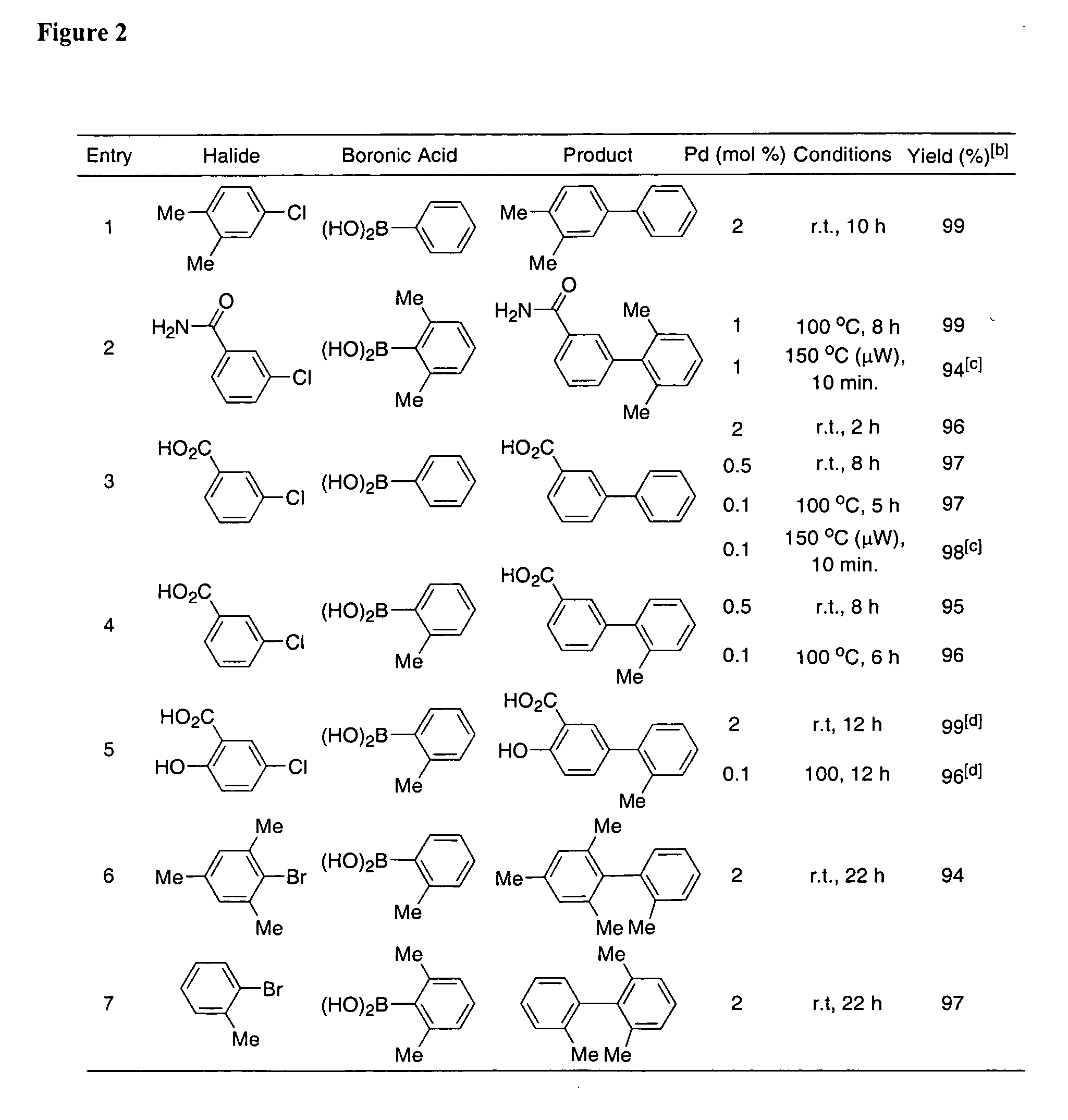
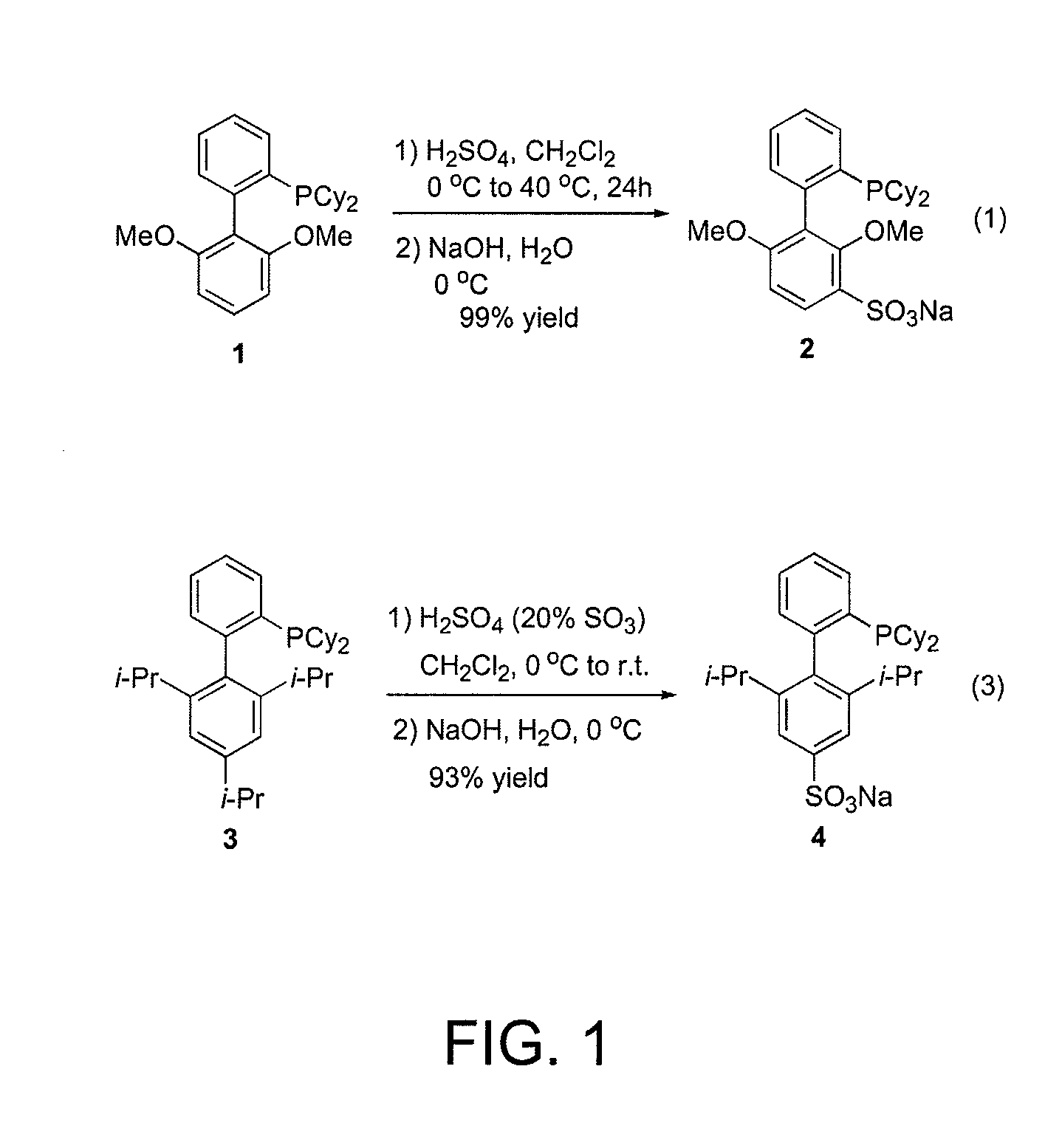
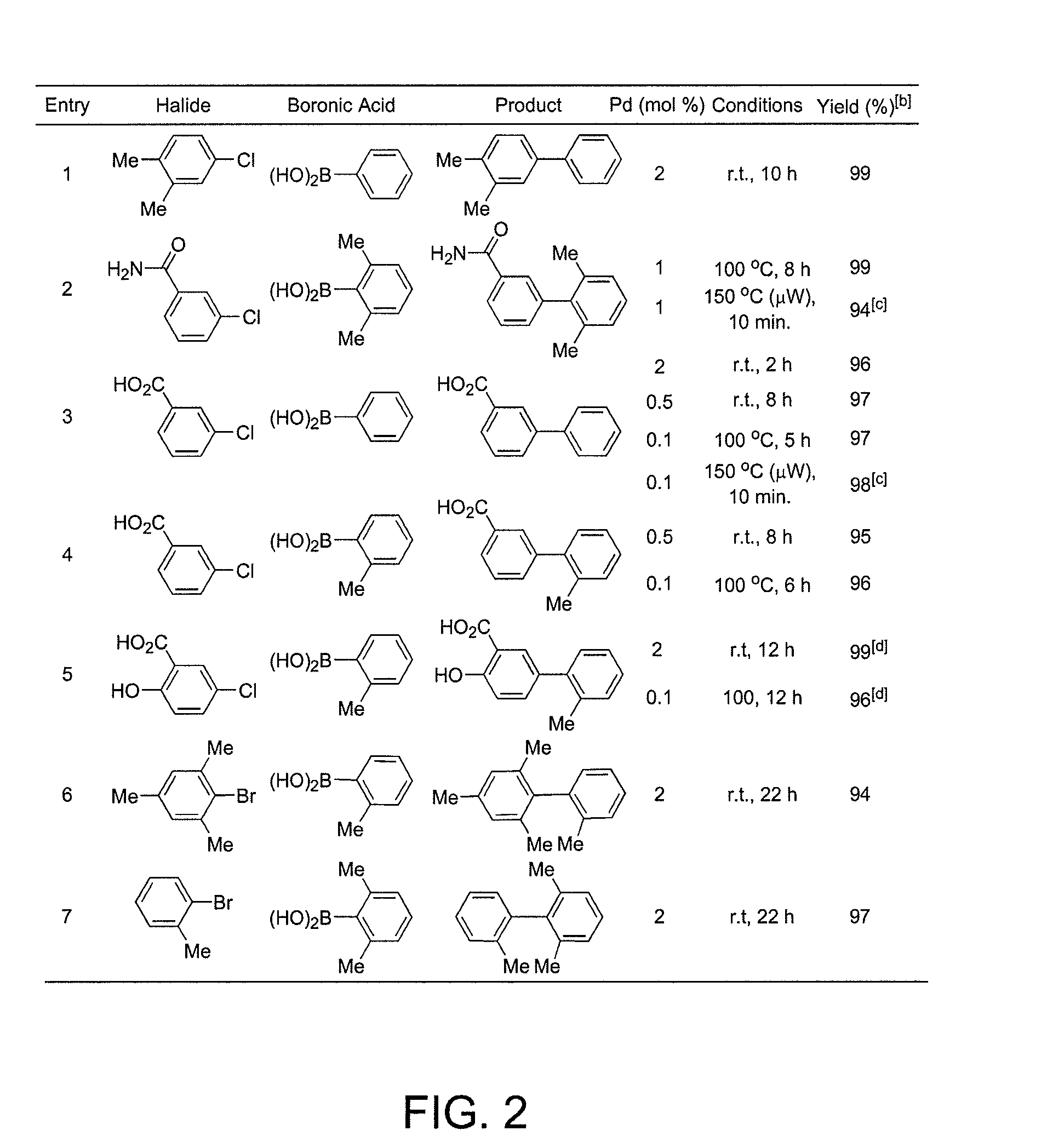
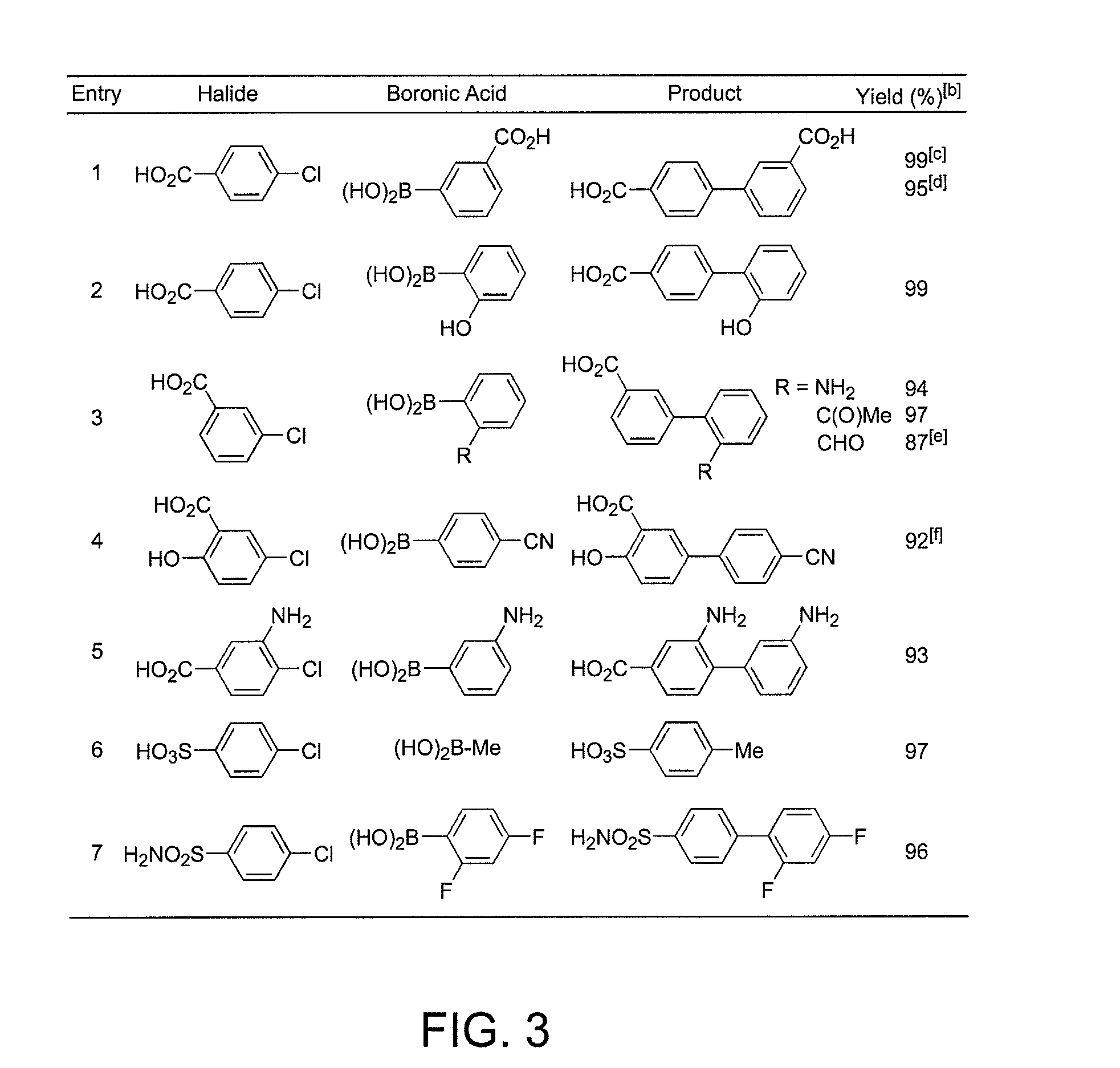
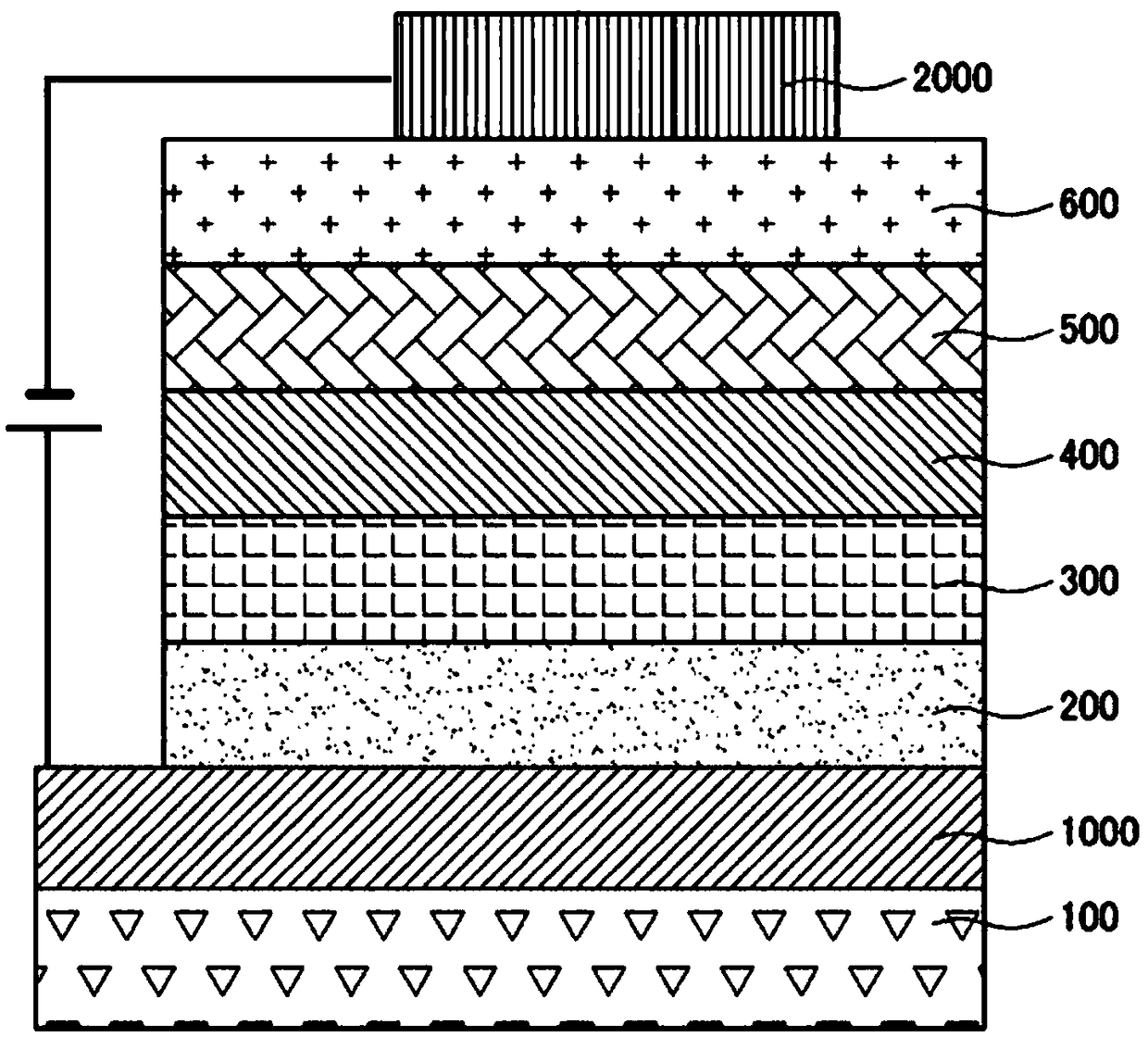
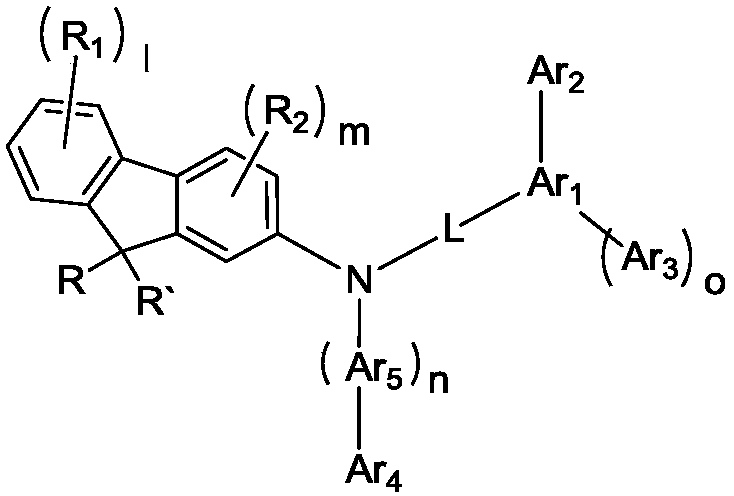
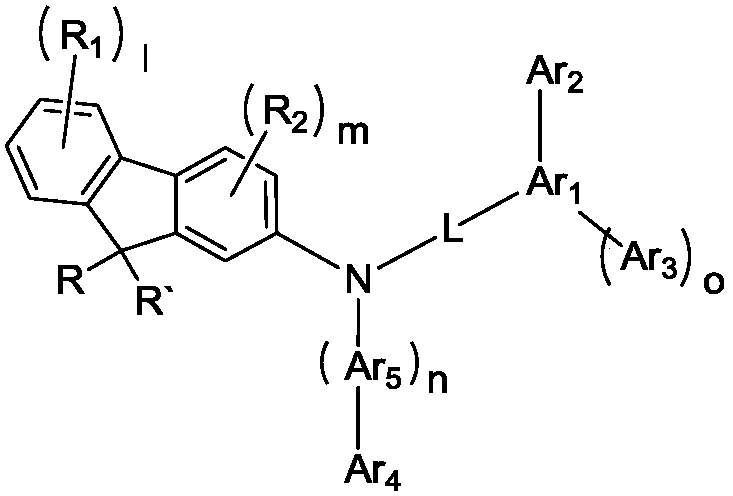
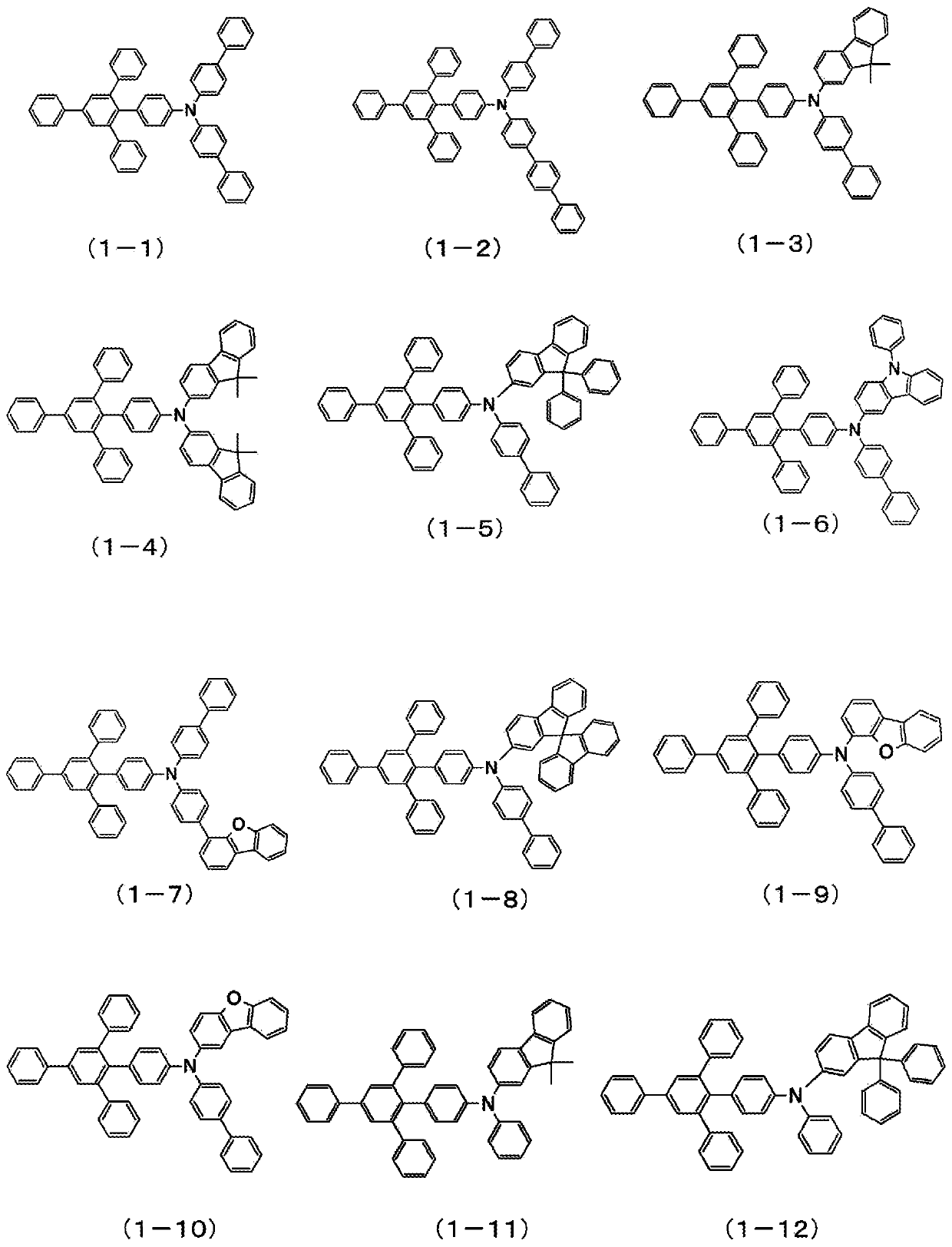
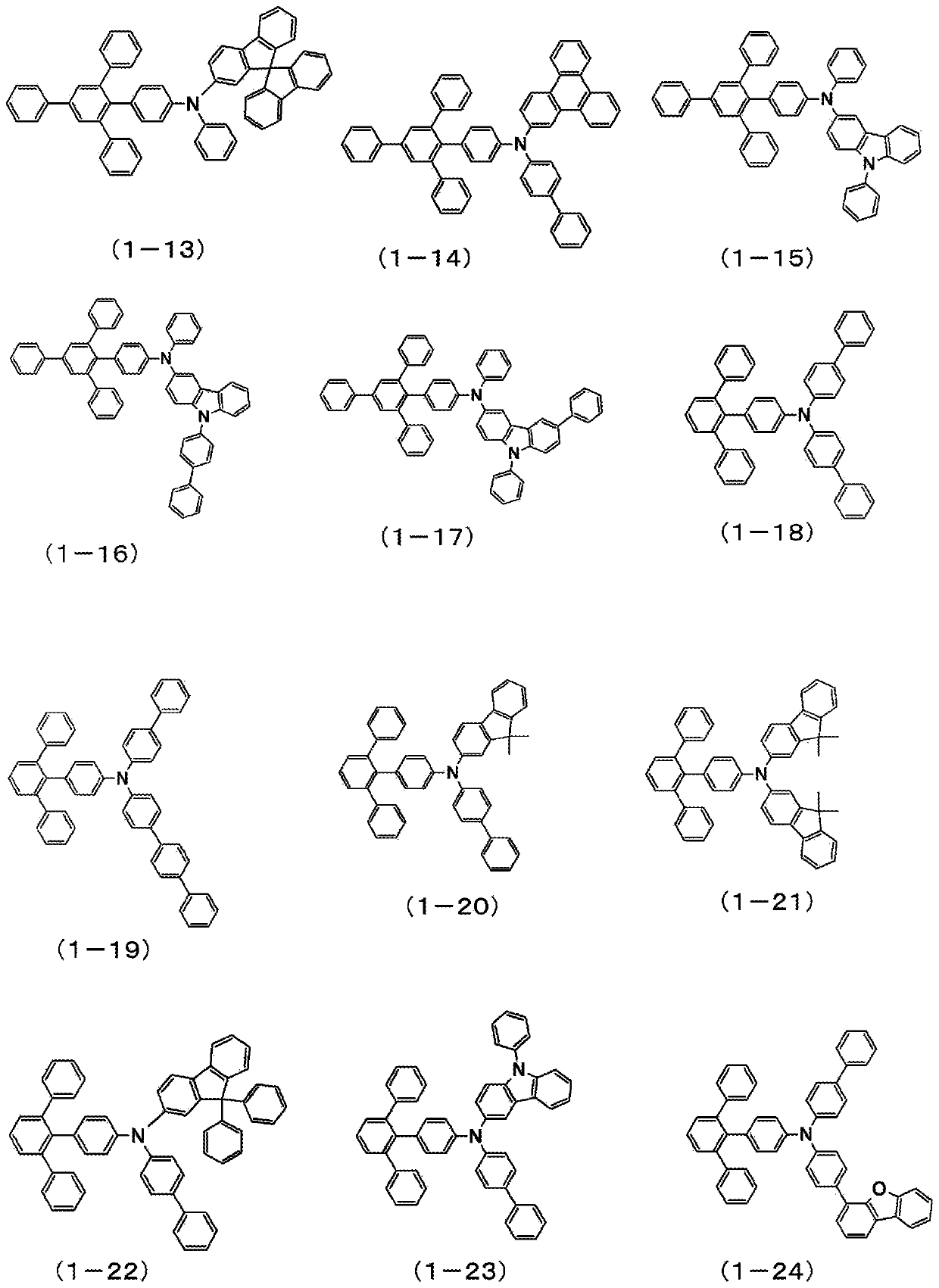
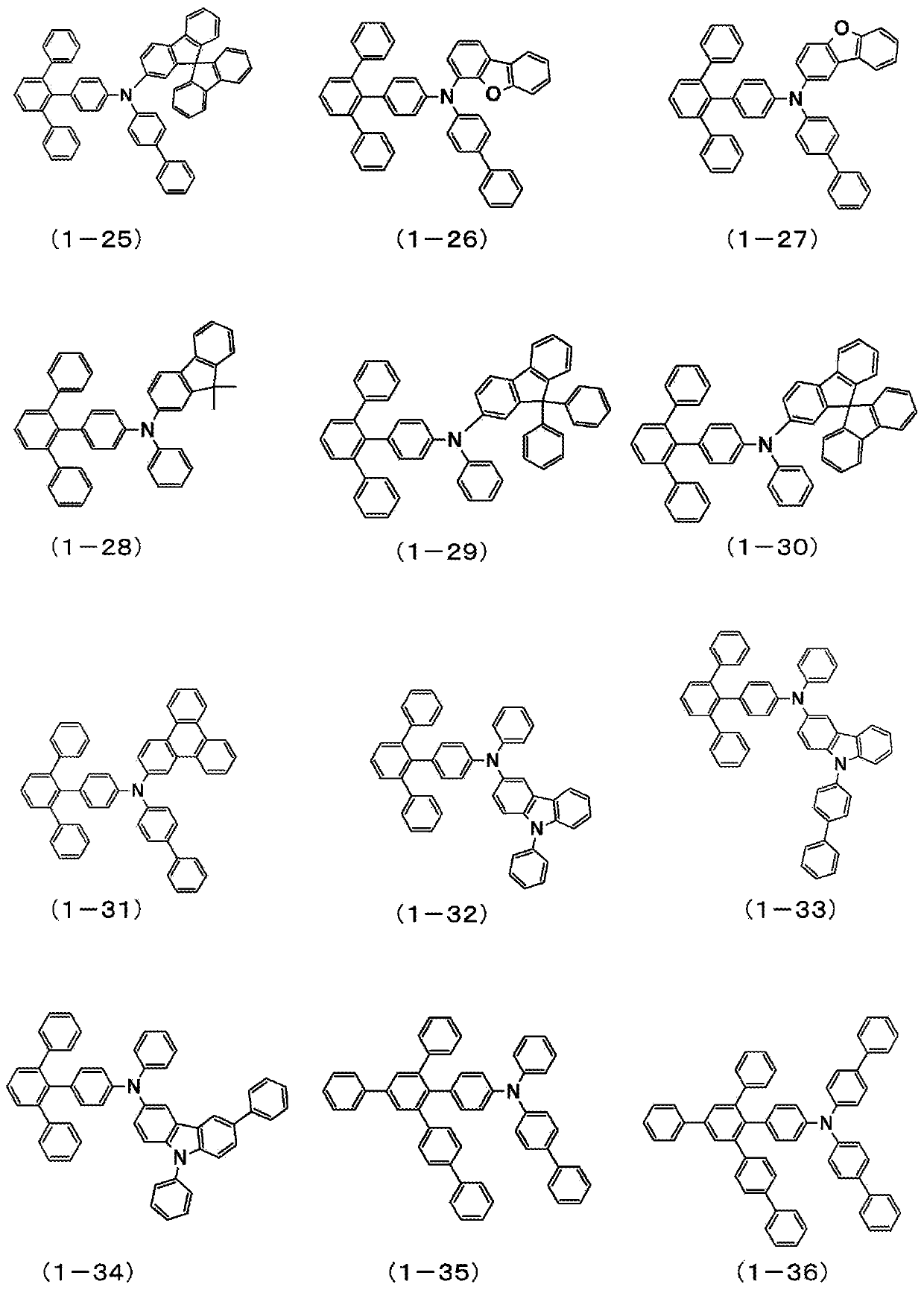
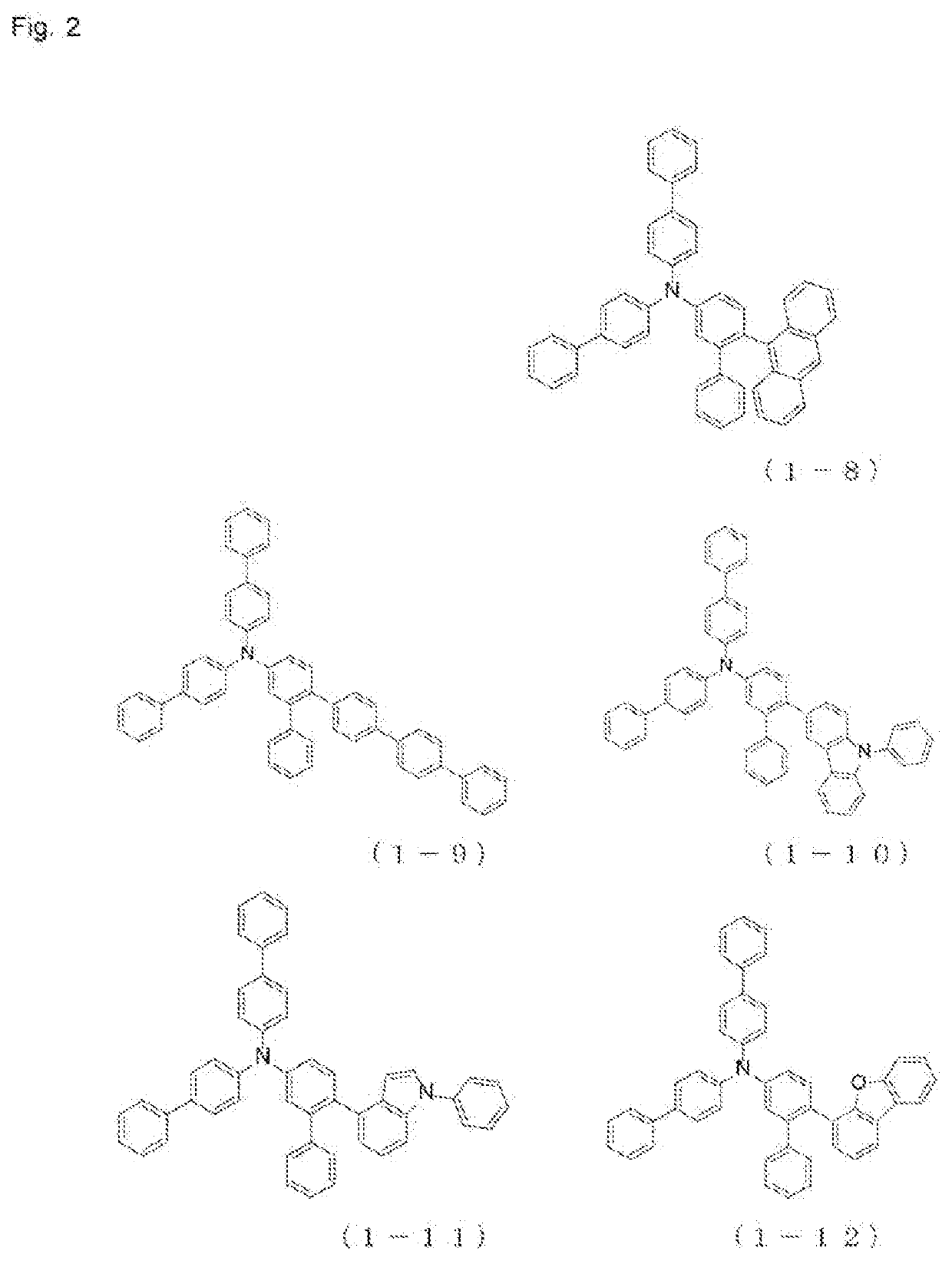
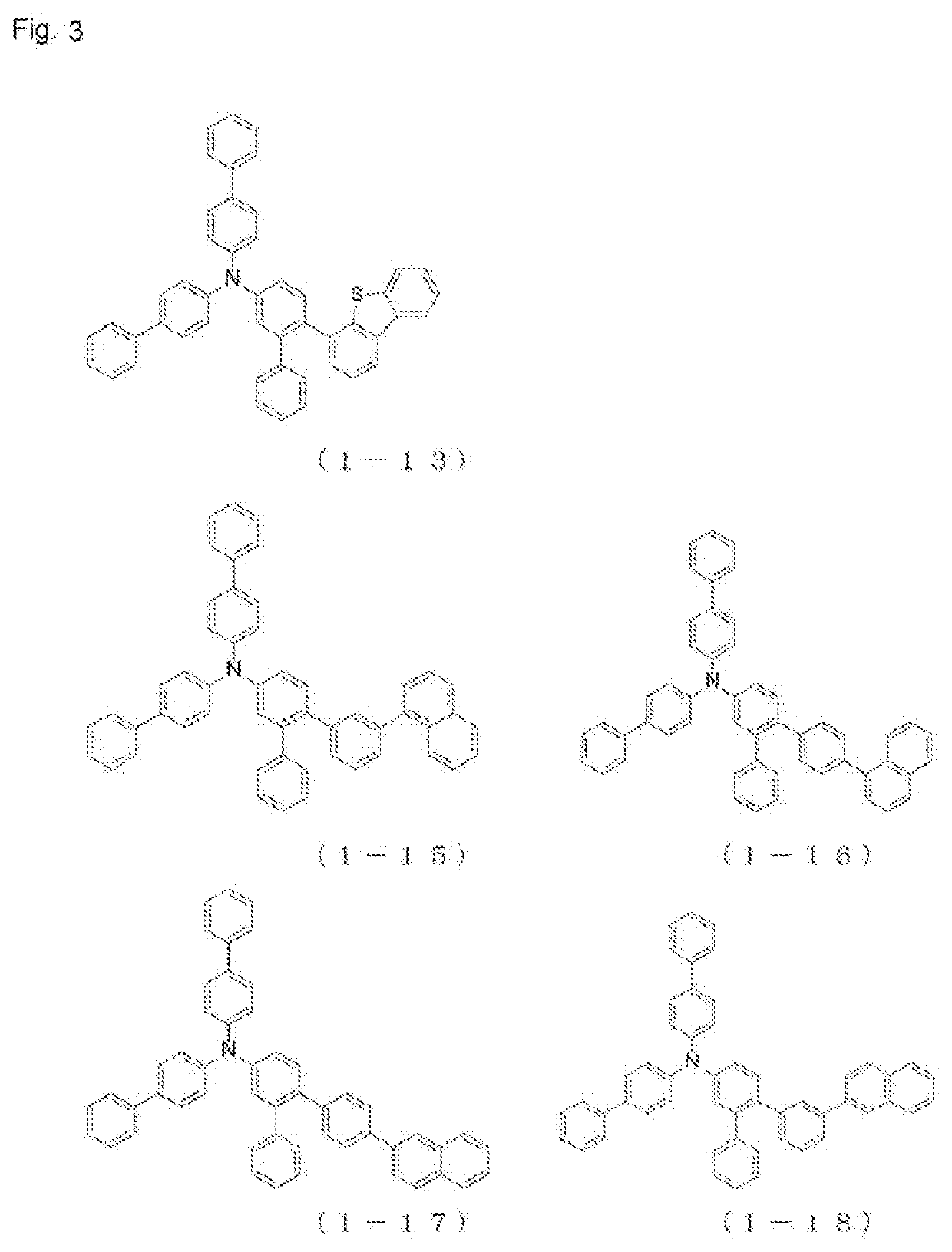
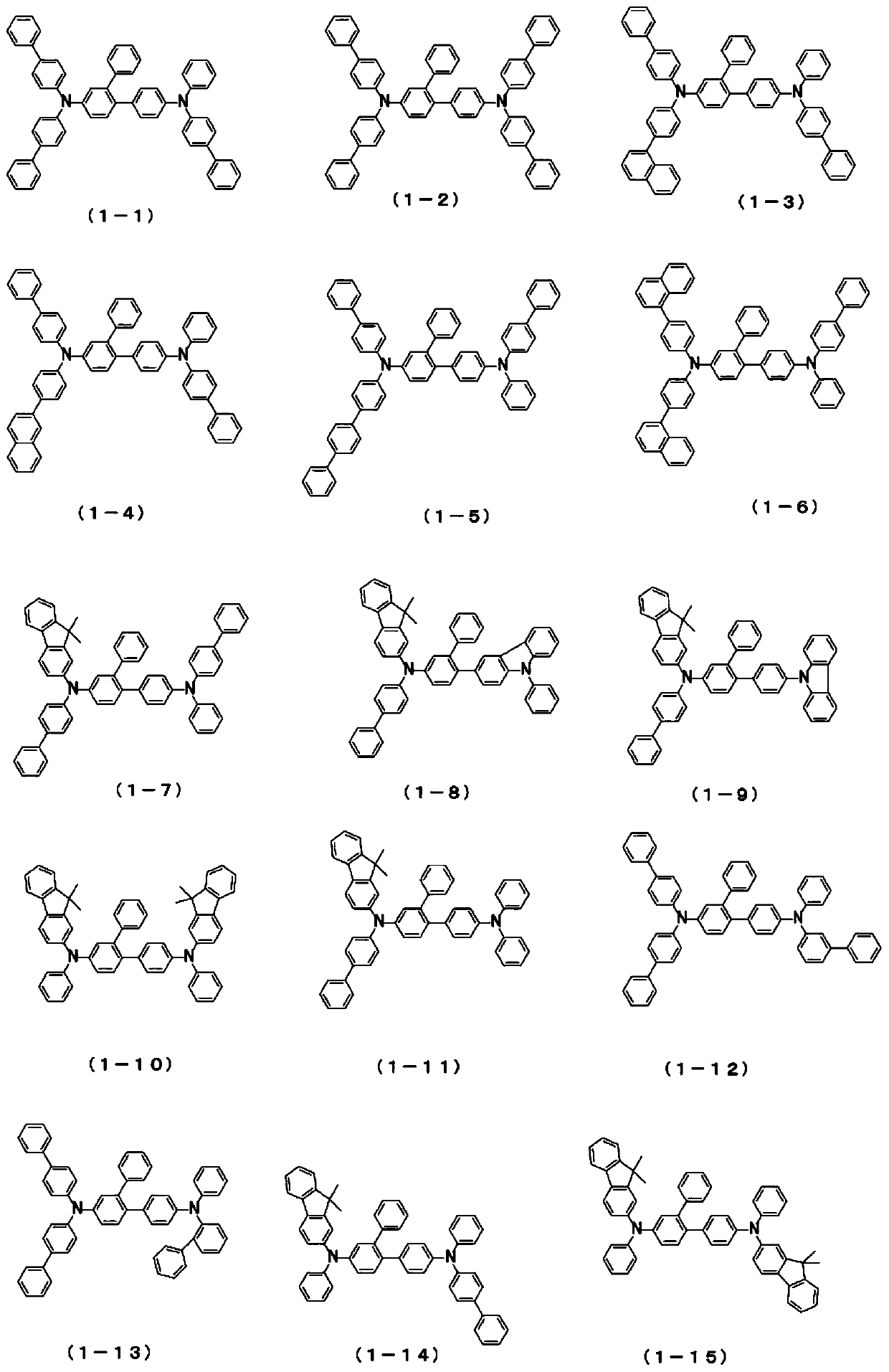
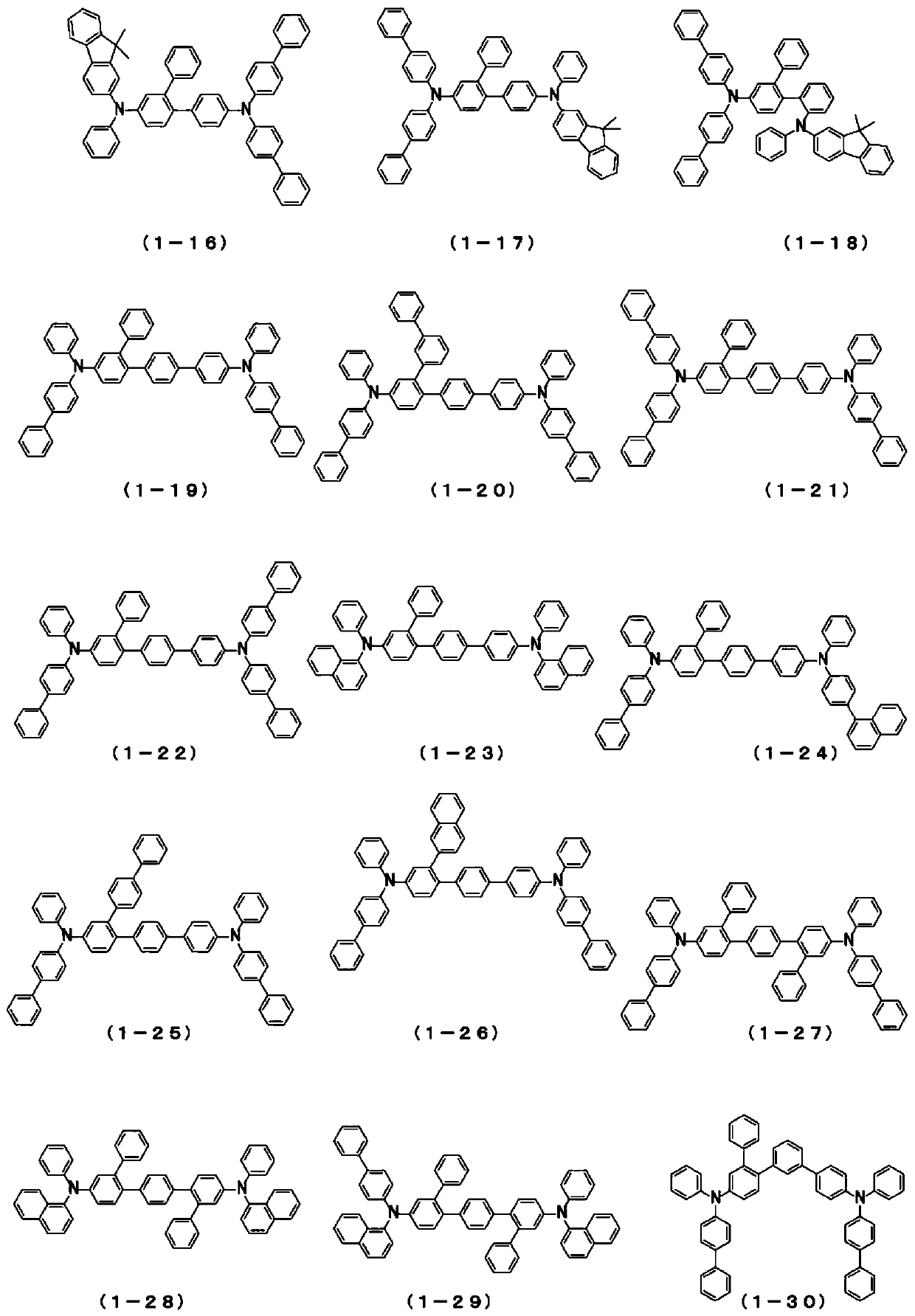
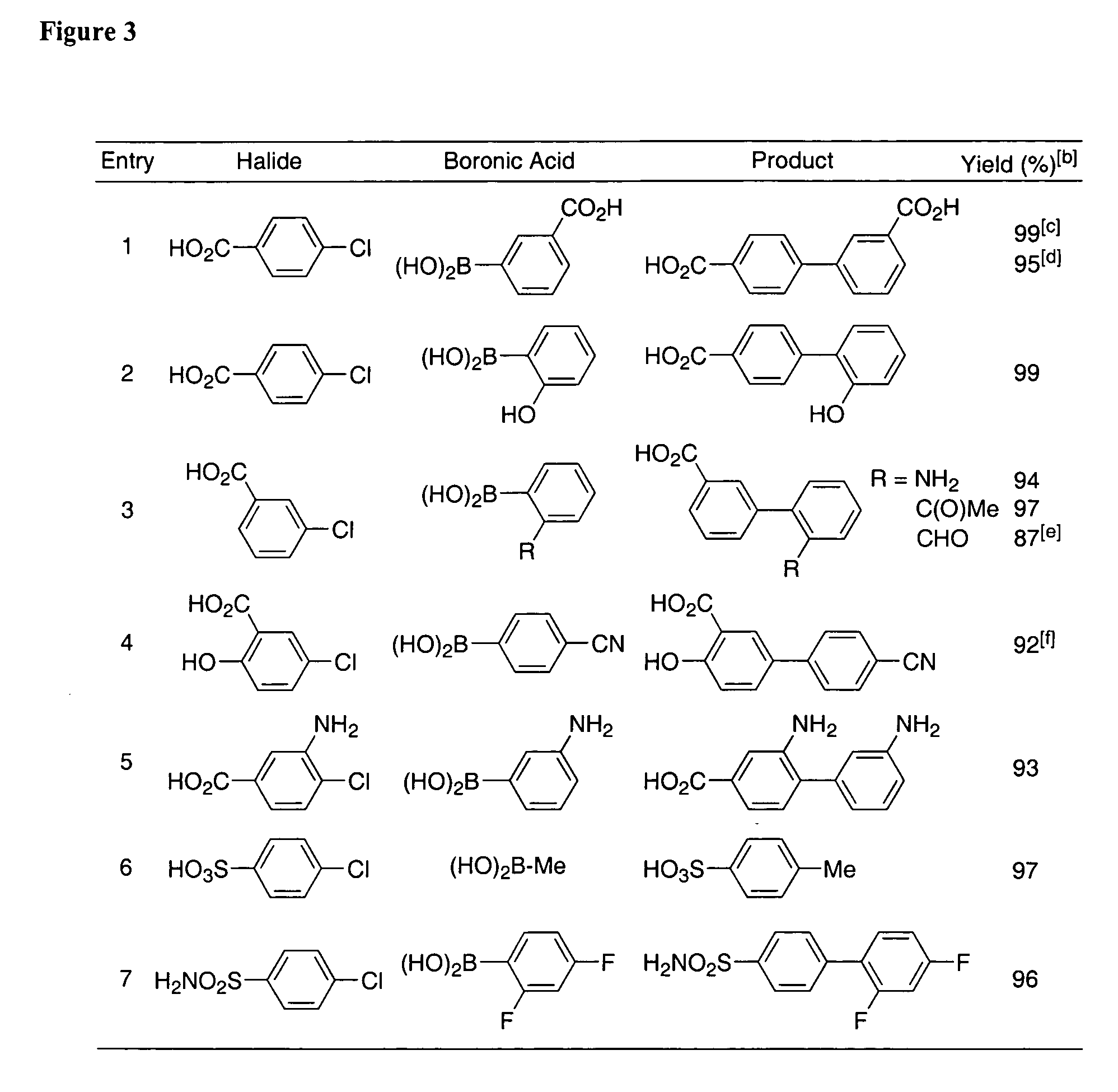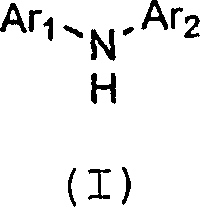Patents
Literature
51 results about "Aryl amination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transition metal-catalyzed process for preparing N-aryl amine compounds
InactiveUS6235938B1Carboxylic acid nitrile preparationOrganic compound preparationChelating ligandsPolymer
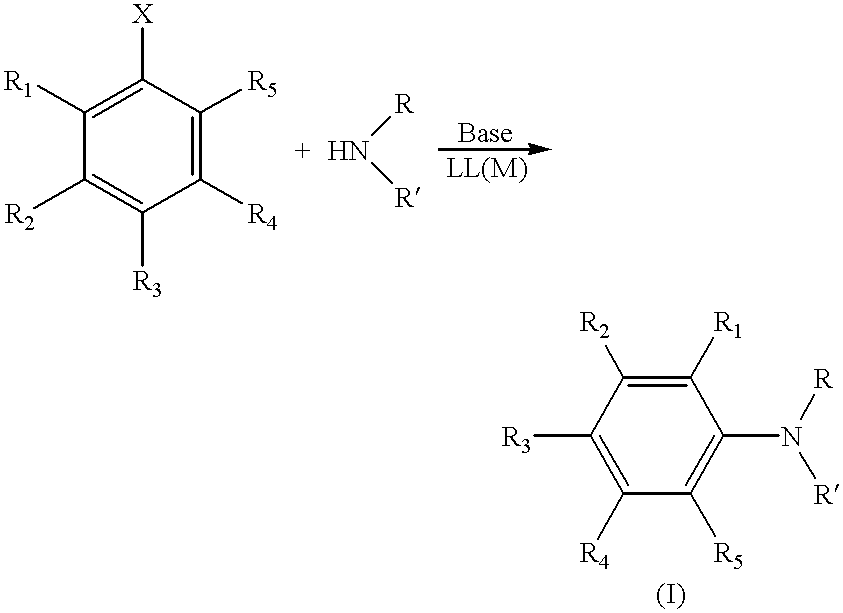
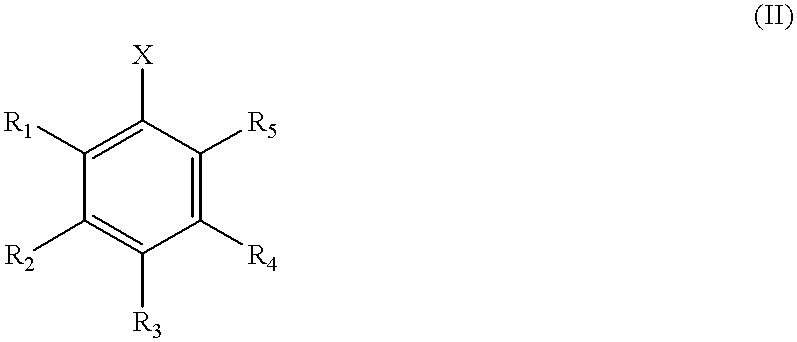
The present invention is directed to a process for the preparation of N-aryl amine compounds. The process of the present invention involves reacting a compound having an amino group with an arylating compound in the presence of a base and a transition