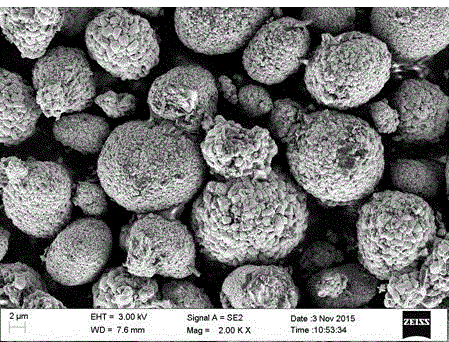
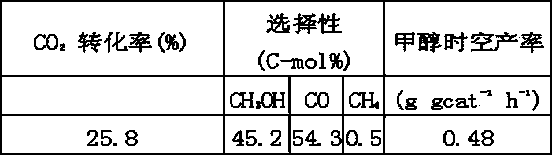
Patents
Literature
620 results about "Divalent metal ions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Divalent metal ions are essential to many enzymatic reactions involving nucleic acids, but their critical and specific role still needs to be uncovered. Restriction endonucleases are a prominent group of such metal-requiring enzymes.
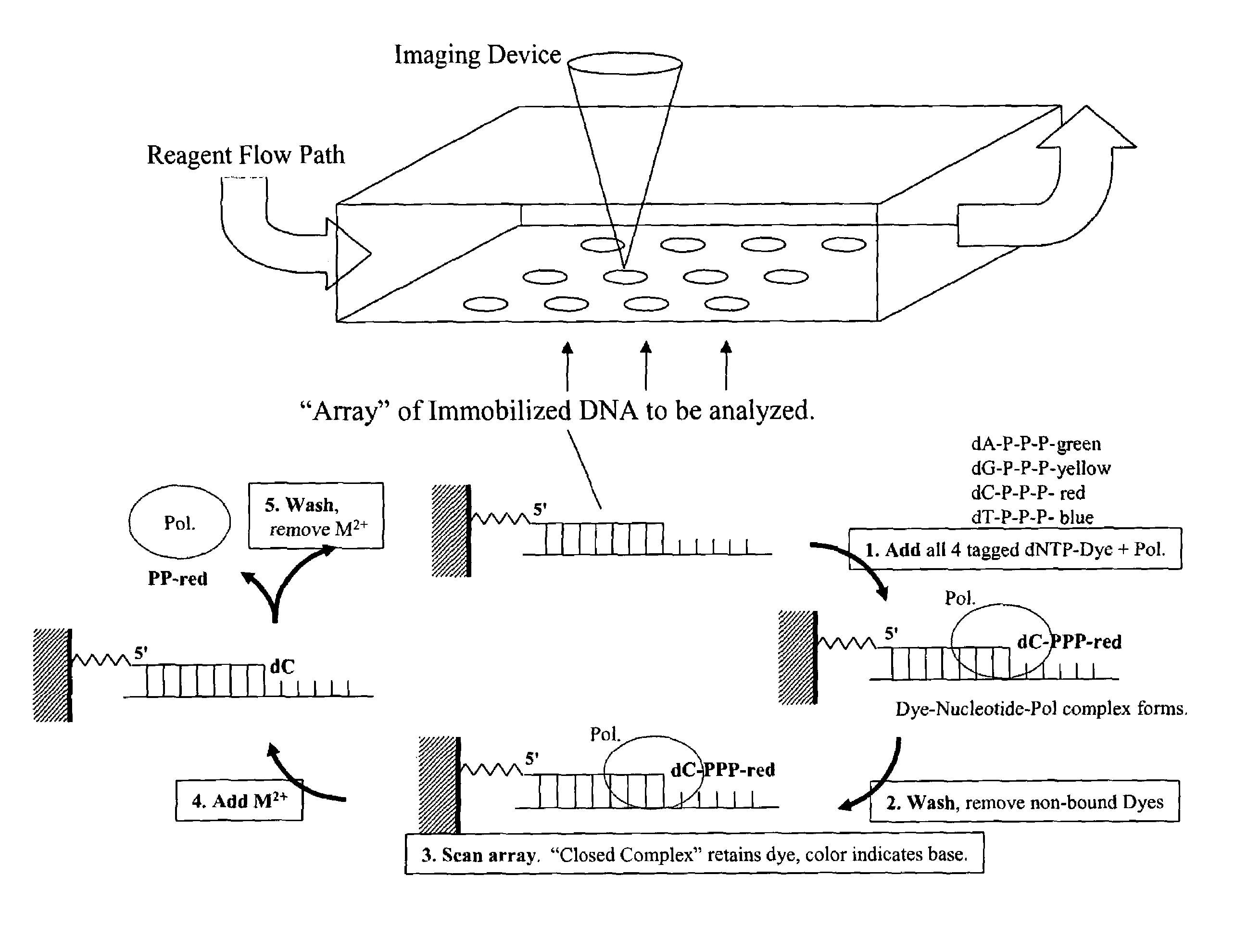
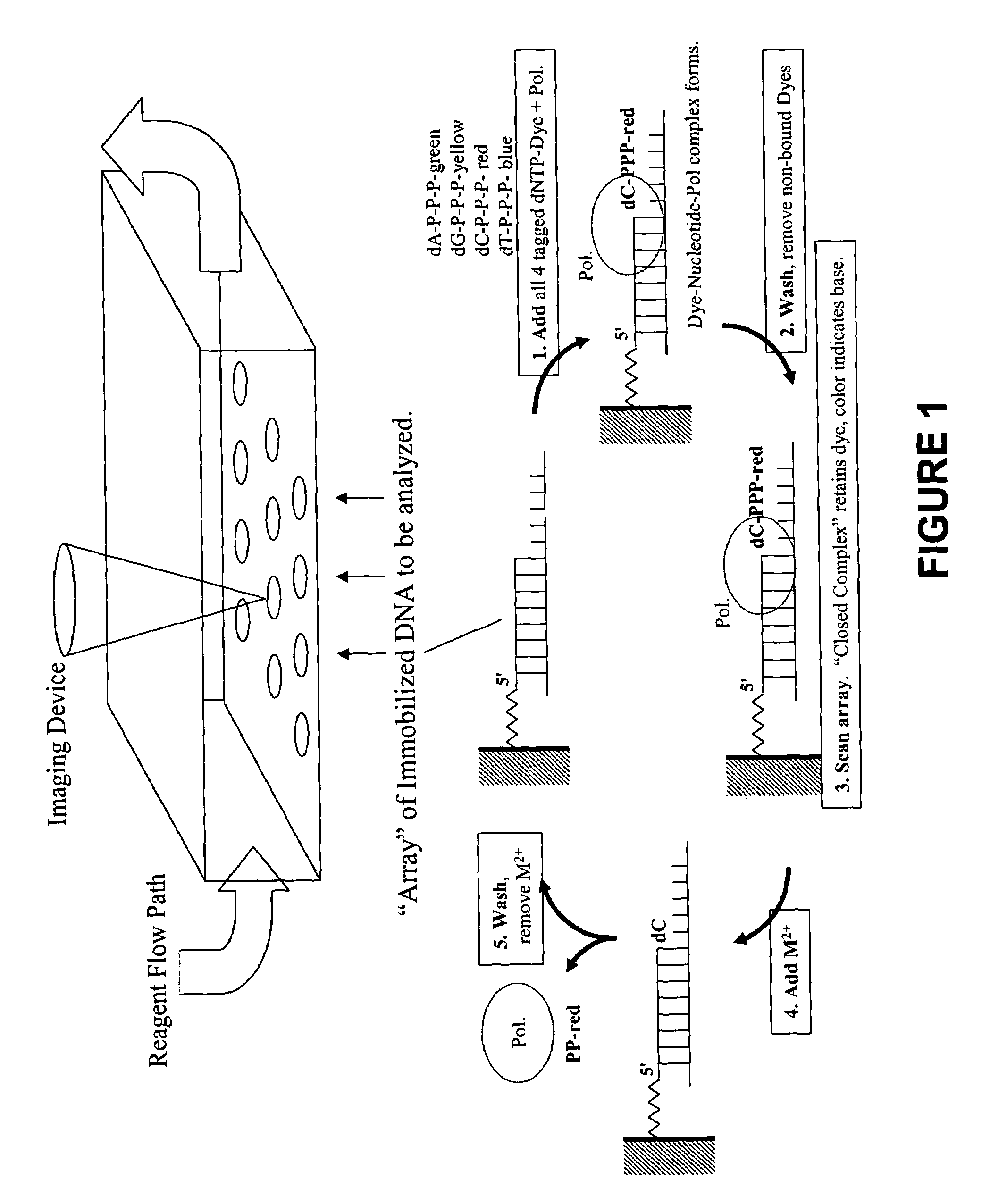
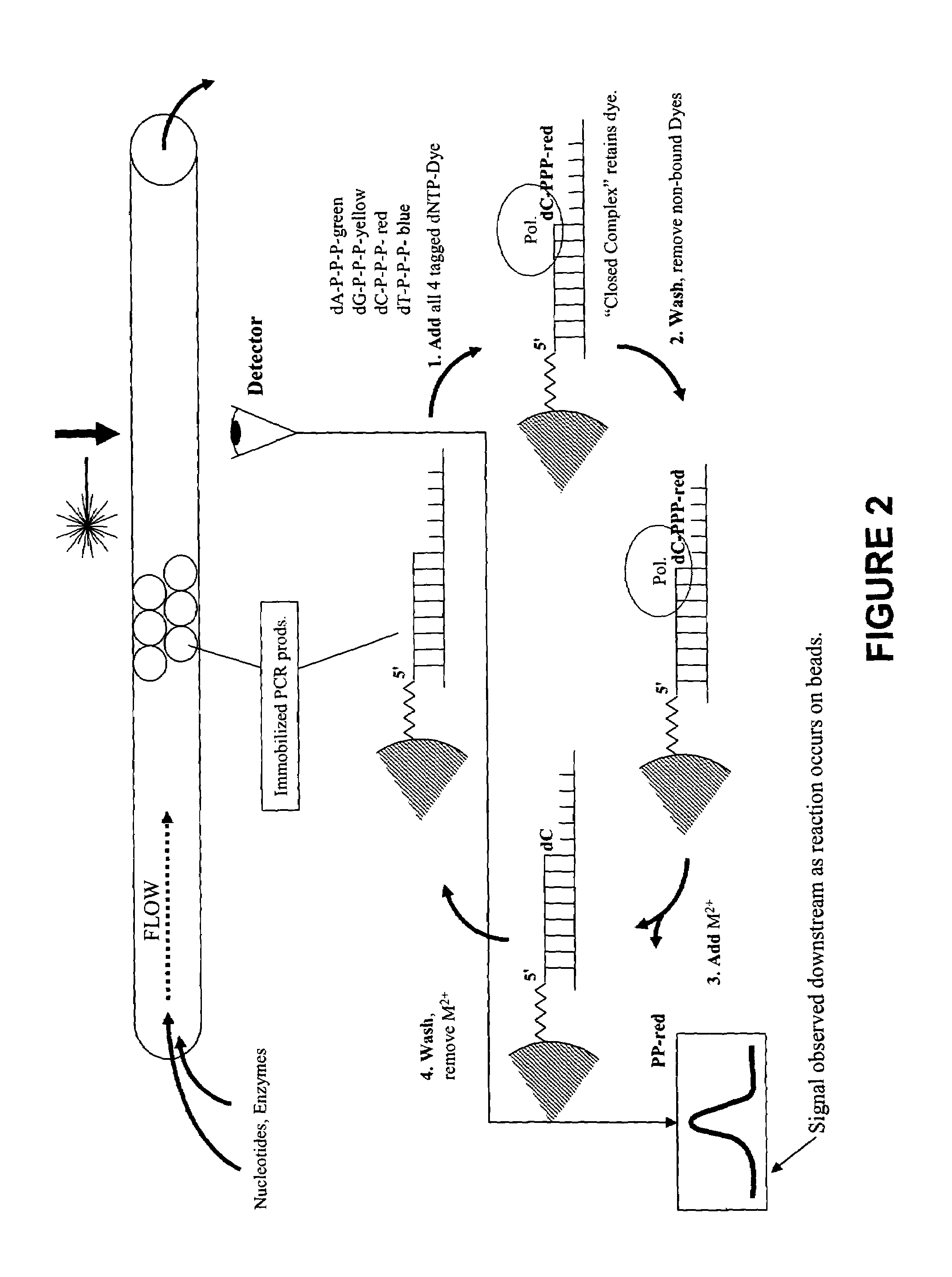
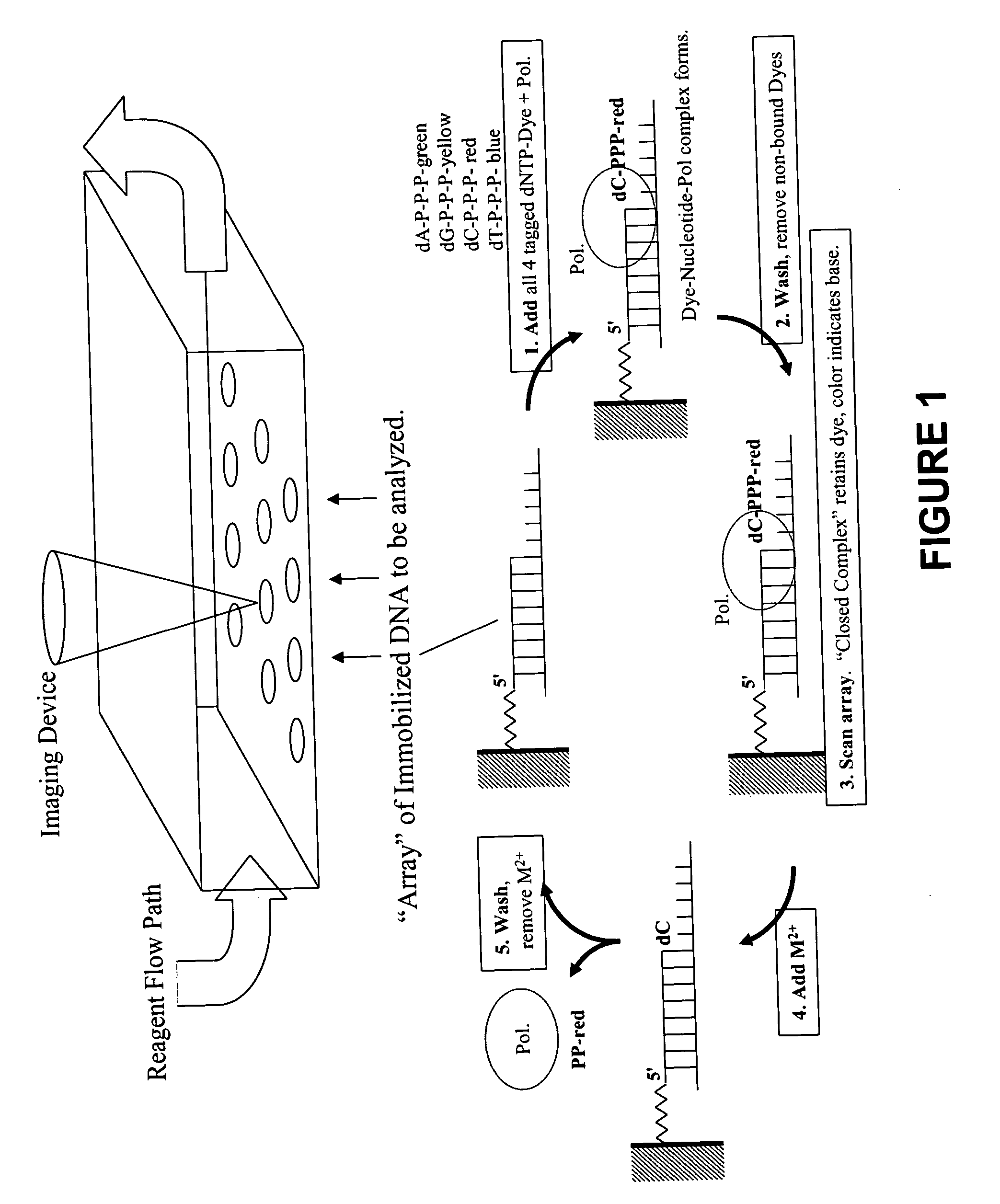
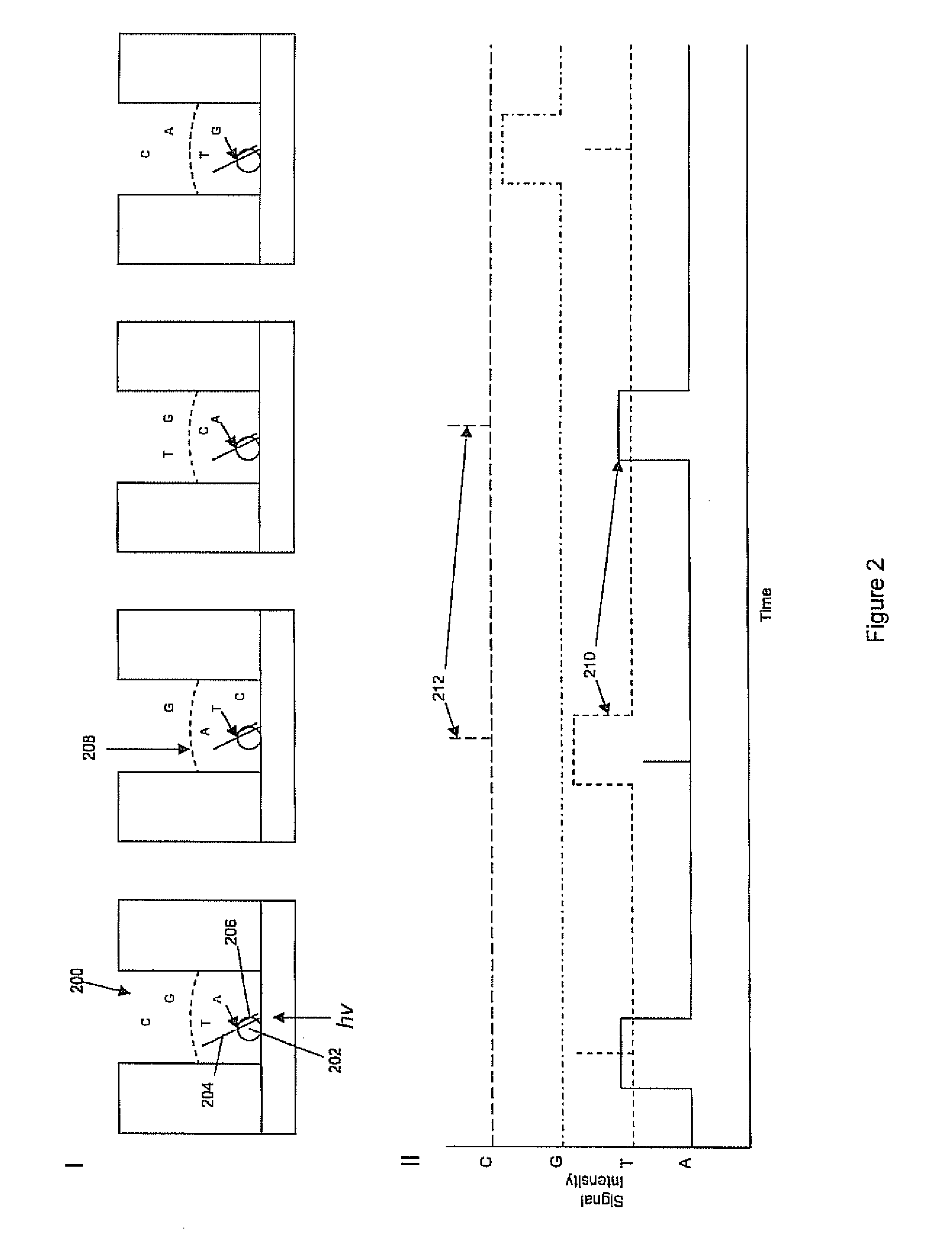
Rapid parallel nucleic acid analysis
ActiveUS7264934B2Efficiently determinedEliminates non-specificitySugar derivativesMicrobiological testing/measurementNucleotidePolymerase L
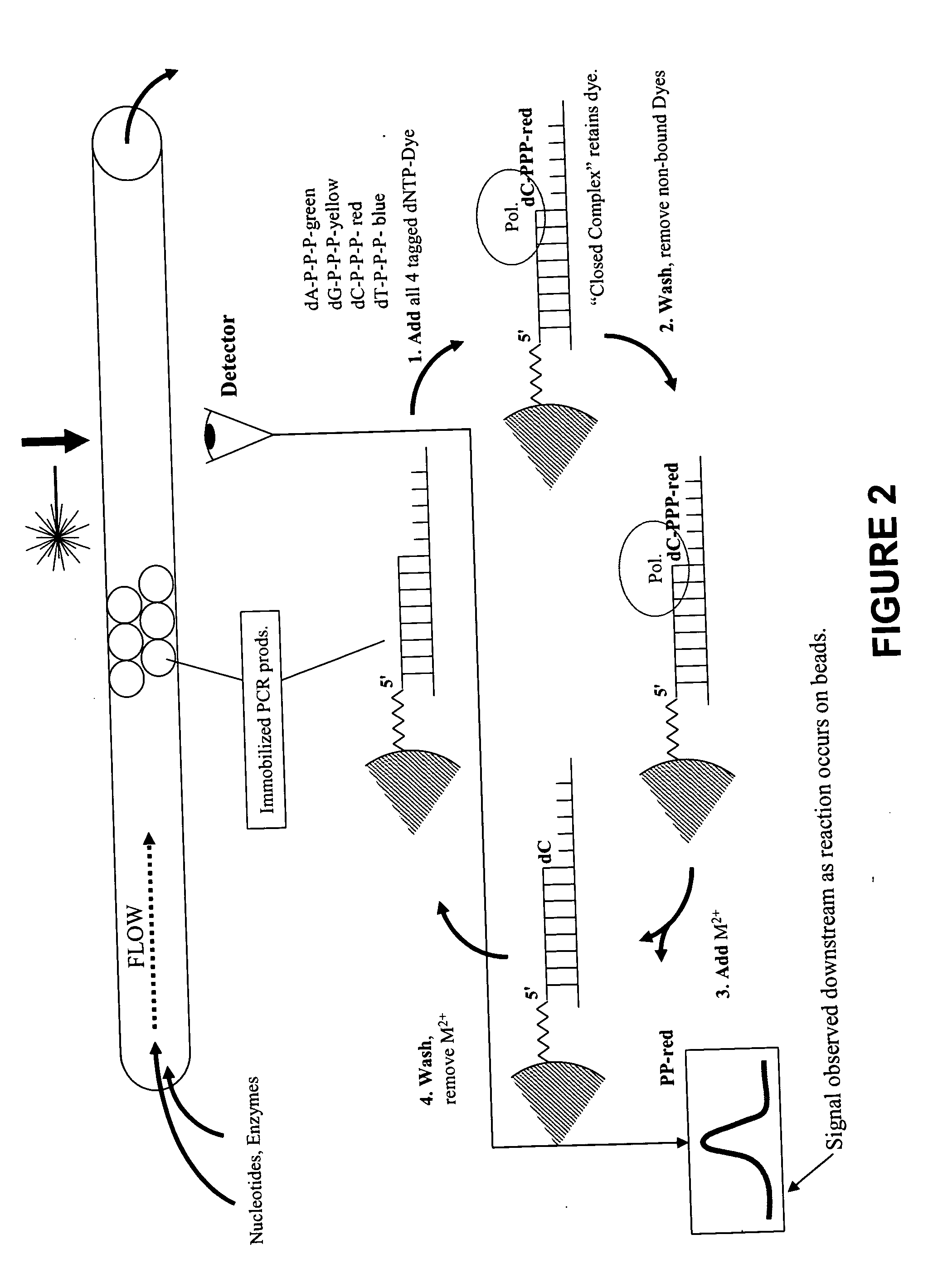
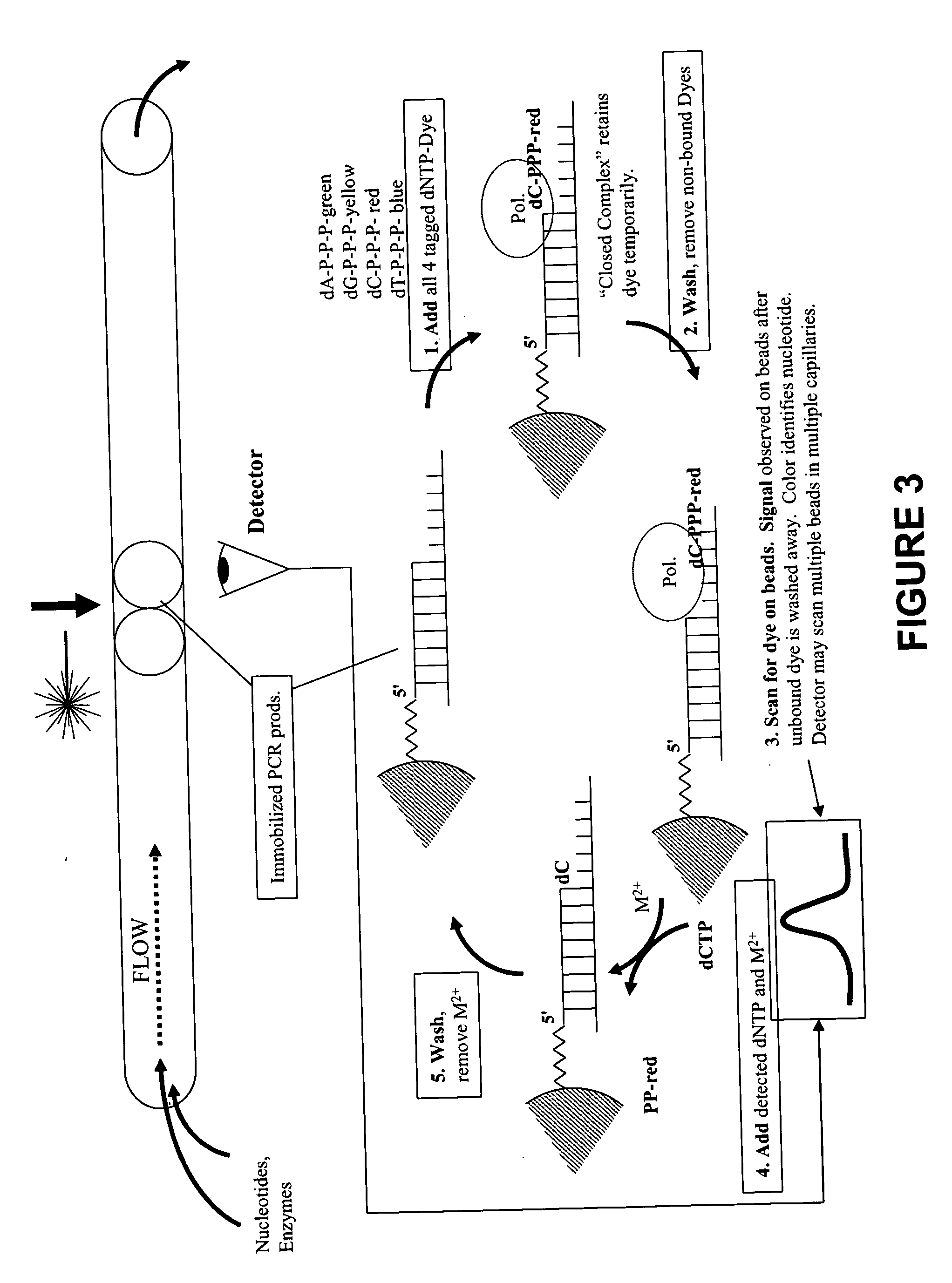
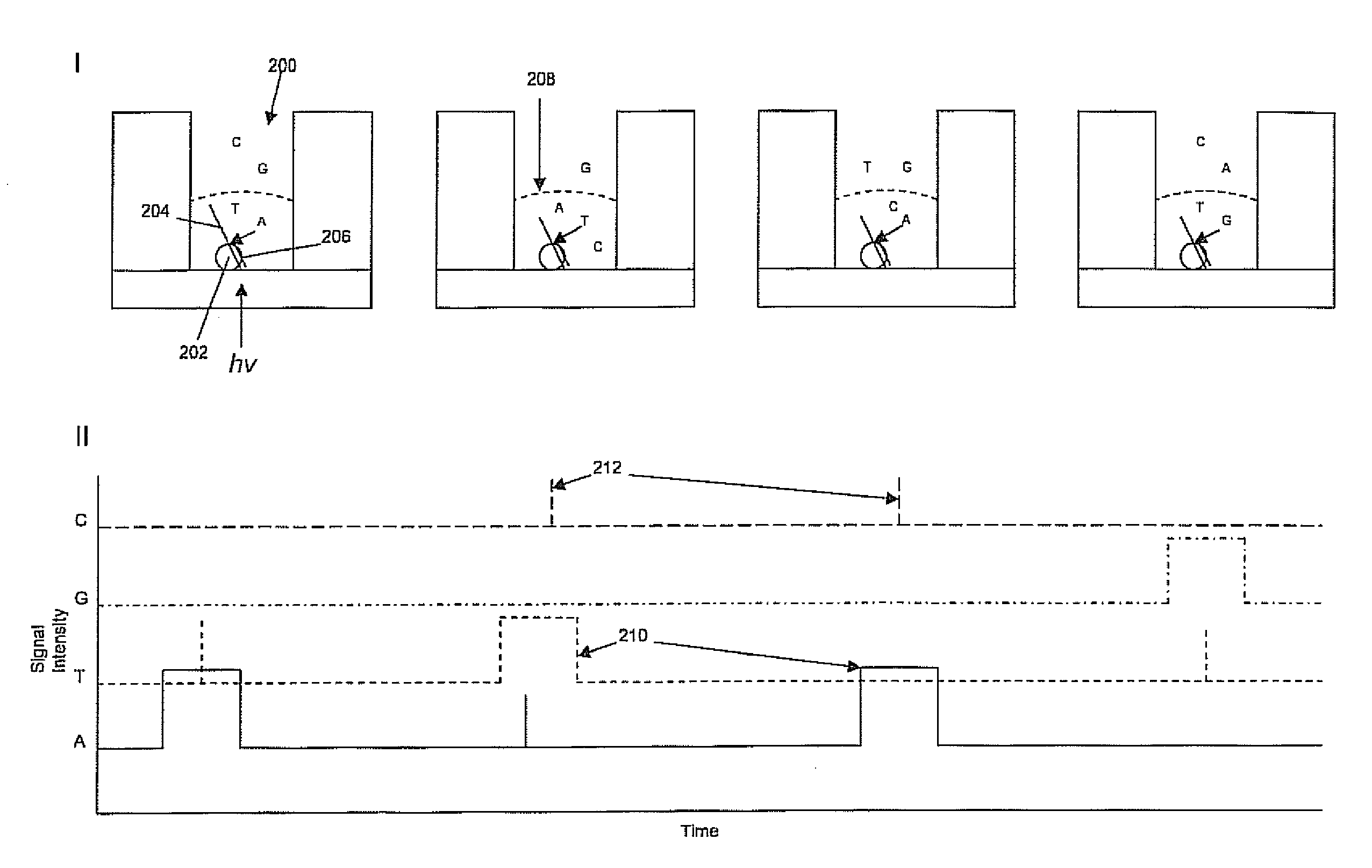
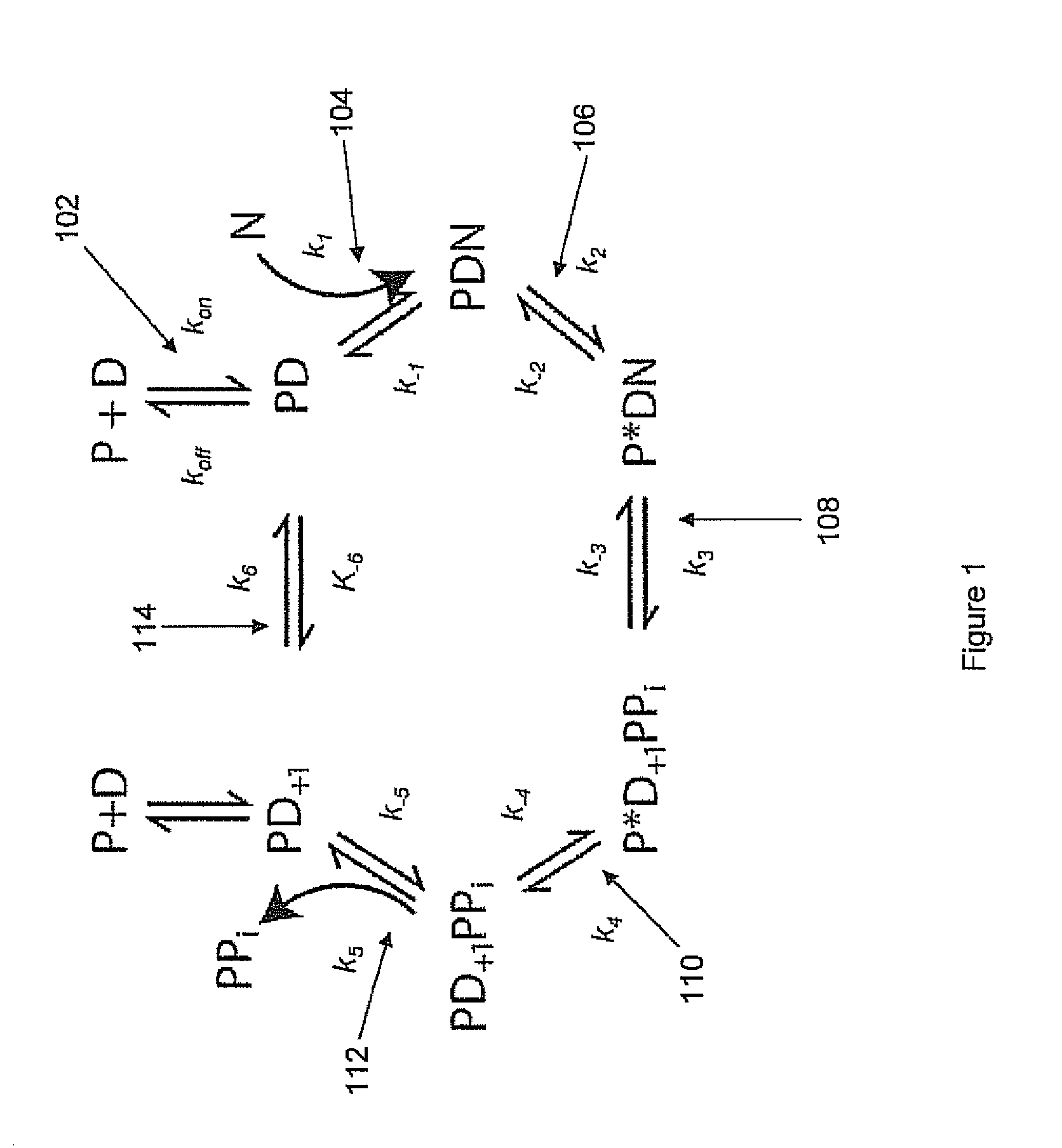
This invention provides methods for massive parallel nucleic acid analysis. A closed complex of nucleic acid template, nucleotide and polymerase can be formed during polymerase reaction, absent divalent metal ion. This is used to trap the nucleotide complementary to the next template nucleotide in the closed complex. Detection of the trapped nucleotide allows determination of the sequence of this next correct nucleotide. In this way, sequential nucleotides of a nucleic acid template can be identified, effectively determining the sequence. This method is applied to sequence multiple templates in parallel, particularly if they are immobilized on a solid support.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Rapid parallel nucleic acid analysis
ActiveUS20060051807A1Efficiently determinedEliminates non-specificitySugar derivativesMicrobiological testing/measurementPolymerase LDivalent metal ions
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Nucleic acid synthesis compositions and methods and systems for using same
ActiveUS20100047802A1Accurate sortingReduce in quantityMicrobiological testing/measurementTransferasesPolymerase LDivalent metal ions
The Application relates to compositions, kits, methods, and systems for nucleotide sequencing comprising producing polymerase reactions that comprise both catalytic and non-catalytic divalent metal ions. Effective ratios and amounts of catalytic and non-catalytic divalent metal ions are described.
Owner:PACIFIC BIOSCIENCES
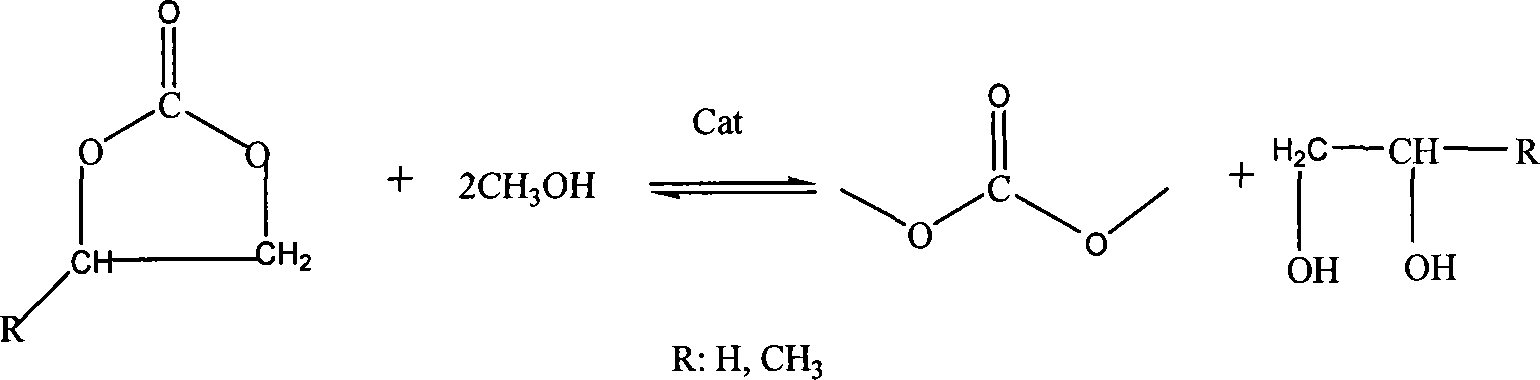
Load type solid body base catalyst of synthesizing dimethyl carbonate and method of preparing the same
InactiveCN101249452AEasy to makeEasy to preparePhysical/chemical process catalystsPreparation from organic carbonatesMethyl carbonatePotassium fluoride
