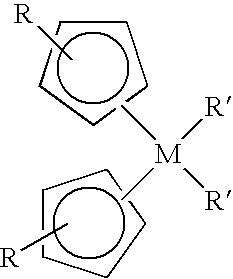
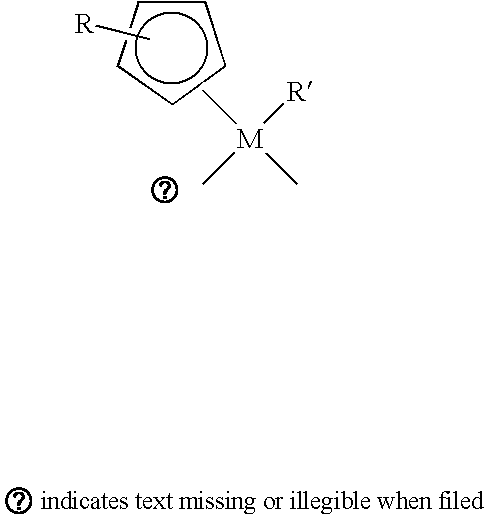
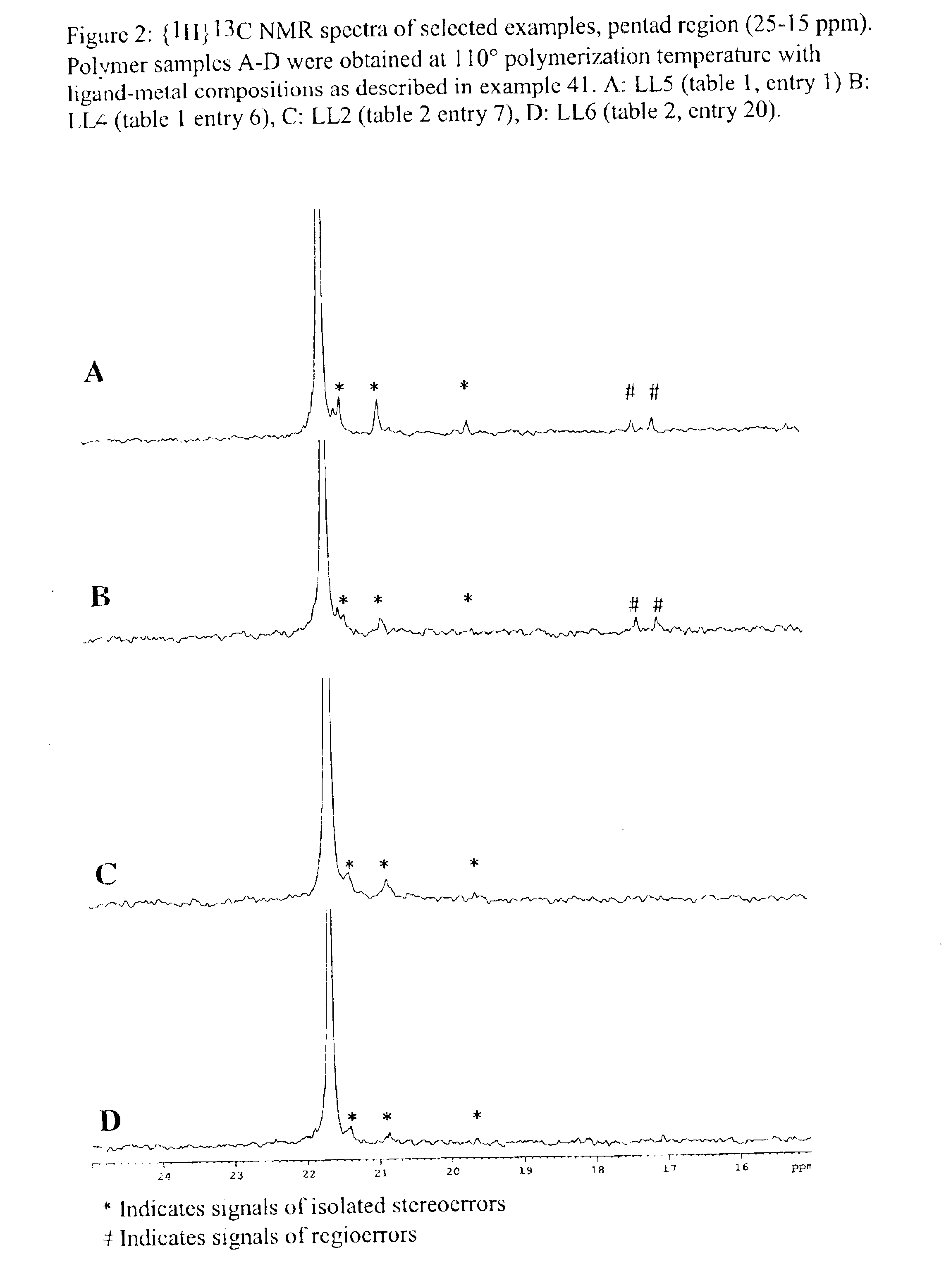
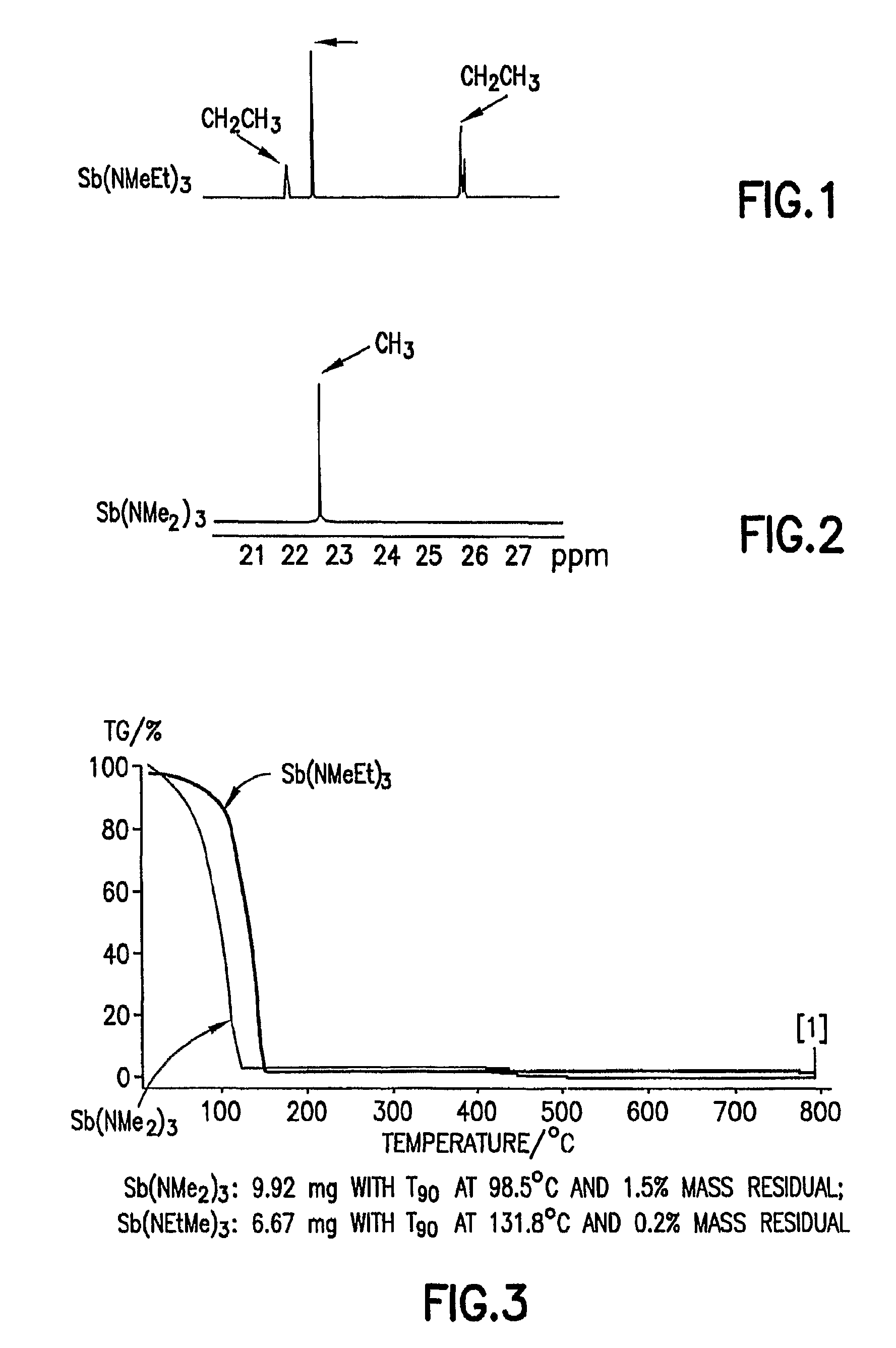
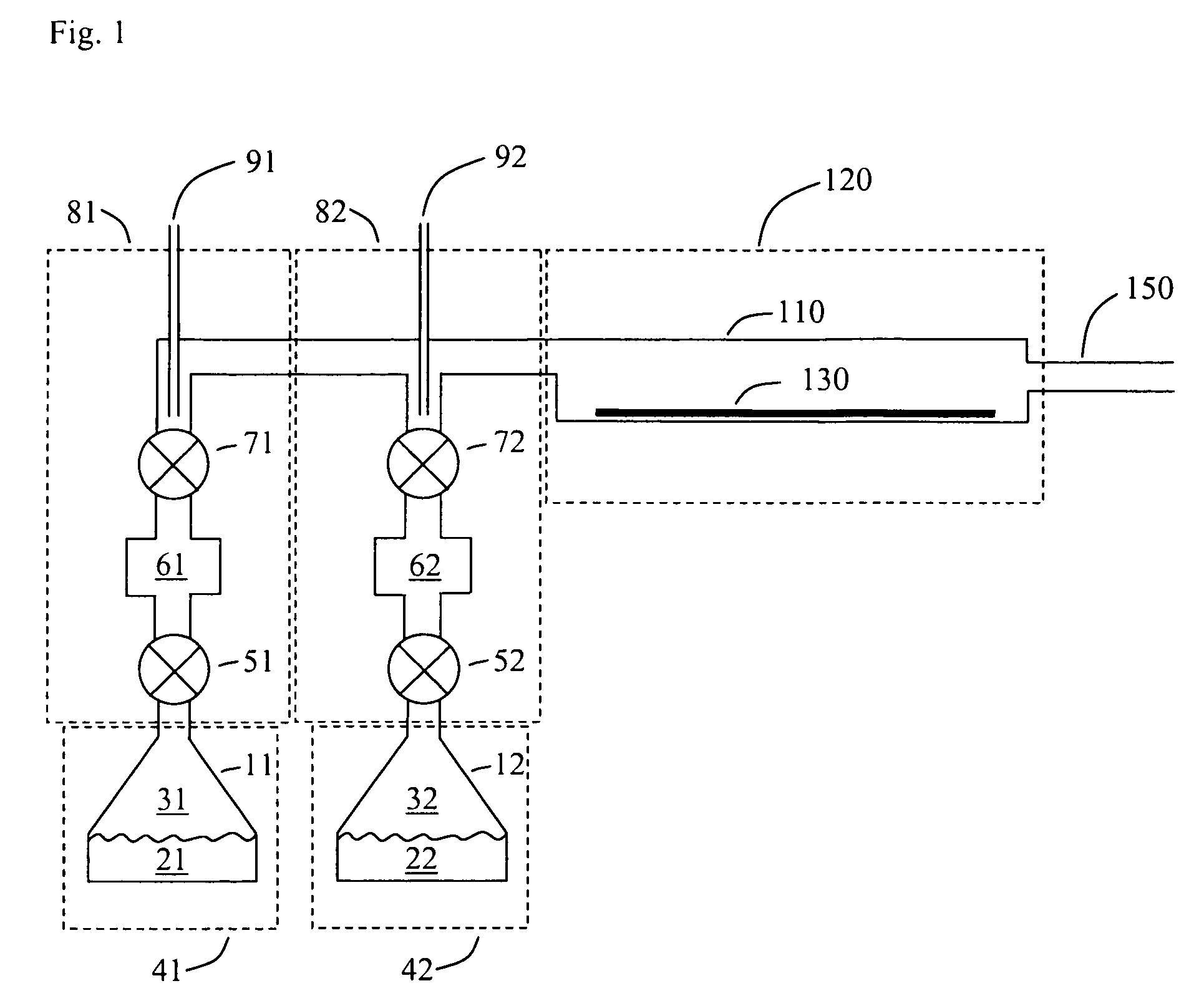
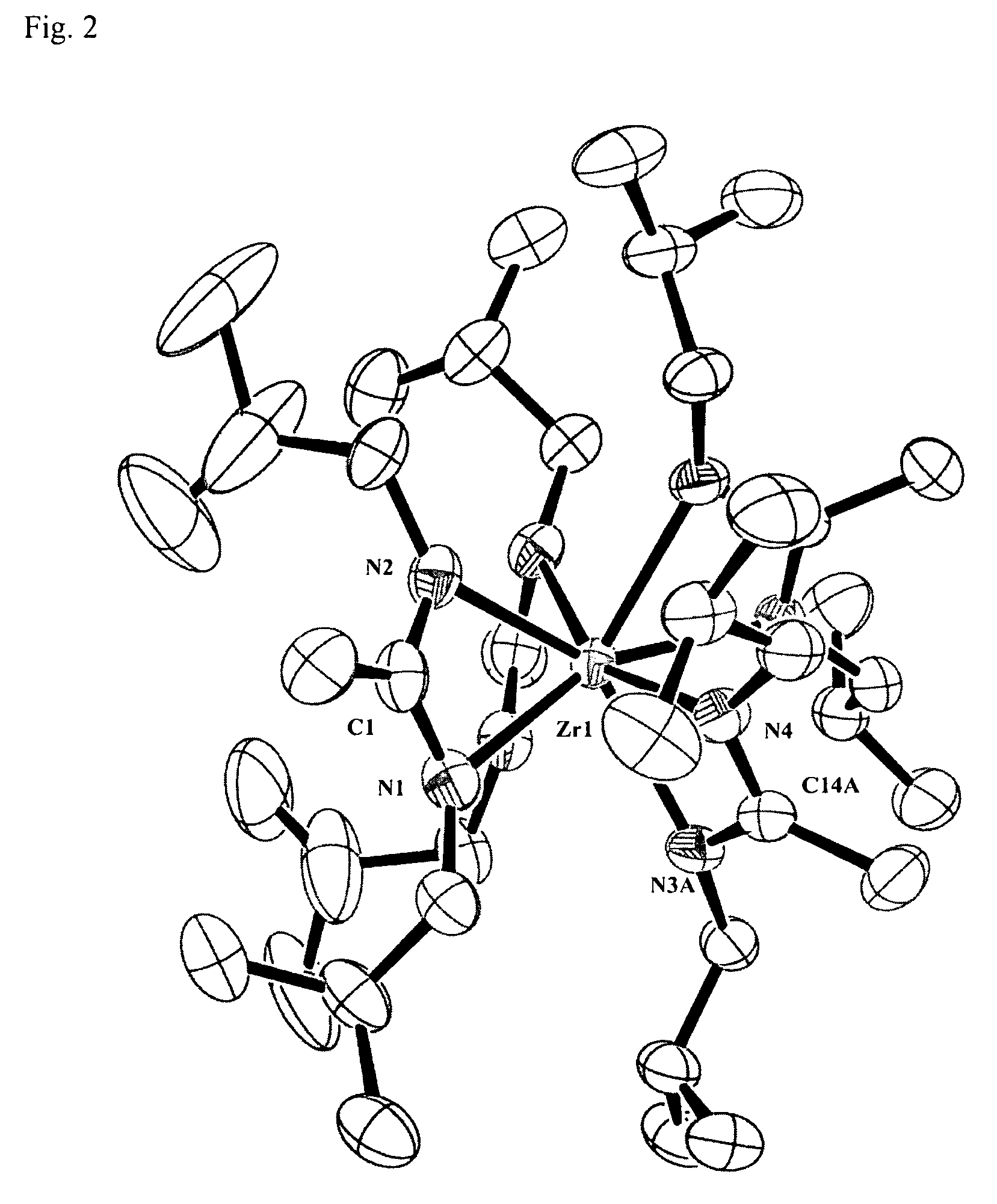
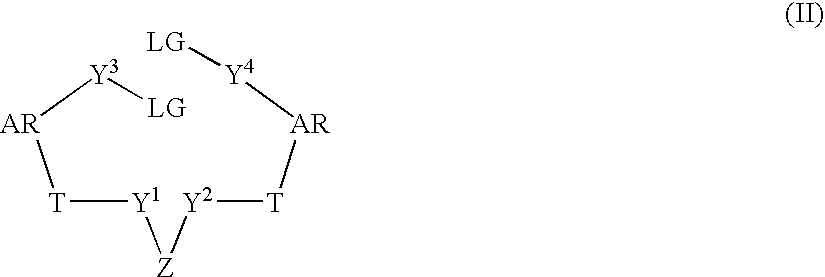
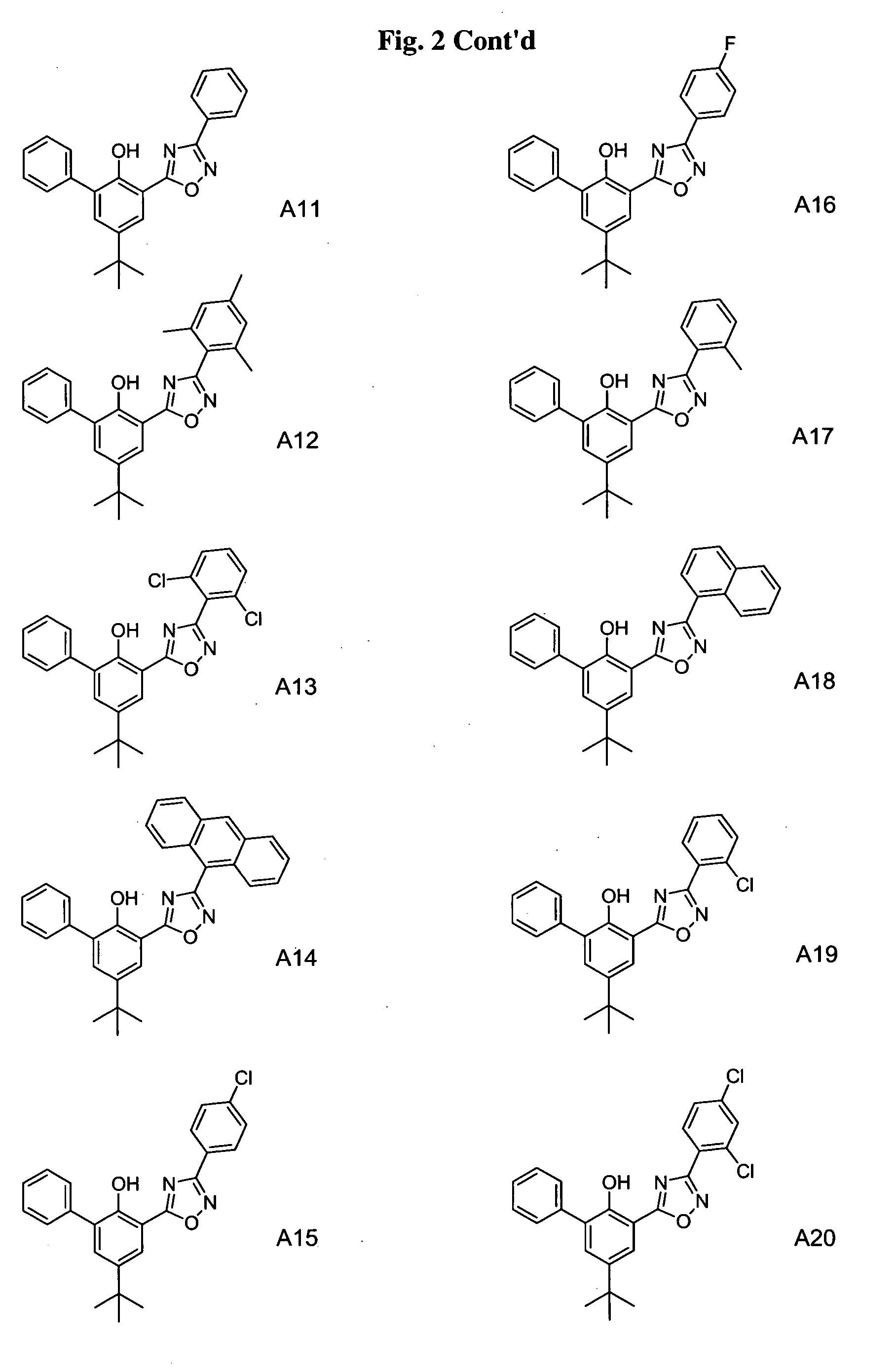
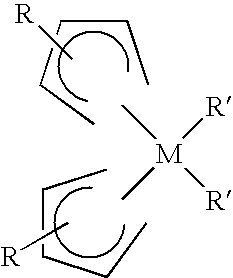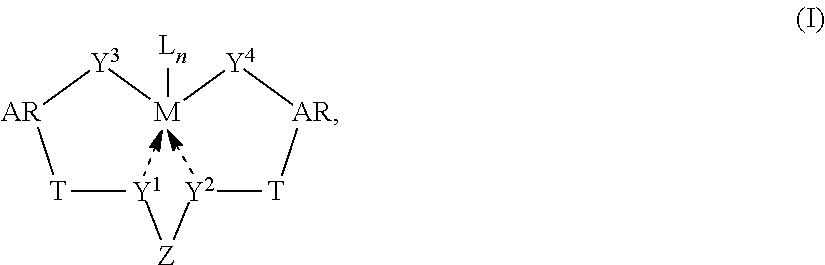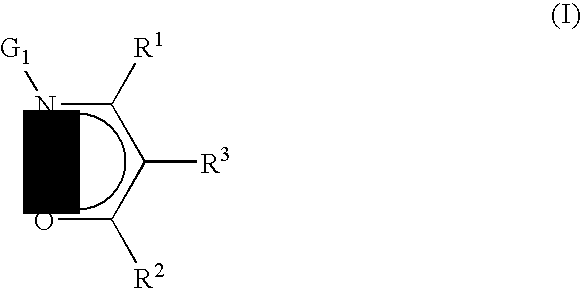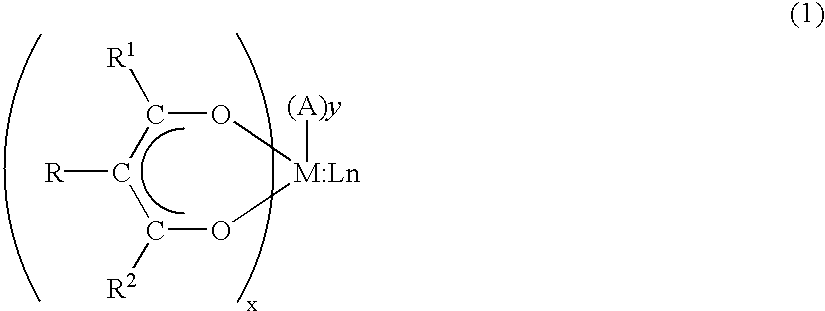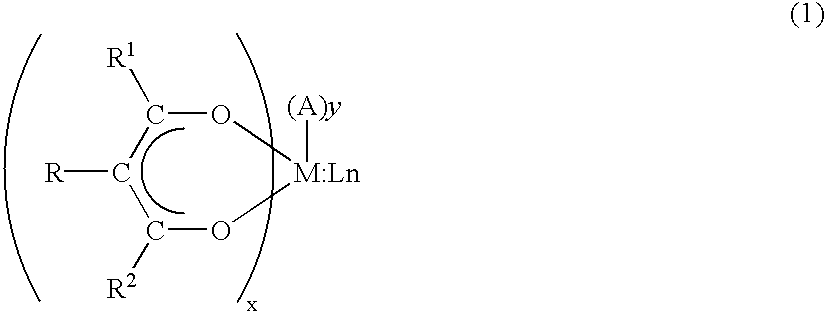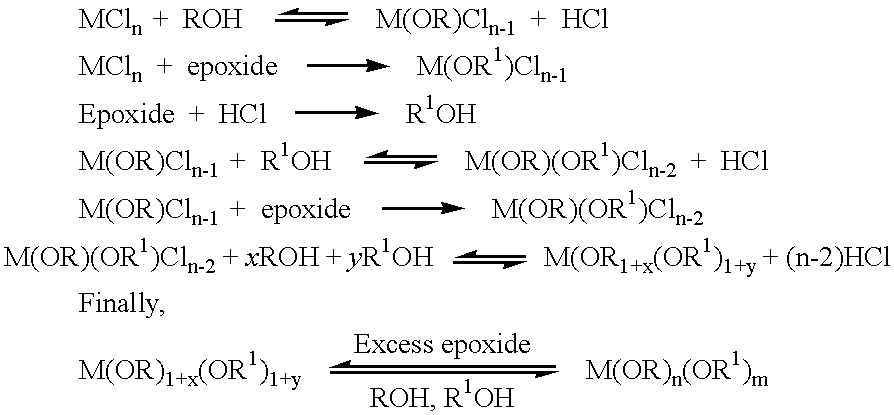Patents
Literature