metal catalyst under reaction conditions effective to form an N-aryl amine compound, the transition metal catalyst comprising a Group 8 metal and at least one chelating ligand selected from the group consisting of bisphosphines having at least one stearically hindered alkyl substituent. The formed products are valuable intermediates in the pharmaceutical and polymer fields.
Owner:MITSUBISHI RAYON CO LTD +1
Transition-metal-catalyzed carbon-nitrogen and carbon-carbon bond-forming reactions
ActiveUS20060173186A1More featureGroup 8/9/10/18 element organic compoundsCarboxylic acid amides preparationCarbon–carbon bondCoupling
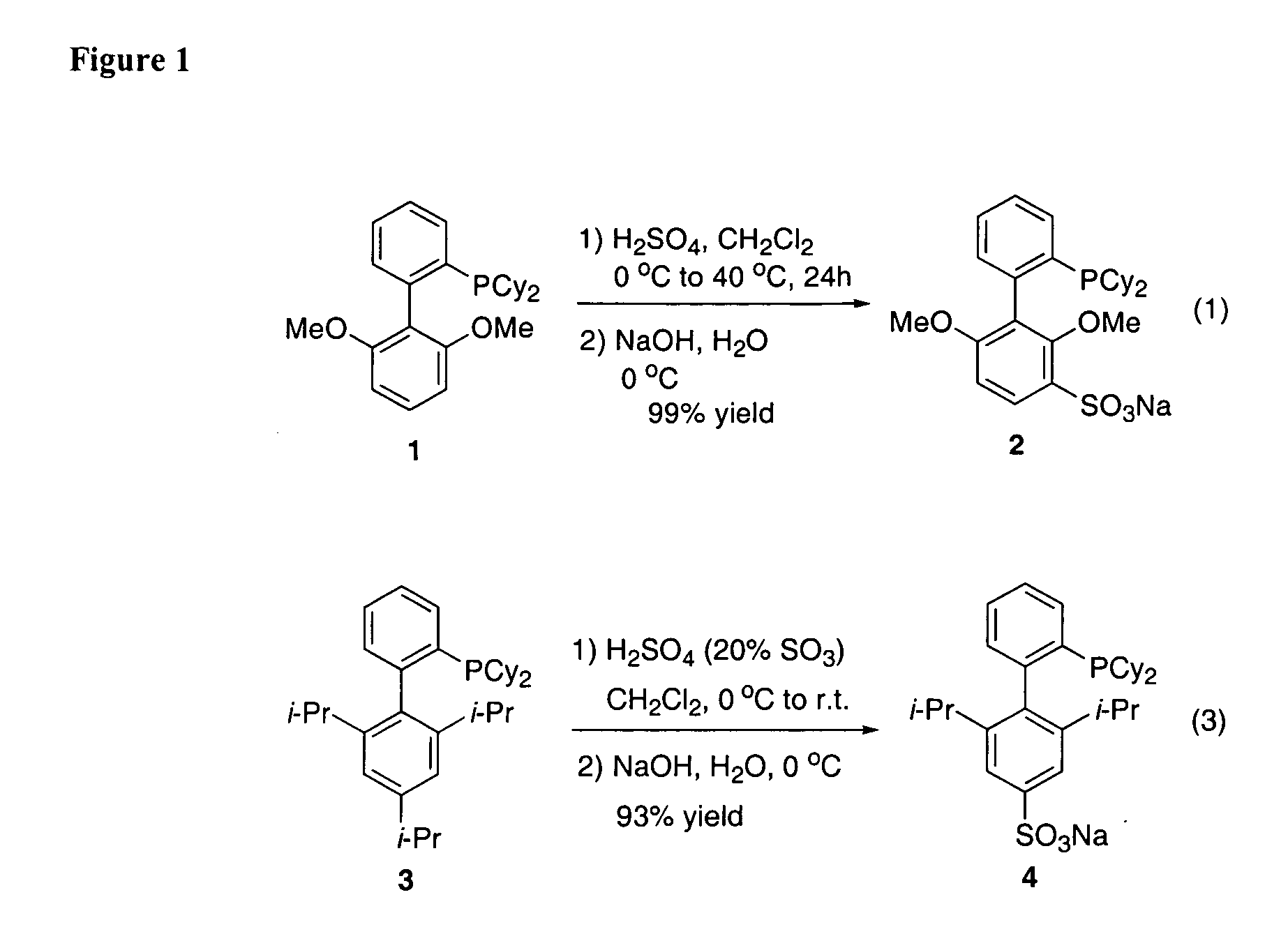
One aspect of the present invention relates to ligands for transition metals. A second aspect of the present invention relates to the use of catalysts comprising these ligands in various transition-metal-catalyzed carbon-heteroatom and carbon-carbon bond-forming reactions. The subject methods provide improvements in many features of the transition-metal-catalyzed reactions, including the range of suitable substrates, number of catalyst turnovers, reaction conditions, and efficiency. For example, improvements have been realized in transition metal-catalyzed: aryl amination reactions; aryl amidation reactions; Suzuki couplings; and Sonogashira couplings. In certain embodiments, the invention relates to catalysts and methods of using them that operate in aqueous solvent systems.
