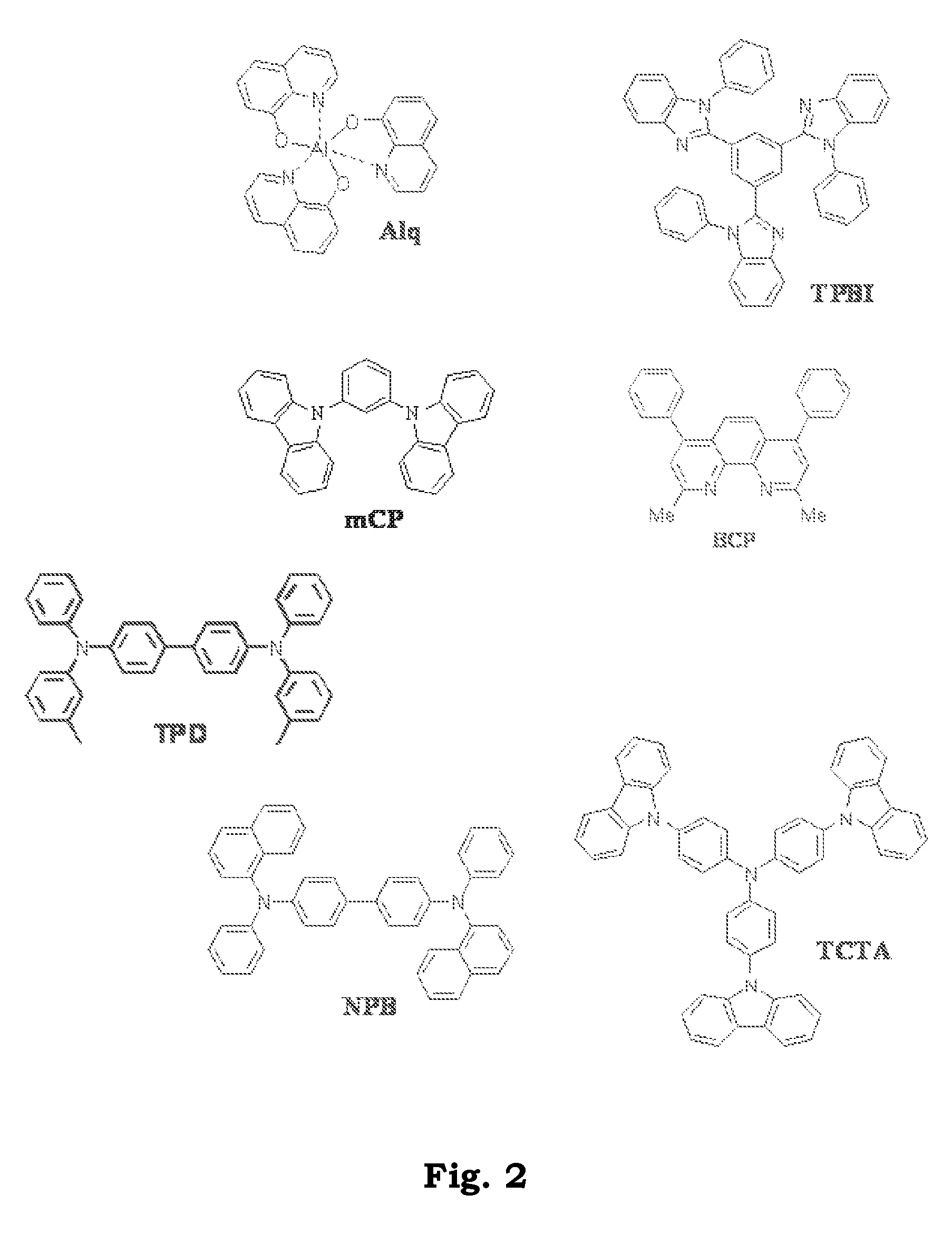
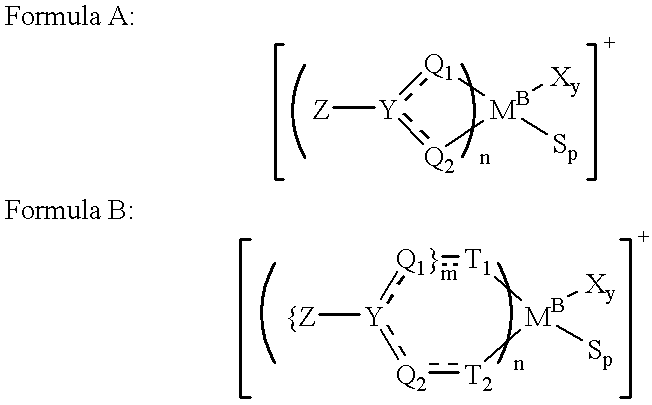
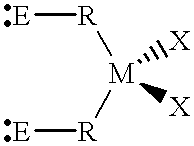
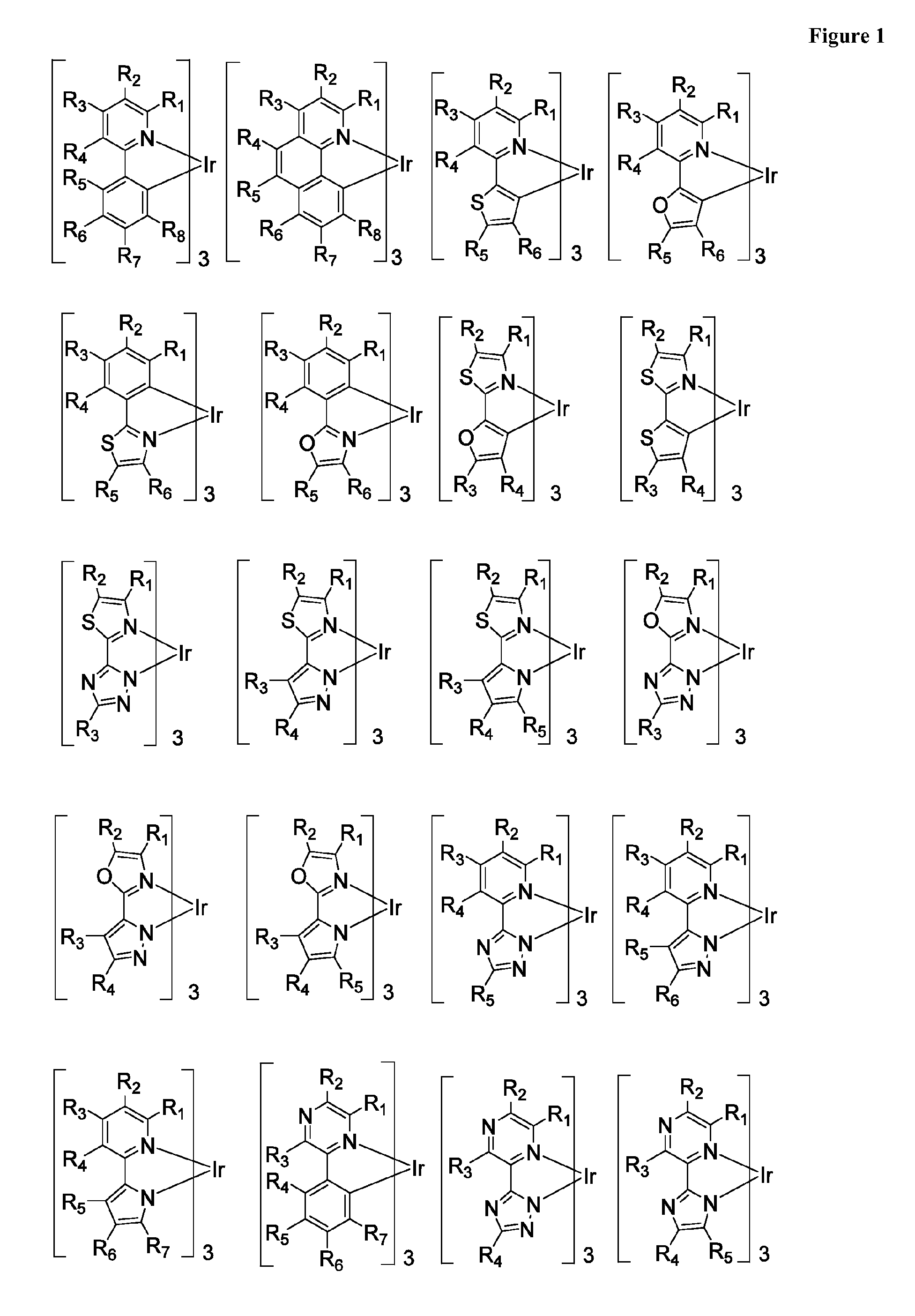
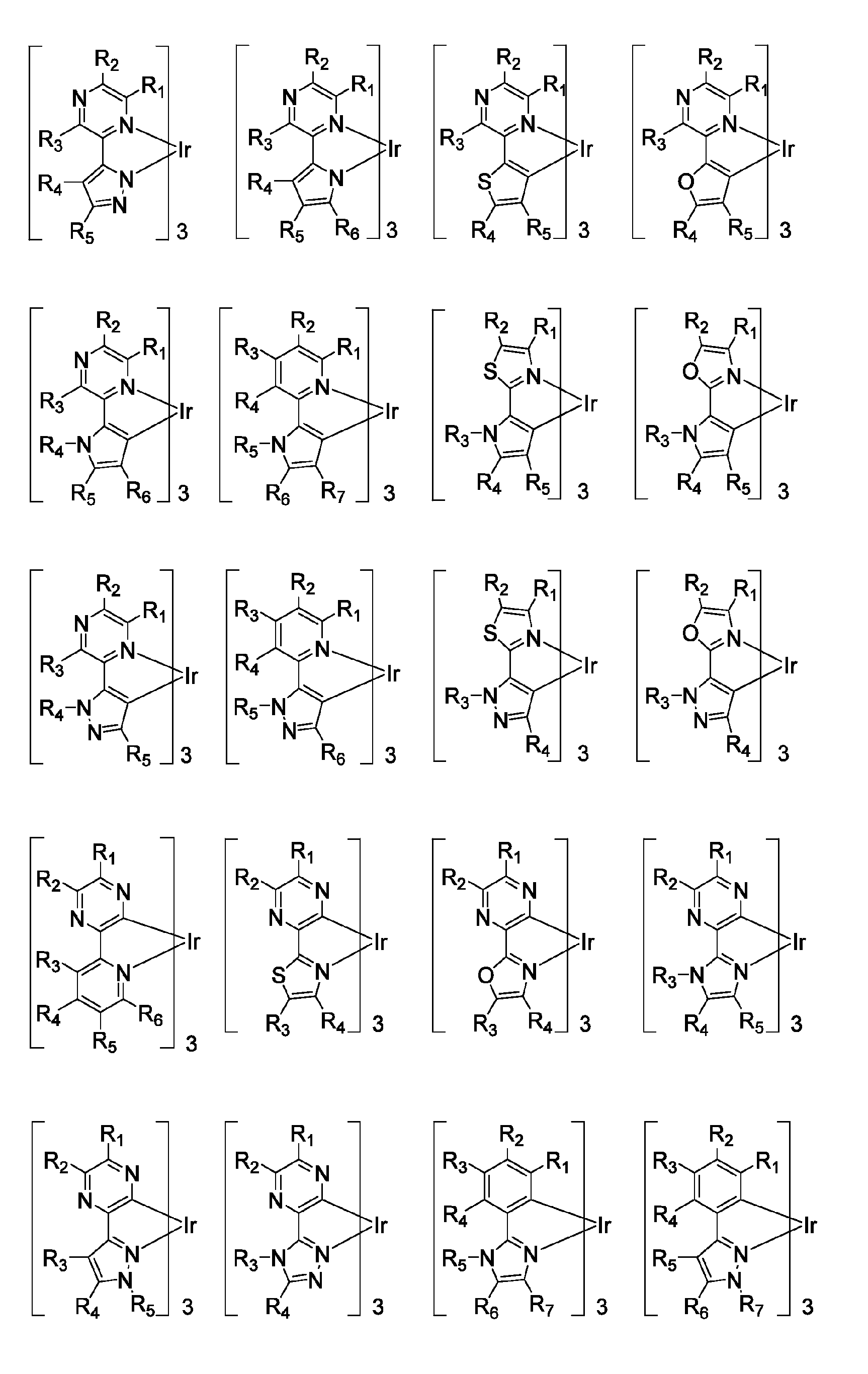
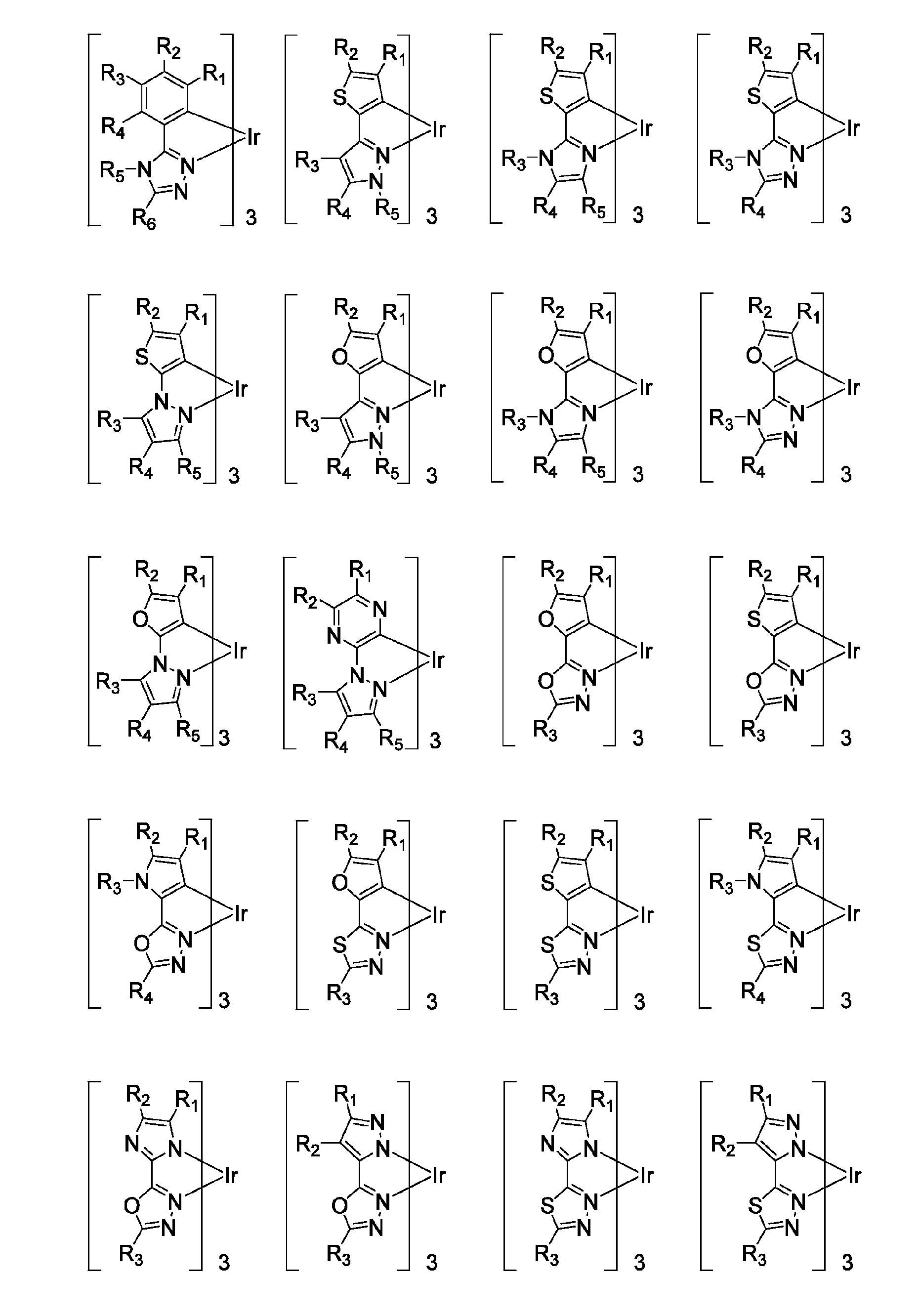
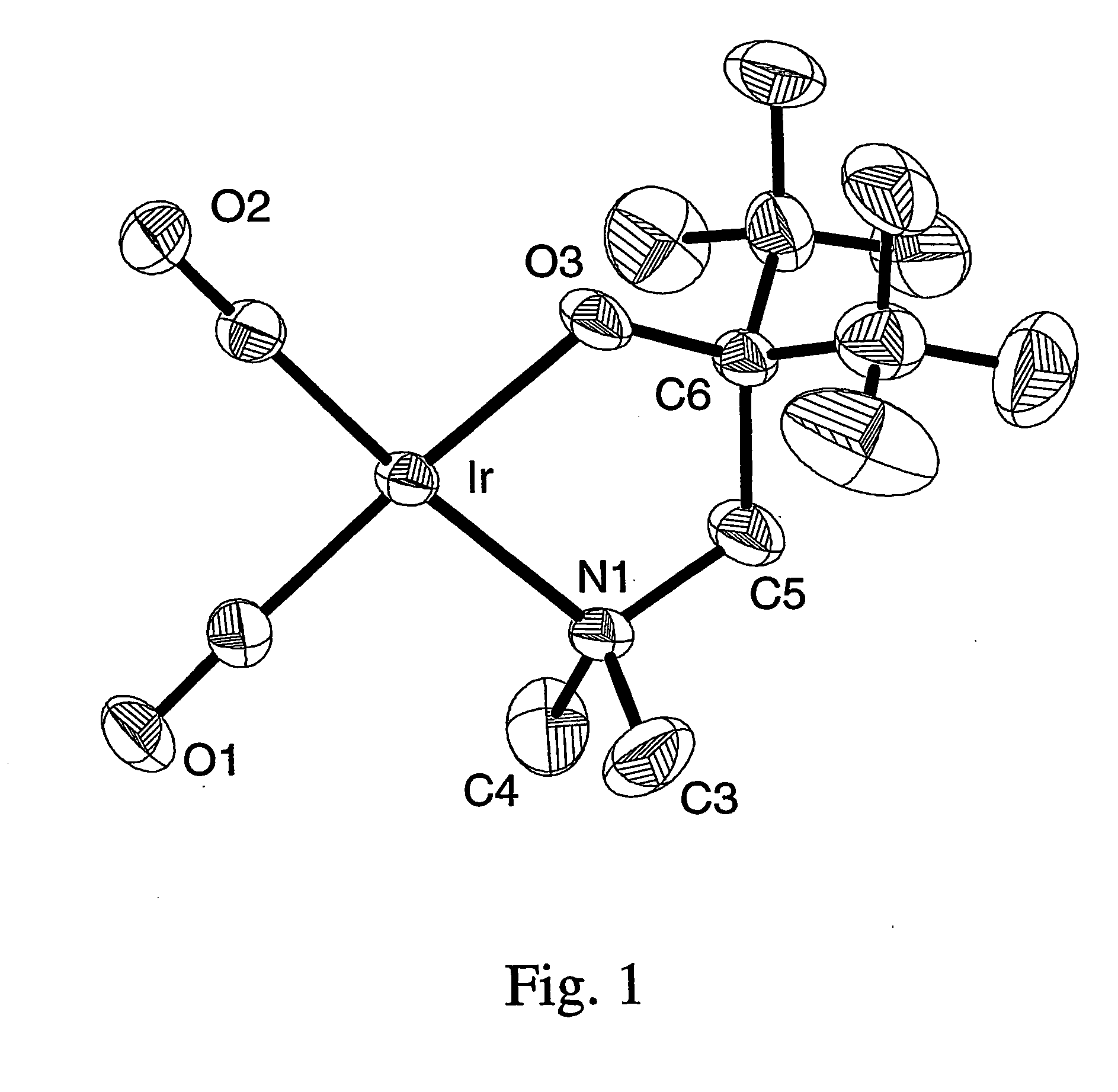
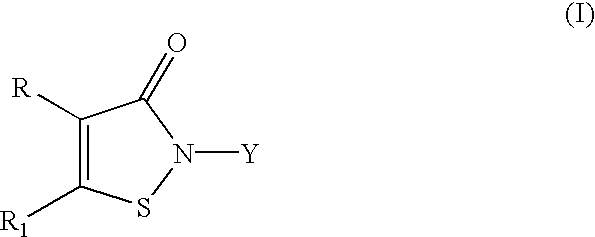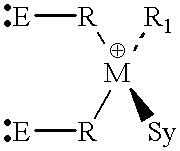Patents
Literature
210 results about "Chelating ligands" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chelating ligand: a ligand that is attached to a central metal ion by bonds from two or more donor atoms.