569results about "Group 4/14 organic compounds without C-metal linkages" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
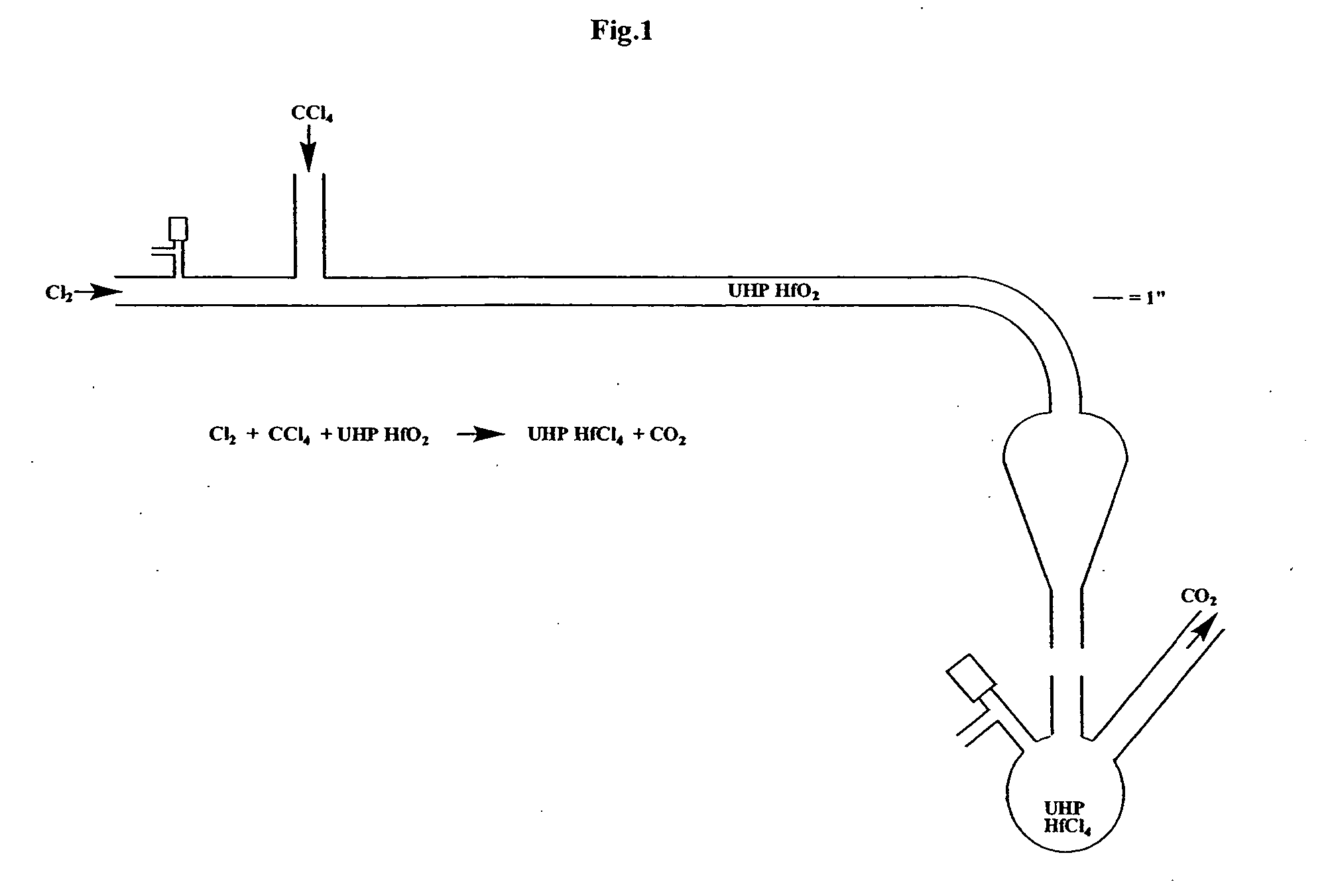
Low zirconium, hafnium-containing compositions, processes for the preparation thereof and methods of use thereof
InactiveUS20060193979A1Improve propertiesDesired morphologyGroup 4/14 organic compounds without C-metal linkagesPretreated surfacesHafniumChemical vapor deposition
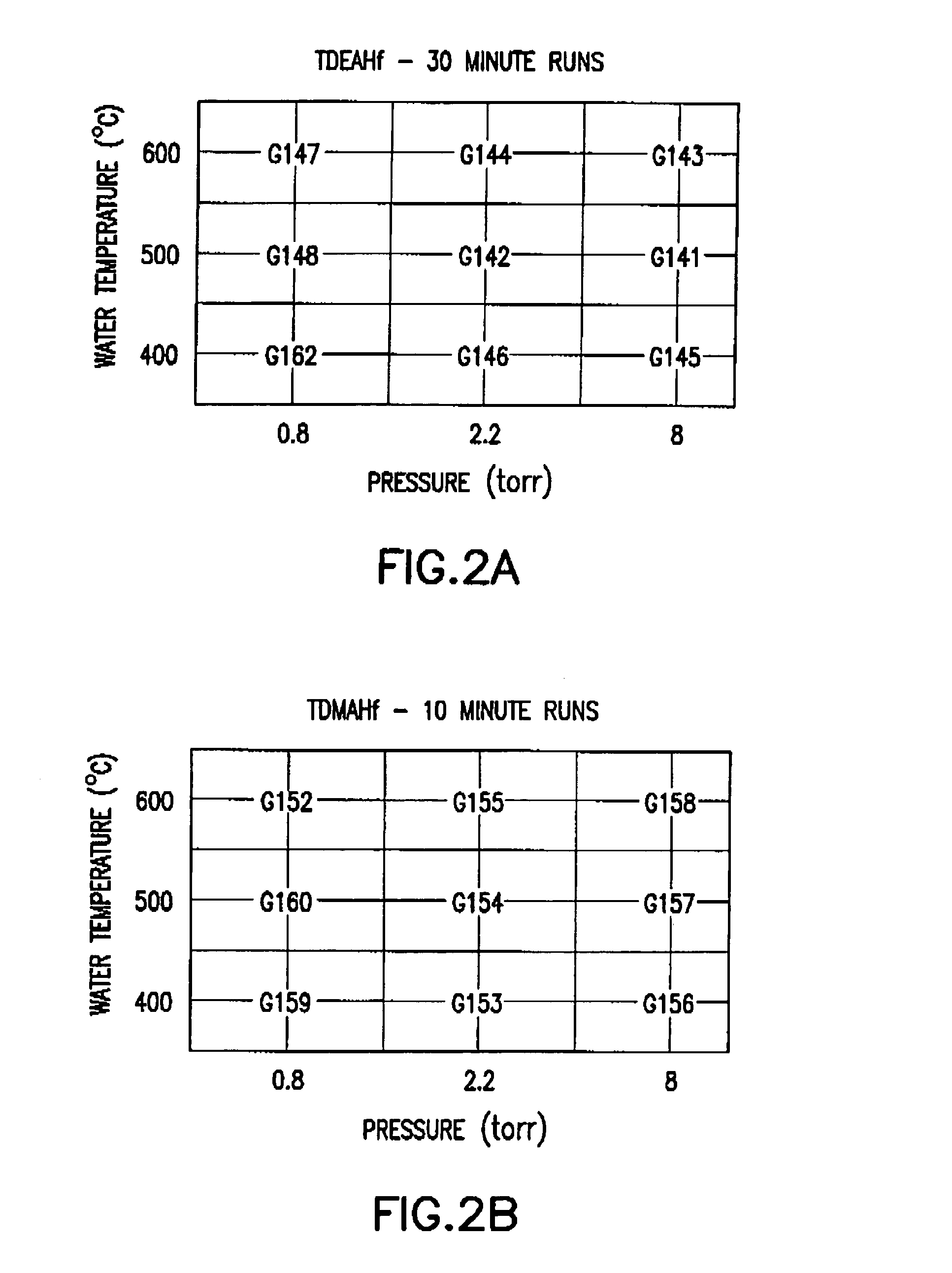
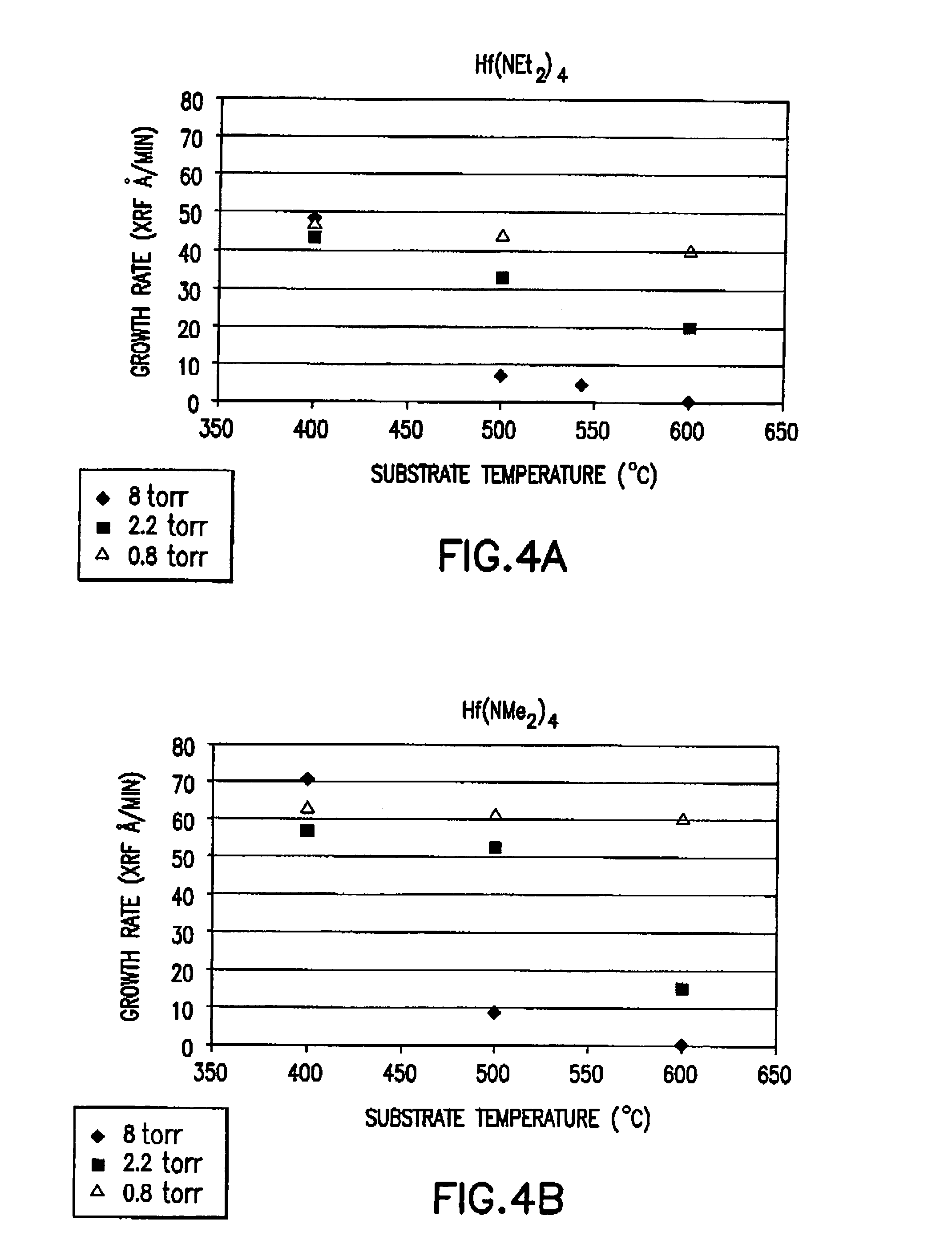
This invention relates to hafnium-containing compositions having a zirconium concentration of less than about 500 parts per million, a process for producing the hafnium-containing compositions, organometallic precursor compositions containing a hafnium-containing compound and having a zirconium concentration of less than about 500 parts per million, a process for producing the organometallic precursor compositions, and a method for producing a film or coating from the organometallic precursor compositions. The organometallic precursor compositions are useful in semiconductor applications as chemical vapor deposition (CVD) or atomic layer deposition (ALD) precursors for film depositions.
