heat at 90°C Insulated in an oven for 3h, filtered to obtain a reaction mixture;
[0079] (3) The obtained reaction mixture was washed 7 times with deionized water and 1 time with ethanol, and then dried in air at 60° C. for 5 h to obtain 1.785 g of the product.
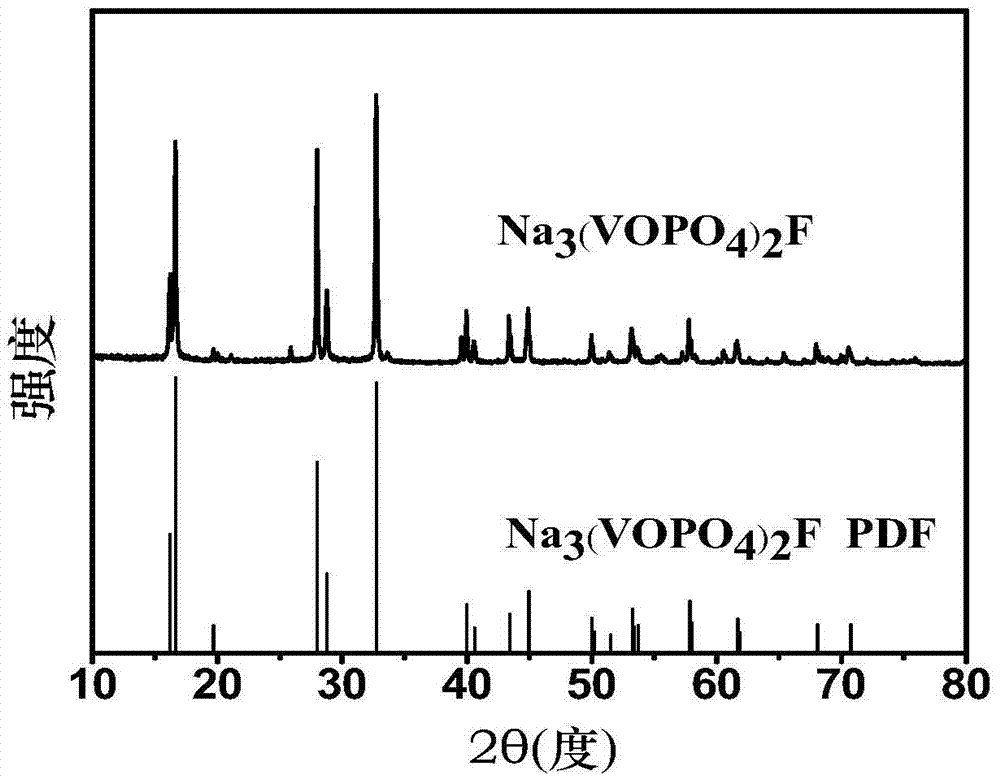
[0080] Gained product is carried out XRD test, test result is as follows figure 1 As shown, the test results show that the obtained product is Na 3 (VOPO 4 ) 2 F, and it has good crystallinity and high purity.
[0081] The resulting product was analyzed by a scanning electron microscope (SEM), and the characterization results...
Embodiment 2
[0084] A preparation method of vanadium sodium salt of fluorophosphate, said method comprising the steps of:
[0085] (1) 0.326g vanadyl sulfate and 0.158g vanadium trichloride were dissolved in 8mL deionized water to obtain a vanadium source solution;
[0086] (2) Add 416uL phosphoric acid and 0.143g sodium fluoride (V:P:F:Na about 1:3:1.7:1.7) to the vanadium source solution, stir and adjust the pH to 3.3 with solid sodium hydroxide, and the obtained The suspension containing the precipitate was heated in a water bath at 80°C for 1 hour, and then filtered to obtain a reaction mixture;
[0087] (3) The reaction mixture was washed 6 times with deionized water and 1 time with ethanol, and dried in air at 70° C. for 5 hours to obtain 0.295 g of the product.
[0088] Carry out XRD and SEM test to gained product, test result is as follows Figure 4 with Figure 5 shown. The XRD test result shows that the obtained product is Na 3 (VO 0.5 PO 4 ) 2 f 2 , The SEM image shows ...
Embodiment 3
[0090] A preparation method of vanadium sodium salt of fluorophosphate, said method comprising the steps of:
[0091] (1) 1.630g of vanadyl sulfate was dissolved in 20mL of deionized water to obtain a vanadium source solution;
[0092] (2) Add 7.242g disodium hydrogen phosphate dodecahydrate and 1.071g sodium fluoride (V:P:F:Na about 1:4:5:13) to the vanadium source solution, filter to obtain the reaction mixture;
[0093] (3) The reaction mixture was washed 6 times with deionized water, and then dried in air at 100° C. for 5 h to obtain 1.483 g of the product.
[0094] Carry out XRD and SEM test to gained product, test result is as follows Image 6 with Figure 7 shown. XRD showed that the obtained product was Na 3 (VOPO 4 ) 2 F, and the product has good crystallinity and high purity; the SEM figure shows that the morphology of the product obtained is a micron-scale aggregate.
PUM
| Property | Measurement | Unit |
|---|---|---|
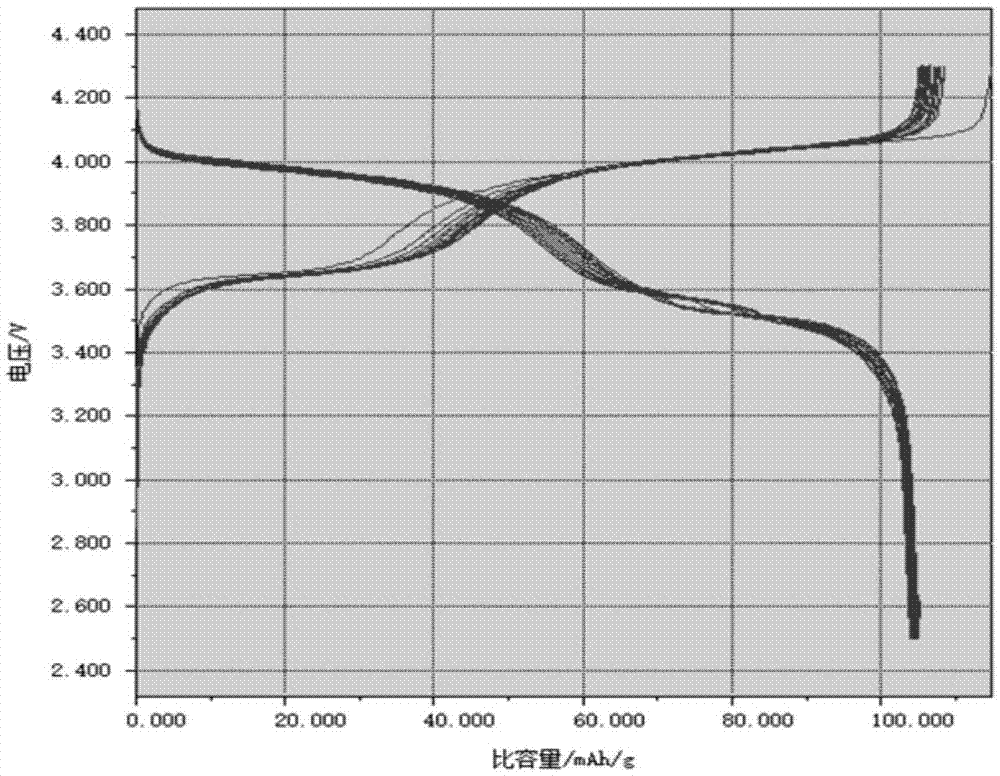
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com