Oxide catalyst for selective reduction of nitrogen oxide, preparation and uses thereof
A technology of nitrogen oxides and manufacturing methods, applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
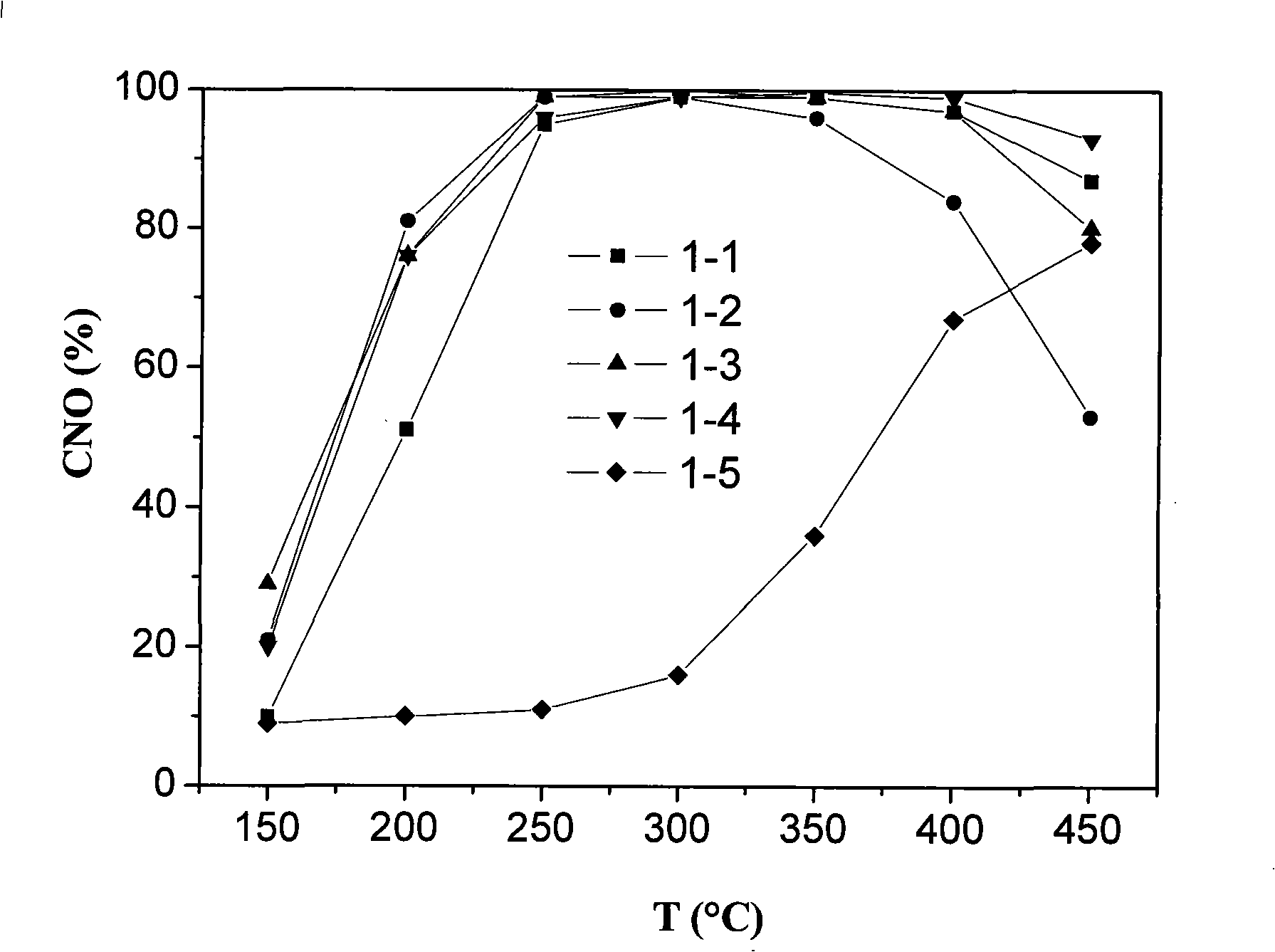
[0171] Example 1 Preparation of WO by impregnation method 3 / C x Zr 1-x o 2 Particulate Catalysts and Their Characterization
[0172] The second component Ce x Zr 1-x o 2 (x=0.2; 0.66; 0.8) The composite oxide was prepared as follows.
[0173] Prepare 2 mol / L Ce(NO 3 ) 3 and Zr(NO 3 ) 4 aqueous solution. Take quantitatively the Ce(NO) prepared above 3 ) 3 solution and Zr(NO 3 ) 4 solution, urea and deionized water to make 2000 milliliters of total cation concentration is a solution of 0.1 mol / liter, and make the molar ratio Ce 3+ : Zr 4+ : urea = x: (1-x): 15 (x = 0.2; 0.66; 0.8). The solution was heated to its boiling point with stirring until a precipitate appeared. The resulting mixture was then aged at boiling point for 2 hours and then stirred at room temperature for 2 hours. The precipitate was filtered and washed with deionized water for 15 minutes with stirring. This step is repeated three times. The filter cake was then rinsed with isopropanol o...