Owner:MASSACHUSETTS INST OF TECH
Transition-metal-catalyzed carbon-nitrogen and carbon-carbon bond-forming reactions
ActiveUS7560596B2More featureGroup 8/9/10/18 element organic compoundsCarboxylic acid amides preparationCarbon–carbon bondCoupling
Owner:MASSACHUSETTS INST OF TECH
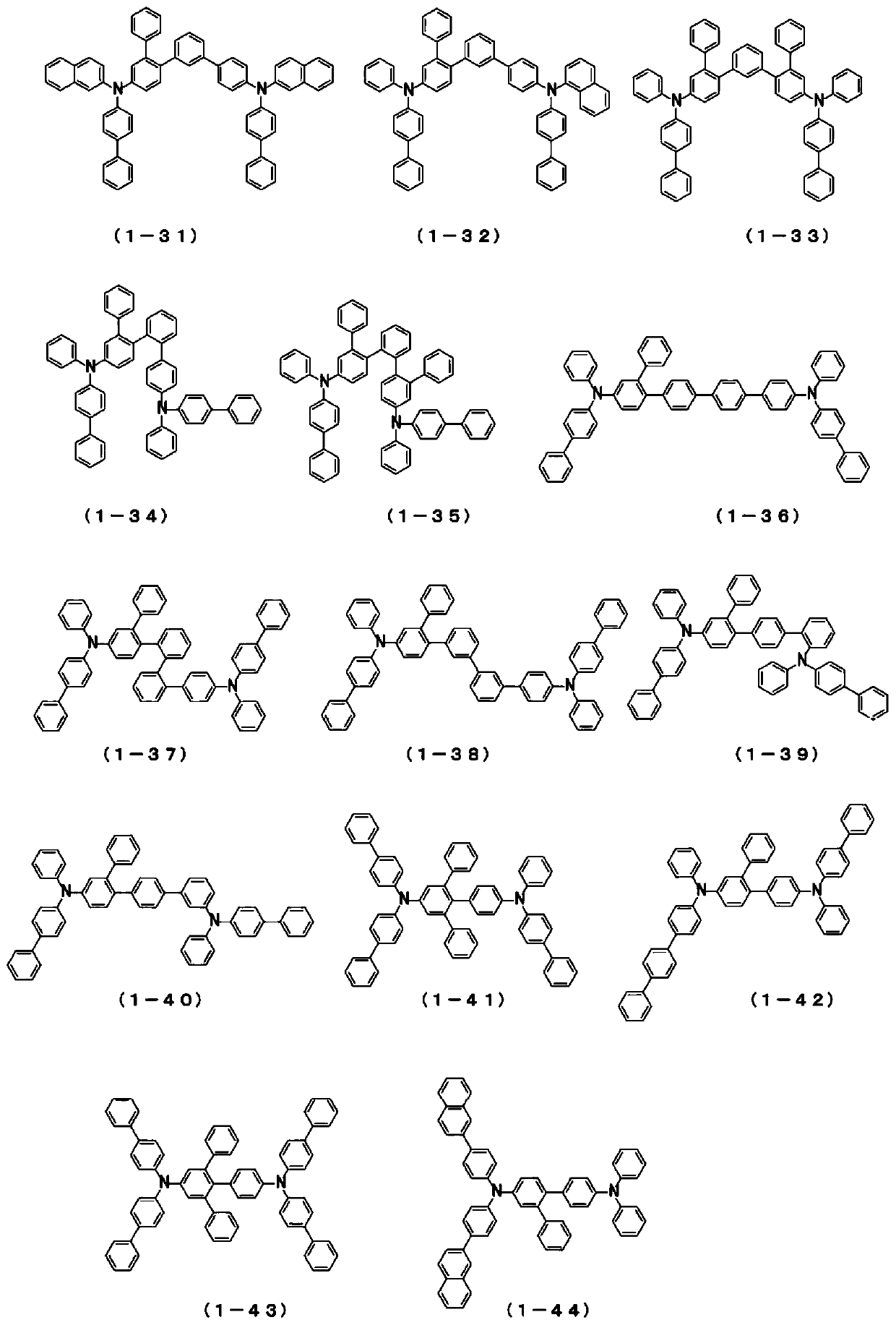
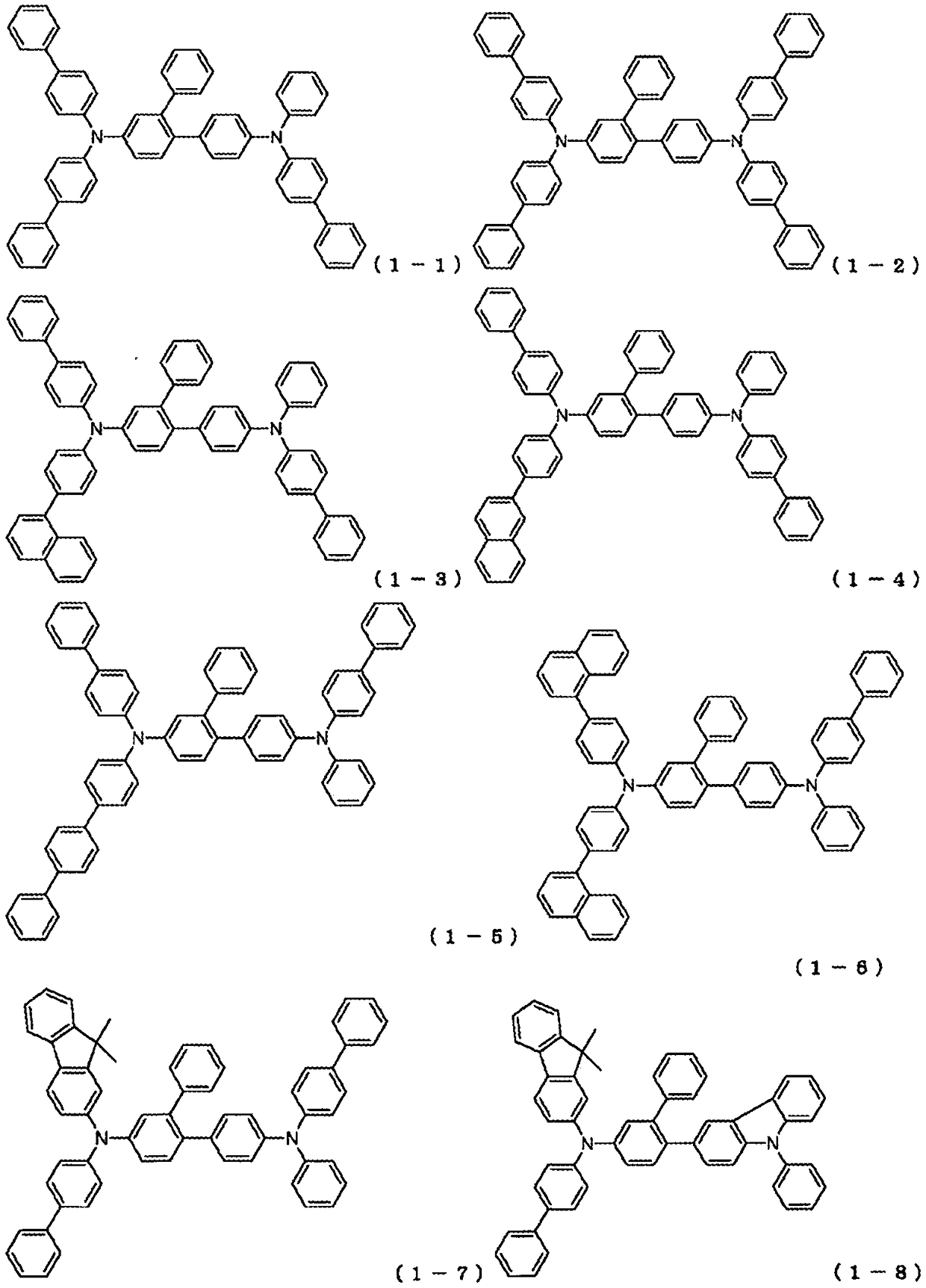
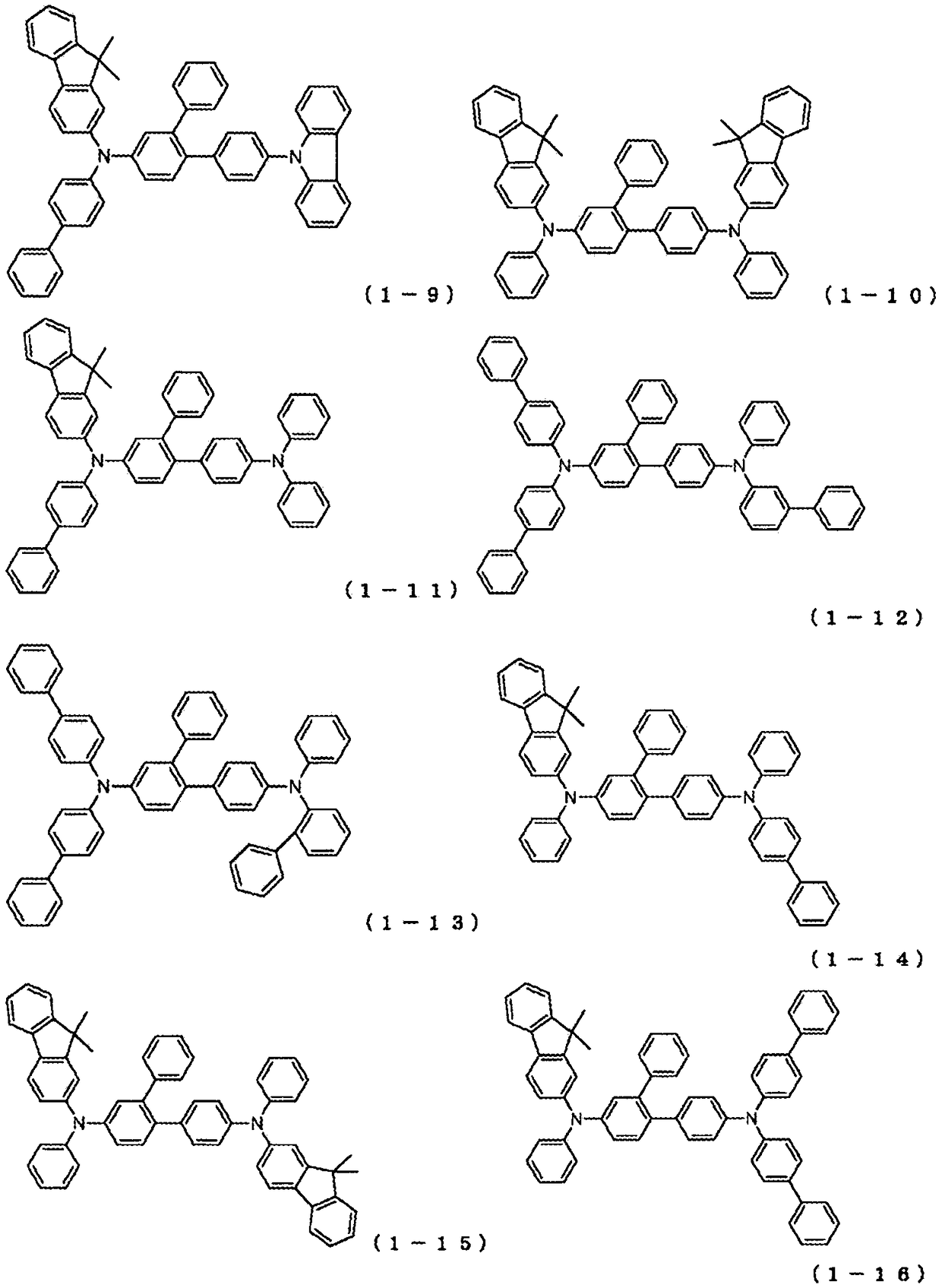
Novel compound and organic electroluminescent device including the same
PendingCN109206327AHigh triplet energyImprove efficiencyOrganic chemistrySolid-state devicesHole injection layerOrganic light emitting device
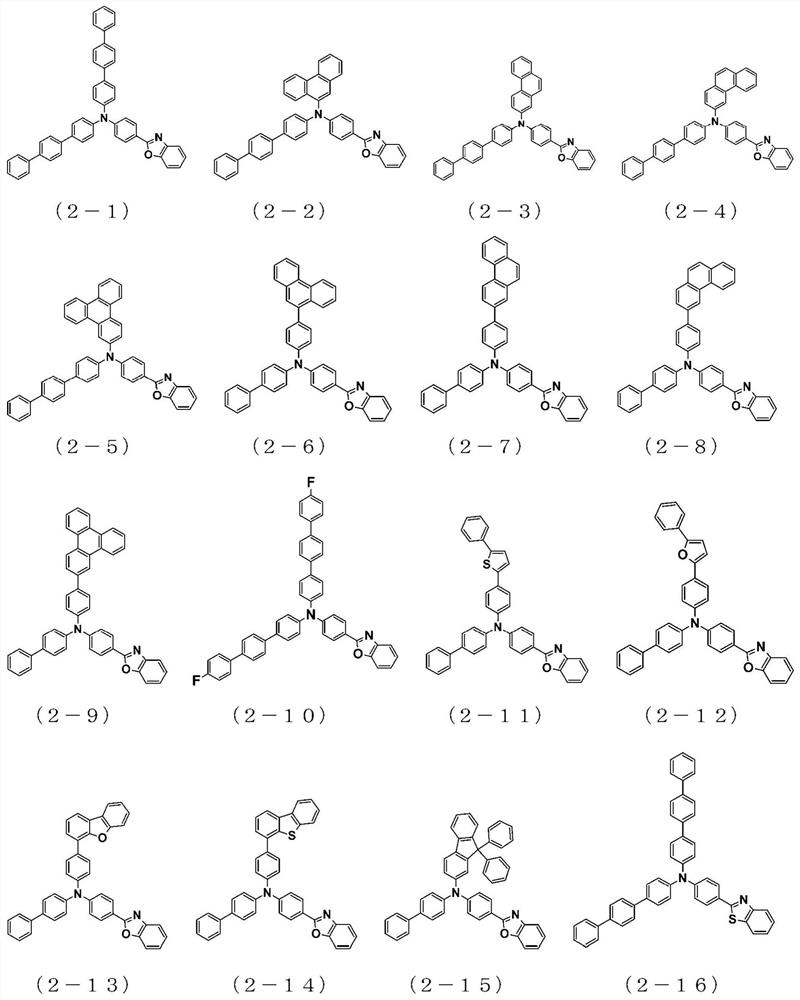
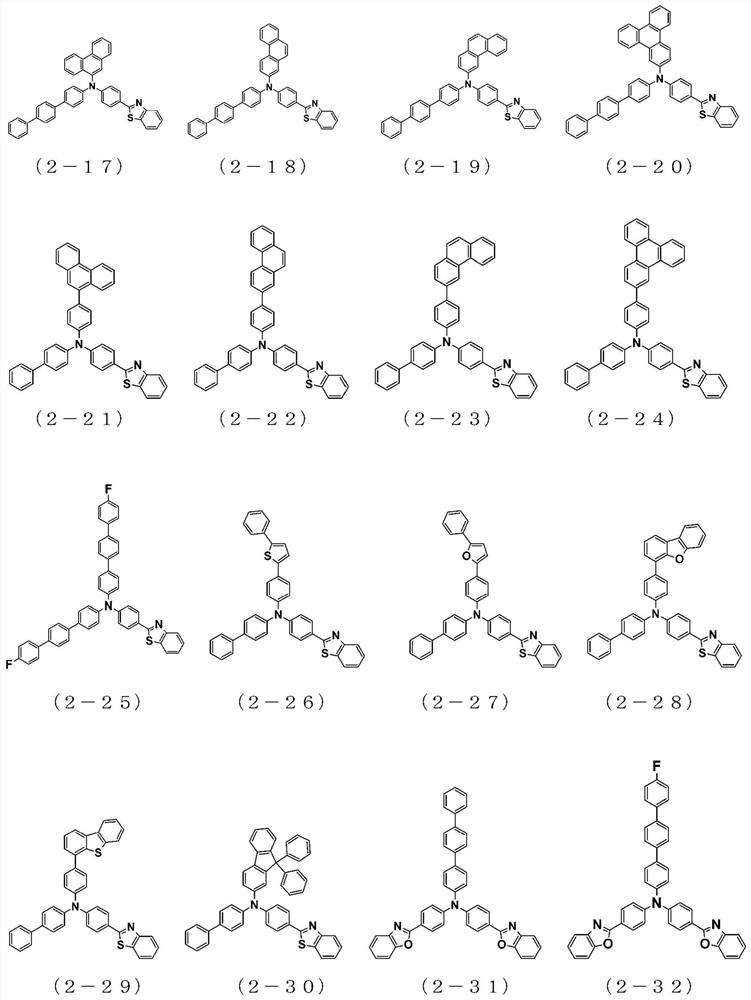
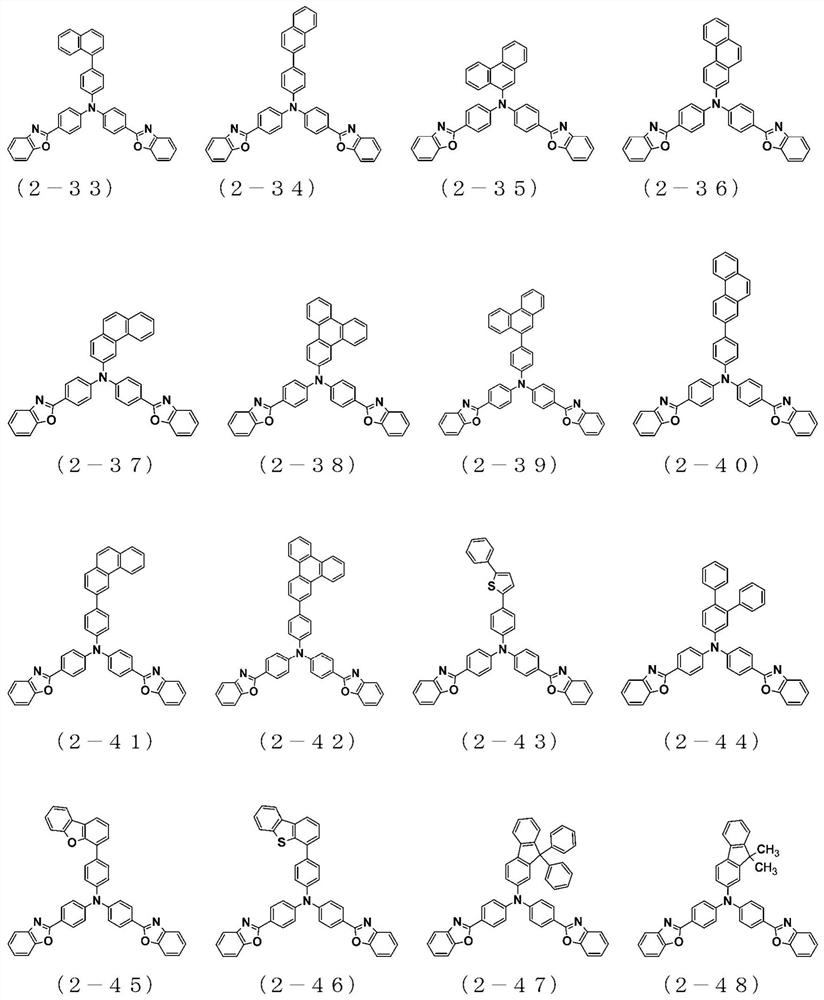
The present invention relates to a novel arylamine compound, when used as a hole transport layer (HTL), a light emitting auxiliary layer (HT prime) or a hole injection layer (HIL), hole injection andtransport properties can be increased, and high efficiency and long life effects of the device can be provided.
Owner:DONGJIN SEMICHEM CO LTD
