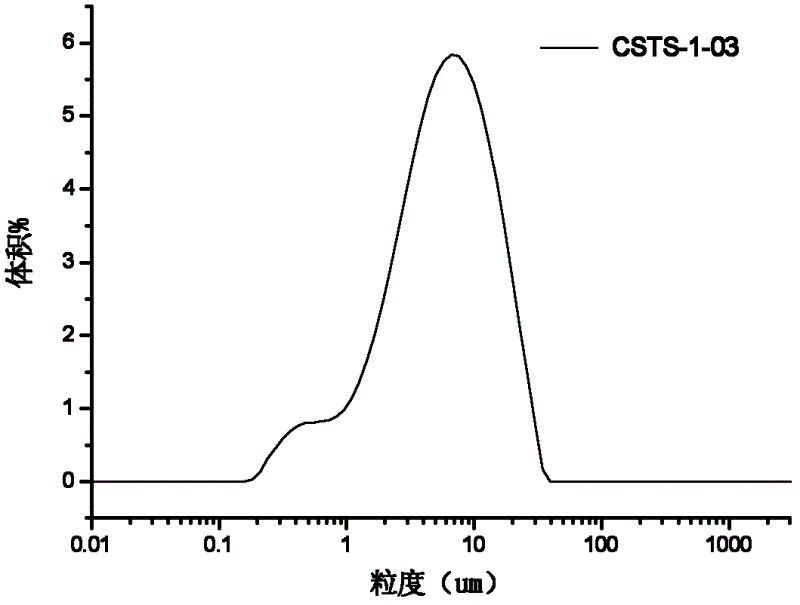
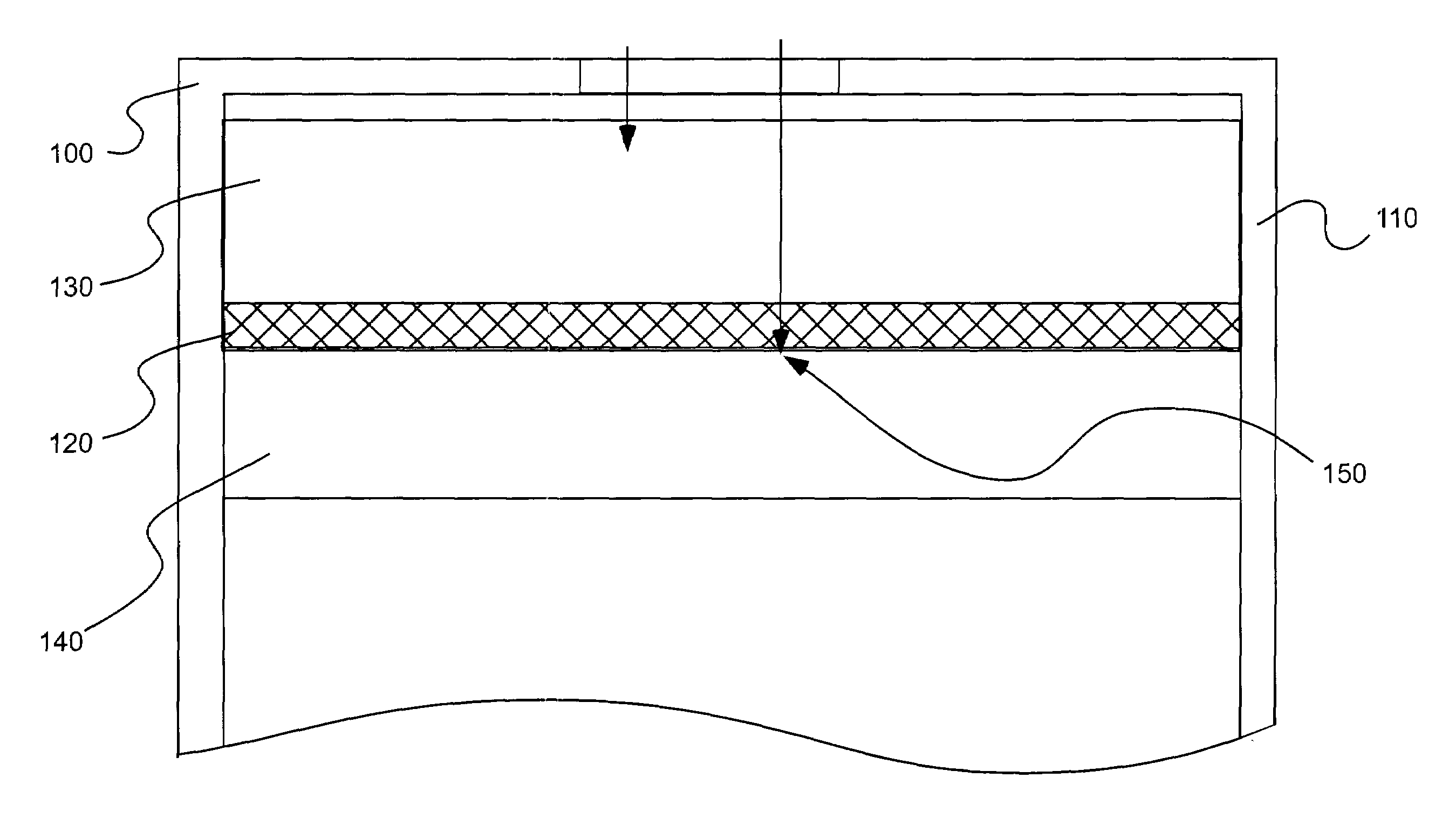
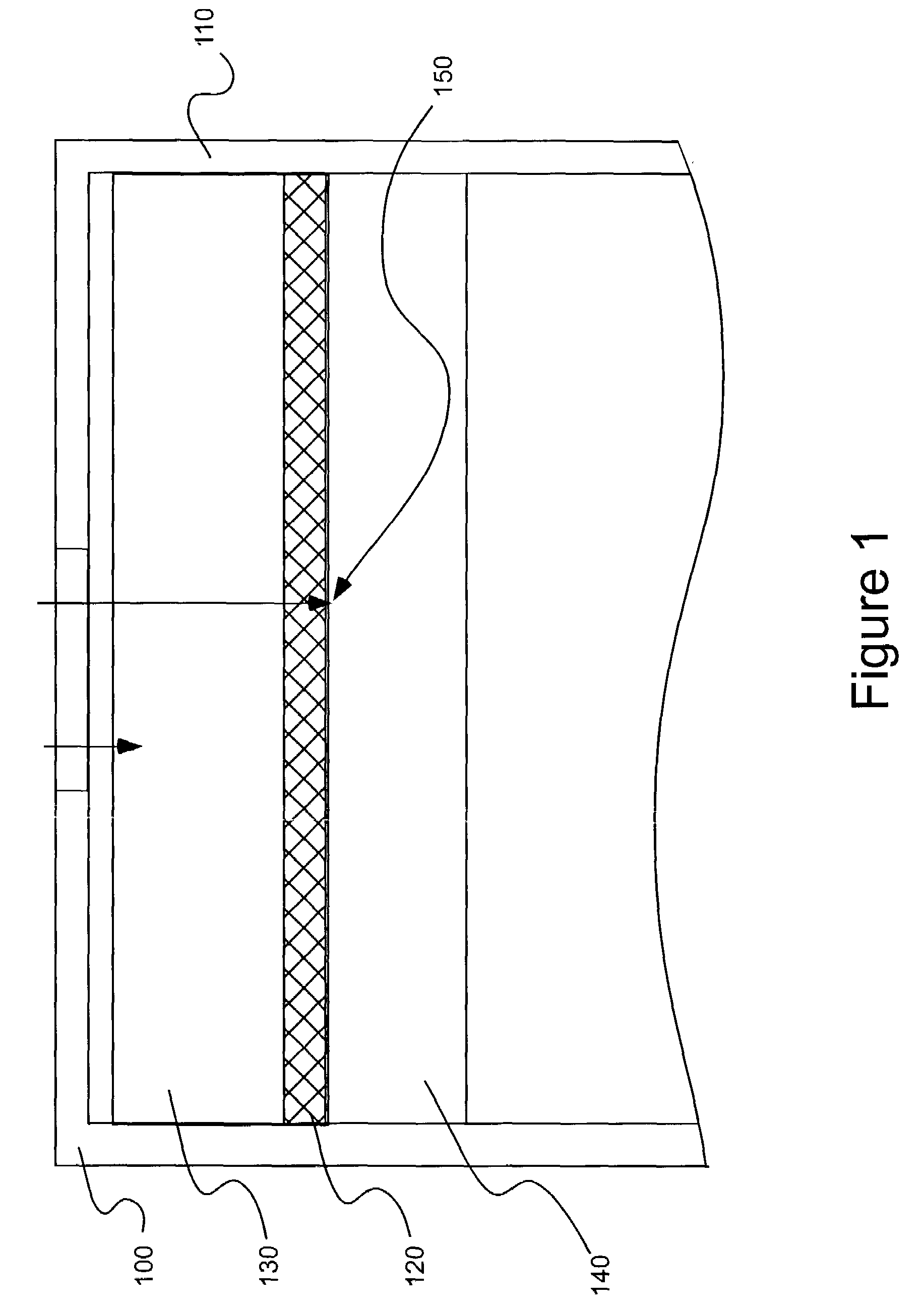
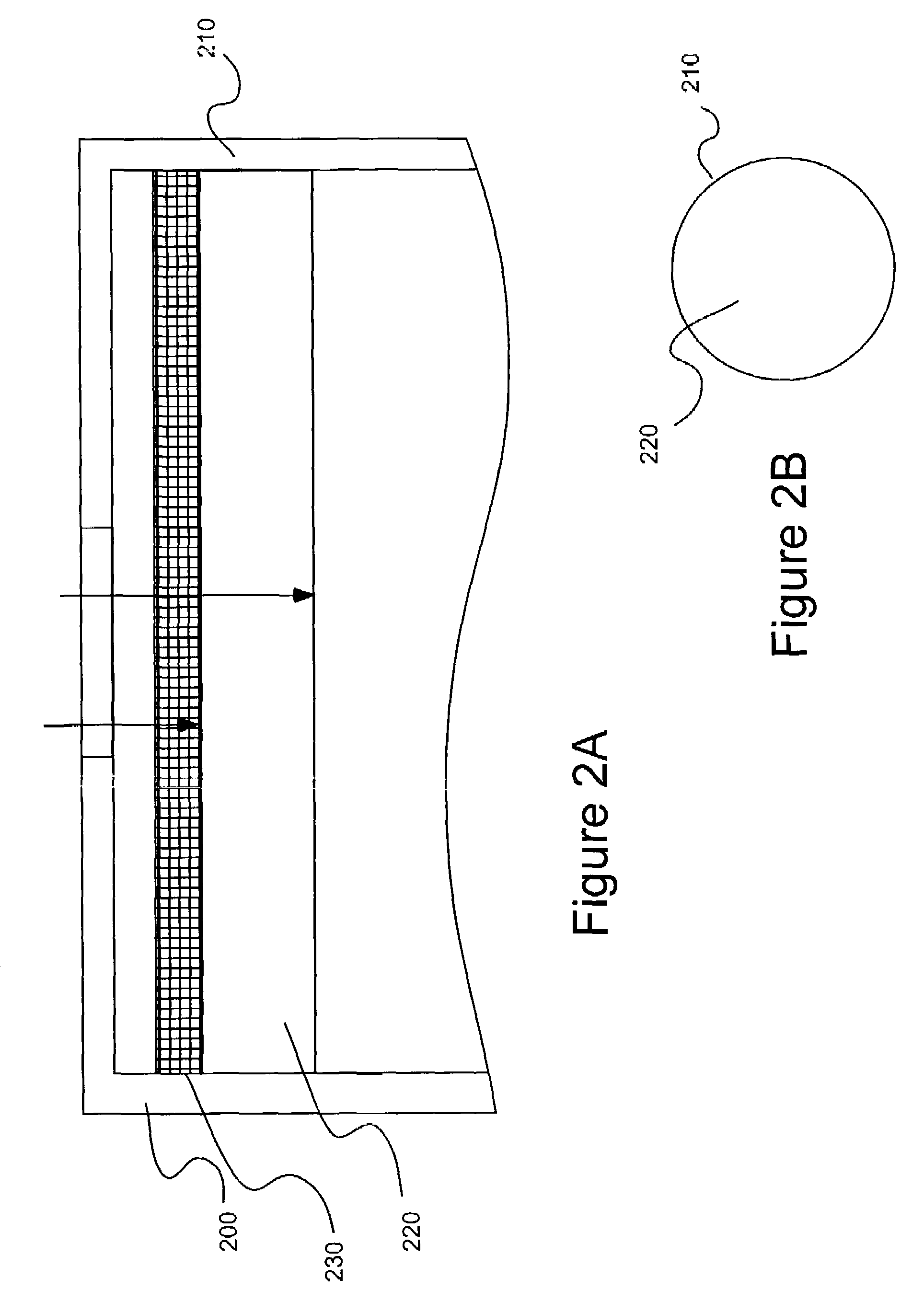
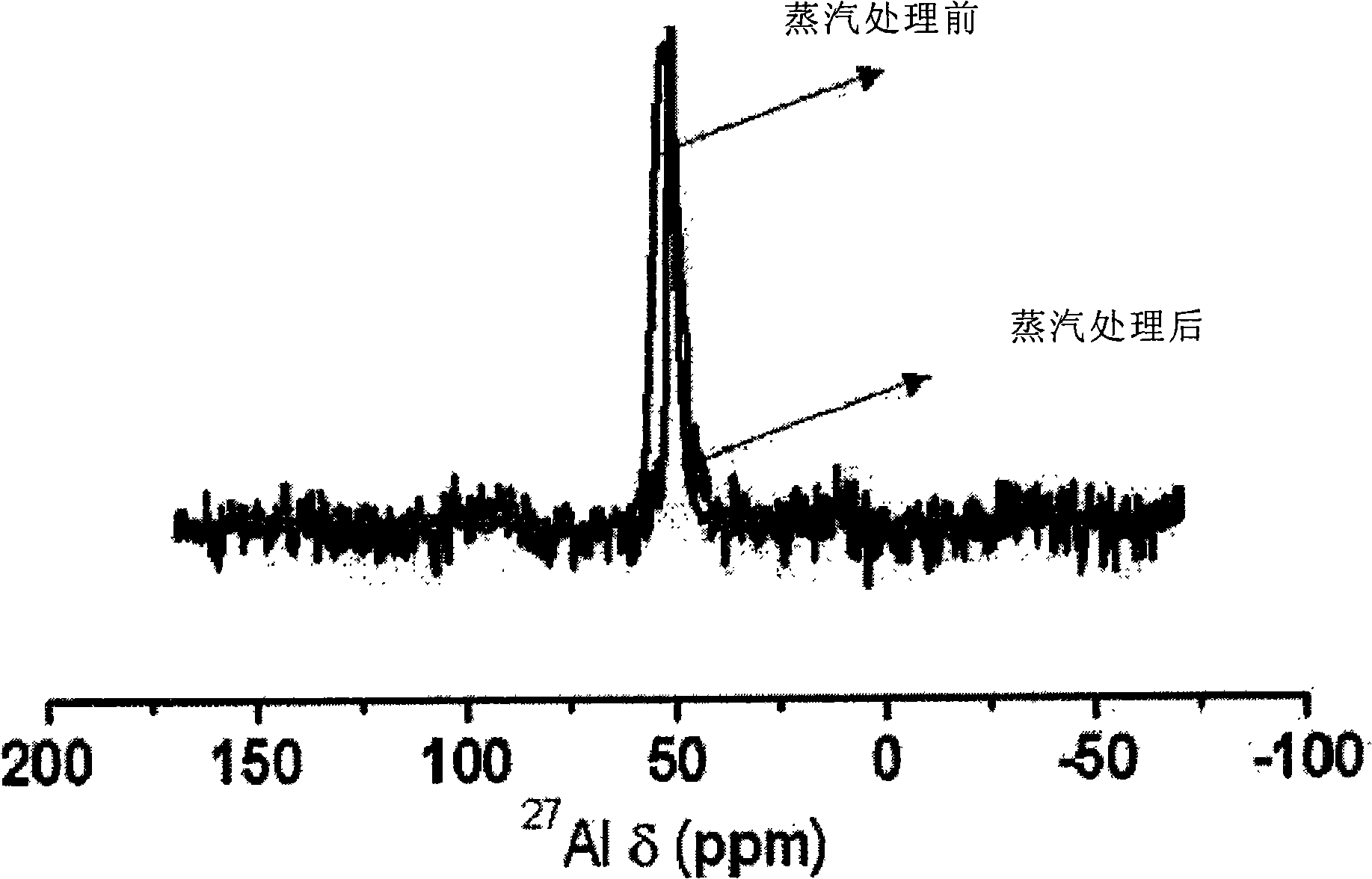
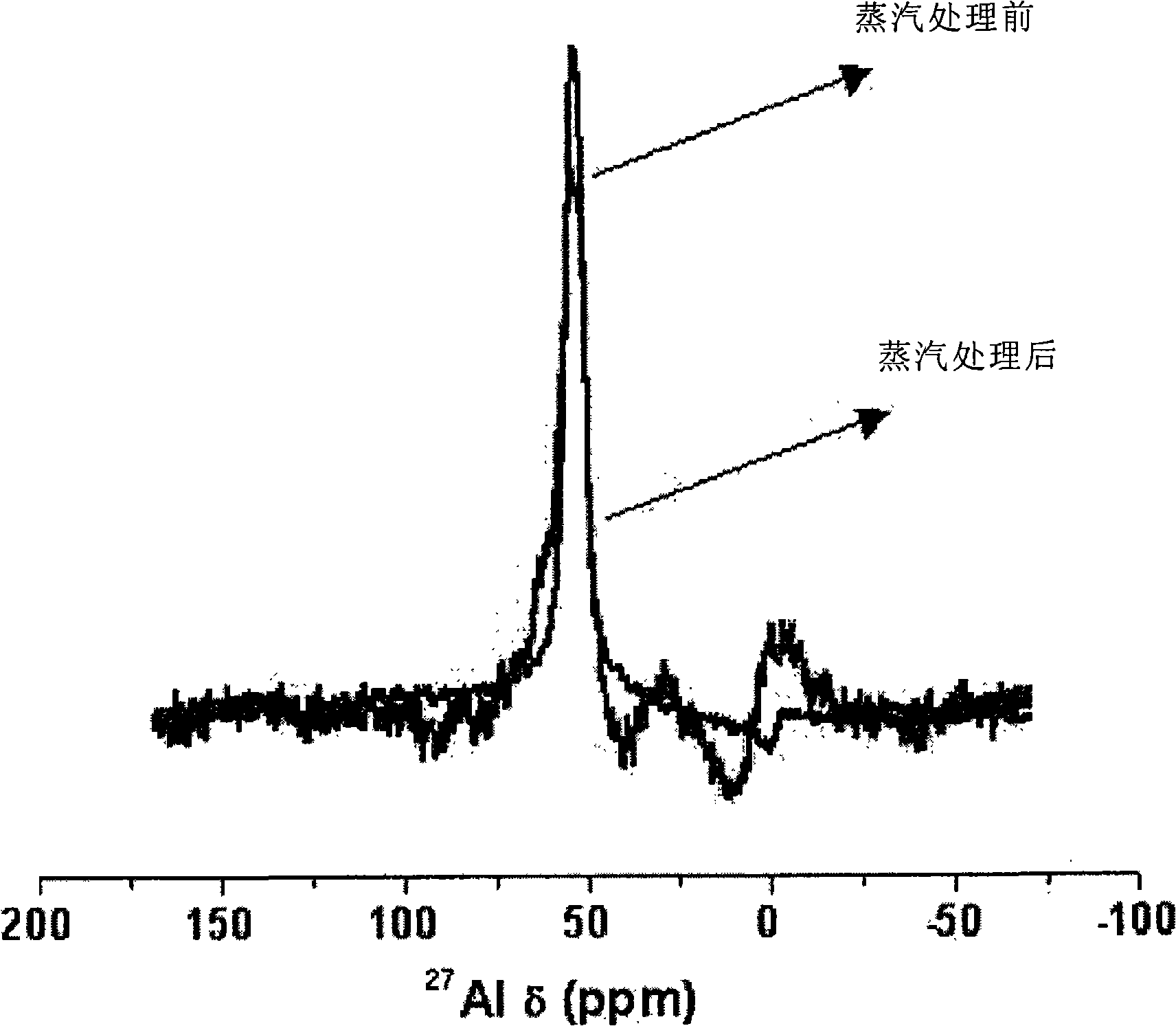
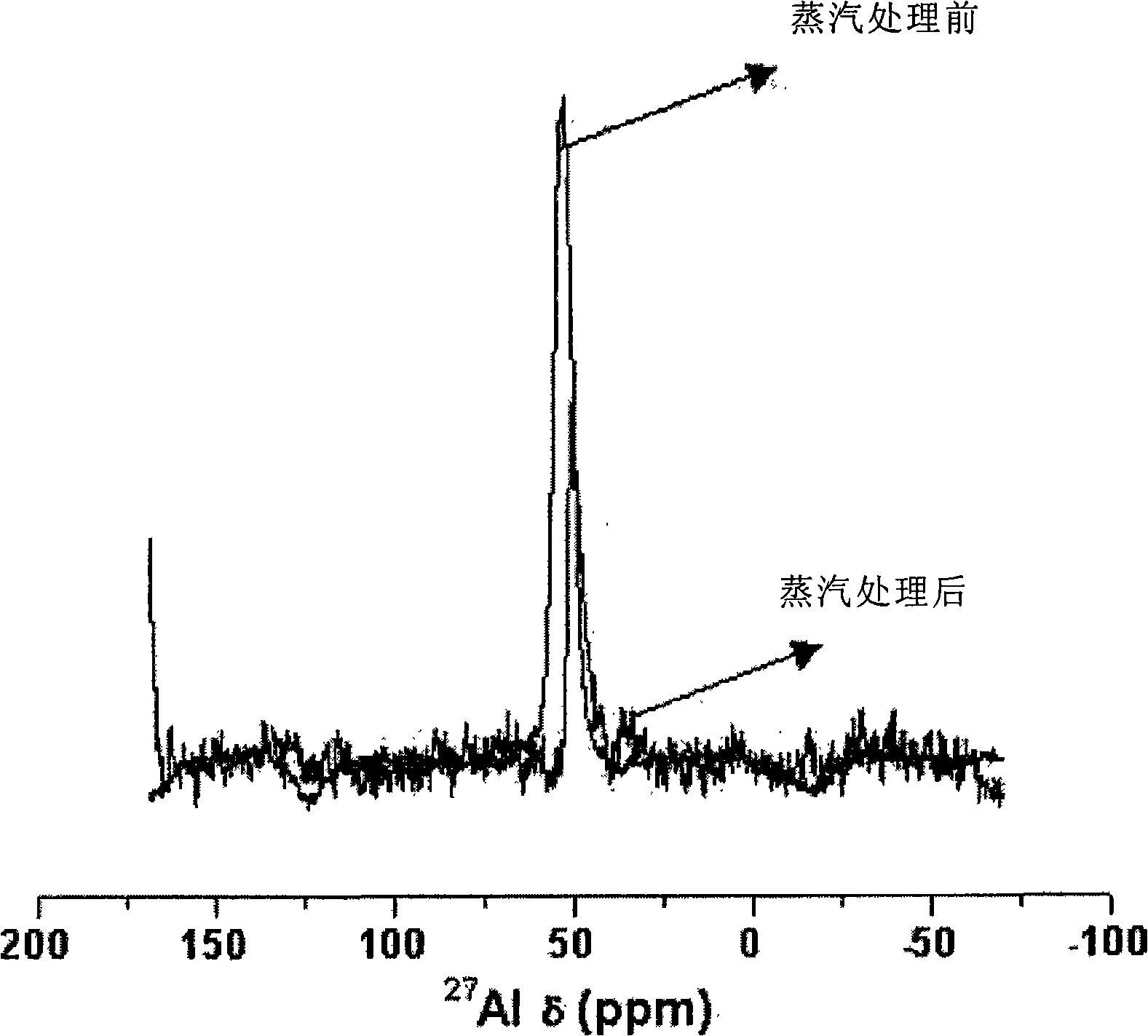
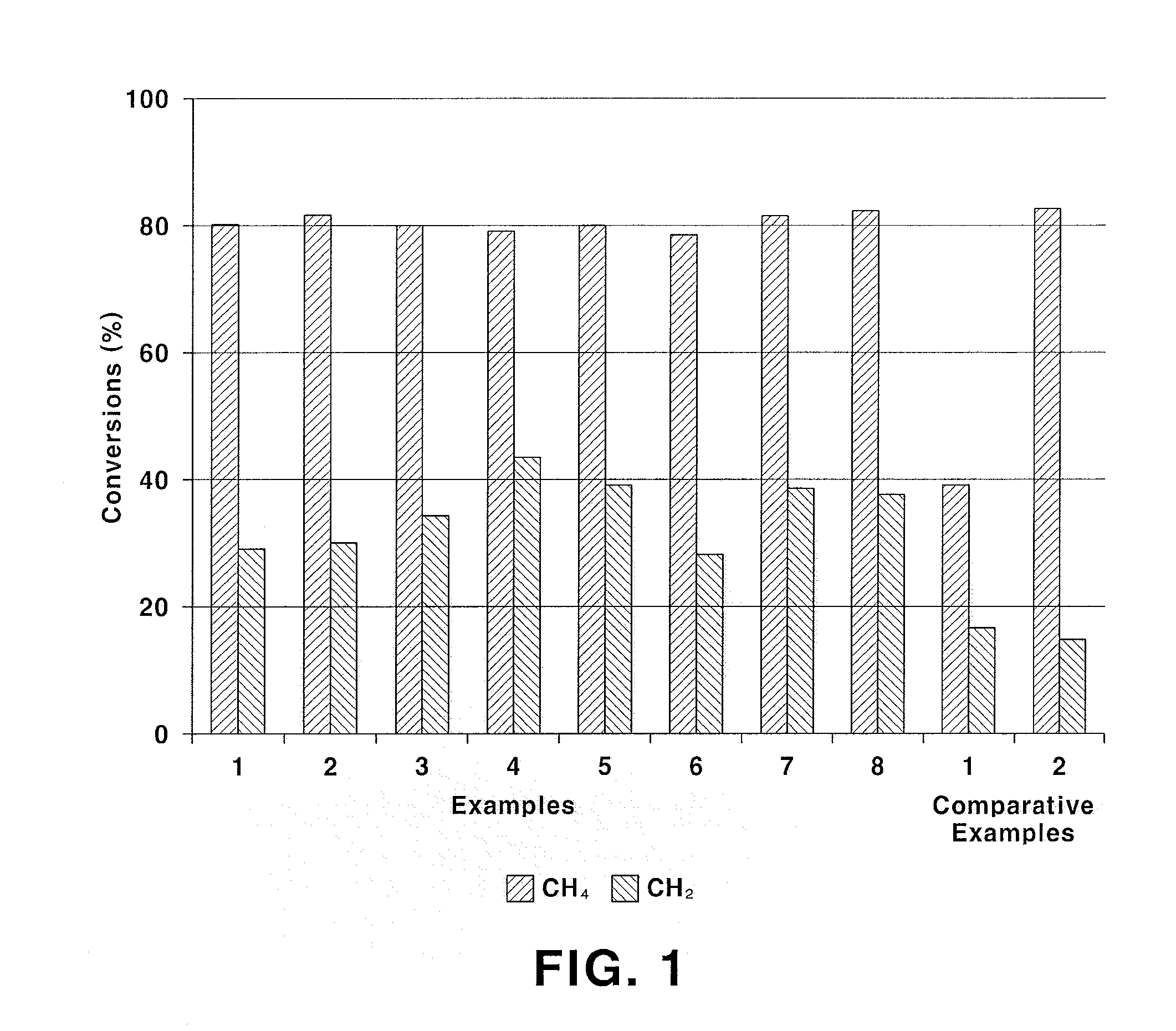
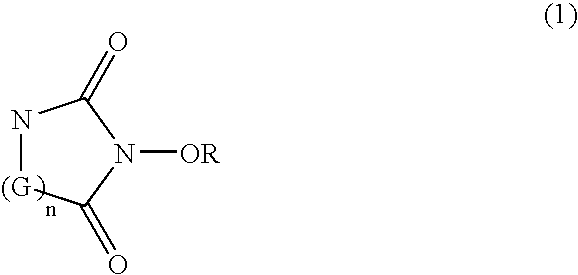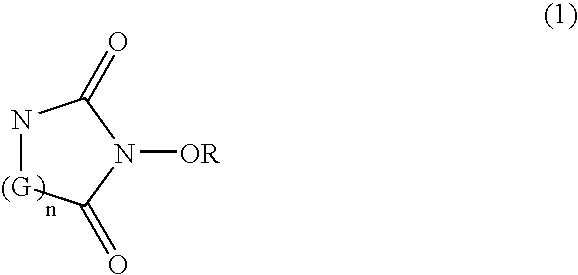