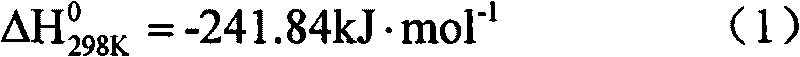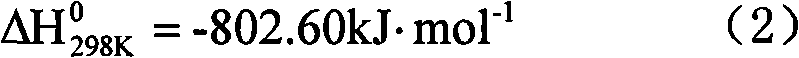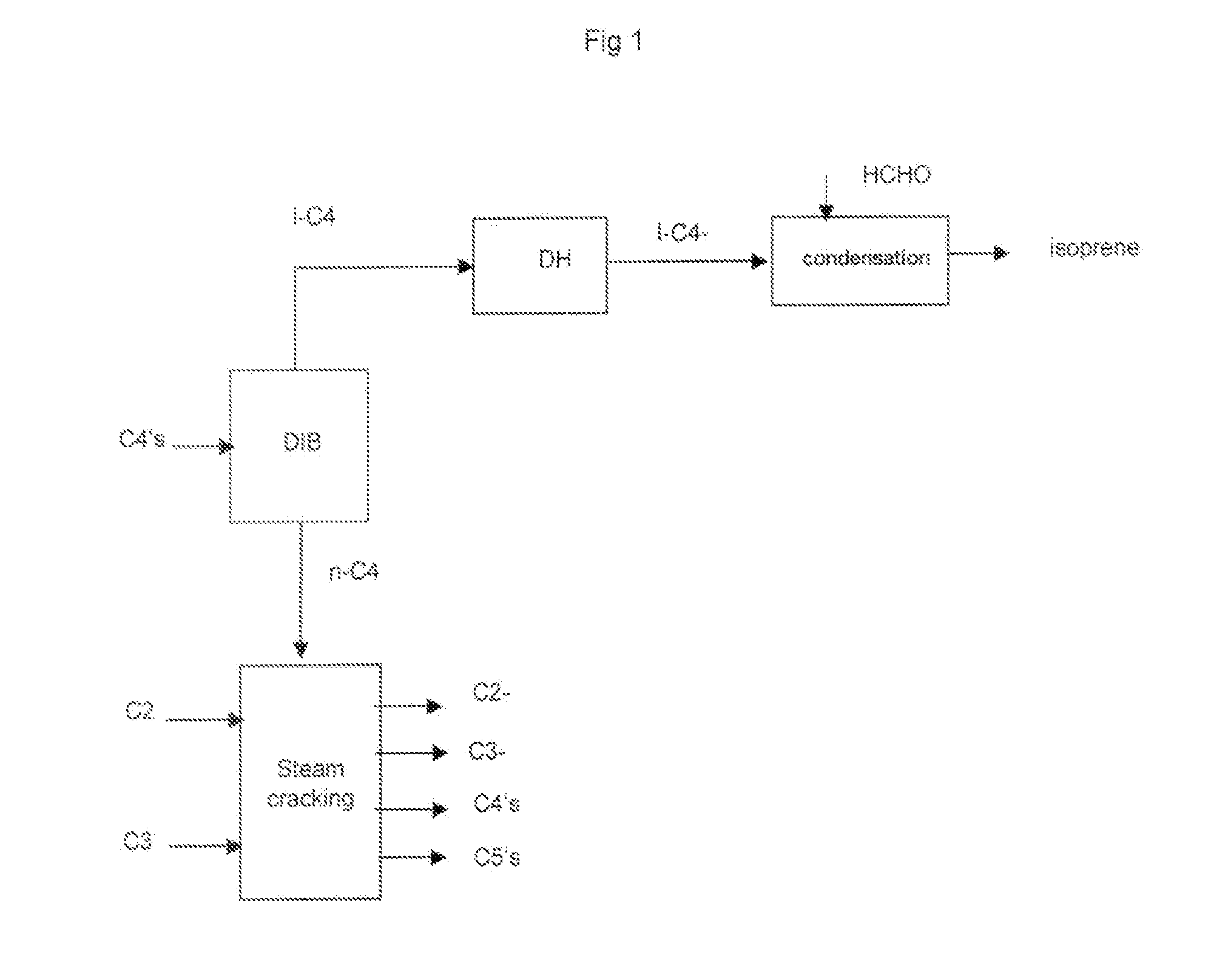Patents
Literature
425 results about "Non catalytic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non catalytic reactions are chemical reactions in which a catalyst does not involve in the reaction process. Therefore, in these reactions, the reaction rate does not increase by any external influence.
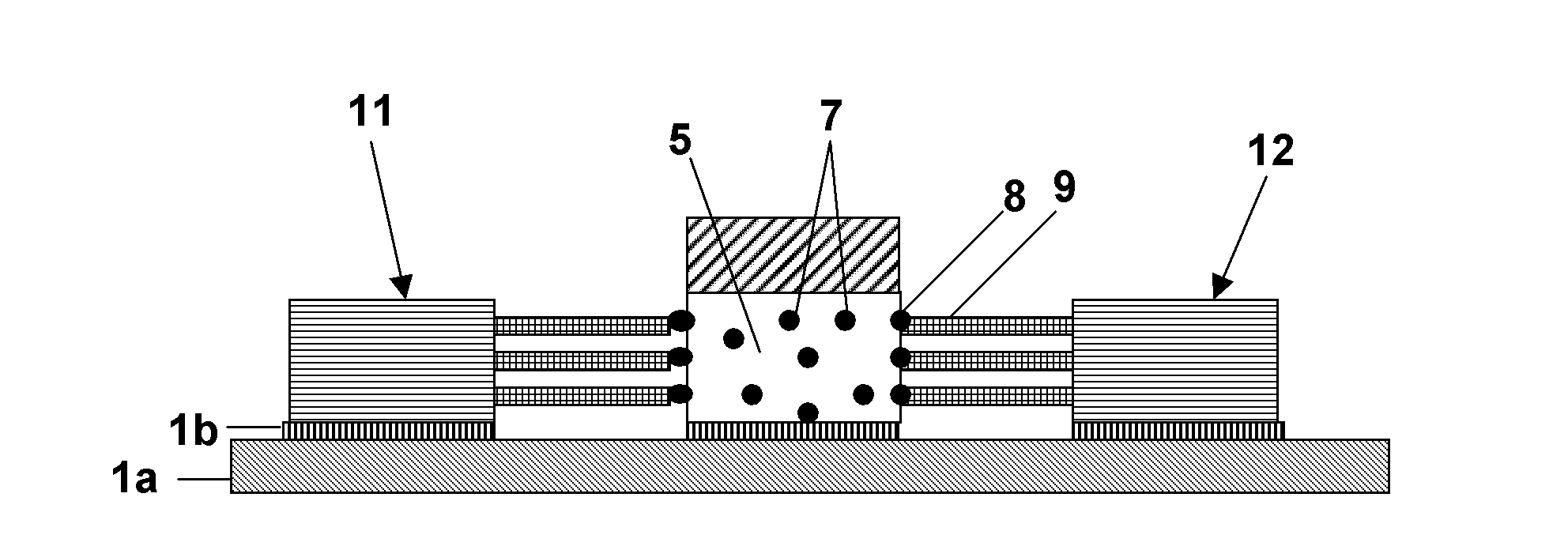
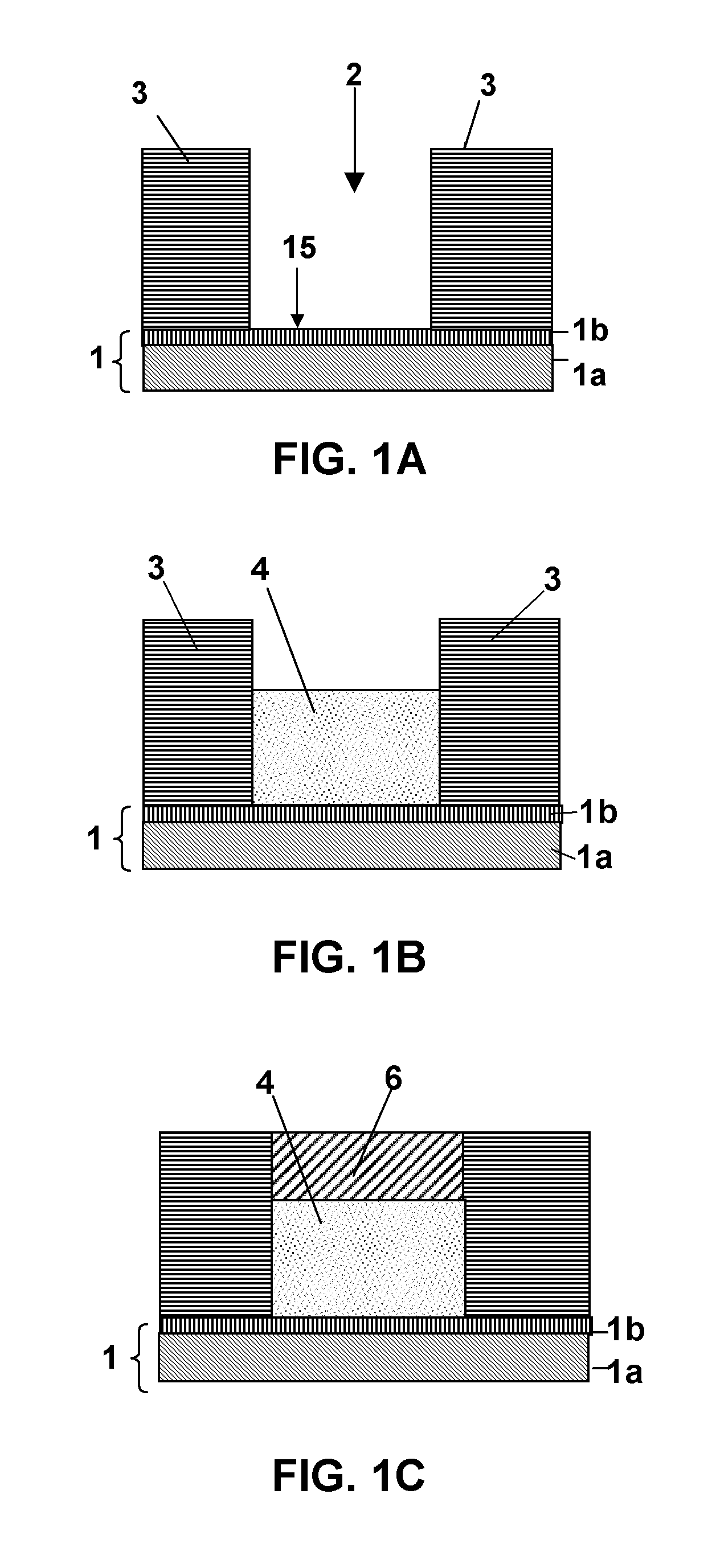
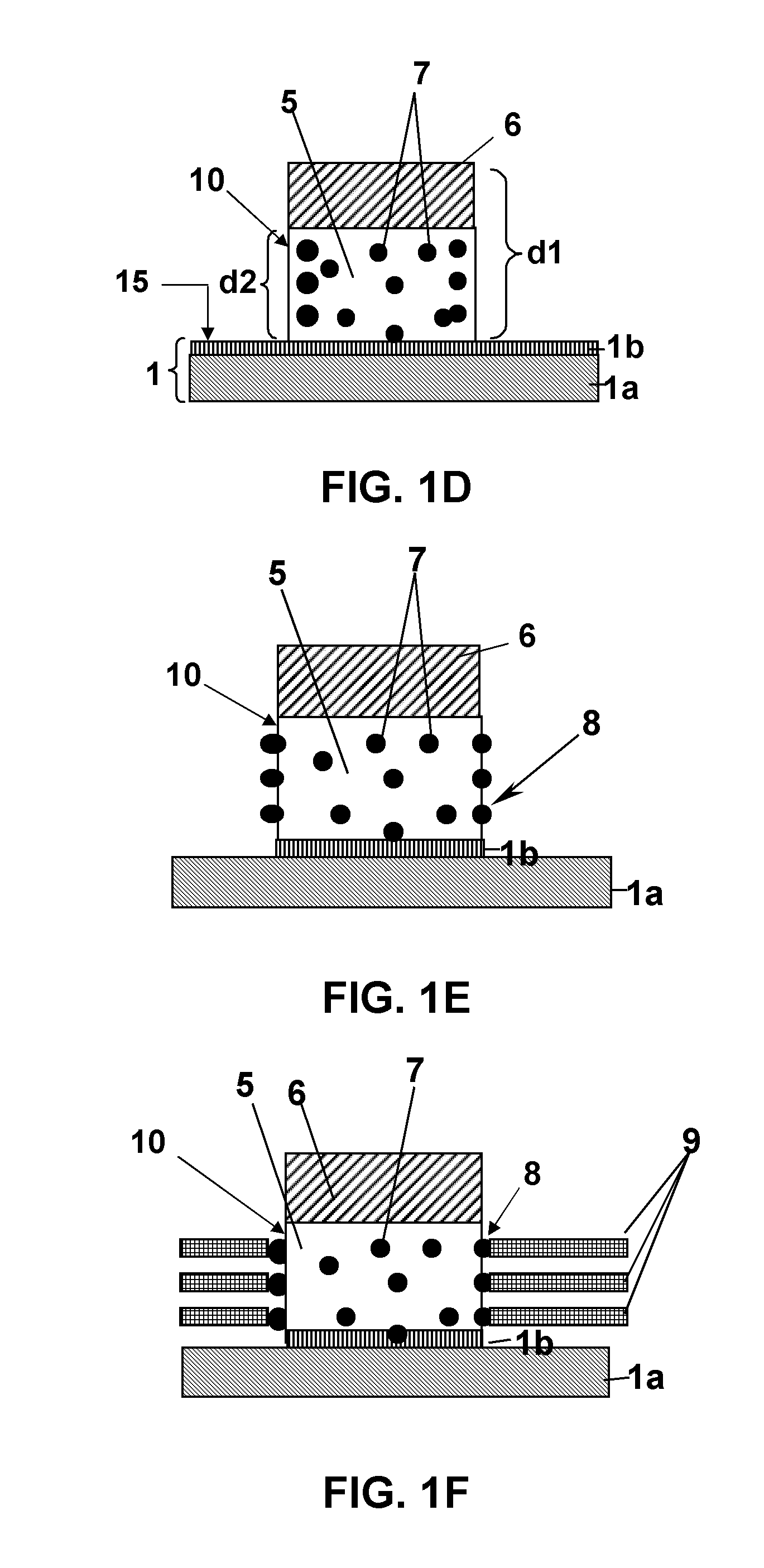
Polycrystalline compacts having material disposed in interstitial spaces therein, cutting elements and earth-boring tools including such compacts, and methods of forming such compacts
Polycrystalline compacts include smaller and larger hard grains that are interbonded to form a polycrystalline hard material. The larger grains may be at least about 150 times larger than the smaller grains. An interstitial material comprising one or more of a boride, a carbide, a nitride, a metal carbonate, a metal bicarbonate, and a non-catalytic metal may be disposed between the grains. The compacts may be used as cutting elements for earth-boring tools such as drill bits, and may be disposed on a substrate. Methods of making polycrystalline compacts include coating smaller hard particles with a coating material, mixing the smaller particles with larger hard particles, and sintering the mixture to form a polycrystalline hard material including interbonded smaller and larger grains. The sizes of the smaller and larger particles may be selected to cause the larger grains to be at least about 150 times larger than the smaller grains.
Owner:BAKER HUGHES INC
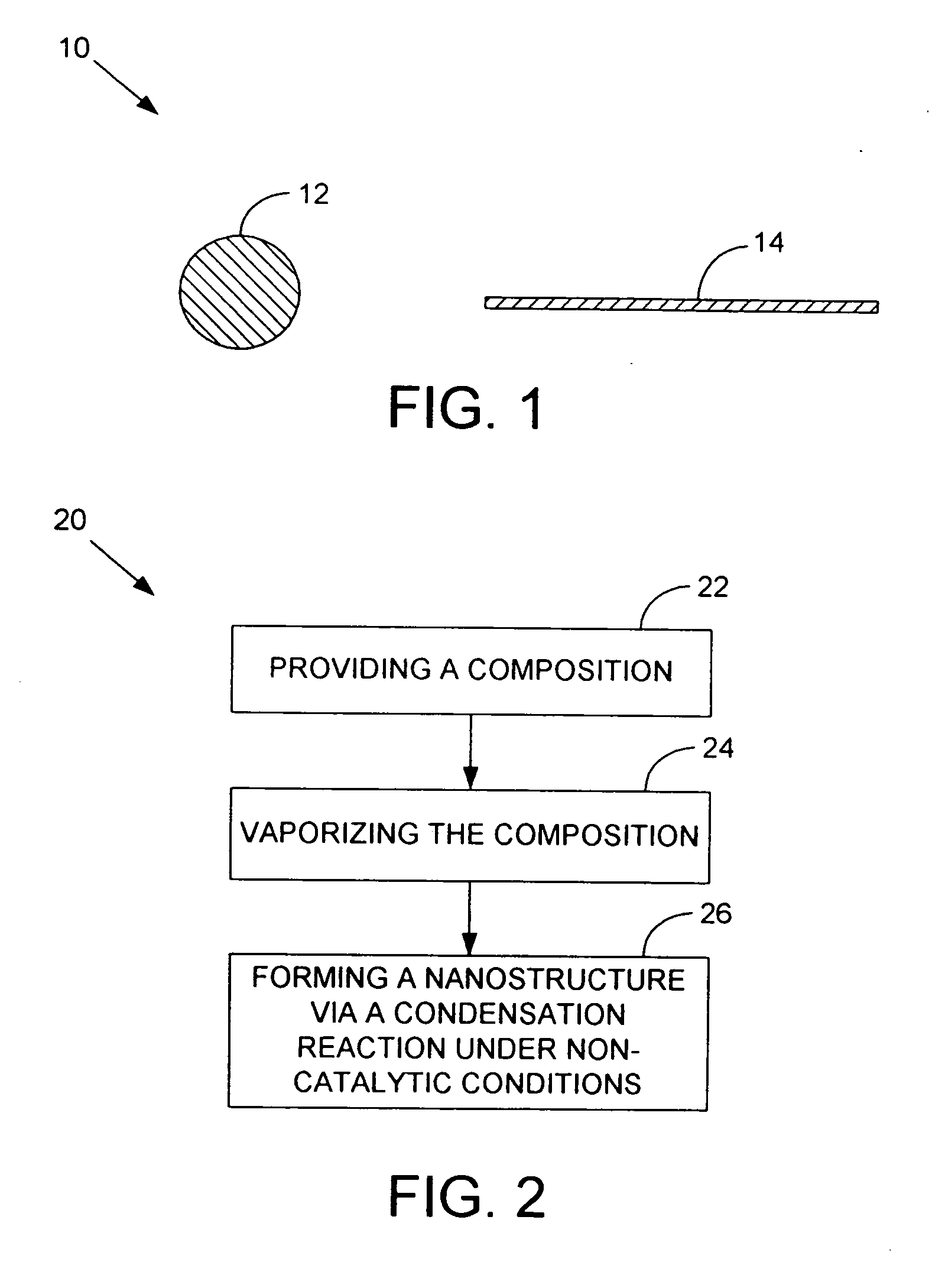
Silicon based nanospheres and nanowires
InactiveUS20070178673A1Material nanotechnologyPolycrystalline material growthNanowireOptoelectronics
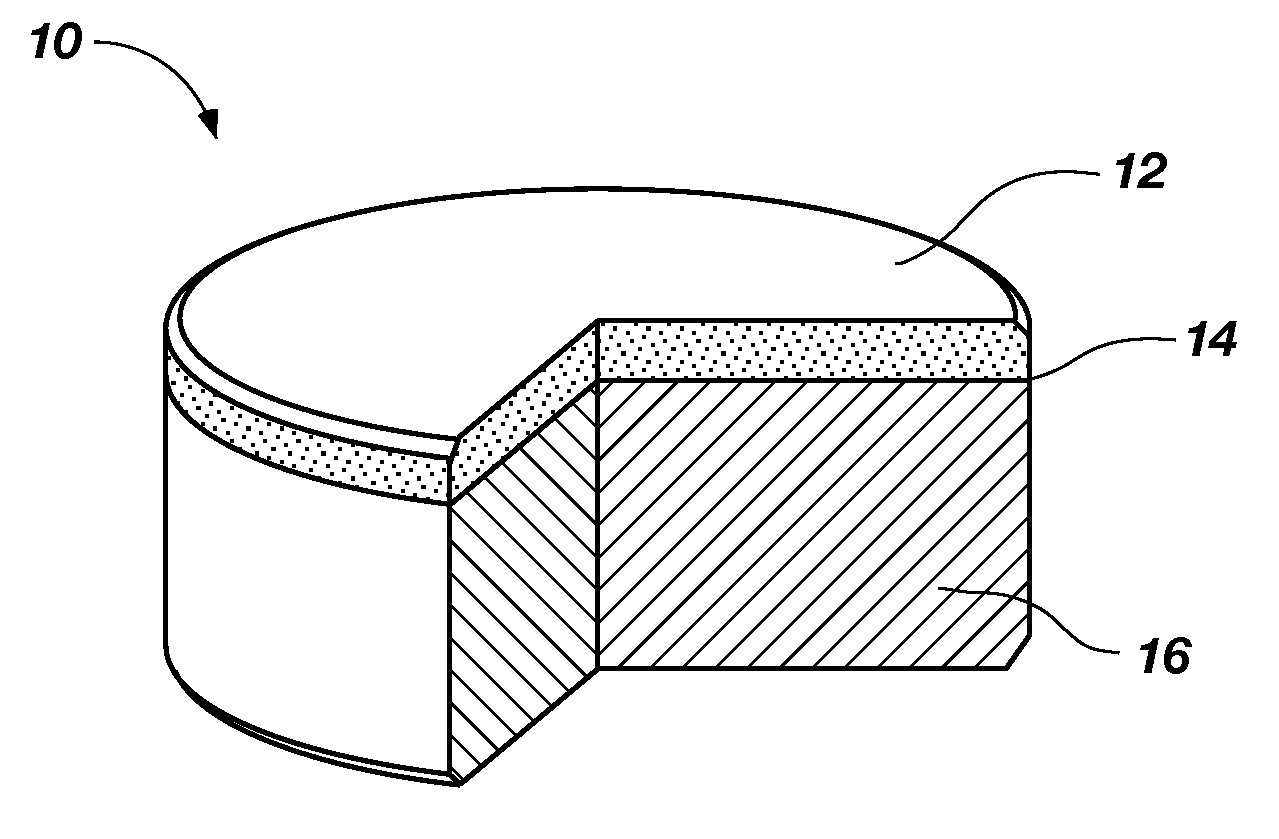
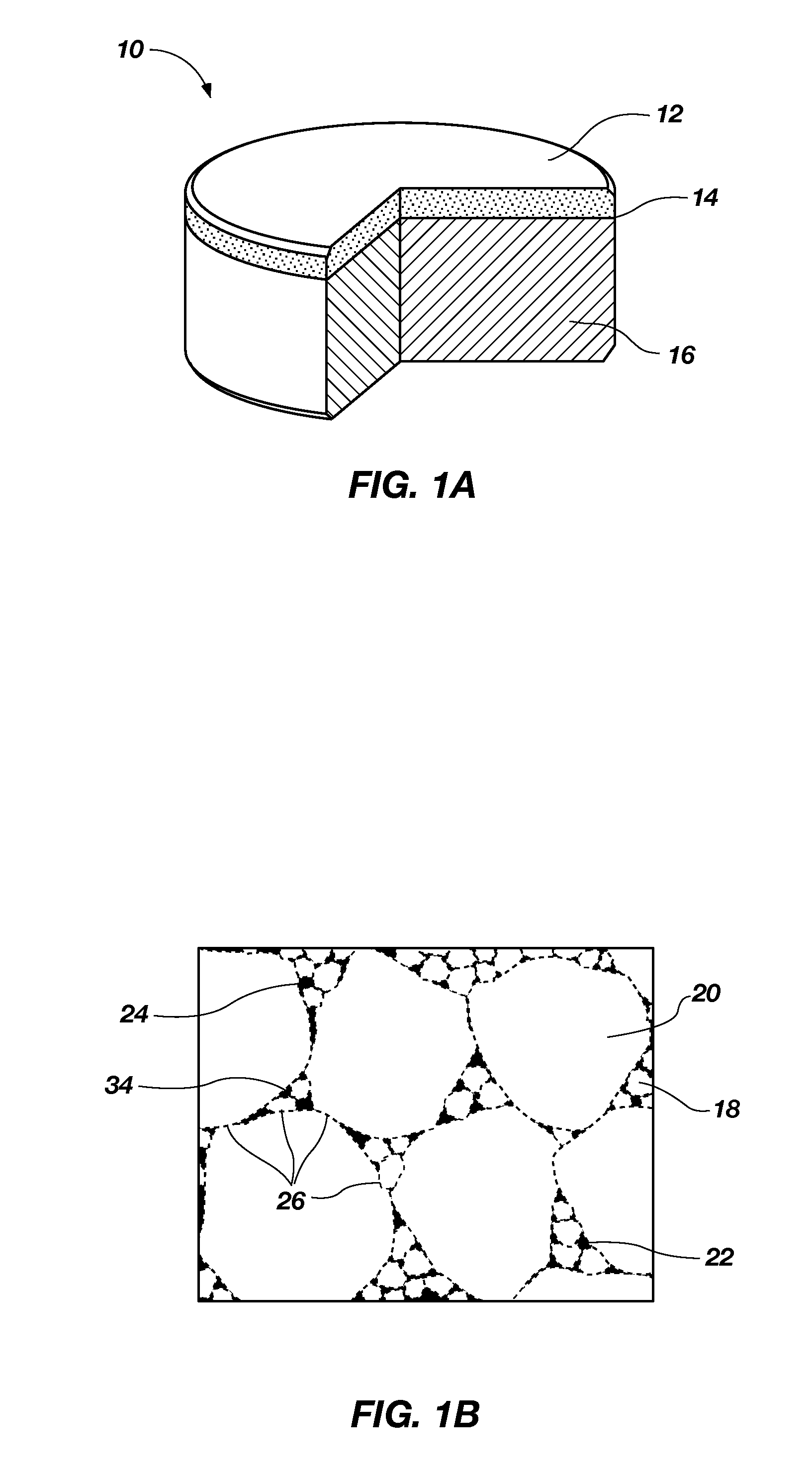
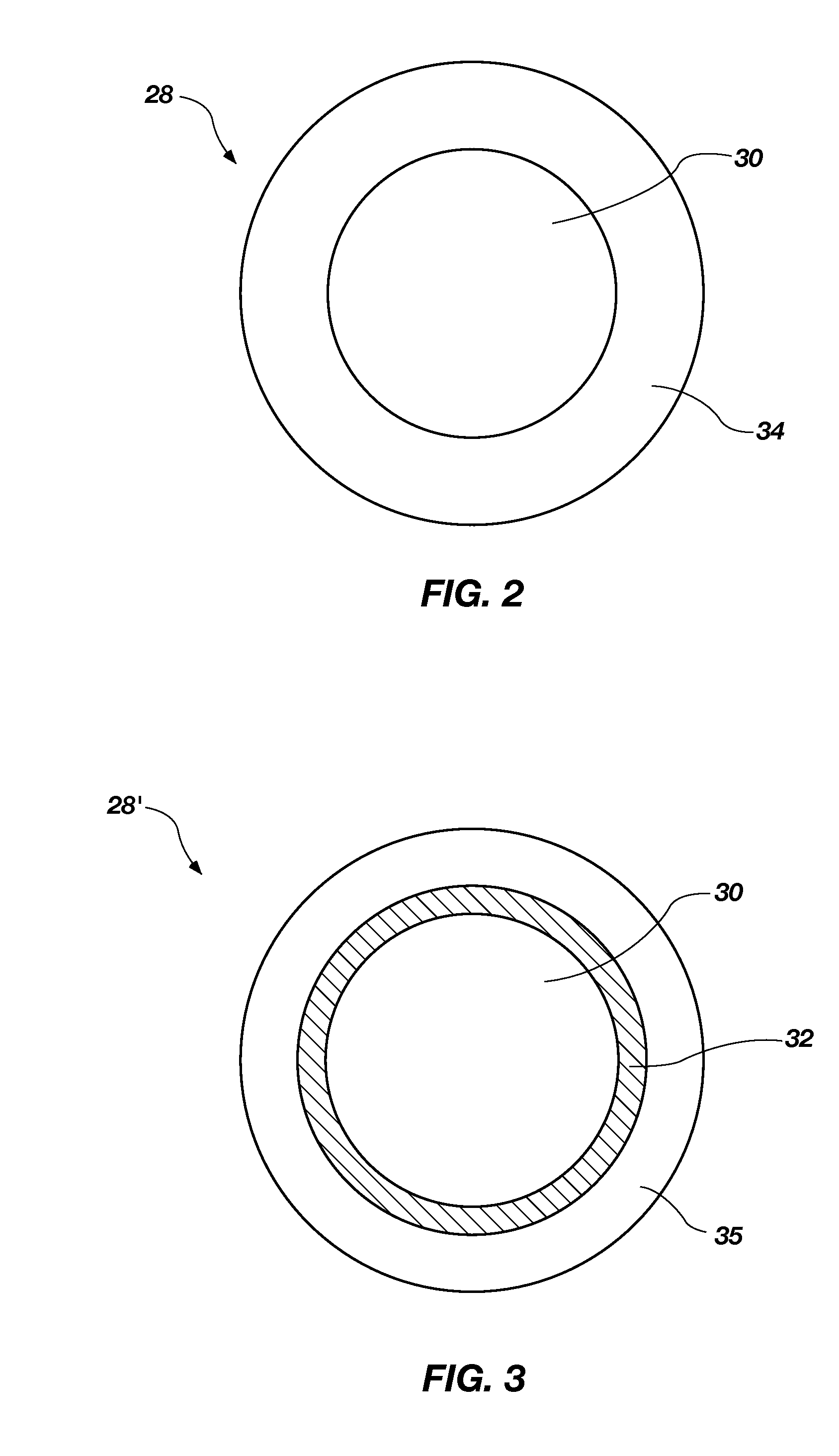
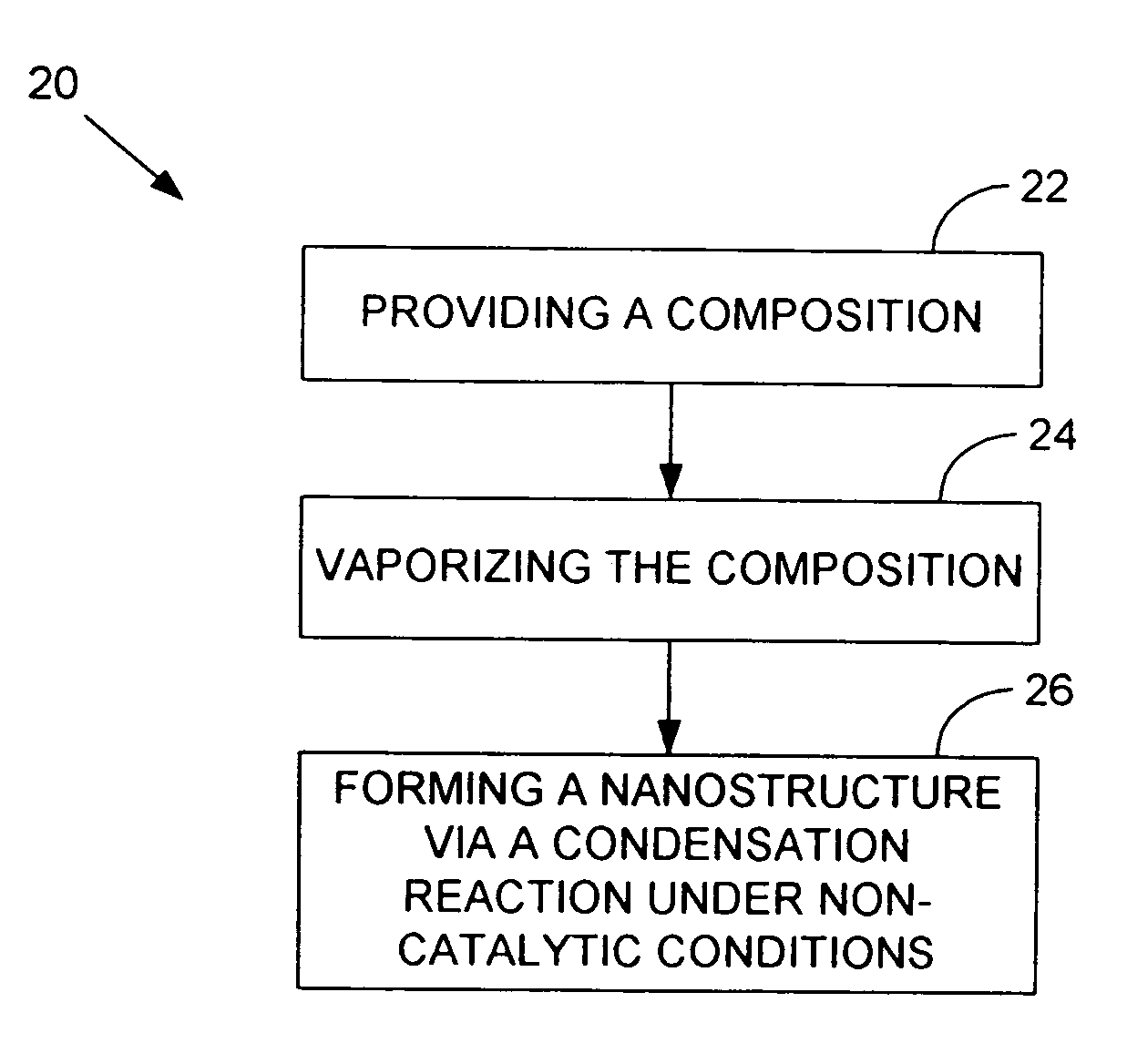
A nanowire, nanosphere, metallized nanosphere, and methods for their fabrication are outlined. The method of fabricating nanowires includes fabricating the nanowire under thermal and non-catalytic conditions. The nanowires can at least be fabricated from metals, metal oxides, metalloids, and metalloid oxides. In addition, the method of fabricating nanospheres includes fabricating nanospheres that are substantially monodisperse. Further, the nanospheres are fabricated under thermal and non-catalytic conditions. Like the nanowires, the nanospheres can at least be fabricated from metals, metal oxides, metalloids, and metalloid oxides. In addition, the nanospheres can be metallized to form metallized nanospheres that are capable as acting as a catalyst.
Owner:GEORGIA TECH RES CORP
Nucleic acid synthesis compositions and methods and systems for using same
