Peroxidase catalyzed cell surface protein labeling method
A peroxidase and cell surface technology, applied in biological testing, material analysis through optical means, fluorescence/phosphorescence, etc., can solve the problem of slow reaction rate and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
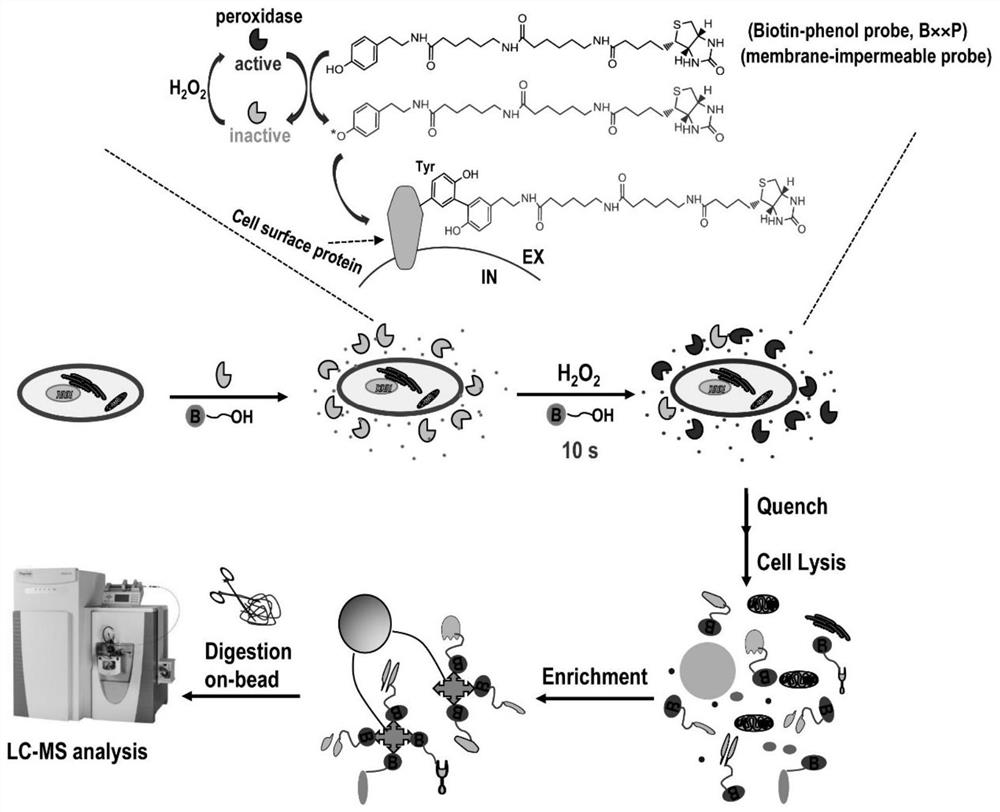
[0058]HeLa cells were cultured to cover 80% of the surface area of a 10 cm culture dish (purchased from Corning). water, pre-equilibrated to room temperature) and washed twice, adding 2 ml containing 0.5 mg / ml horseradish peroxidase (150-250U / mg, Sigma-Aldrich, product number: P8250) and 200μM BXXP (Peptide Biochemical Synthetic) PBS labeling solution (pre-equilibrated to room temperature) was placed on a slowly oscillating destaining shaker to ensure that the labeling reaction solution covered all cells. Immediately add 2 ml of PBS labeling solution (pre-equilibrated to room temperature) containing 2 mM hydrogen peroxide (Sigma-Aldrich, catalog number: 88597) and 200 μM BXXP to the experimental group to initiate the labeling reaction in the reaction system. At the same time, 2 ml of PBS labeling solution containing 200 μM BxxP (pre-equilibrated to room temperature) was added to the cells of the control group. Slowly oscillate on a decolorizing shaker and react at room temp...
