Transition metal-catalyzed process for preparing N-aryl amine compounds
a metal catalyzed process and naryl amine technology, applied in the preparation of amino compounds, organic chemistry, carboxylic acid nitrile, etc., can solve the problems of inefficient or economically unattractive methods for producing naryl amines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
1-19
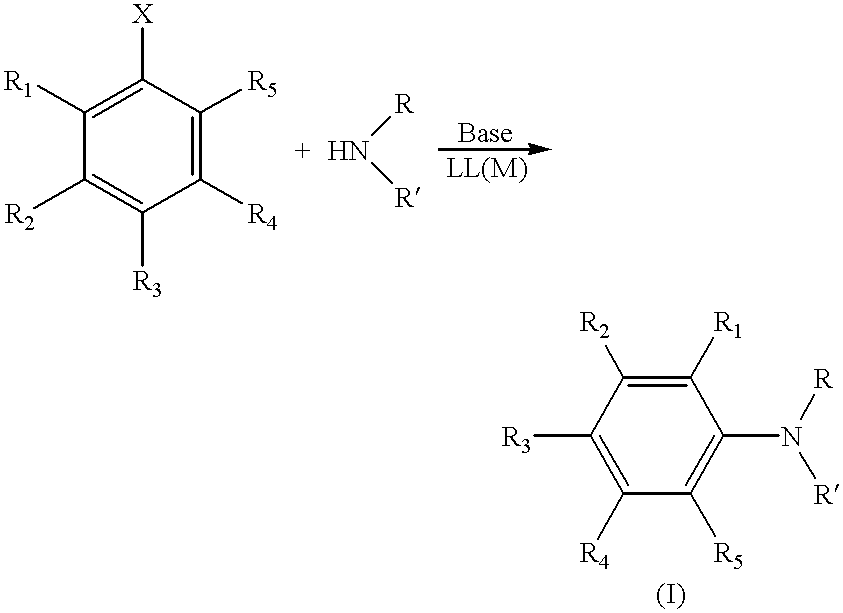
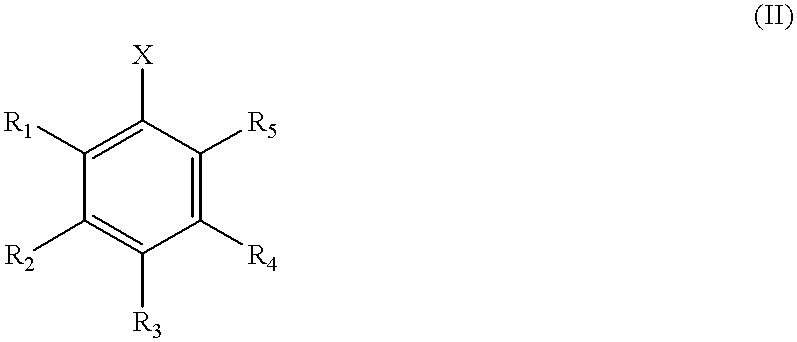
The known ligand DB.sup.t PF (1,1'-bis-(di-t-butylphosphino)ferrocene, Ligand 1) was evaluated in the amination reactions. DB.sup.t PF is air sensitive over long periods of time in solution, but can be handled and weighed in air. Table 1 summarizes the results with this ligand and others discussed below in the general reaction presented in Scheme I. In Table 1, yields are for pure isolated product and are an average of 2 runs on a 1 mmol scale using 0.5-1.0M concentrations, except for entry 15 (0.1 mmol scale).
The results of the amination reactions illustrate (1) remarkable rate enhancements for reactions with sterically hindered alkylphosphine ligands, (2) mild conditions for aminations of aryl chlorides, (3) amination of aryl tosylates, and (4) preparation of mixed alky arylamines in high yields by the metal-catalyzed amination of unactivated aryl chlorides with primary alkylamines.
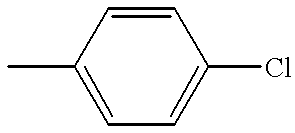
DB.sup.t PF leads to exceptionally high-yield amination of activated aryl chlorides with aniline...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| Ph | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com