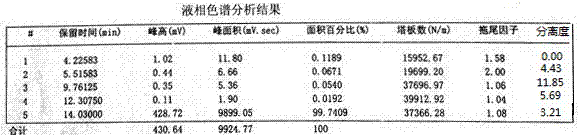
Method for preparing ultraviolet light absorber UVT-150
A technology of UVT-150 and absorbent, applied in the direction of organic chemistry, etc., can solve the problems of difficult esterification reaction, difficult to increase yield, waste water pollution, etc., and achieve the effect of simple production process, mild reaction conditions and simple preparation route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
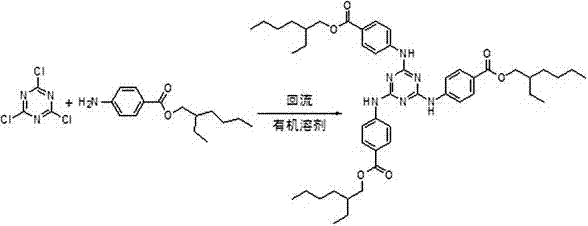
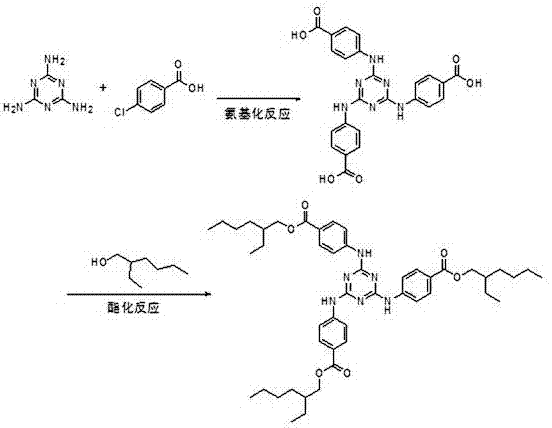
[0031] ①Aromatic amination reaction: Add 950g of methyl p-aminobenzoate and 2000 mL of xylene to the reactor, raise the temperature to 60-150°C and stir to dissolve, and slowly drop the pre-dissolved in 1500 mL of xylene under this condition 380g of cyanuric chloride, the dropwise addition is completed, and the reaction is carried out at 120~125°C for 5-6h. The hydrogen chloride gas generated during the reaction is introduced into the lye absorption device. After the reaction is completed, the solvent is recovered by distillation, cooled and crystallized to obtain the intermediate methyl Triazinone 1036g, yield 98%, directly used for the next step transesterification reaction without refining.
[0032] ②Transesterification reaction: Add 2000 mL of toluene, 103.6 g of sodium methoxide and 1210 mL of isooctyl alcohol into the reactor, heat and stir to dissolve, and raise the temperature to 110~115 °C, slowly add 1500 mL of toluene containing 1036 g of methyl triazone Solution, a...
Embodiment 2
[0036] ①Aromatic amination reaction: Add 850g of methyl p-aminobenzoate and 1500 mL of benzene into the reactor, raise the temperature to 60-150°C and stir to dissolve, and slowly add dropwise the pre-dissolved in 800 mL of xylene under this condition 369g of cyanuric chloride, after the dropwise addition, keep warm at 120~125°C for 5-6h, the hydrogen chloride gas generated during the reaction is introduced into the lye absorption device, after the reaction is completed, the solvent is recovered by distillation, cooled and crystallized to obtain the intermediate methyl trichloride Azinonone 951g, yield 90%, directly used for the next step transesterification reaction without refining.
[0037]②Transesterification reaction: Add 1000 mL of toluene, 80 g of sulfuric acid and 800 mL of isooctyl alcohol into the reactor, heat and stir to dissolve, and raise the temperature to 110~115 °C, slowly add 1200 mL of toluene solution of 951 g of methyl triazone, dropwise After the addition...
Embodiment 3
[0039] ①Aromatic amination reaction: Add 800g of methyl p-aminobenzoate and 1800 mL of toluene to the reactor, raise the temperature to 60-150°C and stir to dissolve, and slowly add dropwise the pre-dissolved in 900 mL of xylene under this condition After the addition of 369g cyanuric chloride, the dropwise reaction was carried out at 120~125°C for 5-6 hours. The hydrogen chloride gas generated during the reaction was introduced into the lye absorption device. After the reaction was completed, the solvent was recovered by distillation, cooled and crystallized to obtain the intermediate methyl trichloride Azinonone 850g, yield 80%, directly used for the next step transesterification reaction without refining.
[0040] ②Transesterification reaction: Add 1000 mL of toluene, 70 g of toluenesulfonic acid and 800 mL of isooctyl alcohol into the reactor, heat and stir to dissolve, and raise the temperature to 110~115 °C, slowly add 850 g of methyl triazone in 1000 mL of toluene Solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com