Preparation method of eldecalcitol A ring intermediate
A technology of idecalcidol and intermediates, which is applied in chemical instruments and methods, organic chemistry, bulk chemical production and other directions, can solve the problems of long synthesis route, high production cost, high difficulty and the like, and achieves simple preparation method, Inexpensive raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
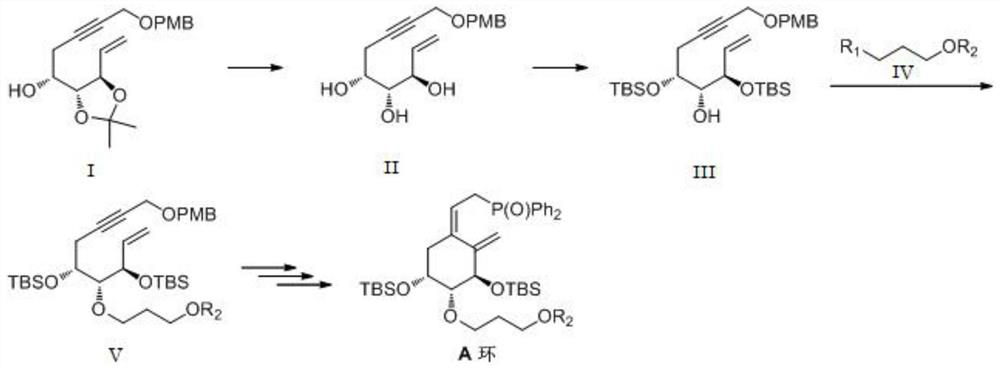
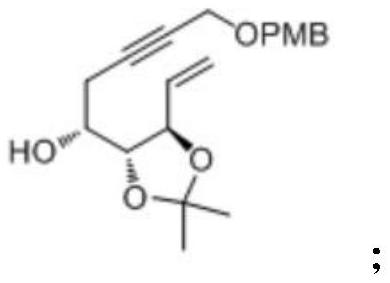
[0042] This embodiment provides a kind of preparation method of idecalcitol A ring intermediate, and synthetic route is as follows figure 1 shown, including the following steps:
[0043] (1) Preparation of Compound II
[0044] Compound I (100.0g, 288.9mmoL) was dissolved in 500.0mL of methanol and 50.0mL of water in a 1000mL three-necked flask, hydrochloric acid (216.7g, 433.3mmoL) was added at room temperature, stirred at room temperature for 15 hours, and the reaction solution was concentrated and dried to obtain The crude product was subjected to column chromatography with ethyl acetate and n-heptane to obtain compound II (60.2 g) with a yield of 67.9%.
[0045] (2) Preparation of Compound III
[0046] Dissolve compound II (60.0g, 196.0mmoL) in 500.0mL of N,N-dimethylformamide in a 1000mL three-neck flask, cool down to -5°C-0°C, add imidazole (53.3g, 783.9mmoL), Add tert-butyldimethylsilyl chloride (88.2g, 588.0mmoL) in batches, heat up to room temperature and stir for 1...
Embodiment 2
[0051] (1) Preparation of Compound II
[0052] Compound I (100.0g, 288.9mmoL) was dissolved in 500.0mL ethanol in a 1000mL three-necked flask, 50mL of 10% sulfuric acid solution was added at room temperature, stirred at room temperature for 11 hours, the reaction solution was concentrated and dried to obtain the crude product, which was washed with ethyl acetate and n-heptane column chromatography to obtain compound II (59.0 g) with a yield of 65%.
[0053] (2) Preparation of Compound III
[0054]Dissolve compound II (60.0g, 196.0mmoL) in 500.0mL of N,N-dimethylformamide in a 1000mL three-neck flask, cool down to -5°C-0°C, add imidazole (53.3g, 783.9mmoL), Add tert-butyldimethylsilyl chloride (88.2g, 588.0mmoL) in batches, heat up to room temperature and stir for 15 hours, add water, extract with ethyl acetate, dry and concentrate to obtain the crude product, which is washed with ethyl acetate and n-heptyl Through alkane column chromatography, compound III (85.0 g) was obtai...
Embodiment 3
[0059] (1) Preparation of Compound II
[0060] Dissolve compound I (100.0g, 288.9mmoL) in 500.0mL methanol and 50.0mL water in a 1000mL three-necked flask, add hydrochloric acid (216.7g, 433.3mmoL) at room temperature, stir at room temperature for 15 hours, and concentrate the reaction solution to dryness to obtain The crude product was subjected to column chromatography with ethyl acetate and n-heptane to obtain compound II (60.2 g) with a yield of 67.9%.
[0061] (2) Preparation of Compound III
[0062] Dissolve compound II (60.0g, 196.0mmoL) in 500.0mL of N,N-dimethylformamide in a 1000mL three-neck flask, cool down to -5°C-0°C, add imidazole (53.3g, 783.9mmoL), Add tert-butyldimethylsilyl chloride (88.2g, 588.0mmoL) in batches, heat up to room temperature and stir for 15 hours, add water, extract with ethyl acetate, dry and concentrate to obtain the crude product, which is washed with ethyl acetate and n-heptyl Through alkane column chromatography, compound III (85.0 g) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com