Volatile noble metal organometallic complexes
InactiveUS20050033075A1Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIridiumIodide
A series of noble metal organometallic complexes of the general formula (I): MLaXb(FBC)c, wherein M is a noble metal such as iridium, ruthenium or osmium, and L is a neutral ligand such as carbonyl, alkene or diene; X is an anionic ligand such as chloride, bromide, iodide and trifluoroacetate group; and FBC is a fluorinated bidentate chelate ligand such as beta diketonate, beta-ketoiminate, amino-alcoholate and amino-alcoholate ligand, wherein a is an integer of from zero (0) to three (3), b is an integer of from zero (0) to one (1) and c is an 10 integer of from one (1) to three (3). The resulting noble metal complexes possess enhanced volatility and thermal stability characteristics, and are suitable for chemical vapor deposition(CVD) applications. The corresponding noble metal complex is formed by treatment of the FBC ligand with a less volatile metal halide. Also disclosed are CVD methods for using the noble metal complexes as source reagents for deposition of noble metal-containing films such as Ir, Ru and Os, or even metal oxide film materials IrO2, OsO2 and RuO2.
Owner:NATIONAL TSING HUA UNIVERSITY +1
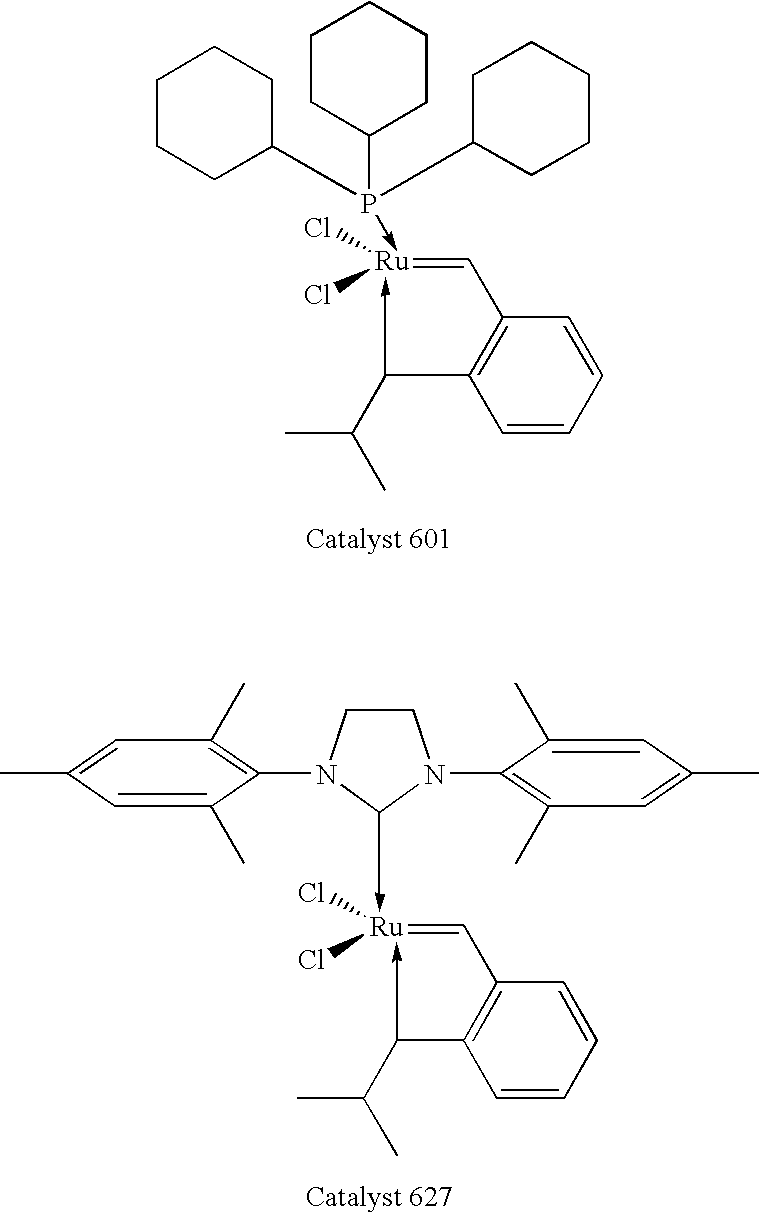
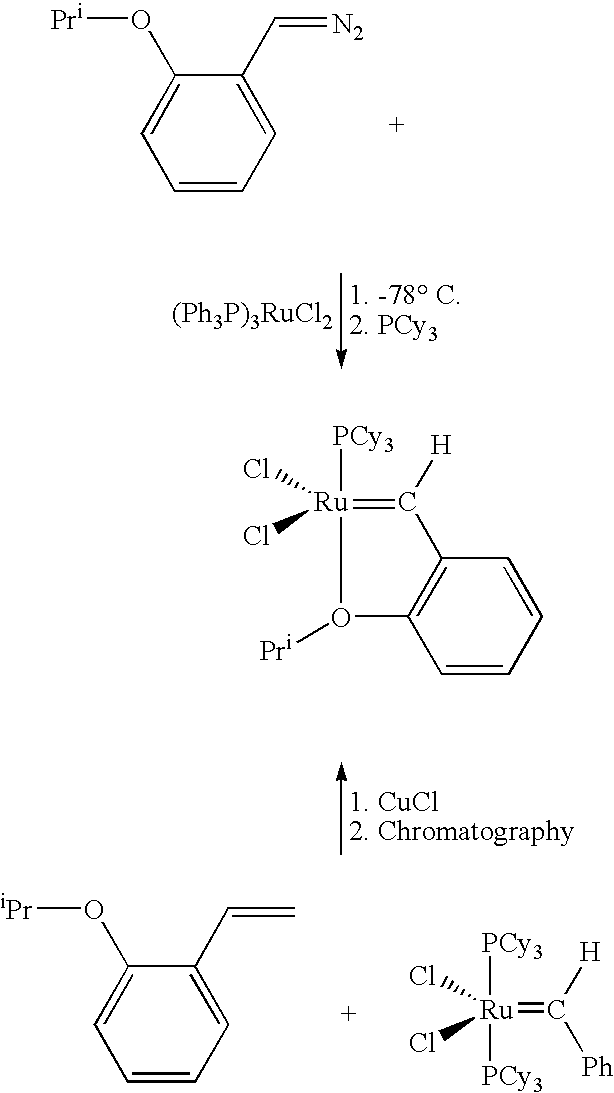
Chelating carbene ligand precursors and their use in the synthesis of metathesis catalysts
InactiveUS7026495B1High yieldImprove isolationRuthenium organic compoundsGroup 5/15 element organic compoundsHigh concentrationCarbene
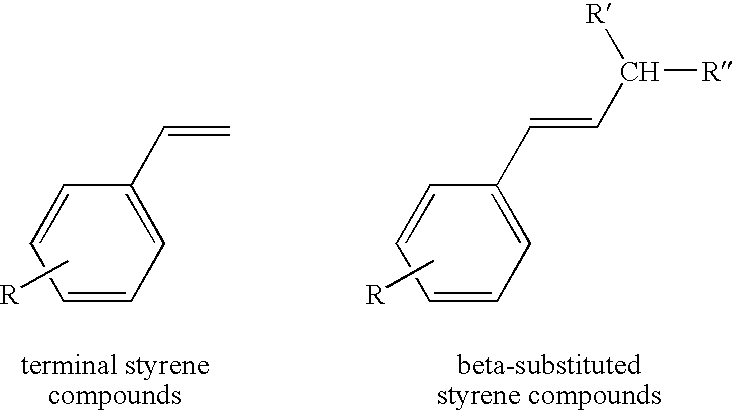
Chelating ligand precursors for the preparation of olefin metathesis catalysts are disclosed. The resulting catalysts are air stable monomeric species capable of promoting various metathesis reactions efficiently, which can be recovered from the reaction mixture and reused. Internal olefin compounds, specifically beta-substituted styrenes, are used as ligand precursors. Compared to terminal olefin compounds such as unsubstituted styrenes, the beta-substituted styrenes are easier and less costly to prepare, and more stable since they are less prone to spontaneous polymerization. Methods of preparing chelating-carbene metathesis catalysts without the use of CuCl are disclosed. This eliminates the need for CuCl by replacing it with organic acids, mineral acids, mild oxidants or even water, resulting in high yields of Hoveyda-type metathesis catalysts. The invention provides an efficient method for preparing chelating-carbene metathesis catalysts by reacting a suitable ruthenium complex in high concentrations of the ligand precursors followed by crystallization from an organic solvent.
Owner:UMICORE AG & CO KG
Preparation method of hydro-treatment catalyst
ActiveCN103769125AModerate decrease in viscosityImprove permeabilityMetal/metal-oxides/metal-hydroxide catalystsRefining to eliminate hetero atomsPtru catalystUltrasonic cavitation
The invention discloses a preparation method of a hydro-treatment catalyst. The preparation method comprises the following steps: preparing an alumina carrier, preparing an impregnation solution containing the VIII group metal and VIB group metal, wherein the impregnation solution comprises a proper amount of an organic auxiliary agent containing chelating ligand, then impregnating the alumina carrier into the impregnation solution under a proper ultrasonic treatment condition, and drying so as to obtain the hydro-treatment catalyst. The preparation method utilizes the ultrasonic cavitation effect to properly reduce the viscosity of the impregnation solution; at the same time the complexing capacity between the chelating ligand in the organic auxiliary agent and nickel / cobalt is improved by the catalytic function of the ultrasonic, the interaction force between the chelating ligand and nickel / cobalt is strengthened, thus the active metal component is promoted to highly disperse on the carrier surface, furthermore, the existing state of the active metal on the catalyst surface is improved at the same time, the sulfurization degree and sulfurization uniformity of the active metal are both improved, so the activity and stability of the catalyst are both improved. The catalyst is especially suitable for being used in the hydro-denitrogenation process and hydro-desulfurization process of heavy distillate oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
Reforming catalyst with chelated promotor
A process for preparing a naphtha reforming catalyst has been developed. The process involves the use of a chelating ligand such as ethylenediaminetetraacetic acid (EDTA). The aqueous solution of the chelating ligand and a tin compound is used to impregnate a support, e.g., alumina extrudates. A platinum-group metal is also an essential component of the catalyst. Rhenium may also be a component. A reforming process using the catalyst has enhanced yield, activity, and stability for conversion of naphtha into valuable gasoline and aromatic products.
Owner:UOP LLC
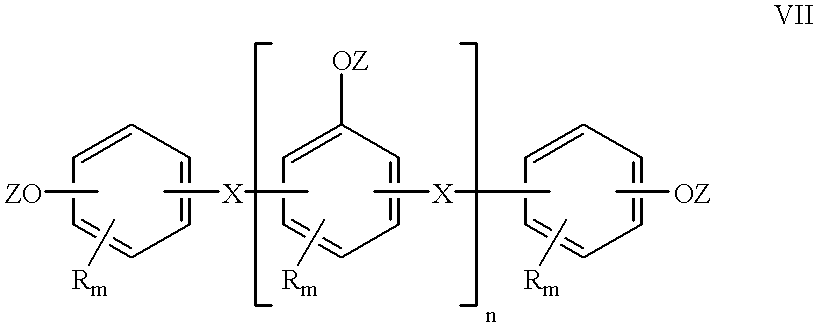
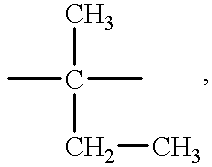
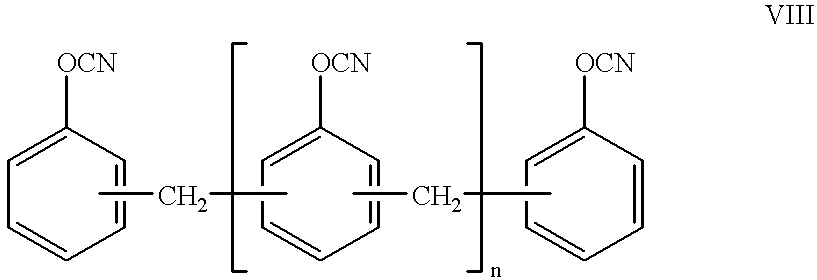
Cyanate resin, polyepoxide and metal complex curing agent
A curable mixture comprising at least one cyanate resin, at least one epoxide compound with more than one epoxy group per molecule, and a metal formula selected from the group consisting of MLxBy, M[SR]xBy, M[SR]x(N)y, M(PHal)m and M(PHal)m(N)n wherein M is a cation of a complexing metal, SR is an organic or inorganic acid residue, L is a chelating ligand, B is a Lewis base, PHal is an ion of a pseudohalide, N is a nitrogen base, x is an integer from 1 to 8, y is an integer from 1 to 5, z is an integer from 7 to 8, m is an integer from 2 to 3 and n is an integer from 1 to 2.
Owner:BAKELITE
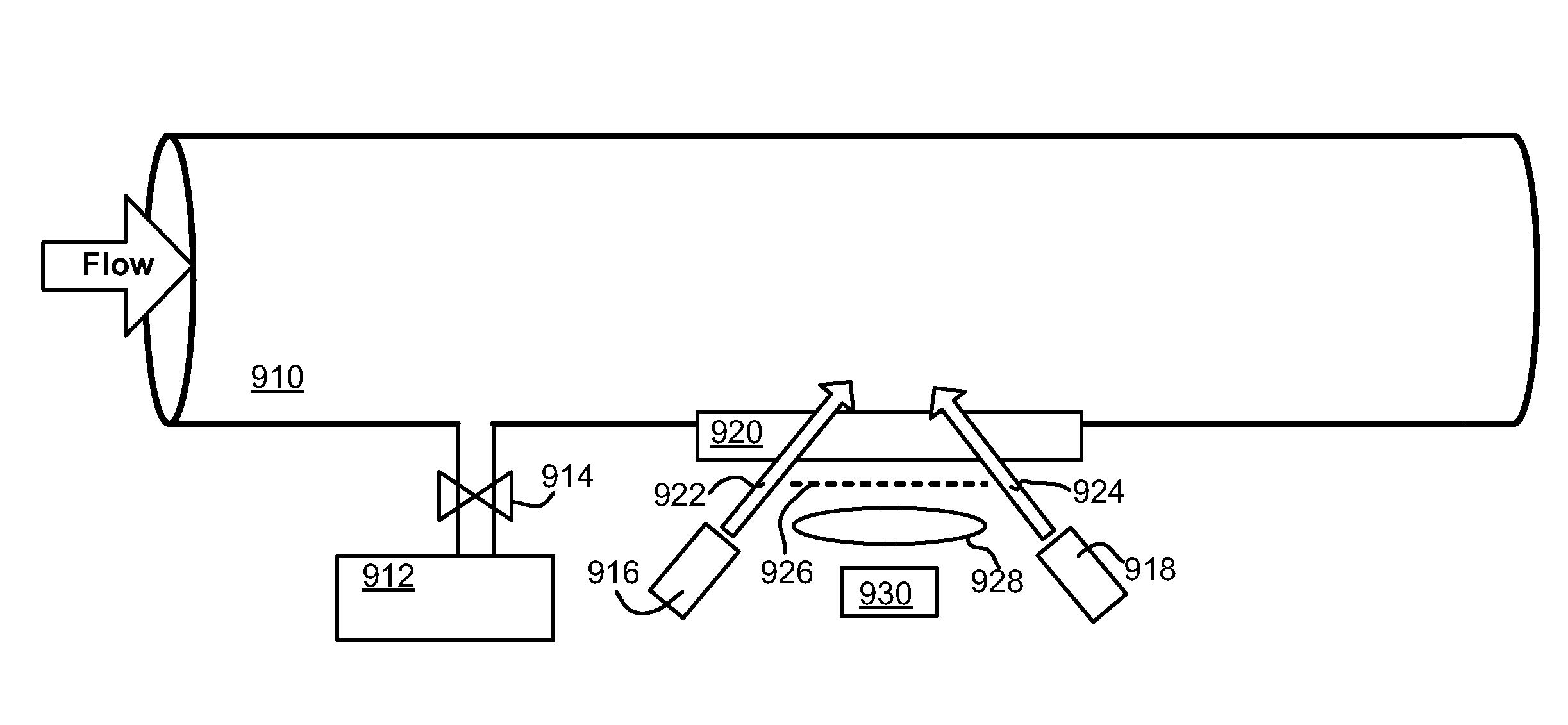
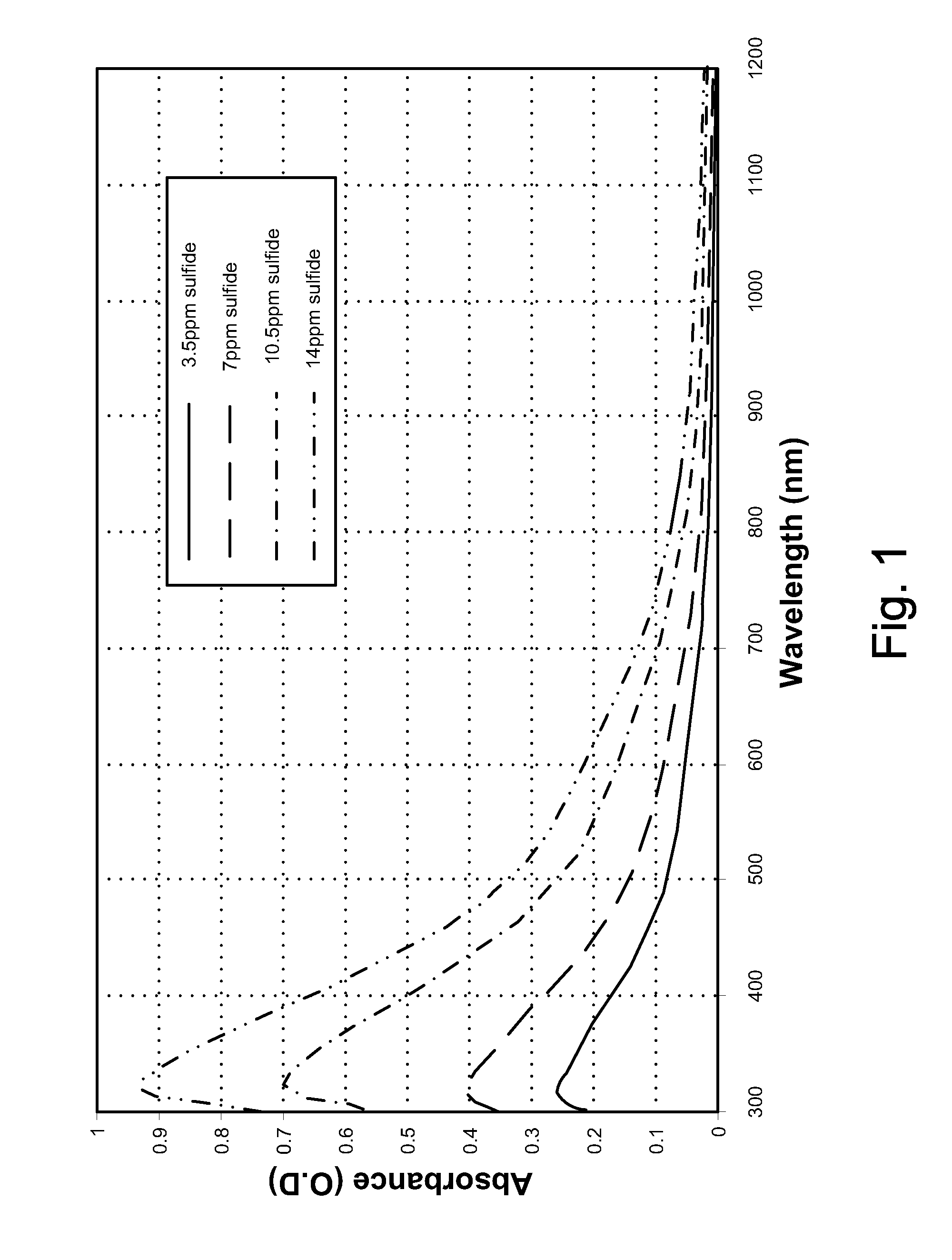
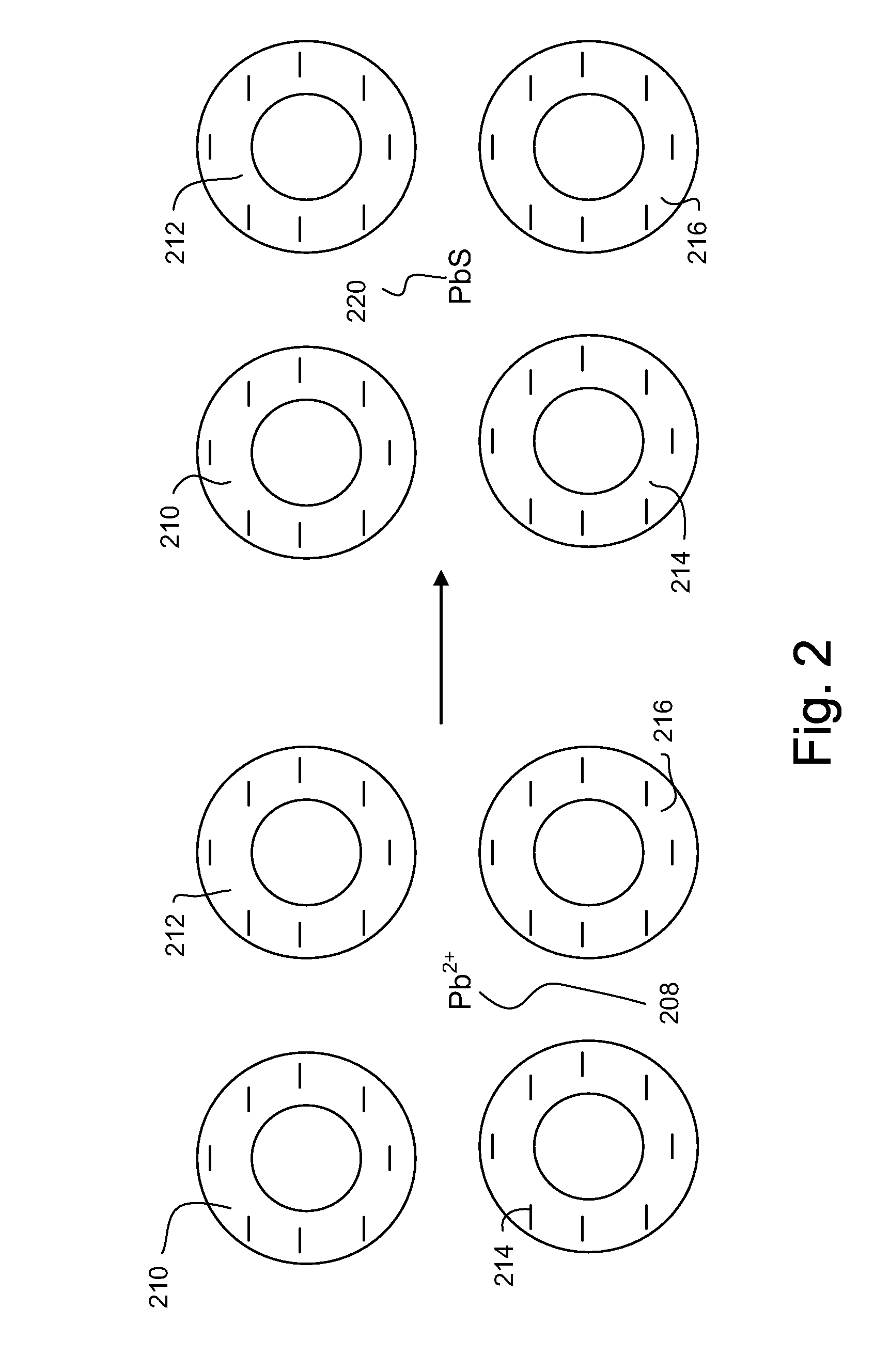
Downhole spectroscopic hydrogen sulfide detection
ActiveUS20090107667A1SurveyMaterial analysis by observing effect on chemical indicatorHeat resistanceNanoparticle
Methods and related apparatuses and mixtures are described for detecting hydrogen sulfide in a formation fluid downhole. A detection mixture is combined with the formation fluid downhole. The detection mixture includes metal ions for reacting with hydrogen sulfide forming a metal sulfide, and charged nanoparticles sized so as to inhibit significant aggregation of the metal sulfide so as to enable spectroscopic detection of the metal sulfide downhole. The combined mixture and formation fluid is then spectroscopically interrogated so as to detect the presence of the metal sulfide thereby indicating the presence of hydrogen sulfide in the formation fluid. The mixture also includes chelating ligands for sustaining thermal endurance of the mixture under downhole conditions.