Embodiment 2
[0178] Example 2 WO 3 / C 0.8 Zr 0.2 o 2 (1 / 10) Long-term Stability Characterization of Granular Catalysts at 300℃
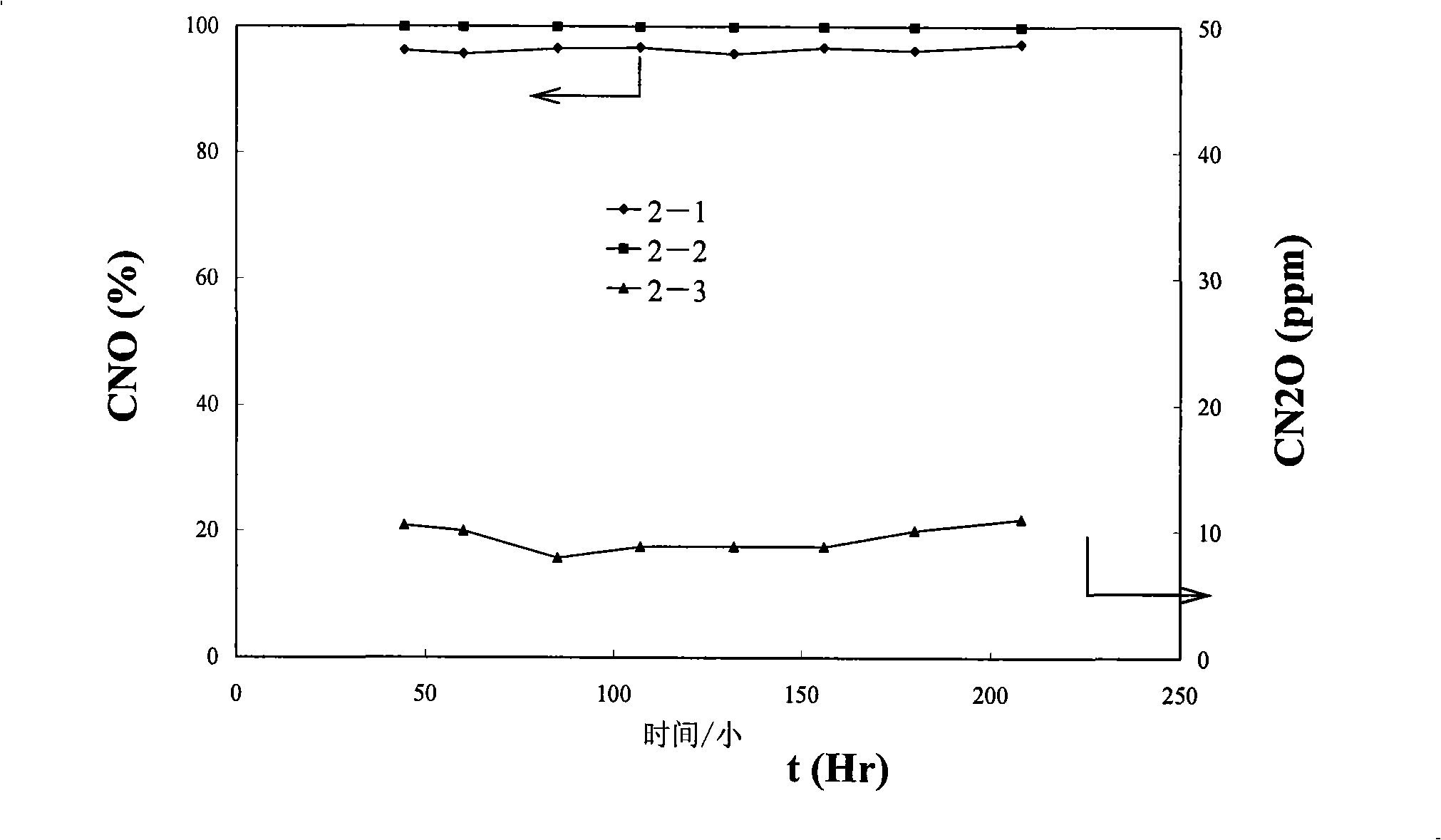
[0179] WO 3 : Ce 0.8 Zr 0.2 o 2 = 1:10 (weight ratio) of WO 3 / C 0.8 Zr 0.2 o 2 Catalyst is prepared with above-mentioned embodiment 1. The stability of the catalyst was investigated by SCR reaction at 300°C for 200 hours. The test conditions are the same as those in Example 1 above. See the test results figure 2 . figure 2 Middle: Curve 2-1 is the conversion rate of NO during the 200-hour test period; Curve 2-2 is the NH conversion rate during the 200-hour test period 3 conversion rate; Curve 2-3 is the N in the reaction tail gas during the 200-hour test 2 O concentration.