Owner:PRAXAIR TECH INC
Metal-containing compound, its production method, metal-containing thin film, and its formation method
ActiveUS20100105936A1Appropriate thermal stabilityAppropriate volatilityGroup 3/13 organic compounds without C-metal linkagesGroup 4/14 organic compounds without C-metal linkagesLithiumHydrogen
Owner:TOSOH CORP +1
Bridged bi-aromatic ligands, catalysts, processes for polymerizing and polymers therefrom
InactiveUS6869904B2Improve catalytic performanceOrganic compound preparationGroup 4/14 organic compounds without C-metal linkages1-OctenePolypropylene
New ligands, compositions, metal-ligand complexes and arrays with bridged bis-aromatic ligands are disclosed that catalyze the polymerization of monomers into polymers. These catalysts with metal centers have high performance characteristics, including higher comonomer incorporation into ethylene / olefin copolymers, where such olefins are for example, 1-octene, propylene or styrene. The catalysts also polymerize propylene into isotactic polypropylene.
Owner:DOW GLOBAL TECH LLC
Substituted pyridyl amine complexes, and catalysts
InactiveUS6900321B2Improve catalytic performanceIncrease temperatureSilicon organic compoundsMacromolecular libraries1-OcteneHafnium
New ligands, compositions, metal-ligand complexes and arrays with pyridylamine ligands are disclosed that catalyze the polymerization of monomers into polymers. Certain of these catalysts with hafnium metal centers have high performance characteristics, including higher comonomer incorporation into ethylene / olefin copolymers, where such olefins are for example, 1-octene, isobutylene or styrene. Certain of the catalysts are particularly effective at polymerizing propylene to high molecular weight isotactic polypropylene in a solution process at a variety of polymerization conditions.
Owner:FREESLATE
Source reagent compositions for CVD formation of gate dielectric thin films using amide precursors and method of using same
InactiveUS6869638B2Group 4/14 organic compounds without C-metal linkagesGroup 8/9/10/18 element organic compoundsGate dielectricHydrogen
A CVD Method of forming gate dielectric thin films on a substrate using metalloamide compounds of the formula M(NR1R2)x, or wherein M is Zr, Hf, Y, La, Lanthanide series elements, Ta, Ti, or Al; N is nitrogen; each of R1 and R2 is same or different and is independently selected from H, aryl, perfluoroaryl, C1-C8 alkyl, C1-C8 perfluoroalkyl, alkylsilyl; and x is the oxidation state on metal M; and an aminosilane compound of the formula HxSiAy(NR1R2)4-x-y or wherein H is hydrogen; x is from 0 to 3; Si is silicon; A is a halogen; Y is from 0 to 3; N is nitrogen; each of R1 and R2 is same or different and is independently selected from the group consisting of H, aryl, perfluoroaryl, C1-C8 alkyl, and C1-C8 perfluoroalkyl; and n is from 1-6. By comparison with the standard SiO2 gate dielectric materials, these gate dielectric materials provide low levels of carbon and halide impurity.
Owner:ENTEGRIS INC
Antimony and germanium complexes useful for cvd/ald of metal thin films
ActiveUS20090305458A1Group 4/14 organic compounds without C-metal linkagesGermanium organic compoundsPhase-change memoryTe element
Antimony, germanium and tellurium precursors useful for CVD / ALD of corresponding metal-containing thin films are described, along with compositions including such precursors, methods of making such precursors, and films and microelectronic device products manufactured using such precursors, as well as corresponding manufacturing methods. The precursors of the invention are useful for forming germanium-antimony-tellurium (GST) films and microelectronic device products, such as phase change memory devices, including such films.
Owner:ENTEGRIS INC
Method of forming high-k dielectric films based on novel titanium, zirconium, and hafnium precursors and their use for semiconductor manufacturing
ActiveUS20090311879A1Group 4/14 organic compounds without C-metal linkagesConductive materialDielectricFilm base
A method of forming on at least one support at least one metal containing dielectric films having the formula (M11-a M2a) Ob Nc, wherein: 0≦a<1, 01 and M2 being metals Hf, Zr or Ti using precursors with pentadienyl ligands and / or cyclopentadienyl ligands.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Source reagent compositions for CVD formation of gate dielectric thin films using amide precursors and method of using same
InactiveUS7005392B2Group 4/14 organic compounds without C-metal linkagesVacuum evaporation coatingArylGate dielectric
A CVD Method of forming gate dielectric thin films on a substrate using metalloamide compounds of the formula M(NR1R2)x, wherein M is selected from the group consisting of: Zr, Hf, Y, La, Lanthanide series elements, Ta, Ti, Al; N is nitrogen; each of R1 and R2 is same or different and is independently selected from the group consisting of H, aryl, perfluoroaryl, C1–C8 alkyl, C1–C8 perfluoroalkyl, alkylsilyl and x is the oxidation state on metal M; and an aminosilane compound of the formula HxSi(NR1R2)4-x, wherein H is hydrogen; x is from 0 to 3; Si is silicon; N is nitrogen; each of R1 and R2 is same or different and is independently selected from the group consisting of H, aryl, perfluoroaryl, C1–C8 alkyl, and C1–C8 perfluoroalkyl. By comparison with the standard SiO2 gate dielectric materials, these gate dielectric materials provide low levels of carbon and halide impurity.
Owner:ADVANCED TECH MATERIALS INC
Preparation method of thermal conduction enhanced metal organic framework gas storage material
ActiveCN104624160AImprove adsorption capacityHigh thermal conductivityOther chemical processesGroup 4/14 organic compounds without C-metal linkagesIndustrial gasSorbent
The invention relates to a preparation method of a thermal conduction enhanced metal organic framework gas storage material and belongs to the field of nanocomposites. The preparation method comprises the following steps: firstly selectively preparing a metal organic framework material with a large surface area and a high micropore proportion; performing synthesis post-modification on the metal organic framework material by a 'one-pot' method, regulating the polarity and contained functional groups of pores, immobilizing metal nanoparticles inside the pores to enhance the thermal conduction property of the metal organic framework material; adsorbing industrial gas by utilizing the ultra-large specific surface area and the nano duct structure of the metal organic framework material, wherein the thermal conduction enhanced adsorption material can be used for quickly transmitting the heat generated in the adsorption and desorption process of the industrial gas. The metal organic framework industrial gas adsorber prepared by the invention can be used for efficiently adsorbing and desorbing the industrial gas and effectively improving the thermal conduction property of the adsorber, and avoiding the influence of the heat effect on the adsorption quantity in the adsorption and desorption process. The preparation method provided by the invention has the advantages of use of readily available and inexpensive raw materials, simple process, and mild reaction conditions and is suitable for large-scale production.
Owner:UNIV OF SCI & TECH BEIJING
Bridged bi-aromatic ligands, catalysts, processes for polymerizing and polymers therefrom
InactiveUS6841502B2Improve catalytic performanceOrganic compound preparationGroup 4/14 organic compounds without C-metal linkages1-OcteneAlkene
New ligands and compositions with bridged bis-aromatic ligands are disclosed that catalyze the polymerization of monomers into polymers. These catalysts with metal centers have high performance characteristics, including higher comonomer incorporation into ethylene / olefin copolymers, where such olefins are for example, 1-octene, propylene or styrene. The catalysts also polymerize propylene into isotactic polypropylene.
Owner:DOW GLOBAL TECH LLC
Antimony and germanium complexes useful for CVD/ALD of metal thin films
ActiveUS7838329B2Group 4/14 organic compounds without C-metal linkagesGermanium organic compoundsPhase-change memoryTe element
Antimony, germanium and tellurium precursors useful for CVD / ALD of corresponding metal-containing thin films are described, along with compositions including such precursors, methods of making such precursors, and films and microelectronic device products manufactured using such precursors, as well as corresponding manufacturing methods. The precursors of the invention are useful for forming germanium-antimony-tellurium (GST) films and microelectronic device products, such as phase change memory devices, including such films.
Owner:ENTEGRIS INC
Drying agent
InactiveUS20030110981A1Group 8/9/10/18 element organic compoundsSemiconductor/solid-state device detailsArylDesiccant
An organic EL device is disclosed in which is placed a transparent water-capturing film comprising an easy-to-use organometallic compound illustrated by the chemical formula (1): wherein, R1, R2 and R3 are selected from the group consisting of alkyl group, aryl group, cycloalkyl group, heterocyclic group and acyl group having one carbon atom and above, and M is a trivalent metallic atom.