Owner:SCHLUMBERGER TECH CORP
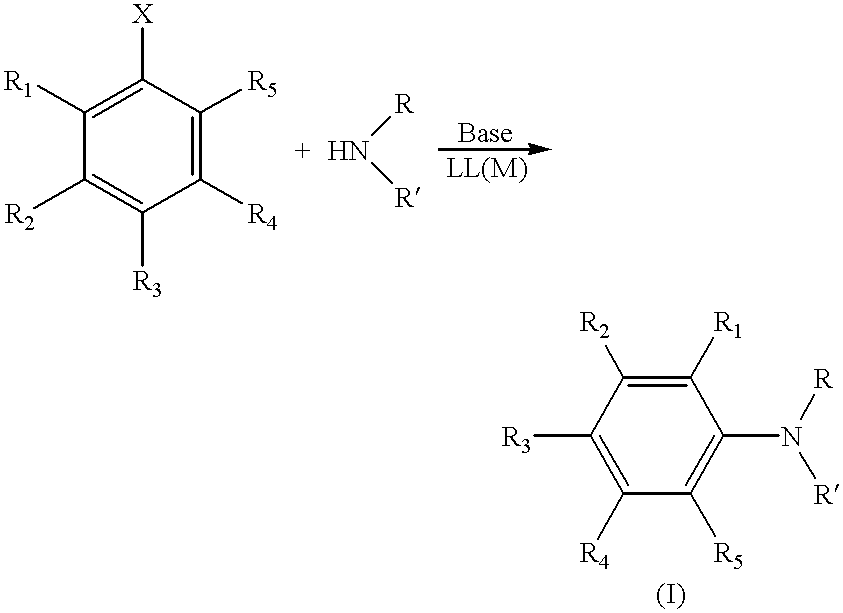
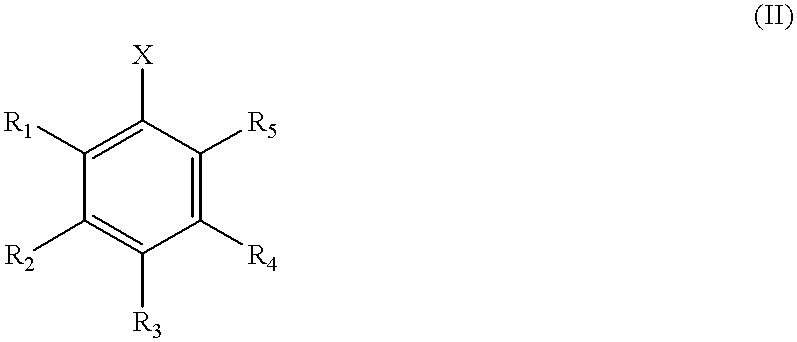
Transition metal-catalyzed process for preparing N-aryl amine compounds
InactiveUS6235938B1Carboxylic acid nitrile preparationOrganic compound preparationChelating ligandsPolymer
The present invention is directed to a process for the preparation of N-aryl amine compounds. The process of the present invention involves reacting a compound having an amino group with an arylating compound in the presence of a base and a transition metal catalyst under reaction conditions effective to form an N-aryl amine compound, the transition metal catalyst comprising a Group 8 metal and at least one chelating ligand selected from the group consisting of bisphosphines having at least one stearically hindered alkyl substituent. The formed products are valuable intermediates in the pharmaceutical and polymer fields.
Owner:MITSUBISHI RAYON CO LTD +1
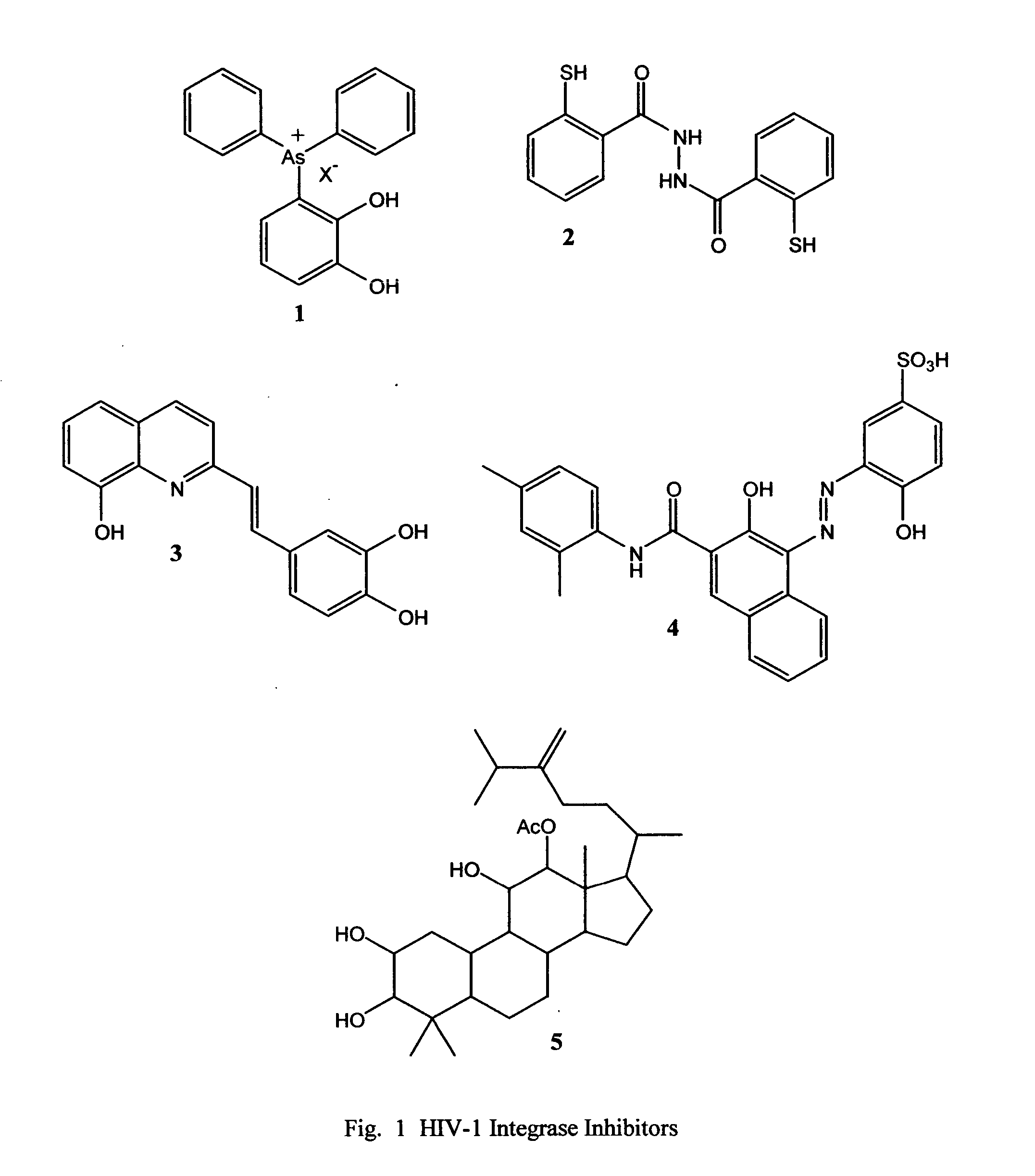
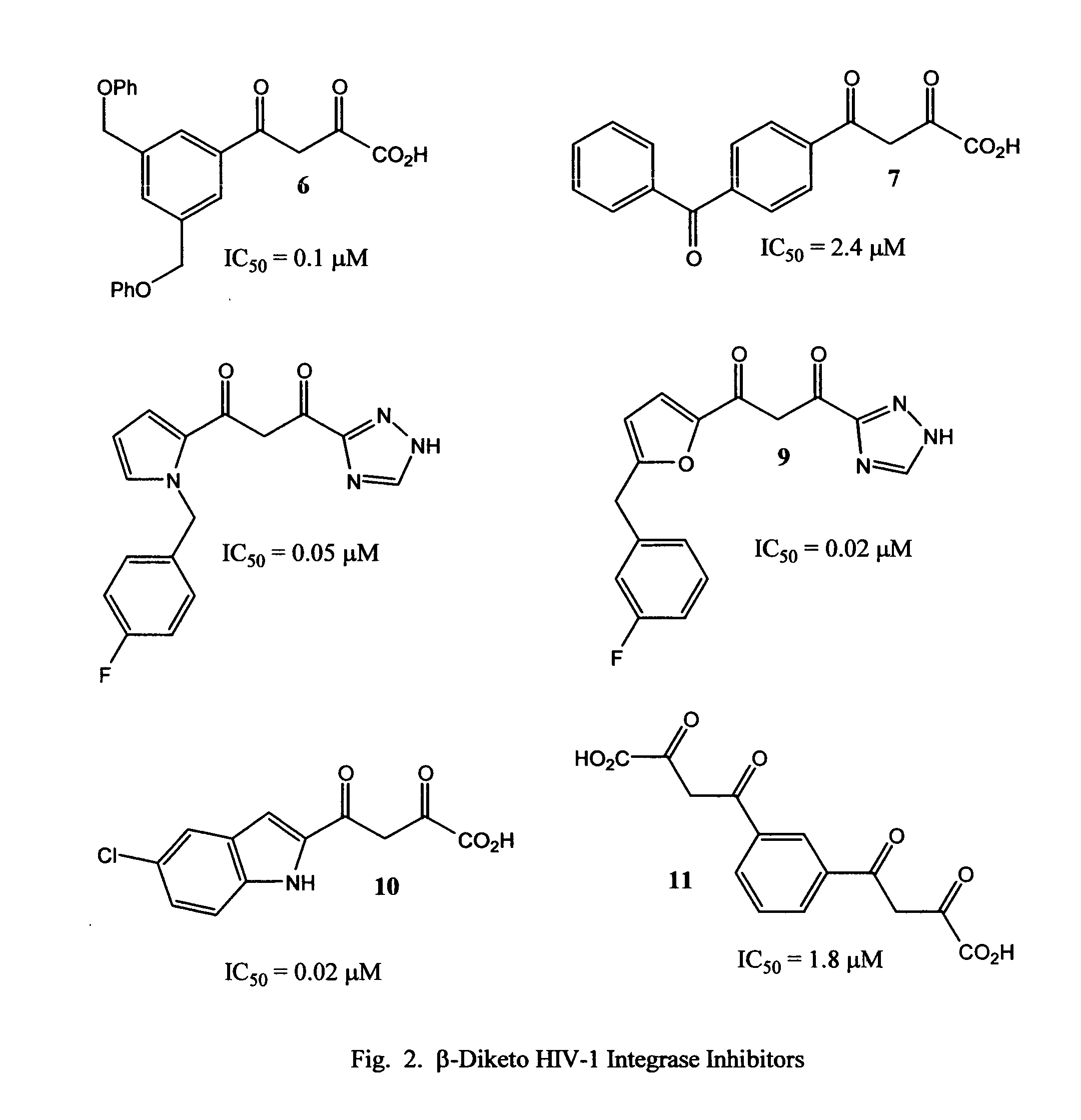
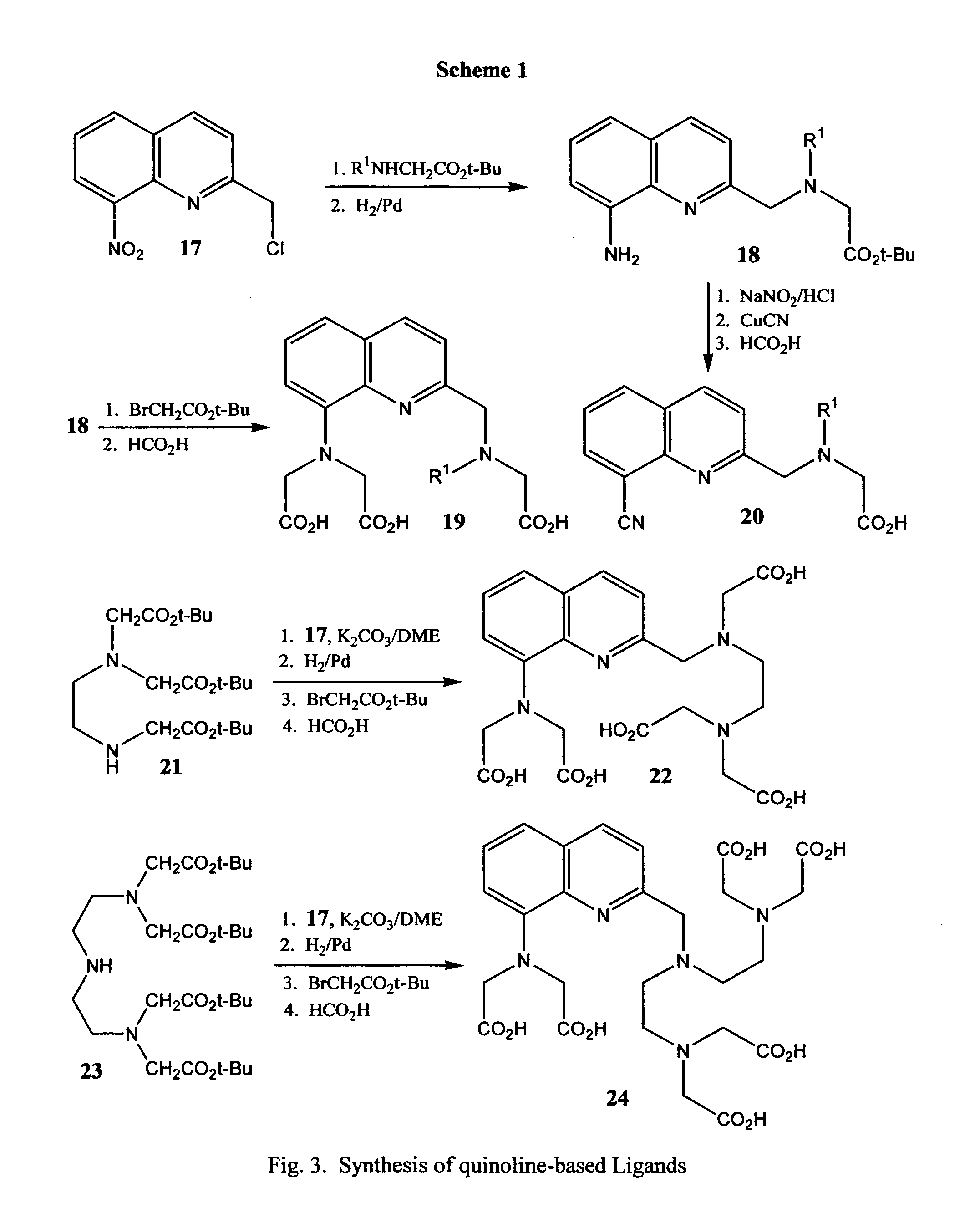
Novel quinoline-based metal chelators as antiviral agents
This invention relates to novel quinoline-based divalent metal ion chelating ligands of Formula I, wherein A or B are independently —CR7R8, or —CH(R9)CH(R10). X is hydrogen, C1-C10 alkyl; —OH, or —NR11R12. R1 to R12 are various substituents selected to optimize the physicochemical and biological properties such as enzyme binding, tissue penetration, lipophilicity, toxicity, bioavailability, and pharmacokinetics of compounds of Formula 13. R1 to R12 may include, but are not limited to hydrogen, alkyl, acyl, hydroxyl, hydroxyalkyl, substituted or unsubstituted aryl, amino, aminoalkyl, alkoxyl, aryloxyl, carboxyl, halogen, alkoxycarbonyl, cyano, and other suitable electron donating or electron withdrawing groups. The compounds of the present invention are useful for inhibiting the activity of viral enzymes responsible for the proliferation of human immunodeficiency virus (HIV).