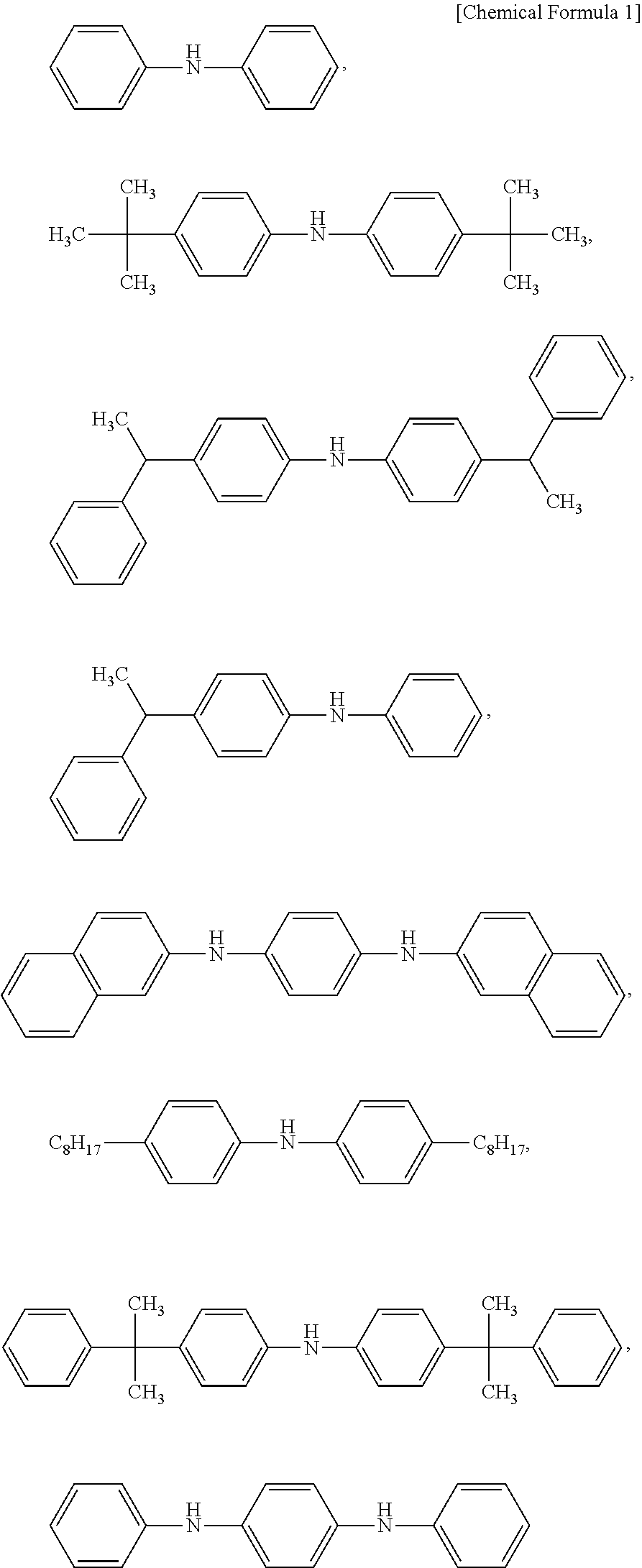
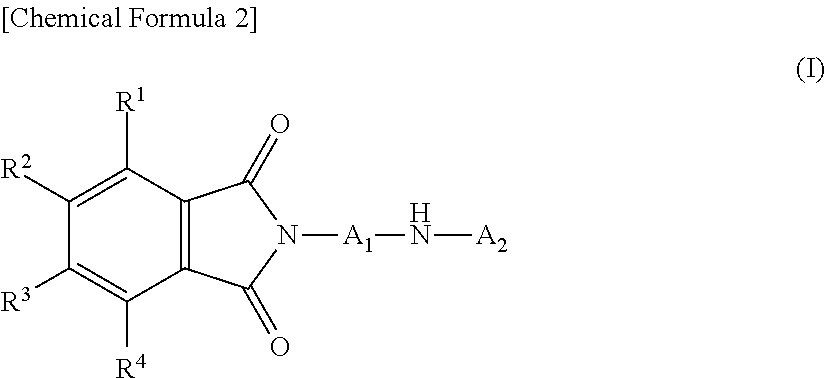
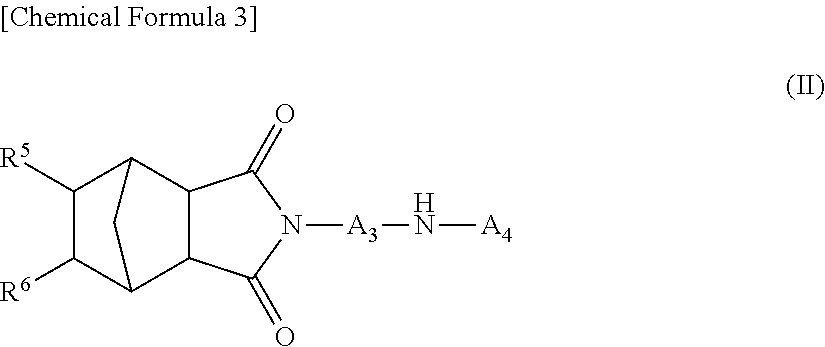
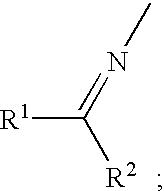
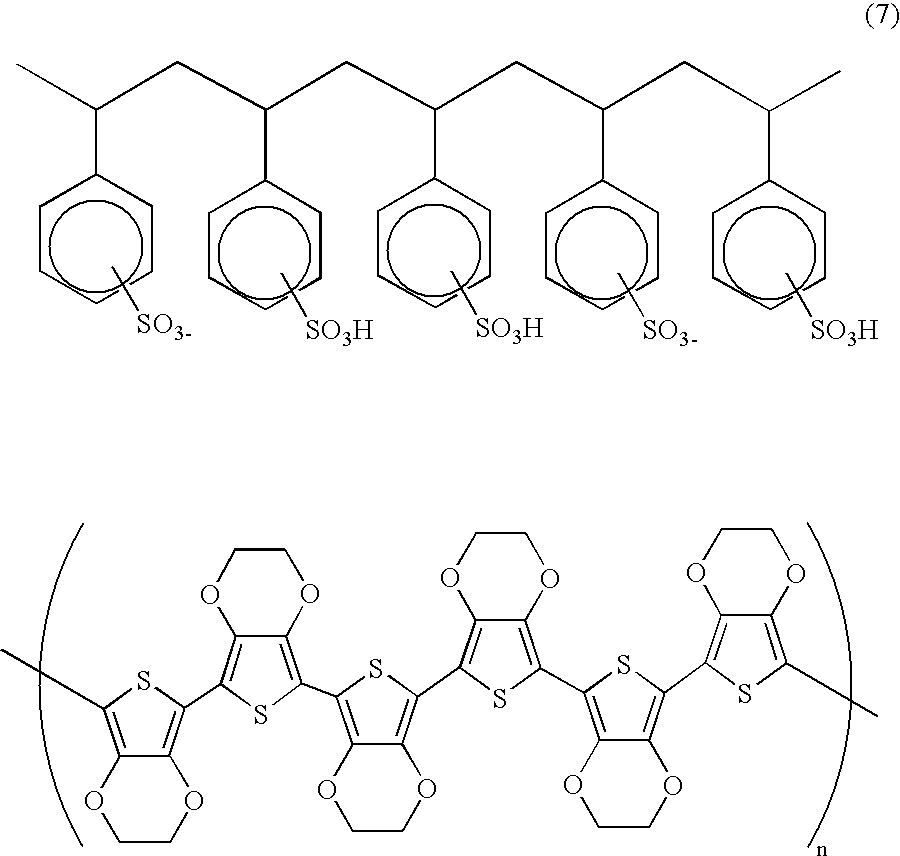
Novel diarylamine compounds, aging inhibitor, polymer composition, crosslinked rubber product and molded article of the crosslinked product, and method of producing diarylamine compound
ActiveUS20120101196A1Oxidative deteriorationImprove heat resistanceOrganic chemistryPolymer scienceMoiety
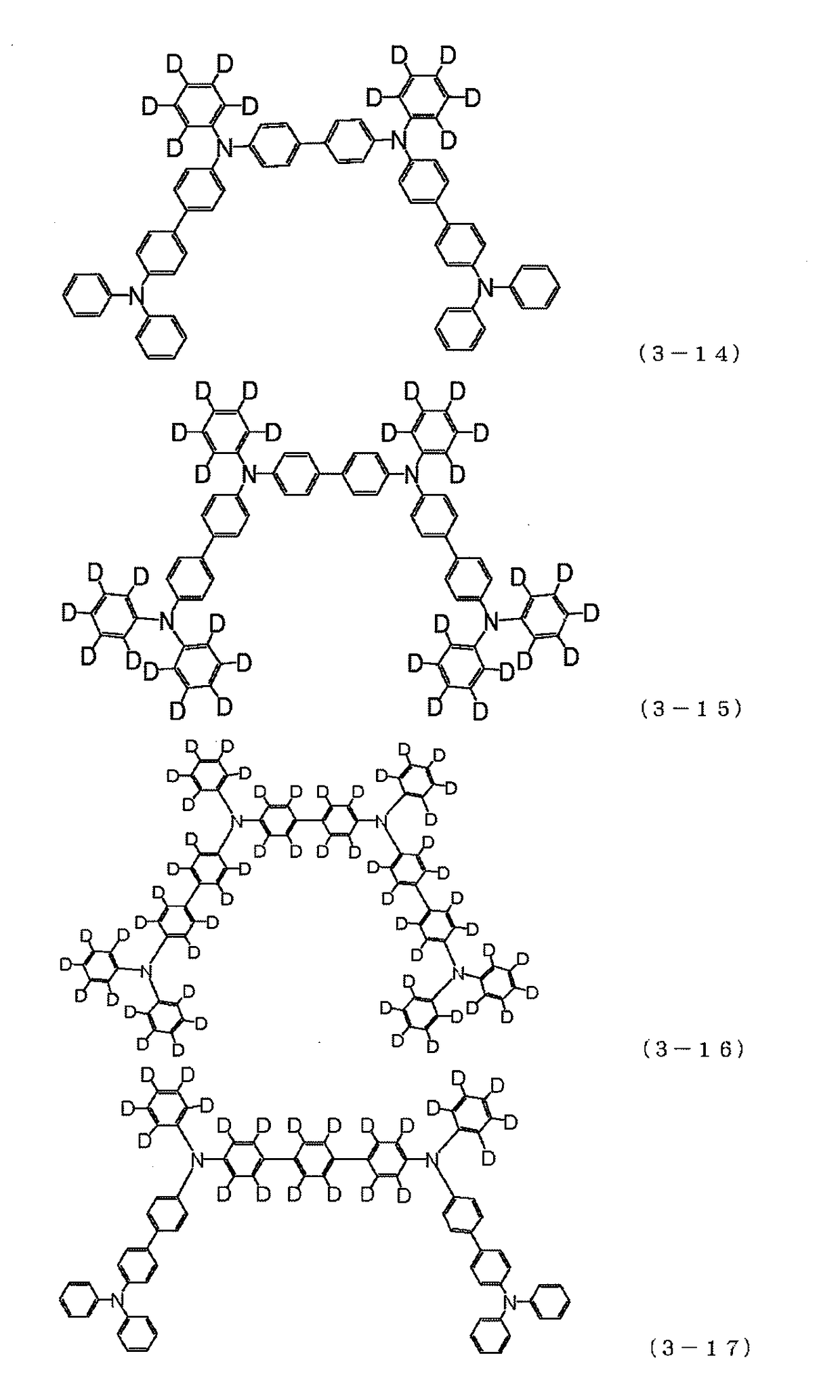
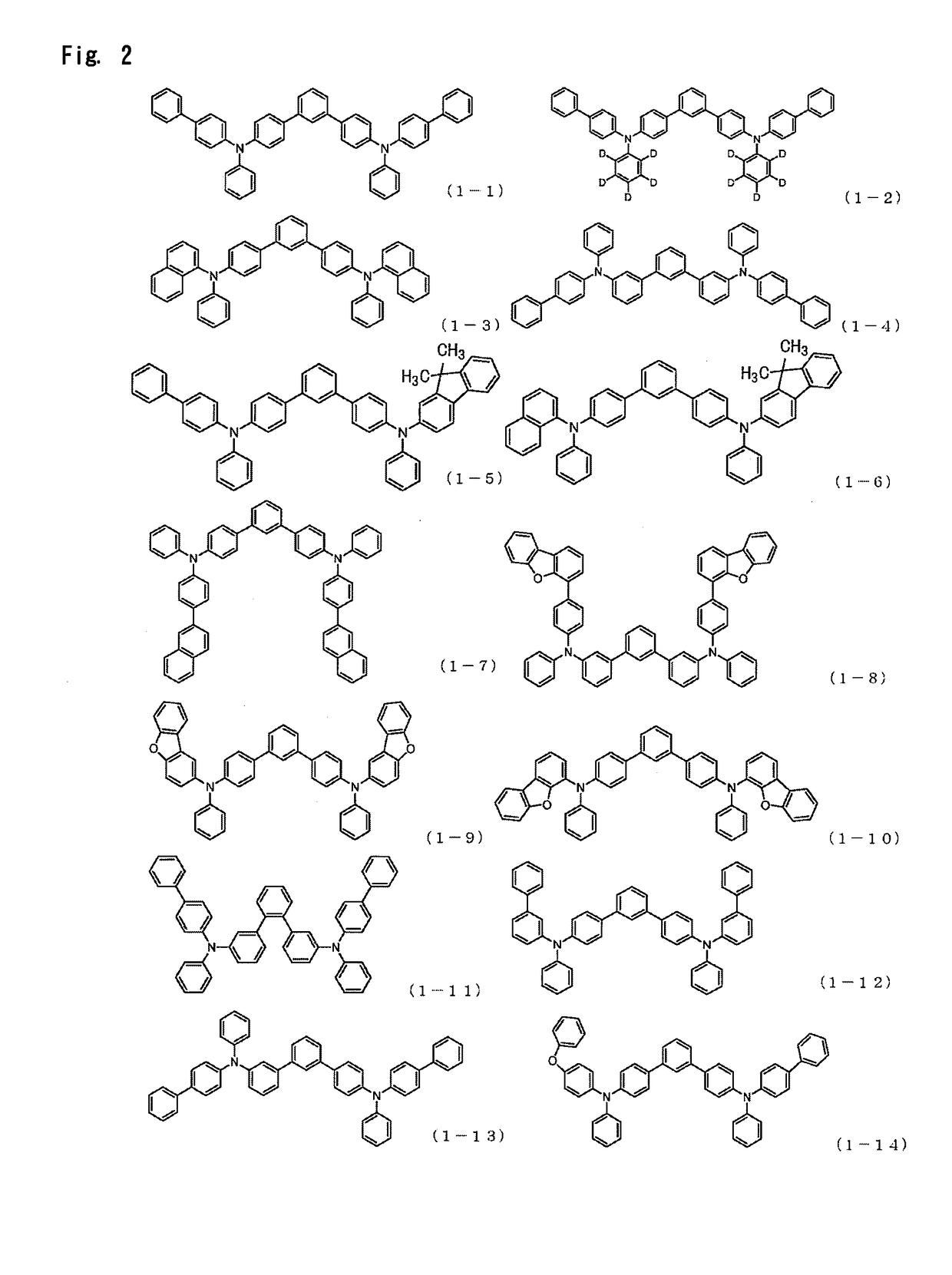
There are provided a novel diarylamine compound represented by the following formula (I), (II) or (III), which has at least one signal attributable to the hydrogen of the N—H moiety at 8.30 ppm to 9.00 ppm when a deuterated dimethyl sulfoxide solution of the diarylamine compound is analyzed by 1H-NMR; and an aging inhibitor, a polymer composition, a crosslinked rubber product and a molded article thereof, and a method of producing a diarylamine compound. In the formulas, A1 to A6 each represent an aromatic group which may have a substituent; A represents an aromatic group or a cyclic aliphatic group, which may both have a substituent; L represents 1 or 2; and n represents 0 or 1.