Owner:FUTABA CORPORATION
Method for catalytic polymerization of alpha-olefin monomers using an ultra-high activity non-metallocene pre-catalyst
InactiveUS6596827B2High molecular weightLow molecularGroup 4/14 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsPolymer sciencePolyolefin
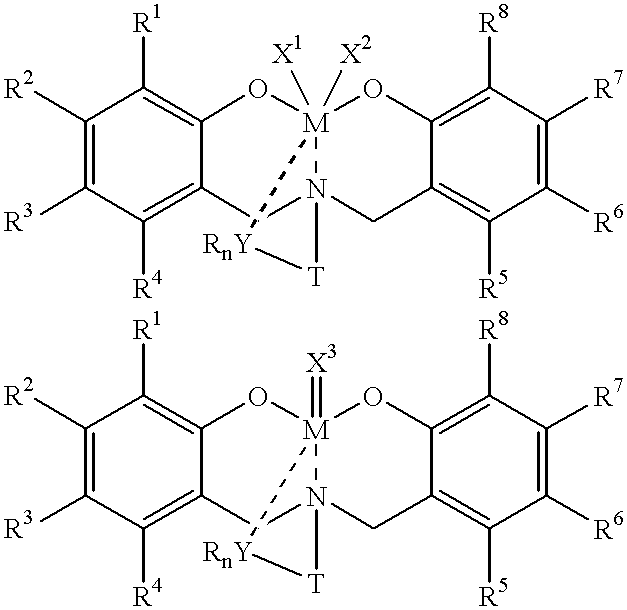
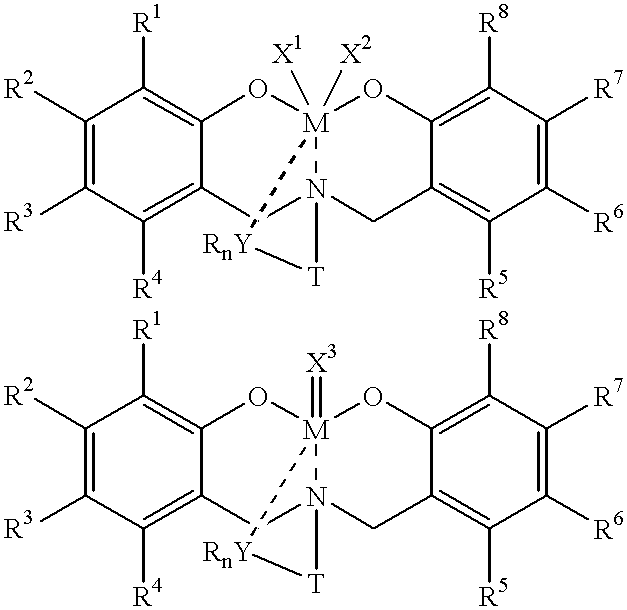
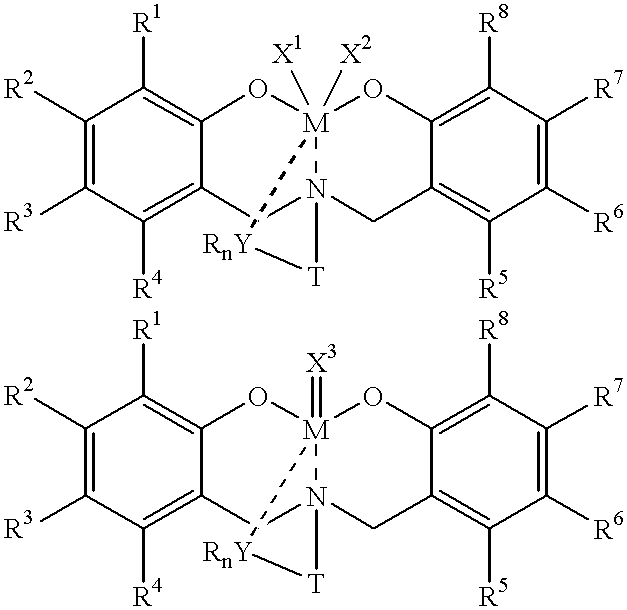
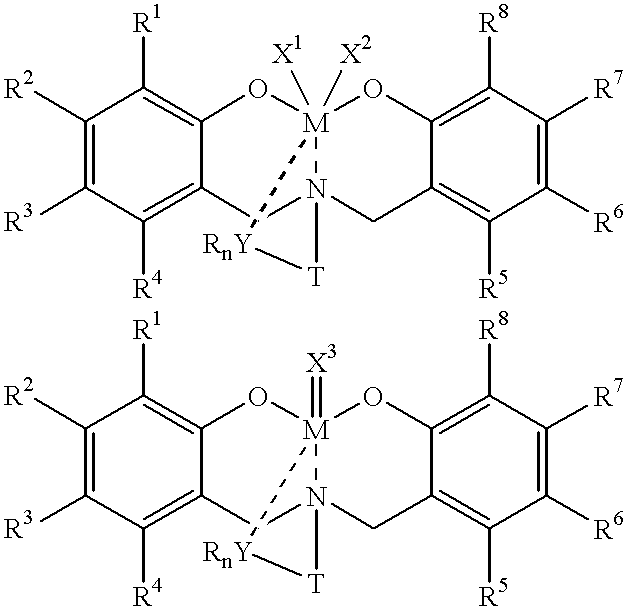
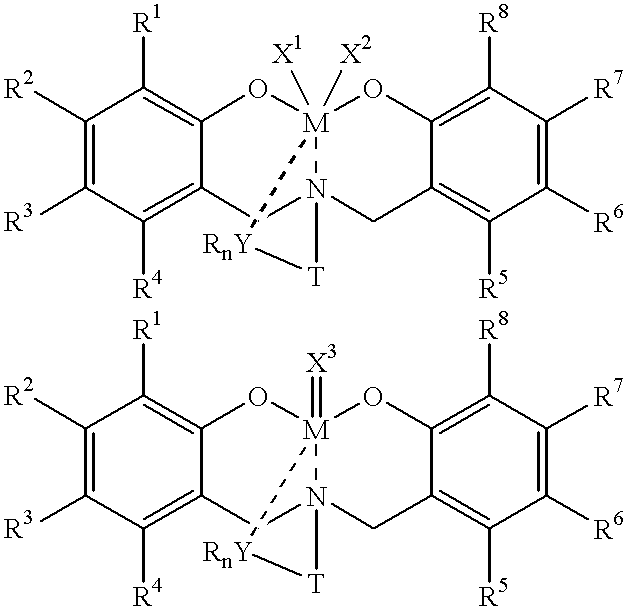
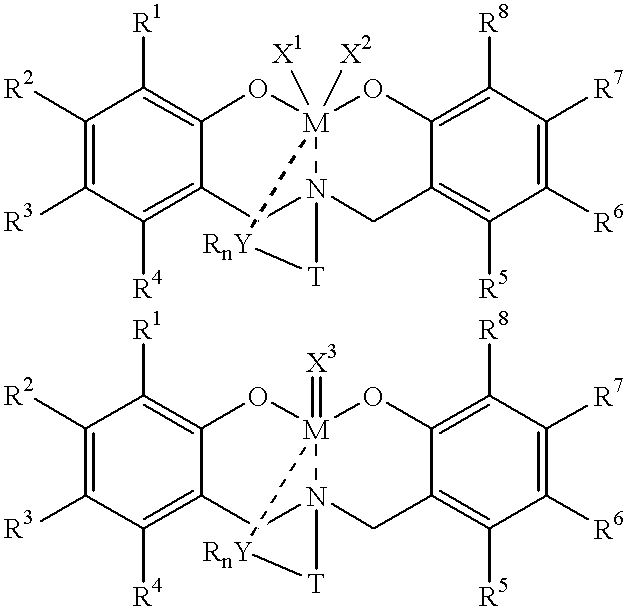
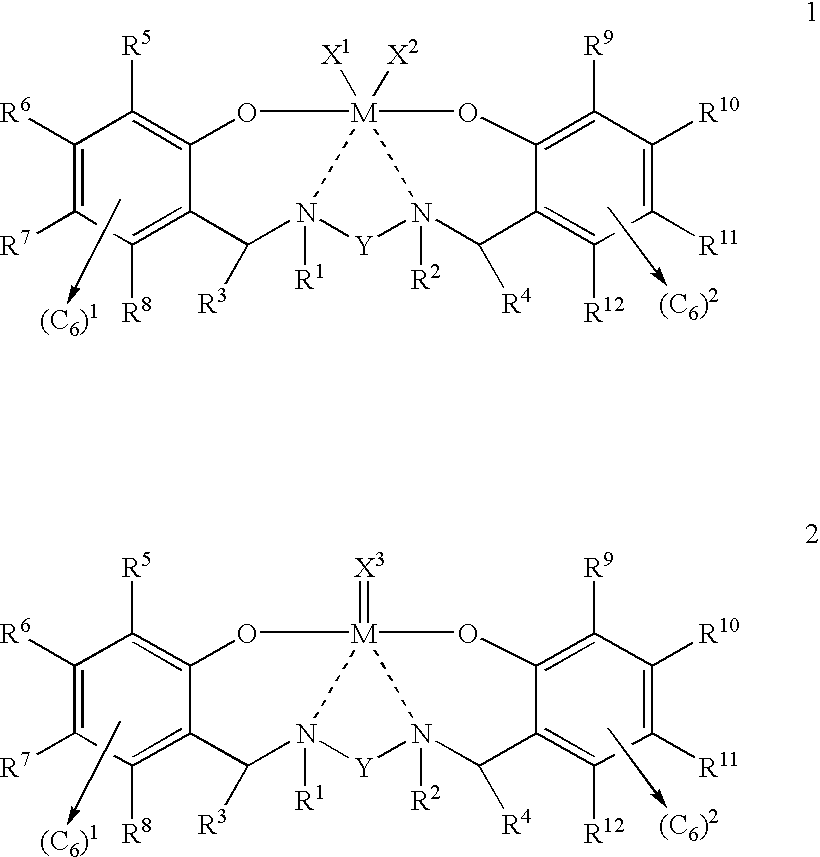
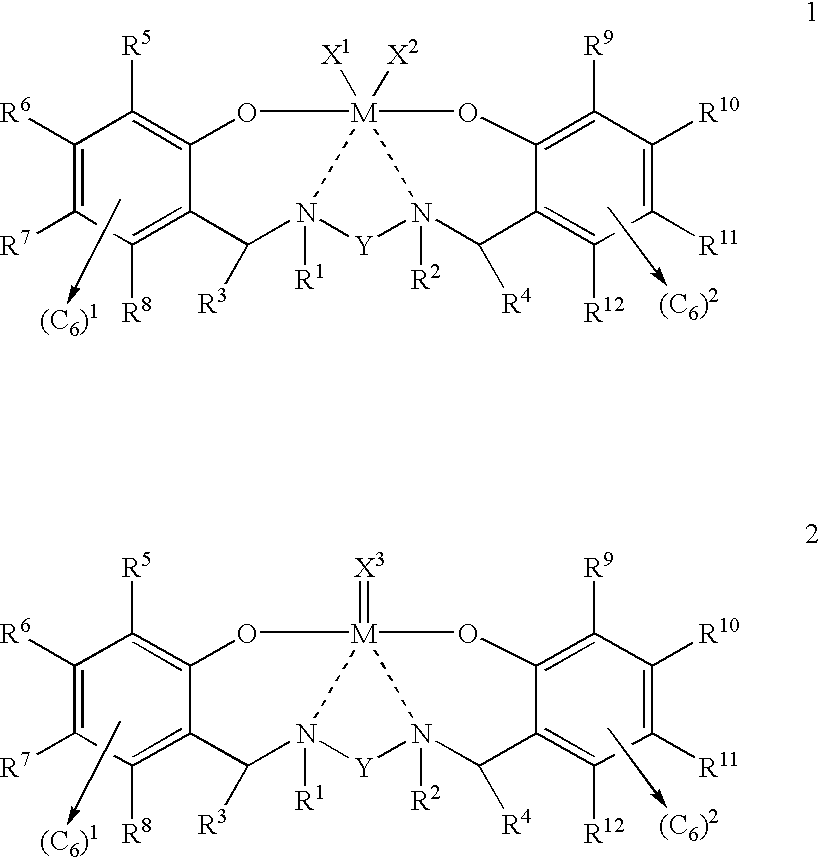
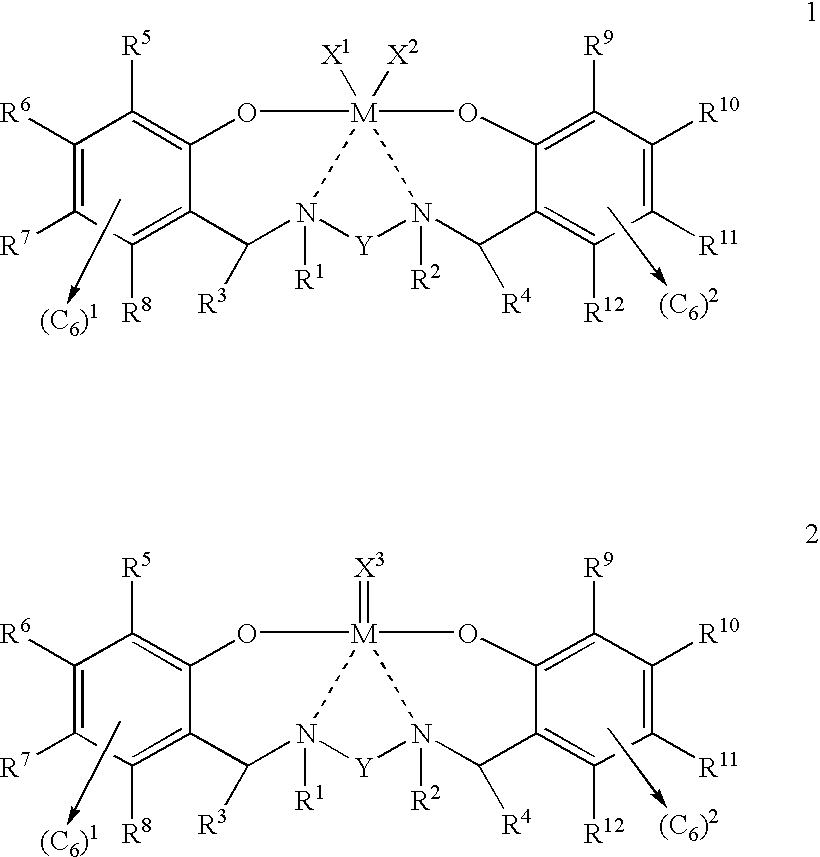
Disclosed are olefin polymerization methods comprising the use of the following compounds:wherein M is a metal atom, R1-R8 are univalent radicals, X1 and X2 are univalent ligands, X3 is a divalent ligand, and (RnY-T) is an optional donor or non-donor group. The relatively stable and simply synthesized pre-catalyst is activated by a co-catalyst under mild reaction conditions, producing exceptionally reactive polymerization of a wide variety of alpha-olefin monomers, and forming a variety of poly(alpha-olefin) products, having high molecular weight and low molecular weight distribution (PDIs close to 1). Living polymerization is performed at or above room temperature, along with achieving block co-polymerization of alpha-olefin monomers at room temperature, and producing polymers and oligomers having a wide range of molecular weights. The catalyst formed during reaction remains "alive' for as long as 31 hours, for producing a polymer with a molecular weight as high as 450,000 grams / mole.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Source reagent compositions and method for forming metal films on a substrate by chemical vapor deposition
InactiveUS7323581B1Group 4/14 organic compounds without C-metal linkagesCobalt organic compoundsCoordination complexChemical vapor deposition
A metalorganic complex composition comprising a metalorganic complex selected from the group consisting of: metalorganic complexes comprising one or more metal central atoms coordinated to one or more monodentate or multidentate organic ligands, and complexed with one or more complexing monodentate or multidentate ligands containing one or more atoms independently selected from the group consisting of atoms of the elements C, N, H, S, O and F; wherein when the number of metal atoms is one and concurrently the number of complexing monodentate or multidentate ligands is one, then the complexing monodentate or multidentate ligand of the metalorganic complex is selected from the group consisting of beta-ketoiminates, beta-diiminates, C2-C10 alkenyl, C2-C15 cycloalkenyl and C6-C10 aryl.