Owner:BIOFLEXIS
Preservation of wood products
InactiveUS6753016B2Maintain stabilityMaintain surfaceBiocideHeavy metal active ingredientsInorganic saltsIron salts
A method for the protection of wood and other wood materials without affecting dimensional stability or surface integrity of the treated material is described. The method involves treating wood material with an iron salt and selected oxidants where the iron salt is preferably complexed with organic chelating ligands. Preferably, a microbicidal agent is also incorporated into the method to provide treated wood products that demonstrate excellent surface integrity, dimensional stability and retention of the infused microbicidal agents for extended periods of time without incurring the detrimental environmental effects of conventional chromium or copper-based inorganic salt preservation methods.
Owner:ROHM & HAAS CO
Phosphorescent Osmium (II) complexes and uses thereof
InactiveUS20070001166A1Discharge tube luminescnet screensElectroluminescent light sourcesPhenanthrolineCarbene
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds. Compounds of interest include: wherein A is Os or Ru The anionic chelating chromophores NˆN, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage. L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene. L can occupy either cis or trans orientation. When L occupies the trans position, the preferred structure contains both the hexagonal fragment of NˆN as well as its pentagonal fragment located at the trans position respect to their counterparts of the second NˆN chromophore. When L occupies the cis position, the preferred structure consists of the pentagonal unit of NˆN chromophores residing opposite to the L. X,1 X2 and X3 independently are C or N; when X2 is N, R1 is omitted, when X3 is N, R2 is omitted, R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl, R2 is H, F or cyano substituent, X4 is either C or N; X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4, R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:TAO YE +3
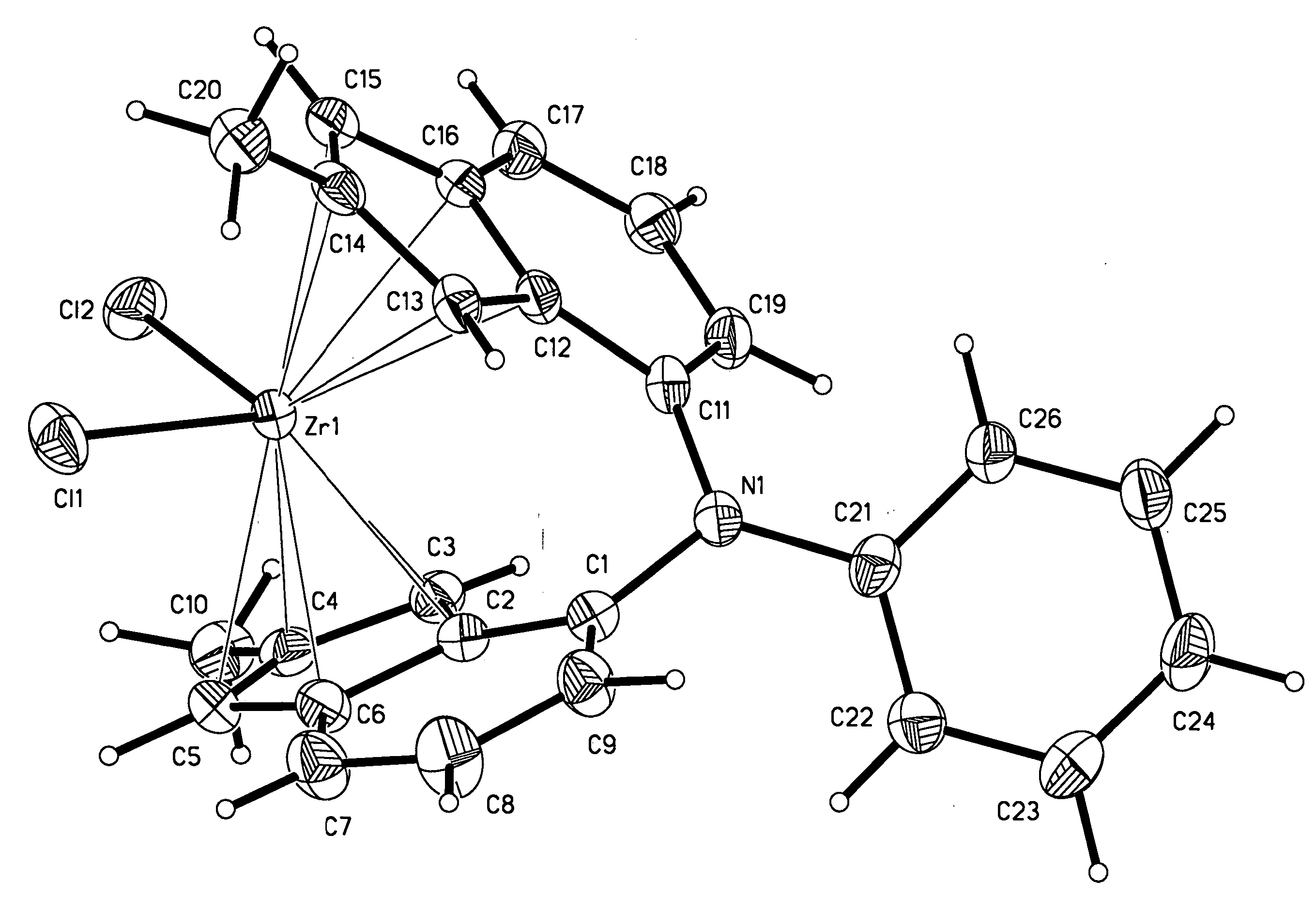
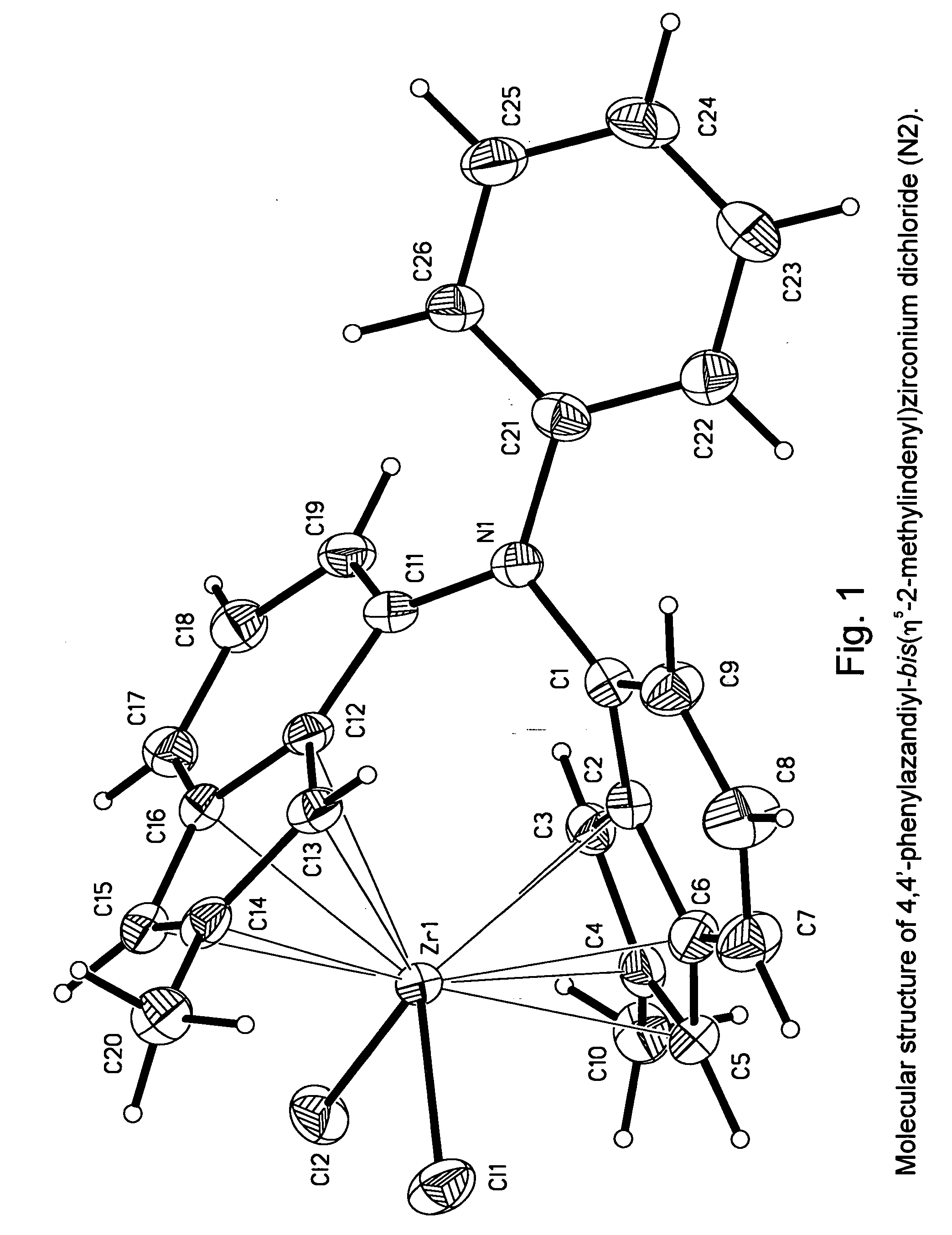
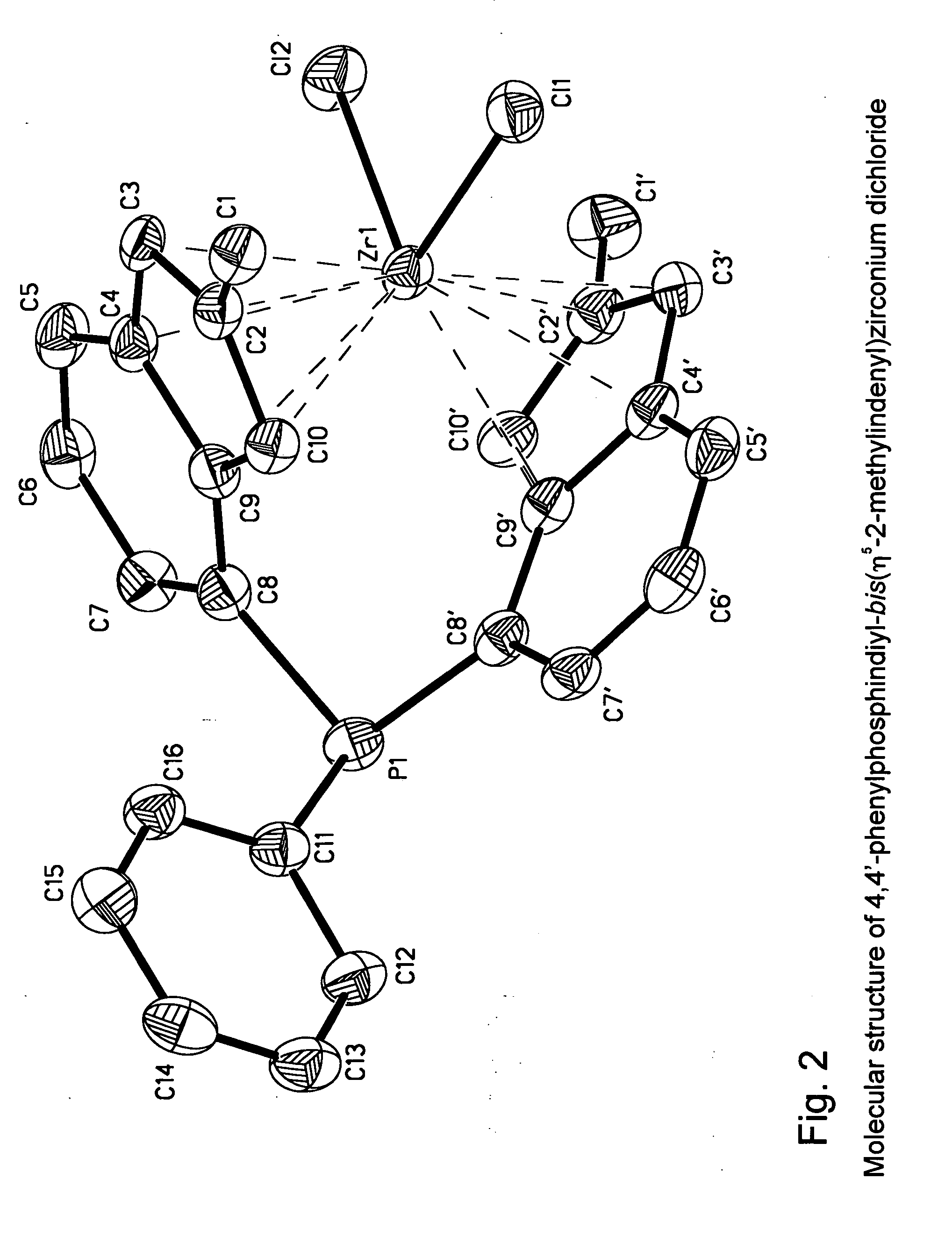
Heteroatom bridged metallocene compounds for olefin polymerization
InactiveUS20070135597A1Organic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsPolymer sciencePolyolefin
This invention relates to a transition metal compound represented by the formula: wherein M is a group 3, 4, 5 or 6 transition metal atom, or a lanthanide metal atom, or actinide metal atom; E is: 1) a substituted or unsubstituted indenyl ligand that is bonded to Y through the four, five, six or seven position of the indenyl ring, or 2) a substituted or unsubstituted heteroindenyl ligand that is bonded to Y through the four, five or six position of the heteroindenyl ring, provided that the bonding position is not the same as the position of the ring heteroatom, or 3) a substituted or unsubstituted fluorenyl ligand that is bonded to Y through the one, two, three, four, five, six, seven or eight position of the fluorenyl ring, or 4) a substituted or unsubstituted heterofluorenyl ligand that is bonded to Y through the one, two, three, four, five or six position of the heteroindenyl ring, provided that the bonding position is not the same as the position of the ring heteroatom; A is a substituted or unsubstituted cyclopentadienyl ligand, a substituted or unsubstituted heterocyclopentadienyl ligand, a substituted or unsubstituted indenyl ligand, a substituted or unsubstituted heteroindenyl ligand, a substituted or unsubstituted fluorenyl ligand, a substituted or unsubstituted heterofluorenyl ligand, or other mono-anionic ligand; Y is a Group 15 or 16 bridging heteroatom substituent that is bonded via the heteroatom to E and A; and X are, independently, univalent anionic ligands, or both X are joined and bound to the metal atom to form a metallocycle ring, or both X join to form a chelating ligand, a diene ligand, or an alkylidene ligand. This invention further relates to catalyst systems comprising the above transiotioon metal compounds, activators and optional supports and their use to polymerize or oligomerize olefins.
Owner:EXXONMOBIL CHEM PAT INC
Volatile noble metal organometallic complexes
InactiveUS7112690B2Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIodideChelating ligands
Owner:NATIONAL TSING HUA UNIVERSITY +1
Binuclear Metal Complex, Metal Complex Dye, Photoelectric Conversion Element, and Photochemical Battery
InactiveUS20080015356A1High absorbance indexImproved short-circuit current densityRuthenium organic compoundsElectrolytic capacitorsHeteroatomPhotoelectric conversion
A novel binuclear metal complex according to the present invention is an asymmetric binuclear metal complex represented by the general formula: (L1)2M1(BL)M2(L2)2(X)n, wherein M1 and M2, which may be identical or different, represent a transition metal; L1 and L2, which are different, represent a chelate ligand capable of polydentate coordination and two L1s may be different and two L2s may be different; BL represents a bridge ligand having at least two heteroatom-containing cyclic structures, the heteroatoms contained in the cyclic structures being ligand atoms coordinating to M1 and M2; X represents a counter ion; and n is the number of counter ions needed to neutralize the charge of the complex. And the binuclear metal complex is useful as a metal complex dye.