Owner:ZEON CORP
Amination process
InactiveUS20020128490A1Isocyanic acid derivatives preparationOrganic compound preparationCarbon–nitrogen bondPyrrolidine
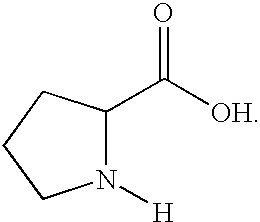
The present invention provides methodology for carbon-nitrogen bond formation via vinyl or aryl amination. In the process of the invention, an sp.sup.2 hybridized radical is reacted with an azomethine moiety to form pyrrolidine and indole compounds. The methodology provides a facile process for the synthesis of compounds having the pyrrolidine or indole subunit and is especially advantageous for compounds having acid or base labile functional groups andor is comprised of chiral centers susceptible to acidbase epimerization.
Owner:ADVANCED RES TECH INST
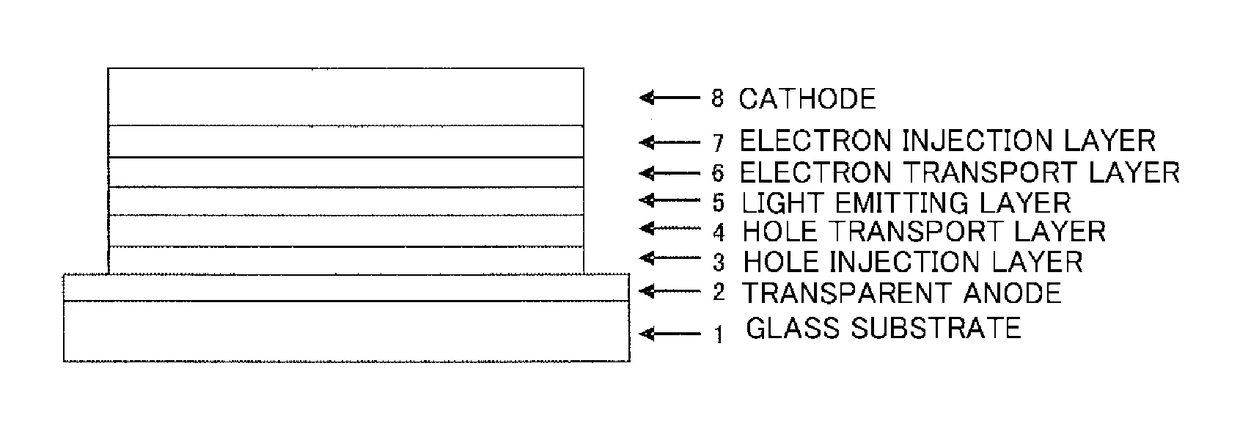
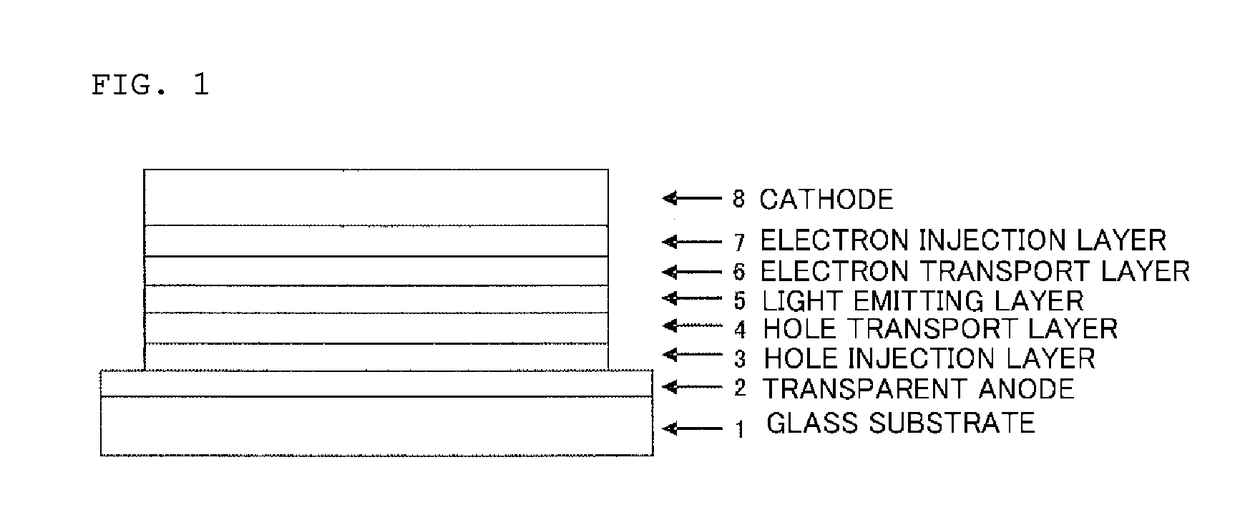
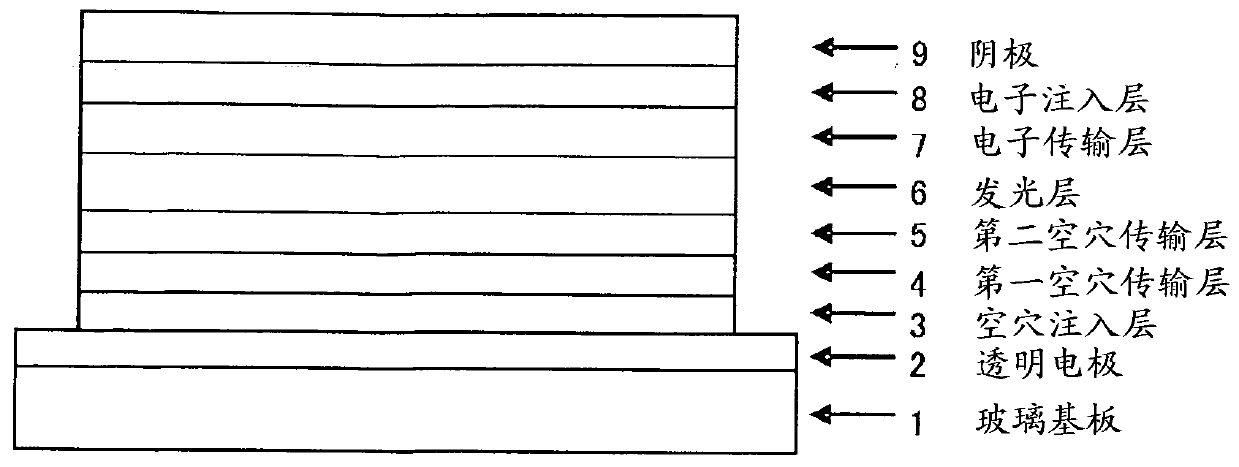
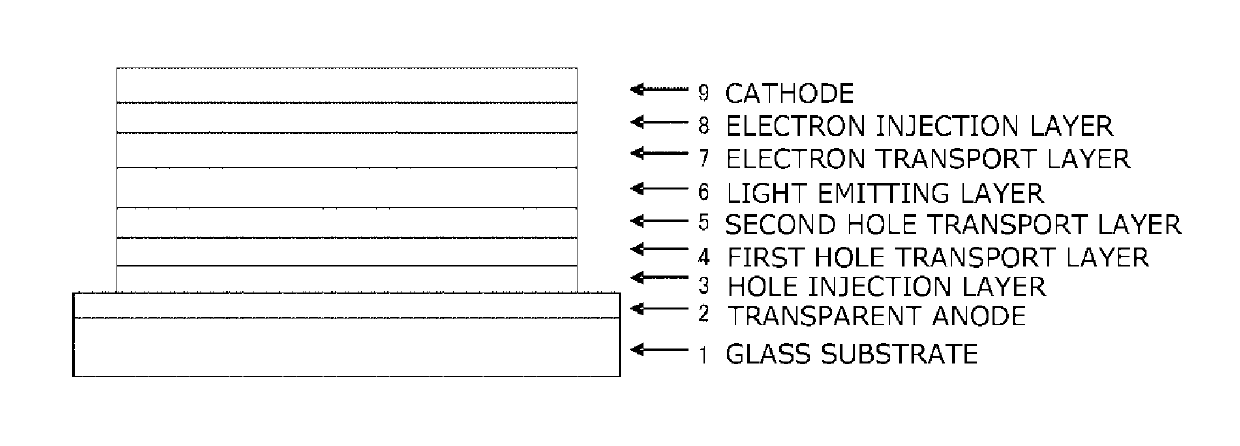
Organic electroluminescent device
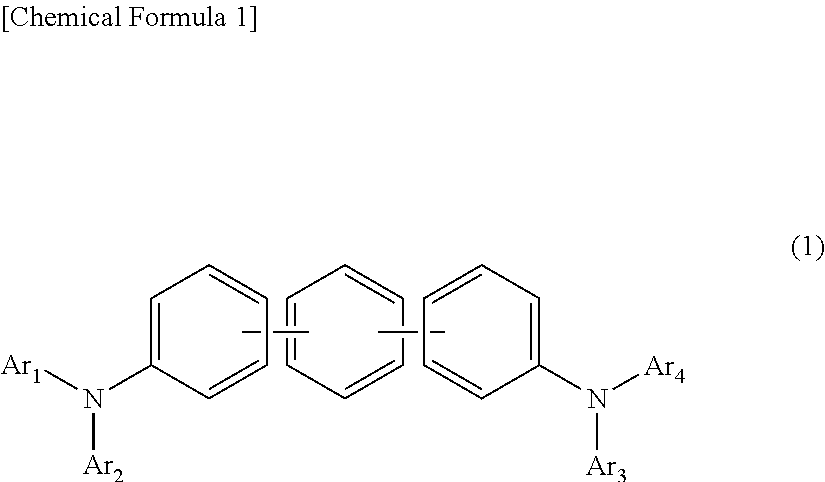
ActiveUS20180006235A1Excellent hole injection/transportReduce the driving voltageOrganic chemistrySolid-state devicesElectron holeHole injection layer
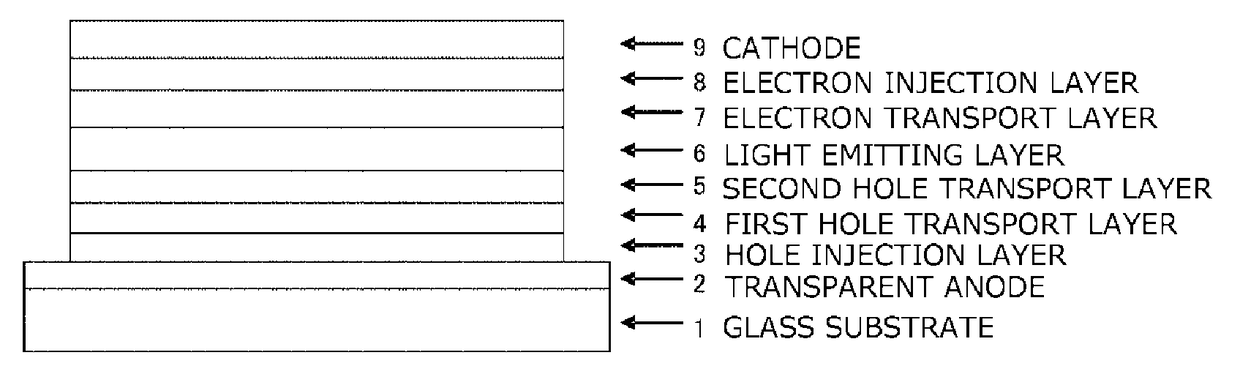