The invention relates to a carrier type solid base catalyst for synthesizing dimethyl carbonate, which belongs to the technical field of catalytic material. The catalyst's formula is KF / M<2+>-N<3+>-(O), wherein M<2+>-N<3+>-(O) represents the composite metal oxide, M<2+> can be divalent metal ion (Mg<2+> or Ca<2+> ) and N<3+> can be trivalent metal ion (Fe<3+> and / or Al<3+>). The catalyst is prepared from potassium fluoride and composite metal oxide at a certain weight ratio by immersing the potassium fluoride into the composite metal oxide, wherein the composite metal oxide is prepared from hydrotalcite precursor and in nucleation / crystallization isolation method by calcining at a certain temperature. The catalyst has the advantages of nanoscaled particle size, high specific surface area and high activity, selectivity in catalytic reaction and simple preparation, and can be reused as it can be centrifugally separated after reacting.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing corrosion inhibition anion intercalated layered double hydroxides/oxide composite material and application
InactiveCN101418154AFacilitated releaseImprove anti-corrosion performanceAnti-corrosive paintsEpoxy resin coatingsOxide compositeReaction temperature
The invention provides a corrosion-inhibiting anionic intercalated hydrotalcite / nano oxide composite material, a preparation method thereof and application of the corrosion-inhibiting anionic intercalated hydrotalcite / nano oxide composite material. The preparation method is as follows: corrosion-inhibiting anions are directly inserted between hydrotalcite layers by the one-step coprecipitation method and the roasting restoring method; the nano oxide is generated by means of in situ synchronization during the process of generation of hydrotalcite crystals by controlling the mixture ratio of divalent metal ions to trivalent metal ions, the pH value of a reaction solution and the reaction temperature; and the corrosion-inhibiting anionic intercalated hydrotalcite / nano oxide composite material with the anti-corrosive function is prepared. The invention is mainly characterized in that the in situ preparation method for the hydrotalcite / oxide composite material provided with a nano lamellar structure improves the release amount of a corrosion inhibitor and reduces the water absorption of a coating by leading into the corrosion inhibitor with the anti-corrosive function and the nano oxide which is generated in situ during the reaction process. The composite material can be used to be pigment of an anti-corrosive metallic coating system, and particularly has potential application value in improving the anti-corrosive performance of a magnesium alloy anti-corrosive coating.
Owner:HARBIN ENG UNIV
Oral Stannous Compositions
ActiveUS20090136432A1High activityLow levelCosmetic preparationsToilet preparationsPhosphateDivalent metal ions
The present invention relates to an aqueous oral composition comprising:a) from 0.2% to 3% divalent metal ions comprising:i. from 0.1% to 1.5% of zinc ions;ii. from 0.1% to 2% of tin (II) ions; andb) a source of fluoride ions;c) a silica dental abrasive;d) one or more chelants having a MW of less than 1000 and capable of forming water-soluble complexes with the zinc ions, wherein the chelants comprise less than 0.2% linear polyphosphates having a chain length of