Embodiment 2
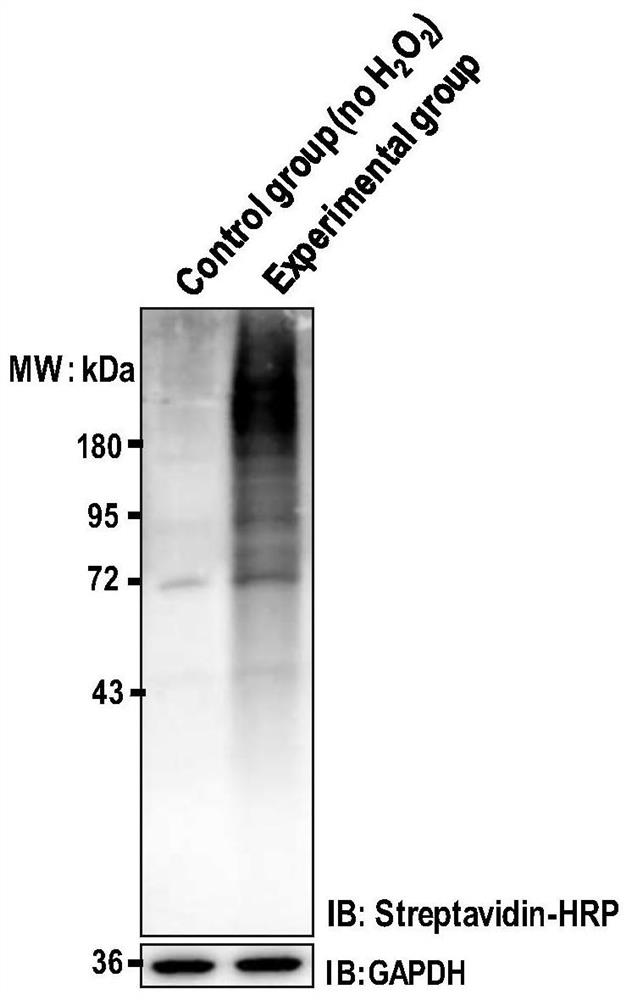
[0062] The operation process is the same as in Example 1, except that the technique for detecting the labeling effect after the cell surface labeling reaction is completed is different from Example 1.
[0063] HeLa cells were cultured to cover 80% of the surface area of a 10 cm culture dish (purchased from Corning). After removing the culture medium, PBS phosphate buffer (containing 0.01M phosphate, 0.15M sodium chloride, pH 7.4, the balance was water, pre- Equilibrate to room temperature) and wash twice, add 2 ml of a solution containing 0.5 mg / ml horseradish peroxidase (150-250 U / mg, Sigma-Aldrich, catalog number: P8250) and 200 μM BXXP (medium peptide biochemical synthesis) PBS labeling solution (pre-equilibrated to room temperature) was placed on a slowly shaking decolorizing shaker to ensure that the labeling reaction solution covered all cells. Immediately add 2 ml of PBS labeling solution (pre-equilibrated to room temperature) containing 2 mM hydrogen peroxide (Sigma-...
Embodiment 3
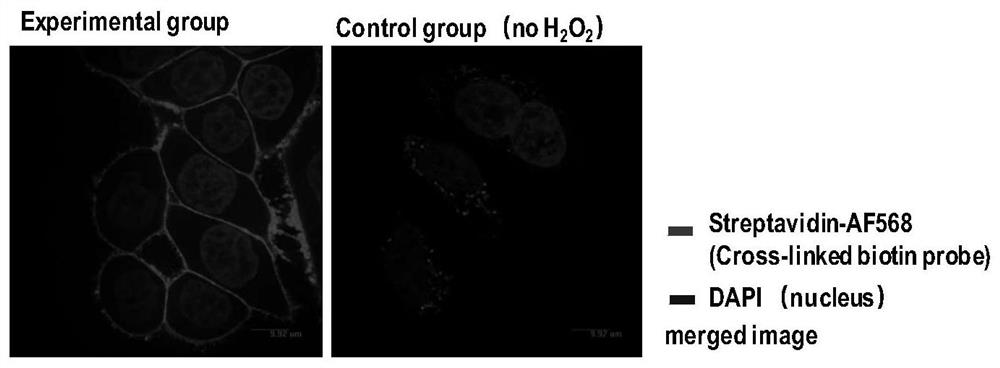
[0066] The operation process is the same as that in Example 1, except that the technique for detecting the labeling effect after the cell surface labeling reaction is completed is different from Example 1.
[0067] HeLa cells were cultured to cover 80% of the surface area of a 10cm culture dish (purchased from Corning). , pre-equilibrated to room temperature) and washed twice, adding 2 ml containing 0.5 mg / ml horseradish peroxidase (150-250U / mg, Sigma-Aldrich, product number: P8250) and 200 μM BXXP (medium peptide biochemical synthesis ) PBS labeling solution (pre-equilibrated to room temperature) was placed on a destaining shaker that oscillated slowly to ensure that the labeling reaction solution covered all cells. Immediately add 2 ml of PBS labeling solution (pre-equilibrated to room temperature) containing 2 mM hydrogen peroxide (Sigma-Aldrich, catalog number: 88597) and 200 μM BXXP to the experimental group to initiate the labeling reaction in the reaction system. At ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com