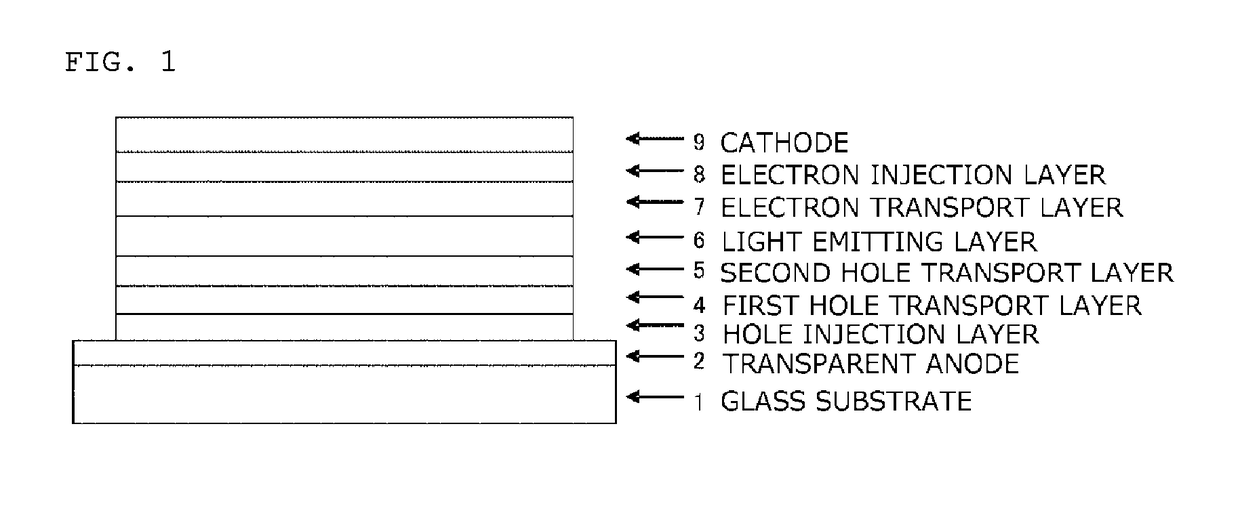
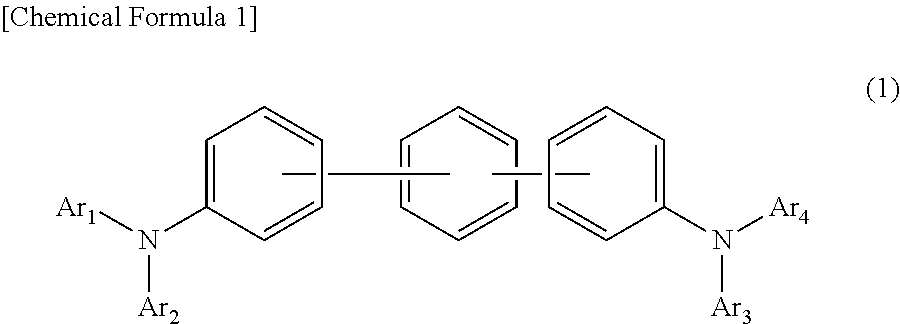
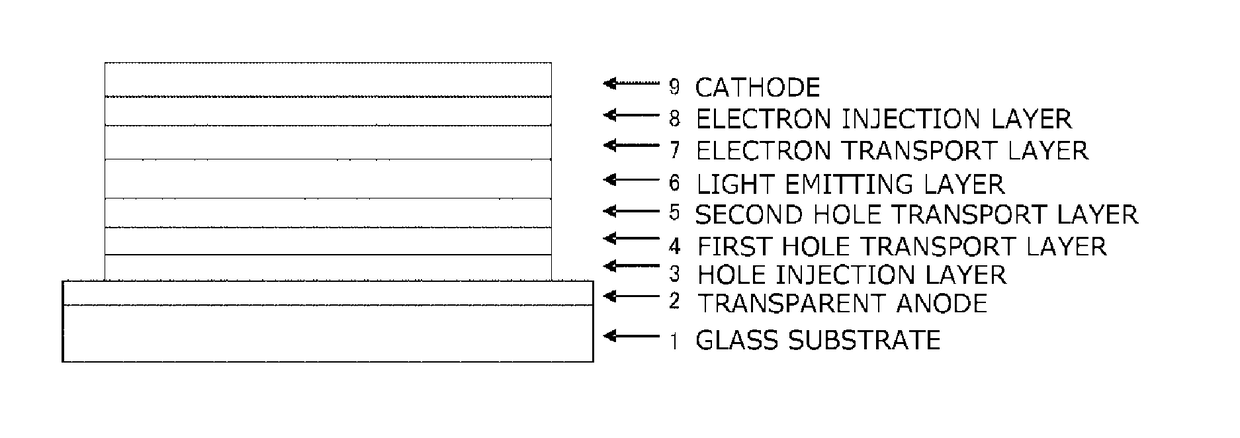
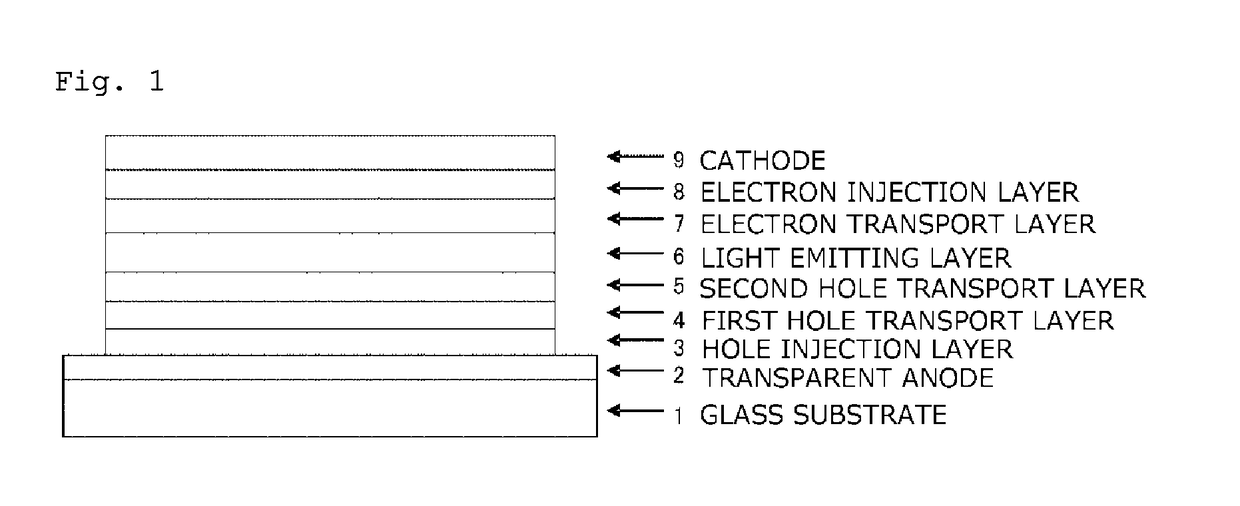
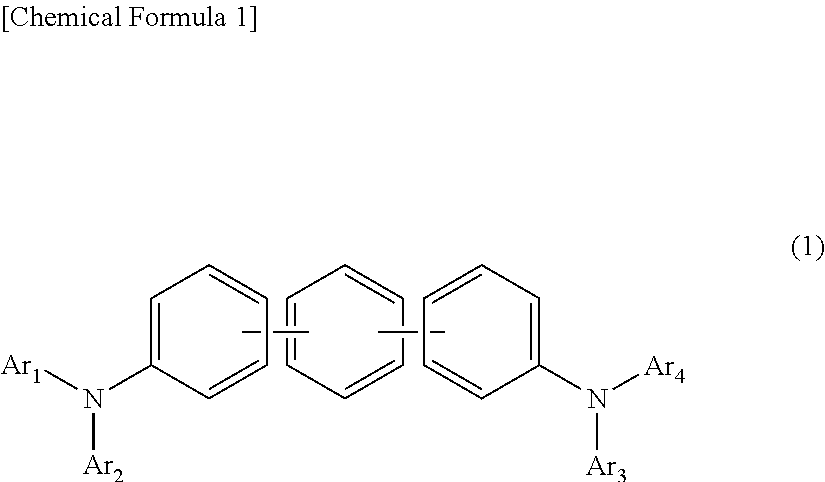
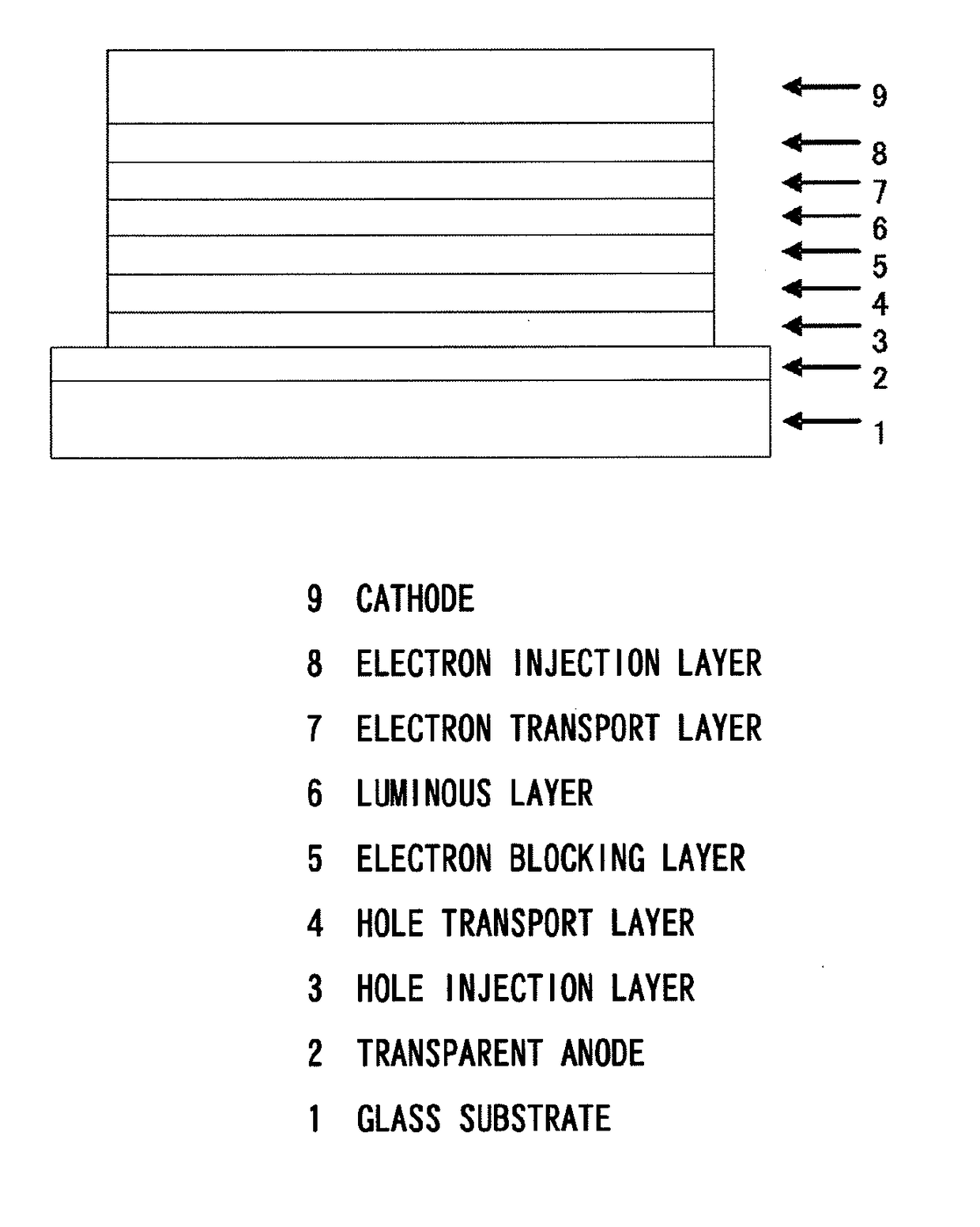
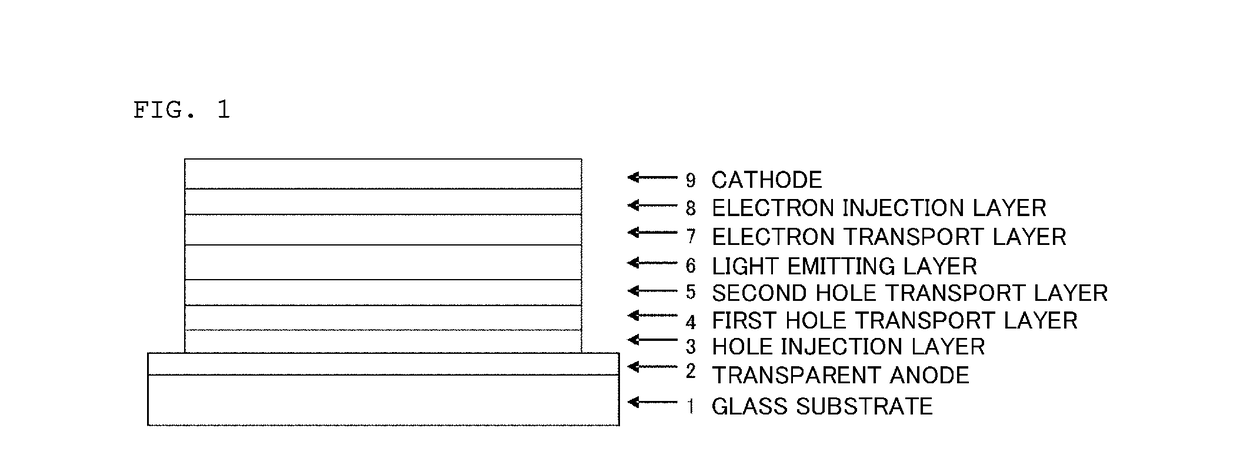
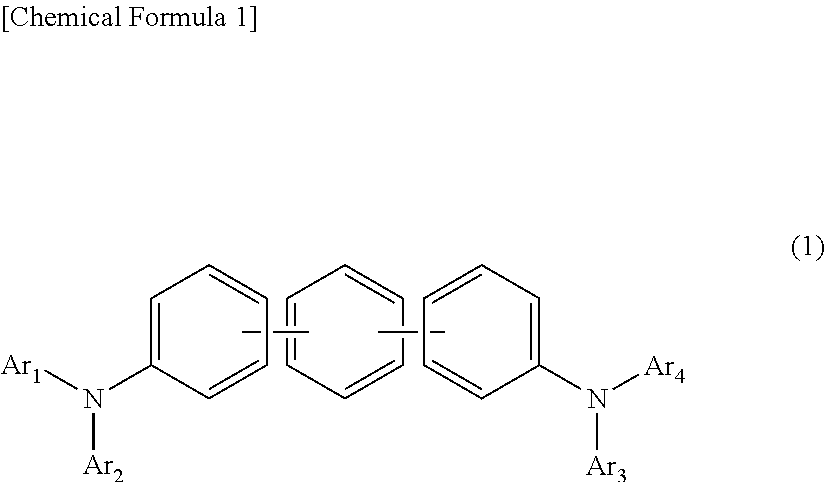
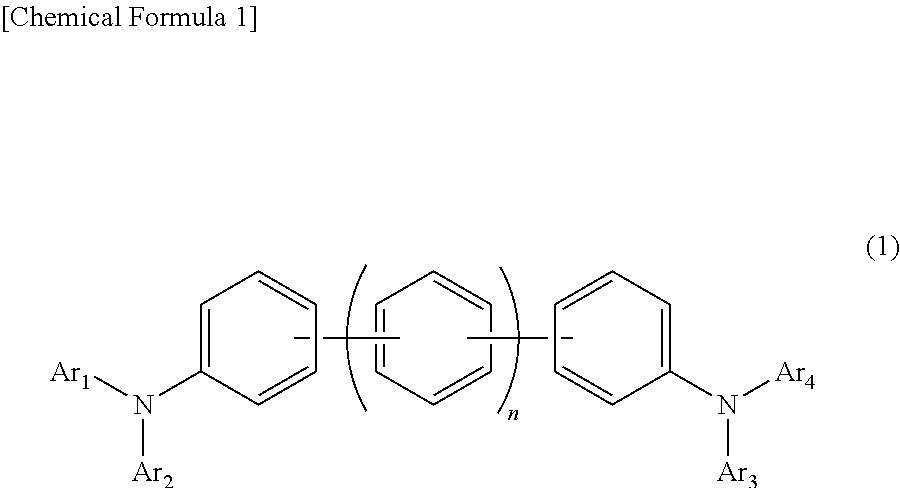
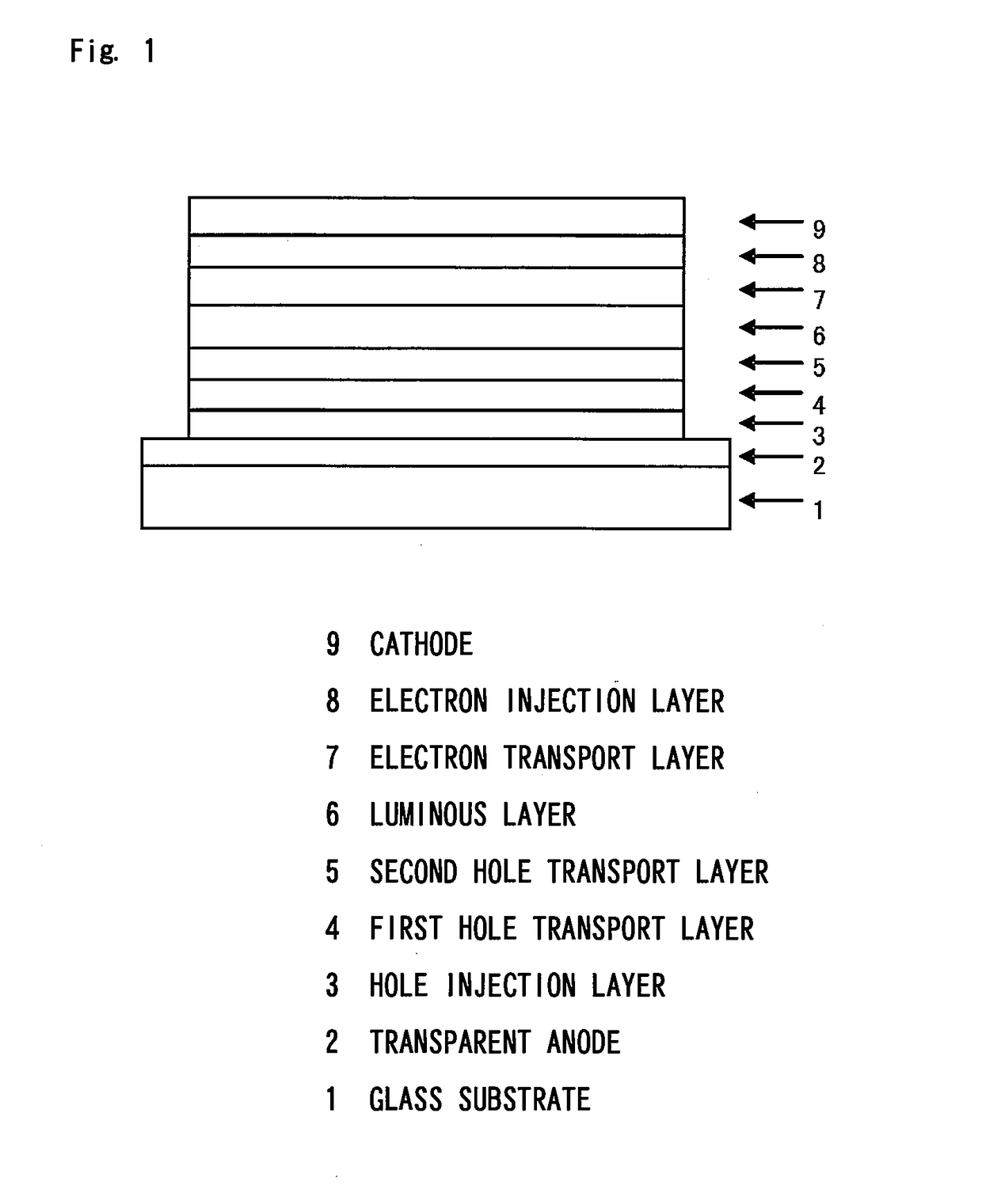
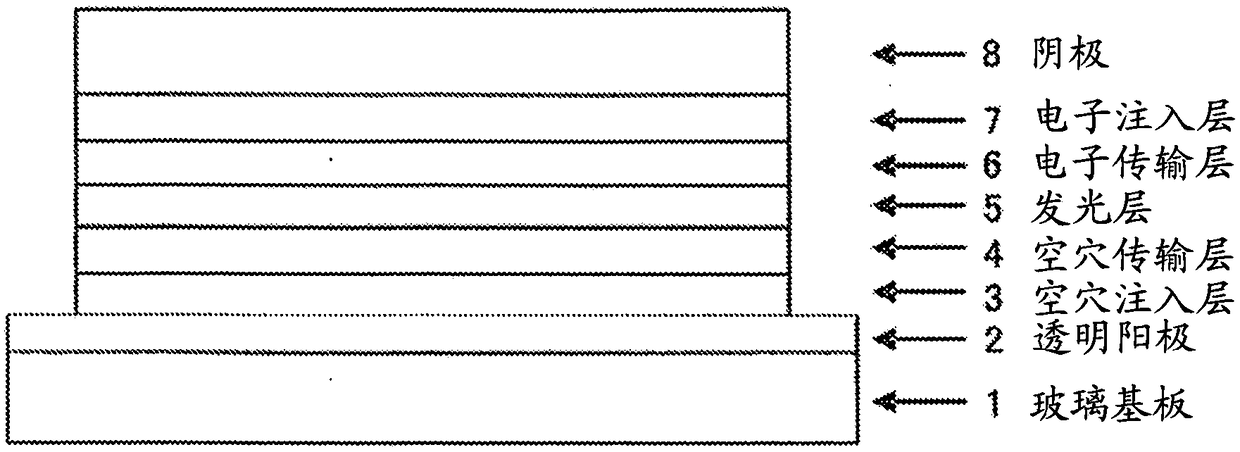
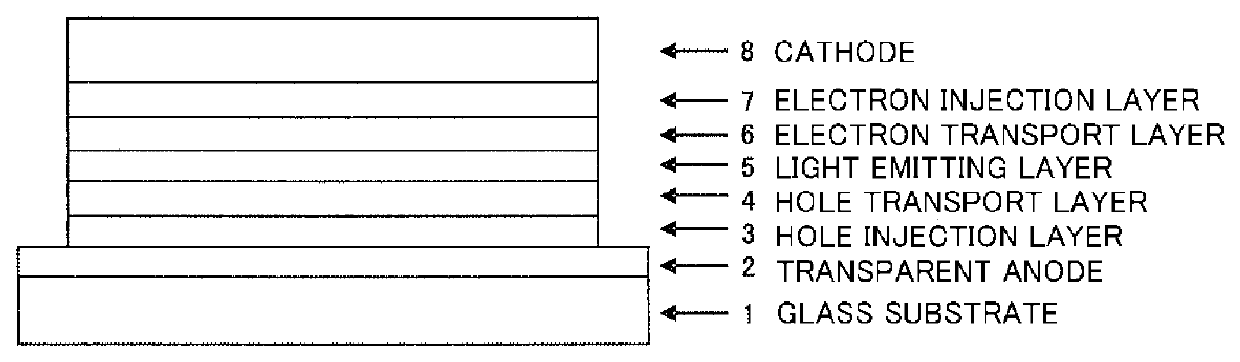
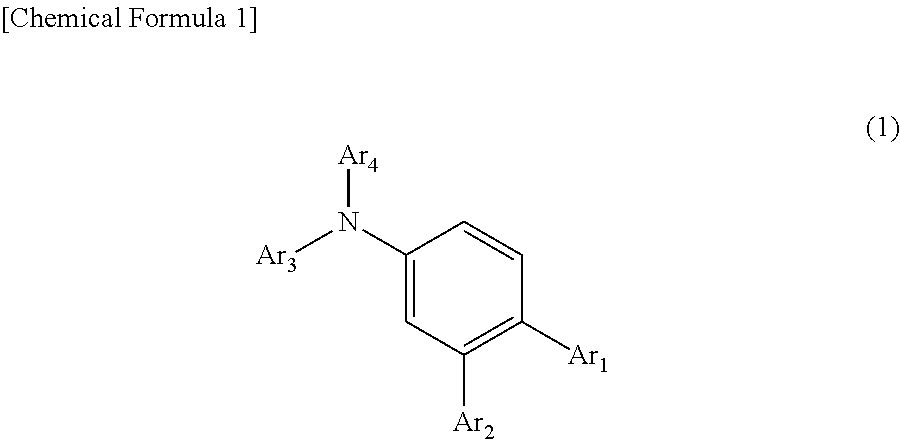
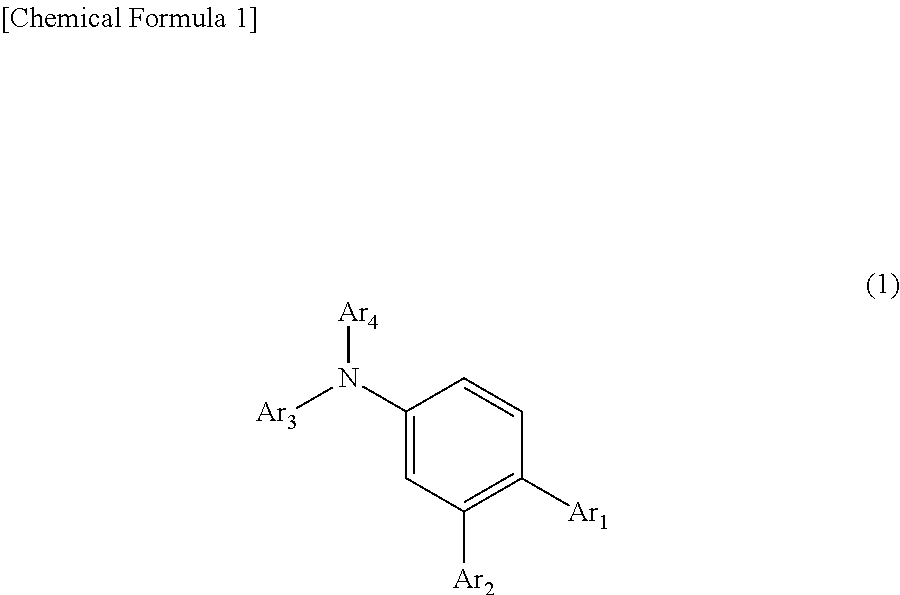
In the organic electroluminescent device having at least an anode, a hole injection layer, a first hole injection layer, a second hole injection layer, a light emitting layer, an electron transport layer and a cathode in this order, the hole injection layer includes an arylamine compound of the following general formula (1) and an electron acceptor.In the formula, Ar1 to Ar4 may be the same or different, and represent a substituted or unsubstituted aromatic hydrocarbon group, a substituted or unsubstituted aromatic heterocyclic group, or a substituted or unsubstituted condensed polycyclic aromatic group.