ActiveUS20100047802A1Accurate sortingReduce in quantityMicrobiological testing/measurementTransferasesPolymerase LDivalent metal ions
The Application relates to compositions, kits, methods, and systems for nucleotide sequencing comprising producing polymerase reactions that comprise both catalytic and non-catalytic divalent metal ions. Effective ratios and amounts of catalytic and non-catalytic divalent metal ions are described.
Owner:PACIFIC BIOSCIENCES
Method for forming catalyst nanoparticles for growing elongated nanostructures
InactiveUS20090072222A1Fully compatibleGrowth inhibitionMaterial nanotechnologyCarbon compoundsNanowireCatalyst nanoparticles
Preferred embodiments provide a method for forming at least one catalyst nanoparticle on at least one sidewall of a three-dimensional structure on a main surface of a substrate, the main surface lying in a plane and the sidewall of the three-dimensional structure lying in a plane substantially perpendicular to the plane of the main surface of the substrate. The method comprises obtaining a three-dimensional structure on the main surface, the three-dimensional structure comprising catalyst nanoparticles embedded in a non-catalytic matrix and selectively removing at least part of the non-catalytic matrix at the sidewalls of the three-dimensional structure to thereby expose at least one catalyst nanoparticle. According to preferred embodiments a method is also provided for forming at least one elongated nanostructure, such as e.g. a nanowire or carbon nanotube, using the catalyst nanoparticles formed by the method according to preferred embodiments as a catalyst. The methods according to preferred embodiments may be used in, for example, semiconductor processing. The methods according to preferred embodiments are scalable and fully compatible with existing semiconductor processing technology.
Owner:INTERUNIVERSITAIR MICRO ELECTRONICS CENT (IMEC VZW)
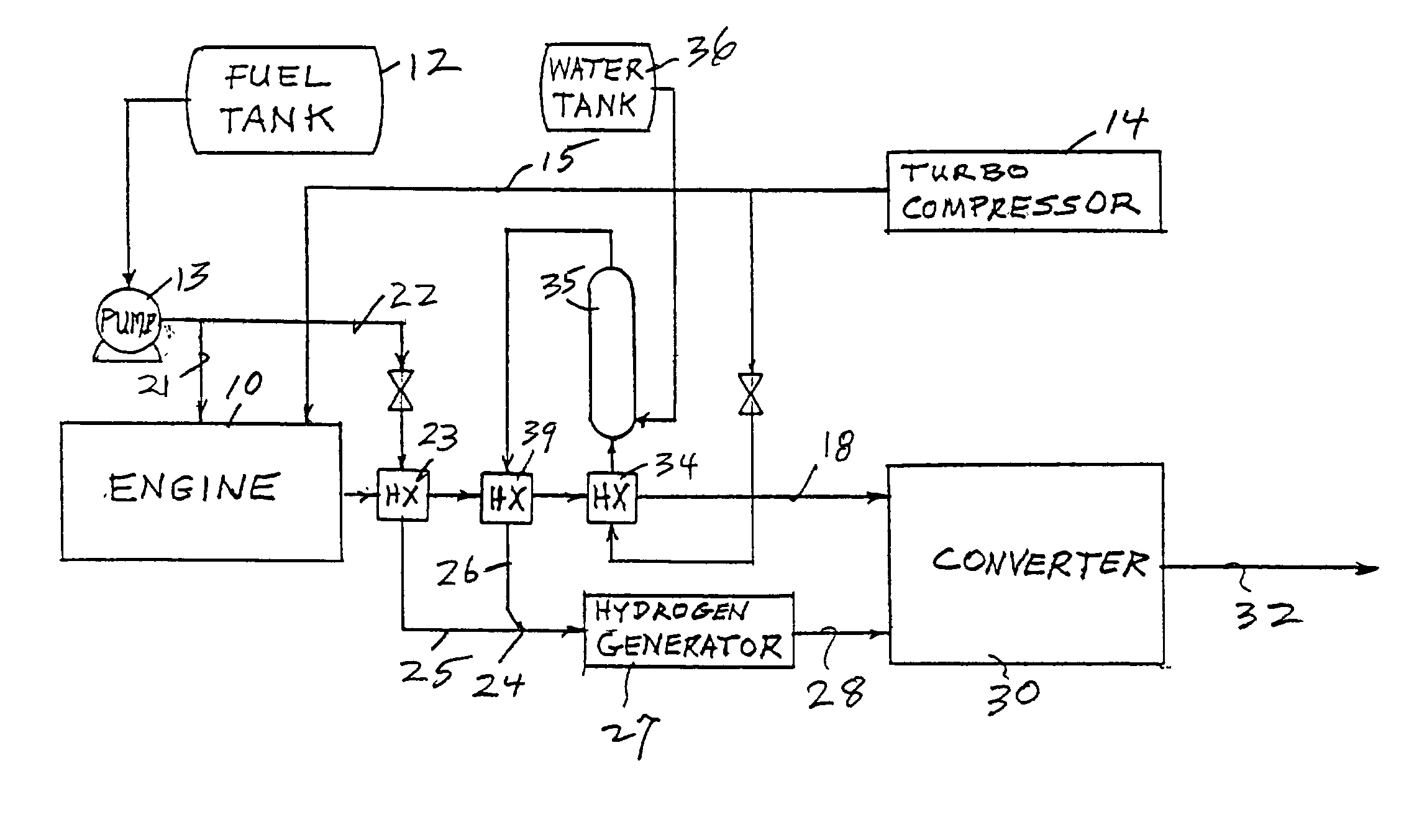
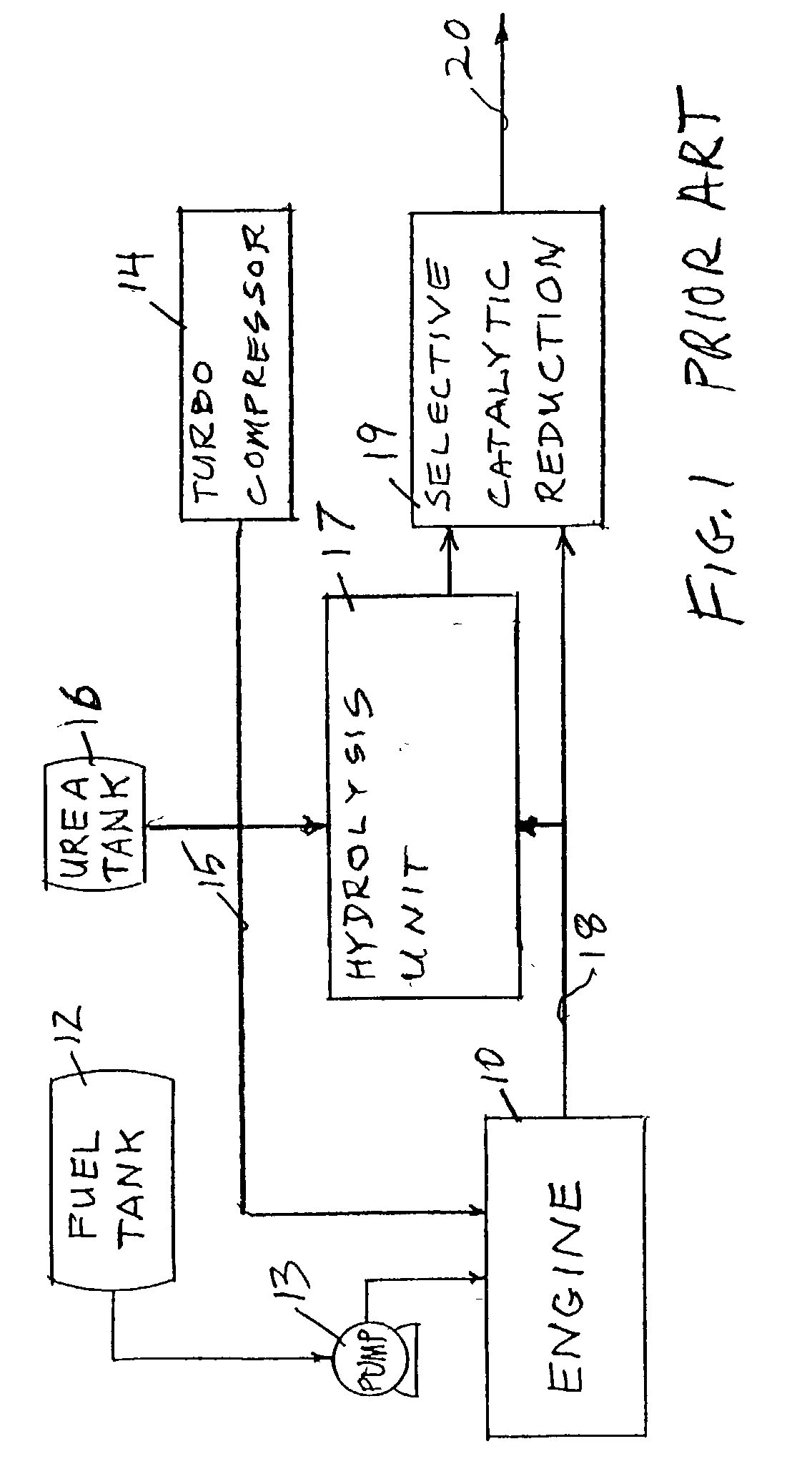
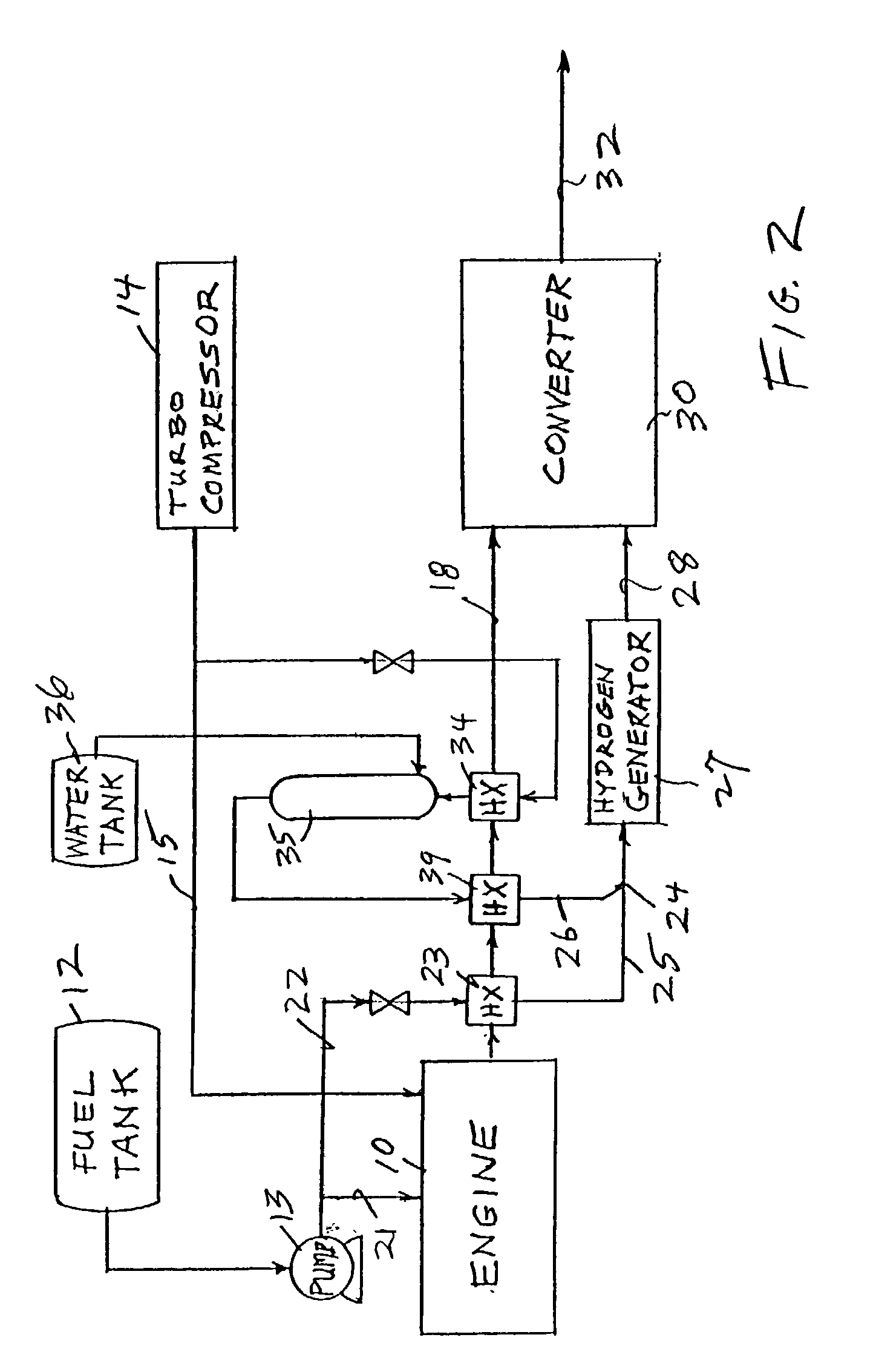
Reducing oxides of nitrogen using reformate generated from engine fuel, water and/or air
InactiveUS20030226350A1Reduce additionalSimple and low-cost hydrogen generationHydrogenInternal combustion piston enginesMethaneCatalytic converter
Inlet air (15) humidified in an air bubbling (or other) humidifier (35) that receives water from a tank (36) is sent to a hydrogen generator (27) along with vaporized (23) diesel fuel (22) to produce hydrogen and carbon monoxide (28) for either (a) mixing with the mainstream of exhaust (18) fed to a catalytic converter (30) or (b) regenerating a pair of NOx adsorption traps (38, 39), thereby reducing oxides of nitrogen (NOx), to provide system exhaust (32) which may have less than 0.40 grams / bhp / hr of NOx and 0.28 grams / bhp / hr of non-methane hydrocarbons. In other embodiments, unhumidified air mixed with fuel feeds a homogeneous non-catalytic partial oxidizer (27) to provide the required hydrogen and carbon monoxide.
