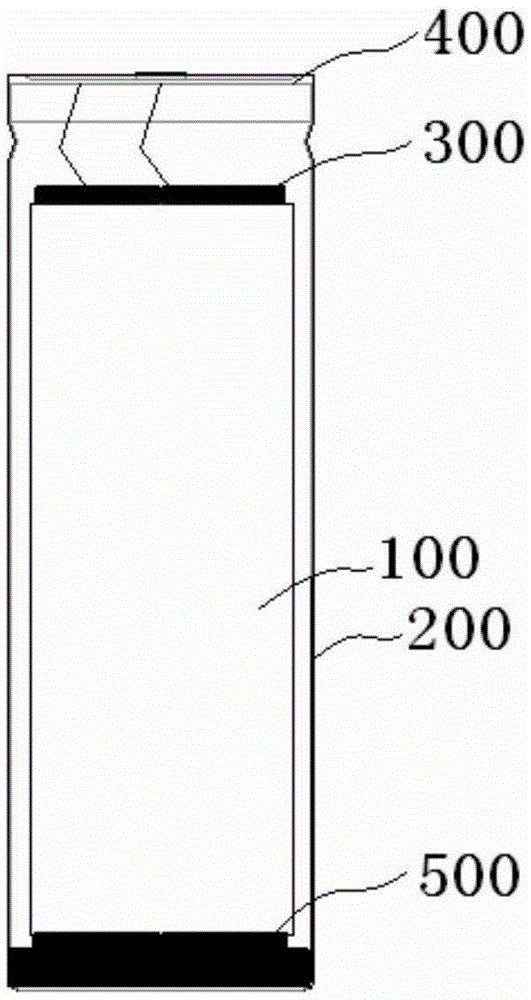
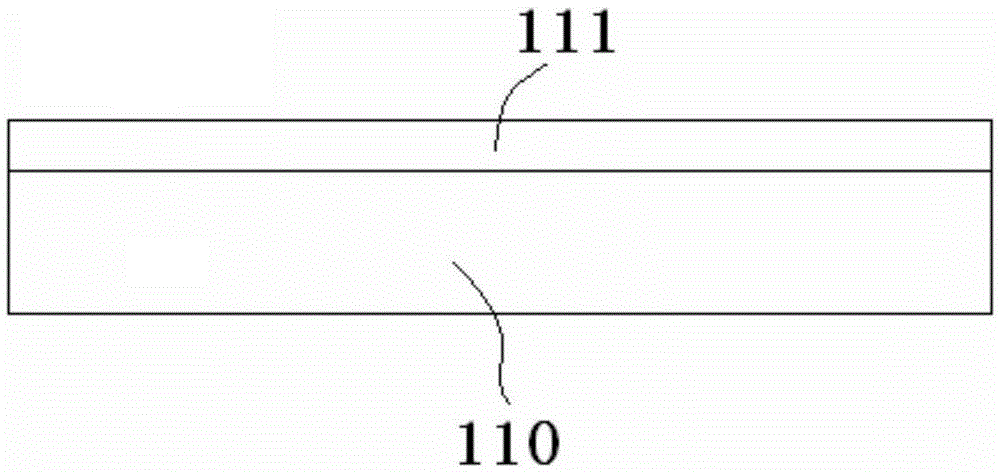
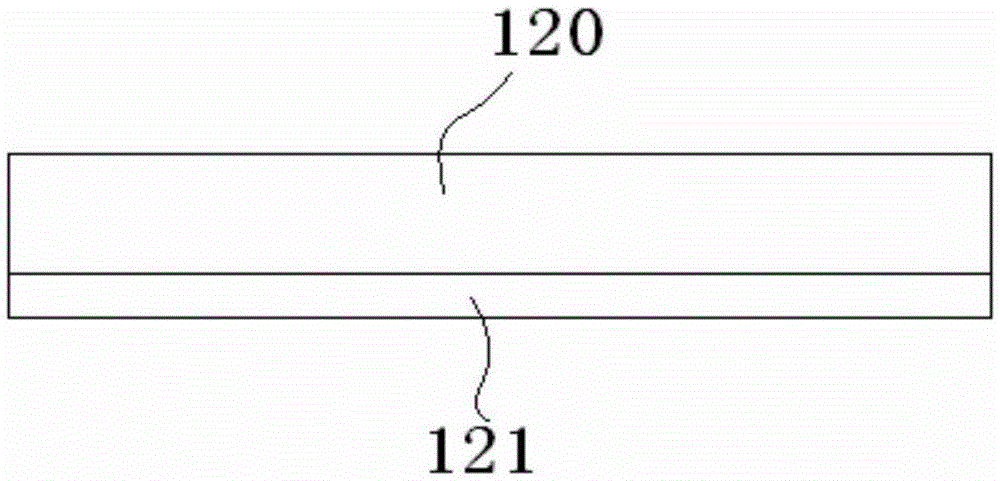
Super-wide-temperature-range nickel-hydrogen battery and manufacturing method therefor
A nickel-metal hydride battery and the technology of its manufacturing method are applied in the direction of nickel storage battery, alkaline storage battery manufacturing, battery, etc., which can solve the problems of decreased battery discharge capacity and battery failure to discharge electricity, etc., and achieve the reduction of ohmic internal resistance, small internal resistance, The effect of high output power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Positive active material Ni(OH) 2 The preparation adopts the complexation precipitation method, the concentration is 2.0mol / L ammoniacal liquor, the sodium hydroxide solution and the nickel sulfate, zinc sulfate, cobalt sulfate mixed salt solution and the mixed salt solution of nickel sulfate, zinc sulfate, cobalt sulfate and the mixing reaction under stirring, described mixing The concentration of nickel ions in the salt solution is 0.911mol / L, the concentration of zinc ions is 0.057mol / L, the concentration of cobalt ions is 0.032mol / L, the pH value is controlled at 10, the temperature is at 50°C, and the reaction time is 12h. The curing time is 12h, and the green Ni(OH) is obtained 2 Precipitation, pouring out the reaction solution, static layering, pouring off the upper clear night, washing with distilled water, and drying at 120 ° C until the water content of the sample is ≤ 0.5%, that is, the green positive electrode active material Ni(OH) is obtained 2 . The pos...
Embodiment 2