Owner:HODOGOYA CHEMICAL CO LTD
Organic electroluminescent device
ActiveUS20180315928A1Excellent hole injection and transport performanceReduce the driving voltageOrganic chemistrySolid-state devicesElectron holeHole injection layer
An organic electroluminescent device having low driving voltage, high luminous efficiency, and a long lifetime is provided by combining various materials for an organic electroluminescent device. In the organic electroluminescent device having at least an anode, a hole injection layer, a first hole transport layer, a second hole transport layer, a light emitting layer, an electron transport layer, and a cathode in this order, the hole injection layer includes an arylamine compound of the following general formula (1) and an electron acceptor.In the formula, Ar1 to Ar4 may be the same or different, and represent a substituted or unsubstituted aromatic hydrocarbon group, a substituted or unsubstituted aromatic heterocyclic group, or a substituted or unsubstituted condensed polycyclic aromatic group.
Owner:HODOGAYA KAGAKU IND
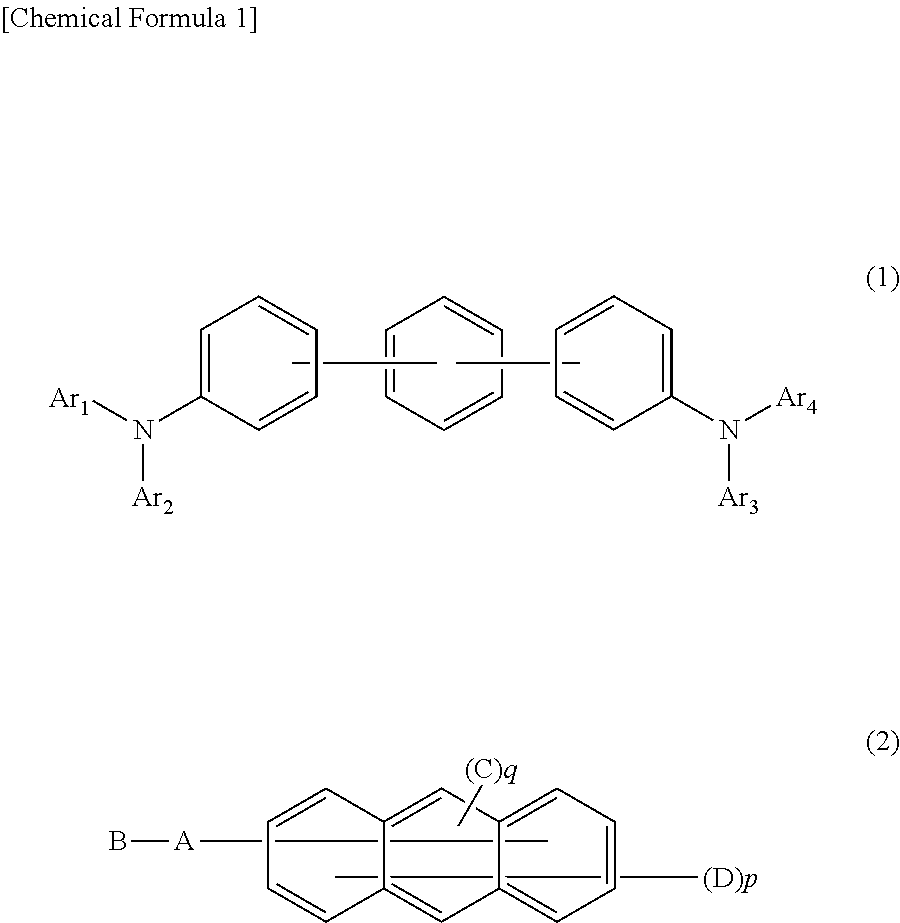
Organic electroluminescent element
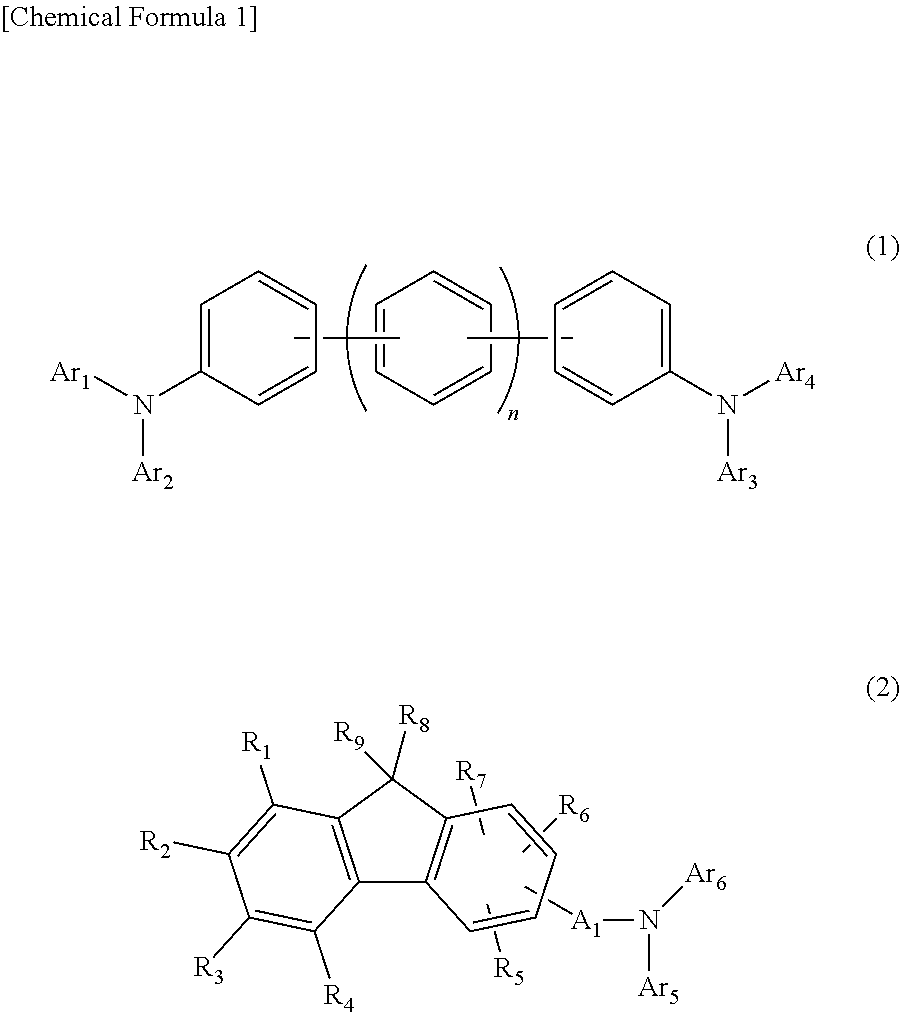
The present invention aims to provide an organic compound which functions as a material for a highly efficient and highly durable organic EL element, exhibits the excellent hole injection and hole transport performance, has electronic stopping power, and exhibits the excellent properties of being highly stable as a thin film and having high light emission efficiency; and the organic EL element which uses the compound and exhibits high durability and high efficiency. The organic EL element is characterized by being provided with a first hole transport layer, a second hole transport layer, a green light-emitting layer, and an electron transport layer which are positioned between a positive electrode and a negative electrode orderly from the positive electrode side, and comprising an arylamine compound represented by general formula (1) and contained in one or more layers among the layered films positioned between the electron transport layer and the first hole transport layer or the second transport layer. (In the formula, Ar1, Ar2, Ar3 and Ar4 may be identical to or different from one another, and represent a substituted or unsubstituted aromatic hydrocarbon group, a substituted orunsubstituted aromatic heterocyclic group, or a substituted or unsubstituted condensed polycyclic aromatic group. L1 represents a substituted or unsubstituted aromatic hydrocarbon divalent group, a substituted or unsubstituted aromatic heterocyclic divalent group, or a substituted or unsubstituted condensed polycyclic aromatic divalent group. R1, R2 and R3 represent a hydrogen atom, a deuterium atom, a fluorine atom, a chlorine atom, a cyano group, a nitro group, a C1-6 straight-chain or branched alkyl group which may have a substituent group, a C5-10 cycloalkyl group which may have a substituent group, a C2-6 straight-chain or branched alkenyl group which may have a substituent group, a C1-6 straight-chain or branched alkyloxy group which may have a substituent group, a C5-10 cycloalkyloxy group which may have a substituent group, a substituted or unsubstituted aromatic hydrocarbon group, a substituted or unsubstituted aromatic heterocyclic group, a substituted or unsubstituted condensed polycyclic aromatic group, or a substituted or unsubstituted aryloxy group. n is an integer of 1-3, inclusive.)
Owner:HODOGOYA CHEMICAL CO LTD
Processes for the preparation of N-heteroaryl-N-aryl-amines by reacting an N-aryl carbamic acid ester with a halo-heteroaryl and analogous processes
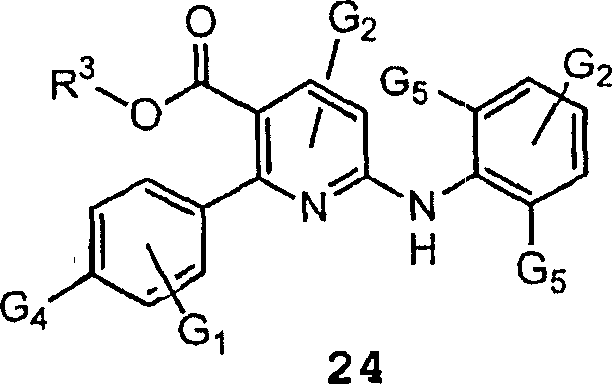
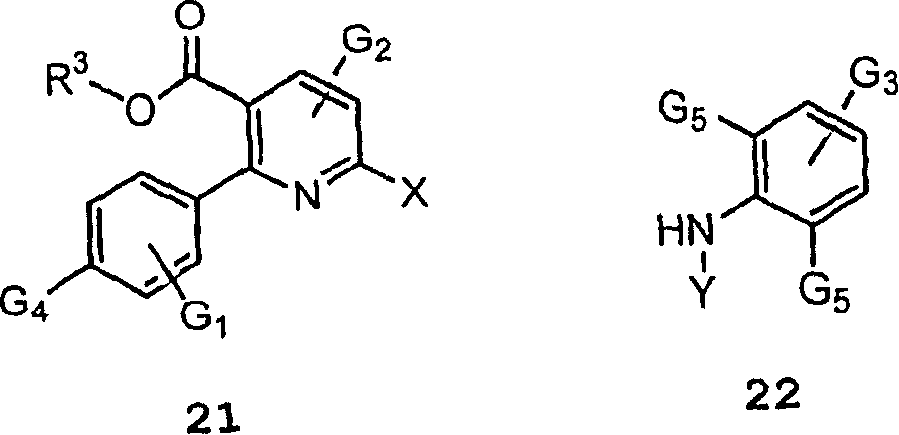
InactiveCN1761653ASimple reaction conditionsUrea derivatives preparationOrganic compound preparationFormic Acid EstersPtru catalyst
The present invention relates to processes for producing a diaryl amine compound of the formula (I); or a salt thereof, said process comprising the step of coupling a compound of formula (II) with an amine of formula (III) in the presence of an alkali metal salt or a transition metal catalyst, wherein: Ar1 and Ar2 are independently Q; wherein each Q is an aryl or heteroaryl ring system optionally fused to a saturated or unsaturated 5-8 membered ring having 0-4 heteroatoms; wherein Q is optionally substituted as defined in claim 1, wherein: X is a leaving group; and Y is -C(O)-O-Z; and Z is selected from C1-C6 aliphatic, benzyl, Fmoc, -SO2R' and Q, provided that Q is not substituted with X or alkyne; wherein R' is as defined in claim 1.
Owner:VERTEX PHARMA INC
Organic electroluminescent element
InactiveCN110226240AImprove mobilityExcellent electron injection and transport propertiesOrganic chemistry methodsSolid-state devicesHole transport layerLight emission
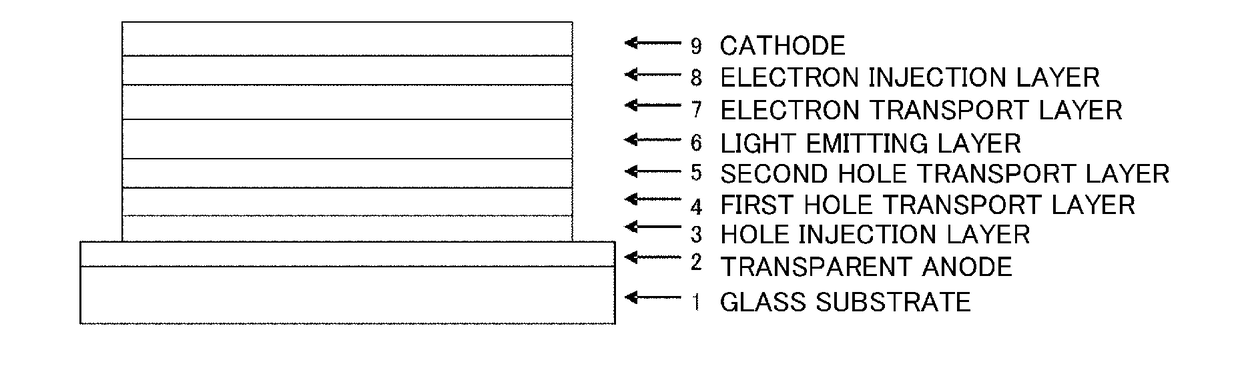
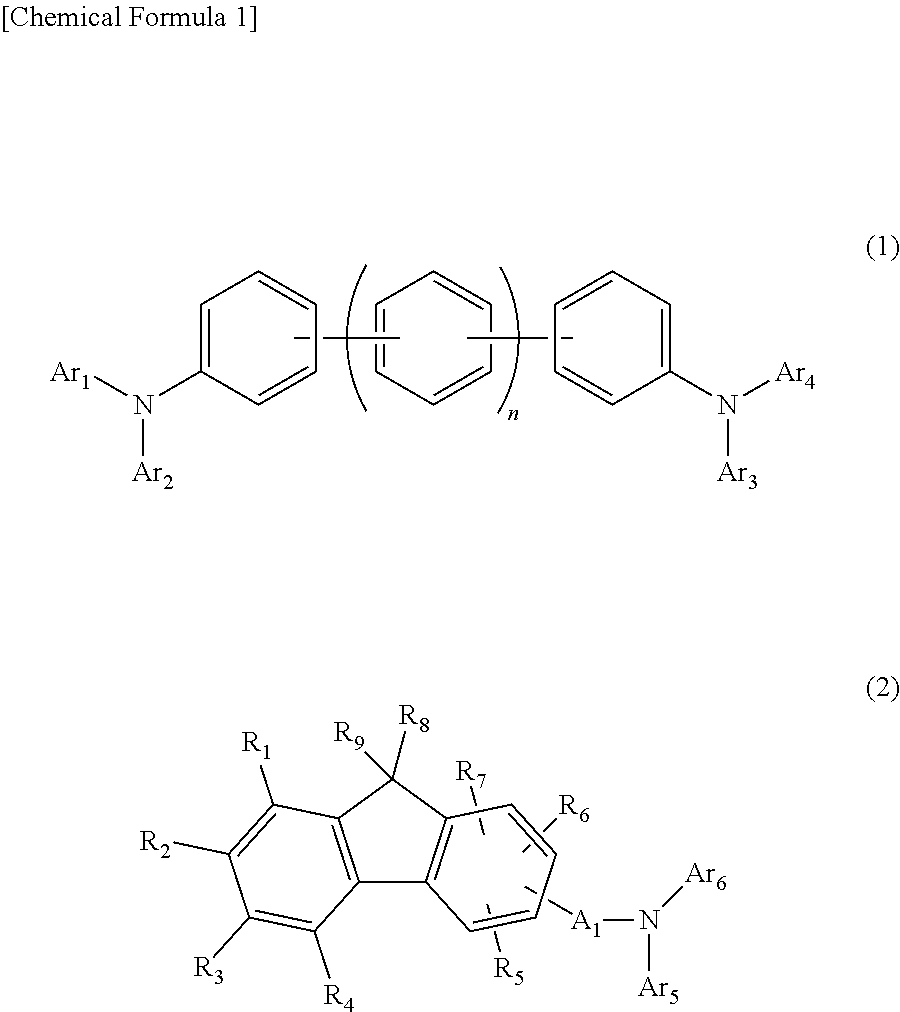
The purpose of the present invention is to provide an organic EL element which (1) has high luminous efficiency and high power efficiency, (2) has a low light emission start voltage, (3) has a low practical driving voltage, and (4) particularly has a long service life. According to the present invention, an organic electroluminescent (EL) element is provided, which is provided with at least a positive electrode, a hole injection layer, a first hole transport layer, a second hole transport layer, a light-emitting layer, an electron transport layer and a negative electrode in this order and is characterized in that the second hole transport layer contains an arylamine compound represented by general formula (1) and the electron transport layer contains a pyrimidine derivative represented bygeneral formula (2). In general formula (2), A represents a monovalent group represented by structural formula (3).
Owner:HODOGOYA CHEMICAL CO LTD
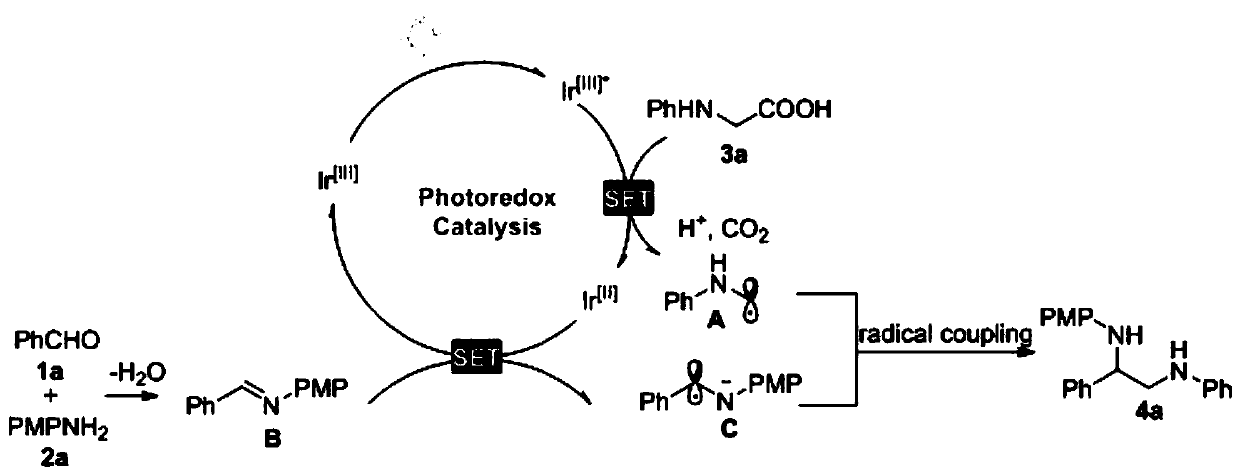
Method for green synthesis of 1,2-diamine compound under catalysis of visible light
PendingCN111285776AThe synthetic route is simpleSimple and fast operationOrganic compound preparationAmino-hyroxy compound preparationOrganic synthesisEthyl acetate
The invention discloses a method for green synthesis of a 1,2-diamine compound through visible light catalysis, and belongs to the technical field of organic synthesis. The method comprises the following steps: (1) adding N-phenylglycine or (4-fluorophenyl) glycine, a photocatalyst, an aldehyde compound or an imine compound, an arylamine compound and a solvent into a dry Schlenk tube; (2) sealingafter three times of air suction and exchange treatment; (3) carrying out stirring reaction under the irradiation of a Blue LED, and ending the reaction when TLC detects that the aldehyde compound disappears; and (4) adding water to quench, extracting with ethyl acetate, collecting an organic phase, drying with anhydrous sodium sulfate, filtering, adding silica gel, concentrating under reduced pressure, and purifying by column chromatography to obtain the diamine compound. Compared with the prior art, the method is simple in synthetic route, easily available in raw materials, simple and convenient to operate, environment-friendly, mild in reaction condition, good in reaction condition controllability and wide in substrate application range, and the preparation of the compound can realize an amplification reaction of gram level or above.