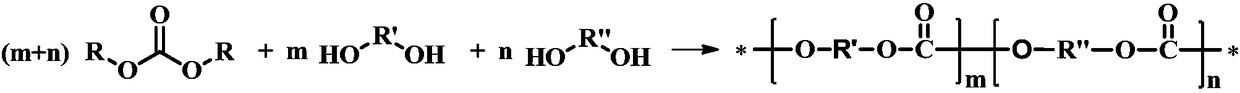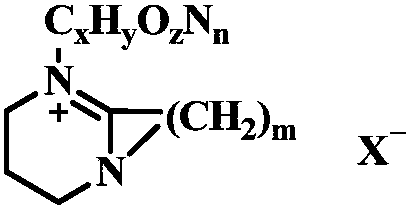Patents
Literature
283results about How to "Maintain catalytic activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
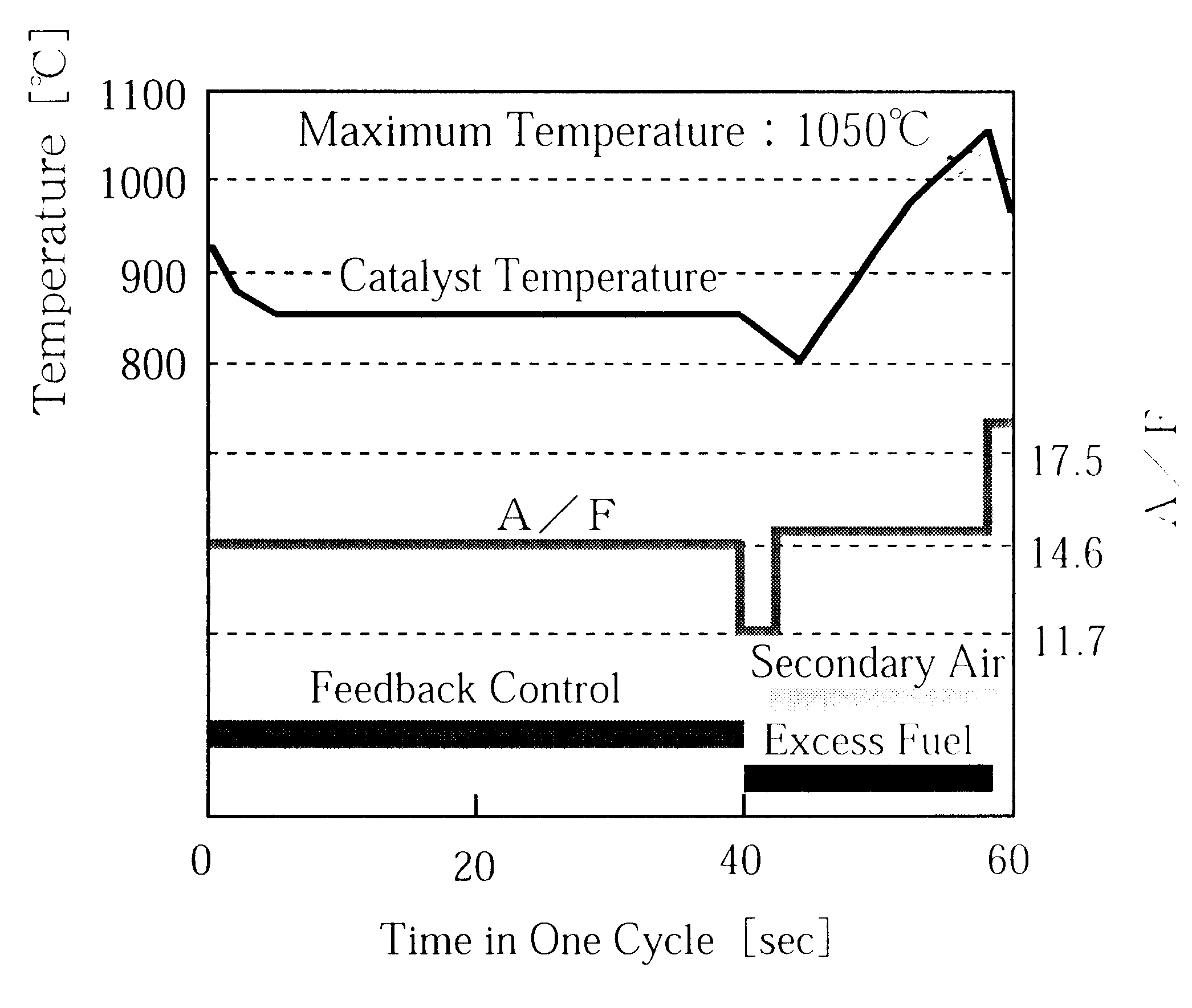
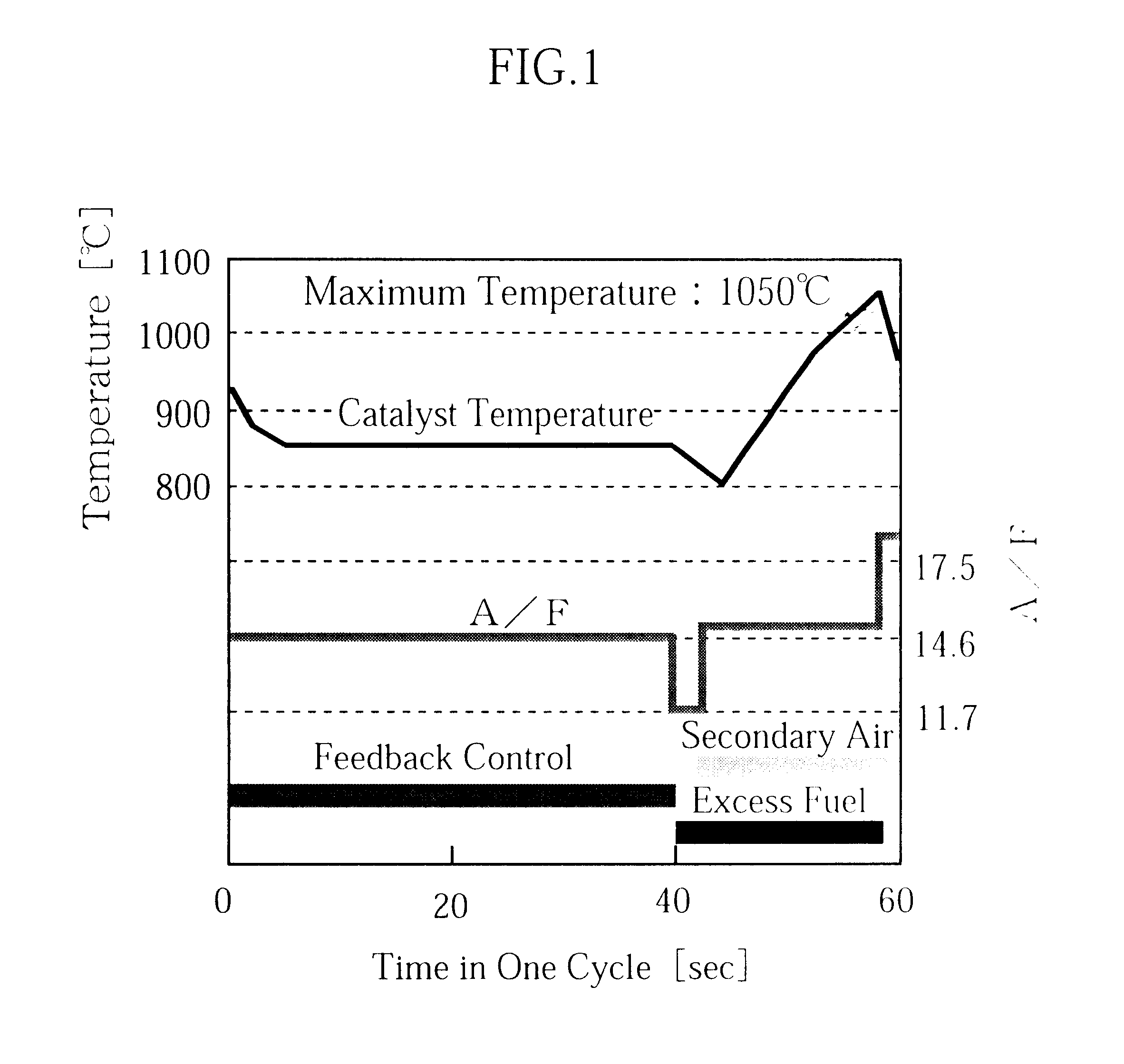
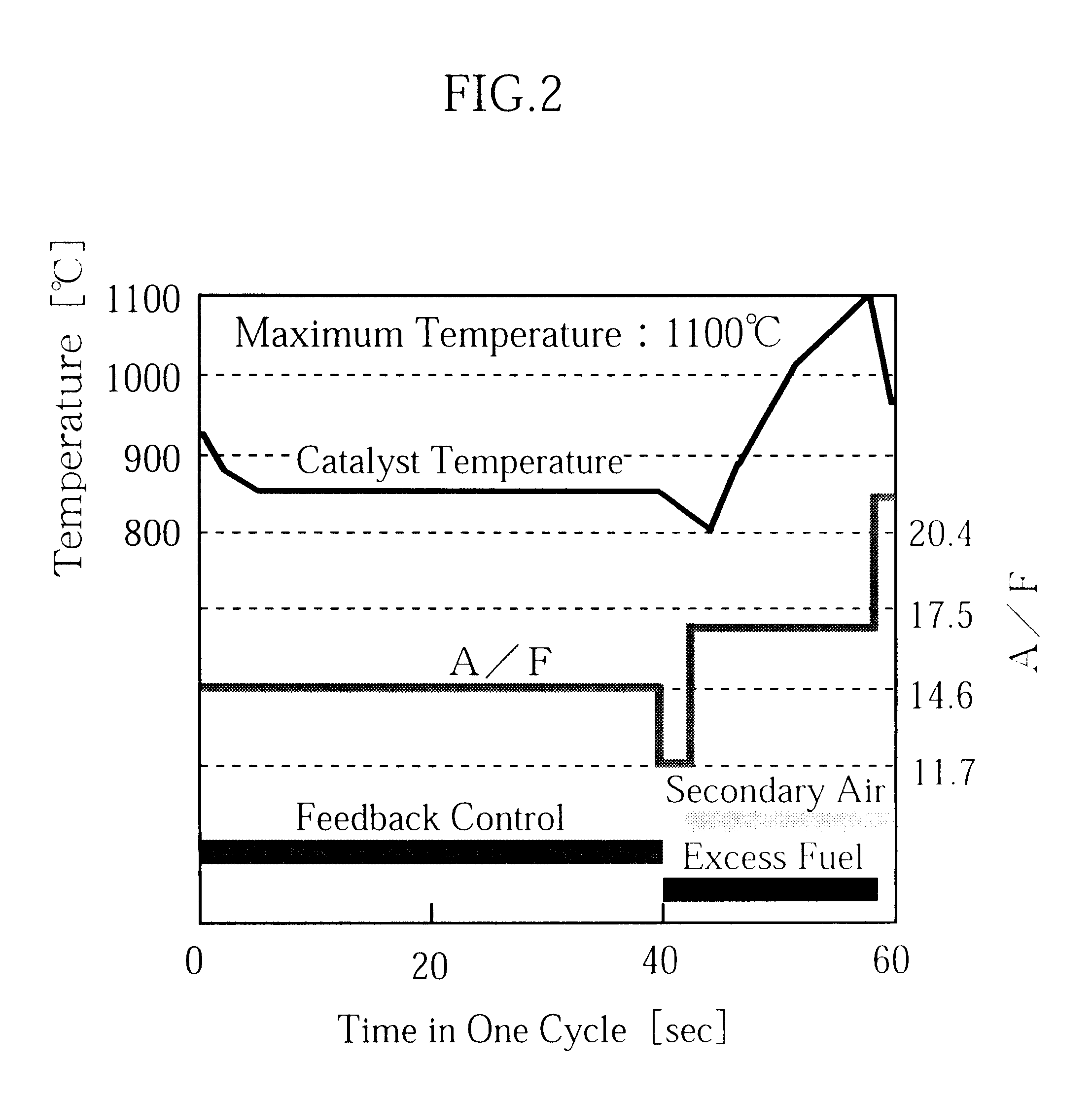
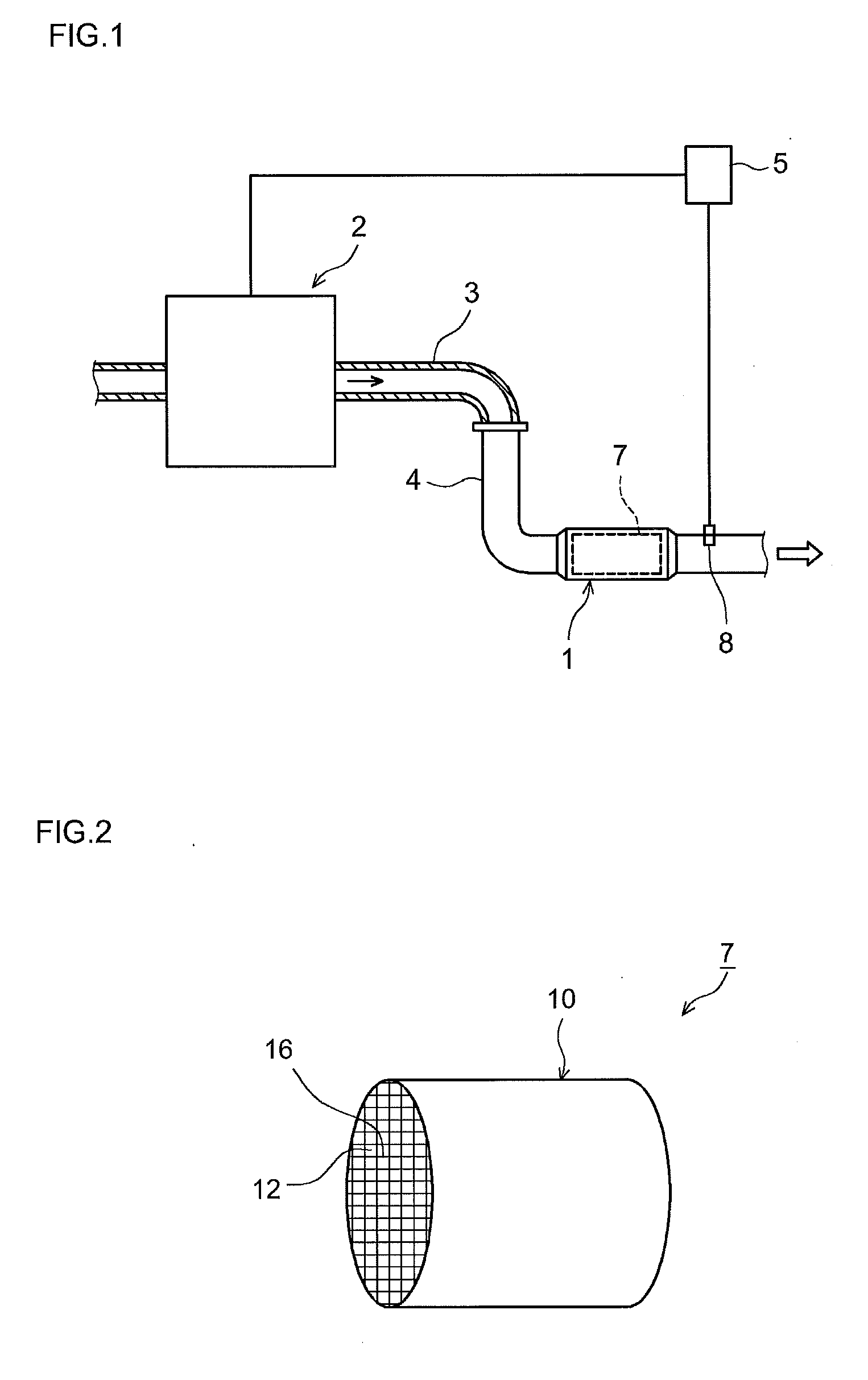
Catalytic converter for cleaning exhaust gas
InactiveUS6261989B1Reduce and preventEasy to cleanNitrogen compoundsInternal combustion piston enginesCeriumCe element
A catalytic converter for cleaning exhaust gas includes a heat-resistant support, and a catalytic coating formed on the heat-resistant support. The catalytic coating contains Pd-carrying particles of a cerium complex oxide, Pt & Rh-carrying particles of zirconium complex oxide, and particles of a heat-resistant inorganic oxide.