Owner:SHELL OIL CO
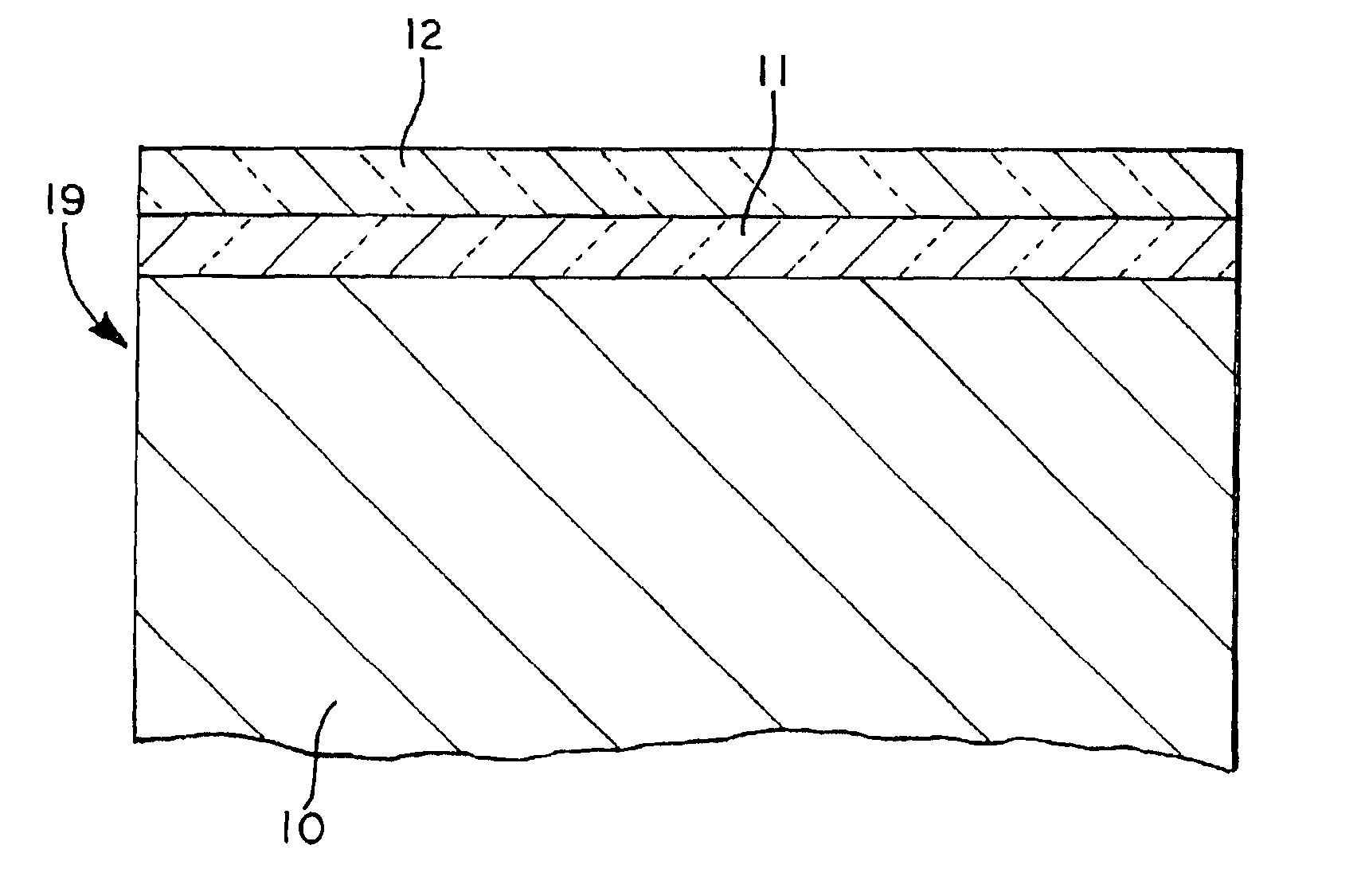
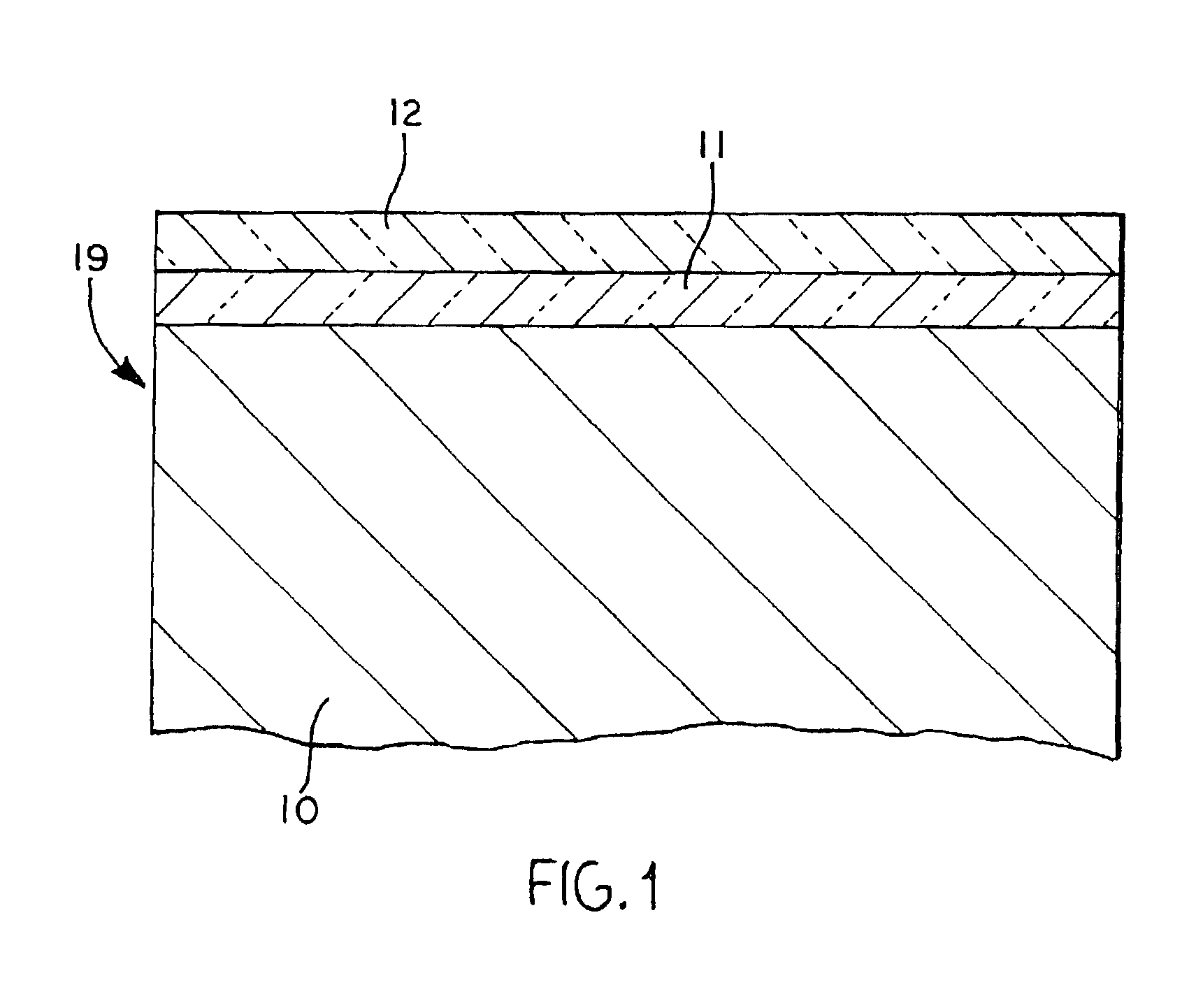
Method for simultaneously protecting carbon-containing components against catalytic oxidation and high temperature non-catalytic oxidation
Coated articles (19) that comprise components, made of carbon fiber or carbon-carbon composites which may be configured, for example, as aircraft landing system brake discs. The components (10) are coated with a system that includes a phosphorus-containing undercoating (11) having a specified formulation and a boron-containing overcoating (12) having specified formulation. The coated articles of the invention, e.g., aircraft brake discs, are protected against catalytic oxidation when the article is subjected to temperatures of 800° C. (1472° F.) or greater. Also, a method of protecting a component made of a carbon fiber or carbon-carbon composite simultaneously against catalytic oxidation (e.g., catalyzed by de-icer compositions) and high temperature non-catalytic oxidation.
Owner:HONEYWELL INT INC
Process for the production of biodiesel in continuous mode without catalysts
InactiveUS20070010681A1Fatty oils/acids recovery from wasteFatty acid esterificationBiodieselVegetable oil
A continuous, non catalytic process for producing biodiesel from vegetable oils and ethanol or methanol includes pumping a mixture of vegetable alcohol through a pump towards a tube shaped reactor, wherein the mixture is submitted to high pressure and temperature, where the resulting product is non reacted alcohol, glycerin and a mixture of esters (biodiesel) which is directed to the reservoir at the reactor outlet where an upper phase of alcohol is redirected through an alcohol return pipe to the pump inlet, and the intermediate phase, biodiesel, and the lower phase (mostly of glycerin) are led to the separation reservoir or decantation tank, where the alcohol is removed through the alcohol return pipe, being biodiesel and glycerin the final products, which are then collected for the end to which they were aimed.
Owner:INTECNIAL +1
Application of manganese dioxide for preparing microorganism fuel cell cathode
InactiveCN101355170AGuaranteed uptimeHigh power outputCell electrodesMicrobial fuel cellMicroorganism
The invention discloses application of manganese dioxide for preparing a cathode of a microorganism fuel battery. The method comprises the following steps: the manganese dioxide is taken as a catalyst, the mixture of the catalyst, a conductive carbon material and a caking agent is coated on a conductive substrate to prepare the cathode of the microorganism fuel battery and a membrane composite cathode applied to the microorganism fuel battery. Compared with a non-catalytic electrode, MnO2 is taken as a cathode catalyst for remarkably increasing the reducing speed, reducing the polarization of the cathode and improving the energy output of the microorganism fuel battery; compared with the prior Pt catalyst, MnO2 has low price and wide source, and the microorganism fuel battery assembled by the catalyst of the cathode can operate stably for a long time and has high power output. The manganese dioxide for preparing the microorganism fuel battery electrode provides solid foundation for the commercial application of the microorganism fuel battery.
Owner:GUANGDONG INST OF ECO ENVIRONMENT & SOIL SCI
Polycrystalline compacts including nanoparticulate inclusions, cutting elements and earth-boring tools including such compacts, and methods of forming same
Polycrystalline compacts include non-catalytic, non-carbide-forming particles in interstitial spaces between interbonded grains of hard material in a polycrystalline hard material. Cutting elements and earth-boring tools include such polycrystalline compacts. Methods of forming polycrystalline compacts include forming a polycrystalline material including a hard material and a plurality of particles comprising a non-catalytic, non-carbide-forming material. Methods of forming cutting elements include infiltrating interstitial spaces between interbonded grains of hard material in a polycrystalline material with a plurality of non-catalytic, non-carbide-forming particles.
Owner:BAKER HUGHES INC
High-temperature heat exchanger
ActiveUS20080072425A1Convenient coatingCheap offerCombination devicesPhysical/chemical process catalystsPlate heat exchangerFuel cells
A low-cost, high-temperature heat exchanger is made from a notched piece of metal, the metal being folded back and forth upon itself to form a monolith. The notches in the metal piece create openings, communicating with distinct sides of the monolith. Ducts are attached to the openings. Cut pieces of corrugated metal, which may have a catalyst coating, are inserted between folds of the monolith. The heat exchanger may be used as part of a fuel cell system, or in other industrial applications, to recover waste heat, or to conduct various catalytic and non-catalytic reactions. The invention also includes an element, or building block, for a high-temperature heat exchanger, including a folded metal monolith with metal combs inserted, the monolith and the combs defining seams which are hermetically sealed.
Owner:JOHNSON MATTHEY PLC
High temperature oxidation inhibitors for carbon-carbon friction materials
Coated articles (19) that comprise components, made of carbon fiber or carbon-carbon composites which may be configured, for example, as aircraft landing system brake discs. The components (10) are coated with a system that includes a phosphorus-containing undercoating (11) having a specified formulation and a boron-containing overcoating (12) having specified characteristics. The coated articles of the invention, e.g., aircraft brake discs, are protected against catalytic oxidation when the article is subjected to temperatures of 800° C. (1472° F.) or greater. Also, a method of protecting a component made of a carbon fiber or carbon-carbon composite simultaneously against catalytic oxidation (e.g., catalyzed by de-icer compositions) and high temperature non-catalytic oxidation.
Owner:HONEYWELL INT INC
Coated substrate and process of preparation thereof
InactiveUS20050054526A1Hot-dipping/immersion processesInternal combustion piston enginesMulti materialSorbent
