Patents
Literature
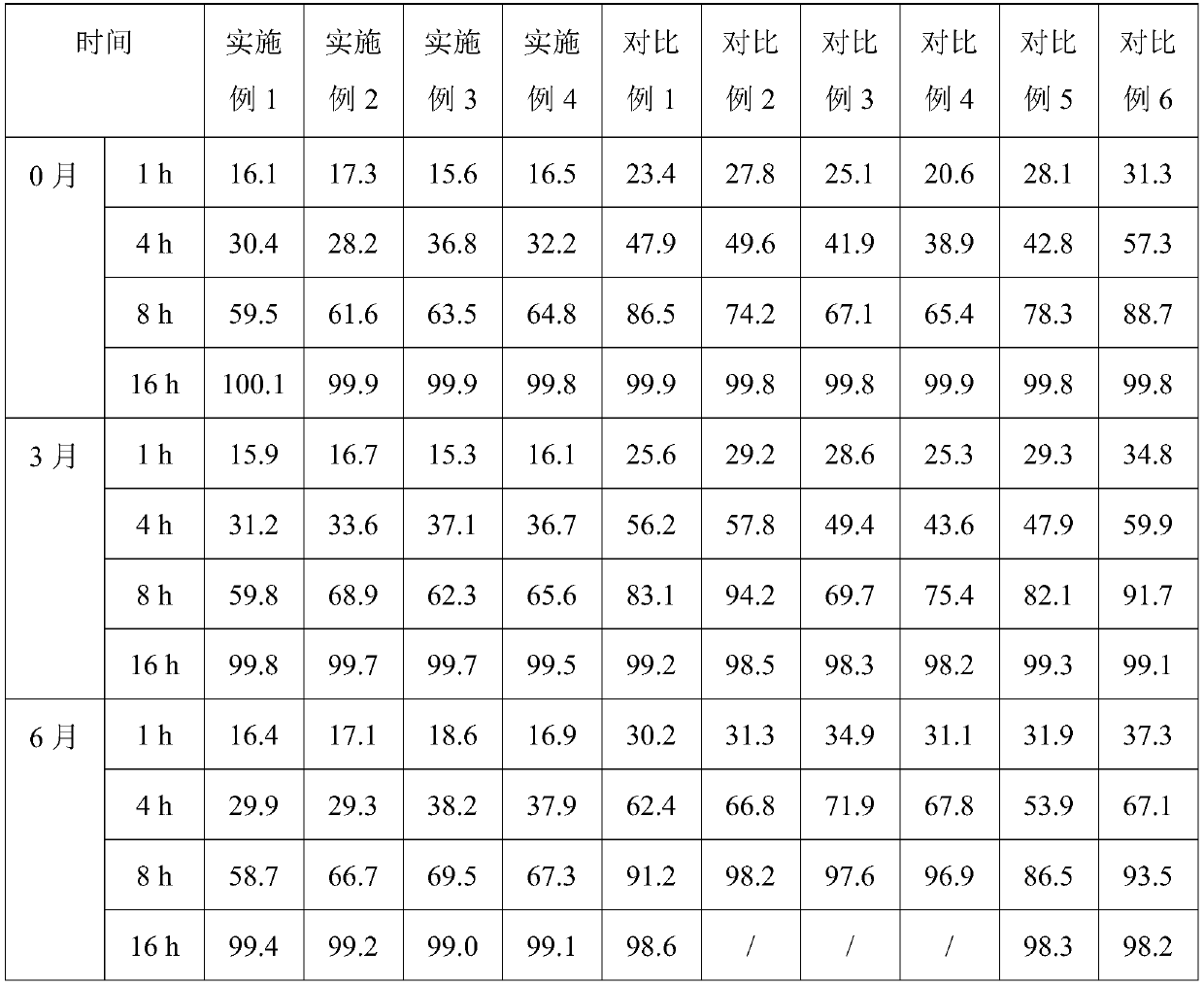
59 results about "Alfacalcidol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
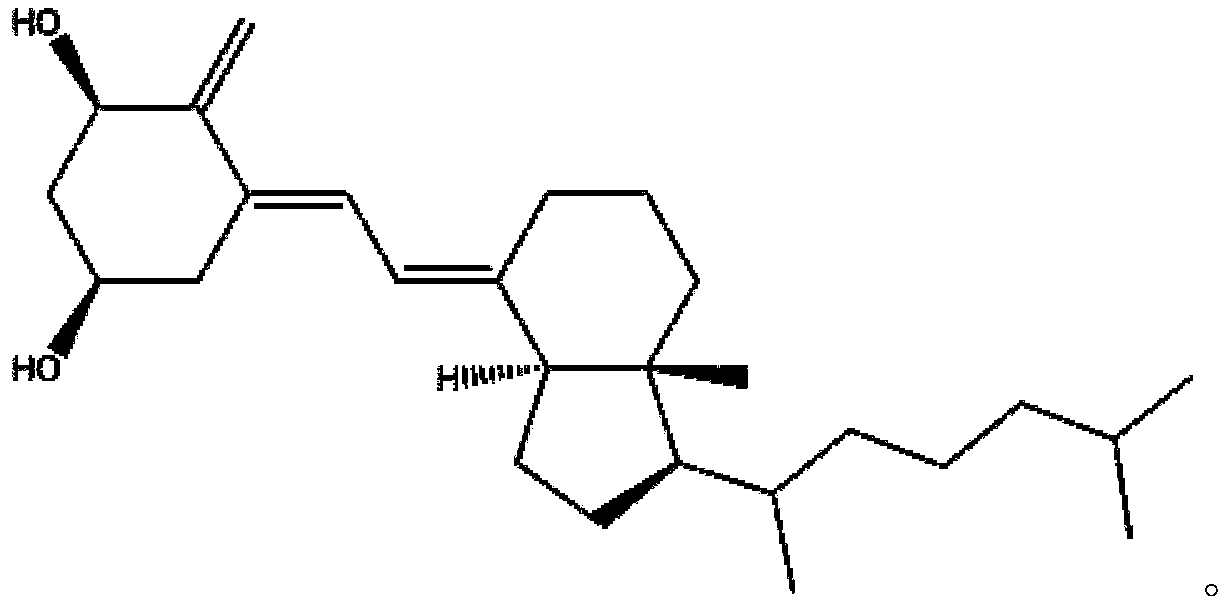
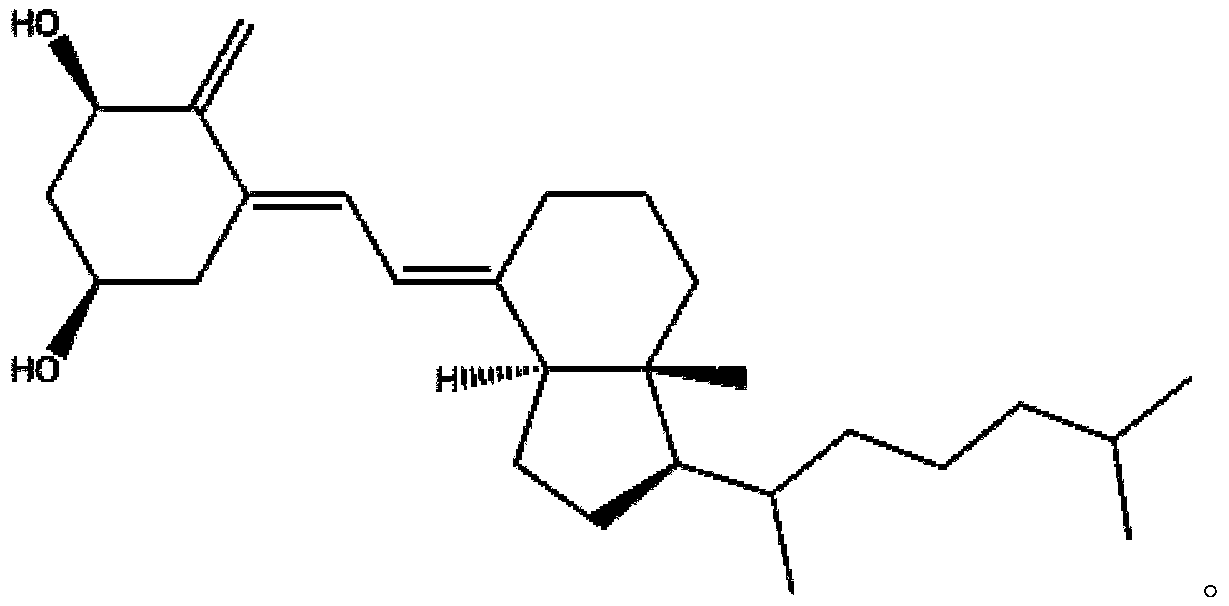
Alfacalcidol (or 1-hydroxycholecalciferol) is an analogue of vitamin D used for supplementation in humans and as a poultry feed additive. Alfacalcidol has a weaker impact on calcium metabolism and parathyroid hormone levels than calcitriol, however alfacalcidol has significant effects on the immune system, including regulatory T cells. It is considered to be a more useful form of vitamin D supplementation, mostly due to much longer half-life and lower kidney load. It is the most commonly prescribed vitamin D metabolite for patients with end stage renal disease, given that impaired renal function alters the ability to carry out the second hydroxylation step required for the formation of the physiologically active form of vitamin D, 1,25-dihydroxyvitamin D3. Alfacalcidol is an active vitamin D3 metabolite, and therefore does not require the second hydroxylation step in the kidney.