Owner:DAIHATSU MOTOR CO LTD +1
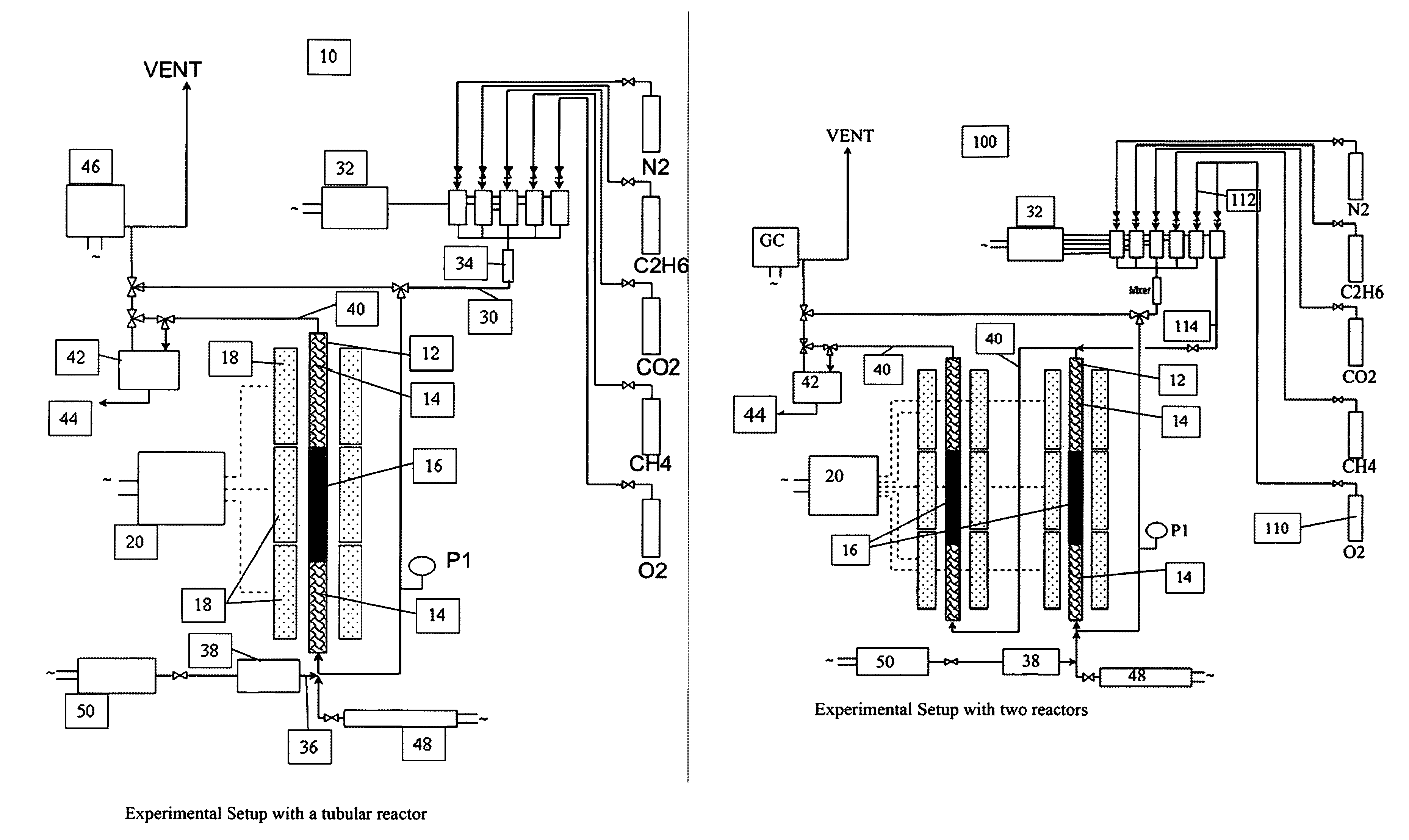
Catalyst and method for converting natural gas to higher carbon compounds
InactiveUS8129305B2Eventual catalyst deactivation is delayed or avoidedMaintain catalytic activityHeterogenous catalyst chemical elementsCatalyst activation/preparationHigh rateHigh carbon
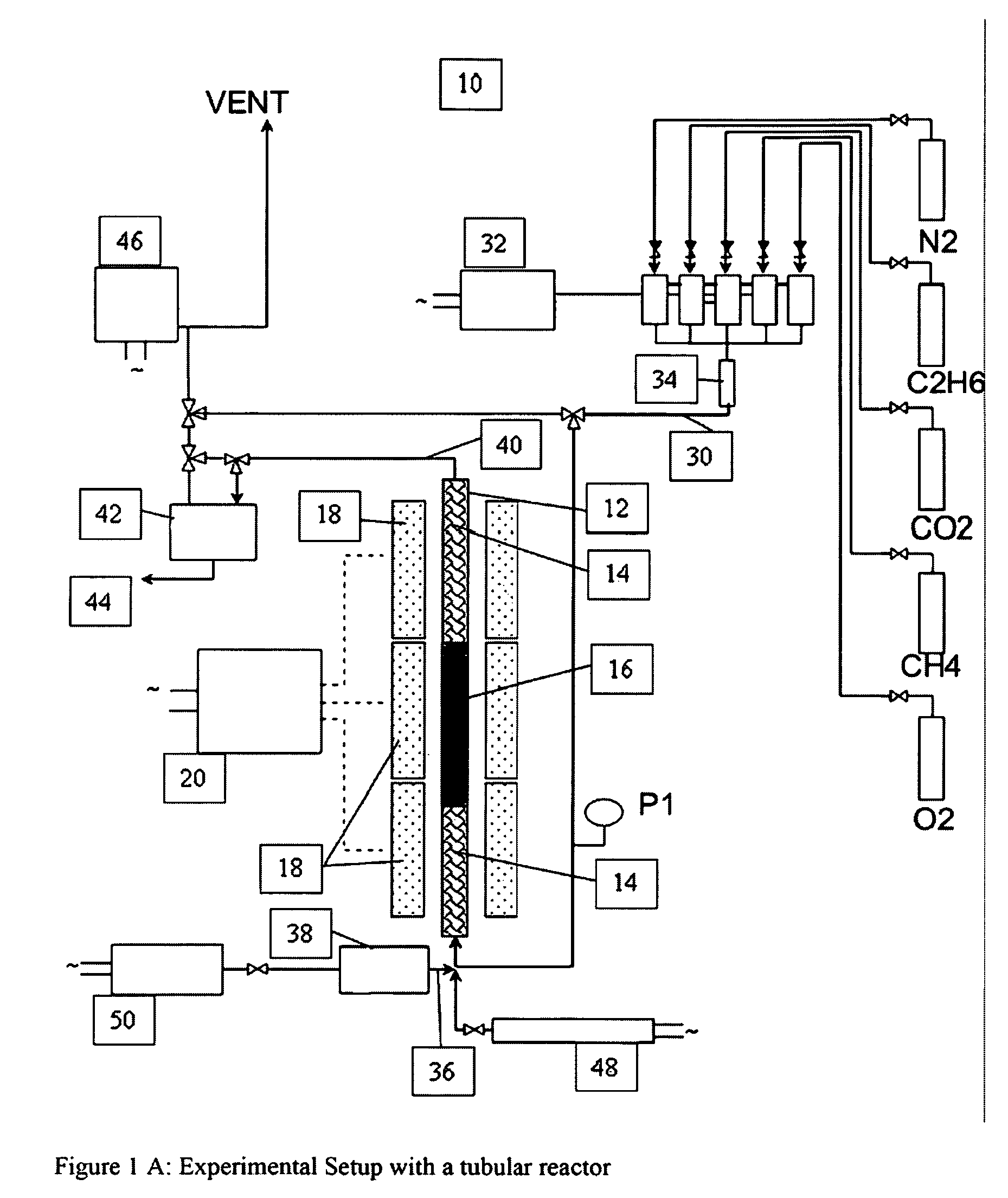
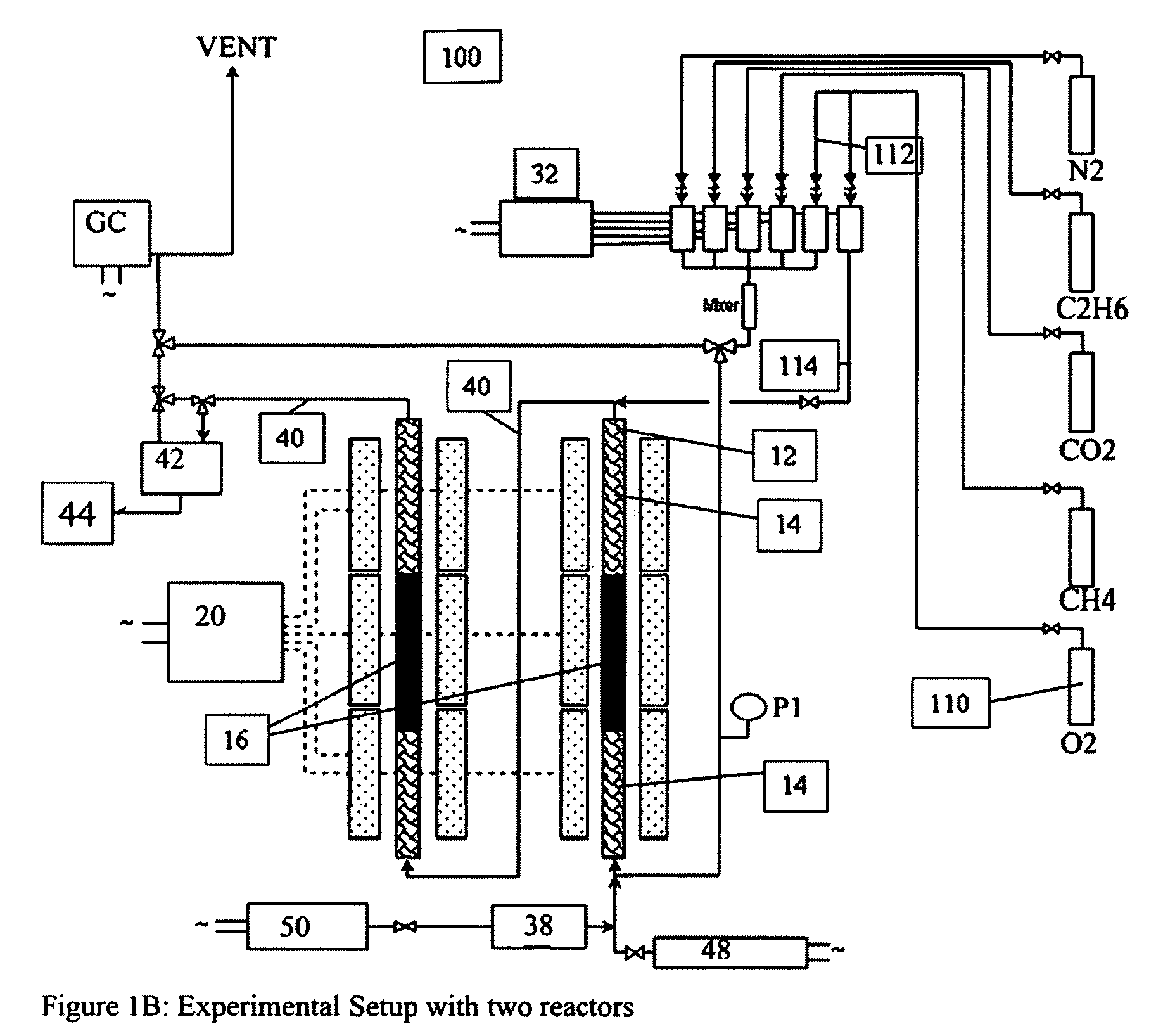
A catalyst composition and process facilitates the oxidative reforming of low molecular weight hydrocarbons, such as methane, to other hydrocarbons having 2 or more carbon atoms (“C2+ compounds”). Compositions having a formula comprising a metal, tungsten, manganese and oxygen effectively catalyze the oxidative reforming of methane with a high rate of conversion and selectivity. Controlling feed gas flow and catalyst bed temperature controls the exothermic OCM reaction, avoiding runaway reactions or coking. A single or multiple reactor system can be utilized for the oxidative reforming reactions. Using two reactors in series, catalyst embodiments produced favorable yields of C2+ compounds, in the presence or absence of a distributed oxygen feed, and with or without interstage effluent cooling. Removal of desirable end products from the reactor effluent, followed by recycling of the residual effluent, increases the conversion to, and ultimate yield of desirable end product.
Owner:HRD CORP
One-step molding method of titanium silicalite molecular sieve
ActiveCN102614911AImprove function and effectImprove adhesionMolecular sieve catalystsCatalyst activation/preparationMolecular sieveCyclohexanone
The invention relates to a one-step molding method of a titanium silicalite molecular sieve. The method comprises the following steps of crystallizing the titanium silicalite molecular sieve subjected to hydro-thermal synthesis; directly adding a matrix substance, a binder, a peptizing agent and a pore-enlarging agent without separating, washing and calcining; pulping; performing spray forming; and calcining the formed microspheres to remove a template agent to obtain large formed titanium silicalite molecular sieve particles which can be applied to ammoximation of cyclohexanone and epoxidation of olefin.
Owner:XIANGTAN UNIV
Electrochemical sensor
ActiveUS7147761B2Increase contactEasy and inexpensive to manufactureMaterial electrochemical variablesElectrodesElectrochemistryHigh surface area
An electrode for use in an electrochemical sensor includes a catalyst dispersed within an electrolyte. Preferably, the catalyst is immobilized within a matrix of the electrolyte. In one embodiment, the electrode of the present invention includes at least one catalyst / electrolyte layer having a mixture of a powdered catalyst, a powdered, quasi-solid electrolyte and a binder material compressed together. The quasi-solid electrolyte can include a liquid electrolyte immobilized by a high-surface-area, high-pore-volume solid.
Owner:MSA TECH
Oxide catalyst for selective reduction of nitrogen oxide, preparation and uses thereof
InactiveCN101314127AHigh hydrothermal stabilityGood hydrothermal stabilityNitrous oxide captureHeterogenous catalyst chemical elementsCeriumCerium oxide
The invention provides a catalyst for selective catalytic reduction of nitrogen oxide, which comprises: a first composition selected from one or a combination of transition metal oxides excluding a second composition, and the second composition selected from one or a combination of cerium oxide, cerium-zirconium compound oxide and cerium-titanium compound oxide. The catalyst can be applied in the form of a granular catalyst, and can also be coated on multiporous integral ceramic to be applied in the form of a honeycomb catalyst. The invention also provides a manufacturing method for the catalyst, which comprises the following steps that: a precursor of the first composition is used to prepare the first composition; the second composition is prepared; and the first composition is loaded onto the second composition. Certain preferable embodiments for the catalyst can ensure that the health of human beings and animals can not be affected when the selective catalytic reduction of nitrogenoxide (NOx) emission is performed.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Microspherical ethylene oxychlorination catalyst preparation method
ActiveCN102049314AMaintain catalytic activityProblems Affecting Catalytic PerformanceCatalyst activation/preparationHalogenated hydrocarbon preparationTime extensionMicrosphere
The invention discloses a microspherical ethylene oxychlorination catalyst preparation method, which comprises the following six steps: solution preparation; precipitation and reaction; peptization; active component and assistant component homogenization; spray forming; and post-treatment. Solution of an active component and solution of an assistant component are dissolved in peptized pulp; a proper amount of dispersant and pore-expanding agent are added into the pulped sol solution to ensure the active component and the assistant component are uniformly distributed in the sol solution; the dispersant can prevent active and assistant component deposition caused by the prolongation of spray time; and the added proper amount of pore expanding agent can expand formed pore passage with a microspherical structure in a spraying process of the sol solution so as to improve the volume of the pore passage of a catalyst carrier and improve the pore volume and specific area of the catalyst.