four or more;e) an orally acceptable carrier comprising at least 20% total water;wherein the pH of the composition is from 5 to 6.5, the molar ratio of the chelants to the divalent metal ions is at least 0.70:1 and at least 80% by weight of the total zinc ions are solubilised within the composition.The composition of the invention has been found to give improved antimicrobial activity from the zinc / stannous combination without significant taste, staining or stability problems, compared to compositions having lower levels of chelants.
Owner:THE PROCTER & GAMBLE COMPANY
Layered double hydroxide and preparation method thereof
InactiveCN101665233AParticle size controlRegular layered structureOxide/hydroxide preparationIndividual molecule manipulationDivalent metal ionsCrystallinity
The invention relates to a layered double hydroxide and a preparation method thereof, belonging to the technical field of metal hydroxide preparation. A chemical formula of the layered double hydroxide is as follows: (M1(1-x)M2x(OH)2)(A<n->)x / n), wherein x is larger than or equal to 0.2 and smaller than or equal to 0.33; M1 represents any one or more of divalent metal ions including Mg<2+>, Zn<2+>, Ni<2+>, Co<2+>, Ca<2+>, Cu<2+>, Fe<2+> and Mn<2+>; M2 represents any one or two of trivalent metal ions including Fe<3+> and Al<3+>; A<n-> represents any one of interlayer anions including CO3<2->,NO3<->, Cl<-> and SO4<2->; and the grain size ranges from 12 nm to 80 nm. The invention has the advantages that the preparation method is a direct method for preparing the layered double hydroxide with high crystallinity, layered structure regularity, wide application range and adjustable particle size. The preparation method comprises the following steps: obtaining highly dispersed metal nanoparticle sol by utilizing the action of colloid mill axial shear force and the sodium borohydride reducibility, and then performing slow oxydrolysis in a hydrothermal system, and the like to generate thenano layered double hydroxide with layered structure regularity and adjustable particle size. The method has the advantages of wide application range, low cost, simple operation and environmental protection.
Owner:BEIJING UNIV OF CHEM TECH
Aqueous dispersions containing ionomer resins and rust-preventive ionomeric coatings made therefrom
The present invention is directed to an aqueous dispersion composition which comprises an ethylene-unsaturated carboxylic acid ionomer resin (A) neutralized with a mixture of ions including at least one divalent metal ion and at least one ammonium ion, (B) a non-water soluble vapor phase corrosion inhibitor, and (C) water. The dispersion exhibits good shelf-life and excellent rust-prevention properties. Durable corrosion resistant ionomeric coatings can be easily formed therefrom, which when applied to metal surfaces and baked, can form a rust-preventive coating layer showing excellent coating adhesion both to the metal surface and to an over coat paint. Such coatings are particularly useful when applied to a vehicle body or part thereof.