A substantially uniformly coated substrate that may be either metallic or ceramic in nature as well as a process for preparing such coated substrate. The substantially uniformly coated substrate typically comprises a plurality of outlets; a plurality of substrate walls; and a plurality of channels defined by the substrate walls, said channels extending directly or indirectly from at least one outlet; and wherein there are a plurality of openings and / or corrugations and / or tabs in the substrate walls that communicate between adjacent channels, said substrate containing at least one substantially uniform layer of a coating material that may be a non-sorbent, non-catalytic material; a sorbent, non-catalytic material; a catalytic material or a mixture of two or more of the foregoing materials. The process for preparing such coated substrate involves immersing the substrate in a slurry of the desired material or materials, centrifuging the substrate so as to thereby distribute the material or materials as a substantially uniform layer on the substrate and remove any material or materials in excess of that desired to be present on the substrate, and thereafter drying and calcining the coated substrate.
Owner:ENGELHARD CORP
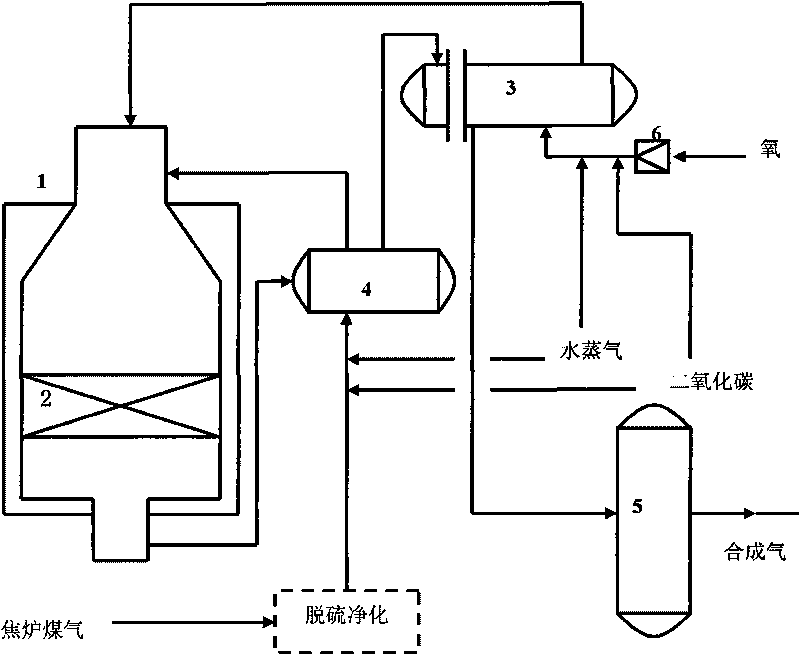
Method for preparing synthesis gas from coke oven gas
InactiveCN101717073AHigh purityLess impuritiesHydrogenBulk chemical productionCatalytic reformingCarbonization
The invention discloses a method for preparing synthesis gas from coke oven gas. Desulfurized and purified high-temperature carbonization gas is taken as a raw material gas, and oxygen, carbon dioxide and vapor are taken as gas transforming agents; and the method comprises the following steps: preheating the raw material gas and the gas transforming agents, introducing into a reactor for mixing, and simultaneously generating autothermal non-catalytic conversion reaction; and then introducing mixed gas after the non-catalytic conversion reaction into a catalyst bed layer for catalytic reforming reaction to prepare the synthesis gas. In the method, the consumption of the catalyst is low, the conversion rate is high, the ratio of hydrogen to carbon of the synthesis gas has strong controllability, the comprehensive utilization of energy is realized, the coke oven gas and the carbon dioxide are utilized as raw material gases, and the method for preparing the synthesis gas from the coke oven gas is economic, environment-friendly and low-carbon.
Owner:TAIYUAN UNIV OF TECH
Asphalt production from solvent deasphalting bottoms
ActiveUS20090301931A1Minimizing conventional waste handling demandEliminate wasteWorking-up pitch/asphalt/bitumen by selective extractionWorking-up tarEffective solutionDesorption
A cost-effective solution is provided for eliminating refinery process waste, including spent catalytic and non-catalytic adsorbent materials, as well as adsorbate process reject materials derived from desorption, while minimizing conventional waste handling demands. An asphalt composition includes asphalt and spent adsorbent material from a solvent deasphalting unit. The asphalt can comprise asphaltic material obtained from a solvent deasphalting unit, and spent adsorbent material in the asphalt composition was previously utilized in the solvent deasphalting unit. The asphalt composition can also include process reject materials.
Owner:SAUDI ARABIAN OIL CO
Method of operating a syngas generator
InactiveUS20080209891A1Maintain working temperatureHydrogenElectrical controlSyngasProcess engineering
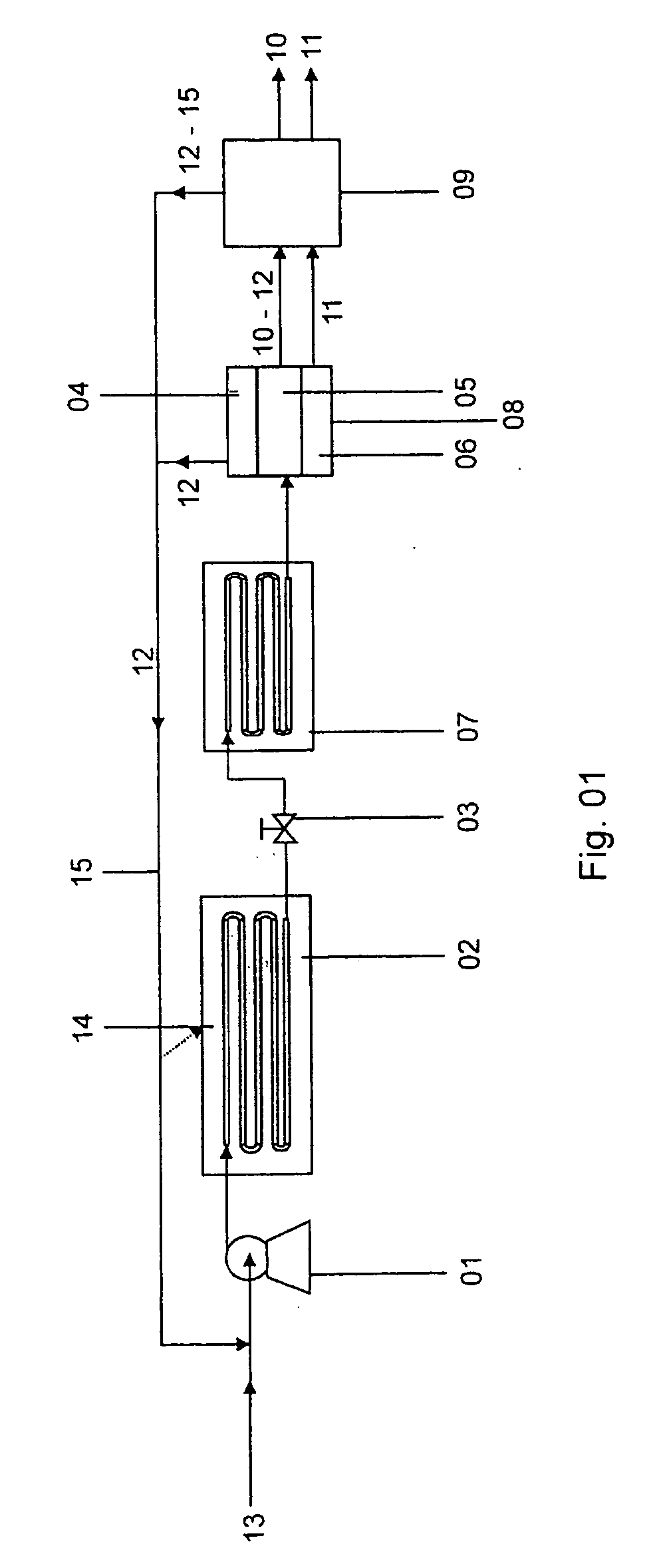
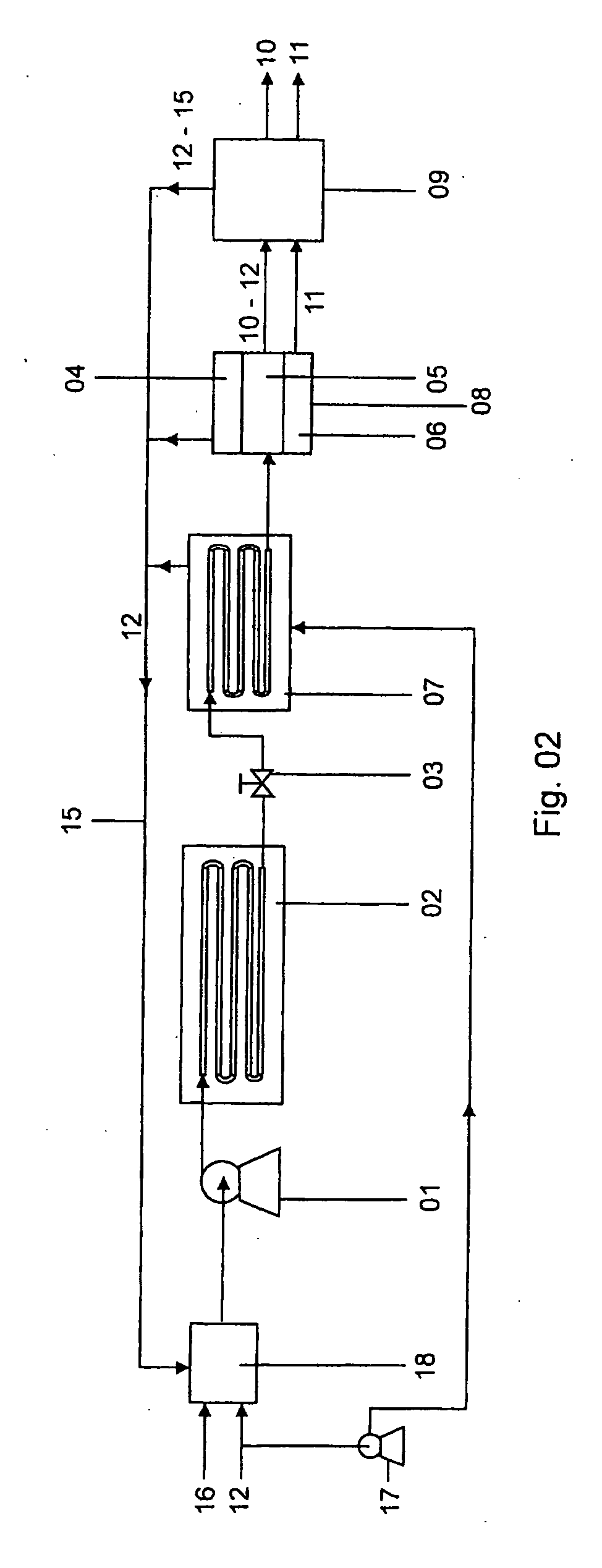
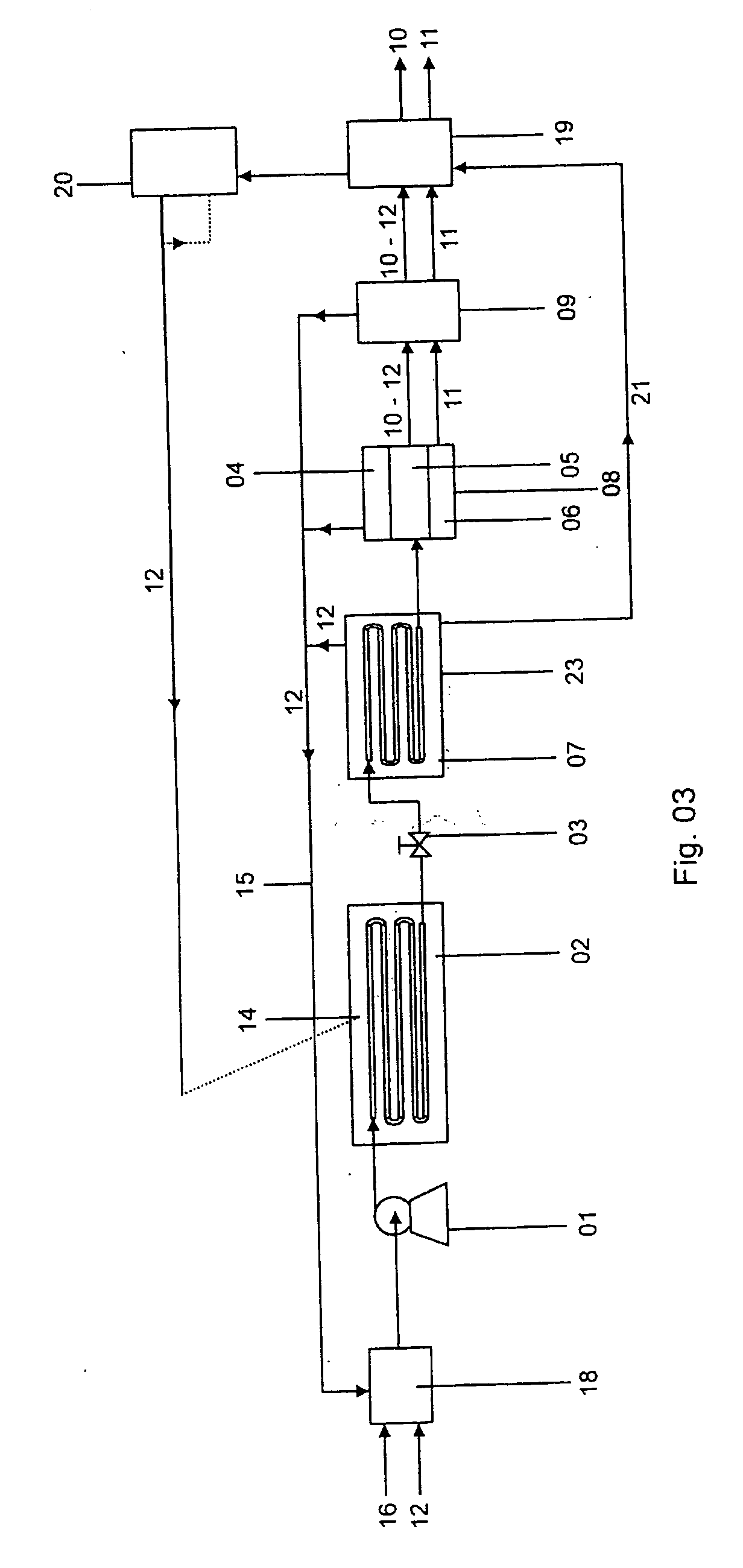
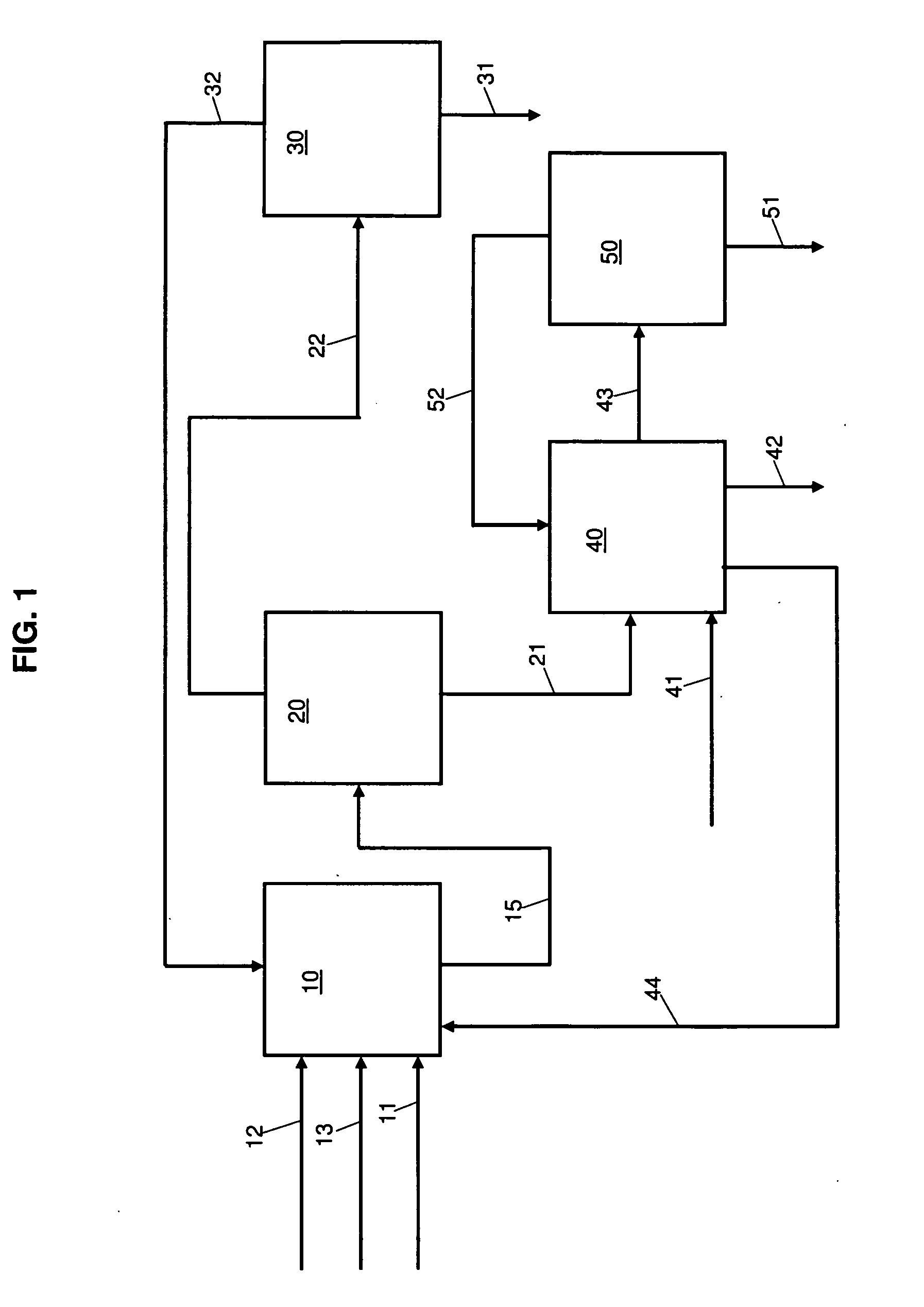
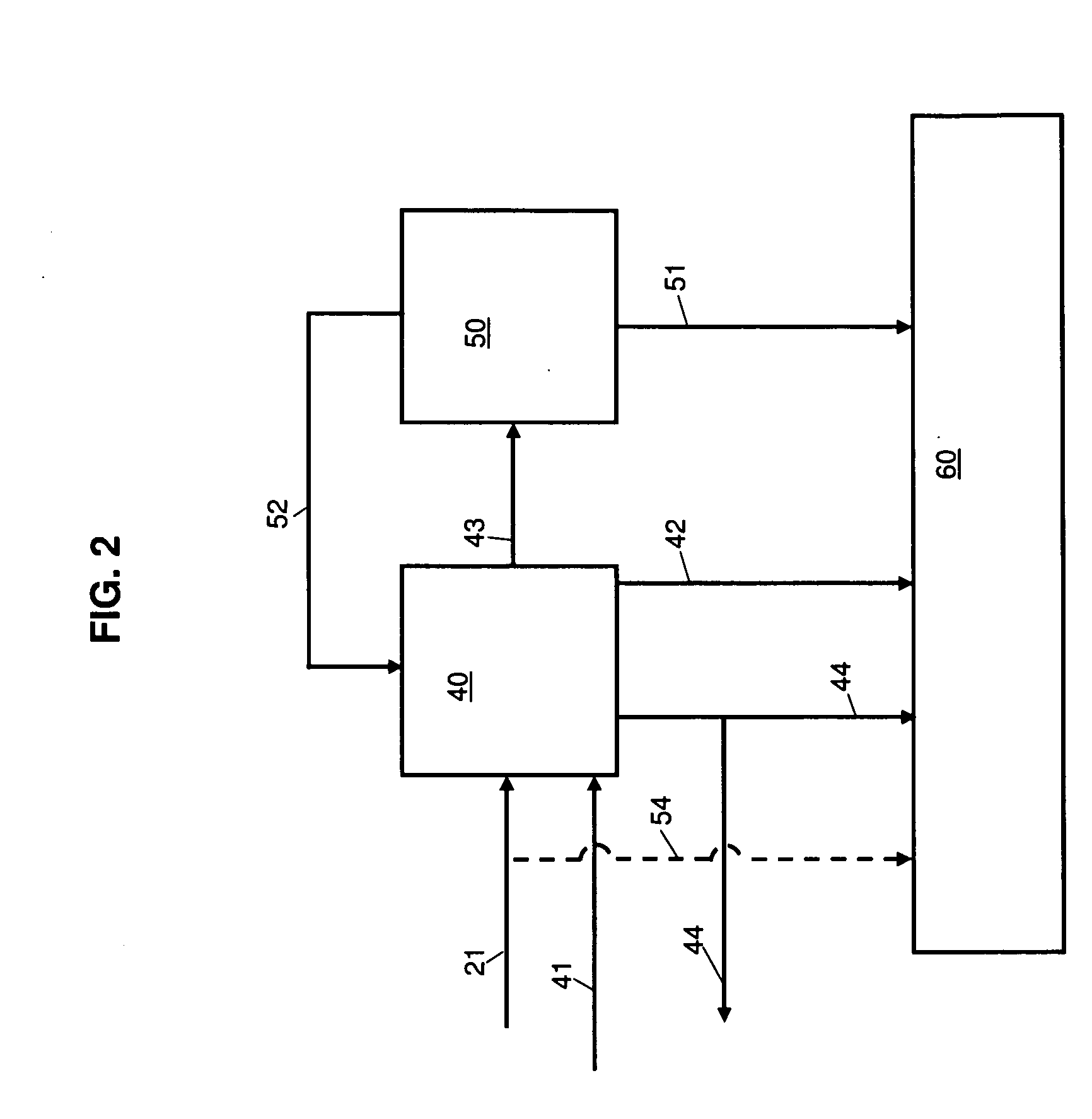
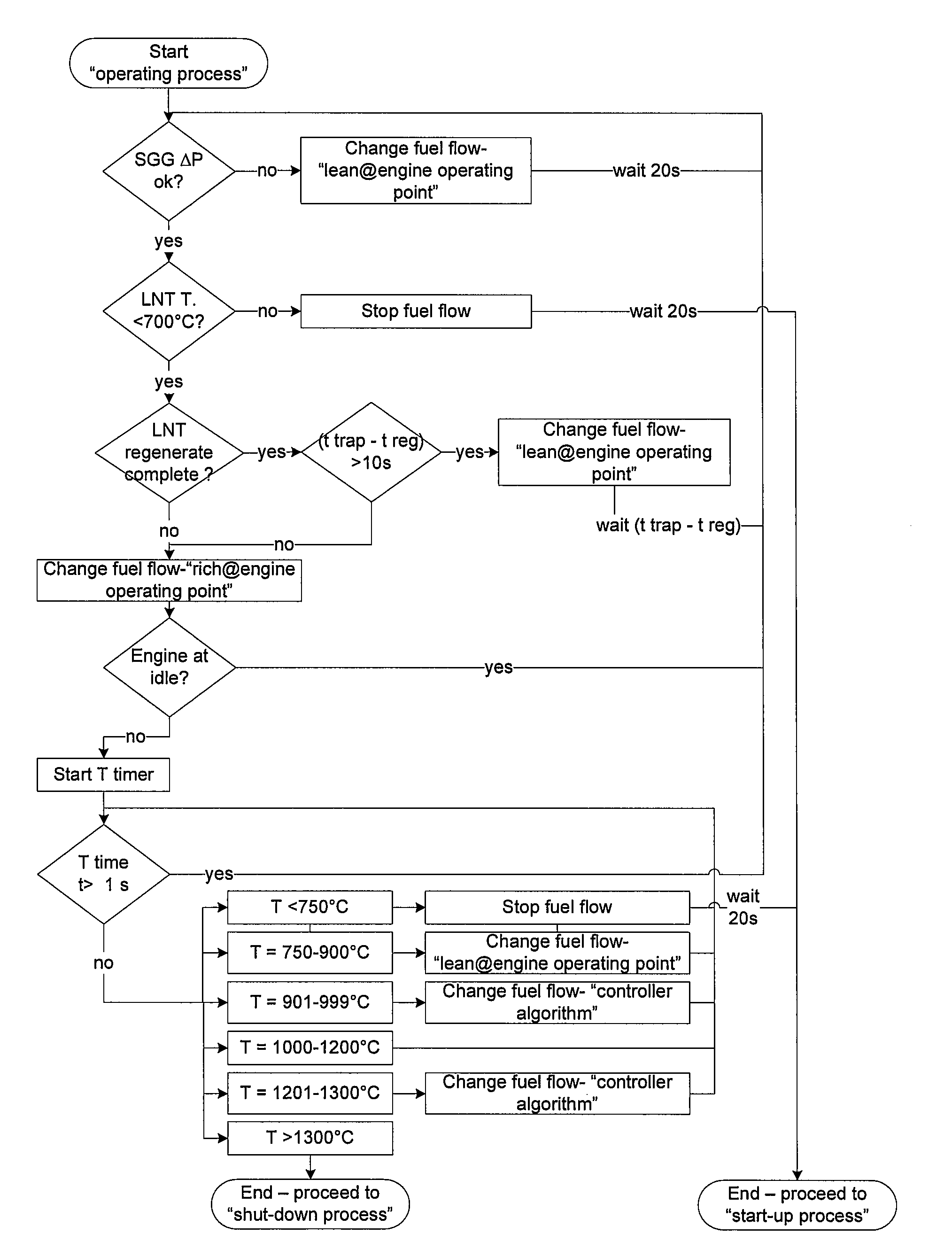
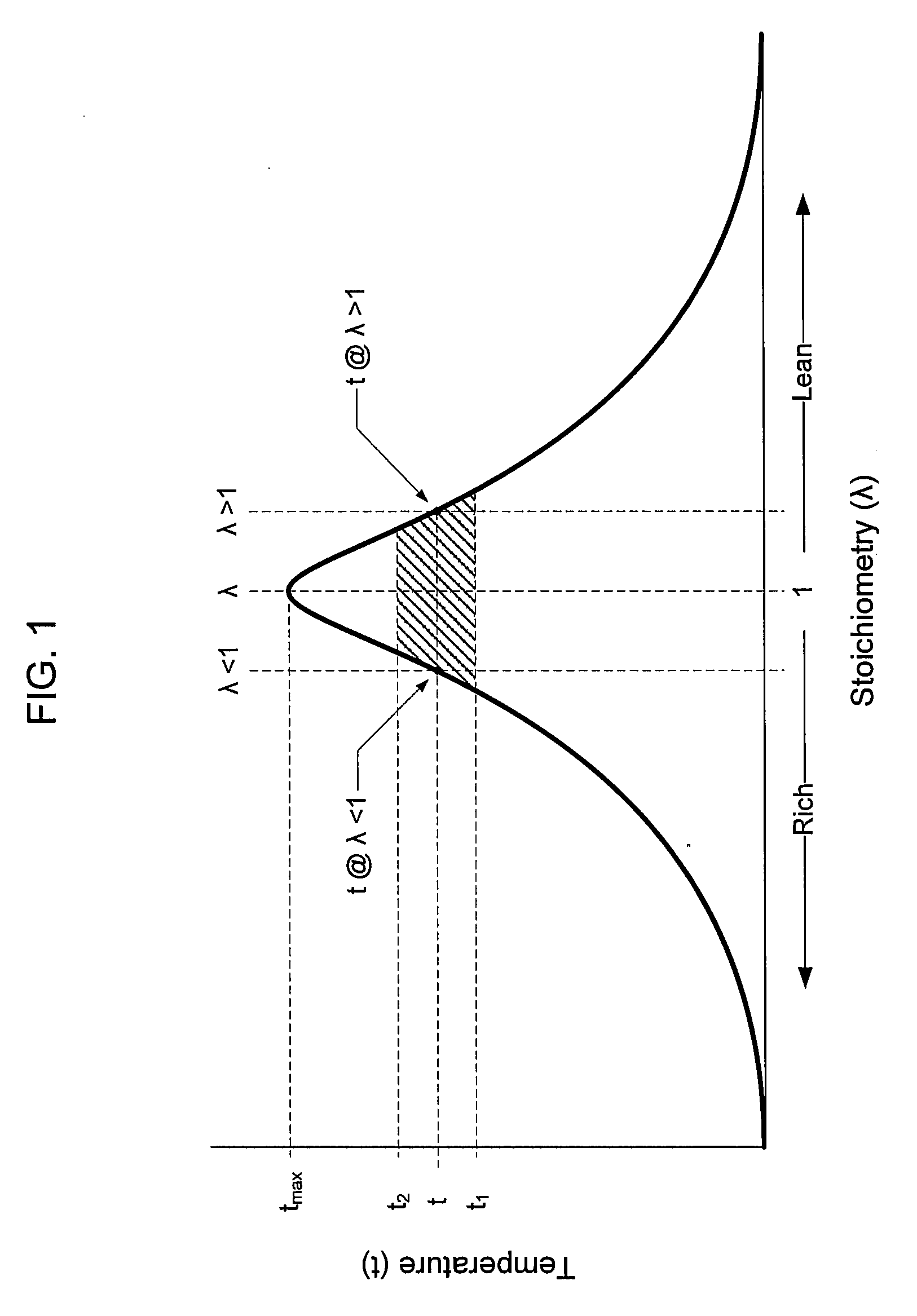
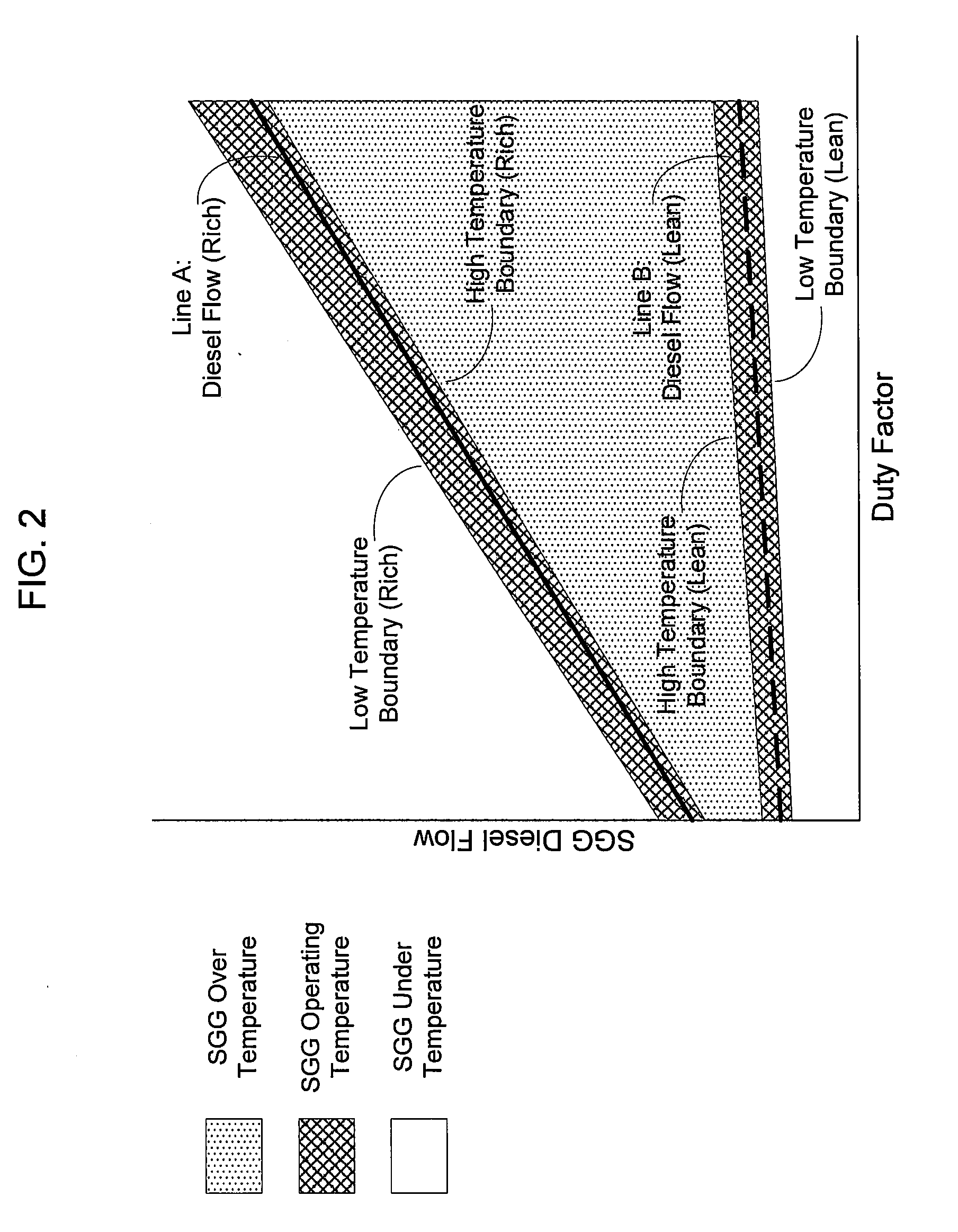
A method of operating a syngas generator within a desired temperature range, despite a need for intermittent syngas output, involves switching between operating the syngas generator in a rich mode and a lean mode. Operation of the syngas generator in both the rich mode and the lean mode sustains the operating temperature of the syngas generator within that desired temperature range, particularly for non-catalytic reactors. The method of switching from the lean mode to the rich mode of operation can include decreasing the oxygen-to-carbon ratio of reactants supplied to the syngas generator. The flow rate of one or more of the reactant streams supplied to the syngas generator can be actively controlled in order to switch operation of the syngas generator between the rich and lean modes.
Owner:WESTPORT POWER
Fuel conversion reactor
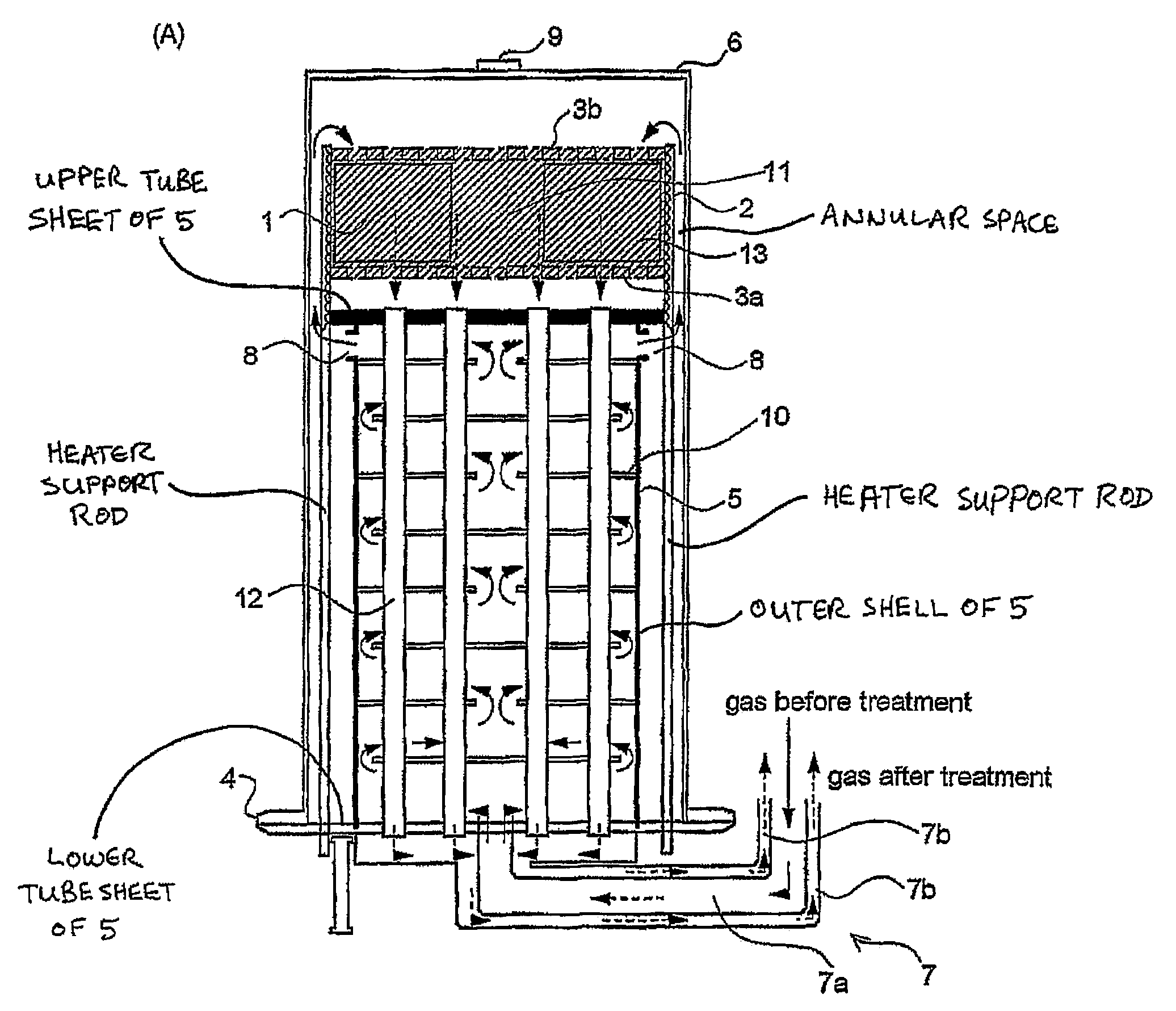
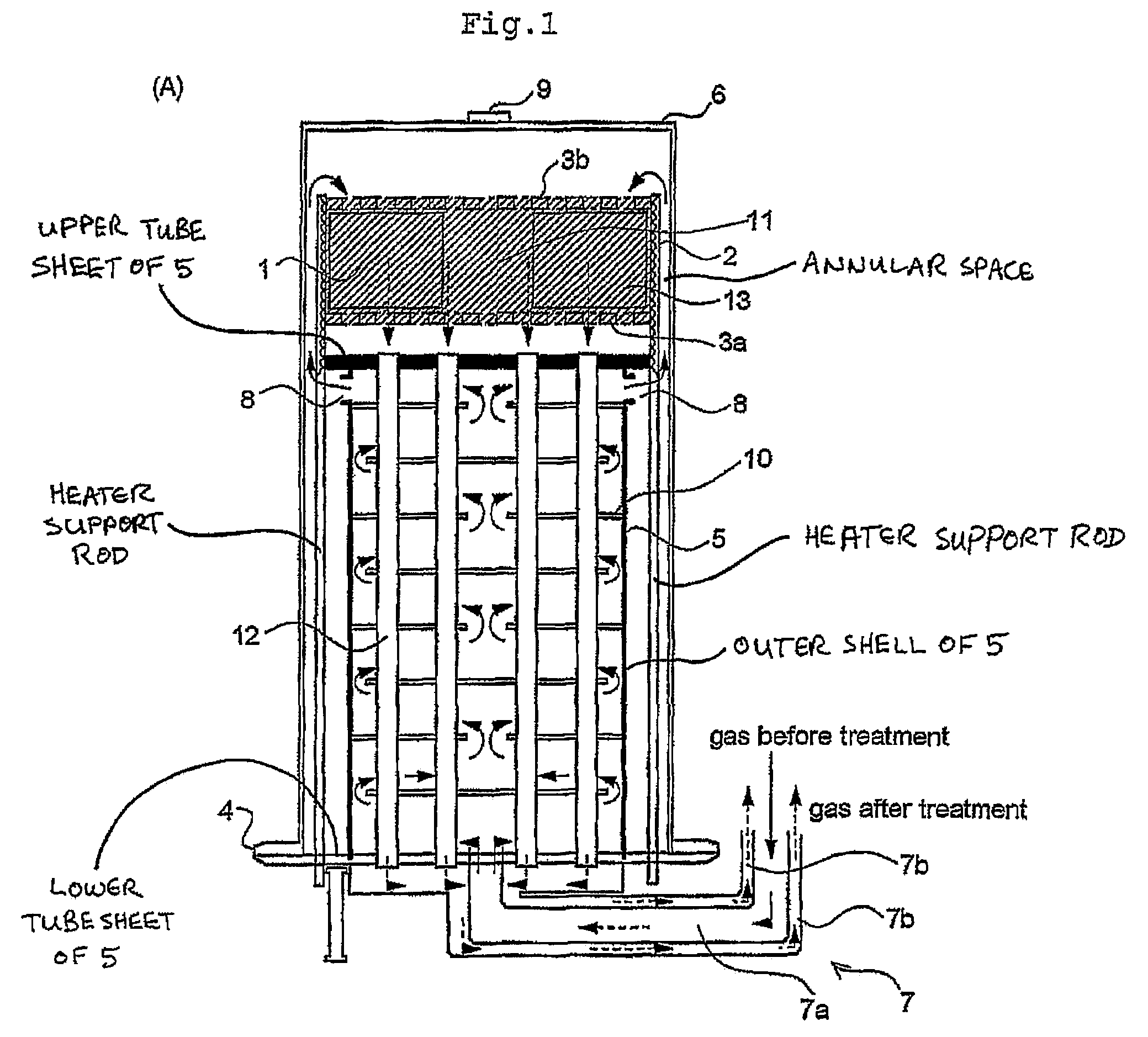
ActiveUS7220392B2Hydrogen/synthetic gas productionHeat exchanger casingsPlate heat exchangerShell and tube heat exchanger
A fuel conversion reactor includes a shell-and-tube heat exchanger for controlling the temperature of a hot gaseous mixture produced by catalytic or non-catalytic reaction of a fuel with a gaseous fluid, and for controlling the temperature of the gaseous fluid and / or the fuel prior to the reaction. The reactor is either a catalytic or non-catalytic burner, or a fuel reformer for converting a fuel to hydrogen. A preferred reactor includes an outer shell having first and second ends and an inner surface, a primary inner shell extending into the outer shell, the primary inner shell defining a heat exchanging chamber and having primary and secondary ends, and a secondary inner shell having a first end located adjacent the secondary end of the primary inner shell. One or more outlet apertures are formed between the two inner shells for passage of the gaseous fluid out of the heat exchanging chamber. There are also a plurality of heat exchange tubes extending through the heat exchanging chamber between first and second tube sheets and connected to same. The first tube sheet is mounted in the primary inner shell while the second tube sheet is connected to the secondary inner shell. The tubes form passages for flow of the hot gaseous mixture in heat exchange contact with the gaseous fluid through the heat exchanging chamber, thereby preheating the gaseous fluid prior to reaction with the fuel. The adjacent ends of the inner shells form a disconnected joint and the secondary inner shell is free to move relative to the primary inner shell upon thermal expansion of the tubes.