Owner:BEIJING SJ ENVIRONMENTAL PROTECTION & NEW MATERIAL CO LTD
Method for preparing porous ceramics supported high activity nano titanium dioxide
InactiveCN1511630ASolve the shortcomings of poor firmness and uneven distributionSolve the shortcomings of easy crackingCatalyst activation/preparationWater vaporAdhesive
The present invention relates to the preparation of high activity TiO2 photocatalyst with porous ceramic base, and is especially the nano titania powder and titania sol combining process of preparing regenerable high activity nano titania carried in porous ceramic. The present invention prepares the high activity nano titania carried in porous ceramic through the combination of two carrying processes, titania powder sintering process and sol-gel process. The process includes preparing titania sol via sol-gel process, mixing titania sol and titania powder in certain ratio while stirring and adding dispersant and adhesive to form new solution, carrying the solution to the surface of porous ceramic, fumigating the carrying ceramic in water vapor atmosphere to eliminate most of the organic matters, and final sintering at high temperature for strong combination between nano titania and carrier and raised activity of titania.
Owner:HUABENGUANG CATALYTIC TECH BEIJING
Method for preparing 5-hydroxymethylfurfural with fructose
InactiveCN102153527AHigh yieldMild reaction conditionsOrganic chemistryChemical recyclingLithium chlorideHeteropoly acid
The invention discloses a method for preparing 5-hydroxymethylfurfural with fructose. The method includes the following steps: taking N, N-dimethyl acetamide as solvent, lithium chloride as additive, fructose as a reaction material, and heteropoly acid loaded by titanium dioxide as a catalyst, reacting at the temperature of 80-150DEG C to generate 5-hydroxymethylfurfural. The method has the characteristics of few by-products, high yield, environment-friendly property, moderate reaction conditions, small corrosion to equipment, recyclability of catalyst, and the like, and has industrial prospect.
Owner:海宁市袁花镇工业投资有限公司
Hydroprocessing catalysts with low surface area binders
ActiveUS20090186754A1Efficient processingMaintain catalytic activityMolecular sieve catalystsCatalyst activation/preparationSulfurNitrogen
Catalysts for dewaxing of hydrocarbon feeds, particularly feeds with elevated sulfur and nitrogen levels, are provided. The dewaxing catalysts include a zeolite with a low silica to alumina ratio combined with a low surface binder, or alternatively the formulated catalyst has a high ratio of zeolite surface area to external surface area.
Owner:EXXON RES & ENG CO
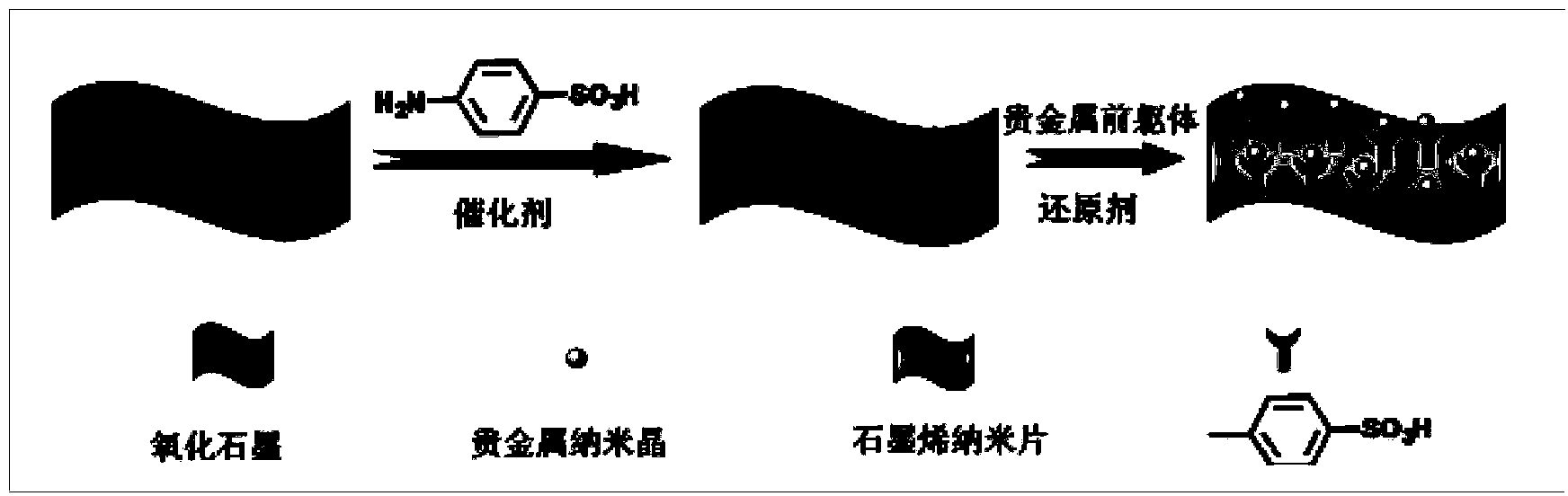
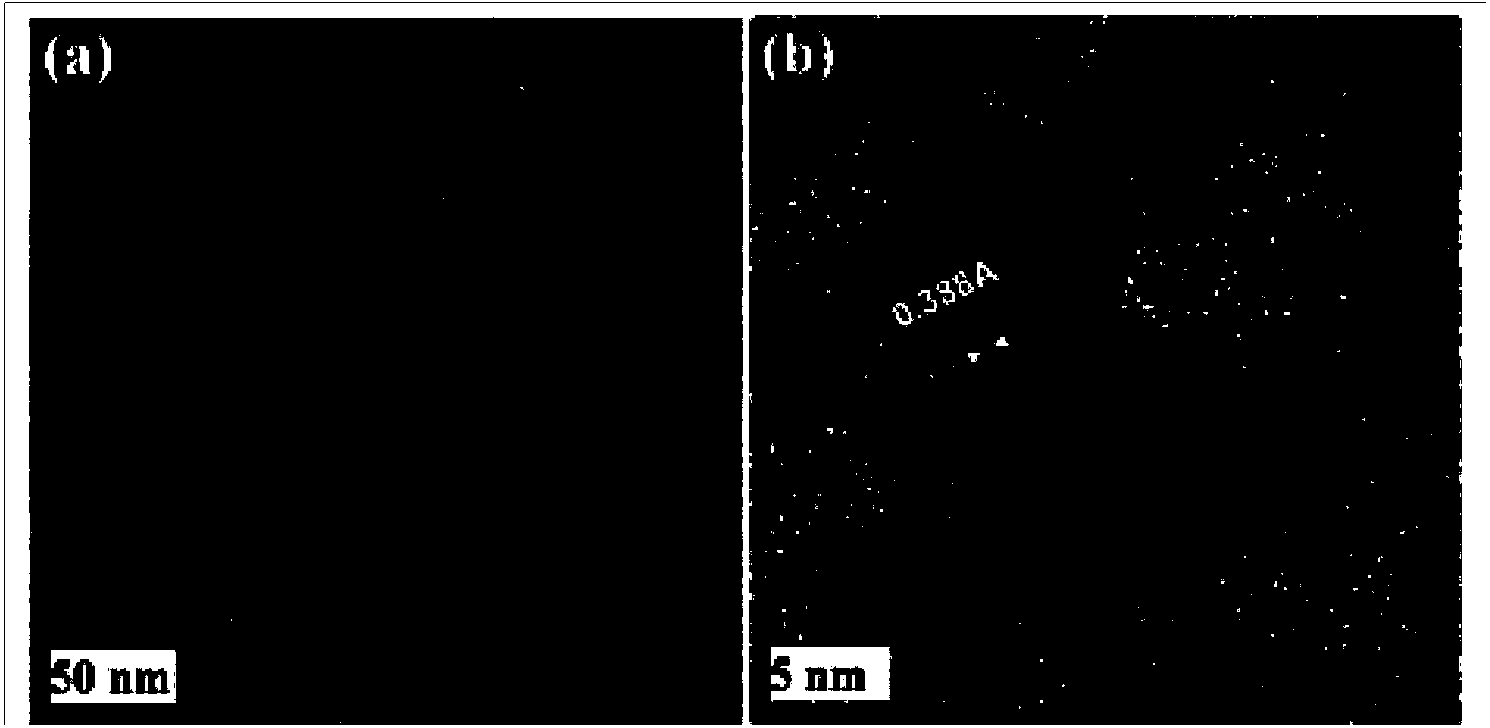
Preparation method for functionalized graphene loaded noble metal nano-crystalline composite catalyst
InactiveCN104043481AExcellent catalytic performanceGood dispersionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCvd grapheneEfficient catalyst
The invention discloses a preparation method for a functionalized graphene loaded noble metal nano-crystalline composite catalyst, which synchronously realizes reduction of a noble metal precursor and graphite oxide to obtain the composite catalyst in a structure that grapheme with surface modified by sulfonic acid is uniformly loaded with noble metal nano-crystalline. The method comprises the following steps: 1, preparing the graphite oxide; 2, carrying out covalent modification on the graphite oxide by benzenesulfonic acid; and 3, synchronously reducing the noble metal precursor and the graphite oxide and stabilizing noble metal nano particles by utilizing a coordination effect between a sulfonic acid group and the noble metal. Meanwhile, the surface sulfonic acid group has the hydrophilcity so that the catalyst can be uniformly dispersed in a water solution. A result shows that the noble metal nano particles with narrow size distribution can be uniformly distributed on the surface of graphene to form the efficient catalyst which has good dispersion in the water solution. Catalytic reduction reaction of p-nitrophenol proves that the catalyst has a very excellent catalysis performance; the catalyst still keeps the previous activity after being recycled and reutilized for five times.
Owner:HENAN AGRICULTURAL UNIVERSITY
High-activity catalyst for synthesizing cyclic carbonate in mild condition
InactiveCN1416952AHigh activityHigh selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDivalent metal ionsHigh activity
The high-activity catalyst for synthesizing cyclic carbonate in mild condition includes main catalyst quadridentate Schiff base metal complex (R1)(R2) SalenMX, where M is metal ion; R1 and R2 is H, Cl-C6 alkyl, alkoxy, C1, Br, NO2 and other group; and X is negative ion; and cocatalyst being inorganic salt and polyether complex M1Yn, where M1 is metal ion or MH4+; Y is Cl, Br, I, OR, NO3, CH3COO, ClO4, BF4 and other one valent negative ion; and n is 1 or 2 with the molar ratio between the cocatalyst and the main catalyst quadridentate Schiff base metal complex being 0.2-5. The two-component catalyst can catalyze the reaction of epoxy compound with CO2 to synthesize cyclic carbonate at mild condition without forming polycarbonate and other side product. The catalyst may be used repeatedly and maintain catalytic activity for long time.
Owner:DALIAN UNIV OF TECH
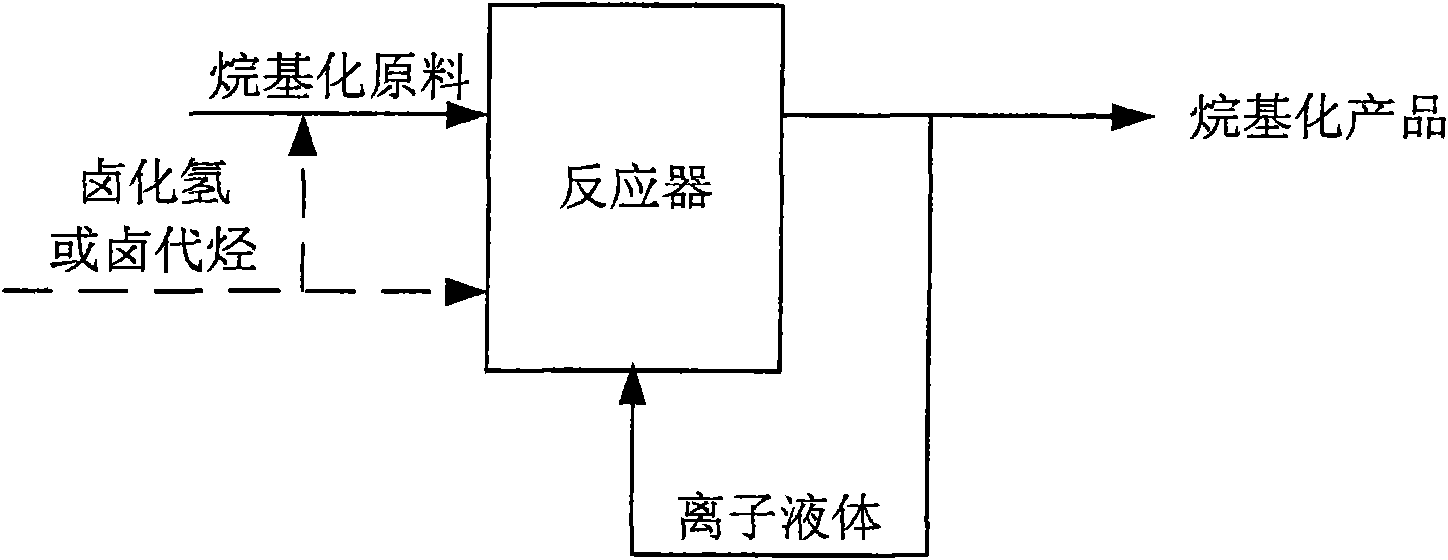
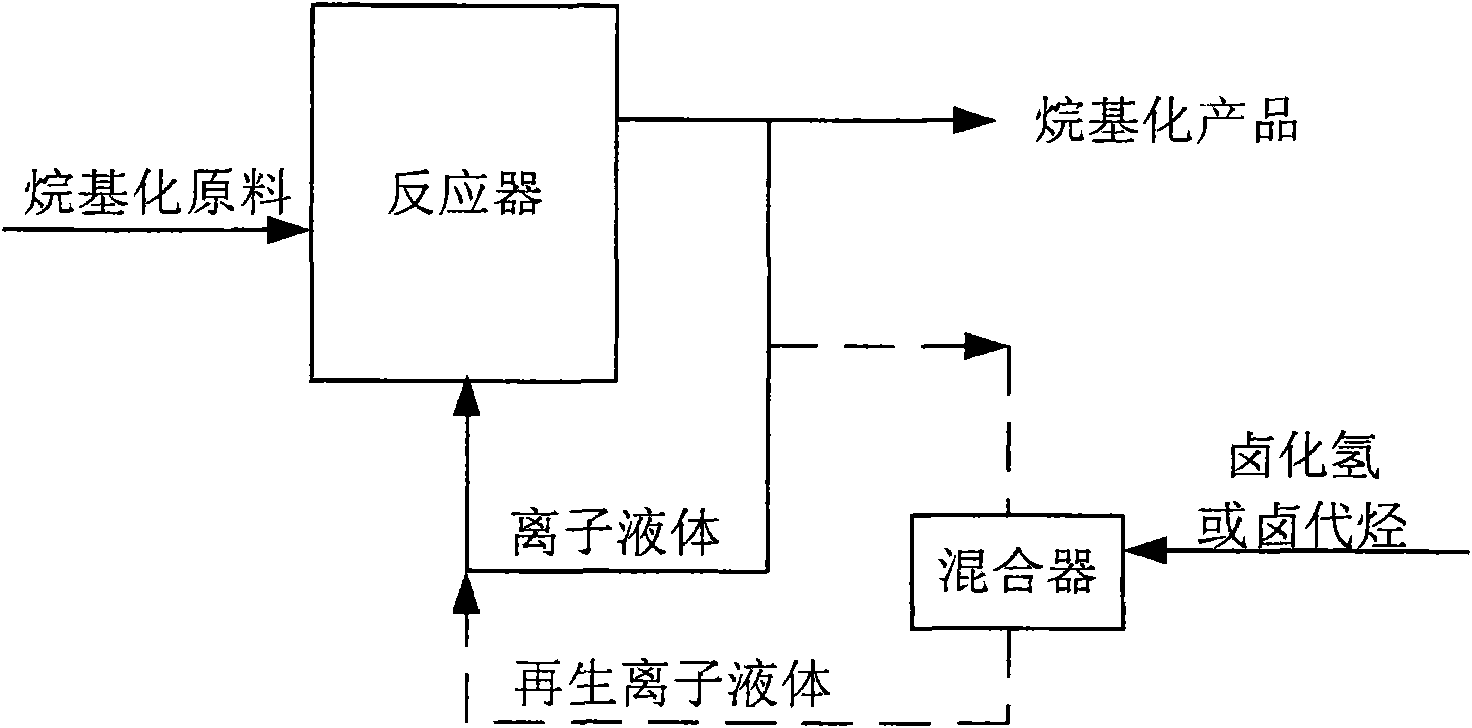
Method for regenerating and maintaining activity of ionic liquid catalyst and method for producing alkylate
ActiveCN101619010AMaintain catalytic activityExtended service lifeOrganic-compounds/hydrides/coordination-complexes catalystsLiquid hydrocarbon mixtures productionHydrogen halideAlkyl transfer
The invention discloses a method for regenerating and maintaining the activity of an ionic liquid catalyst, and the method comprises the following step of adding hydrogen halide or halohydrocarbon in an acidic ionic liquid catalyst or alkylation reaction raw material during the alkylation reaction process, and the ionic liquid catalyst is used for catalyzing alkylation of C4 alkene and alkane. And the invention also provides a method for producing alkylate by using alkylation reaction, and the method comprises the following step of adding the hydrogen halide or the halohydrocarbon in the acidic ionic liquid catalyst or the alkylation reaction raw material during the alkylation reaction process, and the regeneration of the ionic liquid catalyst and the continuous production of the alkylateare realized by adding B acid lost during the alkylation reaction process of the ionic liquid catalyst. The invention can effectively prolong the service life of the acidic ionic liquid catalyst, andthe treatment capacity of the raw materials can reach more than 1000 times of consumption of the ionic liquid; and the method has no influence to the quality of the alkylate, is simple and convenientin operation, and has higher industrial practicability.