Owner:UBE IND LTD
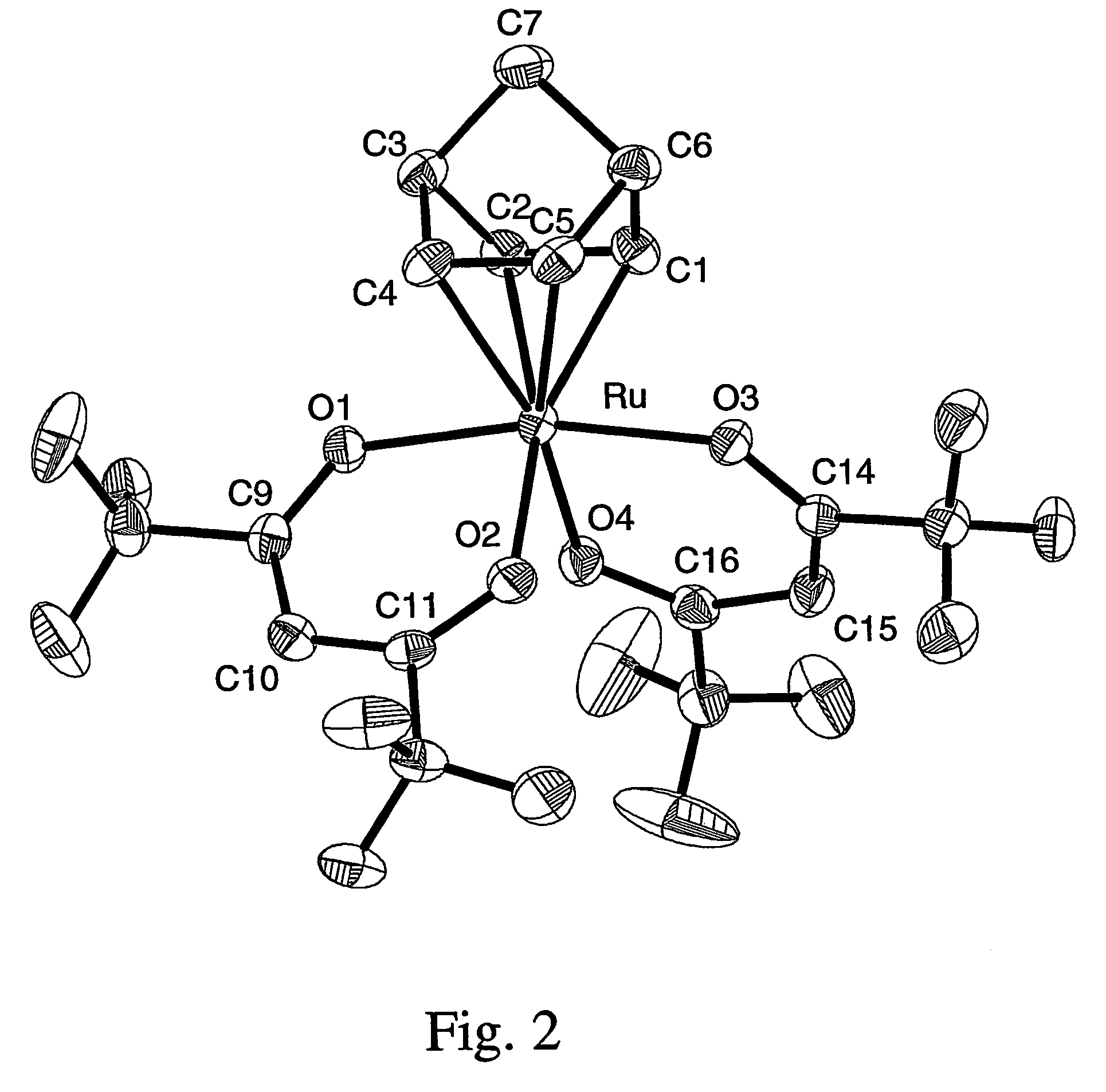
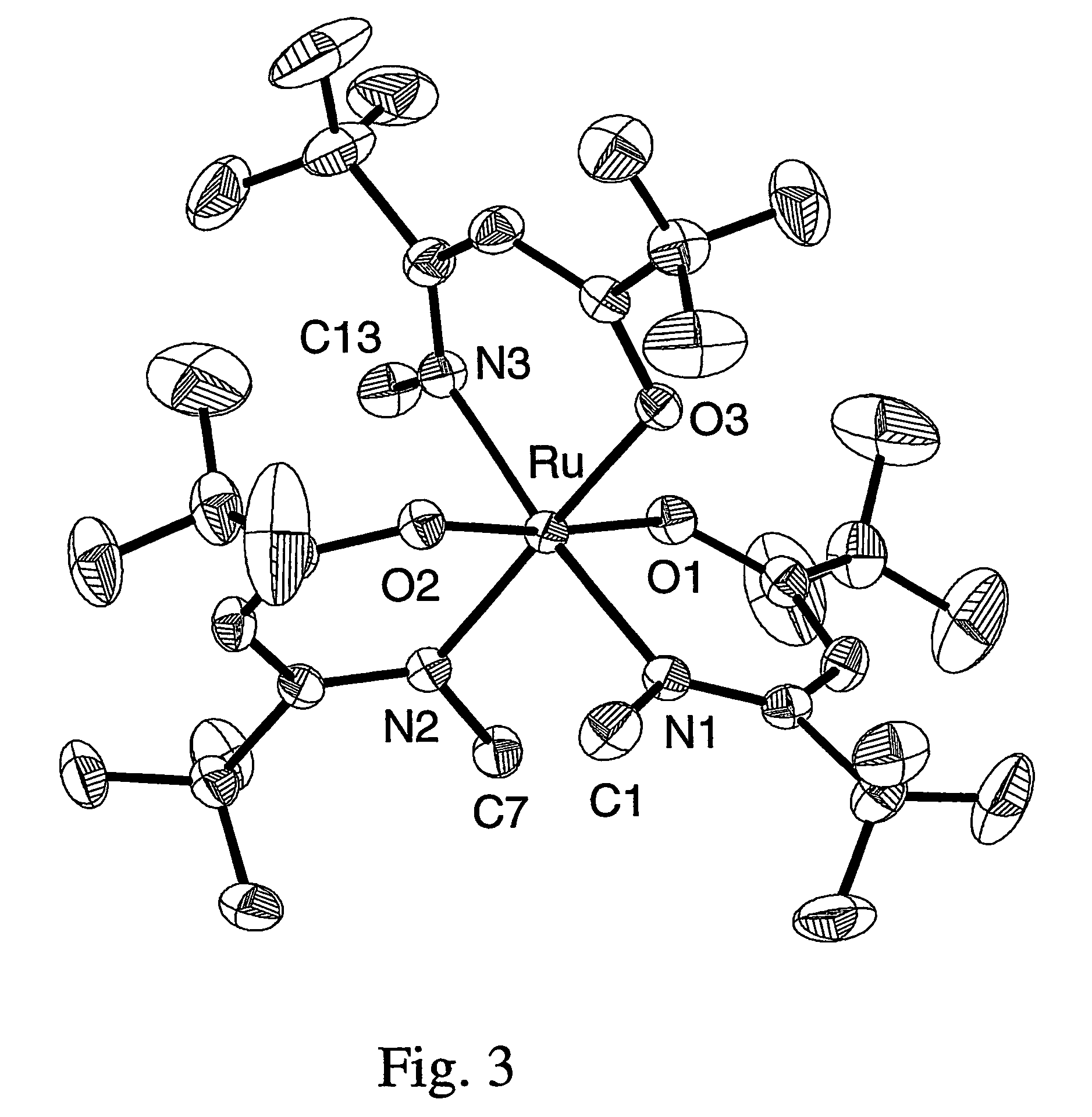
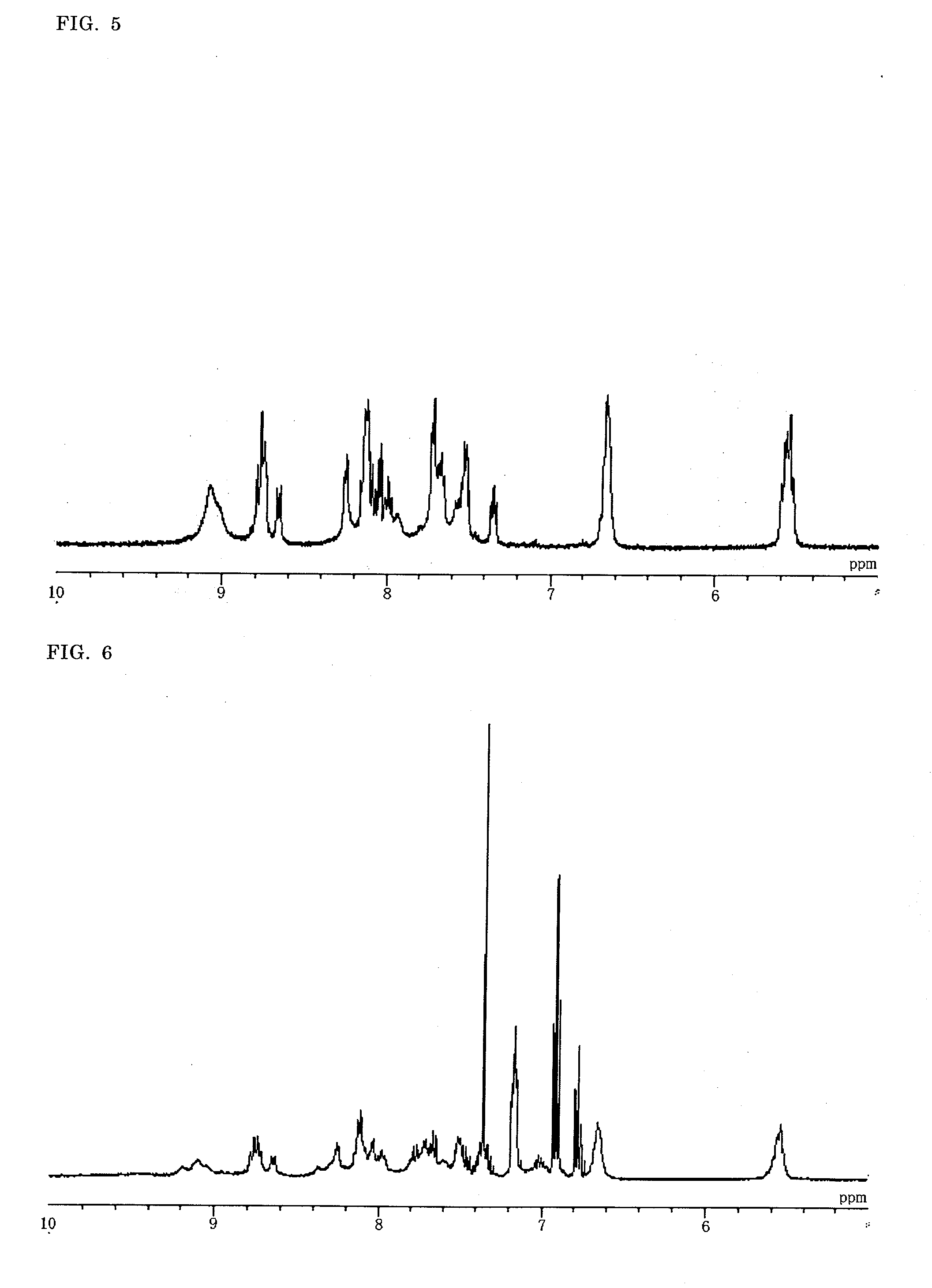
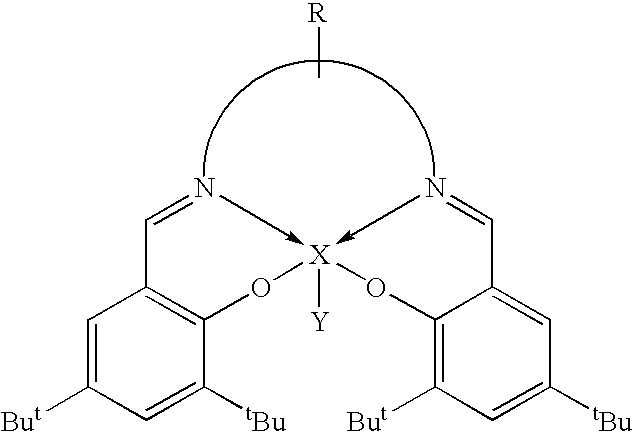
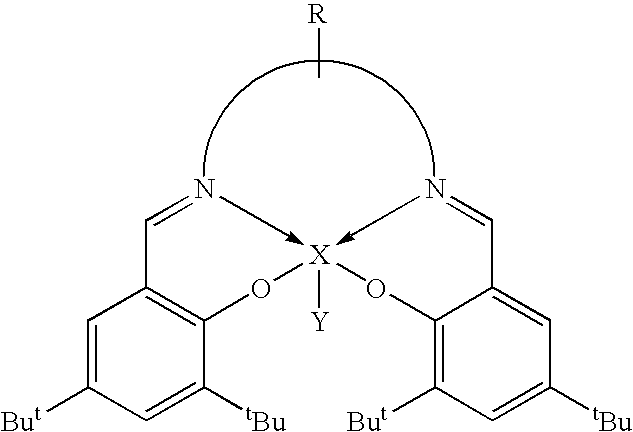
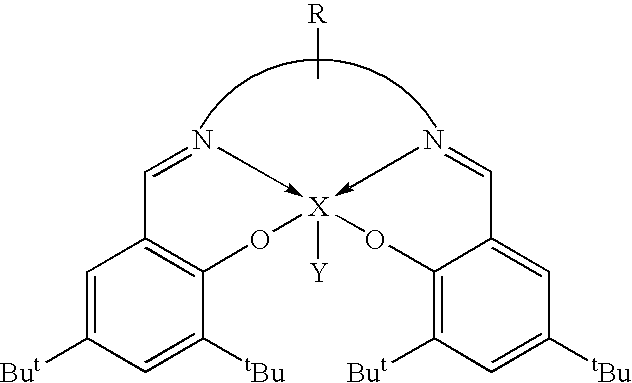
Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof
InactiveCN103951683AGroup 5/15 element organic compoundsCopper organic compoundsPhotoluminescenceDiphosphines
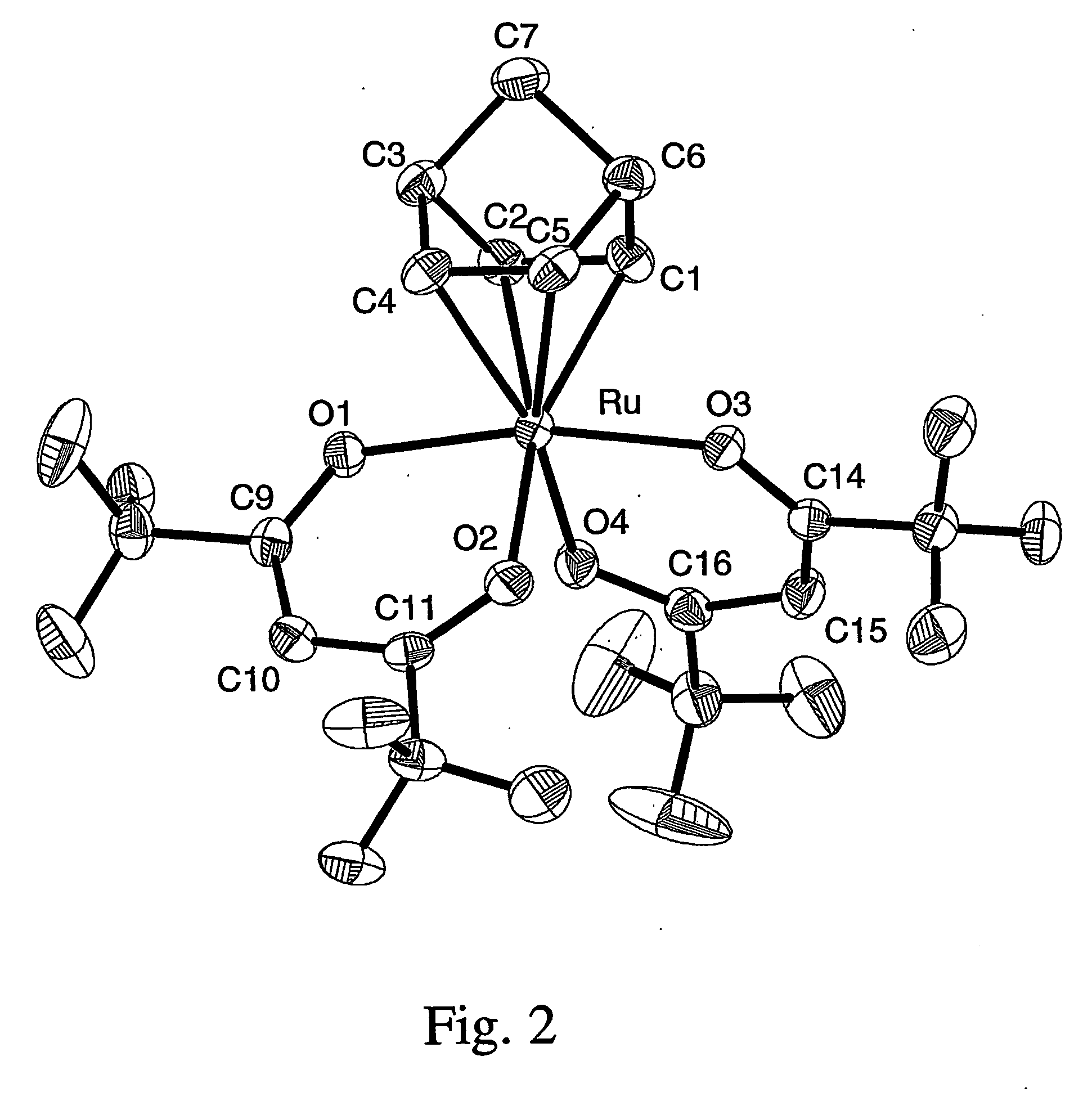
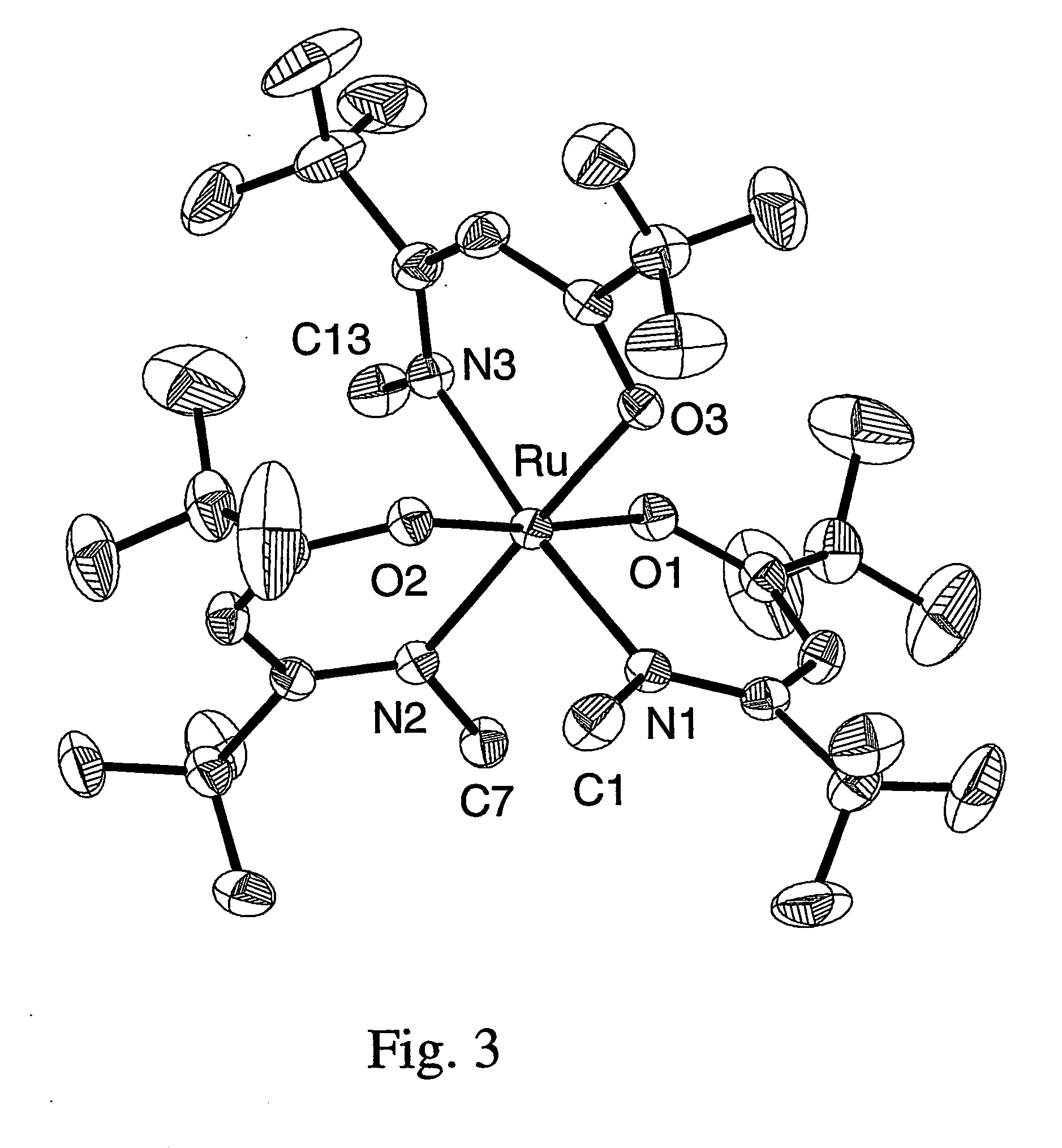
The invention relates to luminescent novel material synthesis technologies and particularly relates to blue-green materials with novel tri / tetra-coordination dual-core copper [I] coordination compounds and a preparation method thereof. According to the preparation method, the novel dual-core copper [I] coordination compound blue-green materials, namely [Cu2[bmptzH][[mu]-dppm]2][ClO4]2 and [Cu2[fptz][[mu]-dppm]2][ClO4], which simultaneously have two coordination bonding manners, namely tri-coordination and tetra-coordination, and have special structures, are synthesized from functionalized pyridine triazole bidentate chelating ligands (5-tert-butyl-3-[6-methyl-pyridine-2-yl]-1,2,4-triazole and 5-trifluoromethyl-3-[pyridine-2-yl]-1,2,4-triazole) and an organic diphosphine auxiliary ligand (bis[diphenylphosphino]methane). The blue-green materials show very good photoluminescence properties in a solid state, and the photoinduced quantum efficiency is 66% and 77% respectively.