Owner:PERFORMANCE MATERIALS NA INC
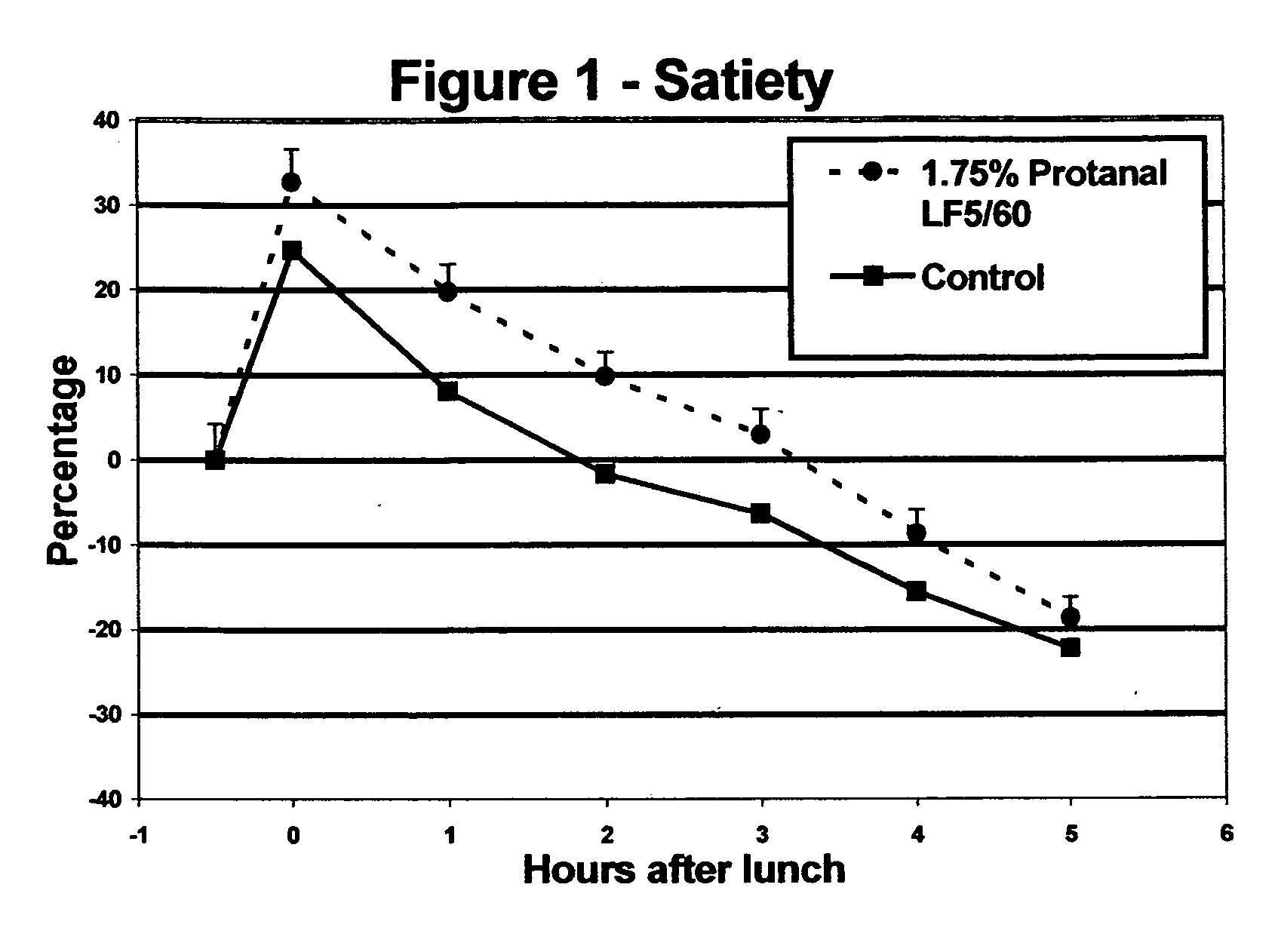
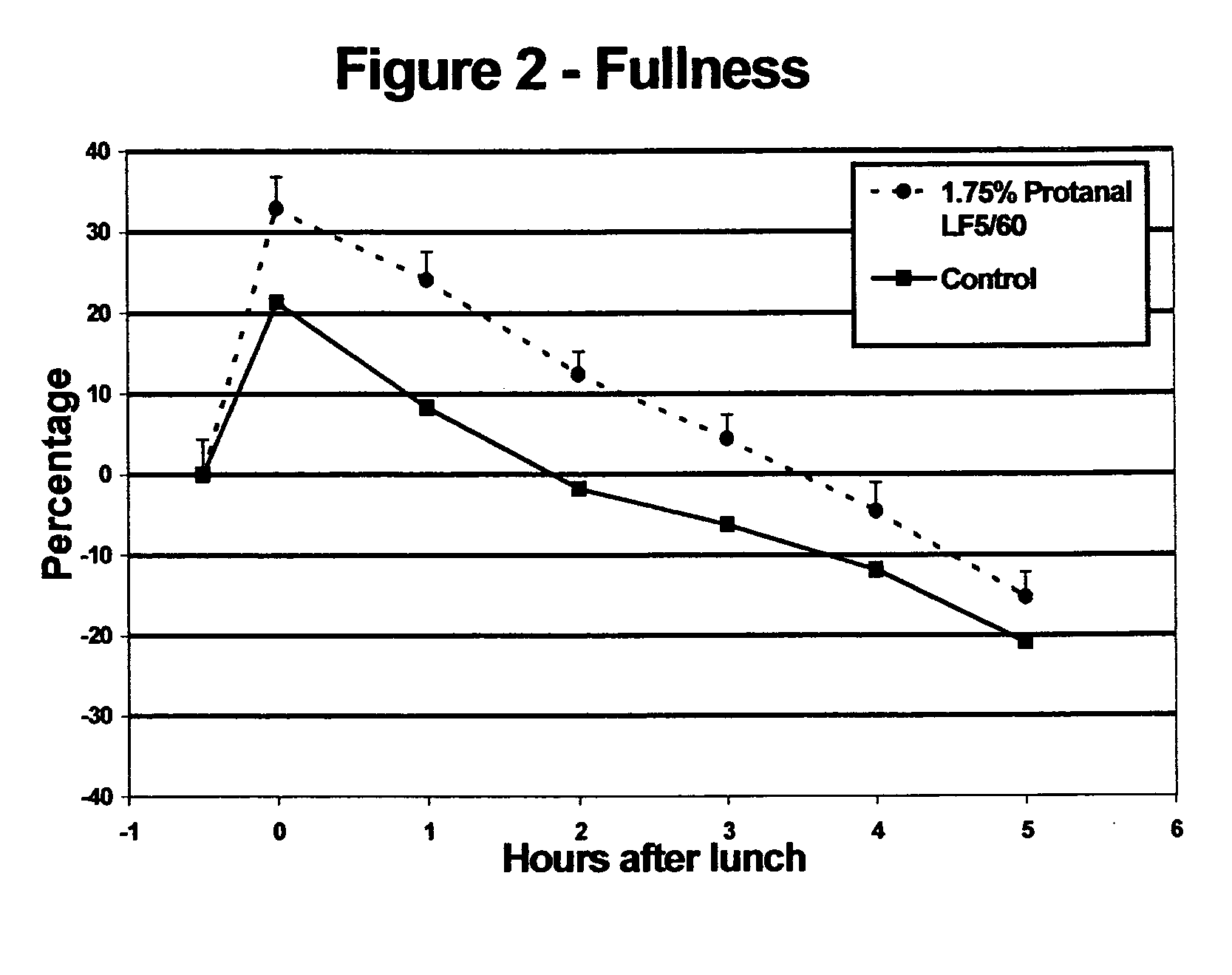
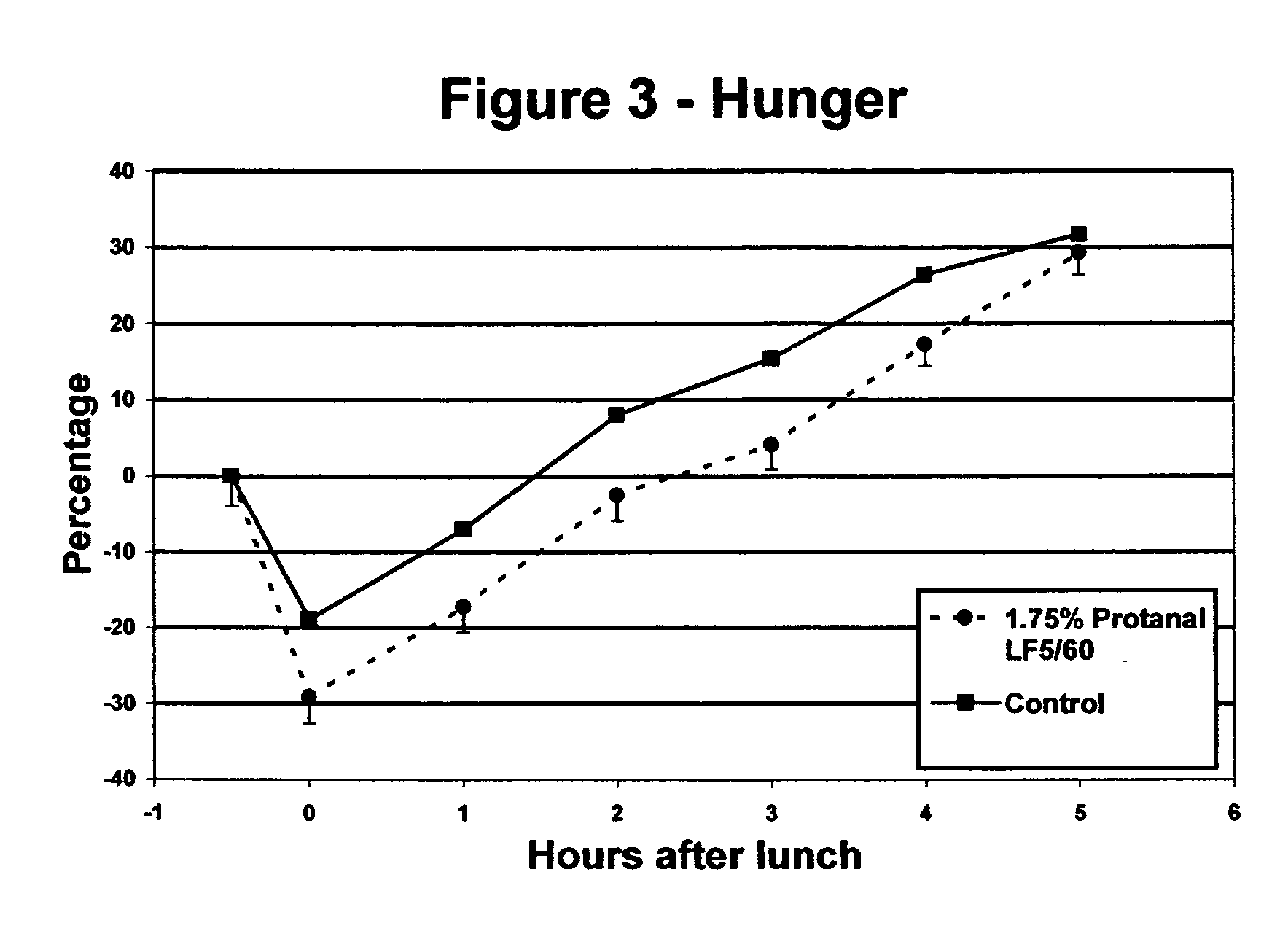
Satiety enhancing food compositions
InactiveUS20050170059A1Promote resultsIncrease satietyFood ingredientsFood preparationDivalent metal ionsUronic acid
The present invention provides edible compositions comprising a source of non-solubilised divalent metal ions and from 0.1 to 6% wt of an alginate having an L-guluronic acid content of at least 60% of the total uronic acid units in the alginate. The compositions of the invention have good satiety effects and are beneficial for use in weight control plans. The edible compositions may be a food compositions intended for use in a weight loss or weight control plan such as a meal replacer food product.
Owner:SLIM FAST FOODS
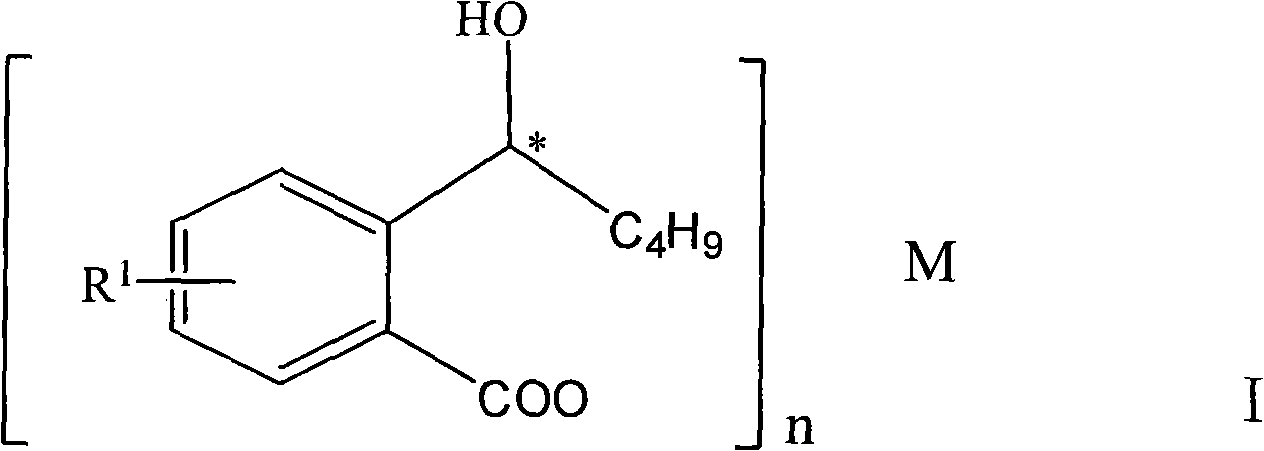
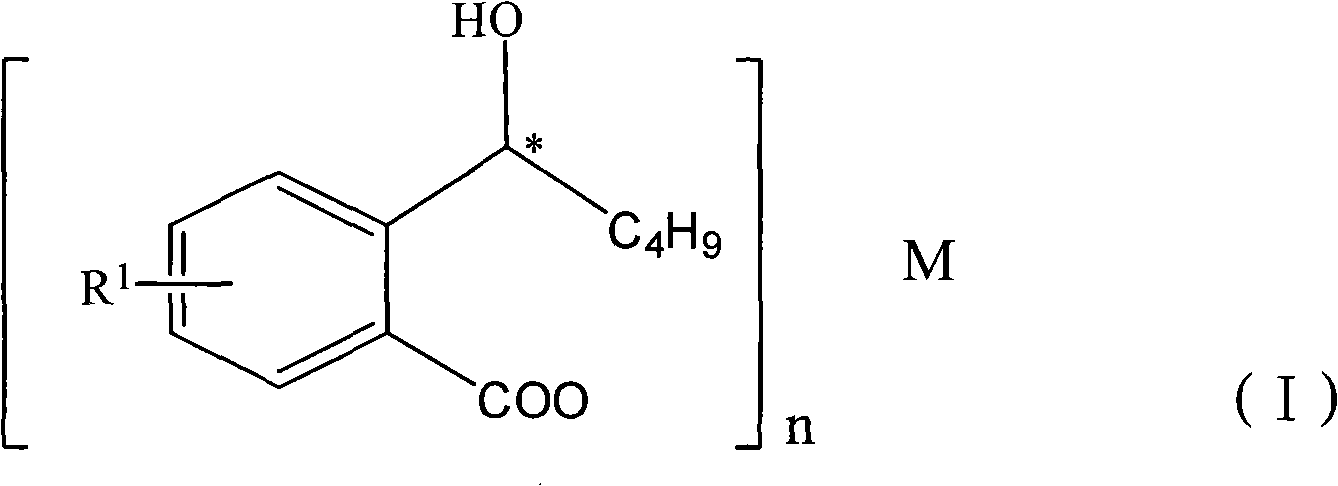
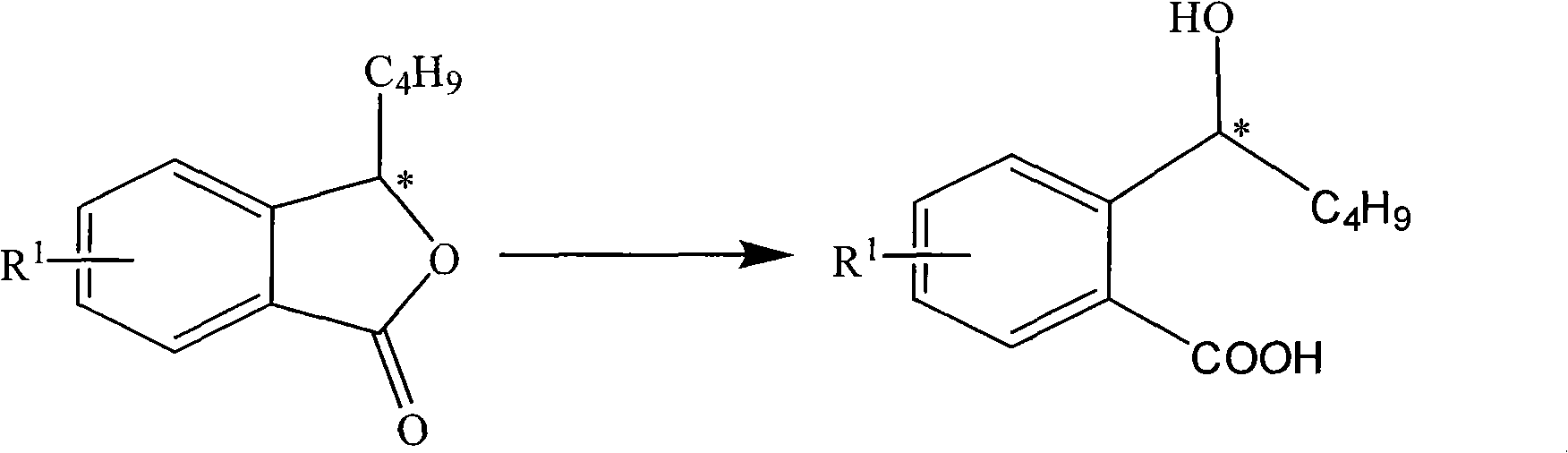
Halogenated 2-(a-hydroxyl pentyl) benzoate, production method and uses thereof
ActiveCN101402565ASuitable for storageSuitable for processingOrganic active ingredientsNervous disorderDiseaseBenzoic acid
The invention discloses a halogen substituted 2-(a-hydroxyl amyl) benzoate compound, a preparation method and medicinal application thereof, which belong to the technical field of organic chemical synthesis. The structural formula of the compound which is showed as above has an antimer structure, wherein <R1> represents a halogen atom; n is equal to 1 to 3; and M represents a univalent metallic ion or a bivalent metallic ion or a trivalent metallic ion or an organic base. The compound has the advantages of better activity on preventing and treating cardio-cerebral ischemia diseases, improving cardio-cerebral circulation disturbance and resisting thrombus, and the like.