Owner:ENTEGRIS INC
Method for catalytic polymerization of alpha-olefin monomers using an ultra-high activity non-metallocene pre-catalyst
InactiveUS20020019503A1High molecular weightLow molecularGroup 4/14 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsMetal chelateAlpha-olefin
A method for catalytic polymerization of alpha-olefin monomers using an ultra-high activity non-metallocene pre-catalyst featuring an amine bis(phenolate) ligand-metal chelate, having general formulas of [{(O)1R1R2R3R4(C6)1(CH2)1(RnY-T)N(CH2)2(C6)2R5R6R7R8(O)2}MX1X2] and [{(O)1R1R2R3R4(C6)1(CH2)1(RnY-T)N(CH2)2(C6)2R5R6R7R8(O)2}MX3], where, M, O, and N, are metal, oxygen, and, nitrogen; X1, X2, and X3, are ligands bonded to M; R1 through R8 are radicals; (RnY-T) is an optional non-donor or donor group bonded to N. The relatively stable and simply synthesized pre-catalyst is activated by a co-catalyst under mild reaction conditions, producing exceptionally reactive polymerization of a wide variety of alpha-olefin monomers, and forming a variety of poly(alpha-olefin) products, having high molecular weight and low molecular weight distribution (PDIs close to 1). Living polymerization is performed at or above room temperature, along with achieving block co-polymerization of alpha-olefin monomers at room temperature, and producing polymers and oligomers having a wide range of molecular weights. The catalyst formed during reaction remains "alive' for as long as 31 hours, for producing a polymer with a molecular weight as high as 450,000 grams / mole.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Zirconium, hafnium, titanium, and silicon precursors for ald/cvd
InactiveUS20100112211A1Group 4/14 organic compounds without C-metal linkagesFibre treatmentHafniumTitanium
Zirconium, hafnium, titanium and silicon precursors useful for atomic layer deposition (ALD) and chemical vapor deposition (CVD) of corresponding zirconium-containing, hafnium-containing, titanium-containing and silicon-containing films, respectively. The disclosed precursors achieve highly conformal deposited films characterized by minimal carbon incorporation.
Owner:ADVANCED TECH MATERIALS INC
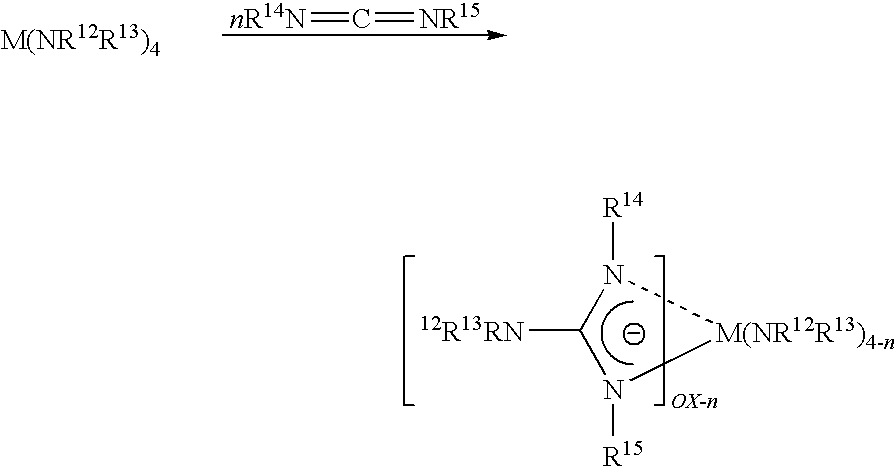
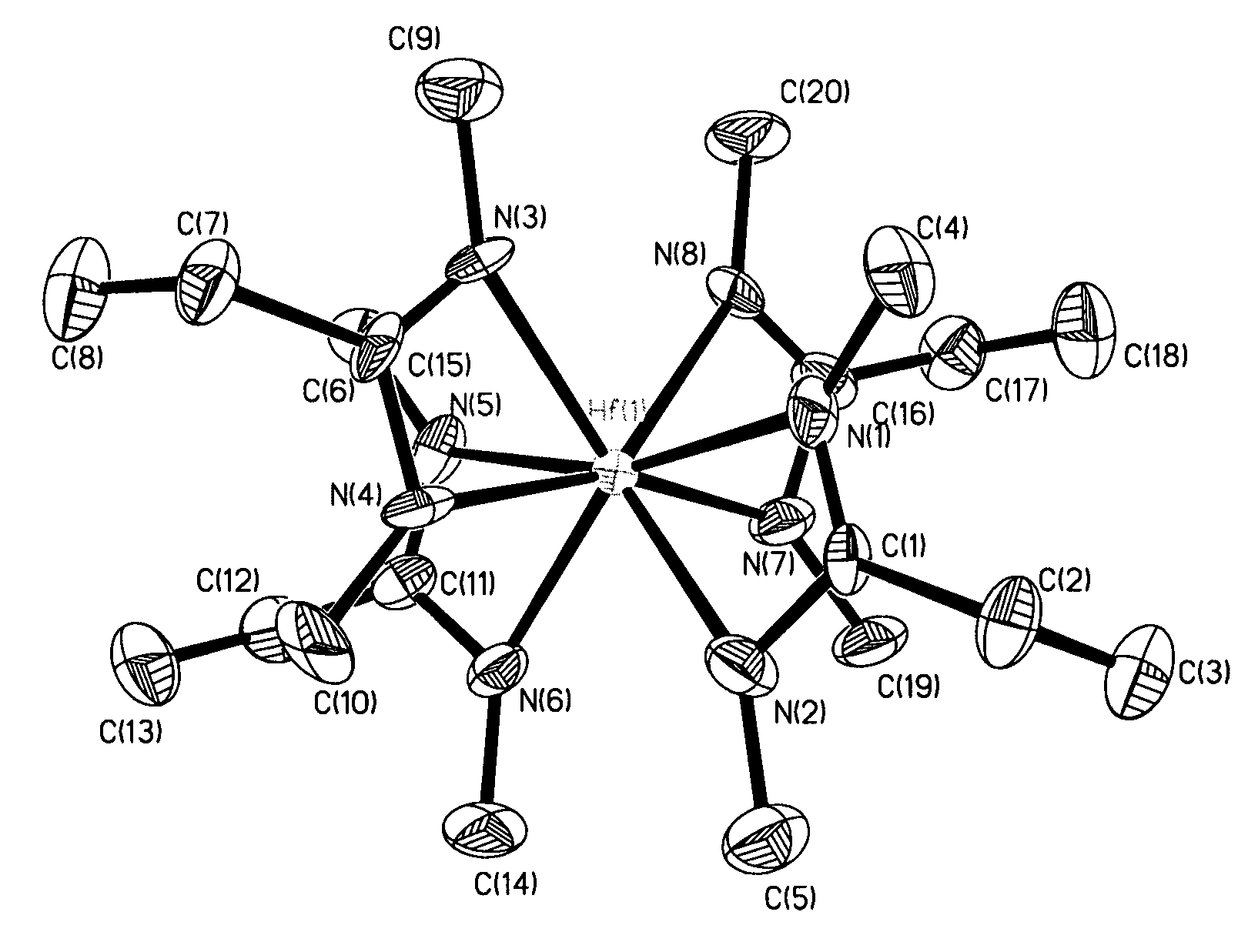
Metal (IV) tetra-amidinate compounds and their use in vapor deposition
ActiveUS7638645B2Group 4/14 organic compounds without C-metal linkagesGroup 8/9/10/18 element organic compoundsGas phaseNiobium
Metal(IV) tetrakis(N,N′-dialkylamidinates) were synthesized and characterized. Exemplary metals include hafnium, zirconium, tantalum, niobium, tungsten, molybdenum, tin and uranium. These compounds are volatile, highly stable thermally, and suitable for vapor deposition of metals and their oxides, nitrides and other compounds.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Polydentate heteroatom ligand containing metal complexes, catalysts and methods of making and using the same
ActiveUS8071701B2Low melting pointHigh molecular weightGroup 4/14 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsCoordination polymerizationHeteroatom
Metal complexes comprising certain polydentate heteroatom containing ligands, catalysts, and coordination polymerization processes employing the same are suitably employed to prepare polymers having desirable physical properties.
Owner:DOW GLOBAL TECH LLC +1
Precursors for cvd/ald of metal-containing films
InactiveUS20100018439A1Improve coordinationGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsGas phaseElectron donor
Precursors useful for vapor phase deposition processes, e.g., CVD / ALD, to form metal-containing films on substrates. The precursors include, in one class, a central metal atom M to which is coordinated at least one ligand of formula (I):wherein:R1, R2 and R3 are each independently H or ogano moieties; andG1 is an electron donor arm substituent that increases the coordination of the ligand to the central metal atom M;wherein when Ga is aminoalkyl, the substituents on the amino nitrogen are not alkyl, fluoroalkyl, cycloaliphatic, or aryl, and are not connected to form a ring structure containing carbon, oxygen or nitrogen atoms. Also disclosed are ketoester, malonate and other precursors adapted for forming metal-containing films on substrates, suitable for use in the manufacture of microelectronic device products such as semiconductor devices and flat panel displays.
Owner:ENTEGRIS INC
Composition and method for bleaching a substrate
InactiveUS20020010120A1Improve stabilityOrganic detergent compounding agentsGroup 4/14 organic compounds without C-metal linkagesPhotochemistryPeroxide
The invention relates to catalytically bleaching substrates, especially laundry fabrics, with a bleaching composition and a peroxyl source.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
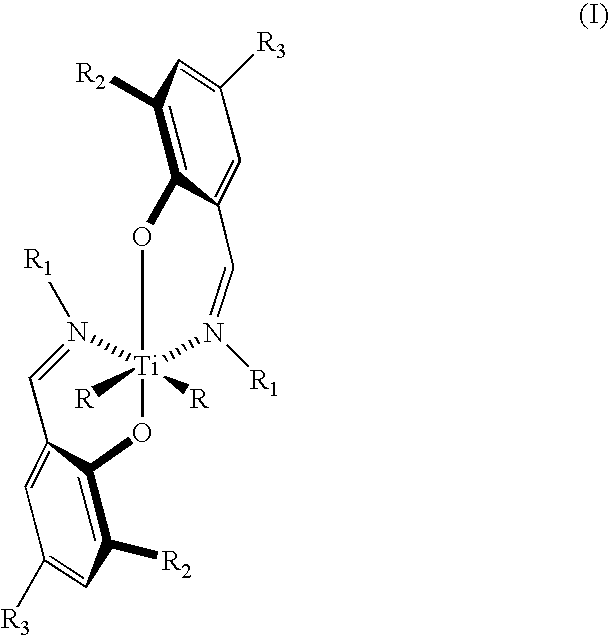
Bis (salicylaldiminato) titanium complex catalysts, highly syndiotactic polypropylene by a chain-end control mechanism, block copolymer containing this
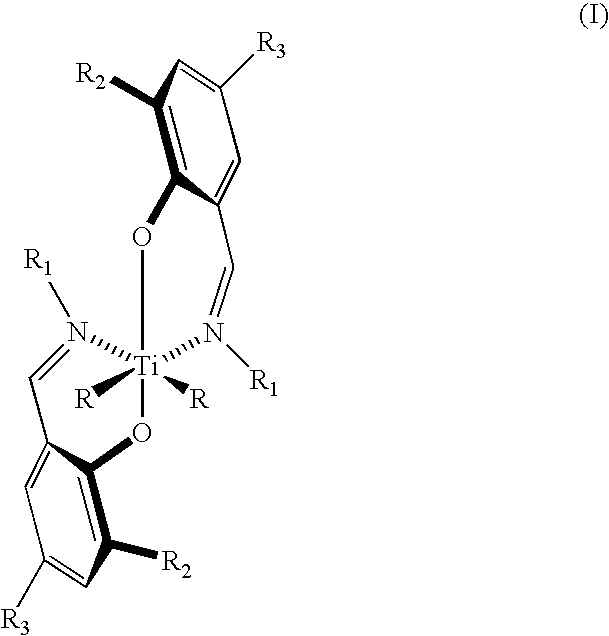
InactiveUS7119154B2Group 4/14 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsOligomerNitrogen
Bis(salicylaldiminato)titanium complex with optionally substituted phenyl or cyclohexyl on nitrogen catalyzes highly syndiospecific polymerization of propylene. Syndiotactic polypropylene with defects of the type rmr having [rrrr] content greater than 0.70 and block copolymer containing block(s) of the syndiotactic polypropylene and block(s) of poly(ethylene-co-propylene) and / or poly(alpha-olefin-co-propylene) are obtained. Certain of the catalysts provide living polymerization. Living olefin polymers and olefin terminated oligomers and polymers are also products.