Owner:DANA CANADA CORP
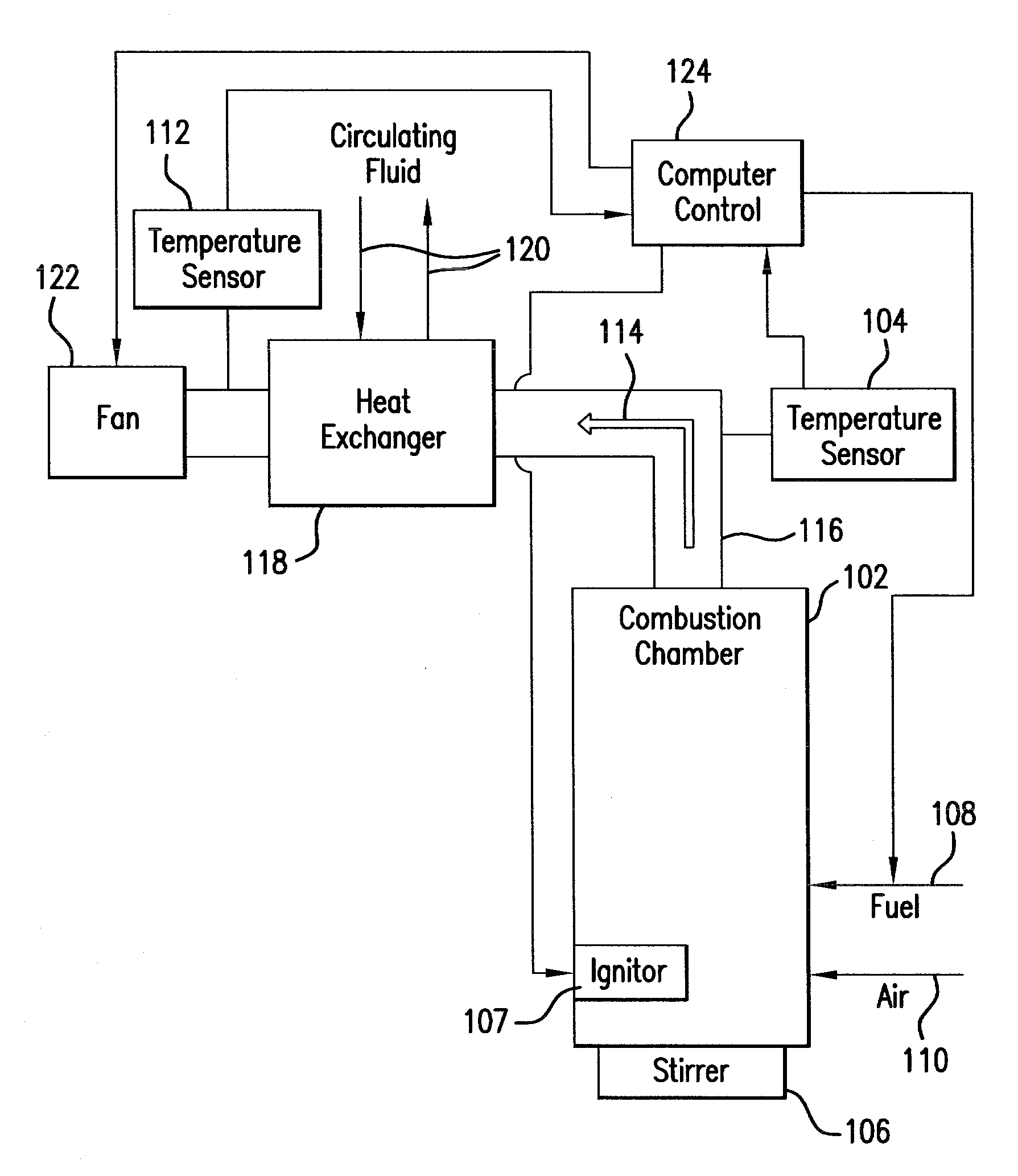
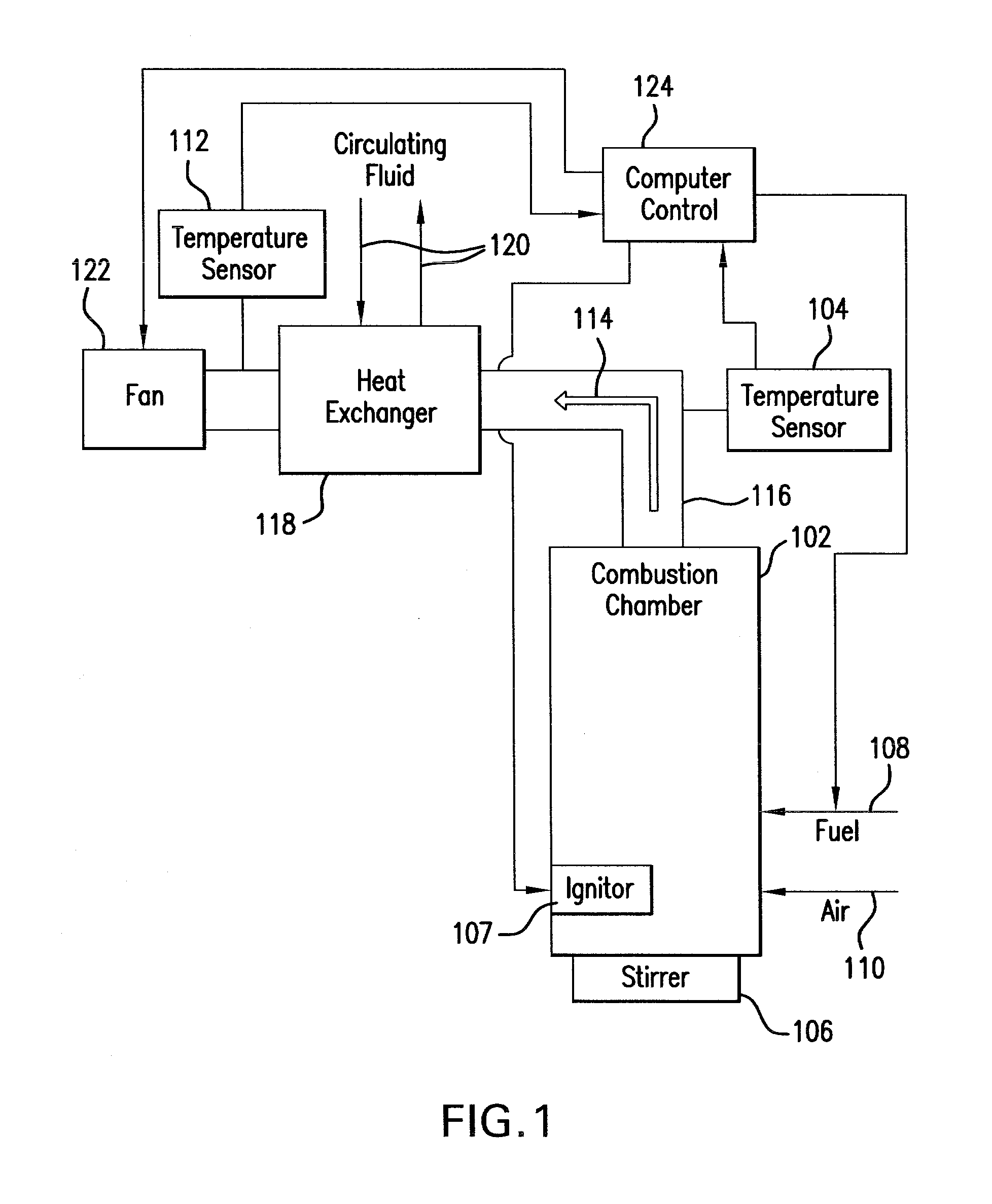
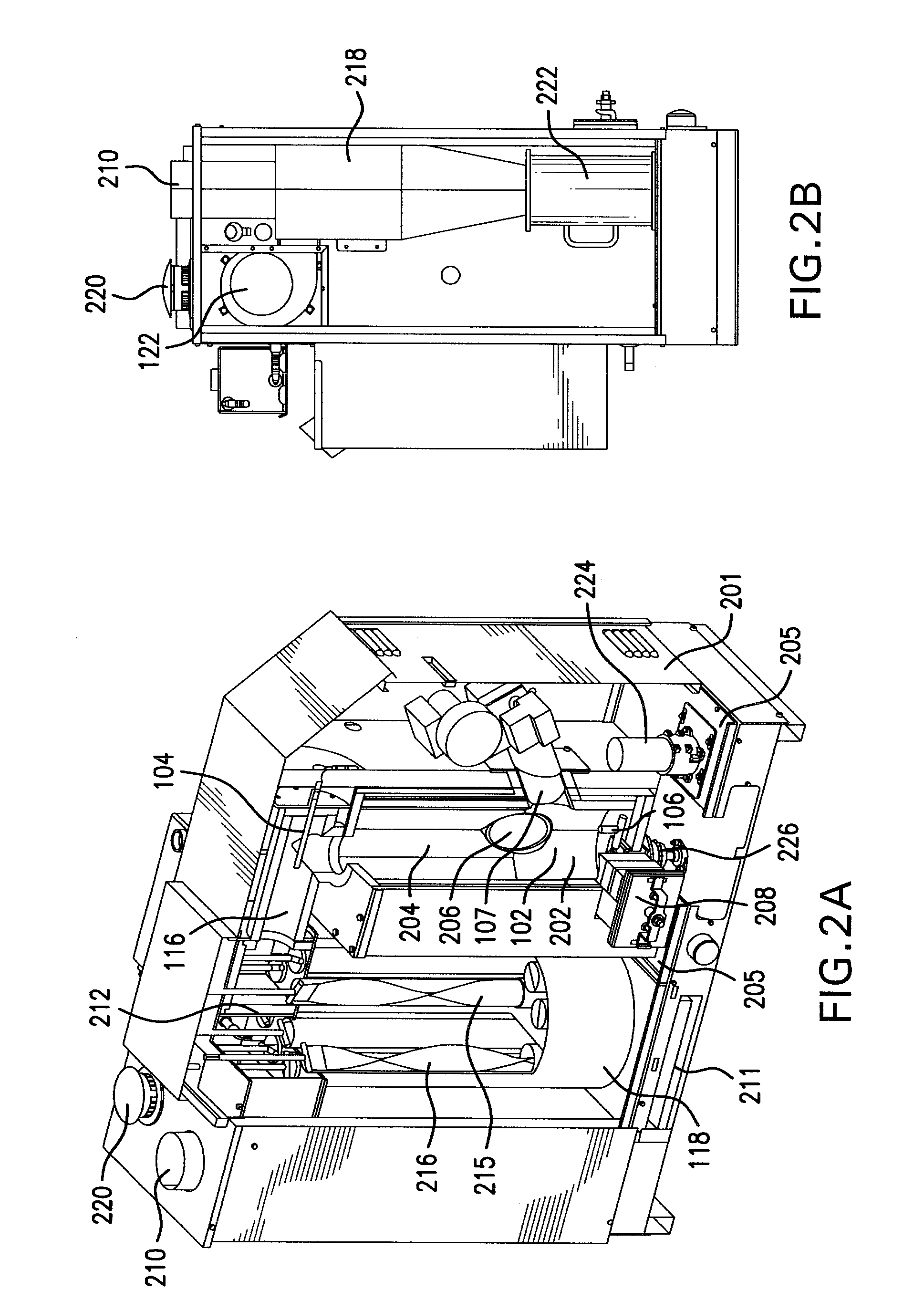
Non-catalytic biomass fuel burner and method
The present invention relates to a non-catalytic biomass burner that may be used to burn a variety of fuel types at high efficiencies. The burner may include a cylindrical combustion chamber with an auxiliary igniter to heat the fuel in the combustion chamber until desirable combustion temperatures are reached. Fuel may be added to the chamber via a fuel feed assembly, and the rate of fuel addition to the chamber by the fuel feed assembly may be controlled by a computer. A fan located on the distal side of a flue pipe from the chamber may also be provided that pulls air into the chamber through one or more air inlets that are designed to encourage cyclonic air and exhaust flow in the chamber. Methods are further provided for controlling the manner of operation of the burner by a computer that may be instructed by a computer program code.
Owner:LASKOWSKI SCOTT +3
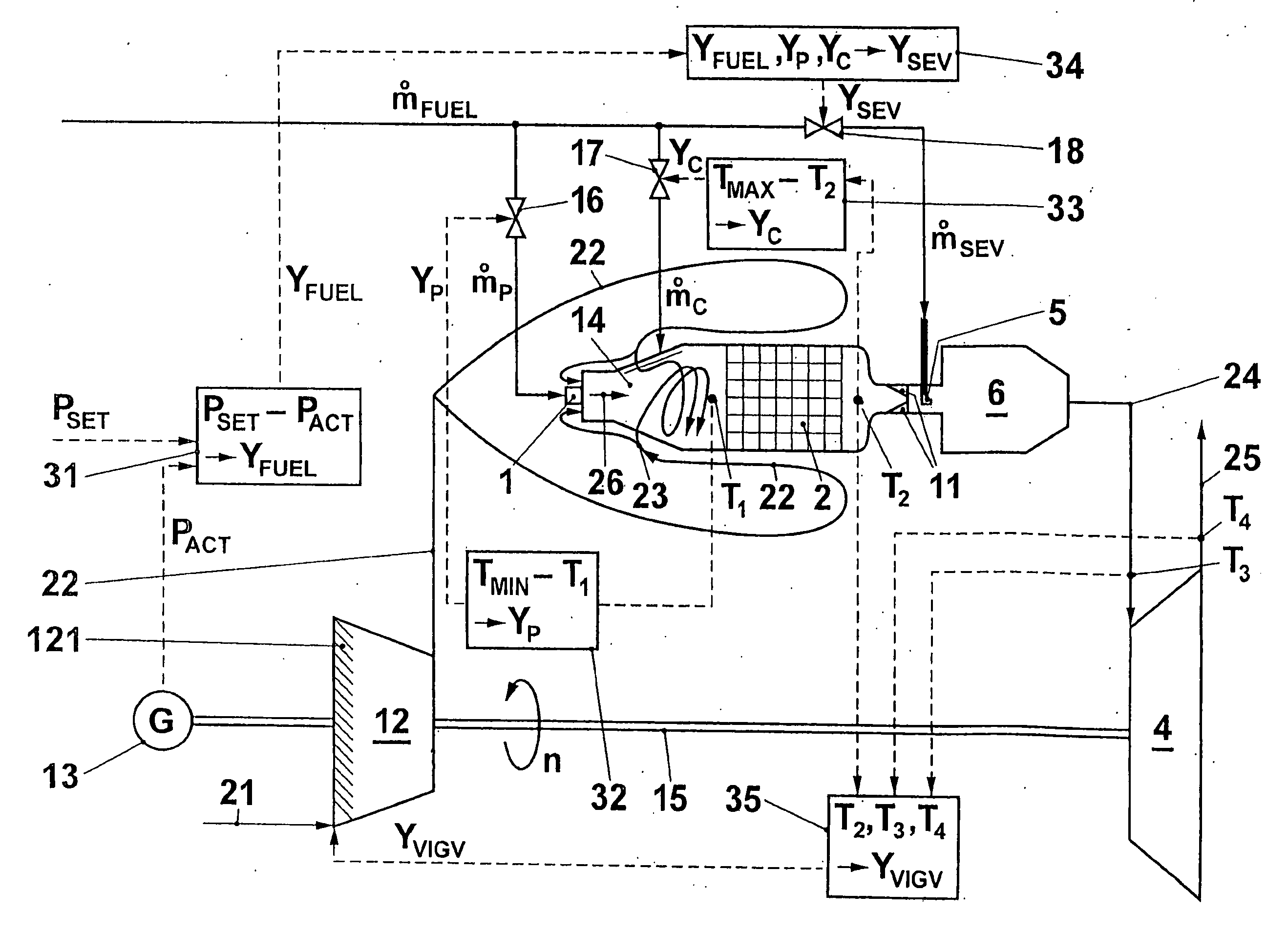
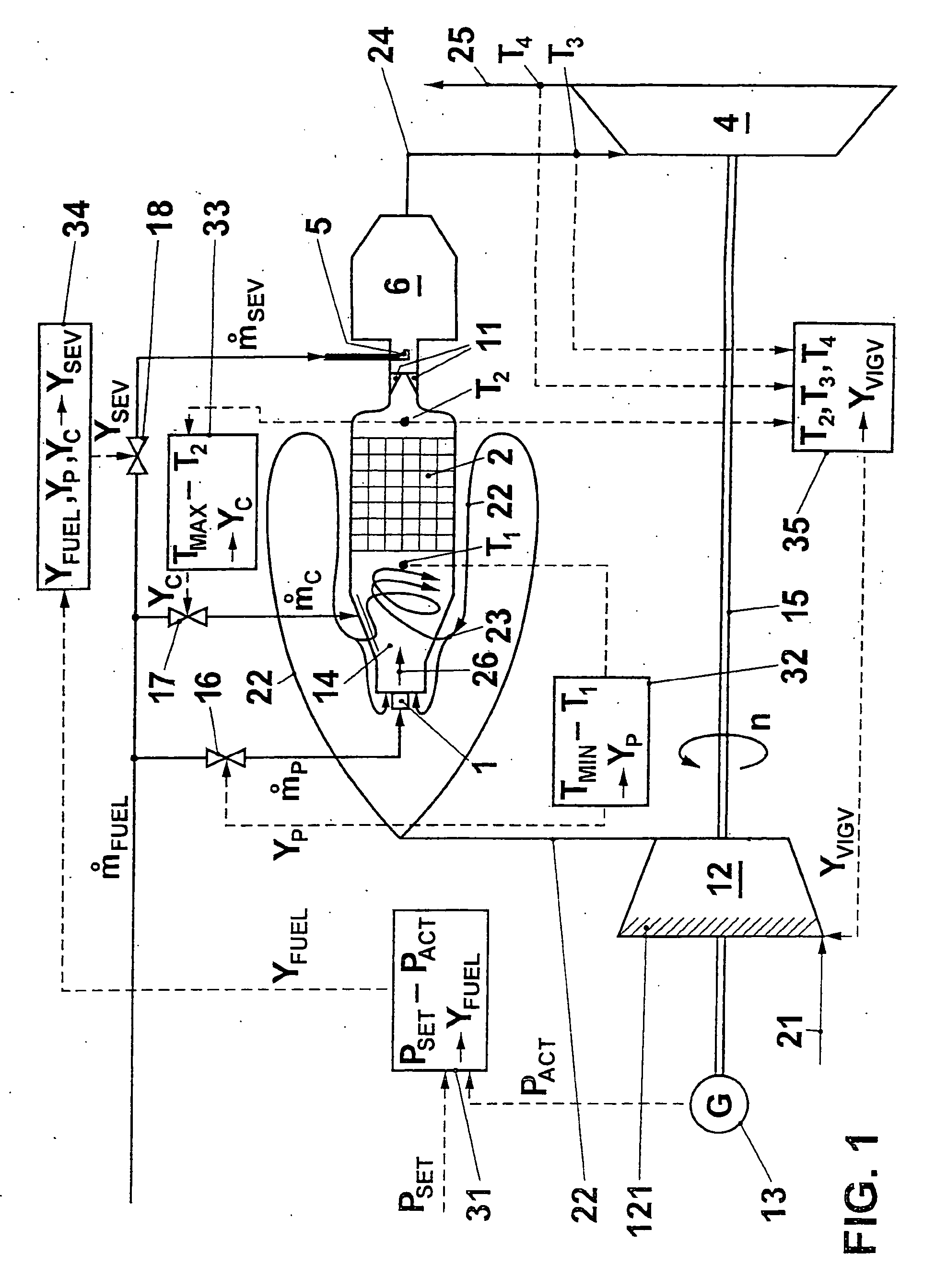
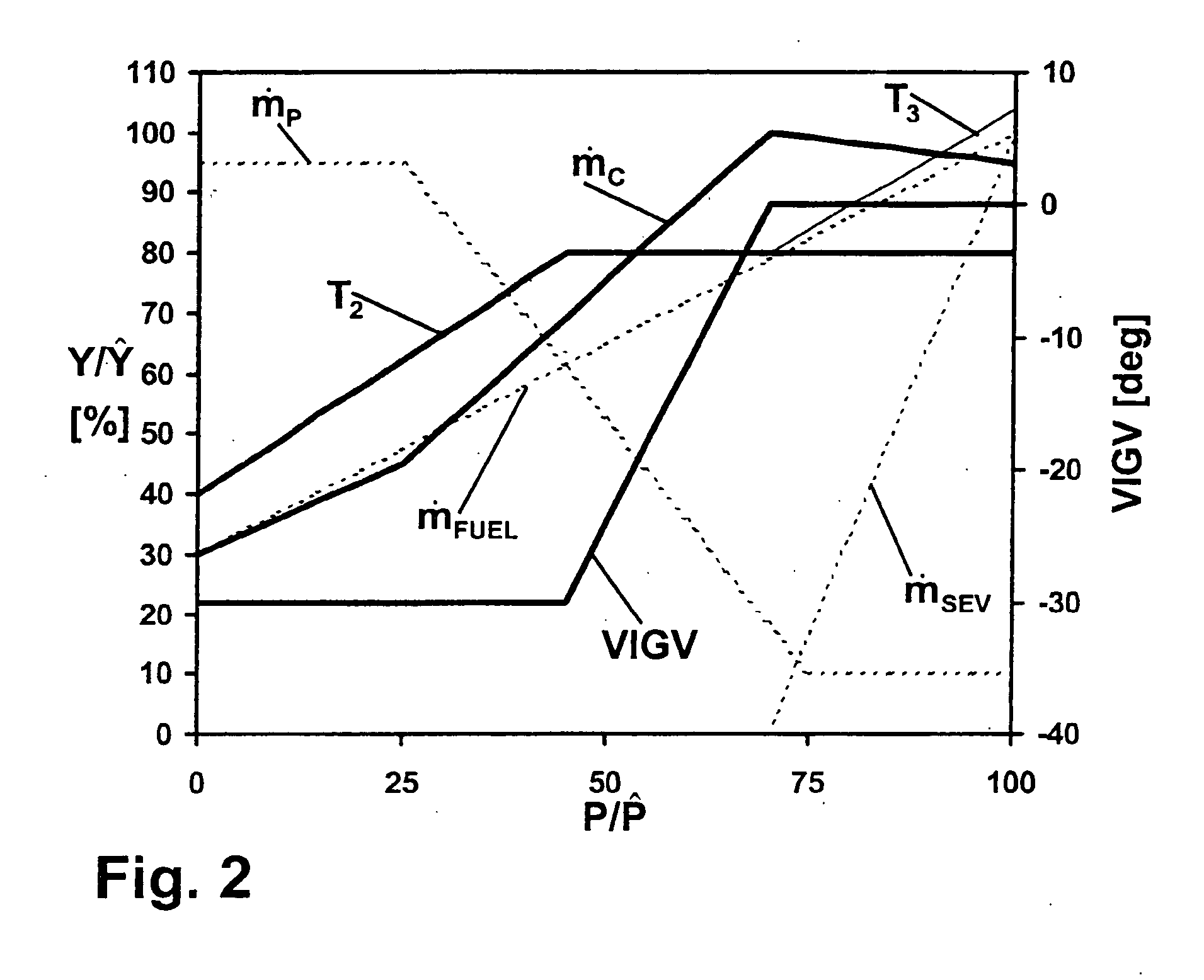
Method for operating a gas turbo group
InactiveUS20040216462A1Speed up the flowReduce cooling powerContinuous combustion chamberTurbine/propulsion engine ignitionCombustion chamberProcess engineering
A gas turbo group has a combustion chamber comprising a catalytic burner stage (2), a preburner stage (1) located upstream from the catalytic burner stage, as well as a non-catalytic burner stage (11, 5, 6) located downstream from the catalytic burner stage. The preburner stage serves to always maintain a temperature (T1) at the inlet into the catalytic stage that corresponds at least to a minimum temperature (TMIN) necessary for operating the catalytic burner stage. According to the invention, the gas turbo group is operated so that the burner stage located downstream from the catalytic combustion chamber is taken into operation only when the temperature (T2) at the outlet from the catalytic stage has reached an upper limit in the presence of a maximum combustion air mass flow.