Owner:JIANGXI UNIV OF SCI & TECH
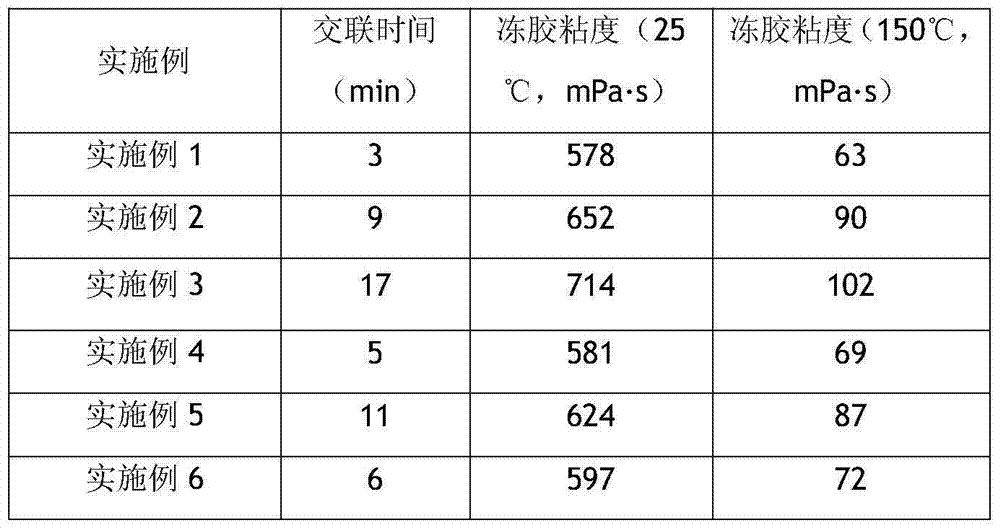
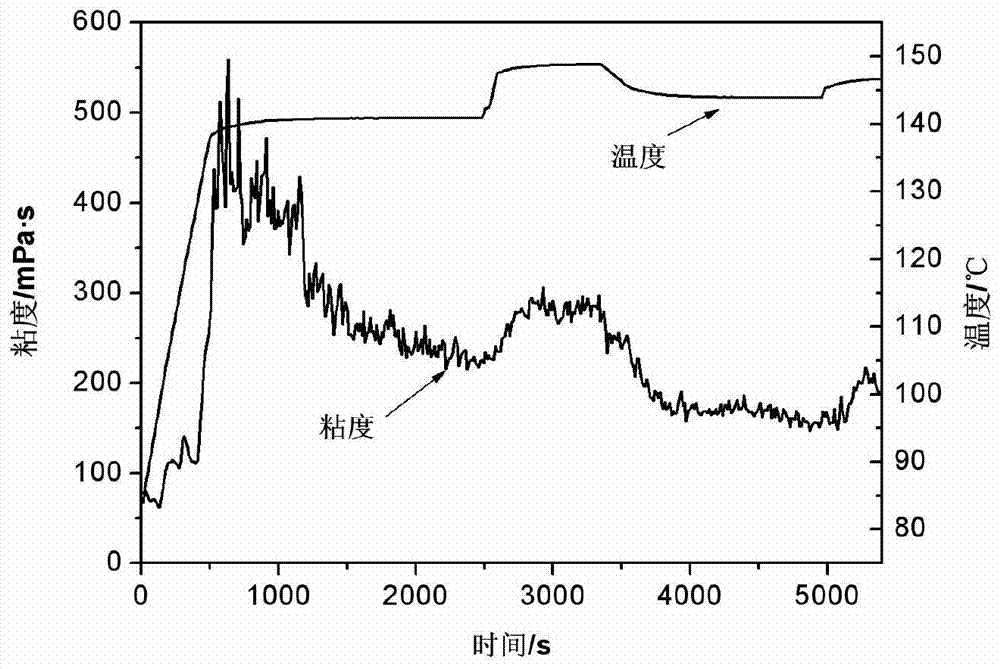
High-temperature hyper-salinity water-base fracturing fluid
ActiveCN103497754AShorten crosslinking timeDisperse completelyDrilling compositionWater basedCross-link
The invention discloses high-temperature hyper-salinity water-base fracturing fluid consisting of thickening liquid, a cross-linking agent and a gel breaker, wherein the thickening liquid comprises the following components in percentage by weight: 0.4-0.7% of a thickening agent, 0.1-0.8% of a cleanup additive, 0.01-0.1% of a sterilizing agent, 0.1-0.3% of a temperature stabilizing agent, and the balance of hyper-salinity seawater or stratum produced water; the thickening agent is sulfonyl hydroxypropyl guar gum, sulfonyl betaine amphoteric guar gum or sulfonyl carboxymethyl guar gum; the cross-linking agent is a stable complex formed from reaction of 9-18% of a zirconium compound, 3-40% of a boron compound, 9-18% of a chelating ligand and 5-19% of a bridging ligand by mass; the gel breaker is either ammonium persulfate or sodium persulfate. The fracturing fluid is directly prepared from hyper-salinity water and realizes effective utilization of seawater and hyper-salinity produced water from land, and is applicable to seaborne and terrestrial high-temperature oil-gas wells.
Owner:SOUTHWEST PETROLEUM UNIV
Cleavage of phosphate ester bonds by use of novel group 13 chelate compounds
InactiveUS7105703B1Organic-compounds/hydrides/coordination-complexes catalystsCobalt organic compoundsPhosphateOxygen donor
A novel chemical compound has a general formula (LX)nY wherein X is selected from a group consisting of a group 13 element other than boron, Y is selected from a group consisting of a halide, a chlorate, a sulfate and a nitrate and L is a chelating ligand containing two nitrogen and two oxygen donor groups where n=1 or 2.
Owner:UNIV OF KENTUCKY RES FOUND
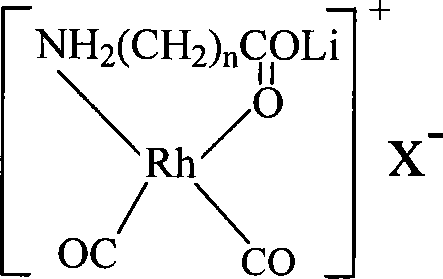
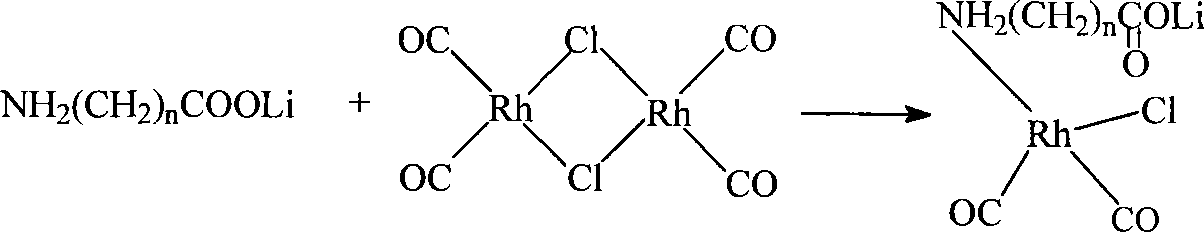
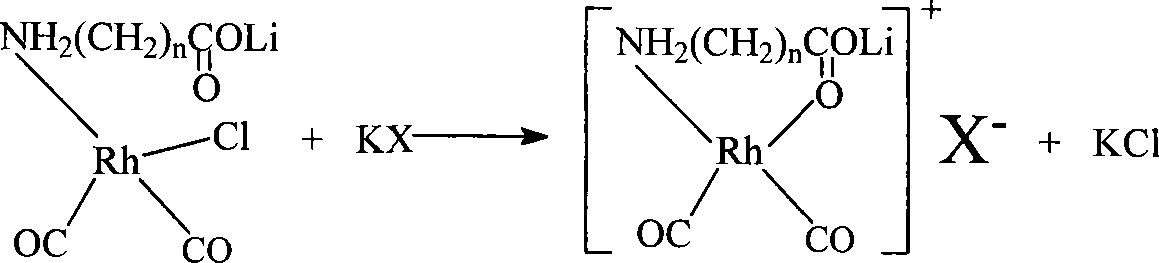
Method for producing acetic acid by carbonylation of methanol as well as special catalyst and preparation method thereof
ActiveCN101182340AHigh selectivityImprove adaptabilityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 8/9/10/18 element organic compoundsPhosphateCarboxylic acid
The invention discloses a method for producing acetic acid by carbonylation of methanol, a special catalyst and a preparation method thereof. The aminocarboxylate lithium rhodium complex of the present invention has a structure as shown in formula I, wherein, X=BPh4 or I; n=1, 2 or 3. In the present invention, lithium aminocarboxylate is used as a ligand and a rhodium complex to form a positive ion active center structure of a strong and weak coordination bond chelation type, and the positive ion part contains a strongly coordinated N→Rh bond and a weakly coordinated O→Rh bond, The stability and activity of the active center are guaranteed. Lithium metal and rhodium metal are co-located in the structure of the active center, which can form a synergistic catalytic effect; moreover, it can also be used in combination with other catalysts to improve its catalytic activity; The strong catalytic effect of the reaction provides a basis for the excellent performance of the catalyst; at the same time, the combination of lithium iodide, lithium acetate and phosphate is used as a promoter, so that the catalyst of the present invention can catalyze the carbonylation of methanol to produce acetic acid. Excellent overall performance.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Pyridine pyrazole dinuclear copper [1] complex luminescent material and preparation method
InactiveCN104877673AEffective control of luminescenceGroup 5/15 element organic compoundsCopper organic compoundsQuantum efficiencyCopper
The invention relates to a dinuclear copper [1] complex luminescent material containing pyridine pyrazole chelating ligand and a preparation method thereof. By the application of the functional pyridine pyrazole two-tooth chelating ligand (3-[2-pyridyl] pyrazole [pypzH for short] or 5-tertiary butyl-3-[2-pyridyl] pyrazole [pybpzH for short] or 5-trifluoromethyl-3-[2-pyridyl] pyrazole [pyfpzH for short] and organic double phosphine auxiliary ligand (1,4-double[diphenylphosphine] butane [dppb for short]), a kind of pyridine pyrazole dinuclear copper [1] complex luminescent material [Cu2[pypzH]2[u-dppb]2][ClO4]2,[Cu2 [pybpzH]2[u-dppb]2][ClO4]2 and [Cu2[pyfpzH]2[u-dppb]2][ClO4]2 is prepared. The solid state luminescence property of the luminescent material can be regulated and controlled through the change of the substituent group on the pyridine and pyrazole ring, the maximum values of the solid state luminescence wave length of the luminescent material are 500 nm, 505 nm and 494 nm, the solid state luminescence life of the luminescent material is 48 us, 19 us and 36 us, and the solid state luminescence quantum efficiency of the luminescent material is 74%, 6% and 85%.
Owner:JIANGXI UNIV OF SCI & TECH
Method for preparing polyvinylidene fluoride/aluminum oxide hybridization film
ActiveCN101698141AImprove hydrophilicityHigh strengthSemi-permeable membranesHYDROSOLAluminium isopropoxide
The invention discloses a method for preparing polyvinylidene fluoride / aluminum oxide hybridization film, comprising taking polyvinylidene fluoride to be dissolved into solvent; after fully dissolving the polyvinylidene fluoride, adding aluminium isopropoxide and coupling agent into the solution in a stirring state; adding water for hydrolyzation, adding acid for catalysis, and then adding pore-forming agent; stirring for at least 20h to obtain even casting film liquid, and standing still at the room temperature; scraping the casting film liquid into a panel film at the room temperature, placing the panel film in the air, soaking in coagulating bath, and forming a hyperfiltration film; and soaking the hyperfiltration film in the water, and taking out to be dried. The method uses strong chelating ligand and high activity aggregation group to respectively carry out chemical modification on PVDF organic macromolecule and Al2O3 inorganic precursor, so that the hybridization film prepared by a sol-gel method maintains the excellent chemical stability and mechanical performance of a PVD film; furthermore, the hydrophilicity and the film contamination resistance of the hybridization film are added, so that the separation property and the stability of the film can be remarkably improved.