Owner:ZHEJIANG AUSUN PHARMA
Catalyst for synthesizing methanol through carbon dioxide hydrogenation as well as preparation method and application thereof
InactiveCN103263926AImprove performanceLarge specific surface areaOrganic compound preparationHydroxy compound preparationDivalent metal ionsHigh carbon dioxide
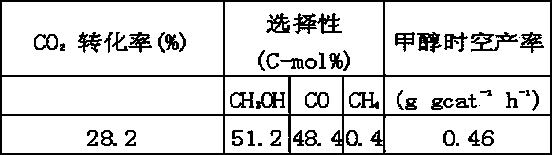
The invention discloses a catalyst for synthesizing methanol through carbon dioxide hydrogenation, which is composed of oxides, wherein measured by metals, the mole ratio of various metals is as follows: [n(Cu)+n(Zn)+n(MA)]:[n(Al)+n(MB)]=2-15, n(Cu):n(Zn)=0.5-4, n(MA):n(Zn)=0-1, and n(MB):n(Al)=0-9, MA represents monovalent or divalent metal ions, and MB represents trivalent or tetravalent metal ions. The catalyst has the advantages of high carbon dioxide conversion ratio, good methanol selectivity and high methanol yield.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Oral stannous compositions
The present invention relates to an aqueous oral composition comprising:a) from 0.2% to 3% divalent metal ions comprising:i. from 0.1% to 1.5% of zinc ions;ii. from 0.1% to 2% of tin (II) ions; andb) a source of fluoride ions;c) a silica dental abrasive;d) one or more chelants having a MW of less than 1000 and capable of forming water-soluble complexes with the zinc ions, wherein the chelants comprise less than 0.2% linear polyphosphates having a chain length of four or more;e) an orally acceptable carrier comprising at least 20% total water;wherein the pH of the composition is from 5 to 6.5, the molar ratio of the chelants to the divalent metal ions is at least 0.70:1 and at least 80% by weight of the total zinc ions are solubilised within the composition.The composition of the invention has been found to give improved antimicrobial activity from the zinc / stannous combination without significant taste, staining or stability problems, compared to compositions having lower levels of chelants.
Owner:PROCTER & GAMBLE CO
Process for dehydrochlorinating 1,1,1,2-tetrafluoro-2-chloropropane to 2,3,3,3-tetrafluoropropene in the presence of an alkali metal-doped magnesium oxyfluoride catalyst and methods for making the catalyst
ActiveUS20090299107A1Preparation by dehalogenationPreparation by hydrogen halide split-offDivalent metal ionsOxygen
A process for making a fluorinated olefin. The process has the step of dehydrochlorinating a hydrochlorofluorocarbon having at least one hydrogen atom and at least one chlorine atom on adjacent carbon atoms in the presence of a catalytically effective amount of a catalyst composition. The catalyst composition is represented by the following: n wt. % MX / M′OyFz, wherein 0<y<1 and 0<z<2 and wherein y+z / 2=1; M is an alkali metal ion selected from the group consisting of Li+, Na+, K+, Rb+, and Cs+; X is a halogen ion selected from the group consisting of F−, Cl−, Br−, and I−, M′ is a bivalent metal ion; wherein n is a weight percentage of about 0.05% to about 50% MX based on the total weight of the MX and M′OyFz, and wherein y and z are the mole fractions of oxygen and fluorine in M′OyFz, respectively. There are also methods for making catalyst compositions.
Owner:HONEYWELL INT INC
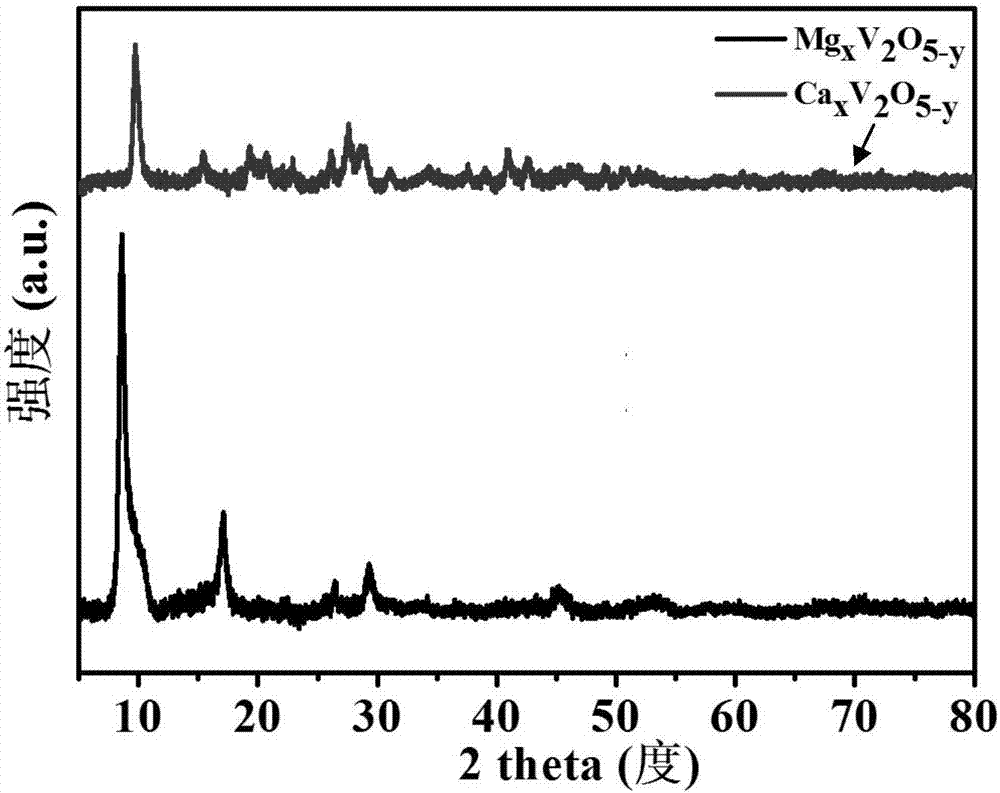
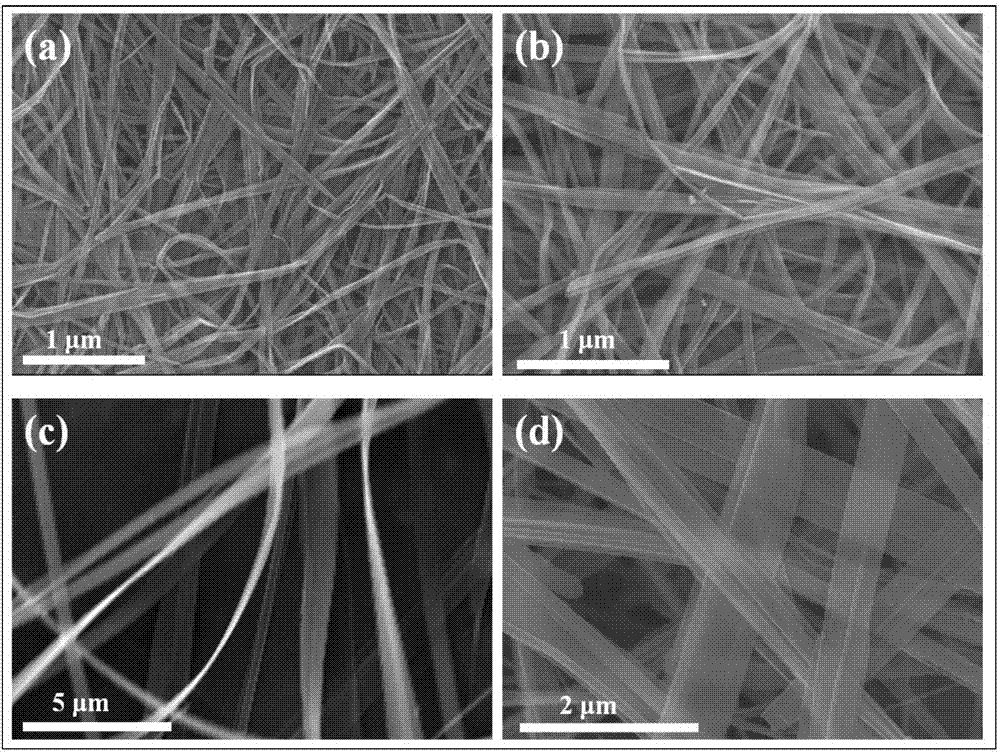
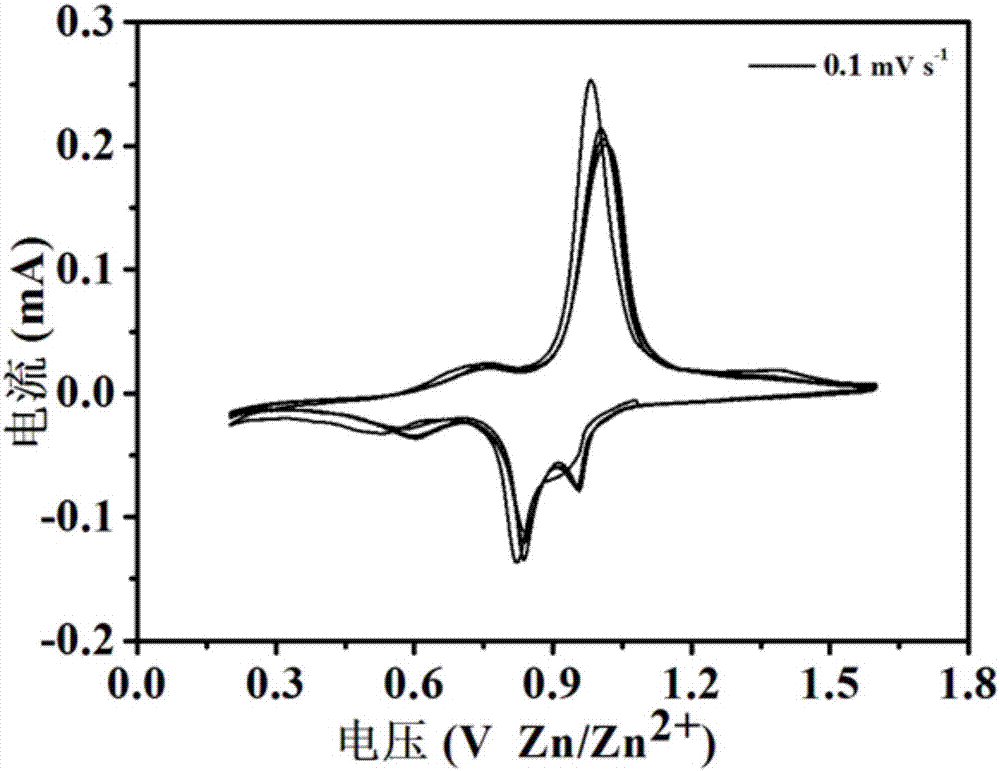
Divalent metal ion pre-embedded layered vanadium oxide nanometer material as well as preparation method and application thereof
ActiveCN107170967ALow priceAchieving controllable synthesisMaterial nanotechnologyCell electrodesDivalent metal ionsVanadium oxide
The invention relates to a divalent metal ion pre-embedded layered vanadium oxide nanometer material as well as a preparation method thereof. The chemical formula of the nanometer material is AxV2O5-y, wherein A is Mg, Ca, Sr or Zn; the MgxV2O5-y has the nanoribbon diameter of 100-1500nm and the length of 5-50 microns or CaxV2O5-y has the nanoribbon diameter of 100-1000nm and the length of 5-50 microns or SrxV2O5-y has the nanoribbon diameter of 100-1000nm and the length of 5-50 microns or ZnxV2O5-y has the nanoribbon diameter of 100-300nm and the length of 5-50 microns or ZnxV2O5-y has the nanoflower diameter of 3-5 microns. The divalent metal ion pre-embedded layered vanadium oxide nanometer material disclosed by the invention has high specific capacity, excellent cycling stability and excellent rate capability and is a potential high-performance commercial zinc ion battery positive electrode material.
Owner:WUHAN UNIV OF TECH
Detergent Composition
InactiveUS20100160202A1Efficient removalImprove enzyme stabilityInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsDivalent metal ionsLotion
A detergent composition comprising; a) a non-phosphorous based builder which is an amino acid based compound or a succinate based compound, b) one or more enzymes which are destabilised by the non-phosphorous containing builder and c) a stabilisation system for the one or more enzymes. The stabilising system comprising one or more divalent metal ion salts and a non-ionic surfactant. Preferably the builder is the tetrasodium salt of glutamic-N,N-diacetic acid and the enzyme comprises protease. The preferred divalent metal ion salts are calcium salts.
Owner:RECKITT BENCKISER NV
Methods of treating subterranean zones using gelled aqueous treating fluids containing environmentally benign sequestering agents
Methods of treating subterranean zones using gelled aqueous treating fluids containing environmentally benign sequestering agents are provided. The methods basically comprise the steps of preparing or providing a treating fluid comprising water containing divalent metal ions, a gelling agent, a borate crosslinking agent and an environmentally benign sequestering agent, and then introducing the treating fluid into the subterranean zone.