Owner:ALSTOM TECH LTD
Application of molybdenum carbide in preparing anode of microbial fuel cell
InactiveCN101656314AIncrease power outputWide variety of sourcesCell electrodesBiochemical fuel cellsMicrobial fuel cellPtru catalyst
The invention discloses the application of molybdenum carbide in preparing the anode of a microbial fuel cell, in particular to a method for preparing the anode of the microbial fuel cell, in which the molybdenum carbide is used as a catalyst and the mixture of the molybdenum carbide and a binder are coated on a conductive base. Compared with a non-catalytic electrode, the molybdenum carbide is used as an anode catalyst to catalyze hydrogen to be oxidized, thereby greatly enhancing the electric energy output of microbial fuel cell; compared with a conventional Pt catalyst, the molybdenum carbide has low price and extensive source, and the microbial fuel cell assembled by taking the molybdenum carbide as the anode catalyst can stably work for a long time and has high power output. The application of the molybdenum carbide in preparing the anode of the microbial fuel cell provides the good foundation for the commercialization application of the microbial fuel cell.
Owner:SOUTH CHINA NORMAL UNIVERSITY
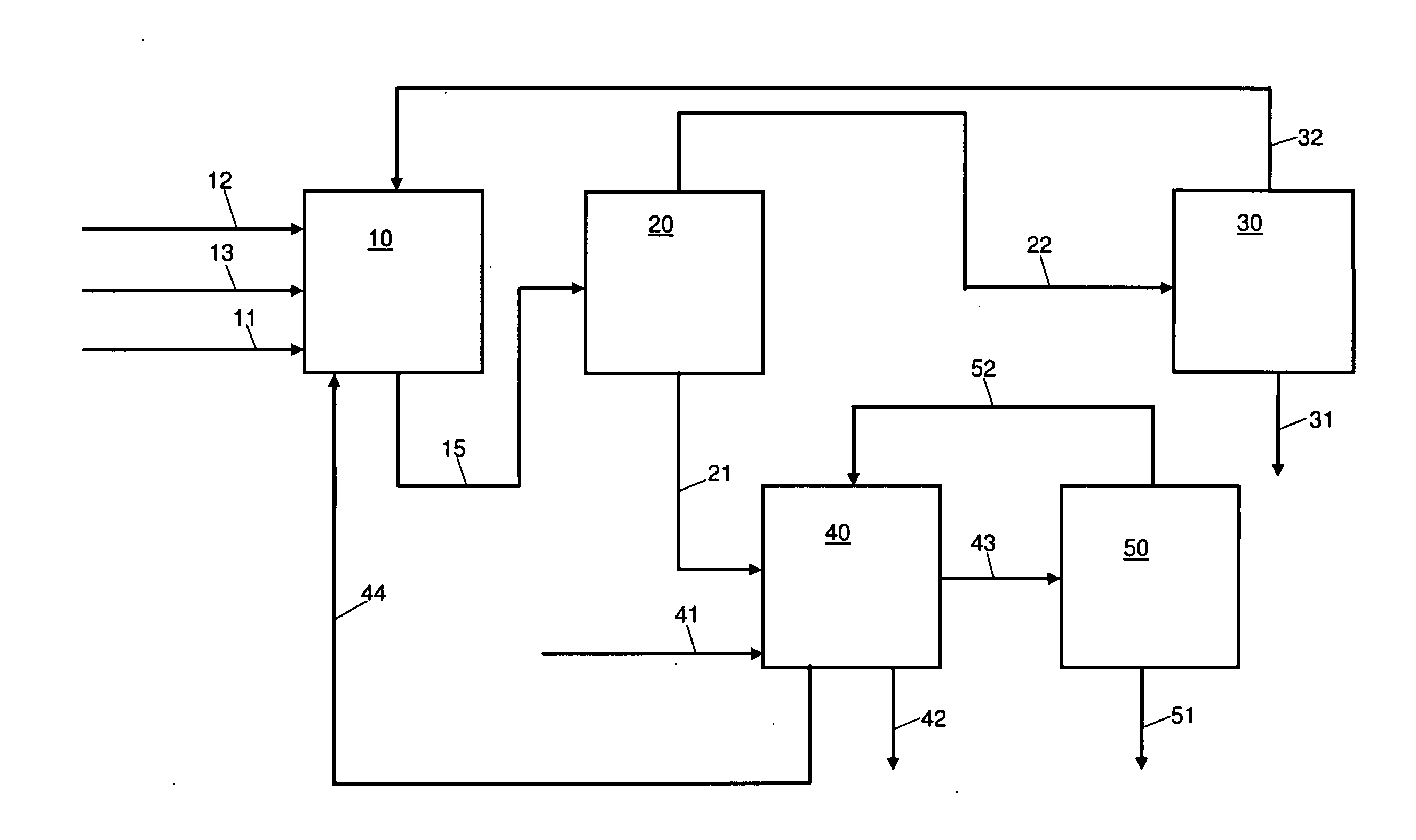
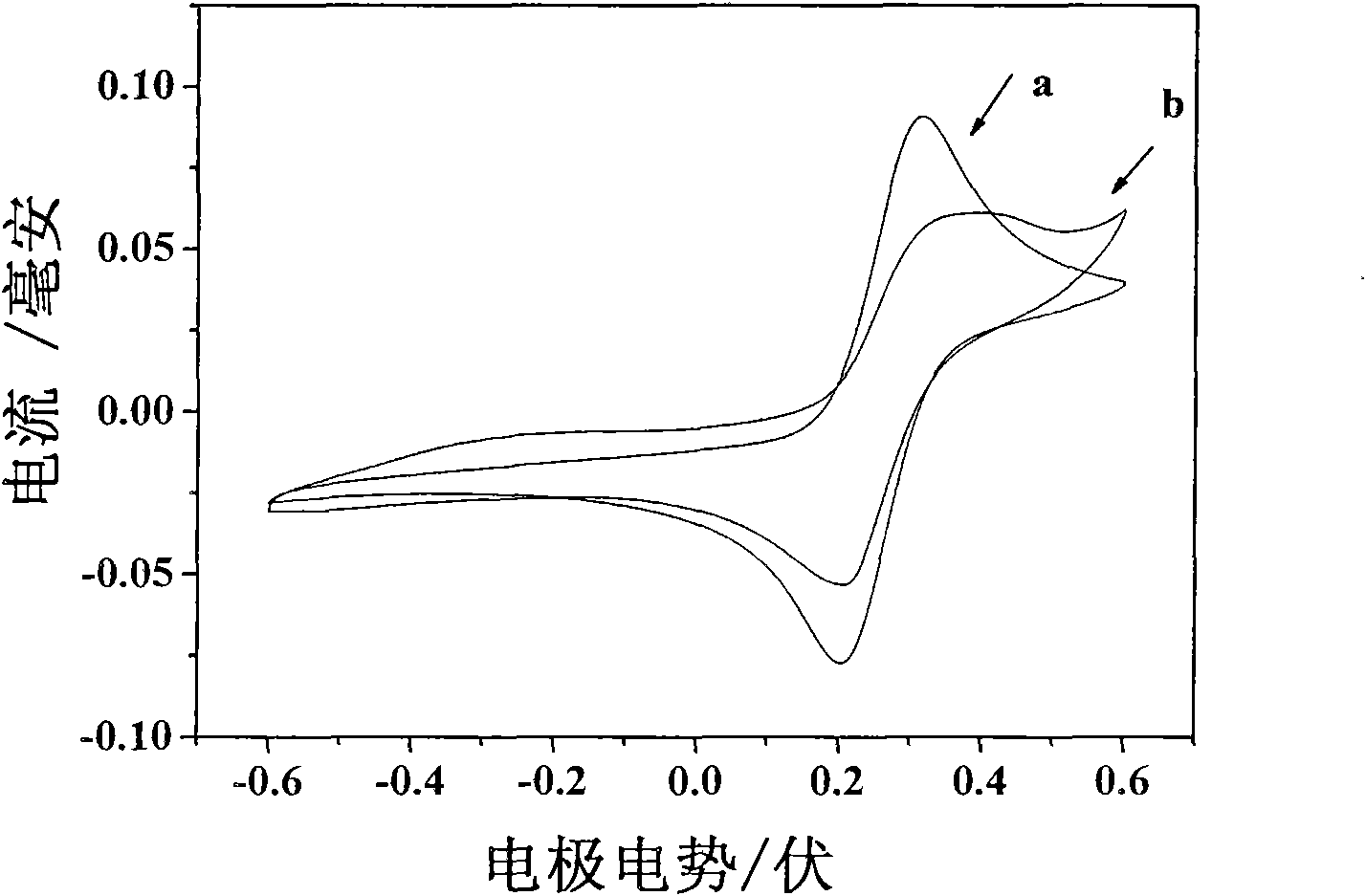
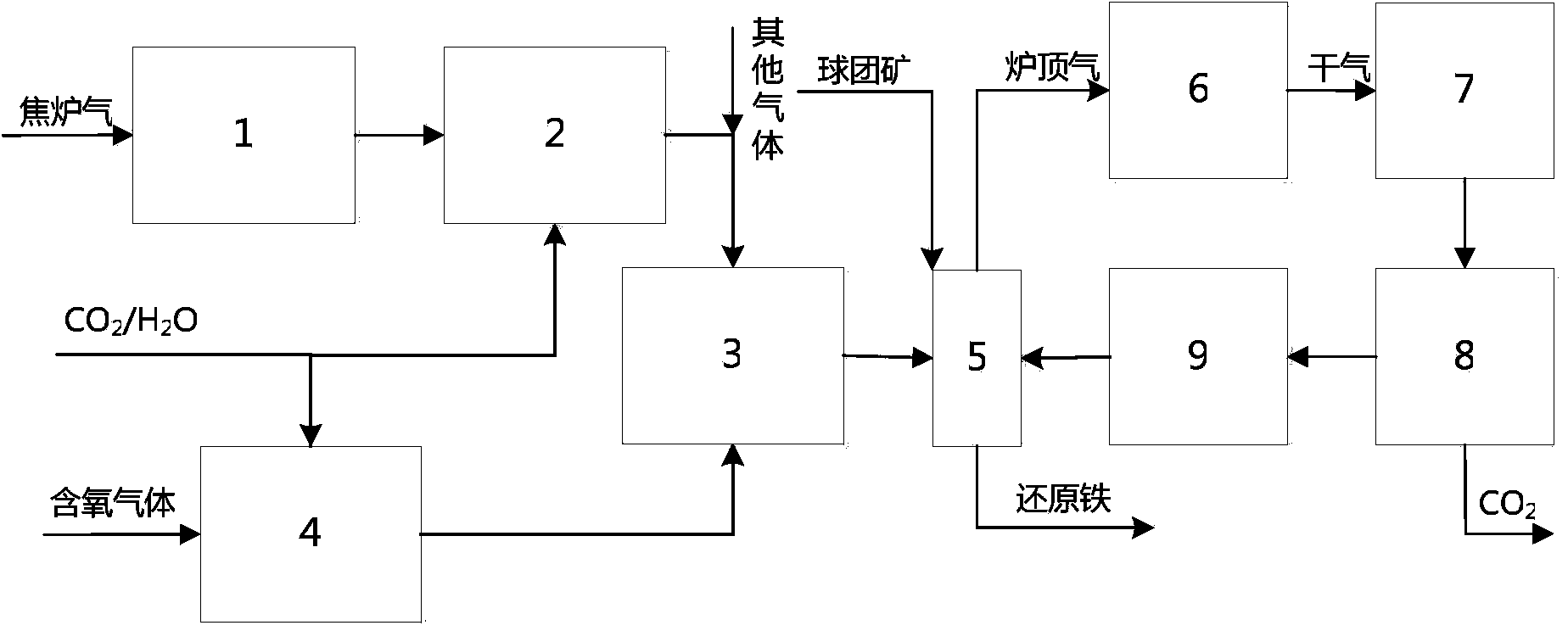
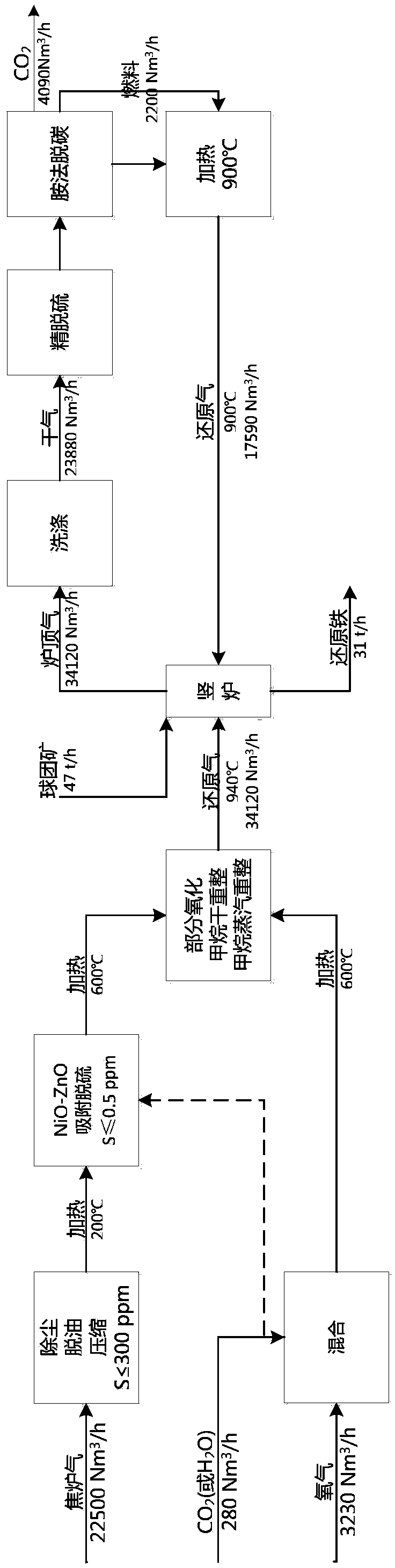
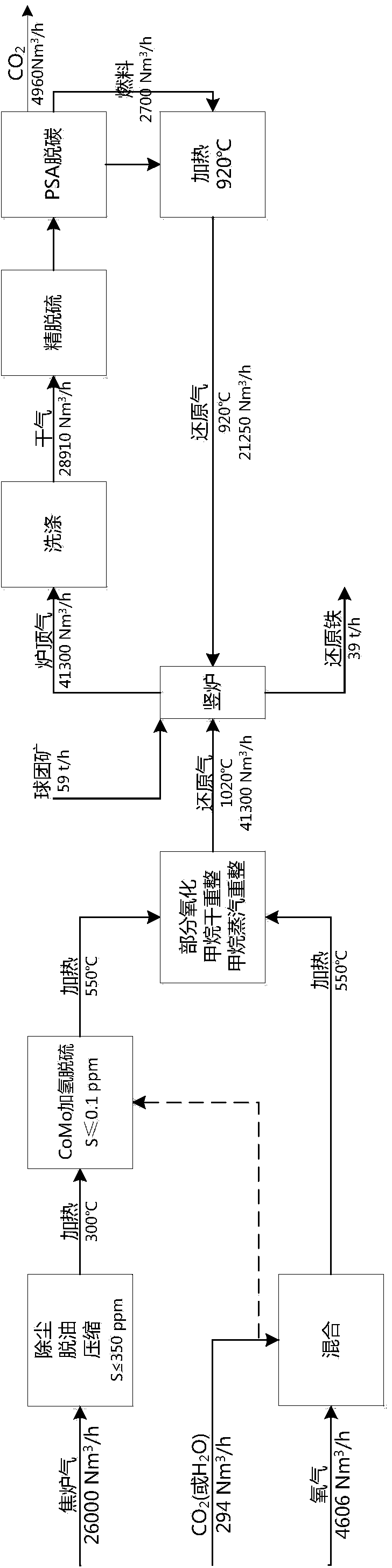
Method for producing gas-based directly reduced iron by utilizing non-catalytic conversion of coke-oven gas, and system thereof
ActiveCN103525965AAvoid inactivationGuaranteed uptimeShaft furnaceProcess efficiency improvementWater vaporEngineering
The invention relates to a method for producing gas-based directly reduced iron by utilizing the non-catalytic conversion of a coke-oven gas, and a system thereof. The method comprises the following steps: mixing the coke-oven gas obtained after routine purification and fine desulfurization treatment with a mixed gas comprising one or above two of a converter gas, a blast furnace gas and a purified tail gas to form a raw material gas mixture, and partially combusting the raw material gas mixture and an oxygen-containing gas at a converter burner outlet at a controlled flame temperature of 1100-2300DEG C for the conversion of hydrocarbons in the coke-oven gas and carbon dioxide or water vapor to generate a synthetic gas containing H2 and CO; and allowing the synthetic gas containing H2 and CO to enter a shaft furnace and reduce iron oxide in order to produce reduced iron, and cooling, washing, decarburizing, desulphurizing and purifying a reduced tail gas discharged from a shaft furnace to obtain a purified tail gas. The invention also provides a system for producing the gas-based directly reduced iron by utilizing the non-catalytic conversion of the coke-oven gas.