Owner:SOUTH CHINA UNIV OF TECH +2
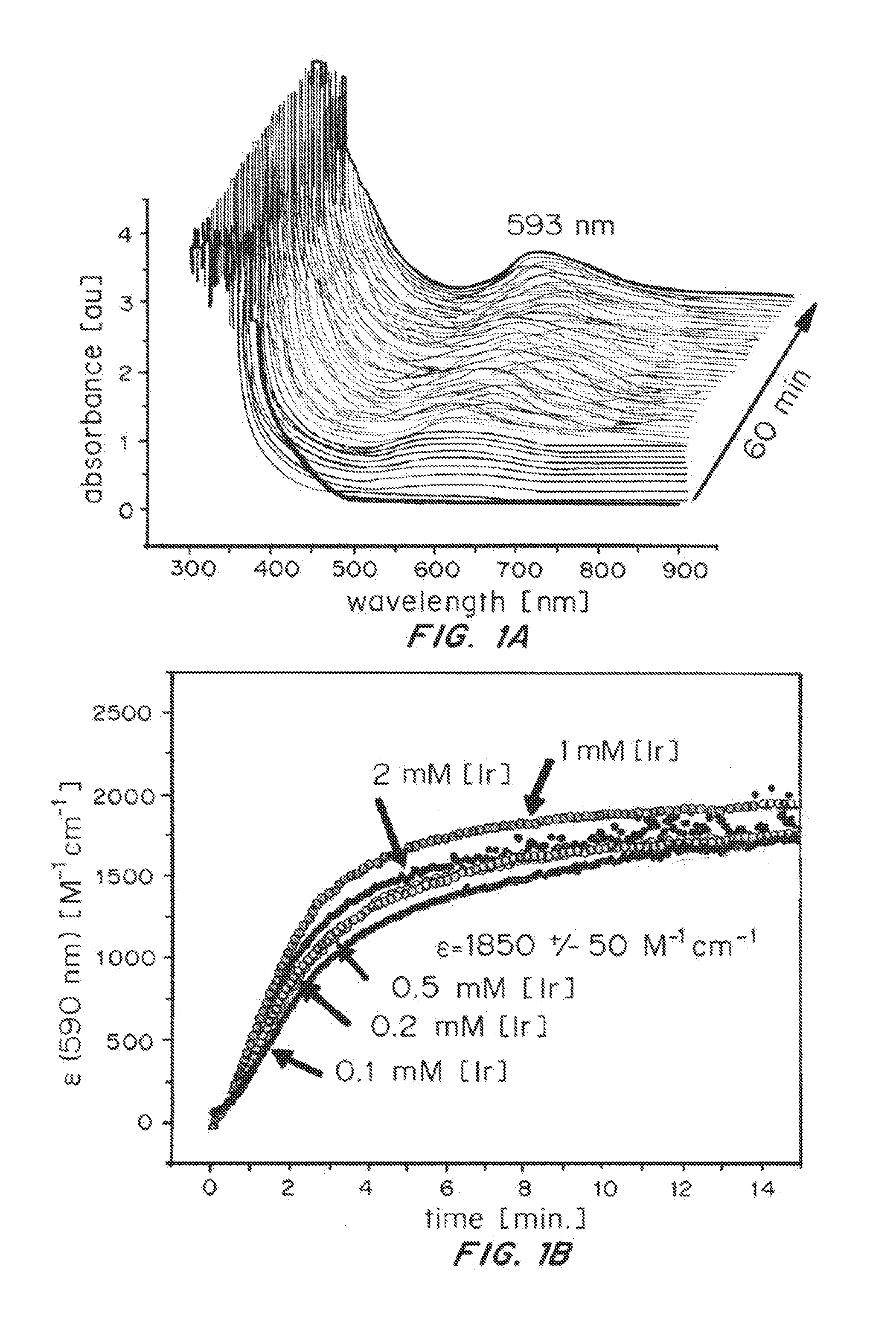
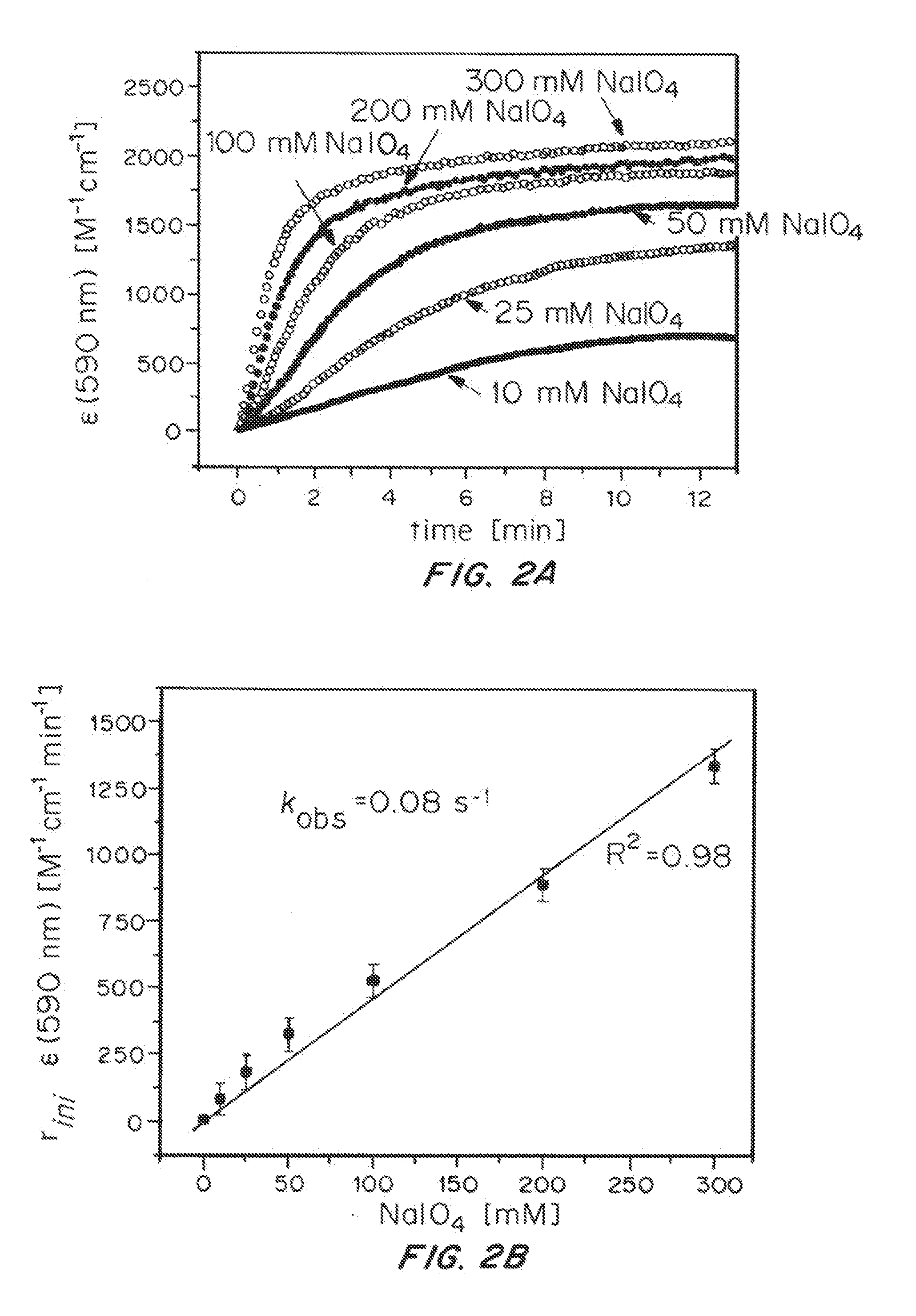
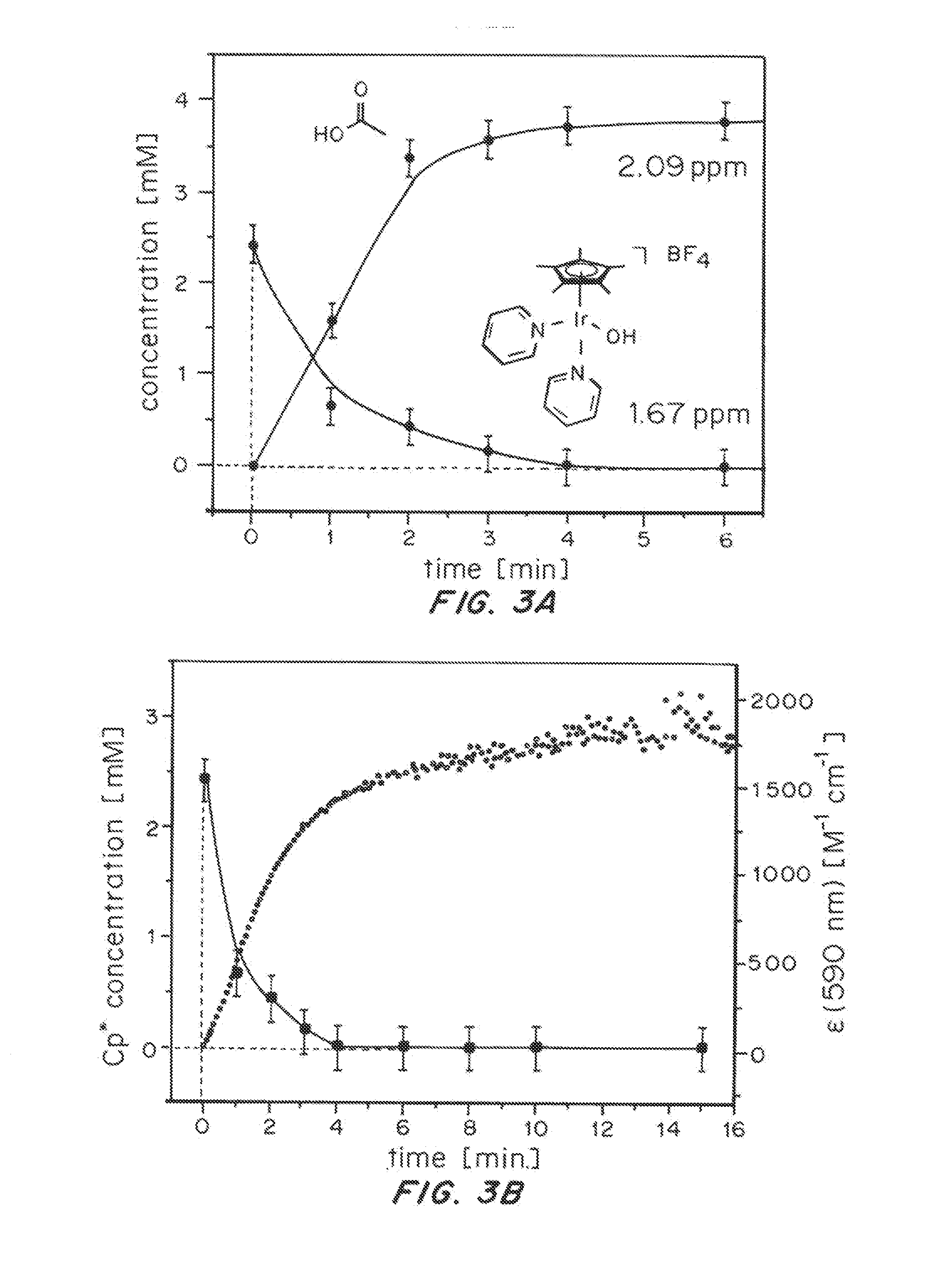
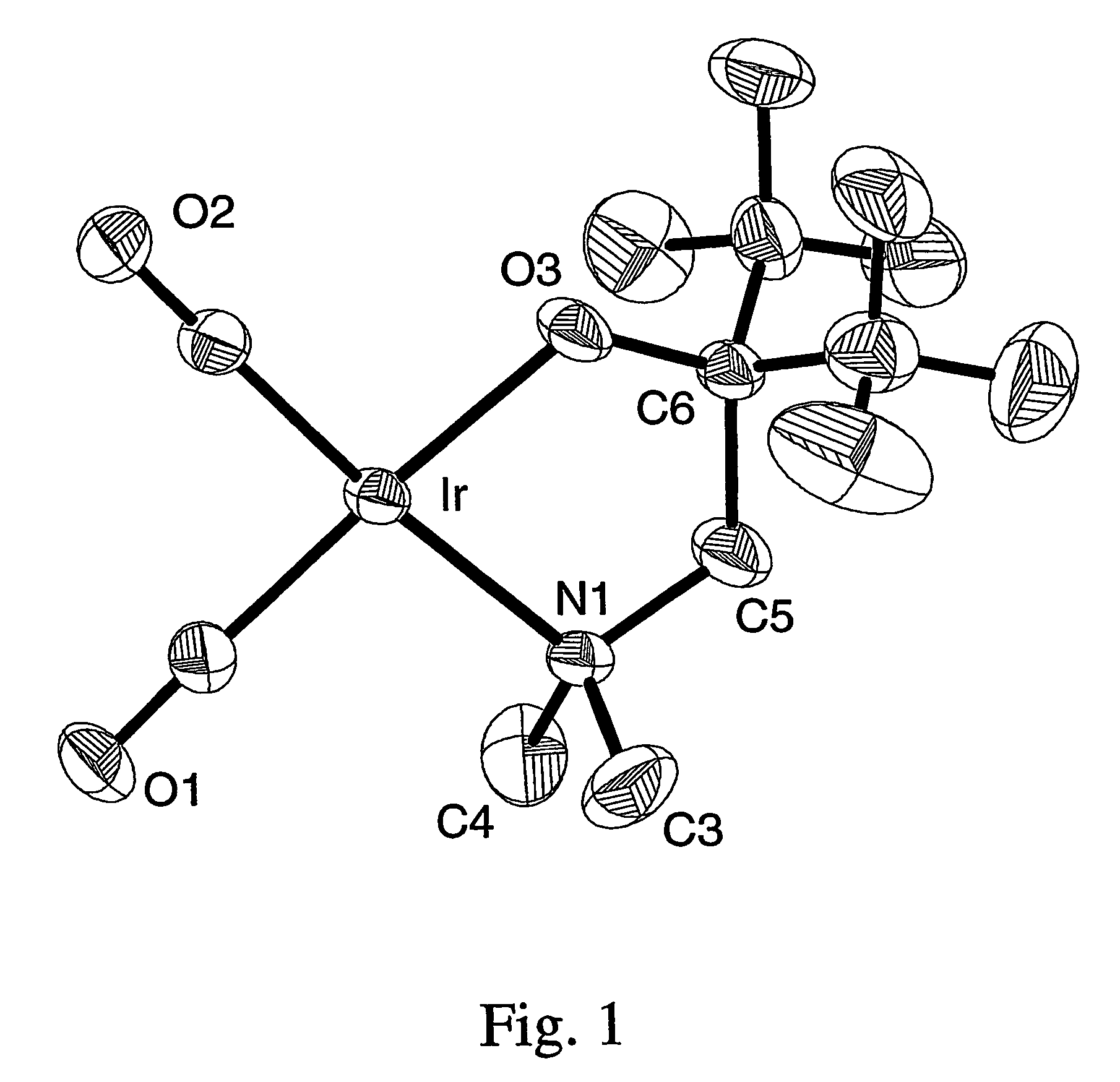
Iridium complexes for electrocatalysis
ActiveUS20150021194A1Improve stabilityConvenient bindingPlatinum group organic compoundsFrom normal temperature solutionsIridiumPentamethylcyclopentadiene
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
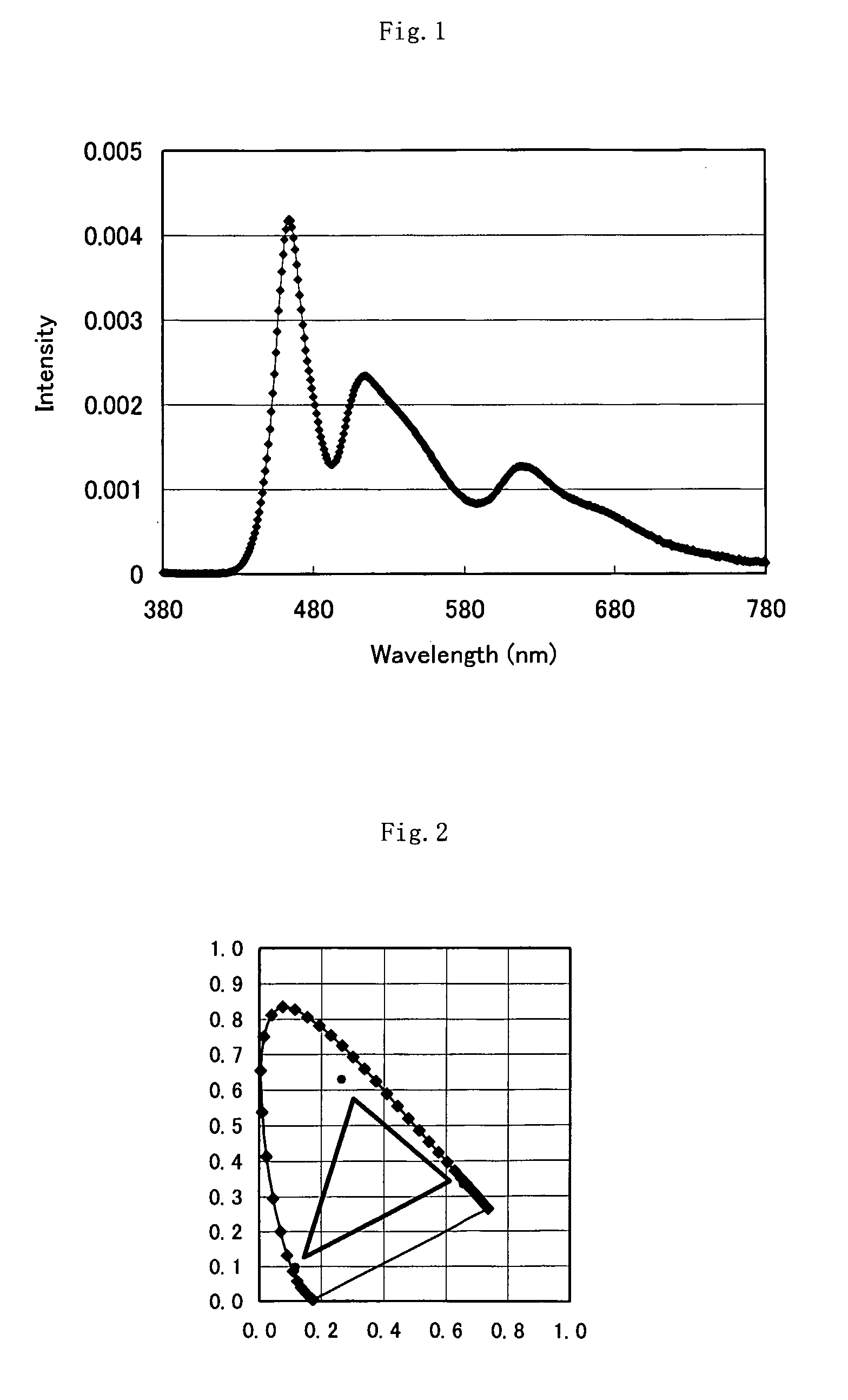
Light -Emitting Body,Lighting Device And Display Device Using The Same
InactiveUS20070292631A1Wide directivityLiquid crystal compositionsIndium organic compoundsControl systemDisplay device
A light-emitting body comprising a primary luminescent unit and a secondary luminescent unit that emits light owing to light emitted from the primary luminescent unit, wherein the secondary luminescent unit contains an iridium compound. As the iridium compound, an iridium complex represented by L1L2L3Ir, (L1, L2 and L3 are organic bidentate ligands coordinated to iridium, and at least one of these ligands coordinates with a nitrogen atom and a carbon atom) preferably, represented by formula (2) can be cited. (In the formula, X represents an atom group that forms an aromatic chelate ligand together with a carbon atom and a nitrogen atom bonded with iridium, n is an integer of 2 or 3, and L represents a bidentate organic ligand wherein the atoms other than carbon atoms are bonded with iridium.) The light-emitting body of the invention contains red (R), green (G) and blue (B) wavelength components to be wide in the color reproduction range, can be driven with a single power supply and control system, and does not cause photo-deterioration of constituent members.
Owner:SHOWA DENKO KK
Biospecific binding reactants labeled with new luminescent lanthanide chelates and their use
ActiveUS7018851B2Simple methodImproved labeling methodMicrobiological testing/measurementChemiluminescene/bioluminescenceLanthanideChelating ligands
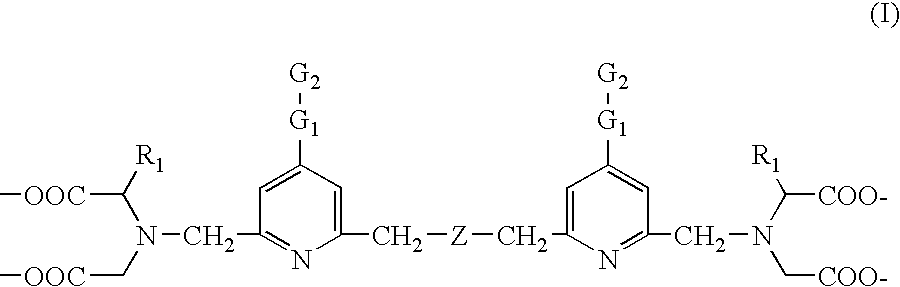
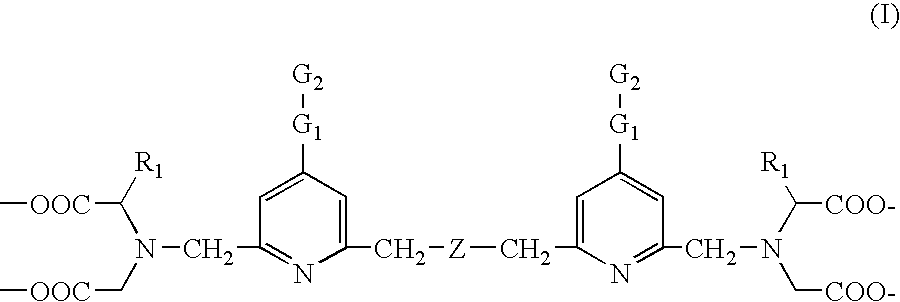
This invention relates to a luminescent lanthanide chelate comprising a lanthanide ion and a chelating ligand of formula (I) whereinR1 is selected from the group consisting H, —COOH, —COO−, —CH2COOH and —CH2COO−; G1 is a group consisting of one or two moieties each moiety being selected from the group consisting of ethynediyl, ethenylene, phenylene, biphenylene, naphthylene, pyridylene, pyrazinylene, pyrimidinylene, pyridazinylene, furylene, thienylene, pyrrolylene, imidazolylene, pyrazolylene, thiazolylene, isothiazolylene, oxazolylene, isoxazolylene, fyrazanylene, 1,2,4-triazol-3,5-ylene and oxadiazolylene; G2 for coupling to a biospecific binding reactant is selected from the group consisting of amino, aminooxy, carbonyl, aldehyde or mercapto groups and activated forms made of them; Z is selected from the group consisting of carboxyalkyl amine, ether, thioether, carbonyl and unsubstituted or substitute methyl (—CR2—) wherein group R2 is selected from the group consisting of H, methyl, ethyl and carboxylalkyl; and the lanthanide ion is europium(III), terbium(III), dysprosiym(III) or samarium(III). This invention further relates to a detectable molecule comprising the lanthanide chelate and the use of the molecule in a method of carrying out a biospecific binding assay.
Owner:INNOTRAC DIAGNOSTISC
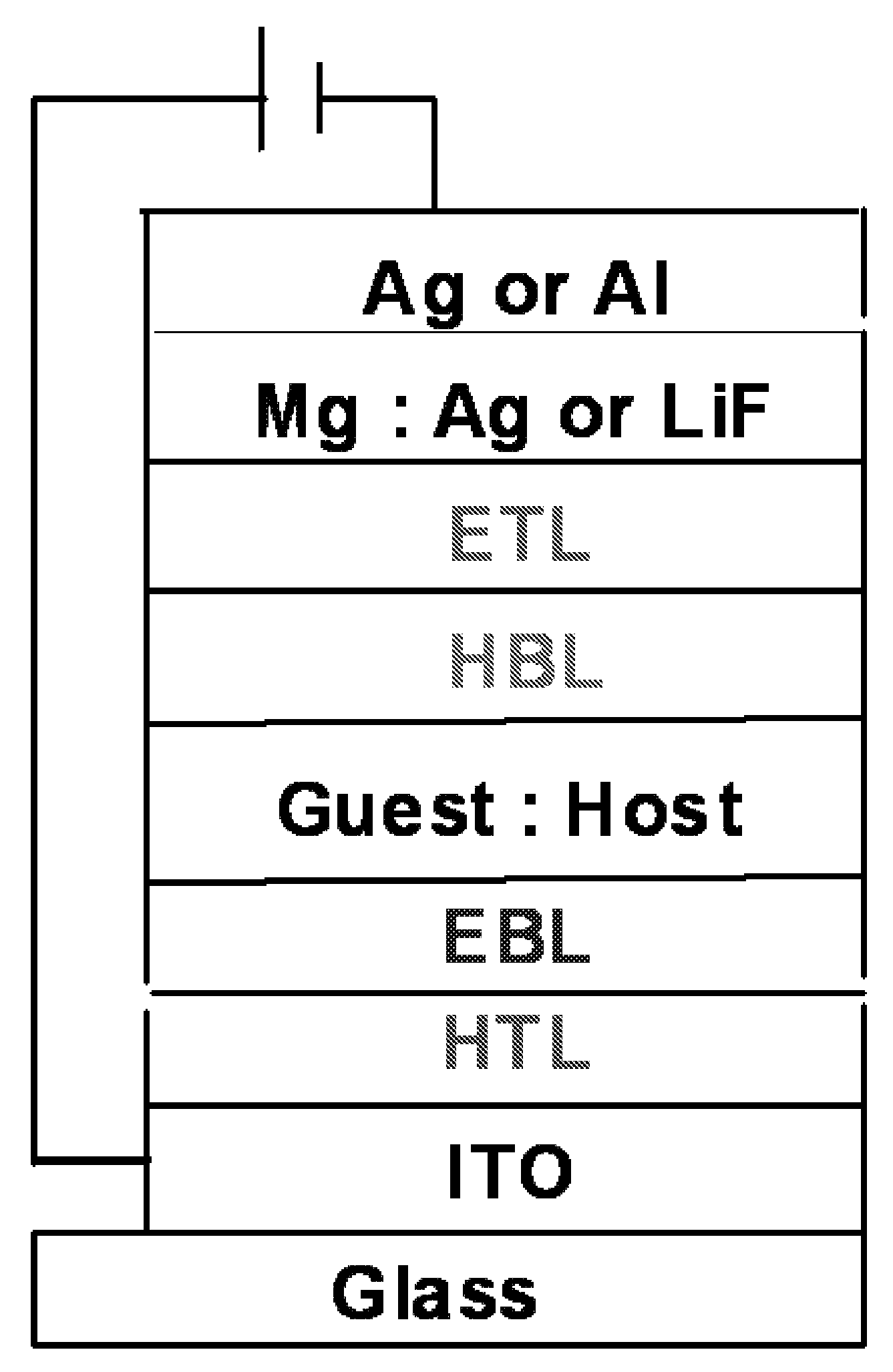
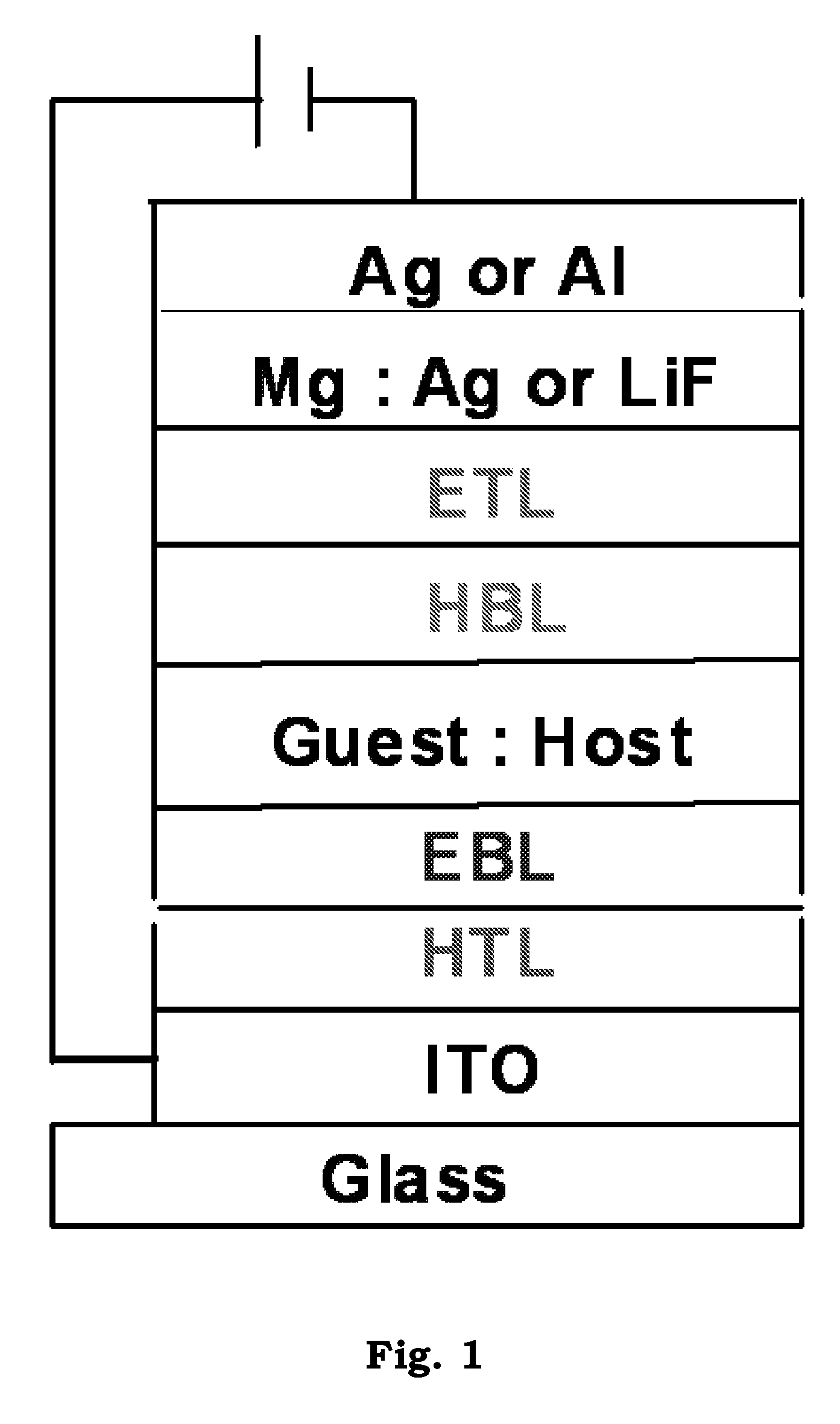
Light emitting device and electronic appliance using the same
InactiveCN1866576AGood current efficiency workIncrease brightnessElectroluminescent light sourcesSolid-state devicesDiketoneHydrogen
The light emitting element includes a first electrode and a second electrode, between which a light emitting layer, a hole transporting layer provide in contact with the light emitting layer, an electron transporting layer provided in contact with the light emitting layer, and a mixed layer provided between the electron transporting layer and the second electrode. The mixed layer includes an electron transporting substance and a substance showing an electron donating property with respect to the electron transporting substance. The light emitting layer includes an organometallic complex represented in General Formula (1) and a host. R1 and R2 each represent an electron-withdrawing substituent group. R3 and R4 each represent any of hydrogen or an alkyl group having 1 to 4 carbon atoms. L represents any of a monoanionic ligand having a beta-diketone structure, a monoanionic bidentate chelating ligand having a carboxyl group, or a monoanionic bidentate chelating ligand having a phenolic hydroxyl grou
Owner:SEMICON ENERGY LAB CO LTD
Preservation of wood products
InactiveUS20030086979A1Shorten treatment timeIncreases level of penetrationBiocideHeavy metal active ingredientsInorganic saltsIron salts
A method for the protection of wood and other wood materials without affecting dimensional stability or surface integrity of the treated material is described. The method involves treating wood material with an iron salt and selected oxidants where the iron salt is preferably complexed with organic chelating ligands. Preferably, a microbicidal agent is also incorporated into the method to provide treated wood products that demonstrate excellent surface integrity, dimensional stability and retention of the infused microbicidal agents for extended periods of time without incurring the detrimental environmental effects of conventional chromium or copper-based inorganic salt preservation methods.