Owner:北京中实奥杰石油科技有限公司
Catalyst Composition
InactiveUS20090023580A1High catalytic activityMaintain catalytic activitySemi-permeable membranesGas treatmentHigh rateSolid solution
An object of the present invention is to provide a catalyst composition containing a perovskite-type composite oxide which exhibits a satisfactory catalytic performance over a long time even in a high temperature atmosphere and has a stable quality in which Rh and / or Pt dissolves to form a solid solution at a high rate.To achieve the object described above, in the present invention the catalyst composition is prepared to comprise an Rh-containing perovskite-type composite oxide represented by the following general formula (I) and / or a Pt-containing perovskite-type composite oxide represented by the following general formula (II) and a thermostable oxide optionally containing a noble metal.A1xA2wB11−(y+z)B2yRhzO3±δ (I)A3rA4sB31−(t+u)B4tPtuO3±δ′ (II)
Owner:DAIHATSU MOTOR CO LTD +1
Preparation method of catalyst for continuous production of succinic anhydride from hydrogenation of maleic anhydride
ActiveCN101502802BHigh yieldHigh activityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsFixed bedHigh activity
Owner:SHANXI UNIV
Graphite-phase carbon nitride sheet material and preparation method thereof
ActiveCN105126895ALarge specific surface areaImprove visible light catalytic performancePhysical/chemical process catalystsNitrogen and non-metal compoundsTube furnaceProduct gas
A preparation method of a graphite-phase carbon nitride sheet material comprises steps as follows: graphite-phase carbon nitride powder is provided, and the graphite-phase carbon nitride powder is flatly spread on a heated carrier; the heated carrier containing the graphite-phase carbon nitride powder is placed in a tubular furnace, the tubular furnace is heated to 150 DEG C-600 DEG C under the atmosphere of protective gas, the heated carrier containing the graphite-phase carbon nitride powder is kept at the temperature for 1 min-20 h in the ammonia gas atmosphere under negative pressure and finally cooled to the room temperature, and the graphite-phase carbon nitride sheet material is obtained. The invention further provides the graphite-phase carbon nitride sheet material prepared with the method.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Catalyst comprising cyclic acylurea compounds and processes for production organic compounds with the same
InactiveUS20050020439A1High selectivityHigh yieldPreparation by oxidation reactionsCarboxylic acid nitrile preparationSimple Organic CompoundsHydrogen atom
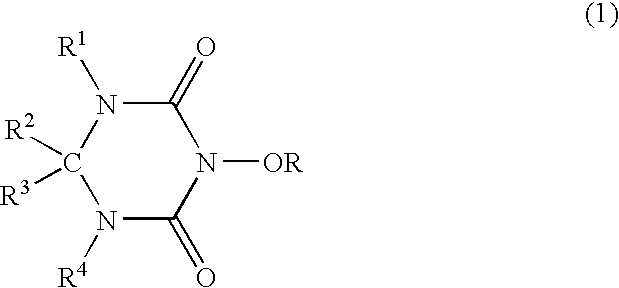
A catalyst of the invention includes a cyclic acylurea compound having a cyclic acylurea skeleton represented by following Formula (I): wherein R is a hydrogen atom or a hydroxyl-protecting group; n is 1 or 2; G is a carbon atom or a nitrogen atom, where two Gs are the same or different when n is 2. The catalyst may include the cyclic acylurea compound and a metallic compound in combination. In the presence of the catalyst, (A) a compound capable of forming a radical is allowed to react with (B) a radical scavenging compound and thereby yields an addition or substitution reaction product of the compound (A) and the compound (B) or a derivative thereof. This catalyst can produce an organic compound with a high selectivity in a high yield as a result of, for example, an addition or substitution reaction under mild conditions.
Owner:DAICEL CHEM IND LTD
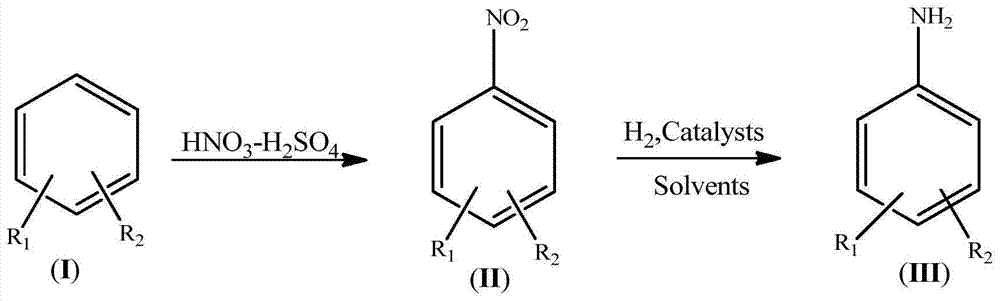
Synthetic process for amino aromatic hydrocarbon compound
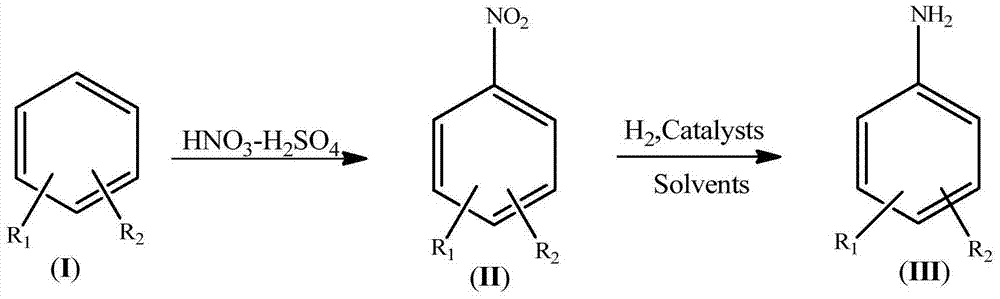
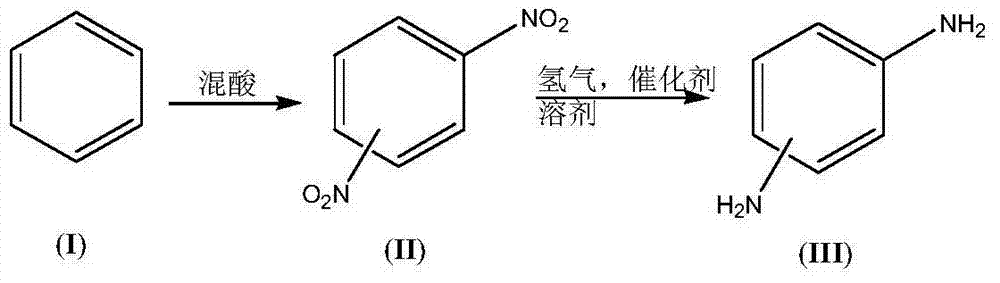
InactiveCN104844461AImprove securityImprove concentrationOrganic compound preparationCarboxylic acid amides preparationEnvironmental resistanceHydrogen
The invention discloses a synthetic process for an amino aromatic hydrocarbon compound. The process comprises the following steps: pre-preparing reaction solutions -1 and -2 by using an aromatic hydrocarbon compound (I), concentrated sulphuric acid and concentrated nitric acid, respectively pumping the solutions in a micro reactor, heating to react, cooling the reaction liquid, and pos-treating to obtain a nitryl aromatic hydrocarbon compound (II); mixing the nitryl aromatic hydrocarbon compound (II), a catalyst and a solvent as a reaction solution -3; respectively pumping the reaction solution -3 and hydrogen into the micro reactor, heating to react, cooling the reaction liquid and post-treating to obtain the amino aromatic hydrocarbon compound (III). The process disclosed by the invention is less in use level of nitric acid, convenient to concentrate and apply waste acid; in the nitrification reaction process, the heat release is little, and the nitration product nitryl aromatic hydrocarbon compound (II) is quickly transferred. The reaction phenomenon is stable without intense heat release and even material punching of a common kettle nitration reaction, so that the safety of nitration reaction is improved; the process is easy to realize industrialization, simple to operate and high in industrial safety degree and meets the environmental requirements.
Owner:ANHUI SHENGYUAN CHEM
Catalyst comprising bimetallic platinum group metal nanoparticles
InactiveUS20190240643A1Improve thermal stabilityMaintain catalytic activityHeterogenous catalyst chemical elementsDispersed particle separationOxidePalladium
The present disclosure provides a three-way conversion (TWC) catalyst composition, and a catalyst article comprising such a catalyst composition suitable for at least partial conversion of gaseous hydrocarbons (HCs), carbon monoxide (CO), and nitrogen oxides (NOx). Generally, the catalyst article includes a catalyst substrate having a plurality of channels adapted for gas flow, each channel having a wall surface and a catalytic coating on the surfaces or inside the pores of the wall. The catalytic coating generally includes a first washcoat with a platinum group metal (PGM) component and a first refractory metal oxide support and a second washcoat having a plurality of palladium-rhodium nanoparticles and a second refractory metal oxide support.
Owner:BASF CORP
Warp knitting jacquard weaving technology based screen cloth manufacturing method
ActiveCN104846536ALow costAvoid influenceWarp knittingArtificial filament heat treatmentPolyesterPolymer science
The invention relates to a warp knitting jacquard weaving technology based screen cloth manufacturing method. The warp knitting jacquard weaving technology base screen cloth manufacturing method is characterized by comprising the specific steps of 1 preparing grey-white ultraviolet-resistant particles, 2 preparing ultraviolet-resistant polyester master batches, 3 preparing ultraviolet-resistant polyester monofilaments, 4 manufacturing warp knitting jacquard screen cloth by adopting an RSJ4 / 2 type jacquard warp knitting machine, performing jacquard layer deign, organization and drawing-in and then performing on-machine warp knitting to obtain warp knitting jacquard screen cloth. The warp knitting jacquard screen cloth manufactured by means of the warp knitting jacquard weaving technology based screen cloth manufacturing method has excellent ultraviolet-resistant, antibacterial and deodorization functions, is applied to the field of shoe materials, mattresses, curtains and the like and has wide prospect.
Owner:FUJIAN JINJIANG HUAYU WEAVING
PbO2 electrode with long service life and high catalytic activity
ActiveCN108217852AExtend your lifeReduce catalytic activityElectrolytic inorganic material coatingWater/sewage treatment using germicide/oligodynamic-processAdhesion forceElectricity
A PbO2 electrode with long service life and high catalytic activity is prepared with SnO2-Sb2O3 as a base layer, alpha-PbO2 as a medium layer, and beta-PbO2 as a surface active layer. The PbO2 in theinvention is compact and uniform and has small particles and large specific surface area; meanwhile, the surface active layer has excellent adhesion force and is not liable to fall off; the surface ofthe PbO2 electrode is smooth and firm and is anti-acid / alkali-corrosion, has high catalytic activity and long service life. In addition, a preparation method is simple in conditions and low in cost;the product has stable performance and is suitable for industrial production, can be widely applied to the field of sewage treatment with electrically catalytic oxidization, and has extensive market prospect.
Owner:CHONGQING UNIV
Method for treating chrome-containing wastewater by catalytic reduction of petaloid magnetic iron oxide/molybdenum sulfide composite
InactiveCN104828902ALow costHigh Photocatalytic Removal EfficiencyPhysical/chemical process catalystsWater/sewage treatment by irradiationFerrous sulfate heptahydrateIon
The invention relates to a method for treating chrome-containing wastewater by catalytic reduction of a petaloid magnetic iron oxide / molybdenum sulfide composite. The method comprises the following steps: weighing ferrous sulfate heptahydrate and sodium chlorate, dissolving in deionized water, stirring until the solution becomes light yellow, reacting in a high-pressure reaction kettle, carrying out centrifugal separation to obtain ferric oxide, drying, and pulverizing into powder; weighing the ferric oxide powder, molybdenum trisulphate dihydrate and thiocarbamide, dissolving in deionized water, stirring uniformly, transferring the mixture into a high-pressure reaction kettle, reacting, and carrying out centrifugal separation; cleaning with ethanol and deionized water; and drying to obtain the ferric oxide / molybdenum sulfide composite finished product, adding the finished product into hexavalent-chrome-containing wastewater with the initial concentration of 5-20 mg / L, stirring uniformly, irradiating with a metal halide lamp for more than 30 minutes, determining the hexavalent chrome concentration after treatment, and calculating the removal rate. The ferric oxide / molybdenum sulfide composite for treating hexavalent-chrome wastewater has the advantages of high removal rate and low treatment cost.
Owner:YANGZHOU UNIV
Air electrode of zinc-air battery
InactiveCN102324527AEasy to operateEven load distributionCell electrodesPtru catalystActivated carbon filtration
The invention relates to an air electrode of a zinc-air battery; the air electrode is molded by compression of a catalysis layer, a diffusion layer and a current collector; a catalyst in the catalysis layer is a TiO2 / AC catalyst. The TiO2 / AC catalyst is prepared by the following method: processing active carbon by dilute nitric acid with a volume concentration of 10%, filtering, washing with distilled water for several times, drying, grinding, sieving, performing a hydrolysis reaction with a titanium tetrachloride solution with a mass ratio of 2-7.6 times of the active carbon at 0 DEG C-5 DEG C, calcining the reaction product at 400 DEG C-800 DEG C, cooling to room temperature, and grinding. Compared with the prior art, the catalyst used by the air electrode of the invention has a simple preparation method, and low price; and with the catalyst, the current density of the air electrode is well increased.