Owner:CHINA UNIV OF PETROLEUM (BEIJING) +1
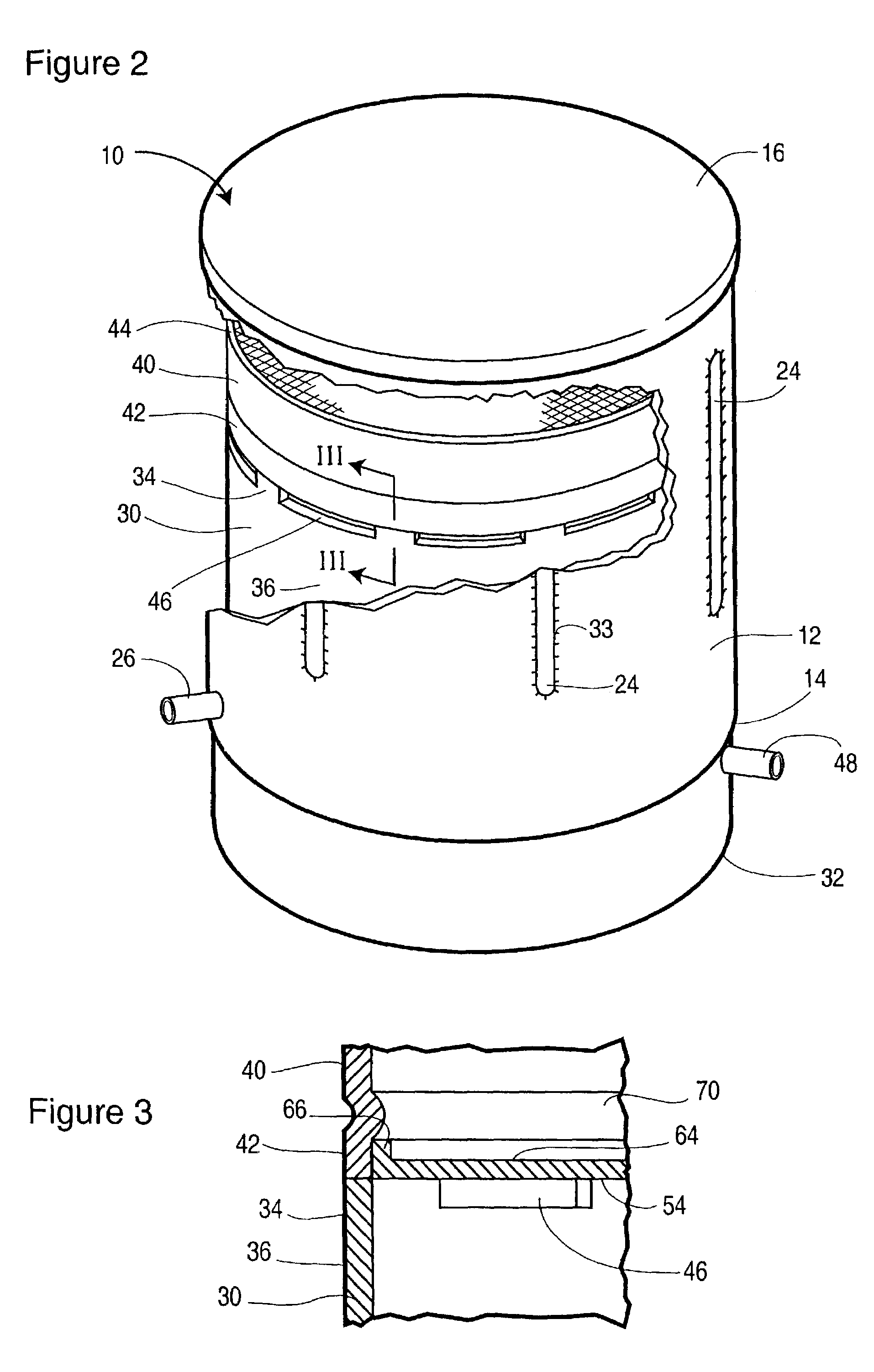
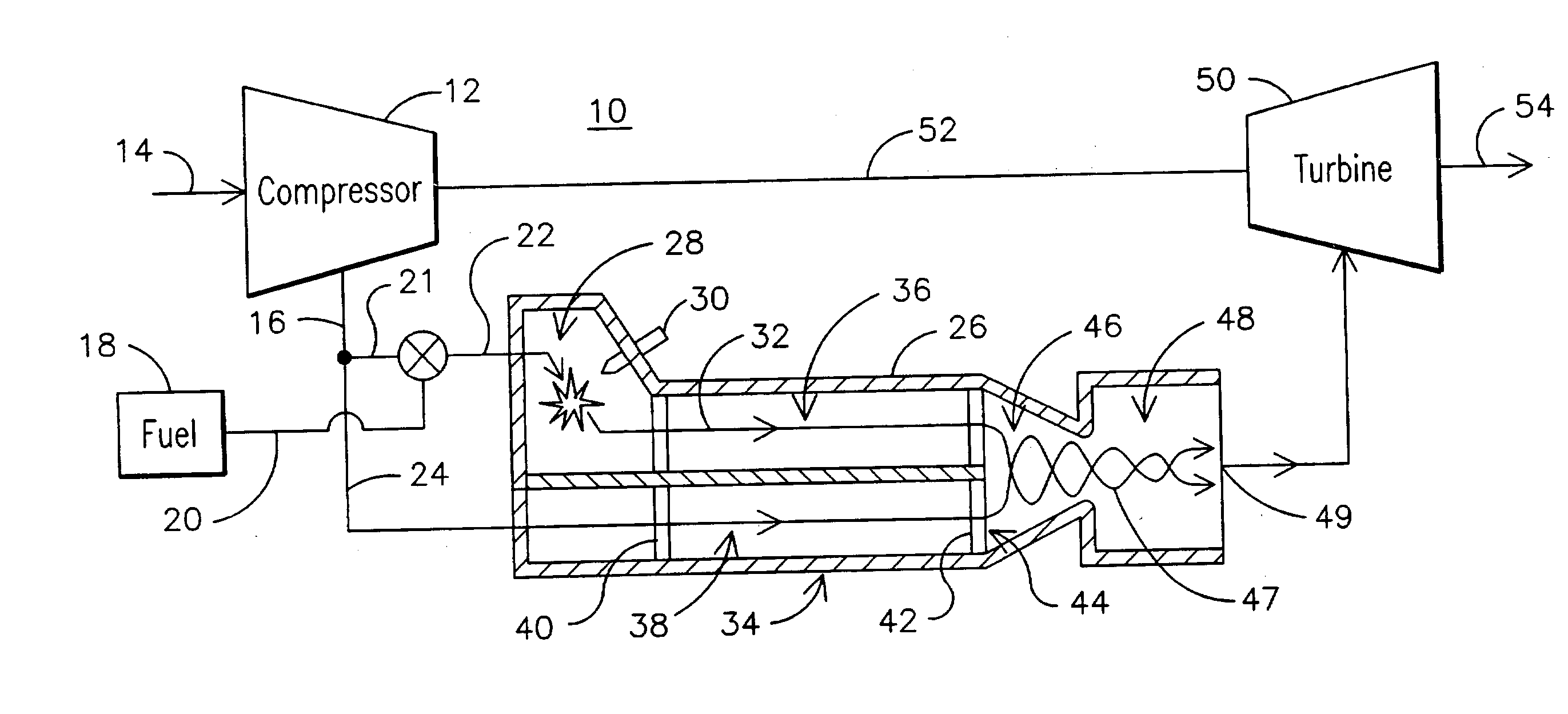
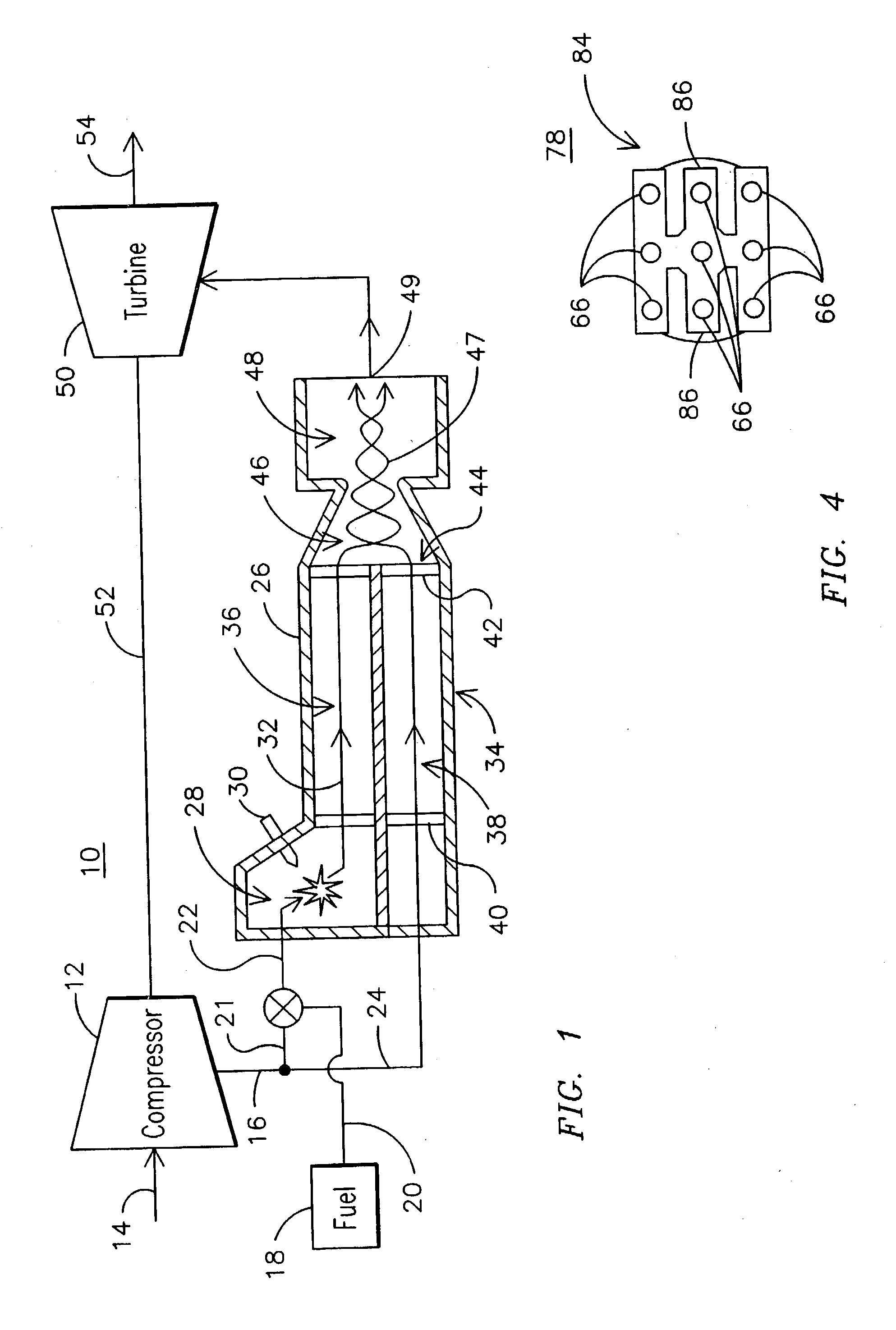
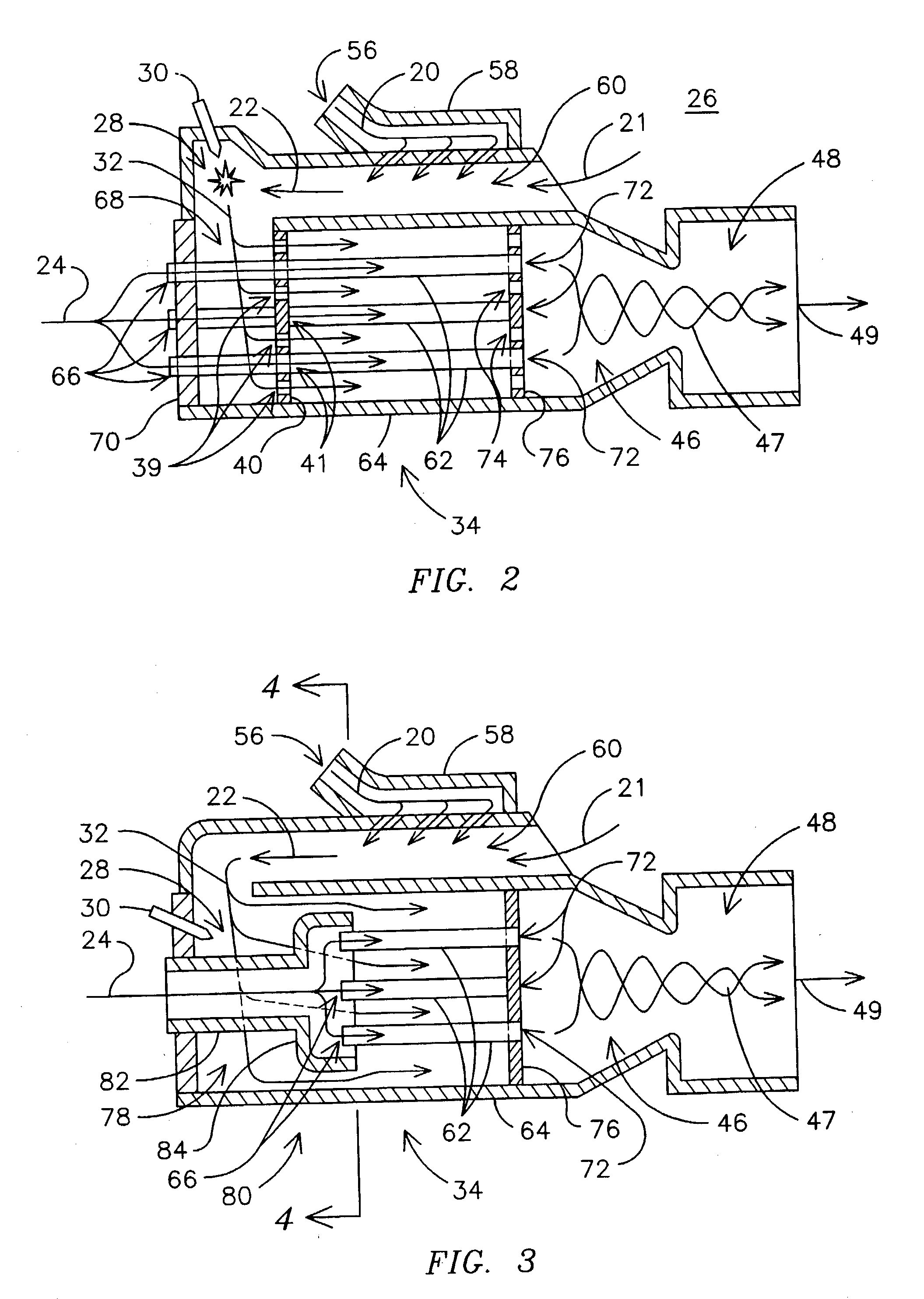
Non-catalytic combustor for reducing NOX emissions
InactiveUS20050188702A1Improved combustor designTurbine/propulsion fuel supply systemsContinuous combustion chamberCombustorCombustion chamber
A gas turbine engine combustor (26) including a primary combustion chamber (28), a mixer element (34), a mixing chamber (46) and a secondary combustion chamber (48). The primary combustion chamber receives a fuel oxidizer mixture flow (22) and discharges a partially oxidized mixture flow (32). The mixer element includes a plurality of flow channels (36, 38) for separating the partially oxidized mixture from a flow of an oxidizer (24) and producing a plurality of partially oxidized mixture flows interspersed with a plurality of oxidizer fluid flows. The mixer element may include a plurality of tubes (62) retained by an upstream tubesheet (70) and a downstream tubesheet (76). The mixer element may function as a heat exchanger to heat the oxidizer fluid flow and to cool the partially oxidized mixture flow upstream of the post-mixing chamber.
Owner:SIEMENS ENERGY INC
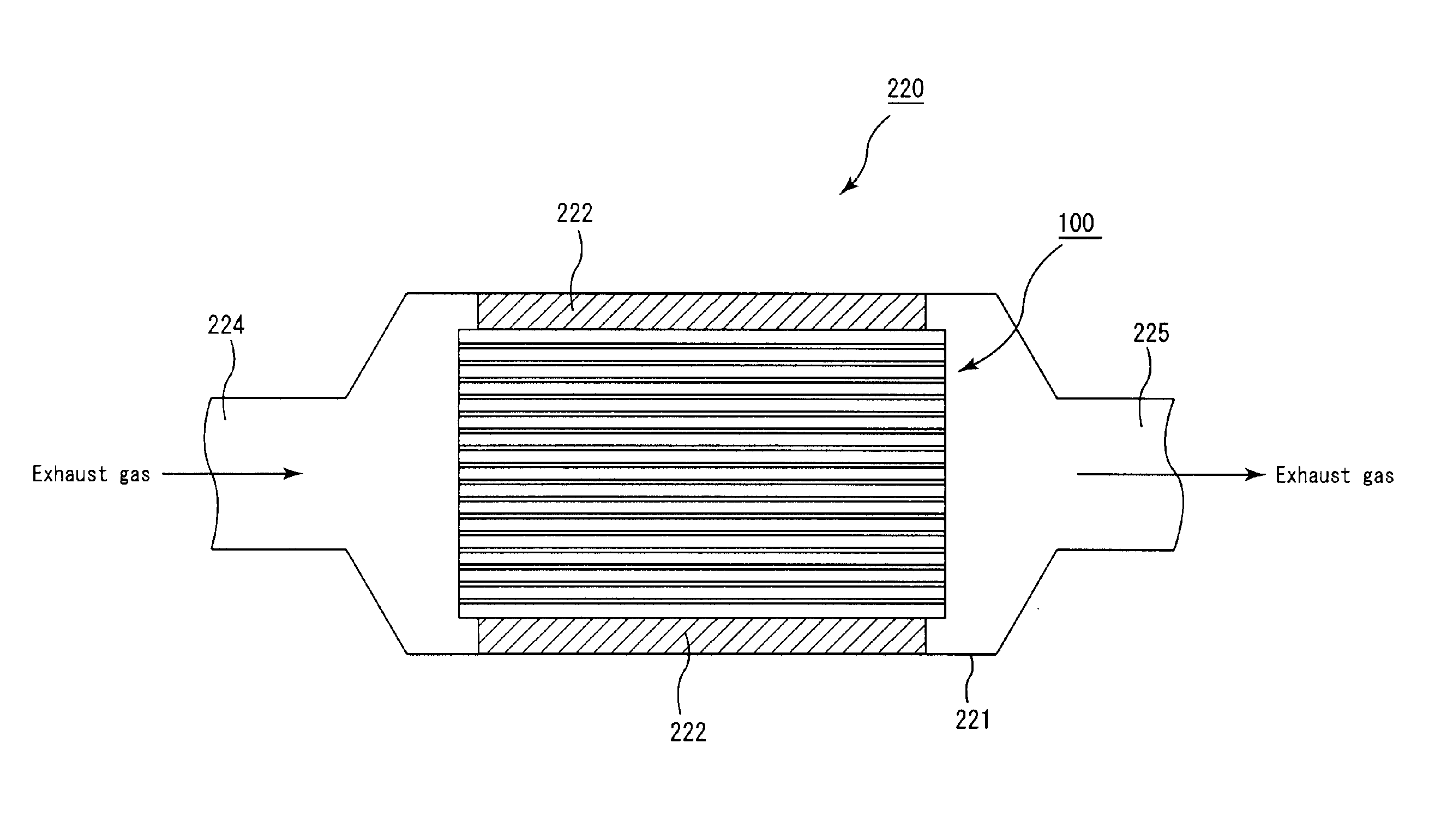
Honeycomb filter and exhaust gas purifying apparatus
A honeycomb filter includes a pillar-shaped honeycomb fired body having first and second end faces on a gas inlet and outlet sides, respectively. A catalyst supporting layer is formed in a catalyst-supporting-layer area covering about 25% to about 90% of an overall length of the honeycomb fired body and abutting the first end face on the gas inlet side. Substantially no catalyst supporting layer is formed in a non-catalyst-supporting-layer area covering about 10% of the overall length on the gas outlet side. A thermal conductivity of the non-catalyst-supporting-layer area is higher than that of the catalyst-supporting-layer area, and inequalities, (a−b)≦about 5, and about 10≦a≦about 20 are satisfied where “a” is a mode of pore diameters obtained by measuring pore distribution of the non-catalyst-supporting-layer area and “b” is a mode of pore diameters obtained by measuring pore distribution of the catalyst-supporting-layer area.
Owner:IBIDEN CO LTD
Non-catalytic reduction and oxidation process for the removal of NOx
InactiveUS20060233688A1Reduce concentrationCatalytic crackingNitrogen compoundsReducing agentScrubber
The present invention relates to a non-catalytic process for reducing NOx concentrations in the regenerator off-gas stream of a fluid catalytic cracking unit. More particularly, the present invention relates to the injection of a reducing agent in combination with a readily-oxidizable gas into a regenerator off-gas stream to reduce at least a portion of the NO in the regenerator off-gas stream, then contacting the regenerator off-gas stream with an effective amount of a treating solution under conditions such that at least a fraction of the oxidizable NOx species present in the regenerator off-gas stream is oxidized to higher oxides, and subsequently removing at least a fraction of these higher oxides from the off-gas stream. One embodiment of this invention also relates to the use of a reacted caustic solution from a wet gas scrubber for the removal of at least a fraction of the higher oxides.
Owner:EXXON RES & ENG CO
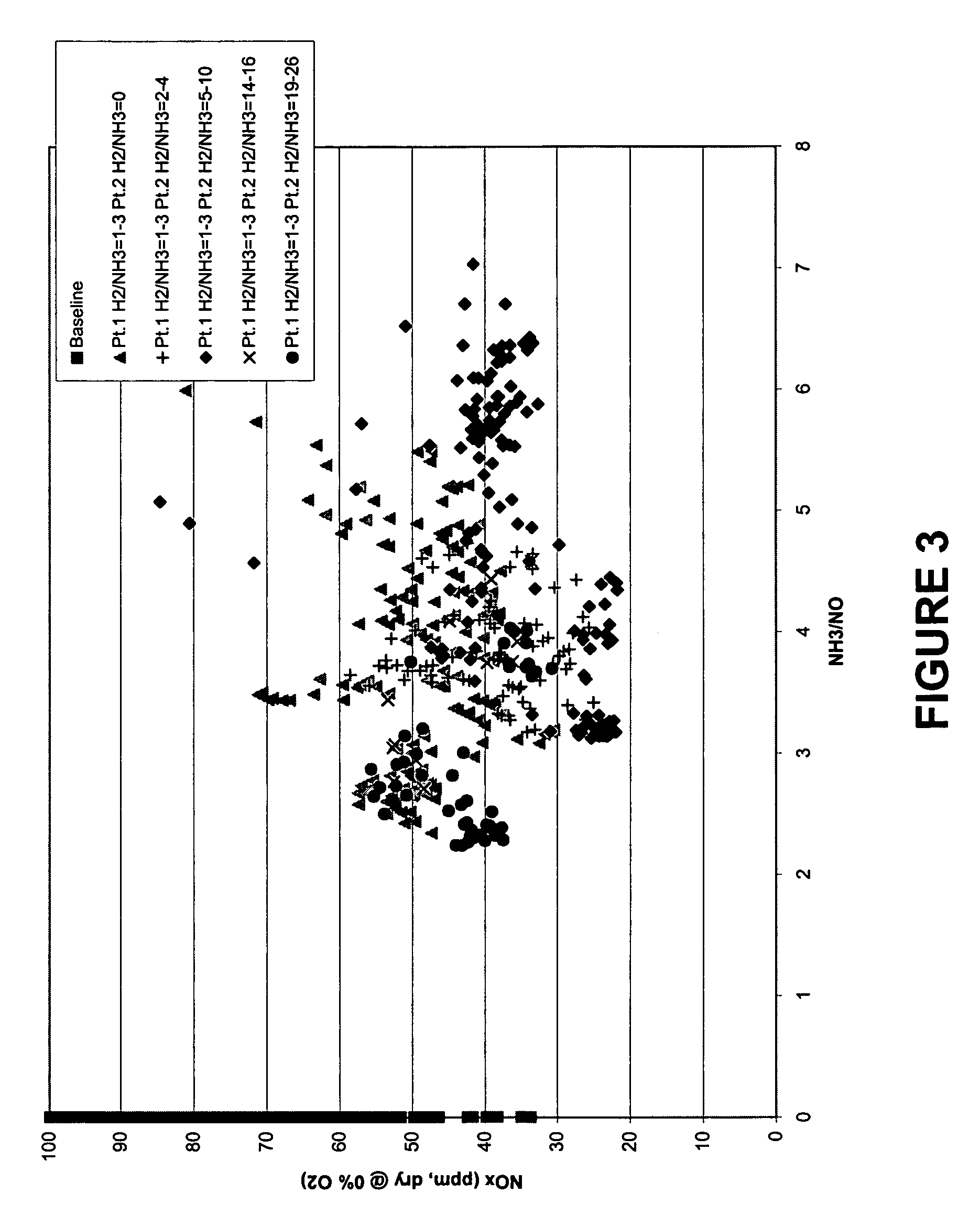
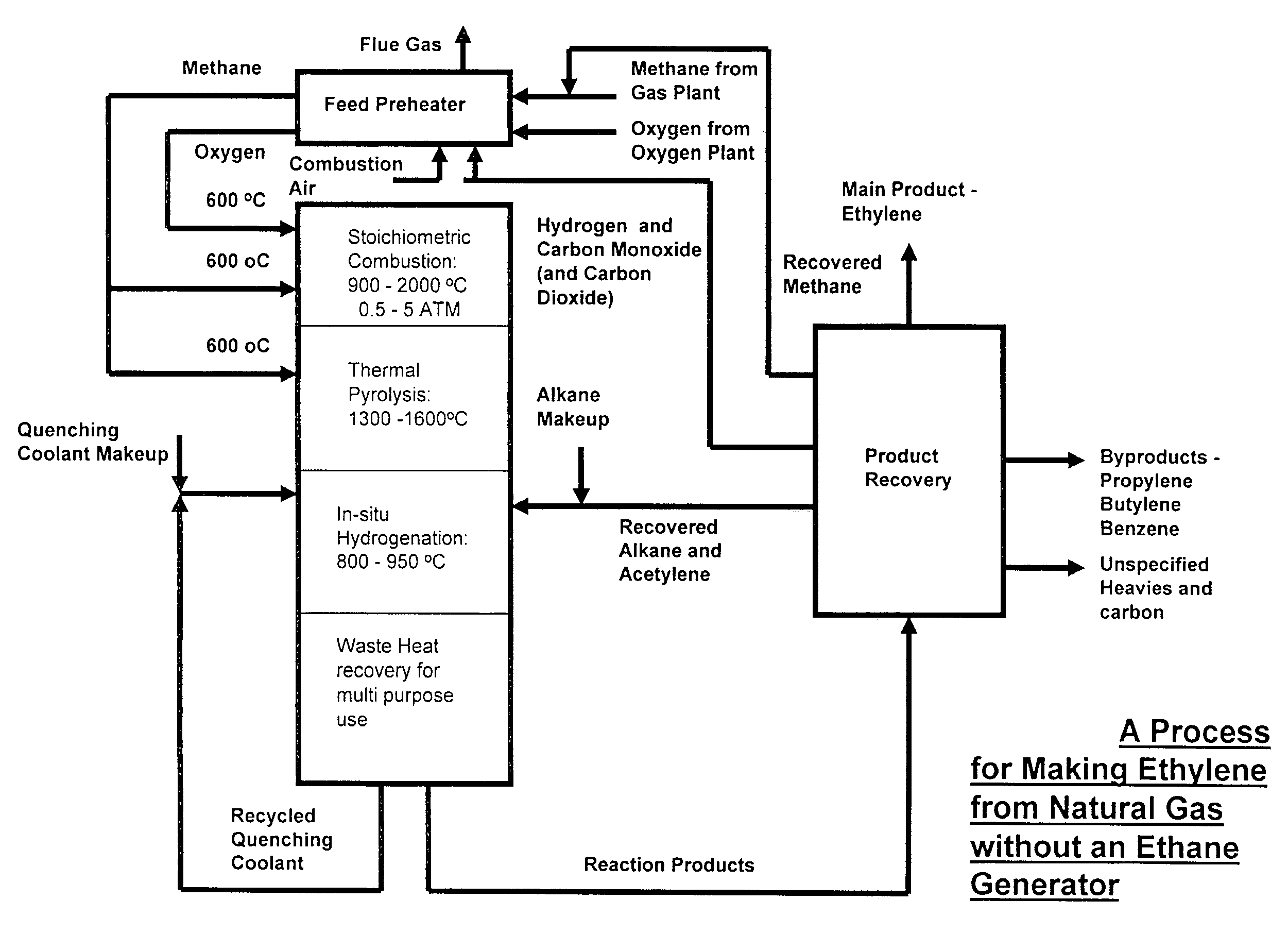
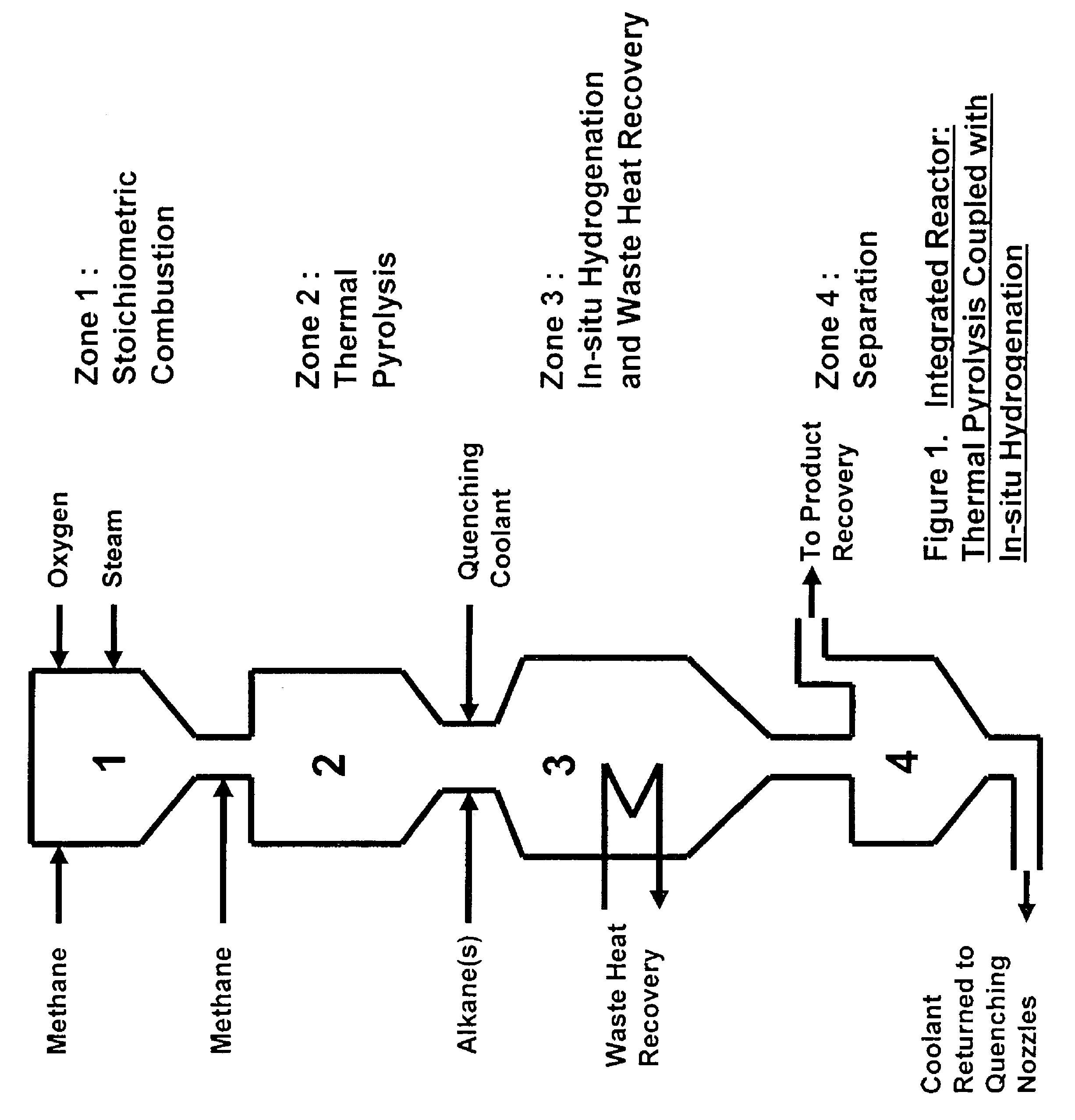
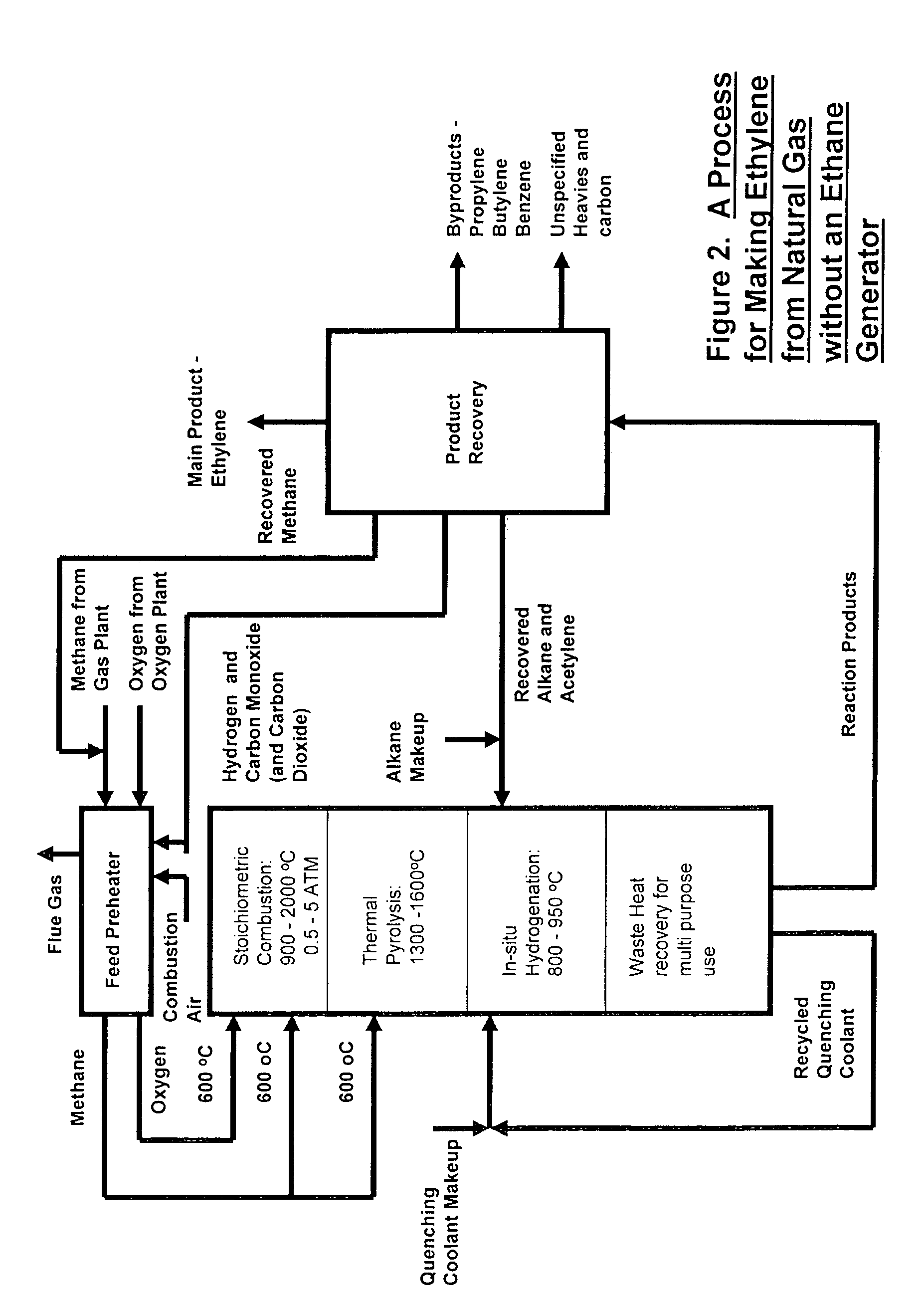
Process for the production of ethylene from natural gas with heat integration
InactiveUS8080697B2Improved economic and energy and environmental aspectHighly effective in conversionHydrocarbon by dehydrogenationHydrocarbon by hydrogenationProcess engineeringAcetylene
The present invention relates to a process for the production of ethylene, comprising the following steps of: (a) thermally converting a feed charge containing methane into acetylene as an intermediate, (b) in-situ hydrogenation of the acetylene produced in step (a) into ethylene by a non-catalytic hydrogen transfer mechanism, characterized by (c) recovering heat from hot effluents obtained in step (b) which may be utilized for different purposes.