Owner:HANGZHOU NORMAL UNIVERSITY
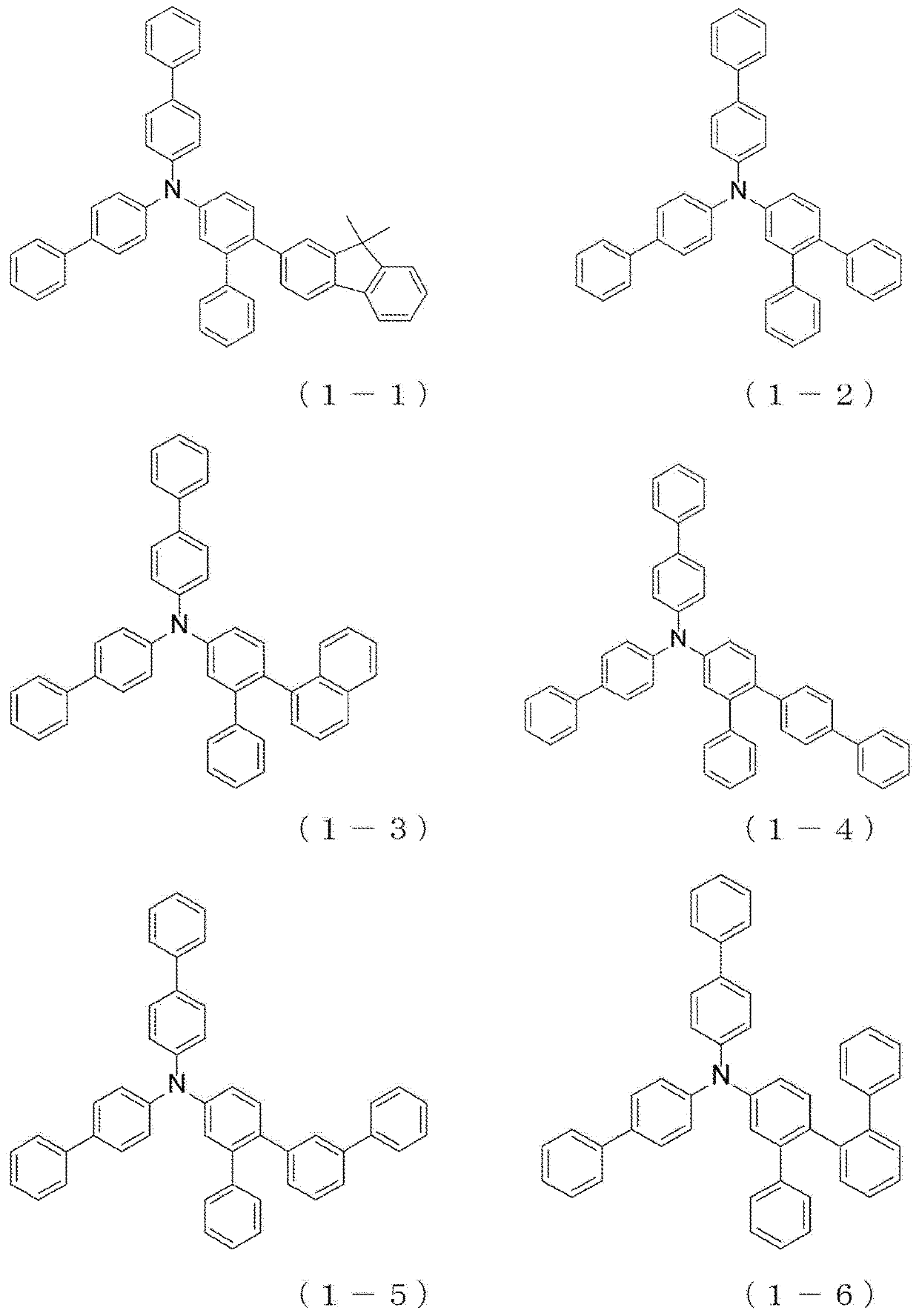
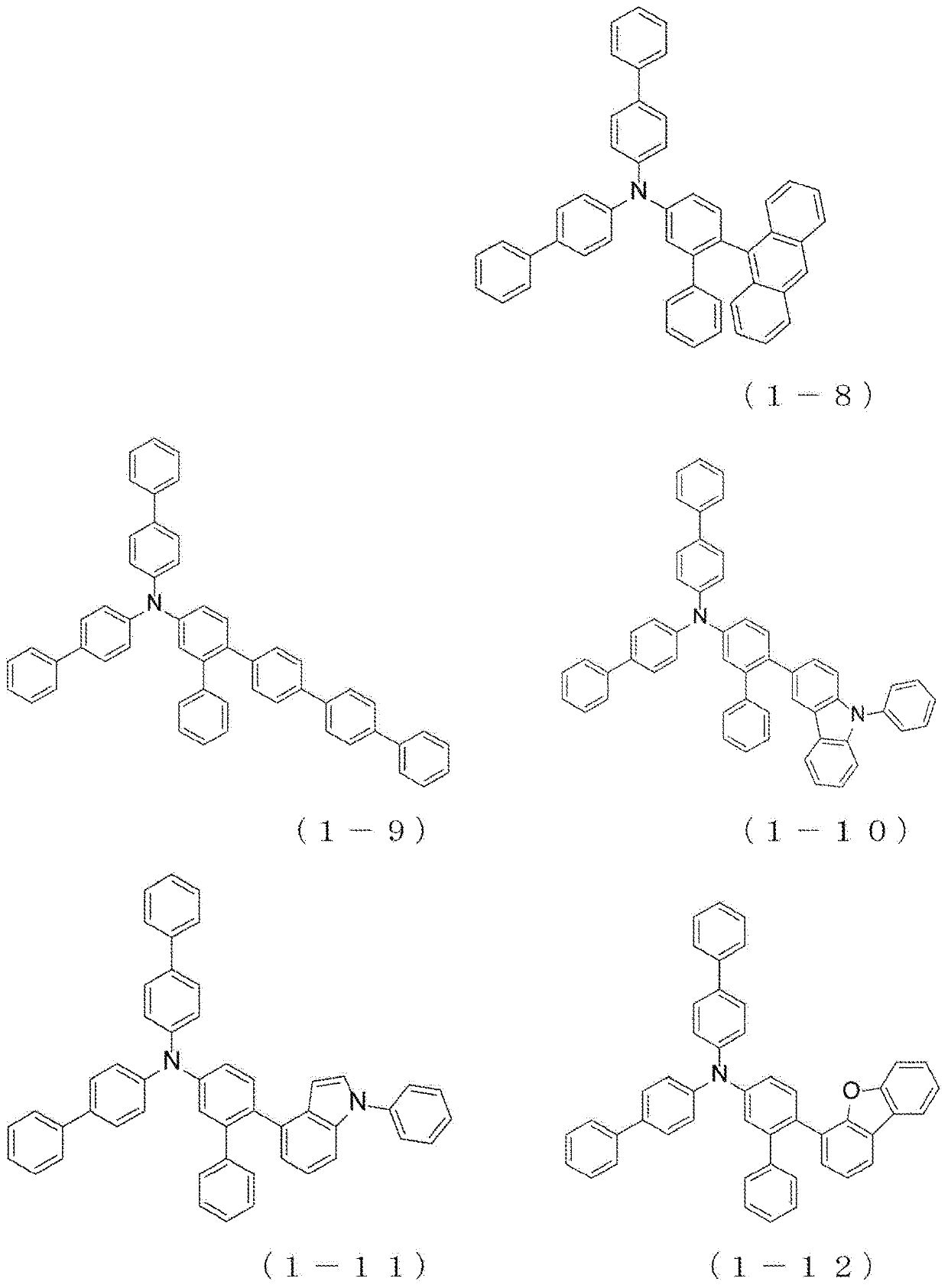
Arylamine compound and organic electroluminescent device
InactiveUS20180175301A1High hole mobilityImprove heat resistanceOrganic chemistrySolid-state devicesPhysical chemistryThin membrane
The present invention provides an arylamine compound represented by the general formula (1) shown below. The arylamine compound of the present invention is a novel compound and, compared with conventional hole transport materials, has high hole mobility, has excellent electron blocking capability, is stable in a thin film state, and is excellent in heat resistance.
Owner:HODOGAYA KAGAKU IND
Organic electroluminescent device
ActiveUS20170207395A1Improve efficiencyReduce the driving voltageSilicon organic compoundsSolid-state devicesElectron injectionHole transport layer
An organic electroluminescent device having high efficiency, low driving voltage and a long lifetime is provided by combining various materials for an organic electroluminescent device, which are excellent, as materials for an organic electroluminescent device having high efficiency and high durability, in hole and electron injection / transport performances, electron blocking ability, stability in a thin-film state and durability, so as to allow the respective materials to effectively reveal their characteristics. In the organic electroluminescent device having at least an anode, a hole transport layer, a light emitting layer, an electron transport layer and a cathode in this order, the hole transport layer includes an arylamine compound represented by the following general formula (1), and the light emitting layer comprises an amine derivative of the following general formula (2) having a condensed ring structure.
Owner:HODOGAYA KAGAKU IND +1
Organic electro-luminescent display device and method for manufacturing the same
InactiveUS7186468B2Discharge tube luminescnet screensElectroluminescent light sourcesOrganic electroluminescenceOptoelectronics
An organic EL display device is provided, which includes a transparent electrode, a counter electrode spaced away from the transparent electrode, a light emitting layer interposed between the transparent electrode and the counter electrode, and a hole transferring layer interposed between the transparent electrode and the light emitting layer. This hole transferring layer comprises a triaryl amine compound which can be represented by any one of general formulas (8), (9), (10) and (11) illustrated in the specification.
Owner:KK TOSHIBA
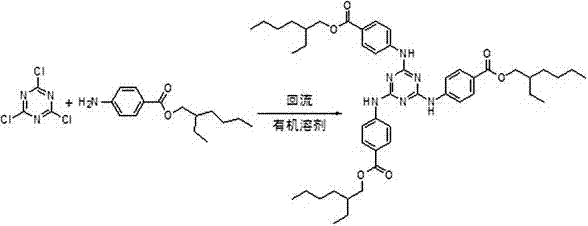
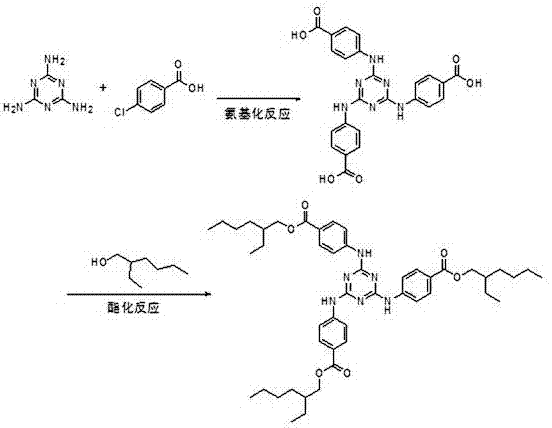
Method for preparing ultraviolet light absorber UVT-150
ActiveCN106986839ASimple preparation routeRaw materials are easy to getOrganic chemistryIsooctyl alcoholOrganic solvent
The invention discloses a method for preparing an ultraviolet light absorber UVT-150. The method is composed of the following process steps: (1) carrying out an aryl amination reaction, namely adding methyl p-aminobenzoate and an organic solvent into a reactor, heating, slowly dripping cyanuric chloride, maintaining the temperature and reacting, distilling, and crystallizing so as to obtain an intermediate methyl triazone; and (2) carrying out an ester exchange reaction, namely adding methylbenzene, a catalyst and isooctyl alcohol into the reactor, heating, dripping a toluene solution of the methyl triazone, maintaining the temperature and reacting, distilling, and crystallizing thereby obtaining the product, namely the ultraviolet light absorber UVT-150. The method disclosed by the invention is simple in process route, readily available in raw materials, convenient to operate and high in yield, and the industrialized production is easily realized.
Owner:HUBEI NORMAL UNIV +1
Organic electroluminescent device
PendingUS20170117481A1Improve efficiencyReduce the driving voltageOrganic chemistryOrganic compound preparationElectron holeElectron injection
An organic electroluminescent device having high efficiency, low driving voltage and a long lifetime is provided by combining various materials for an organic electroluminescent device, which are excellent, as materials for an organic electroluminescent device having high efficiency and high durability, in hole and electron injection / transport performances, electron blocking ability, stability in a thin-film state and durability, so as to allow the respective materials to effectively reveal their characteristics. In the organic electroluminescent device having at least an anode, a hole transport layer, a light emitting layer, an electron transport layer and a cathode in this order, the hole transport layer includes an arylamine compound of the following general formula (1), and the electron transport layer includes a compound the following general formula (2) having an anthracene ring structure.
Owner:HODOGAYA KAGAKU IND
Organic electroluminescent element
ActiveUS20210135116A1High hole mobilityExcellent electron injectabilitySolid-state devicesSemiconductor/solid-state device manufacturingHole transport layerLight emission
An object of the present invention is to provide an organic EL element that has (1) high light emission efficiency and high power efficiency, (2) a low light-emission start voltage, (3) a low actual driving voltage, and (4) an especially long lifespan. According to the present invention, provided is an organic electroluminescent element, or organic EL element, having at least an anode, a hole injection layer, a first hole transport layer, a second hole transport layer, a light emitting layer, an electron transport layer, and a cathode, in this order, wherein the second hole transport layer contains an arylamine compound represented by the general formula (1) below, and the electron transport layer contains a pyrimidine derivative represented by the general formula (2) below. Note that A in the general formula (2) represents a monovalent group represented by the structural formula (3) below.
Owner:HODOGOYA CHEMICAL CO LTD
Organic electroluminescent element
ActiveCN111095588AImprove stabilityIncreased durabilityOrganic chemistrySolid-state devicesElectron injectionHole transport layer
Owner:HODOGOYA CHEMICAL CO LTD
Organic electroluminescent element
ActiveCN110431680AImprove efficiencyReduce the driving voltageSolid-state devicesSemiconductor/solid-state device manufacturingElectron holeElectron injection
An object of the present invention is to provide an organic EL device having (1) high luminous efficiency and high power efficiency, (2) low turn on voltage, (3) low actual driving voltage, and (4) particularly a long lifetime by combining various materials for an organic EL device having excellent hole and electron injection / transport performances, electron blocking ability, stability in a thin-film state, and durability so as to allow the respective materials to effectively reveal their characteristics. The organic EL device has at least an anode, a hole transport layer, a light emitting layer, an electron transport layer and a cathode in this order, the hole transport layer includes an aryl amine compound represented by the following general formula (1), and the electron transport layerincludes a compound having a benzoazole ring structure represented by the following general formula (2).
Owner:HODOGOYA CHEMICAL CO LTD
Organic electroluminescent device
ActiveUS10326079B2Excellent hole injection and transport performanceReduce the driving voltageOrganic chemistrySolid-state devicesHole transport layerPolymer chemistry
An organic electroluminescent device having low driving voltage, high luminous efficiency, and a long lifetime is provided by combining various materials for an organic electroluminescent device. In the organic electroluminescent device having at least an anode, a hole injection layer, a first hole transport layer, a second hole transport layer, a light emitting layer, an electron transport layer, and a cathode in this order, the hole injection layer includes an arylamine compound of the following general formula (1) and an electron acceptor.In the formula, Ar1 to Ar4 may be the same or different, and represent a substituted or unsubstituted aromatic hydrocarbon group, a substituted or unsubstituted aromatic heterocyclic group, or a substituted or unsubstituted condensed polycyclic aromatic group.
Owner:HODOGOYA CHEMICAL CO LTD
Organic electroluminescent device
ActiveUS10693078B2Improve efficiencyReduce the driving voltageSilicon organic compoundsSolid-state devicesElectron injectionHole transport layer
An organic electroluminescent device having high efficiency, low driving voltage and a long lifetime is provided by combining various materials for an organic electroluminescent device, which are excellent, as materials for an organic electroluminescent device having high efficiency and high durability, in hole and electron injection / transport performances, electron blocking ability, stability in a thin-film state and durability, so as to allow the respective materials to effectively reveal their characteristics. In the organic electroluminescent device having at least an anode, a hole transport layer, a light emitting layer, an electron transport layer and a cathode in this order, the hole transport layer includes an arylamine compound represented by the following general formula (1), and the light emitting layer comprises an amine derivative of the following general formula (2) having a condensed ring structure.
Owner:HODOGOYA CHEMICAL CO LTD +1
Organic electroluminescent device
ActiveUS20180294416A1High hole mobilityExcellent in electron injection/transport performanceOrganic chemistrySolid-state devicesElectron holeElectron transporting layer
The present invention provides an organic EL device having at least an anode, a first hole transport layer, a second hole transport layer, a luminous layer, an electron transport layer, and a cathode in this order, wherein the second hole transport layer contains an arylamine compound represented by the following general formula (1), and the electron transport layer contains a pyrimidine derivative represented by the following general formula (2). The organic EL device of the present invention has a high efficiency, and is driven at a low driving voltage. Further, it has a particularly long lifetime.
Owner:HODOGOYA CHEMICAL CO LTD
Organic electroluminescent element
ActiveCN108604643AGood barrierHigh hole mobilitySolid-state devicesSemiconductor/solid-state device manufacturingElectron holeHole injection layer
The present invention provides an organic EL element having, in the indicated sequence, at least an anode, a hole injection layer, a hole transport layer, a light-emitting layer, an electron transportlayer, and a cathode, the organic EL element being characterized in that the hole injection layer contains an arylamine compound represented by general formula (1) and an electron acceptor. The organic EL element according to the present invention provides a higher light emission efficiency and a better durability than heretofore while retaining the same low drive voltage as heretofore.
Owner:HODOGOYA CHEMICAL CO LTD
Organic electroluminescent device
ActiveUS10497876B2Excellent hole injection and transport performanceReduce the driving voltageOrganic chemistrySolid-state devicesElectron holeHole injection layer
In the organic electroluminescent device having at least an anode, a hole injection layer, a hole transport layer, a light emitting layer, an electron transport layer and a cathode in this order, the hole injection layer includes an arylamine compound of the following general formula (1) and an electron acceptor.In the formula, Ar1 to Ar4 may be the same or different, and represent a substituted or unsubstituted aromatic hydrocarbon group, a substituted or unsubstituted aromatic heterocyclic group, or a substituted or unsubstituted condensed polycyclic aromatic group.
Owner:HODOGOYA CHEMICAL CO LTD +1
Arylamine compound having benzoazole ring structure, and organic electroluminescent element
PendingCN113382993AImprove fineAvoid damageOrganic chemistryElectroluminescent light sourcesDopantRefractive index
The purpose of the present invention is to provide an organic EL element in which a capping layer is combined with various materials of the element so that properties of each of the materials of the element can be effectively demonstrated. The capping layer is constituted from a material that does not affect material inside the organic EL element when sunlight having wavelengths of 400-410 nm is absorbed, that has a high absorption coefficient and a high refractive index in order to greatly improve light extraction efficiency, that gives a thin film having excellent stability, durability and lightfastness, and that does not absorb light in the blue, green and red wavelength regions. The invention relates to: an arylamine compound having a benzoazole ring structure; and an organic EL element having a capping layer that contains the arylamine compound, and a luminescent layer that contains a host and a phosphorescent dopant.
Owner:HODOGOYA CHEMICAL CO LTD +1
High molecular weight triarylamine compound comprising terphenyl structure in molecular main chain and organic electroluminescent element comprising said high molecular weight compound
PendingUS20210253785A1High molecular weightExcellent hole injectionSolid-state devicesSemiconductor/solid-state device manufacturingPolymer sciencePolystyrene
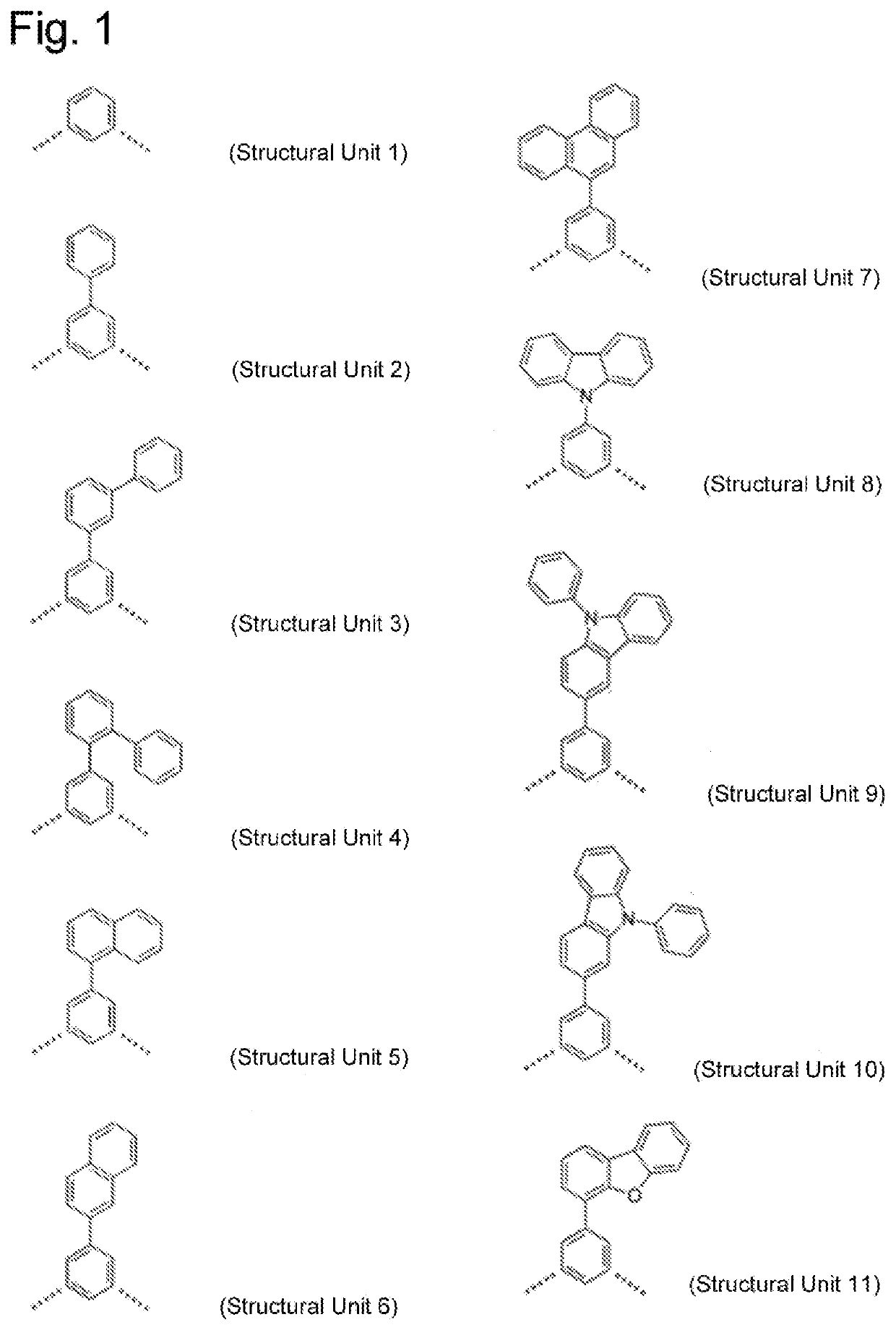
It is an object of the present invention to provide a high molecular weight compound that has excellent hole injection and transport performance, is capable of blocking electrons, and is highly stable as a thin film. It is another object of the present invention to provide an organic EL element that includes an organic layer (thin film) made of the above-described high molecular weight compound, wherein the organic EL element has high light emission efficiency and a long lifespan. The high molecular weight compound according to the present invention includes a repeating unit represented by a general formula (3), that is constituted by a specific triarylamine structural unit and a specific bonding structural unit, and has a weight average molecular weight of 10,000 or more and less than 1,000,000 on a polystyrene basis.
Owner:HODOGOYA CHEMICAL CO LTD
Charge-transporting varnish
InactiveUS10158081B2Excellent brightness characteristic and durabilityHigh yieldSolid-state devicesSemiconductor/solid-state device manufacturingDopantOrganic solvent
Provided is a charge-transporting varnish which includes a charge-transporting material including fluorine atoms, a charge-transporting material not including fluorine atoms, a dopant material comprising heteropoly acid, and an organic solvent, said charge-transporting material including fluorine atoms being a polymer of weight-average 1,000 to 200,000 molecular weight obtained by condensing a triarylamine compound, an aryl aldehyde compound including fluorine atoms, and a fluorine derivative having a carbonyl group, and said charge-transporting material not including fluorine atoms being an oligoaniline compound. The charge-transporting varnish provides a thin film which, even in a case of being used as a single layer in contact with and in between an anode and a luminescent layer, is capable of achieving an organic EL element having superior luminance characteristics and durability.
Owner:NISSAN CHEM IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com