Owner:SHANGHAI YAOYU INDAL
Hydrothermally stable microporous molecular sieve catalyst and preparation method thereof
ActiveCN101282784AStable structureMaintain catalytic activityMolecular sieve catalystsHydrocarbon oils refiningIsomerizationPtru catalyst
Disclosed are a hydrothermally stable porous molecular sieve catalyst and a preparation method thereof. The catalyst consists of a product obtained by the evaporation of water from a raw material mixture comprising a molecular sieve having a framework of Si-OH-Al-, a water- insoluble metal salt and a phosphate compound. The catalyst maintains its physical and chemical stabilities even in an atmosphere of high temperature and humidity. Accordingly, the catalyst shows excellent catalytic activity even when it is used in a severe process environment of high temperature and humidity in heterogeneous catalytic reactions, such as various oxidation / reduction reactions, including catalytic cracking reactions, isomerization reactions, alkylation reactions and esterification reactions.
Owner:SK INNOVATION CO LTD +1
Iron-modified ni-based perovskite-type catalyst, preparing method thereof, and producing method of synthesis gas from combined steam co2 reforming of methane using the same
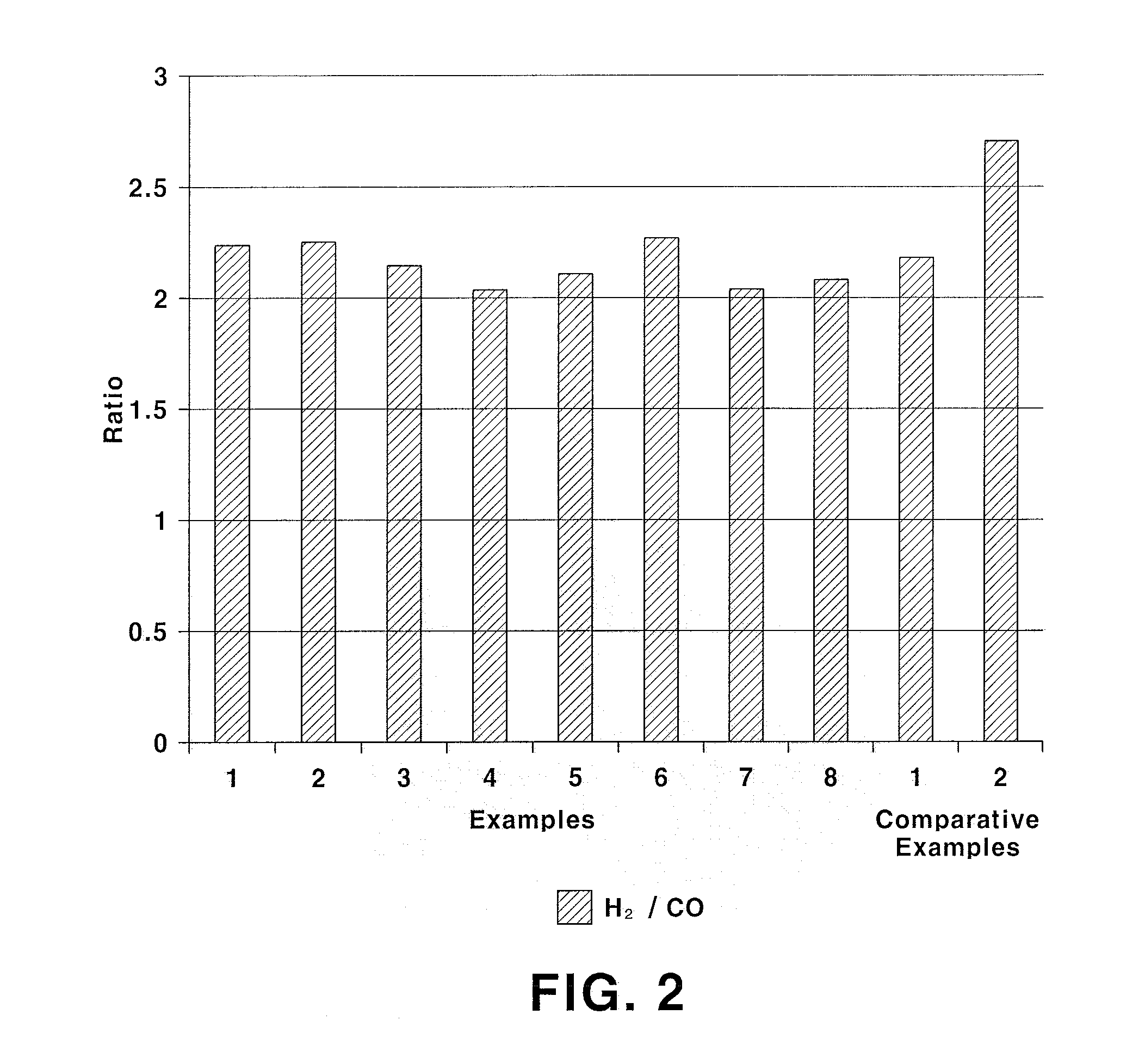
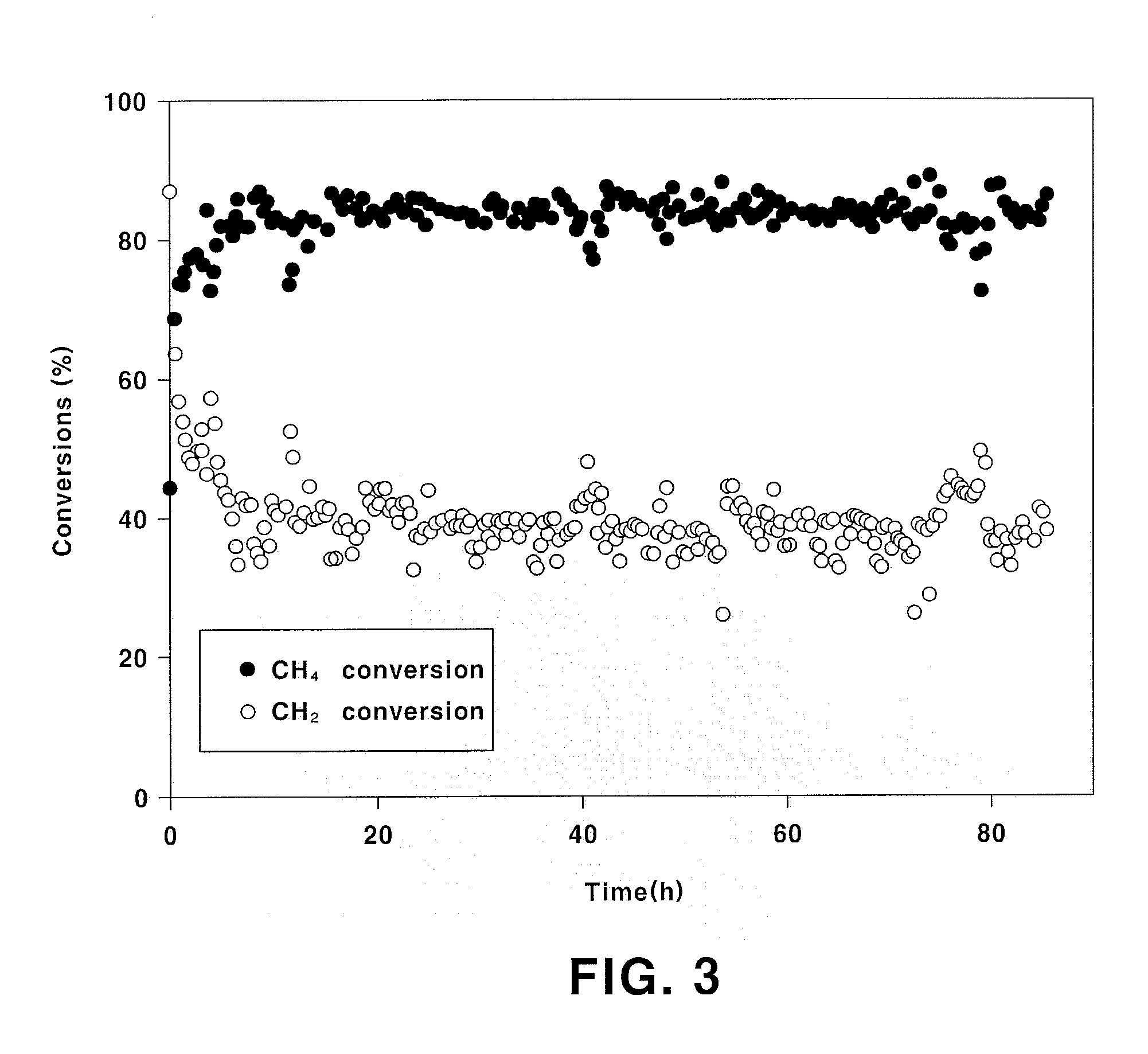
InactiveUS20140145116A1Improve conversion rateResistant to deactivationHeat treatmentsHydrogen/synthetic gas productionHigh carbon dioxideCarbon deposition
The present invention relates to an Fe-modified perovskite-type catalyst, a method for preparing same and a method for preparing a synthesis gas by a combined reforming reaction using same. More particularly, it relates to a catalyst for a combined natural gas / steam / carbon dioxide reforming reaction having a perovskite structure with La and Sr introduced at the A site and Ni and Fe introduced at the B site with specific molar ratios and a method for producing a synthesis gas for Fischer-Tropsch synthesis or methanol synthesis using the catalyst by the combined reforming reaction. The catalyst of the present invention exhibits higher carbon dioxide conversion rate, significantly reduced catalyst deactivation caused by carbon deposition and improved long-term catalyst stability and activity, as compared to the existing catalyst for reforming reaction prepared by the impregnation method.
Owner:KOREA INST OF SCI & TECH
Synthesis process of dimido dipheny compound
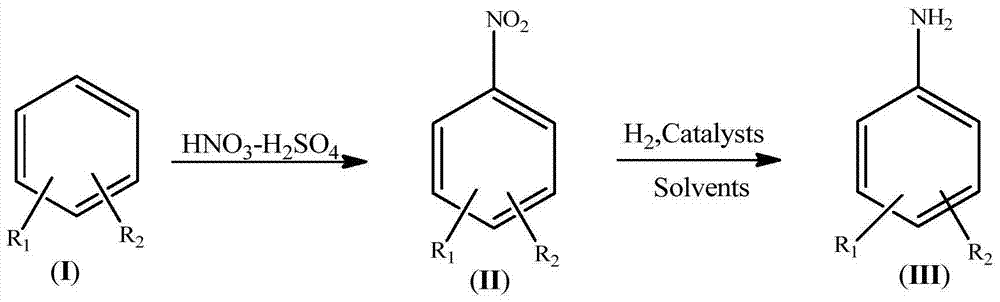
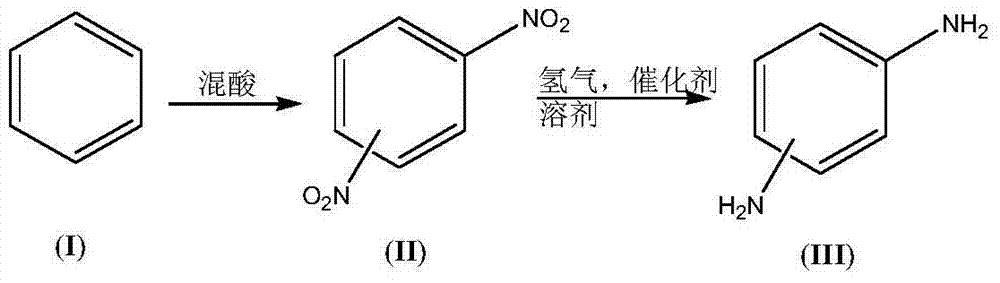
InactiveCN104844462AIncrease dosageReduce dosageOrganic compound preparationAmino compound preparationBenzeneP-Phenylenediamine
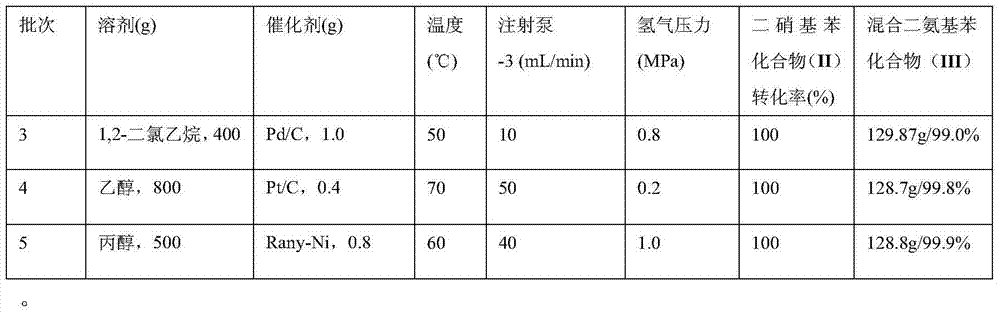
The invention discloses a synthesis process of a dimido dipheny compound. The synthesis process disclosed by the invention comprises the following steps: by taking benzene(I) as a raw material, carrying out continuous nitration on a micro reactor platform to obtain a mixed nitrobenzene compound (II); then obtaining a mixed diaminobenzene compound (III) by carrying out catalytic hydrogenation reduction under a mild condition with temperature of 50~70 DEG C and hydrogen gas pressure not higher than 1.0MPa; and purifying to obtain o-phenylenediamine, p-phenylenediamine and m-phenylenediamine. The synthesis process disclosed by the invention has the benefits that: the use level of nitric acid is low, so that the waste acid is easy to concentrate and apply mechanically; the heat release is less, the product dinitrobenzene compound (II) is quickly transferred, the reaction phenomenon is stable without intense heat release and even punch of a common autoclave nitration reaction, so that the safety of nitration reaction is improved; the rate in nitrification reaction is fast, and the energy consumption is reduced; the reaction phenomenon is stable, so that the safety of catalytic hydrogenation reduction reaction is improved; the synthesis process is a new technique which is high in safety, easy to industrialize and less in environment protection.