Owner:HALLIBURTON ENERGY SERVICES INC
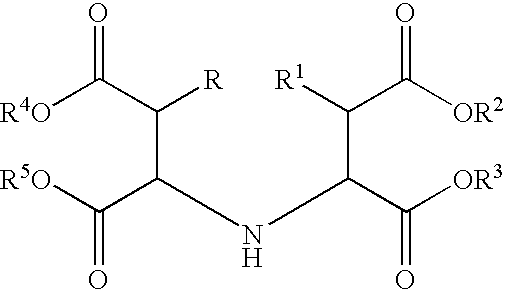
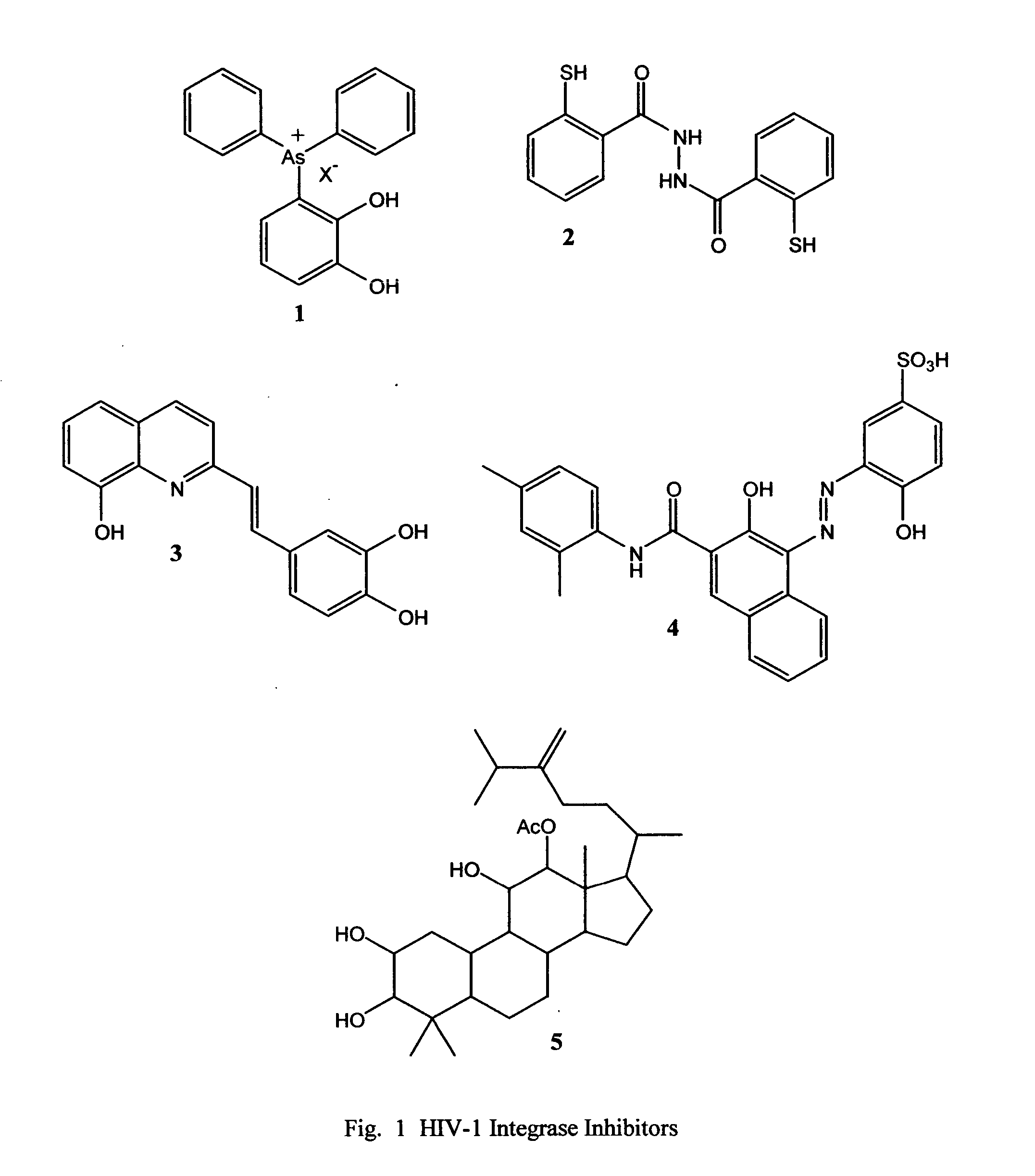
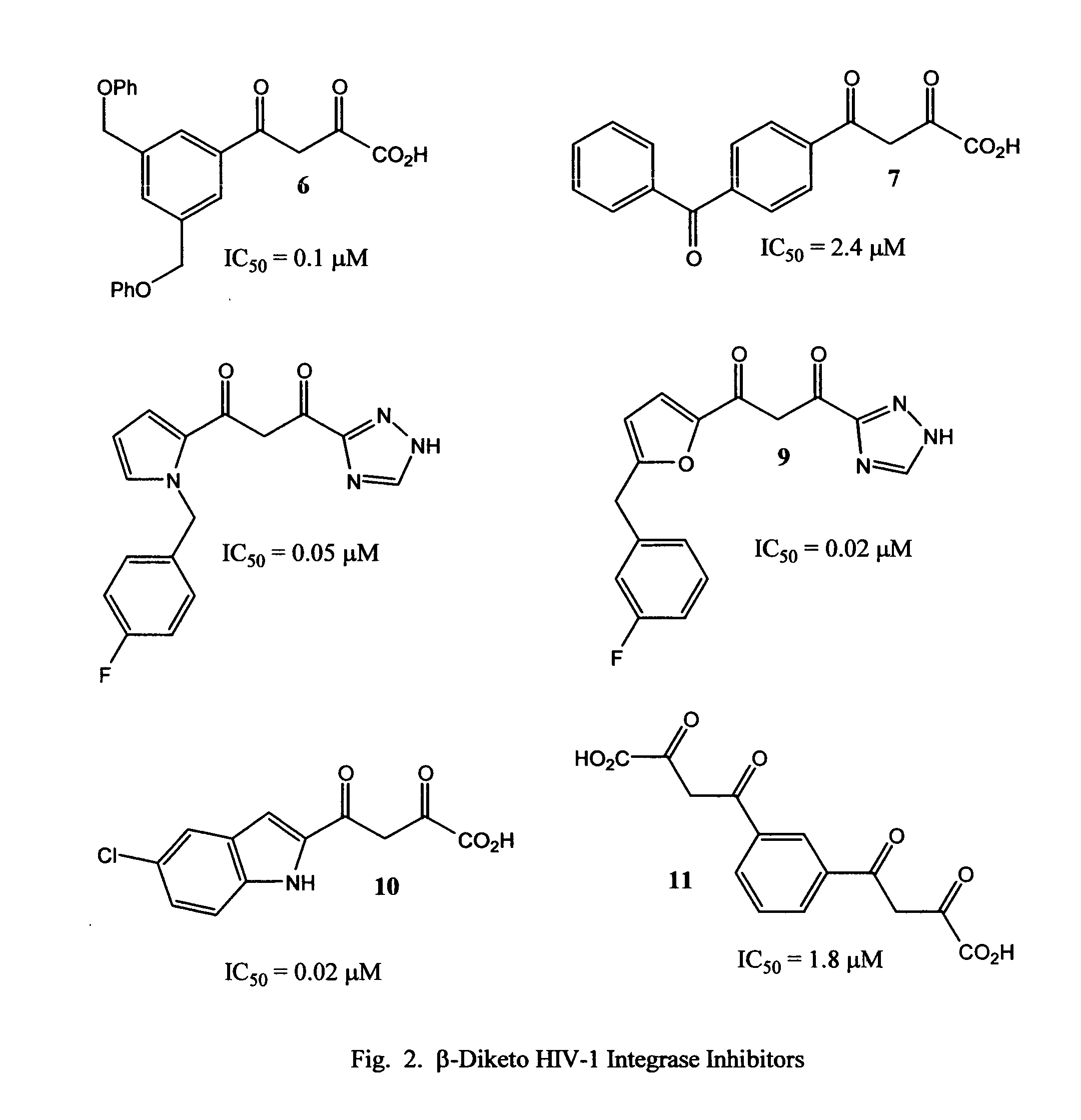
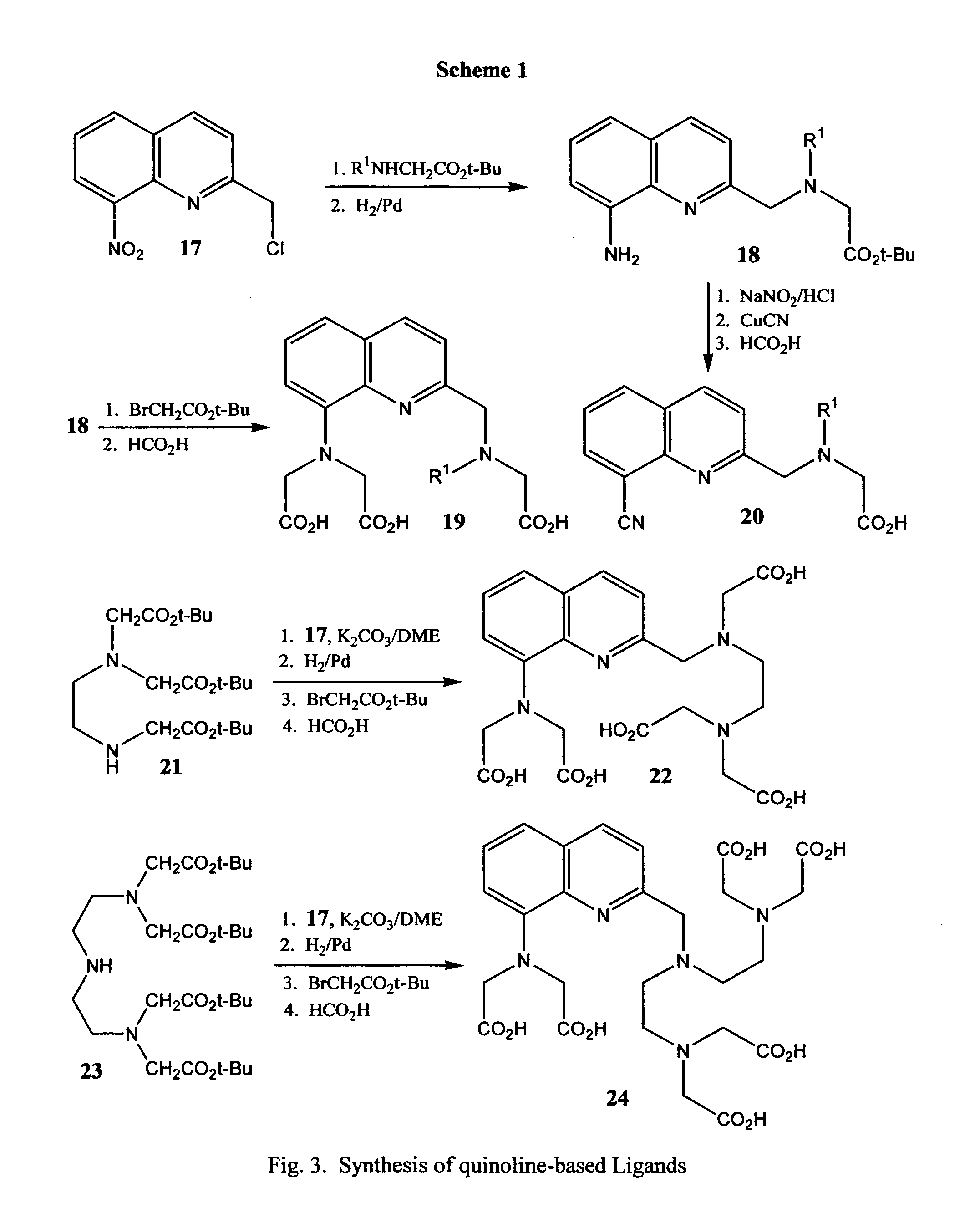
Novel quinoline-based metal chelators as antiviral agents
This invention relates to novel quinoline-based divalent metal ion chelating ligands of Formula I, wherein A or B are independently —CR7R8, or —CH(R9)CH(R10). X is hydrogen, C1-C10 alkyl; —OH, or —NR11R12. R1 to R12 are various substituents selected to optimize the physicochemical and biological properties such as enzyme binding, tissue penetration, lipophilicity, toxicity, bioavailability, and pharmacokinetics of compounds of Formula 13. R1 to R12 may include, but are not limited to hydrogen, alkyl, acyl, hydroxyl, hydroxyalkyl, substituted or unsubstituted aryl, amino, aminoalkyl, alkoxyl, aryloxyl, carboxyl, halogen, alkoxycarbonyl, cyano, and other suitable electron donating or electron withdrawing groups. The compounds of the present invention are useful for inhibiting the activity of viral enzymes responsible for the proliferation of human immunodeficiency virus (HIV).
Owner:BIOFLEXIS
Agent for activation sector cruor activating-enzyme time (APTT)
The invention relates to a detection agent for hemostasis and thrombus belonging to medical clinical detection technical field, in particular to a detection agent for measuring activation partial thromboplastin time (APTT) of blood plasma. The invention is characterized in that the agent is prepared from 0.05-0.5g / L agent solid activator, 20-200mg / L phosphatide, 0.1-10mmol / L divalent metal ion salt, 0.1-0.5g / L stabilizer, 0.1-10g / L polyethylene glycol PEG, 0.05-4.0g / L buffer agent, 2.0-5.0g / L revival agent, while the left is water. The invention provides a high-stability standard agent for meeting APTT measurement, with simple operation, suitable price, short activation time and high sensitivity.
Owner:CHANGAN UNIV
Method for preparing high-strength alginic acid/gelatin cross-blend fiber and its use
ActiveCN1978718AImprove mechanical propertiesImprove water absorptionSurgeryAlginate artificial filamentsCross-linkFiber
The invention discloses high strength alginic acid / gelatin blended fiber manufacturing method and use. The method includes the following steps: making sodium alginate solution with 3-6% mass percent; making gelatin aqueous solution with 6-12% mass percent and 8.0-9.0 pH; mixing them as 10-50:100 volumetric ratio; stirring to homogeneous phase system; decompress; de-bubbling; using wet spinning method to make fiber under 33 degree centigrade of which total draft is 10-30%. The invention makes cross blend spinning fluid be cross linking with Ca2+ divalent metal ion, and makes the fiber under acidity with polyelectrolyte effect which can further increase fiber cross linking level, breaking tenacity to improve fiber physical mechanical properties. Thus it can be used to make non-woven fabrics such as hospital gauze, dressing etc.
Owner:青岛海赛尔新材料科技有限公司
Preparation method for anion-exchangeable, layered double hydroxides
InactiveUS20100279848A1Overcome defectsSimple methodProductsGas treatmentIon exchangeDivalent metal ions
The invention has for its object to provide a preparation method for preparing an anion-exchangeable LDH by decarbonation of a carbonate ion type LDH, which makes sure de carbonation is implemented with safety in a continuous manner while crystal shape, crystal structure and crystallinity are kept intact.The invention provides a preparation method for preparing an anion-exchangeable, layered double hydroxide wherein a carbonate ion type layered double hydroxide (LDH) having a composition represented by a general formula: QxR(OH)z(CO32−)0.5-y / 2(X−)y.nH2O where x is indicative of a numeral range of 1.8≦x≦4.2; z is indicative of 2(x+1); y is indicative of a minimum value of at least 0 that increases to less than 1 when anions (X−) remain or a part of anions is introduced; Q is a divalent metal ion; R is a trivalent metal ion; and n is 2±2 is used as a starting material, and y in said general formula increases to a maximum of 1 by substitution of a minus monovalent anion (X−1) at a carbonate ion site thereby implementing substitution, characterized in that the starting material is dispersed in an aqueous solution mixed with a salt containing minus monovalent anions (X−) in an amount enough for substitution at the carbonate ion site while said aqueous solution is kept at a pH (hydrogen ion exponent) of greater than 4 to less than 7.