[0180] figure 2 The curves clearly show that: in the 200-hour stability experiment, the activity of the tested catalyst remained stable, the NO conversion rate remained at 96%, and the NH 3 The conversion rate is constant at 100%, and only a small amount of N 2 O is ...
Embodiment 3
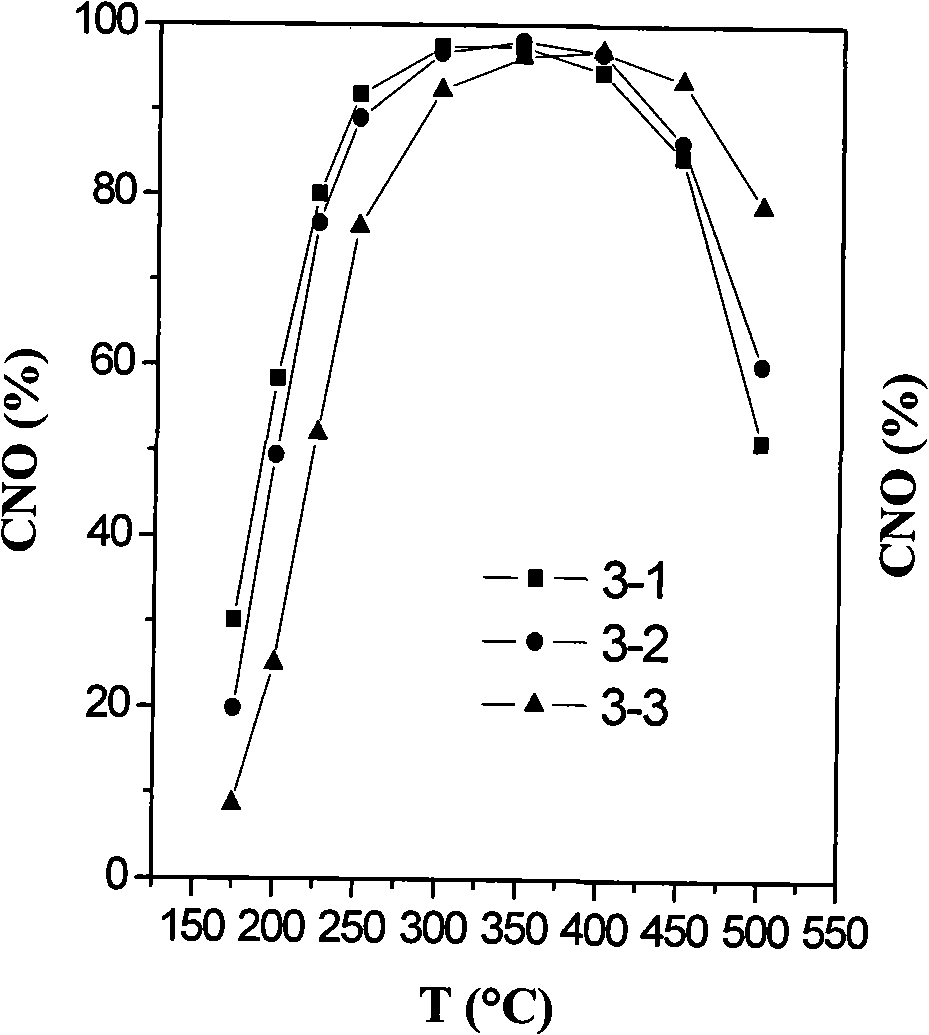
[0181] Example 3 Preparation of WO by impregnation method 3 / C x Zr 1-x o 2 Characterization of granular catalysts and their properties before and after aging
[0182] The second component Ce x Zr 1-x o 2 (x=0.2; 0.5; 0.8) The preparation method of the composite oxide is the same as that of the above-mentioned Example 1, but with (NH 4 ) 2 Ce(NO 3 ) 6 instead of Ce(NO 3 ) 3 as a precursor of Ce. Quantitative (NH 4 ) 2 Ce(NO 3 ) 6 , Zr(NO 3 ) 4 solution and urea in molar ratio Ce 4+ : Zr 4+ : urea = (1-x): x: 15 (x = 0.2; 0.5; 0.8) Prepare 2000 ml of a solution with a cation concentration of 0.2 mol / l. The solution was heated to its boiling point with stirring until co-precipitation was observed. The resulting mixture was then aged at the boiling point for 2 hours and stirred at room temperature for an additional 2 hours. The precipitate was filtered and washed with 1500 ml of deionized water for 15 minutes with stirring. This step is repeated three tim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hole density | aaaaa | aaaaa |
| Hole density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com