[0068] Positive active material Ni(OH) 2 The preparation adopts the complexation precipitation method, the concentration is 2.0mol / L ammoniacal liquor, the sodium hydroxide solution and the nickel sulfate, zinc sulfate, cobalt sulfate mixed salt solution and the mixed salt solution of nickel sulfate, zinc sulfate, cobalt sulfate and the mixing reaction under stirring, described mixing The concentration of nickel ions in the salt solution is 0.880mol / L, the concentration of zinc ions is 0.072mol / L, the concentration of cobalt ions is 0.048mol / L, the pH value is controlled at 11, the temperature is 55 °C, and the reaction time is 12h. The curing time is 12h, and the green Ni(OH) is obtained 2 Precipitation, pouring out the reaction solution, static layering, pouring off the upper clear night, washing with distilled water, and drying at 120 ° C until the water content of the sample is ≤ 0.5%, that is, the green positive electrode active material Ni(OH) is obtained 2 . The posit...
Embodiment 3
[0088] Positive active material Ni(OH) 2 The preparation adopts the complexation precipitation method, the concentration is 1.5mol / L ammoniacal liquor, the sodium hydroxide solution and nickel sulfate, zinc sulfate, cobalt sulfate mixed salt solution and mixed reaction under stirring with concentration, described mixing The concentration of nickel ions in the salt solution is 0.6285mol / L, the concentration of zinc ions is 0.0645mol / L, the concentration of cobalt ions is 0.0570mol / L, the pH value is controlled at 11, the temperature is 55 °C, the reaction time is 12h, and the The curing time is 12h, and the green Ni(OH) is obtained 2 Precipitation, pouring out the reaction solution, static layering, pouring off the upper clear night, washing with distilled water, and drying at 120 ° C until the water content of the sample is ≤ 0.5%, that is, the green positive electrode active material Ni(OH) is obtained 2 . The positive electrode active material Ni(OH) 2 The zinc content is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com