Owner:NAT INST FOR MATERIALS SCI
Nickel-cobalt-aluminum ternary cathode material with high tap-density and preparation method of nickel-cobalt-aluminum ternary cathode material
InactiveCN105489886AStable layered crystal structureIncrease contentCell electrodesSecondary cellsDivalent metal ionsSlurry
The invention relates to a nickel-cobalt-aluminum ternary cathode material with high tap-density and a preparation method of the nickel-cobalt-aluminum ternary cathode material. The method comprises the following steps: adding a nickel-cobalt mixed solution, a precipitant solution and a complexing agent solution to a reaction kettle in parallel flow; controlling the pH value, the temperature, the complexing agent concentration and the stirring speed in the reaction kettle, carrying out co-precipitation reaction and overflow discharge, washing slurry until the pH is smaller than 8.0, and carrying out drying and sieving to obtain spherical nickel-cobalt precursor powder; and mixing the nickel-cobalt precursor powder, an aluminum-containing compound, a doped compound and a lithium salt evenly, presintering the mixture at a low temperature, directly increasing the temperature for heat preservation, naturally cooling the mixture, and crushing an sieving the mixture to obtain the nickel-cobalt-aluminum ternary cathode material with high tap-density. Divalent metal ions are doped, so that a layered crystal structure of the material is stabilized; the content of Ni<3+> is improved; and shuffling of Li<+> and Ni<2+> ions is reduced. According to the method, continuous mass production can be achieved. The nickel-cobalt-aluminum ternary cathode material has the advantages of good rate capability, high discharge capacity, long cycle lifetime and the like.
Owner:SHANDONG YUHUANG NEW ENERGY TECH
Extraction of Sulfate from Water
InactiveUS20120031850A1Reduce metal contentLess soluble in waterSedimentation separationRadioactive contaminantsAluminium chlorideAluminite
Sulfate anions and divalent metal ions, such as magnesium, strontium and barium, in water are removed by treating the water with polyaluminum chloride, usually together with lime, to form ettringite and similar crystalline species which are readily removable by settling, filtration and the like. Iron is also removed by oxidation in a variation of the process. The process is particularly useful for treating aqueous solutions used in well treatment, where flowback fluids can provide some of the divalent metal ions necessary to form the ettringite-like materials, thus reducing the amount of lime otherwise necessary and further facilitating recycling of the fluid.
Owner:SMART CHEM SERVICES LP
Catalyst of load type bimetallic cyaniding complex, preparation method and application
This invention discloses a method for preparing double-metal cyanide complex supported catalyst and its application. The composition is : S-M11a[M(CN)BL1c]íñxM11X2íñy1L2íñy2L3.zH2O, wherein, S is the carrier, and is SiO2, TiO2 or their mixture; M11 is divalent metal ion; M is divalent, trivalent or valency-changeable metal ion; X is halogen anion or analog of halogen anion; L1 is high molecular or low molecular mono-tooth or double-tooth ligand of M; L2 is water-soluble alcohol or cyclic ether; L3 is water-soluble or alcohol-soluble compound, and can be high molecular or low molecular mono-tooth or double-tooth ligand; a and b are positive integers; c is 0 or positive integer; x, y1, y2 and z are 0 or positive numbers. The catalyst is stable in air, and is insensitive to water vapor. The catalyst can be adequately utilized due to supporting, and can be easily removed from reaction products.
Owner:ZHEJIANG UNIV
Catalyst for synthesizing methanol through CO2 hydrogenation as well as preparation method and application
ActiveCN103721719ALarge specific surface areaGood dispersionOrganic compound preparationHydroxy compound preparationPtru catalystHalogen
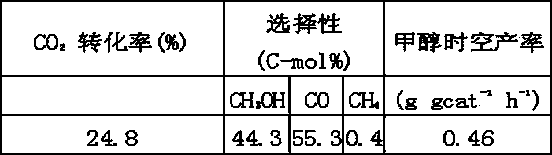
The invention discloses a catalyst for synthesizing methanol through CO2 hydrogenation, comprising Cu, Zn, Al, X, halogen and oxygen elements, oxides and halides, wherein the molar ratio of [Cu+Zn+MA] to [Al+Mb] is 2-18; the molar ratio of Cu to Zn is 0.5-5; the molar ratio of MA to [Cu+Zn] is 0-5; the molar ratio of MB to Al is 0-9; the molar ratio of halogen to Al is 0.05-5; MA and MB cannot be 0 at the same time; MA represents a mono-valent or divalent metal ion in X; MB represents a trivalent and / or tetravalent metal ion in X; X is one or a combination of more elements of Li, K, Mg, B, Ga, In, transition metal elements and rare-earth metal elements. The catalyst has the advantages of high carbon dioxide conversion rate, good methanol selectivity and high methanol yield.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Salt-tolerant resistance-reducing agent applied for shale gas reservoir
InactiveCN103333672AImprove salt toleranceGood resistance reduction effectDrilling compositionBiopolymerDivalent metal ions
The invention relates to a salt-tolerant resistance-reducing agent applied for a shale gas reservoir. The resistance-reducing agent is a biopolymer comprising an acrylamide unit and a propenyl quaternary ammonium salt unit. The weight percent of the acrylamide in the biopolymer is 10%-80%. The propenyl quaternary ammonium salt is one of a propenyl trialkyl ammonium chloride, an alkyl acrylamido propyl trialkyl ammonium chloride or a dialkyl diallyl ammonium chloride. The weight percent of the propenyl quaternary ammonium salt in the biopolymer is 20%-90%, and the molecular weight of the propenyl quaternary ammonium salt is 1*10<6> to 2*10<6>. The resistance-reducing agent provided by the invention can be used as resistance-reducing agents for fracturing flow-back fluid, cavern water and underground produced water, wherein the fracturing flow-back fluid, the cavern water and the underground produced water contain divalent metal ions, and the weight percent resistance-reducing agent provided by the invention for using is 0.05%-0.1%. The resistance-reducing agent provided by the invention has good salt tolerance and resistance-reducing properties, and overcomes problems, of other polypropanamide resistance-reducing agents at present, comprising salt tolerance deficiency in using processes and incapability of being applied for hypersalinity water that comprises Ca<2+> and Mg<2+> at the same time.
Owner:PETROCHINA CO LTD
Inhibitor for enzyme having two divalent metal ions as active center
InactiveUS20100068695A1Inhibit enzymeInhibition is effectiveOrganic chemistryMicrobiological testing/measurementDivalent metal ionsEnzyme inhibitor
Owner:KIYAMA RYUICHI +1
Layer by layer self-assembly compound nanofiltration membrane based on natural cellulose polyelectrolyte and preparation method
ActiveCN103551049AStrong anti-pollutionImprove interception effectSemi-permeable membranesCelluloseUltrafiltration
The invention discloses a layer by layer self-assembly compound nanofiltration membrane based on natural cellulose polyelectrolyte and a preparation method, belonging to the technical field of membrane separation. The preparation method comprises the following steps: firstly, adopting an ultrafiltration membrane as a base membrane for preparing a compound nanofiltration membrane, carrying out chemical modification on the surface of the base membrane to ensure that the surface of the base membrane has a charge property so as to generate static reaction with a polyelectrolyte; and secondly, through alternating depositing anionic and cationic polyelectrolytes, preparing the compound nanofiltration membrane through a layer by layer self-assembly method. According to the preparation method, the adopted cationic polyelectrolyte is a natural cellulose polyelectrolyte; compared with a currently used synthetic polyelectrolyte, the natural cellulose polyelectrolyte is lower in cost, and is an environmental-friendly resource; and the prepared compound nanofiltration membrane containing natural cellulose is good in hydrophilcity and charge performance so that the surface of the membrane is good in pollution resistance; and the compound nanofiltration membrane has a good retaining property for dye molecules such as divalent metal ions such as Ni<2+>, xylenol orange sodium salt, and rhodamine B.
Owner:BEIJING UNIV OF TECH
Synthetic inorganic flame retardants, methods for their preparation, and their use as flame retardants
InactiveUS20110213065A1High flame retardant efficiencyImprove thermal stabilitySilicaAlkali metal silicatesAlkaline earth metalPhosphate
Quite unexpectedly, by suitably modifying the crystal structure of hydrogarnets of the general formula MII3MrI-III2(OH)12 (where MII denotes divalent metal ions, especially alkaline earth metal ions, of Group IIA of the periodic table and MIII denotes trivalent metal ions of Group IIIA of the periodic table, especially aluminum) with suitable amounts of incorporated silicate and / or phosphate, flame retardants having both a higher flame retardant efficiency than such traditional mineral flame retardants as ATH and MDH, and a higher thermal stability than ATH can be produced. It has also been found that synthetic hydrogarnets of the general formula MII3MrIII2(OH)12(where MII and MIII are as defined above) having cubic crystal and these synthetic hydrogarnets also show high flame retardant efficiency.
Owner:ALBEMARLE CORP
Method for coloring ceramic surfaces
InactiveUS6114054APromote formationReduce solubilityPretreated surfacesCoatingsDivalent metal ionsWater soluble
PCT No. PCT / EP98 / 00136 Sec. 371 Date Dec. 15, 1998 Sec. 102(e) Date Dec. 15, 1998 PCT Filed Jan. 13, 1998 PCT Pub. No. WO98 / 31647 PCT Pub. Date Jul. 23, 1998A method for coloring a surface of a ceramic mass to produce a colored ceramic body includes providing a host lattice material composed of a colorless metal oxide compound which crystallizes into one of a spinel lattice or a rutile lattice and which may be water soluble; providing an aqueous coloring solution containing water; a first water soluble compound including a metal ion which is one of a two-valent metal ion or a three-valent metal ion and which colors the host lattice material; and a second water soluble compound including a metal ion which is one of a five-valent metal ion or six-valent metal ion and which provides electrostatic balance; generating a mixed-phase pigment in the surface of the ceramic mass by one of (i.) working a fine powder of the colorless metal oxide into the ceramic mass and applying the aqueous coloring solution onto at least one surface of the ceramic mass, or (i.i.) adding a water soluble colorless metal oxide compound to the aqueous coloring solution in an amount effective to form a mixture having a preselected viscosity and applying this mixture to the surface of the ceramic mass; drying the solution; and firing the ceramic body at a temperature ranging from 300 to 1400 DEG C. for a duration ranging from 0.5 to 5 hours.