Owner:SAUDI BASIC IND CORP SA
Process for the production of ethylene
InactiveUS8013196B2Convenient for design and operationSimple designHydrocarbon by hydrogenationHydrocarbons from unsaturated hydrocarbon additionPartial oxidationProcess design
The invention relates to a process for the production of ethylene, comprising the steps of a) thermally converting, by a pyrolysis or a partial oxidation process, a feed charge containing methane into an acetylene containing effluent, and b) in situ hydrogenating, by a non-catalytic reaction, the acetylene produced in the first step into ethylene by intimately mixing the acetylene containing effluent with an ethane feed. The process according to the invention is more efficient than other synthesis schemes, while simplifying the overall process design. This process thus offers an economically attractive scheme for mass production of ethylene from natural gas, based on a well-known and proven acetylene route.
Owner:SAUDI BASIC IND CORP SA
Enantioselective resolution process for arylpropionic acid drugs from the racemic mixture
InactiveUS6093830AOrganic compound preparationOptically-active compound separationCelsius DegreeOrganic solvent
The invention relates to a novel non-catalytic enantioselective resolution process for the separation of enantiomer of arylpropionic acid drugs from the racemic mixture, which comprises dissolving the racemic mixture of the said drug an organic solvent, reacting this solution with an aqueous phase containing an ionic surfactant with or without a suitable co-surfactant, and an electrolyte in microemulsion / micellar / biphasic medium, reacting this mixture with an appropriate chiral amine at a temperature in the range of 0 to 70 degrees Celsius to obtain a diastereomeric salt, acid hydrolysing the diastereomeric salt to result in the pure enantiomer of the drug which is extracted by known methods.
Owner:COUNCIL OF SCI & IND RES
Processes for the conversion of biomass to oxygenated organic compound, apparatus therefor and compositions produced thereby
ActiveUS20130137151A1Improve concentrationEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsPartial oxidationOrganic compound
Processes are disclosed for the conversion of biomass to oxygenated organic compound using a simplified syngas cleanup operation that is cost effective and protects the fermentation operation. The processes of this invention treat the crude syngas from the gasifier by non-catalytic partial oxidation. The partial oxidation reduces the hydrocarbon content of the syngas such as methane, ethylene and acetylene to provide advantageous gas feeds for anaerobic fermentations to produce oxygenated organic compounds such as ethanol, propanol and butanol. Additionally, the partial oxidation facilitates any additional cleanup of the syngas as may be required for the anaerobic fermentation. Producer gases and partial oxidation processes are also disclosed.
Owner:SYNATA BIO INC
Production of Light Olefins and Isoprene from Butane
InactiveUS20110040133A1Increase valueImprove cokingOrganic compound preparationDistillation purification/separationButeneDehydrogenation
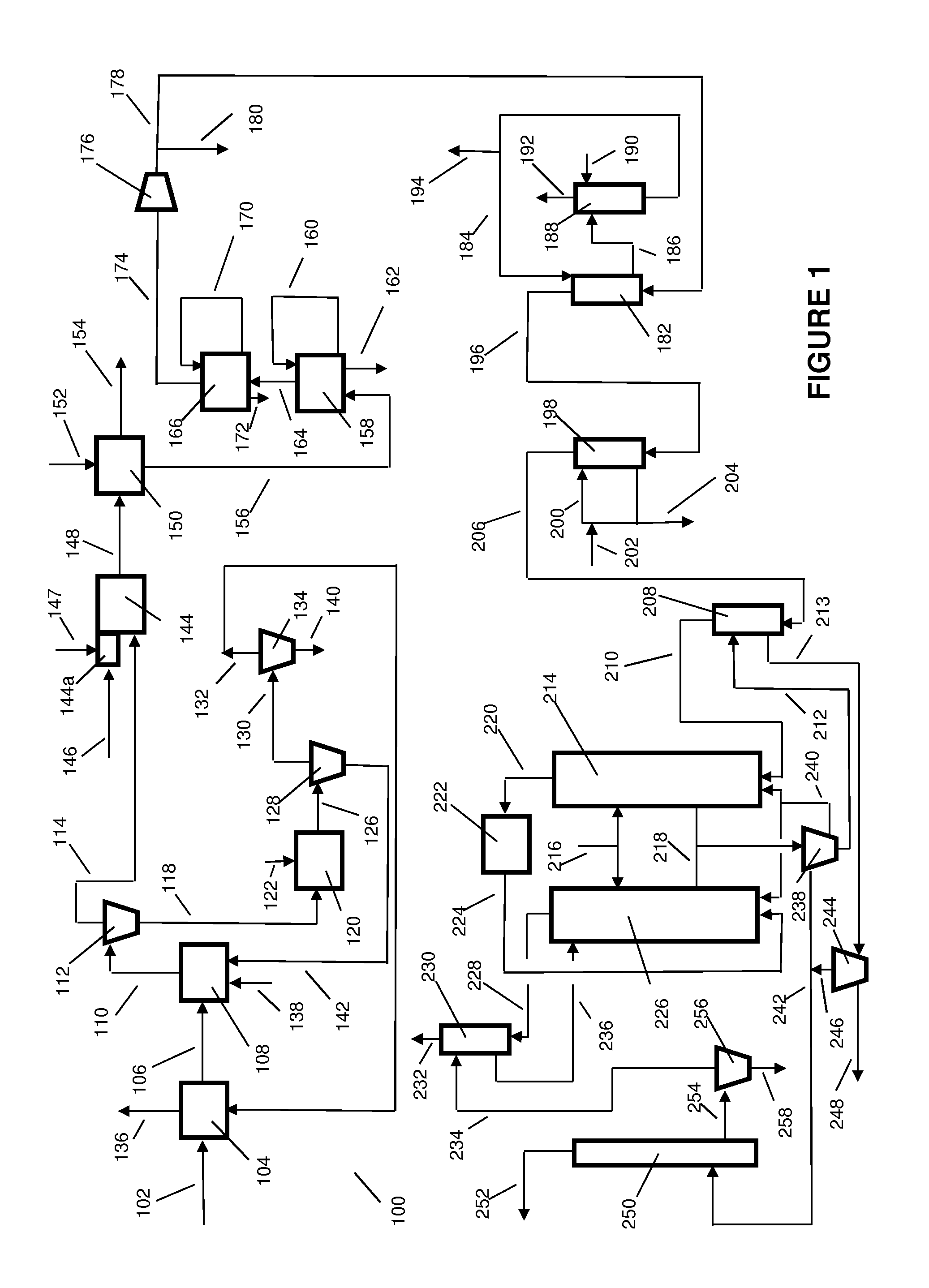
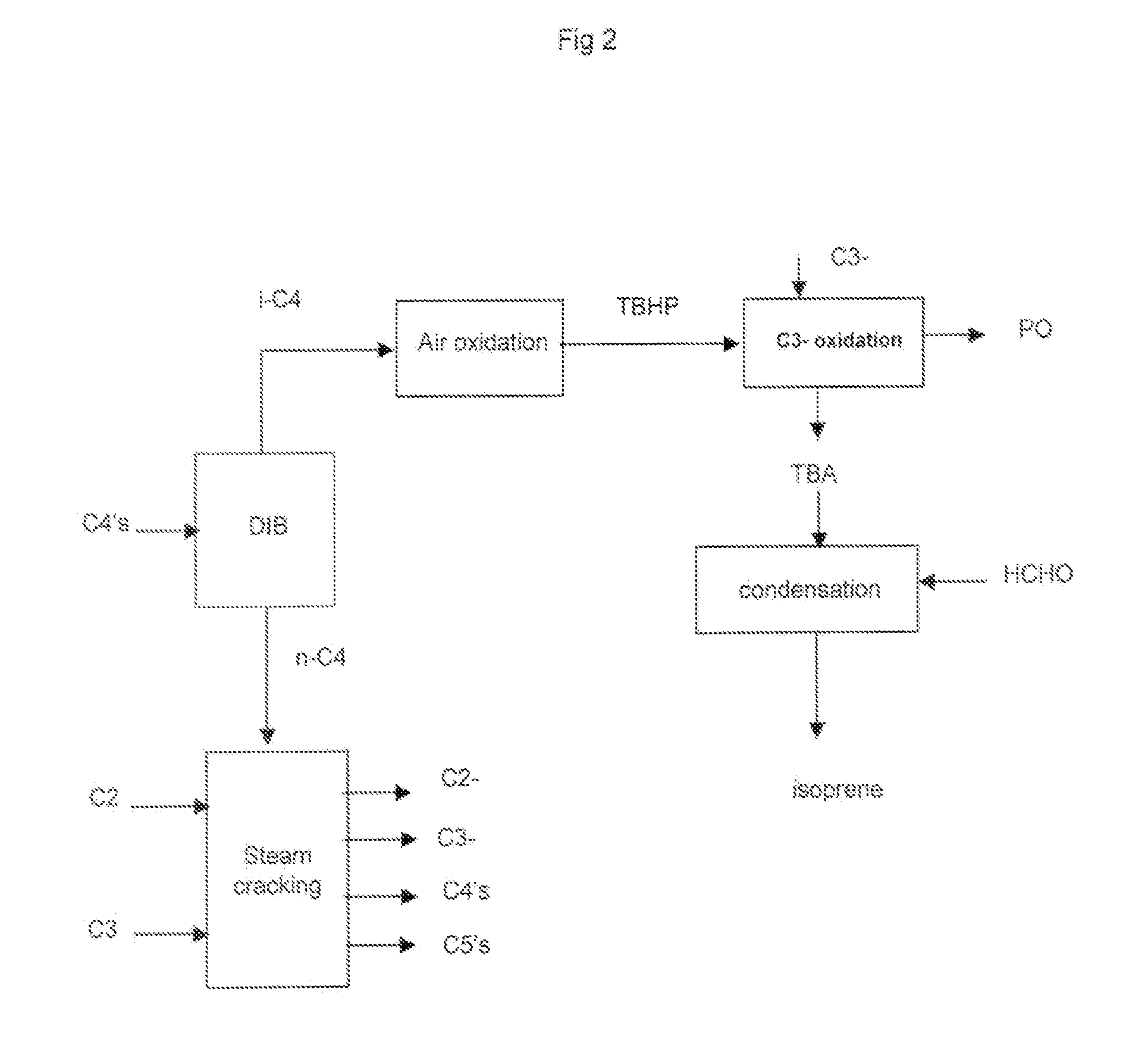
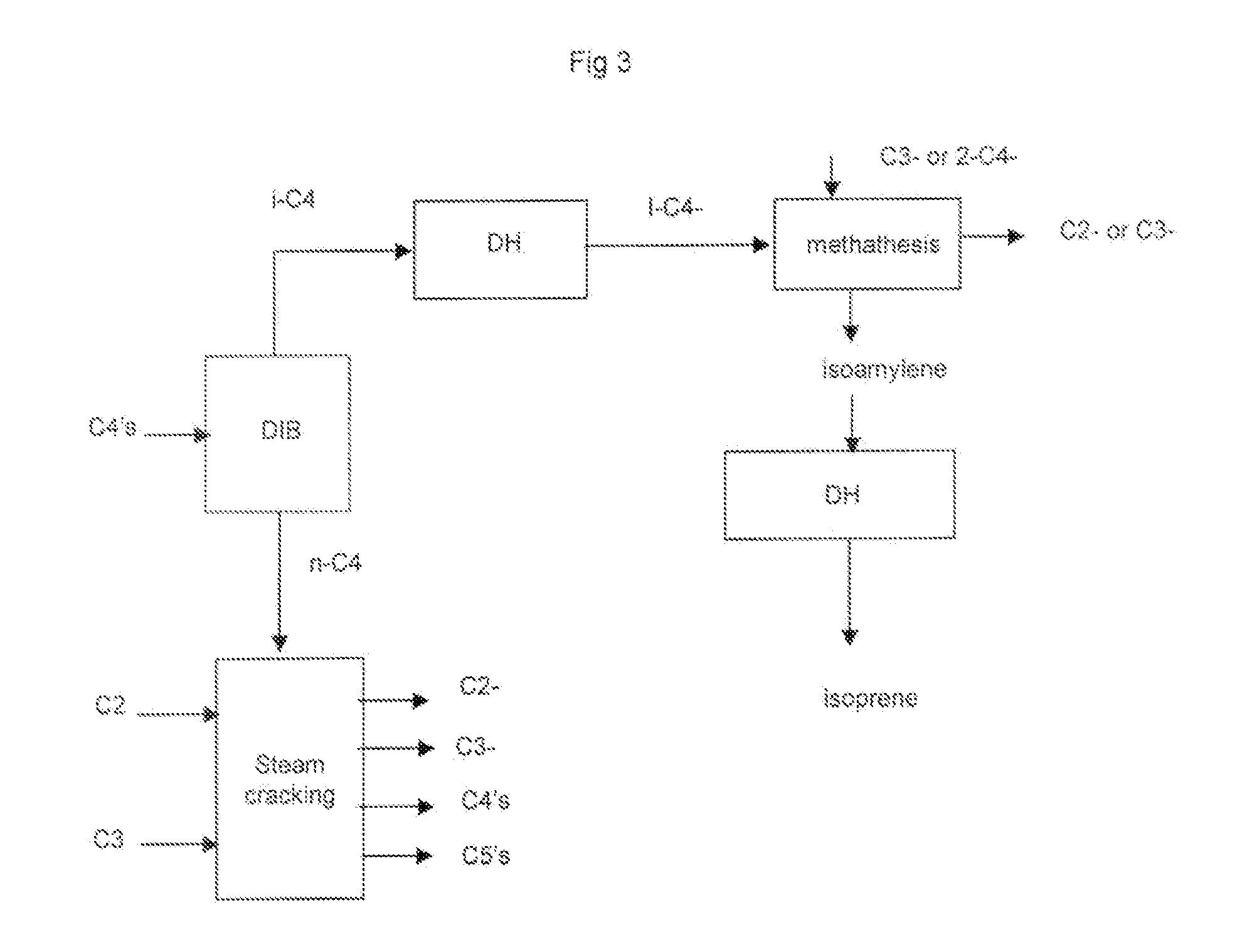
Process for the selective production of ethylene, propylene and isoprene from light hydrocarbons comprising: a) fractionating a butane fraction in a de-isobutanizer to obtain an enriched iso-butane fraction and an enriched normal-butane fraction, b) cracking said normal-butane fraction and optionally an ethane fraction, optionally a propane fraction, in a non-catalytic cracking zone to produce an olefin rich stream, c) treating said olefin rich stream in a separating section to recover: an ethylene stream, a propylene stream, d) transforming the recovered iso-butane of step a) into iso-butene or t-butyl hydroperoxide or partly into iso-butene and partly into t-butyl hydroperoxide, e) optionally reacting iso-butene of step d), if any, with formaldehyde to make isoprene, f) optionally reacting t-butyl hydroperoxide of step d), if any, with an olefin to give an epoxide and t-butanol and further separating t-butanol, or optionally having t-butyl hydroperoxide of step d), if any, decomposed to t-butanol and reacted with formaldehyde to give isoprene, or reacting a part of the t-butyl hydroperoxide of step d) with an olefin and having the remaining part decomposed to t-butanol and reacted with formaldehyde to give isoprene, g) dehydrating the t-butanol recovered at step f), if any, into iso-butene and reacting said iso-butane with formaldehyde to make isoprene, or reacting directly the t-butanol recovered at step f), if any, with formaldehyde to make isoprene, or dehydrating the t-butanol recovered at step f), if any, into iso-butene, hydrogenating said iso-butene to iso-butane and oxidizing said iso-butane into t-butyl hydroperoxide, and recycling said t-butyl hydroperoxide, or dehydrating the t-butanol recovered at step f), if any, into iso-butene, then disproportionating said iso-butene and propylene recovered at step c) (or 2-butene recovered at step c)), separating an isoamylene stream and converting the isoamylene into isoprene by dehydrogenation, or making any combination of above routes of said step g), h) optionally disproportionating iso-butene of step d), if any, and propylene recovered at step c) (or 2-butene recovered at step c)), separating an isoamylene stream and converting the isoamylene into isoprene by dehydrogenation, at least one of steps e), f) and h) is not optional.
Owner:TOTAL RES & TECH FELUY
Direct reduction process for sponge iron production implemented by using non-catalytic conversion of CH4
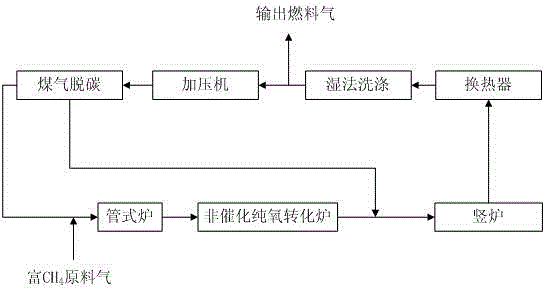
ActiveCN103146866ALower the preheat temperatureLess investmentShaft furnaceGas emission reductionDust controlShaft furnace
The invention discloses a direct reduction process for sponge iron production implemented by using the non-catalytic conversion of CH4, and the direct reduction process comprises the following steps: feeding a feed gas rich in CH4 and subjected to purification and pressure regulation and a stock gas output by a shaft furnace and subjected to cooling, dust removal, pressurization and decarburization into a tube furnace together and preheating; feeding the preheated coal gas into a non-catalytic pure oxygen reforming furnace, performing combustion reaction and heating on the preheated coal gas and oxygen in the non-catalytic pure oxygen reforming furnace, and performing non-catalytic conversion on the CH4 so as to generate CO+H2; after the high-temperature coal gas discharged from the non-catalytic pure oxygen reforming furnace and the stock gas discharged at the front end and subjected to cooling, dust removal, pressurization and decarburization are mixed and cooled, feeding the mixed gas into the shaft furnace and reducing iron ores, thereby producing sponge irons; and after the stock gas discharged from the shaft furnace is subjected to cooling, dust removal, pressurization and decarburization, feeding part of the stock gas and the supplementary feed gas rich in CH4 into the tube furnace and the non-catalytic pure oxygen reforming furnace together, wherein the other part of the stock gas is used as the cold-doped coal gas of the high-temperature coal gas discharged from the non-catalytic pure oxygen reforming furnace. Because the preheating temperature is low, no carbon precipitation occurs, and no strict limitation on the H2S content of the feed gas rich in CH4 exits, so that the bonding of the sponge irons can be prevented.
Owner:CISDI ENG CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com