Owner:ROHM & HAAS CO
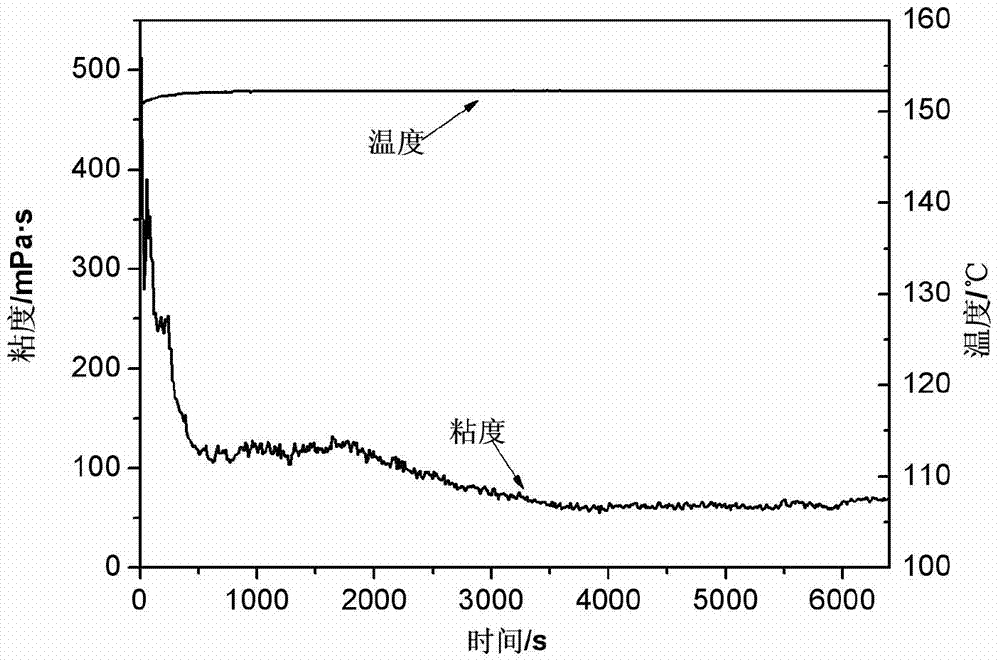
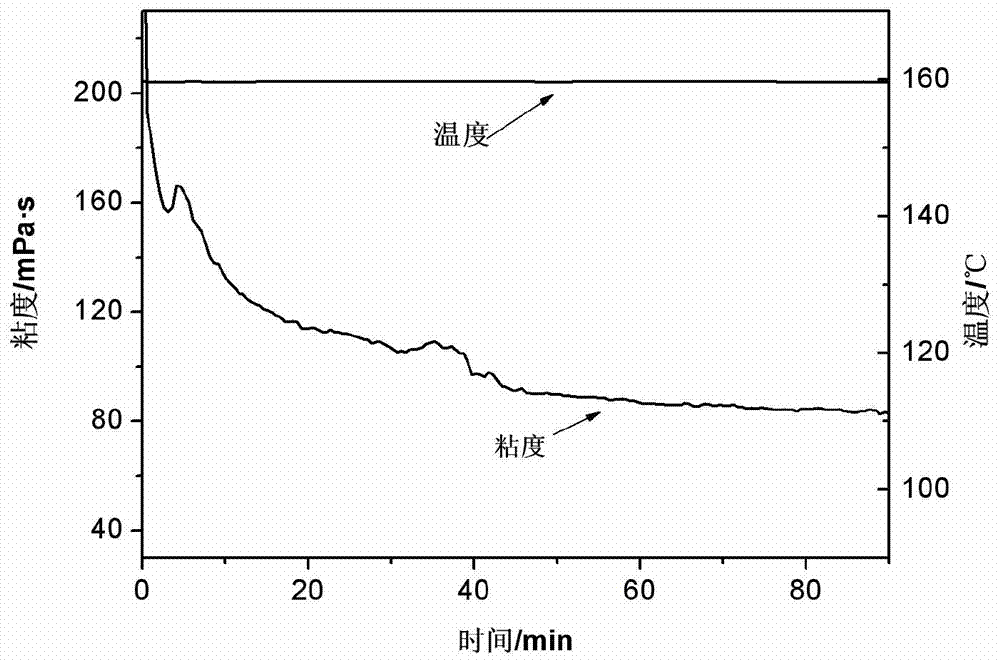
Cross-linking agent applicable to highly-mineralized water fracturing fluid
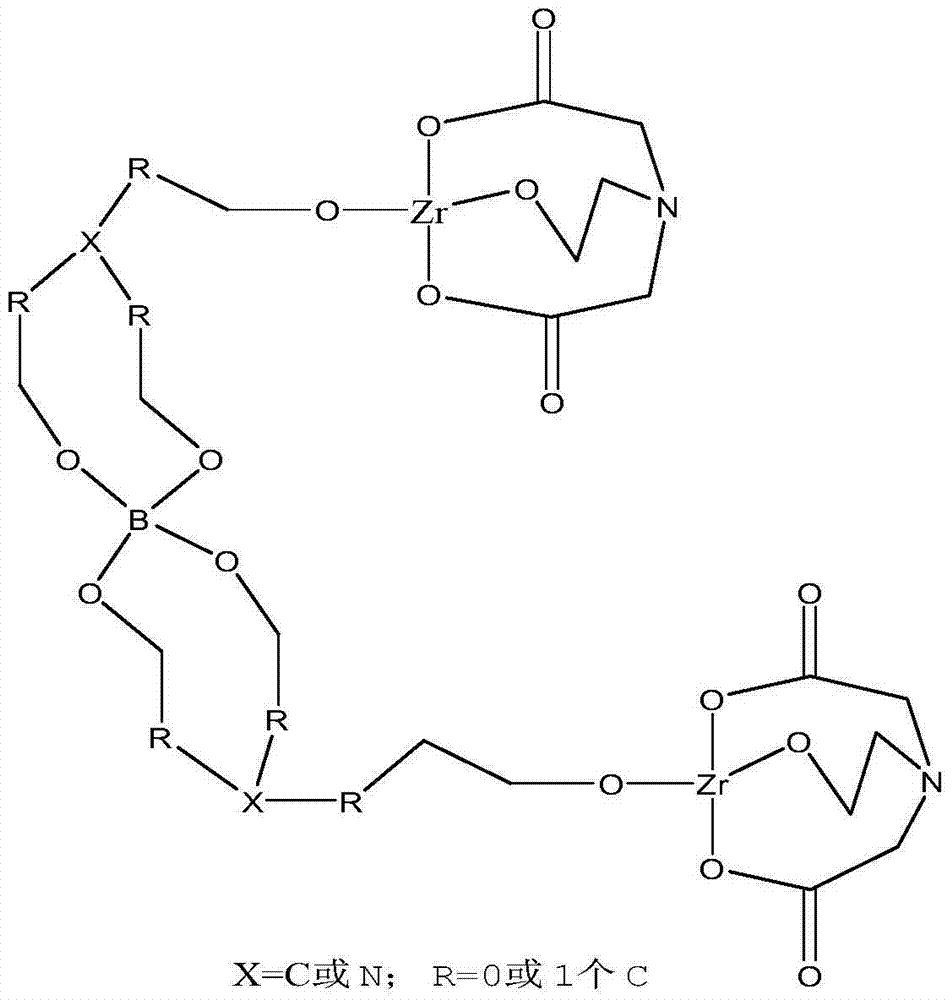
ActiveCN103497753ALow costReduce harmGroup 4/14 organic compounds without C-metal linkagesDrilling compositionCross-linkGlycerol
The invention discloses a cross-linking agent applicable to highly-mineralized water fracturing fluid. The cross-linking agent is stable complex which is formed by reaction of a zirconium compound, a boron compound with a chelating ligand and bridging ligand in aqueous liquor. The zirconium compound is zirconium acetate or zirconium oxychloride with content of 9wt%-18wt%; the boron compound is boric acid or borax with content of 3wt%-40wt%; the chelating ligand is N, N-di(2-hydroxyl ethyl) glycine with content of 9wt%-18wt%; the bridging ligand is one, two or a mixture of more than two of glycerol, mannitol, sorbitol and triethanolamine, with content of 5wt%-19wt%, and the balance of water. The cross-linking agent disclosed by the invention can be cross-linked with a thickening agent under a weakly alkaline condition, not only contains heavy metal zirconium, but also contains boron, so that damages to stratums by the fracturing fluid are effectively lowered; moreover, highly-mineralized water can be adopted to directly prepare the fracturing fluid, so that fresh water resources are saved, and expenditure cost of oilfield enterprises in treating and producing highly-mineralized water is reduced.
Owner:SOUTHWEST PETROLEUM UNIV
Process for the preparation of new transition metal complexes
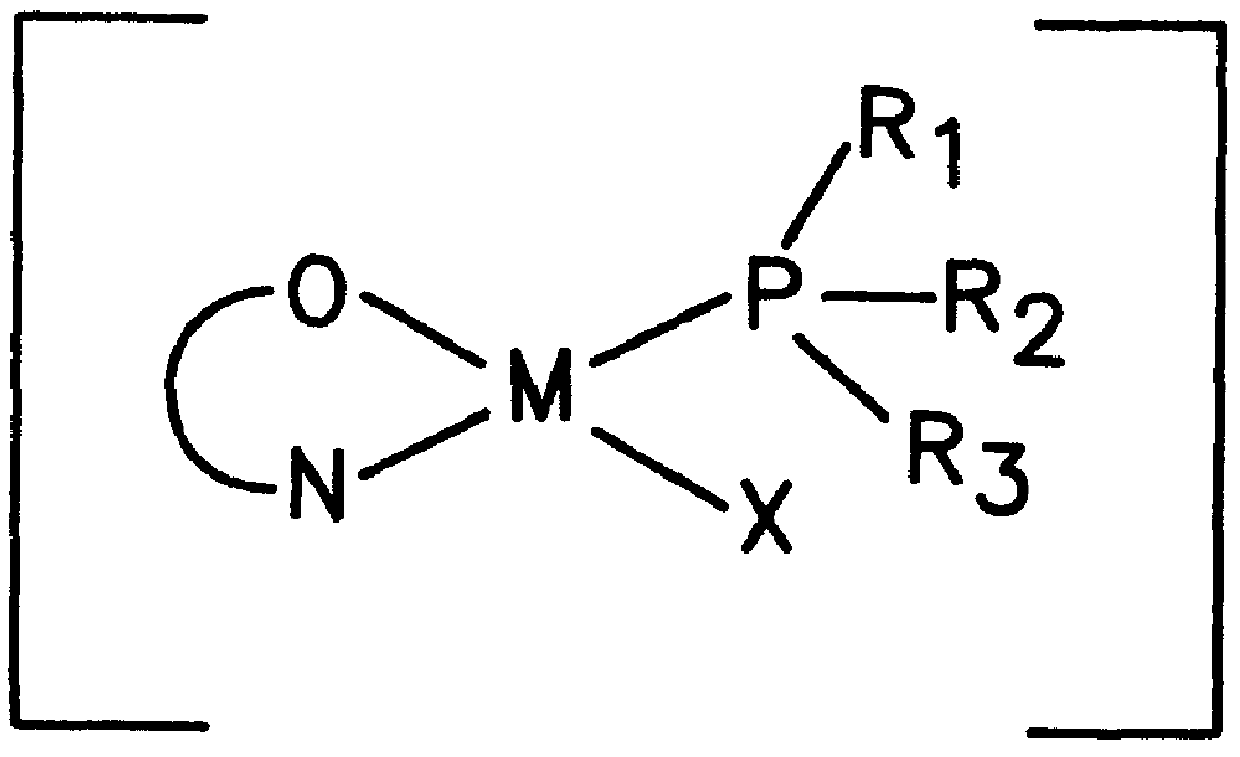
InactiveUS6069253AOrganic-compounds/hydrides/coordination-complexes catalystsPlatinum organic compoundsChelating ligandsPhosphine
The present invention relates to novel group VIII transition metal complexes represented by the formula (I) as: wherein M is the central transition metal; +Z represents a semilabile anionic chelating ligand; R1, R2 & R3 are substituents on the phosphine ligand, X is chosen from sulphonato, carboxylato, formato group or halides and 1<n<10. The invention also provides a process for the preparation of said transition metal complex.
Owner:COUNCIL OF SCI & IND RES
Transition metal complexes with carbene ligands and their application
InactiveUS20090149653A1Improve thermal stabilityExtended service lifeIndium organic compoundsDischarge tube luminescnet screensNitrogenCarbene
The present invention discloses a transition metal complex having carbene ligands. The disclosed transition metal complex has a structure of a center transition metal surrounded by two identical carbene ligands and one double-chilating ligand which is a nitrogen-contain heteroaryl group compound with pyridyl group. The disclosed transition metal complex can be represented by the following formula:
Owner:E RAY OPTOELECTRONICS TECH
Efficient nano negative ion releasing agent and preparation method thereof
The invention provides an efficient nano negative ion releasing agent and a preparation method thereof and relates to the field of negative ion releasing materials. The negative ion releasing materials are widely applied to the fields of ceramics, coatings, plastics, spinning, decoration and the like, currently, most negative ion releasing materials are tourmaline and excite negative ion release by adding a large amount of rare earth or composite salt, radioactivity and heavy metal content of the most negative ion releasing materials exceed standard easily, the negative ion release is unstable, and the release efficiency is low. In order to overcome defects in the prior art, the efficient nano negative ion releasing agent is provided and is characterized by being prepared from raw materials in parts by mass as follows: 50-80 parts of the negative ion releasing material, 30-50 parts of an excitation material, 5-28 parts of an energy transfer material and 1-6 parts of a chelating agent. The efficient nano negative ion releasing agent and the preparation method have beneficial effects as follows: the efficient nano negative ion releasing agent is prepared through comprehensive adoption of a nano grinding technology and combination of piezoelectricity and pyroelectricity of the negative ion releasing material as well as chelating ligands of the excitation material and the energy transfer material with a chelating agent.
Owner:上海前引科技有限公司
Process for the production of stereoregular polymers and elastomers of alpha-olefins and certain novel catalysts therefor
InactiveUS20010029232A1Efficient aggregationEasy to operateOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationElastomerSolvent
A process for the polymerization of one or more alpha-olefins having at least 3 carbon atoms, which comprises contacting the monomer or monomers in a polar or non-polar solvent under polymerization conditions with a homogeneous catalyst system including (a) a cationic form of a racemic mixture of a chiral octahedral transition metal complex or of a non chiral octahedral transition metal complex, comprising 1, 2 or 3 bidentate chelating ligands and no cyclopentadienyl ligands and having C1, C2, or C3 symmetry; and (b) an anion of a Lewis acid or a Brönsted acid; and adjusting the pressure so as to obtain either a highly stereoregular polymer or copolymer or an elastomer.
Owner:TECHNION RES & DEV FOUND LTD
Functionalized triplet emitters for electro-luminescent devices
InactiveUS20140364611A1InteractionTriplet-triplet annihilation and self-quenching can be strongly suppressedIndium organic compoundsElectroluminescent light sourcesChelating ligandsLight-emitting diode
Organo-metallic complexes for opto-electronic and sensory devices and their use in such devices are provided. The organo-metallic complex (triplet emitter) consists of a metal center and chelate ligands. At least one of chelate ligands comprises an aromatic or fused aromatic ring(s). Each ligand is covalently substituted with at least one, preferably two charge transport groups (ctg). The metal center can be coordinated by a spectator ligand. Presence of two ctgs at each ligand is advantageous for applications in organic light emitting diodes (OLEDs). Charge transport units facilitate hole and / or electron transport to the molecular center and allow for efficient exciton formation directly on the complex. Presence of ctgs on each ligand provides a good shielding with respect to interactions with the environment. Emission quenching is strongly reduced and materials with high emission quantum yields are obtained. Presence of ctgs on each ligand reduces undesired quenching by triplet-triplet annihilation or self-quenching effects.
Owner:CYNORA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


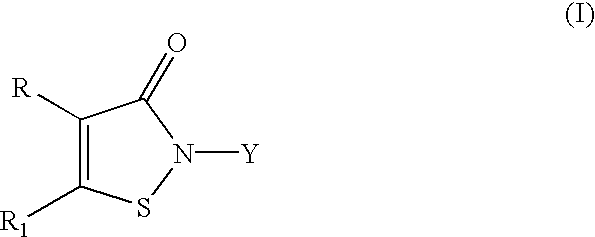
![Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof](https://images-eureka.patsnap.com/patent_img/a12936d2-d316-4ce2-8f0f-eb88c0a93e3b/HDA0000504082740000011.PNG)
![Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof](https://images-eureka.patsnap.com/patent_img/a12936d2-d316-4ce2-8f0f-eb88c0a93e3b/HDA0000504082740000012.PNG)
![Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof](https://images-eureka.patsnap.com/patent_img/a12936d2-d316-4ce2-8f0f-eb88c0a93e3b/HDA0000504082740000021.PNG)
![Pyridine pyrazole dinuclear copper [1] complex luminescent material and preparation method Pyridine pyrazole dinuclear copper [1] complex luminescent material and preparation method](https://images-eureka.patsnap.com/patent_img/3a67f9dc-283f-4fe8-8df6-35e82bd9a8f5/HDA0000743489300000011.PNG)
![Pyridine pyrazole dinuclear copper [1] complex luminescent material and preparation method Pyridine pyrazole dinuclear copper [1] complex luminescent material and preparation method](https://images-eureka.patsnap.com/patent_img/3a67f9dc-283f-4fe8-8df6-35e82bd9a8f5/HDA0000743489300000012.PNG)
![Pyridine pyrazole dinuclear copper [1] complex luminescent material and preparation method Pyridine pyrazole dinuclear copper [1] complex luminescent material and preparation method](https://images-eureka.patsnap.com/patent_img/3a67f9dc-283f-4fe8-8df6-35e82bd9a8f5/HDA0000743489300000021.PNG)