Owner:BK GIULINI CHEM
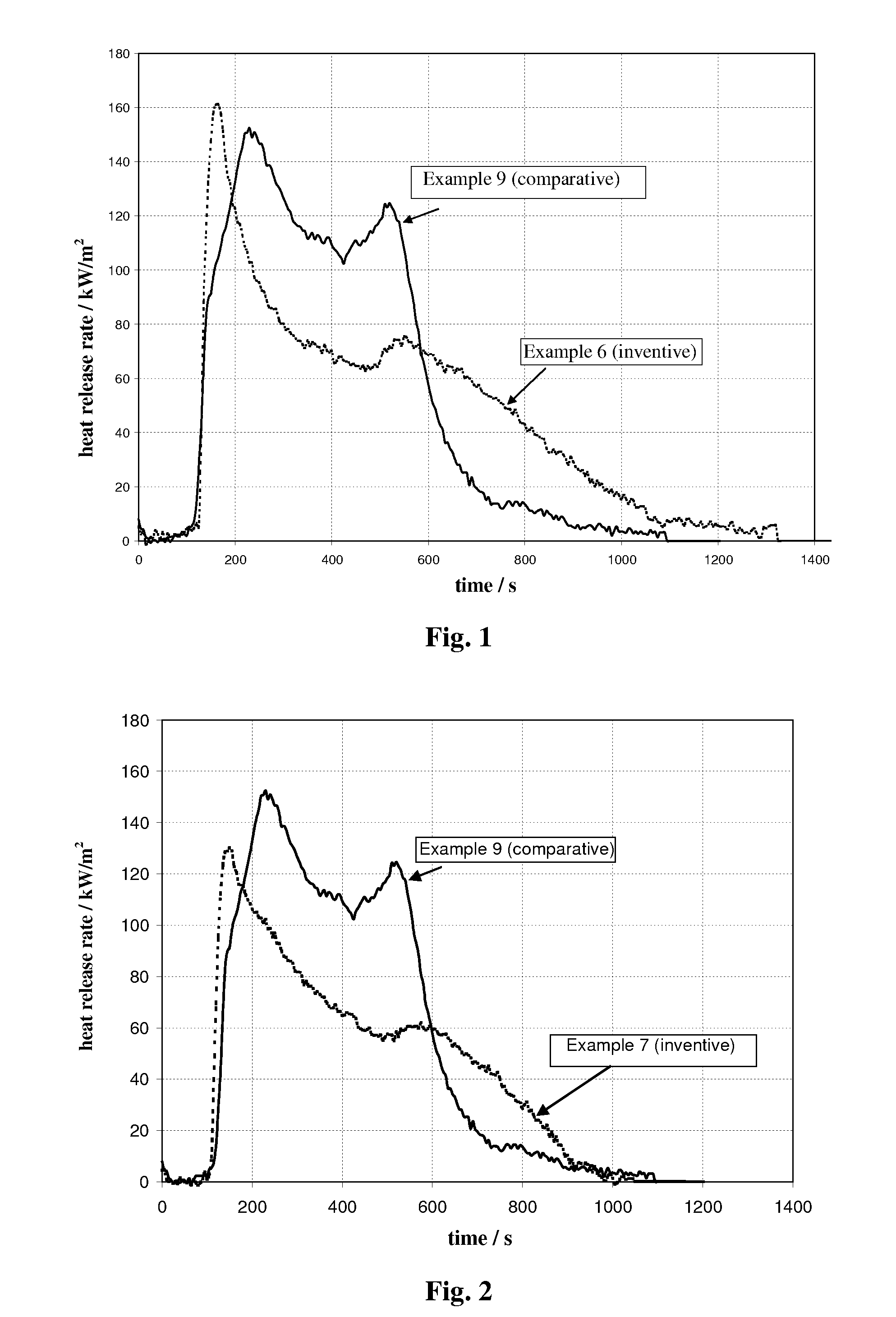
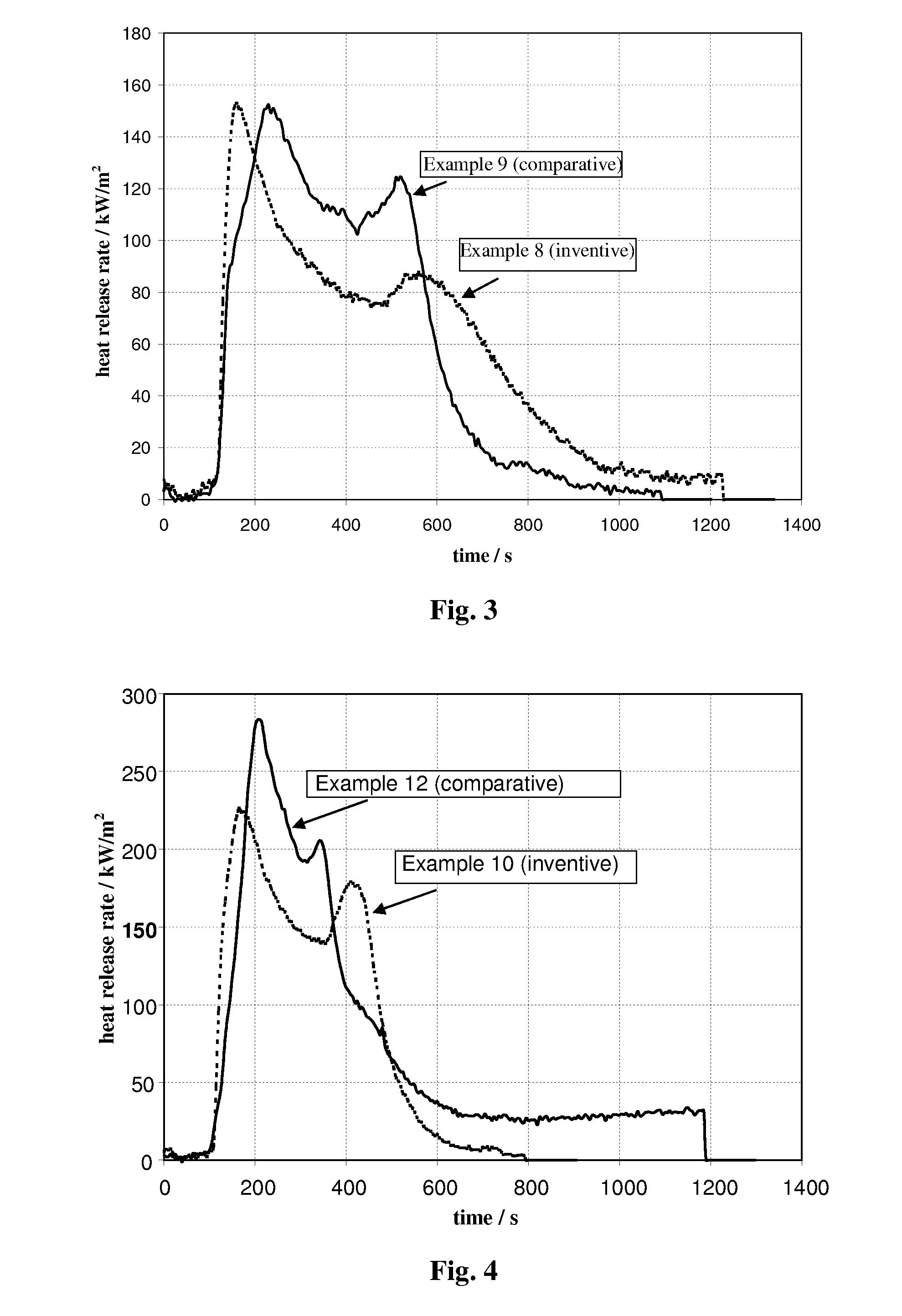
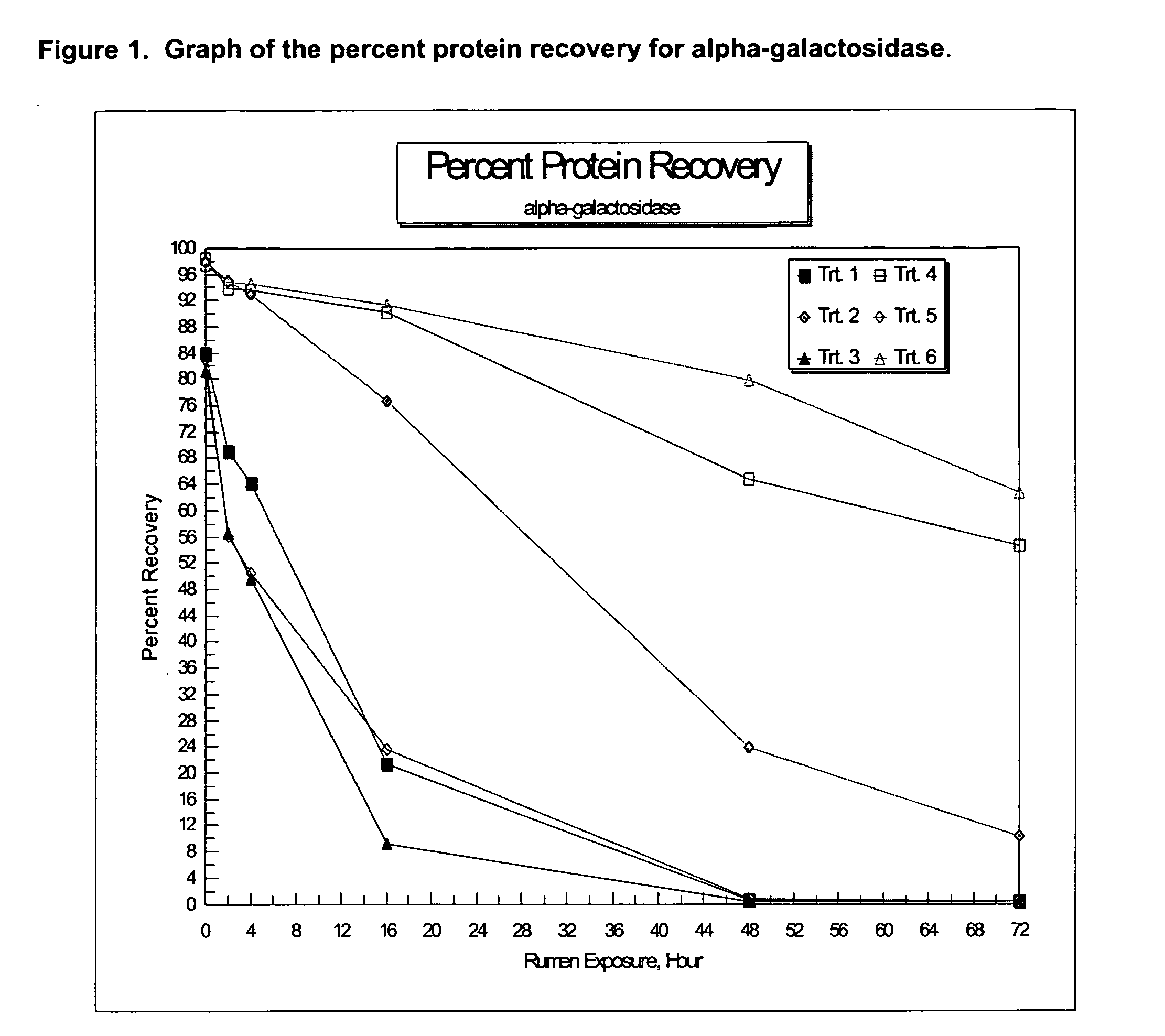
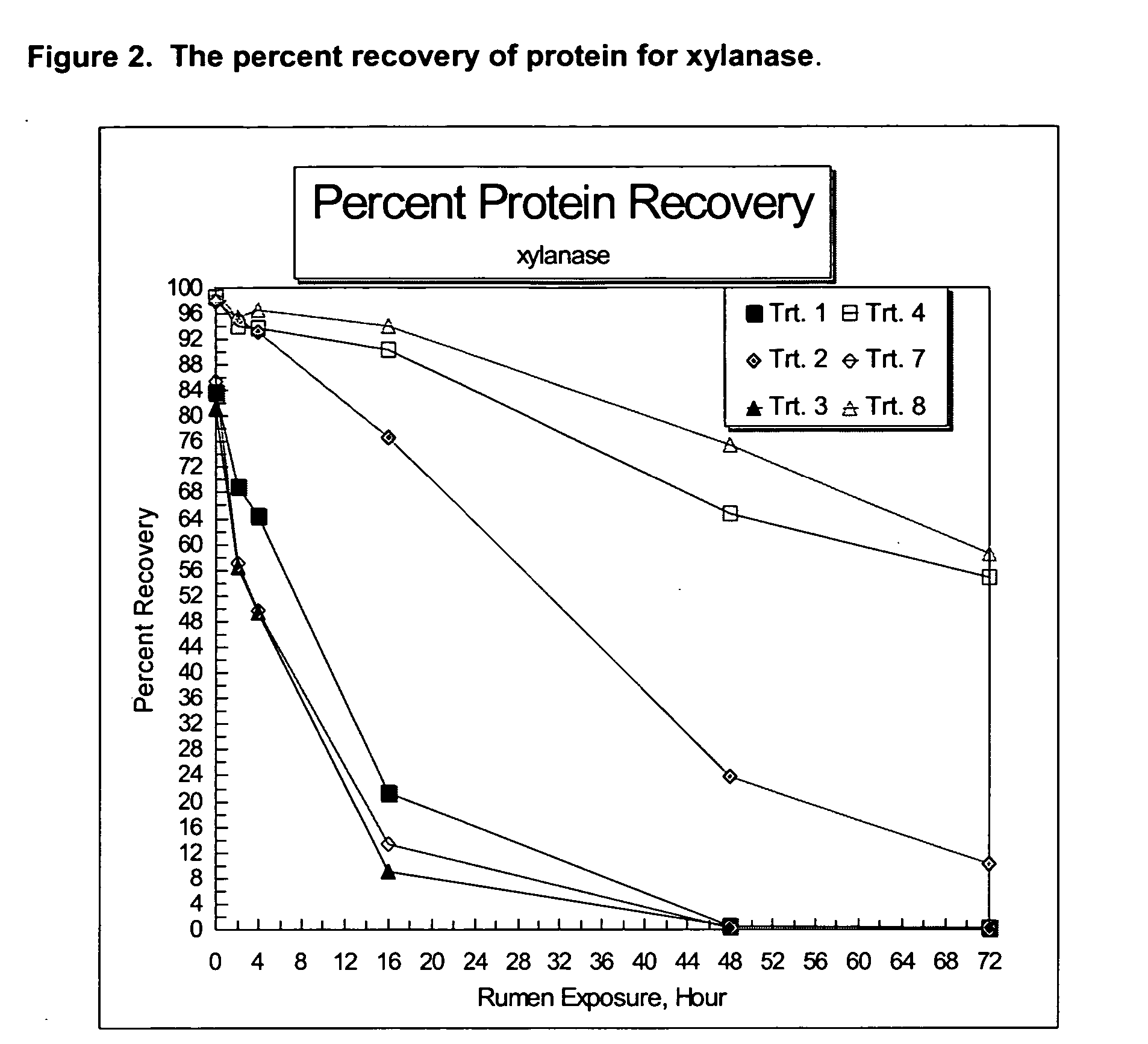
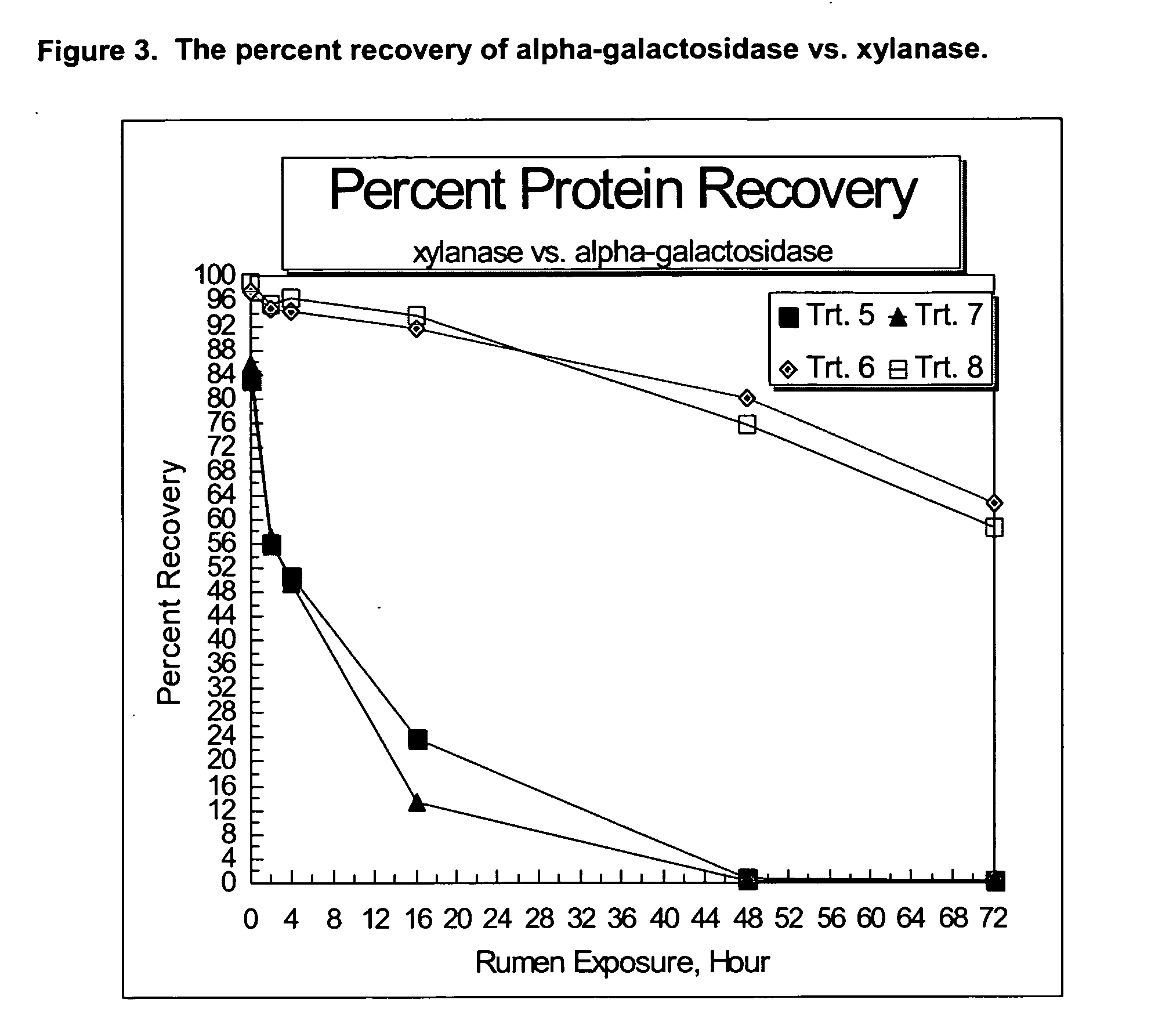
Compositions and methods providing rumen bypass protein in ruminant diets
The present invention is based on the discovery that moist heat treated ruminant animal feed compositions comprising a fermentation biomass, have increased amounts of proteinaceous matter that escapes fermentation within the rumen. The ruminant animal feed compositions may further comprise, alone or in combination, one or more of an isolated enzyme, an organic acid, a gluten protein, at least one divalent metal ion and at least one plant extract. The proteinaceous matter may then be digested or metabolized in the post-rumen portions of the ruminant digestive system, thereby providing further increased energy and protein levels for ruminant animals during times of increased productivity. Compositions and methods of manufacture of the compositions of the embodiments of the present disclosure are disclosed.
Owner:ARCHER DANIELS MIDLAND CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com