Amorphous non-precious metal hydroxide modified perovskite composite catalyst used for oxygen evolution reaction and preparation method thereof
A composite catalyst, hydroxide technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the research without double perovskite material modification, etc. To achieve the effect of maintaining catalytic activity and morphology stability, good cycle stability and high electrochemical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Amorphous Fe 0.75 Ni 0.25 (OH) 2 Preparation of modified PBC catalyst
[0036] Dissolve ferric nitrate and nickel nitrate with a molar ratio of 3:1 into distilled water, wherein 0.25 mmol of nickel nitrate is placed on a magnetic stirrer and stirred to facilitate the dissolution of the salt solution. After the nitrate is fully dissolved, stir to Add 904mg of PBC powder and 124mg of tert-butanol into the solution and continue to stir, the mass of the prepared modified catalyst is about 10wt% of the PBC powder. The excess mixed alkali (in this embodiment, the molar quantities of KOH, ammonia water and urea in the mixed alkali are respectively 1.5mmol, 2mmol and 2mmol) double the molar weight of nitrate ion (the total molar amount of alkali is 5.5mmol) is weighed Measure and dissolve in distilled water, fully dissolve and add to the beaker that is constantly stirring. The iron and nickel ions in the solution will form hydroxides that will precipitate from their salt sol...