Owner:CORNELL RES FOUNDATION INC
Phenol-heterocyclic ligands, metal complexes, and their uses as catalysts
InactiveUS20060135713A1High activityHigh melting pointGroup 4/14 organic compounds without C-metal linkagesGroup 8/9/10/18 element organic compounds1-OctenePHENOL LIQUID
Ligands, compositions, and metal-ligand complexes that incorporate phenol-heterocyclic compounds are disclosed that are useful in the catalysis of transformations such as the polymerization of monomers into polymers. The catalysts have high performance characteristics, including high comonomer incorporation into ethylene / olefin copolymers, where such olefins are for example, 1-octene, propylene or styrene. The catalysts particularly polymerize styrene to form polystyrene.
Owner:FREESLATE
Active non-metallocene pre-catalyst and method for tactic catalytic polymerization of alpha-olefin monomers
InactiveUS6632899B2Group 4/14 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsPDAT enzymeOxygen
Owner:RAMOT AT TEL AVIV UNIV LTD
Precursor compounds for metal oxide film deposition and methods of film deposition using the same
InactiveUS20030118725A1Group 4/14 organic compounds without C-metal linkagesLignin derivativesArylAlkoxy group
Disclosed are precursor compounds useful for deposition of a metal oxide film, which is applied to a capacitor of electronic elements such as semiconductor, to a substrate such as silicon, and a process for depositing such film. The precursor compounds have the formula (1): wherein M is a metal element selected from the Groups 2A, 3A, 4A, 5A, 3B, 4B, 5B and 8B of the Periodic Table; x and y are integers of 1 to 4, provided that the sum of x and y is an integer of 2 to 5; R is hydrogen, fluoro, alkyl group containing 1 to 4 carbon atoms, perfluoroalkyl group or perfluoroaryl group; R1 and R2 independently are an alkyl group containing 1 to 8 carbon atoms, perfluoroalkyl group or alkoxyalkyl group; A is perfluoroalkylalkoxy or alkoxyalkylalkoxy having the formula (2:-O-(CHR3)l-(CR4R5)m-R6 (2)wherein R3 is hydrogen, fluoro, or alkyl or perfluoroalkyl having 1 to 4 carbon atoms; R4 and R5 are the same or different and are hydrogen, fluoro, or alkyl or alkoxy having 1 to 4 carbon atoms; R6 is alkyl or perfluoroalkoxy having 1 to 4 carbon atoms, or an amide group; l and m are integers of 0 to 4; L is a Lewis base; and n is an integer of 0 or more.
Owner:SHIPLEY CO LLC
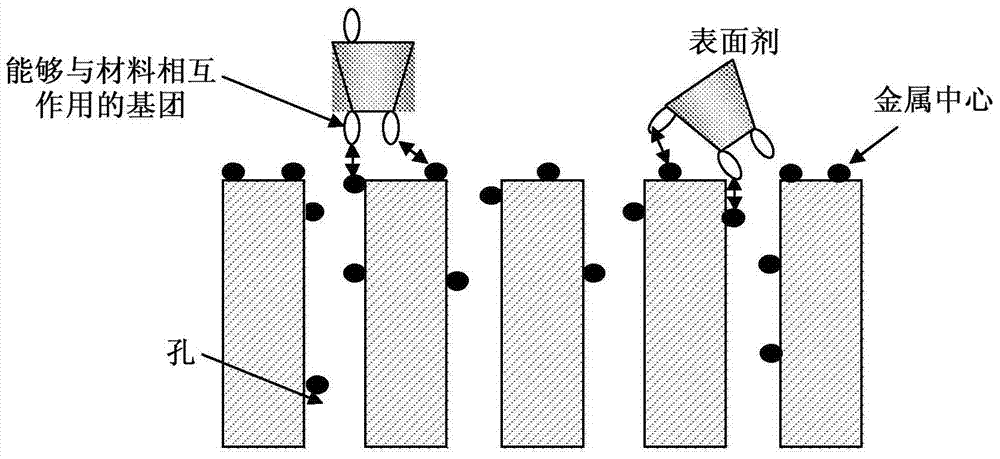
Improved organic-inorganic hybrid solid having a modified outer surface
ActiveCN104718214AImprove thermal stabilityAntibacterial agentsPowder deliveryBiocompatibility TestingBULK ACTIVE INGREDIENT
The present invention concerns metal-organic hybrid solids having a modified outer surface. These solids can be used, for example, for the storage and vectoring of molecules of interest such as pharmaceutically active ingredients, compounds of interest in cosmetics and markers, for example contrast agents. These solids have good results in terms of active drug loading capacities, biocompatibility, stability and controlling the release of the active ingredients encapsulated.
Owner:CENT NAT DE LA RECHERCHE SCI
Preparation method of zirconium-based microporous coordination polymer
ActiveCN105777791APromote formationGuaranteed qualityGroup 4/14 organic compounds without C-metal linkagesCarboxylic acidSolvent
The invention provides a preparation method of a zirconium-based microporous coordination polymer. The preparation method includes: utilizing organic monocarboxylic acid which is simple as a regulator. The organic monocarboxylic acid can promote forming of a Zr6O4(OH)4(CO2)12 cluster, so that influence of water contained on Zr6O4(OH)4 is avoided, forming of Zr-MOF is facilitated, and quality of Zr-MOF is guaranteed. ZrO2+salt containing crystal water, such as ZrOCl2.8H2O is selected as zirconium salt, so that cost can be lowered, water in a trace amount can be guided in to promote generation of the Zr6O4(OH)4(CO2)12 cluster. A periodic adding mode is adopted, so that generation of a reactant can be accelerated, technical process can be shortened effectively, solvent utilization rate is increased, and reaction efficiency is improved finally; more importantly, product quality is guaranteed. The zirconium-based microporous coordination polymer is uniform in grain size, high in crystallinity, complete in shape and suitable for gas storage and separation and serving as a carrier. By using the method, quality such as crystallinity, specific surface area and grain size uniformity of Zr-MOF can be guaranteed, and the zirconium-based microporous coordination polymer is suitable for large-scale production.
