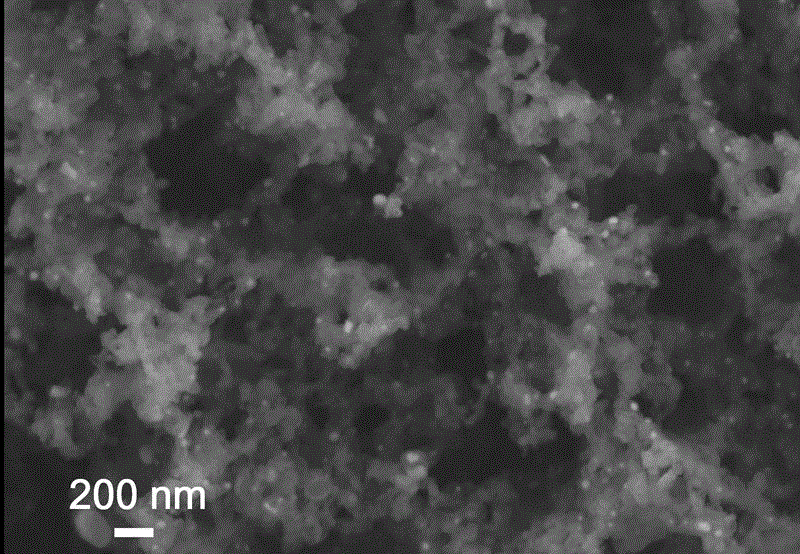
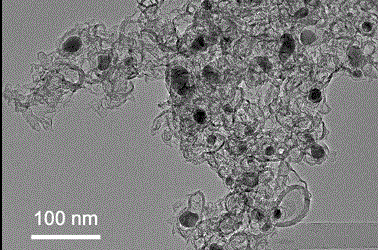
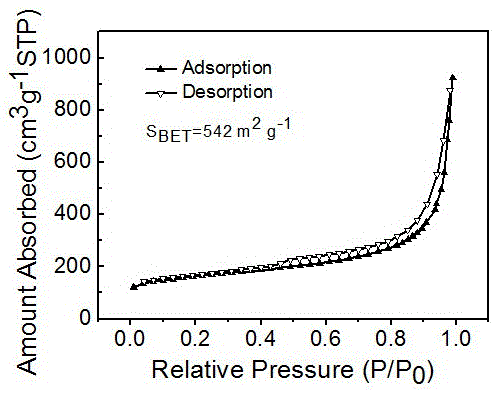
Iron-nitrogen-doped graphene porous material with dual-site catalytic oxygen reduction activity, and preparation method and application therefor
A porous material and graphene technology, applied in the field of nanomaterials, can solve the problems of low specific surface area and limitation, achieve large specific surface area, enrich active sites, and improve electrochemical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Take 10mL of 1mg / mL graphene oxide nanoribbon aqueous solution, add 4vol% of pyrrole, and ultrasonically disperse until a uniform suspension is formed 1.
[0061] (2) The suspension 1 was sealed in a 20mL autoclave, and placed in an oven at 80°C for 12 hours to obtain a graphene oxide nanoribbon / pyrrole composite hydrogel with good mechanical properties.
[0062] (3) Disperse the obtained hydrogel in 25mL of 0.24M ferric chloride solution, stir for 1-12h to complete the polymerization reaction, and then wash the obtained sample with distilled water and absolute ethanol (to remove the by-products of the polymerization reaction and unreacted pyrrole monomer). Then the washed sample was redispersed in 50mL of 0.24M ferric chloride solution, stirred and reacted for 1-12h, and the adsorption of ferric iron was completed. Finally, the wet hydrogel is obtained by filtration.
[0063] (4) Add tert-butanol to the wet hydrogel, pour out the tert-butanol after soaking for 5 ...
Embodiment 2
[0067] (1) Take 10mL of 5mg / mL graphene oxide nanobelt aqueous solution, add 8vol% pyrrole, and disperse ultrasonically until a uniform suspension is formed 2.
[0068] (2) The suspension 2 was sealed in a 20mL autoclave, and reacted in an oven at 100°C for 6h to obtain a graphene oxide nanoribbon / pyrrole composite hydrogel with better mechanical properties.
[0069] (3) Disperse the obtained hydrogel in 50mL of 0.24M ferric nitrate solution, stir the reaction for 1-12h to complete the polymerization reaction, and then wash the obtained sample with distilled water and absolute ethanol (to remove the by-products of the polymerization reaction and unused reacted pyrrole monomer). Subsequently, the washed sample was redispersed in 100mL of 0.24M ferric nitrate solution, stirred and reacted for 1-12h, and the adsorption of ferric iron was completed. Finally, the wet hydrogel is obtained by filtration.
[0070] (4) Add ethanol to the wet hydrogel, pour out the ethanol after soaking...
Embodiment 3
[0074] (1) Take 10mL of 10mg / mL graphene oxide nanobelt aqueous solution, add 12vol% pyrrole, and disperse ultrasonically until a uniform suspension is formed 3.
[0075] (2) The suspension 3 was sealed in a 20mL autoclave, and reacted in an oven at 120°C for 6h to obtain a graphene oxide nanoribbon / pyrrole composite hydrogel with better mechanical properties.
[0076] (3) Disperse the obtained hydrogel in 100mL of 0.24M ferric sulfate solution, stir for 2-10h to complete the polymerization reaction, and then wash the obtained sample with distilled water and absolute ethanol (to remove the by-products of the polymerization reaction and unused reacted pyrrole monomer). Then redisperse the washed sample in 200mL 0.24M ferric sulfate solution, stir and react for 2-10h to complete the adsorption of ferric iron. Finally, the wet hydrogel is obtained by filtration.
[0077] (4) Add tert-butanol to the wet hydrogel, pour out the tert-butanol after soaking for 10 hours, repeat 3 tim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com