Owner:ANHUI SHENGYUAN CHEM
Method for preparing polycarbonate catalyzed by ionic liquid
The invention relates to a method for preparing polycarbonate catalyzed by ionic liquid. The method is characterized in that heterocyclic nitrogen-containing ionic liquid is used as a catalyst, the amount of the catalyst is 5*10<-3>-5% of the mass of a dihydroxy compound, the dihydroxy compound and bis(trichloromethyl) carbonate are taken as raw materials, a feeding mol ratio of the dihydroxy compound to the bis(trichloromethyl) carbonate is 1:0.8-1:10, and the corresponding polycarbonate is obtained by melt polymerization. A synthesis process of the polycarbonate includes two stages of transesterification and polycondensation. In the transesterification stage, a prepolymer is obtained under conditions of a reaction temperature of 90-180 DEG C, an atmospheric pressure and reaction time of0.05-6 h. In the polycondensation stage, the polycarbonate is obtained by synthesizing prepolymer at 200-270 DEG C, a vacuum degree of 4.0*10<-3> MPa-1.0*10<-5> MPa, and reaction time of 0.05-7 h. Thesynthetic method has the advantages of simple catalyst components and molecular structure that can be designed. Moreover, the catalyst is strong in alkalinity and high in activity. By-products can berecycled, which reduces the cost. The synthetic method does not use highly toxic phosgene, dose not require a solvent, is very little in three wastes generation, and conforms to the concept of cleanproduction.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
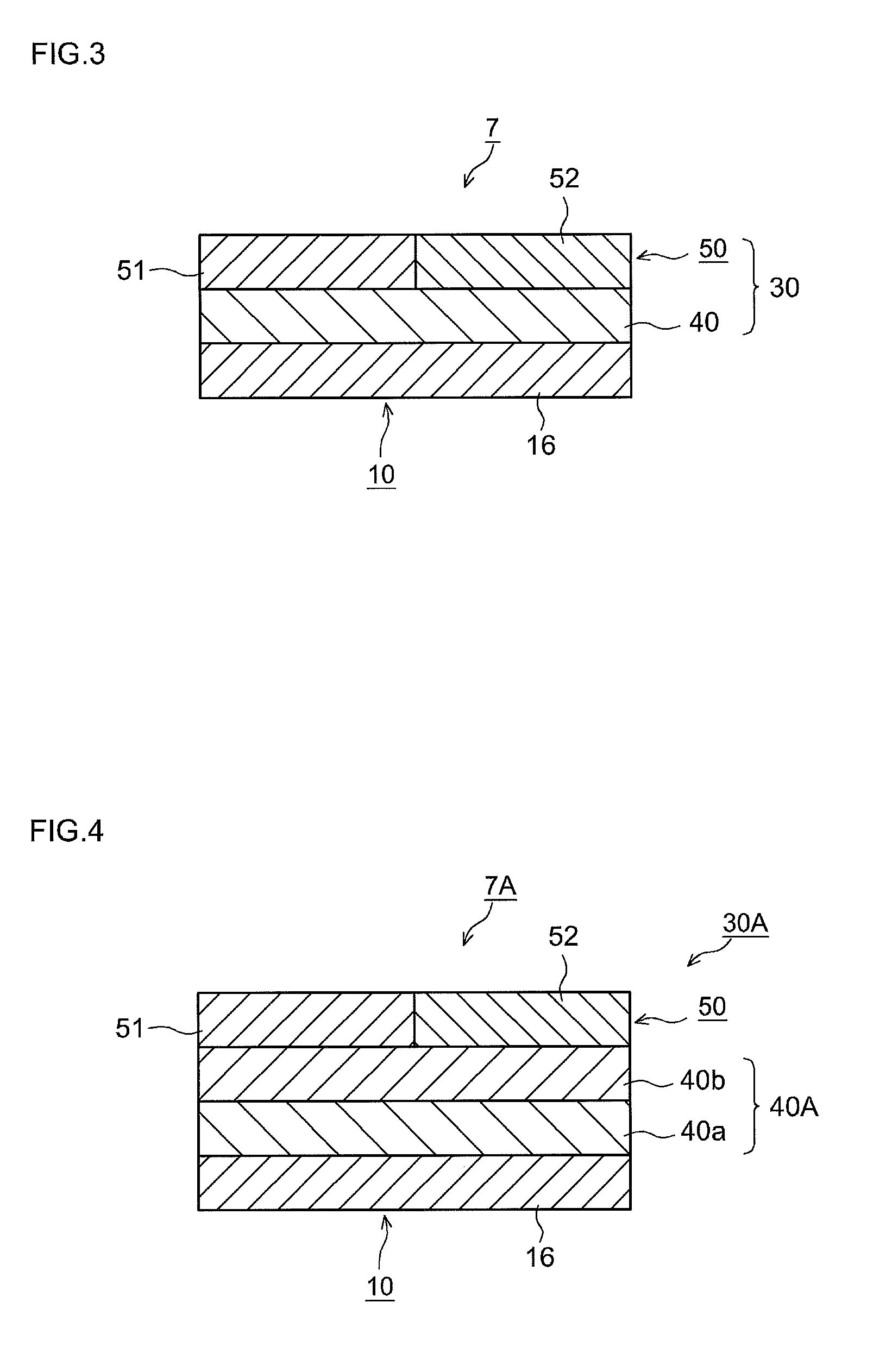
Exhaust cleaning catalyst
ActiveUS20160199815A1Reduce nitrogen oxide emissionsHigh activityGas treatmentInternal combustion piston enginesEngineeringCoating
Provided is an exhaust catalyst that exhibits higher NOX-reducing activities at the time of engine restart while maintaining its catalytic activities during normal traveling. This invention provides an exhaust cleaning catalyst comprising a substrate and a catalyst coating layer that includes CeO2. Catalyst coating layer is constituted in its thickness direction with multiple coating layers. In a top coating layer located at the outermost surface, the CeO2 content in a top coating layer's upstream portion is less than the CeO2 content in a top coating layer's downstream portion; and the CeO2 content in the top coating layer's upstream portion is less than the CeO2 content in a lower coating layer. The CeO2 content per liter of catalyst volume in the entire coating layer is 10 g / L to 30 g / L.
Owner:CATALER CORP
Polyacid-based metal organic framework crystal material and preparation method thereof
InactiveCN107715913AMaintain catalytic activityExcellent electrocatalytic reduction performanceOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationMetal-organic frameworkPorous channel
The invention provides a polyacid-based metal organic framework crystal material and a preparation method thereof and relates to a polyacid-based metal organic framework crystal material with nano porous channels. The invention aims at solving the problems that the polyacid-based metal organic framework material synthesized by adopting the prior art generally has a single porous channel, the finally obtained material does not have the porous channels after a polyacid molecule is immobilized, and therefore, the catalytic activity of the material is low. A chemical formula of the designed and developed flexible ligand assembled polyacid-based metal organic framework material with the nano porous channels is {[CuI(btpe)2][CuII2(H2O)2(btpe)2][BW12]40}}.2H2O. The preparation method comprises the following steps: dissolving borotungstic acid, copper acetate and 1,5-bis(triazole)-pentane into deionized water, regulating pH value, and then carrying out reaction for 3 days at the temperature of160 DEG C. By carrying out the preparation method provided by the invention, the flexible ligand assembled polyacid-based metal organic framework crystal material with the nano porous channels can beobtained.
Owner:HARBIN UNIV OF SCI & TECH
Preparation method of cobalt-cerium composite oxide catalyst and cobalt-cerium composite oxide catalyst
InactiveCN105214675ASimple process conditionsSmall particle sizeGaseous fuelsFuel additivesComposite oxideCerium
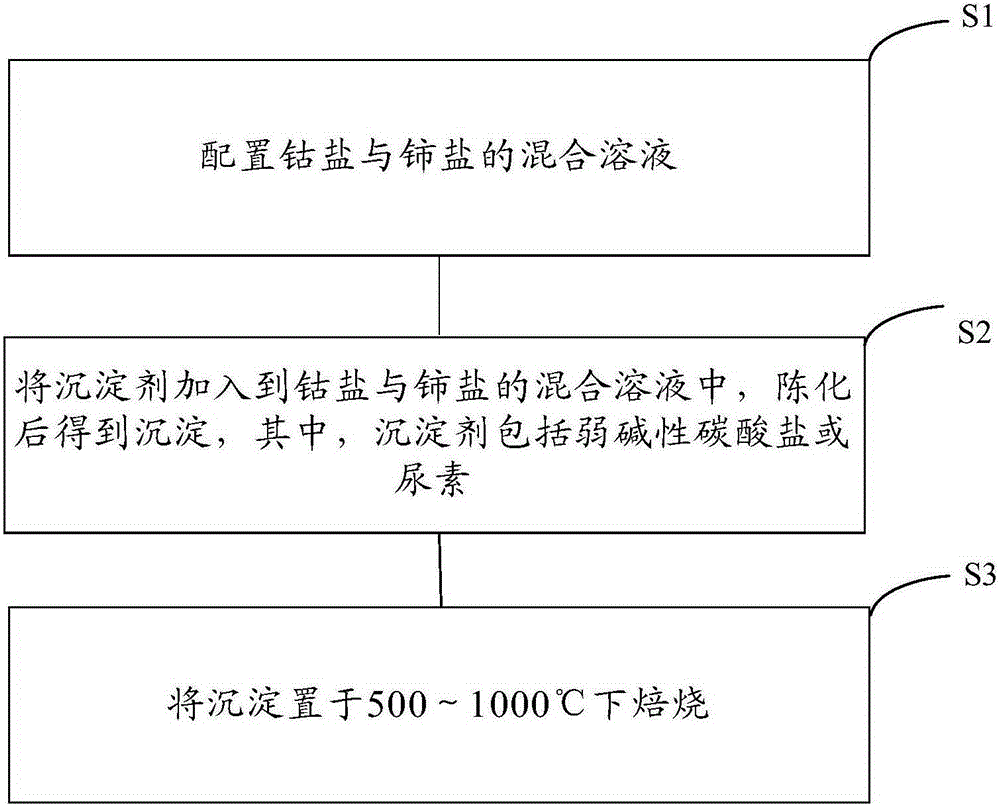
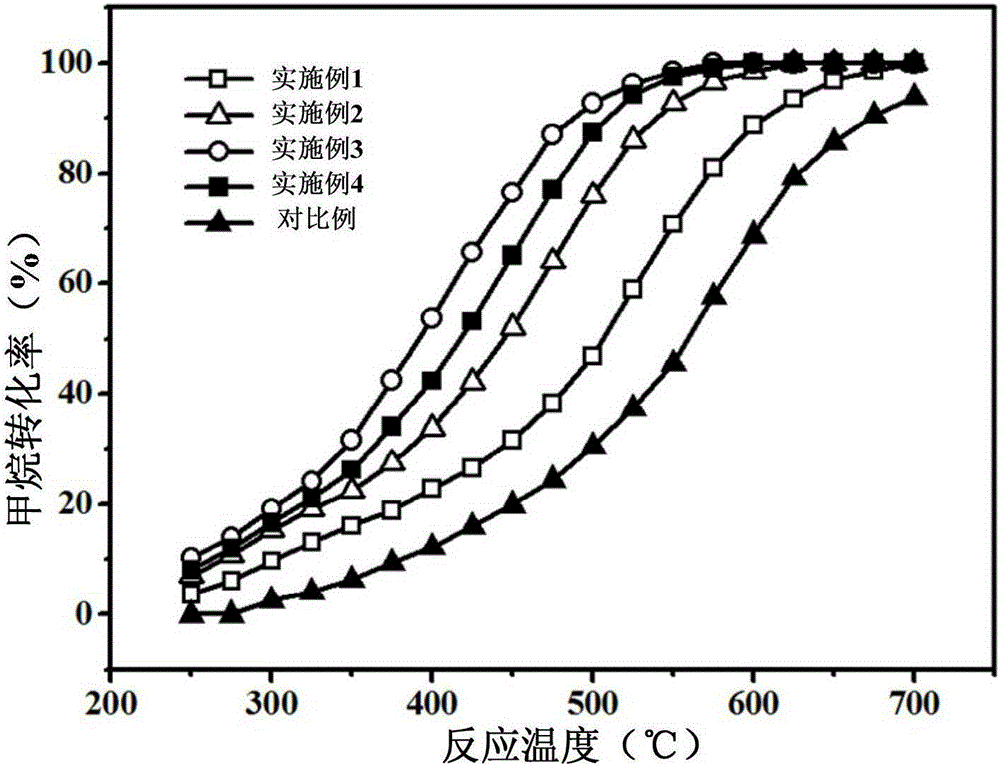
The invention provides a preparation method of a cobalt-cerium composite oxide catalyst and the cobalt-cerium composite oxide catalyst, and belongs to the field of catalysts. The preparation method is simple in technology, the high-temperature stability of the catalyst can be improved, and the catalytic activity of the catalyst can be guaranteed simultaneously. The preparation method of the cobalt-cerium composite oxide catalyst comprises the following steps that S1, a mixed solution of cobalt salt and cerium salt is prepared; S2, precipitating agents are added to the mixed solution of the cobalt salt and the cerium salt, and precipitation is obtained after aging, wherein the precipitating agents comprise alkalescent carbonate or urea; S3, the precipitation is calcinated at the temperature ranging from 500 DEG C to 1000 DEG C. The method is used for preparing the cobalt-cerium composite oxide catalyst for catalyzing methane combustion, and the low-temperature catalytic activity and the high-temperature stability of the catalyst are high.
Owner:ENN SCI & TECH DEV
Amorphous non-precious metal hydroxide modified perovskite composite catalyst used for oxygen evolution reaction and preparation method thereof
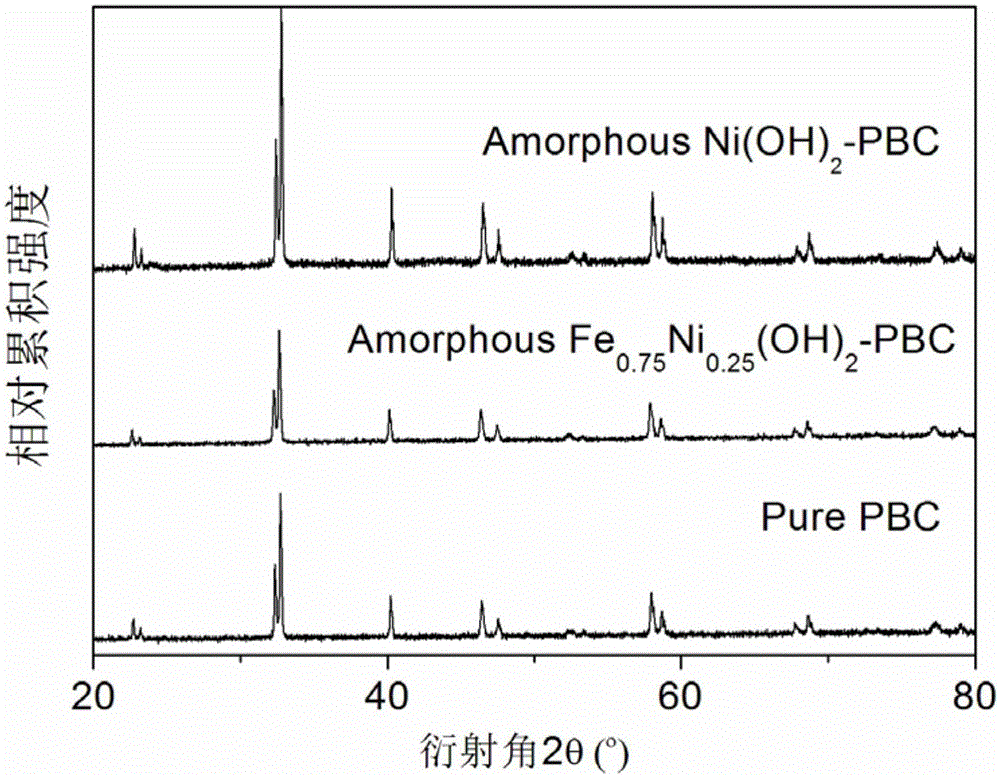
ActiveCN105056961AImprove electrochemical activityMaintain catalytic activityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsChemical compositionAmorphous silicon
The invention discloses an amorphous non-precious metal hydroxide modified perovskite composite catalyst used for oxygen evolution reaction and a preparation method thereof. The composite catalyst is composed of a main body material and a modification material, wherein the main body material is perovskite oxide and the modification material is amorphous non-precious metal hydroxide. The chemical composition of the amorphous non-precious metal hydroxide modification material is MxA1-x(OH)y, wherein M and A are selected from transition metal elements, x is no less than 0 and no more than 1, and y is no less than 2 and no more than 3. The main body material A-position ordered double perovskite oxide and has a molecular formula of Ln0.5Ba0.5CoO3-delta. The hydroxide modification material and the perovskite main body in the composite catalyst produce cooperative effect; the catalytic activity of the composite catalyst is greatly improved compared with catalytic activity of a main body catalyst; and after long-time electrochemical performance test, the composite catalyst can maintain catalytic activity and morphological stability.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com