Owner:李亚丰
Acid, solvent, and thermal resistant metal-organic frameworks
ActiveCN106029674ASemi-permeable membranesGroup 4/14 organic compounds without C-metal linkagesCatalytic methodSolid acid
The disclosure provides for thermal, solvent, and / or acid resistant metal organic frameworks and the use of these frameworks in devices and methods for gas separation, gas storage, and catalysis. The disclosure further provides for MOFs that are strong solid acids, and the use of these strong solid acid MOFs in catalytic devices and catalytic methods.
Owner:RGT UNIV OF CALIFORNIA
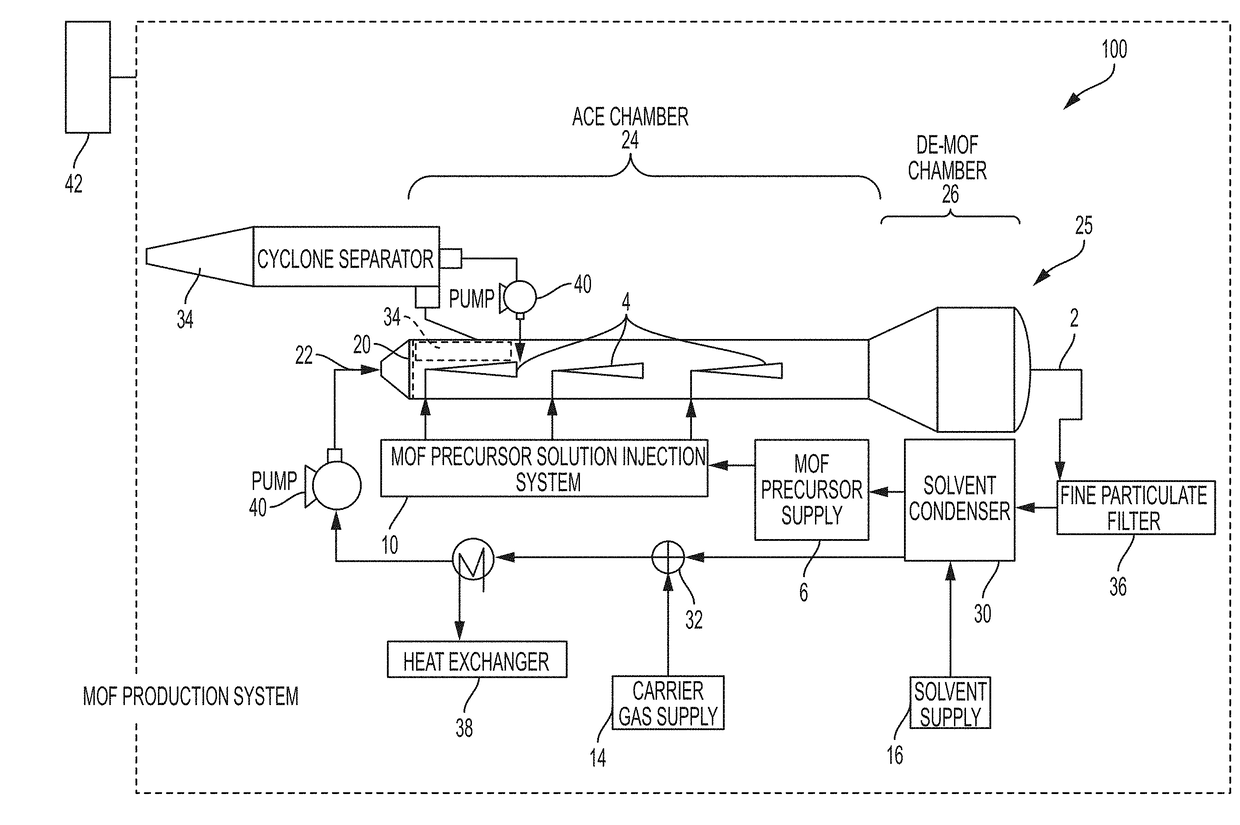
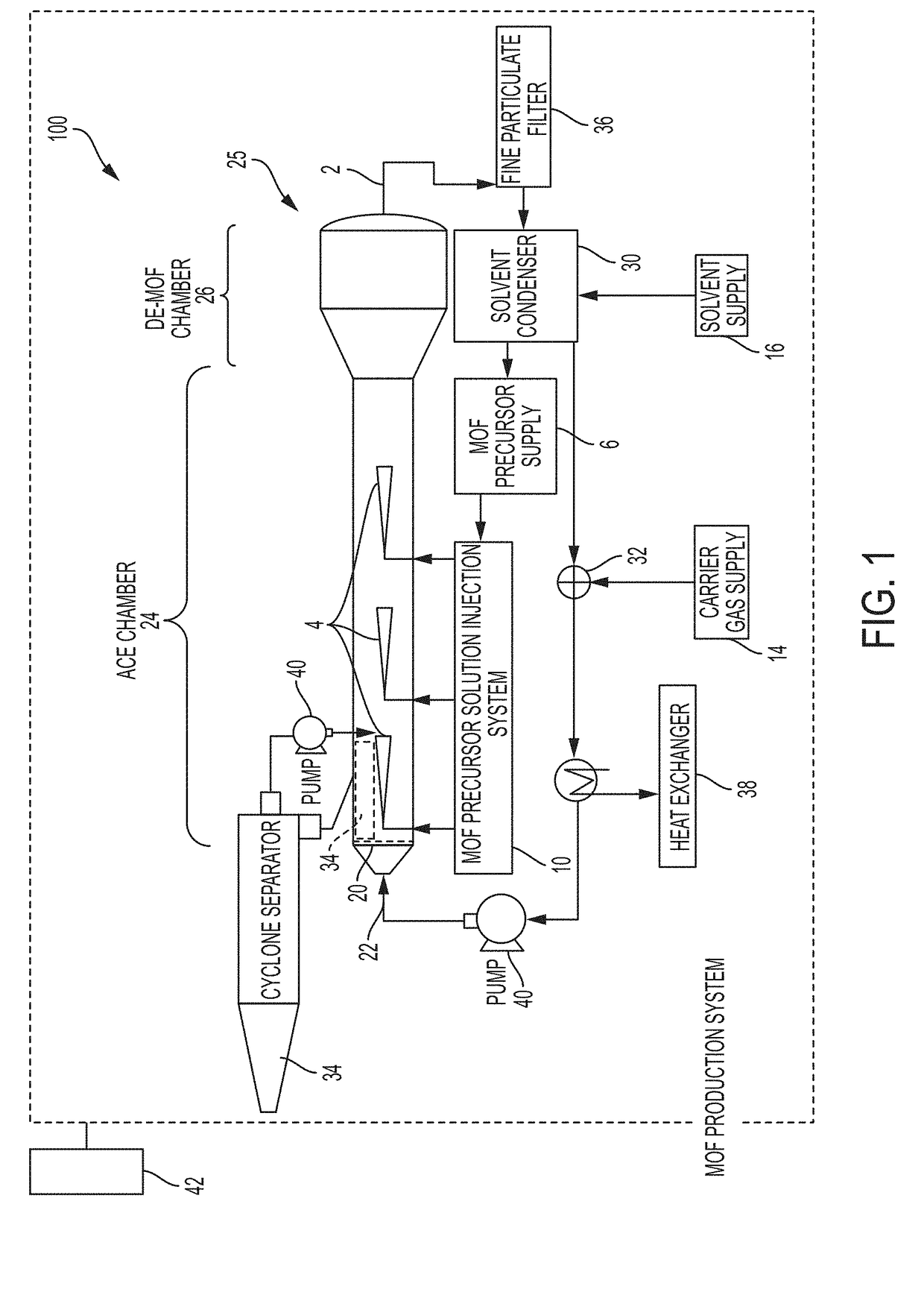
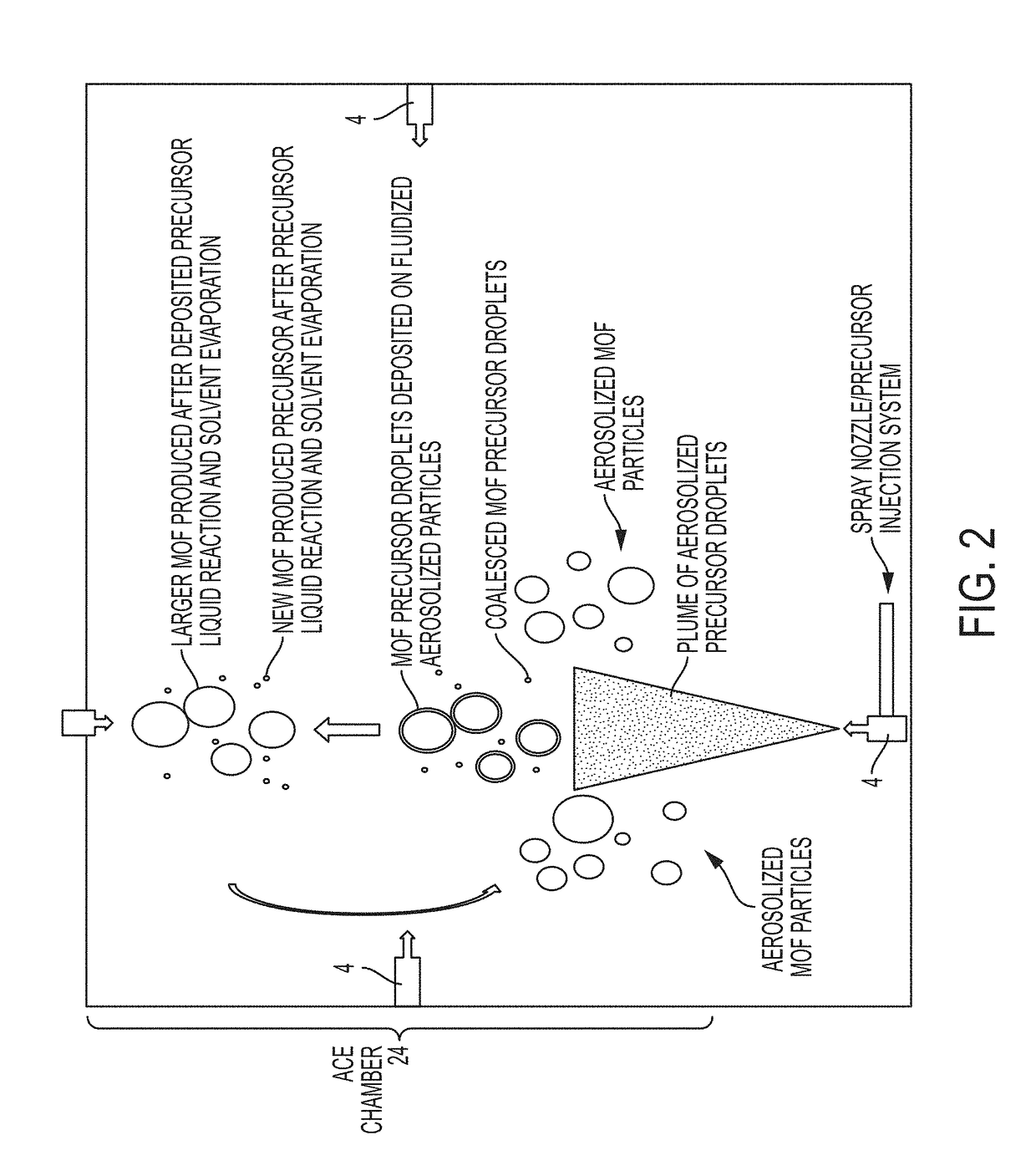
System and process for continuous and controlled production of metal-organic frameworks and metal-organic framework composites
ActiveUS20170361300A1Efficient scalable synthesisEffective and efficient MOF productCopper organic compoundsNickel organic compoundsMetal-organic frameworkMaterials science
Owner:BATTELLE MEMORIAL INST
Metal compound, material for thin film formation, and process of forming thin film
InactiveUS20070122947A1Group 4/14 organic compounds without C-metal linkagesSemiconductor/solid-state device manufacturingChemical reactionLiquid state
A metal compound represented by general formula (I): wherein R1, R2, R3, and R4 each represent an alkyl group having 1 to 4 carbon atoms; A represents an alkanediyl group having 1 to 8 carbon atoms; M represents a lead atom, a titanium atom or a zirconium atom; n represents 2 when M is a lead atom or 4 when M is a titanium or zirconium atom. The metal compound has a low melting point and is therefore deliverable in a liquid state, has a high vapor pressure and is therefore easy to vaporize, and, when mixed with other metal compound, undergoes no denaturation due to a chemical reaction. The metal compound is suitable as a material of thin film formation processes involving vaporization of a metal compound, such as CVD.
Owner:ADEKA CORP
Preparation of metal alkoxides
InactiveUS6355821B1Heat dissipationReduce thermal decompositionPlatinum group organic compoundsCopper oxides/halidesSolventEthanol
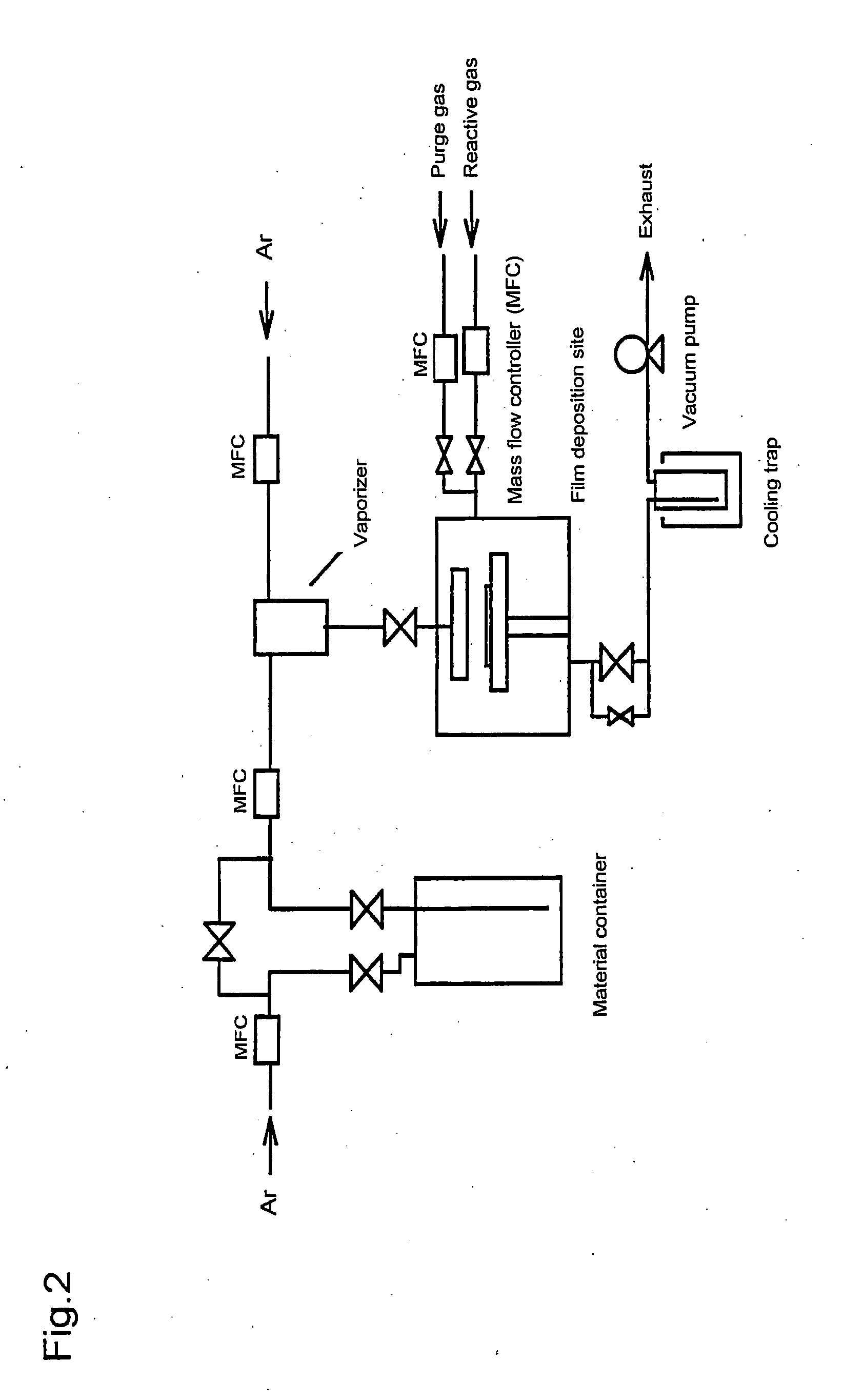
Methods of forming metal alkoxides and methods of forming precursor solutions of metal alkoxides suitable for the coating of glass in the manufacture of electrochromic devices are disclosed. The method of forming metal alkoxides involves dissolving the metal halide in an anhydrous solvent and reacting it with an alcohol and (together with the addition of the alcohol or subsequently) adding an epoxide, and then evaporating-off the volatile components of the reaction product to leave a solid metal alkoxide that is substantially free of halide. The alkoxide may then be dissolved in a solvent including an alcohol (preferably ethanol) containing a small proportion of water to produce a precursor solution suitable for coating glass, the coating then being hydrolyzed to form a sol-gel and then baked to remove volatile components and to yield a thin layer of metal oxide.
Owner:DYESOL PTY LTD
Popular searches
Titanium organic compounds Liquid/solution decomposition chemical coating Chemical vapor deposition coating Arsenic organic compounds Catalyst activation/preparation Group 3/13 element organic compounds Ether preparation by ester reactions Organic chemistry methods Sputtering coating Special surfaces
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com