Embodiment 2
[0038] Amorphous Ni(OH) 2 Preparation of modified PBC catalyst
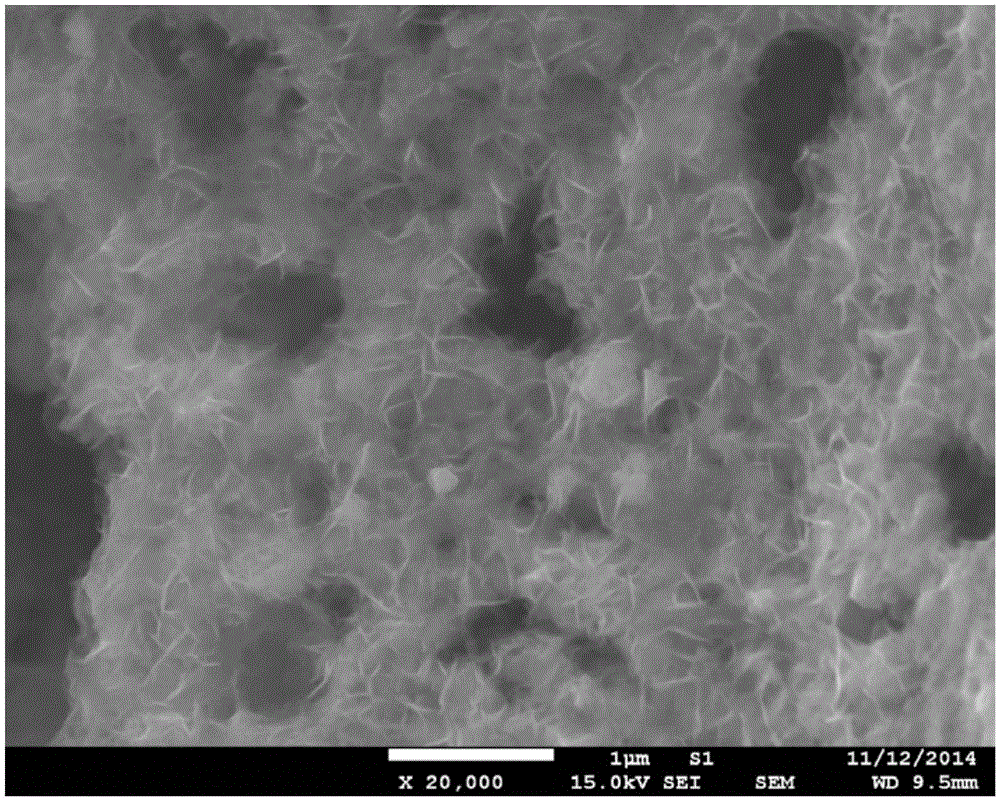
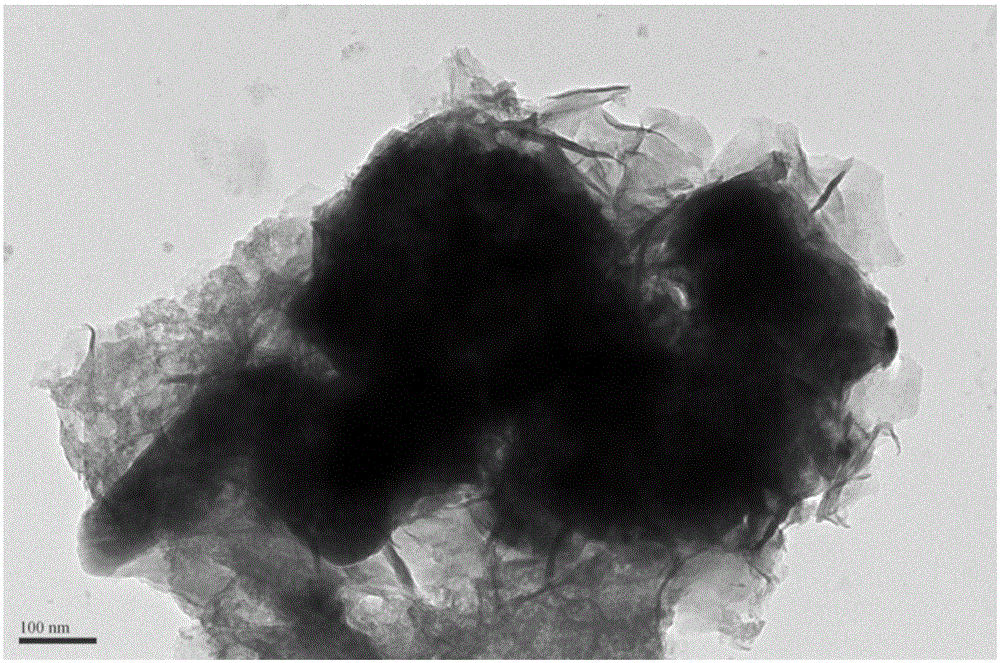
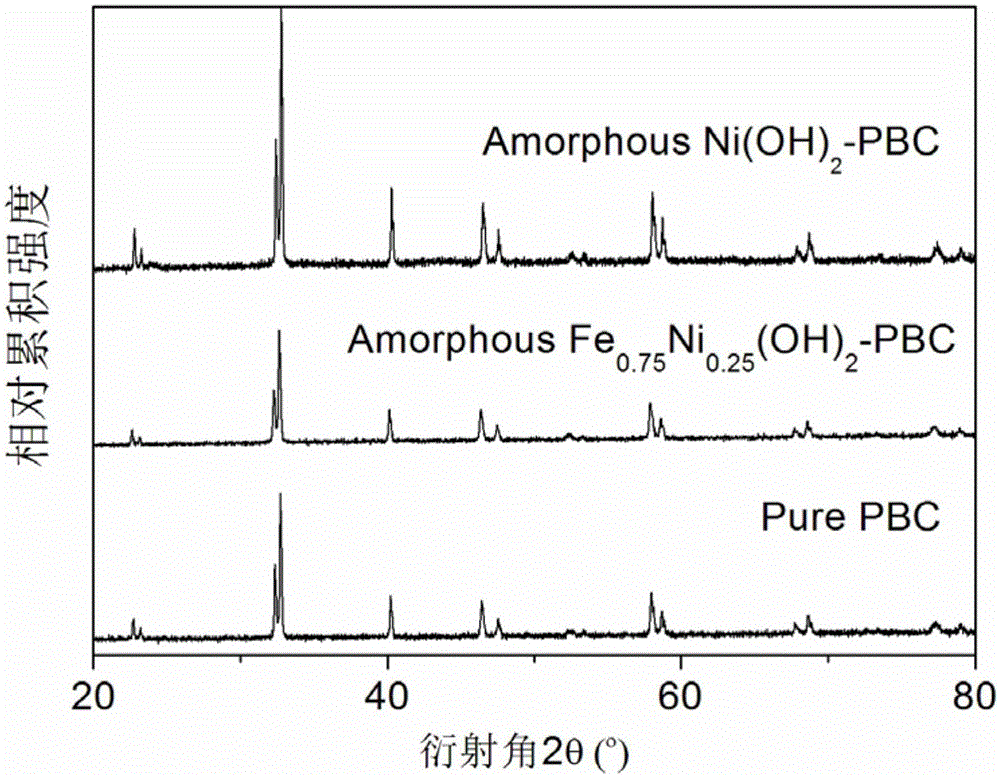
[0039] Unlike Example 1, except that the modified material is selected, only nickel nitrate is used to impregnate the PBC powder in this example, and the rest of the synthesis process and testing methods are the same as in Example 1. image 3 The results of the phase structure containing the catalyst in the PBC are not significantly different from that of pure PBC, confirming that Ni(OH) 2 The modification layer is in an amorphous state. Figure 6 is Ni(OH) 2 Scanning electron microstructure image of the modified PBC catalyst, with amorphous Fe 0.75 Ni 0.25 (OH) 2 The modified PBC is similar, and the modified material has a flake-like loose structure; Figure 7 , 8 The electrochemical performance and stability of its oxygen evolution are given in 9 and 9. It can be seen that its catalytic activity is greatly improved compared with pure PBC and corresponding oxide-modified PBC, but its performance is slight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com