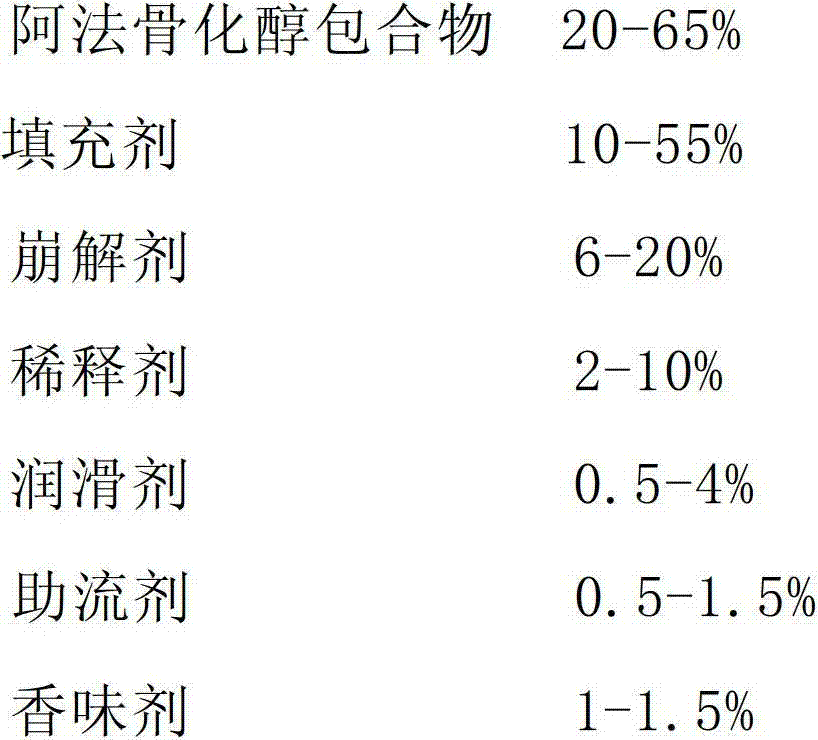
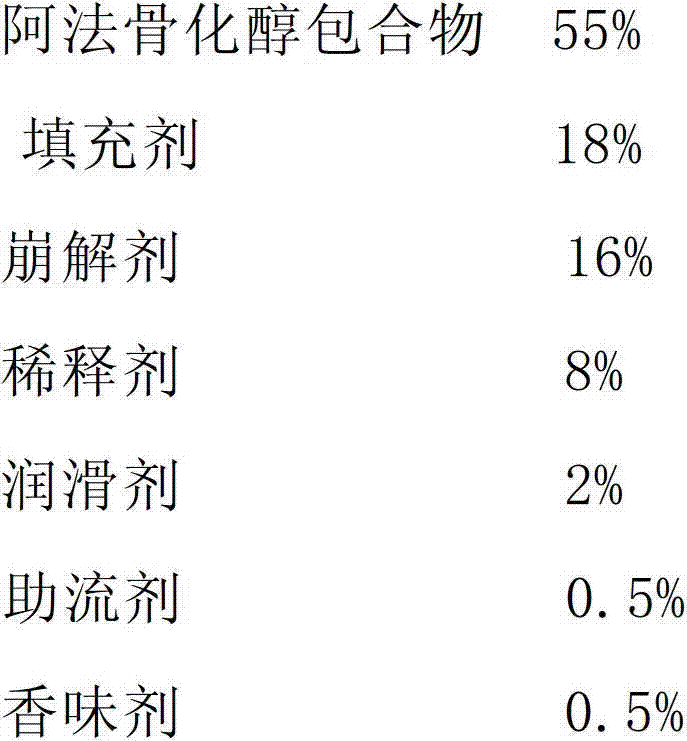
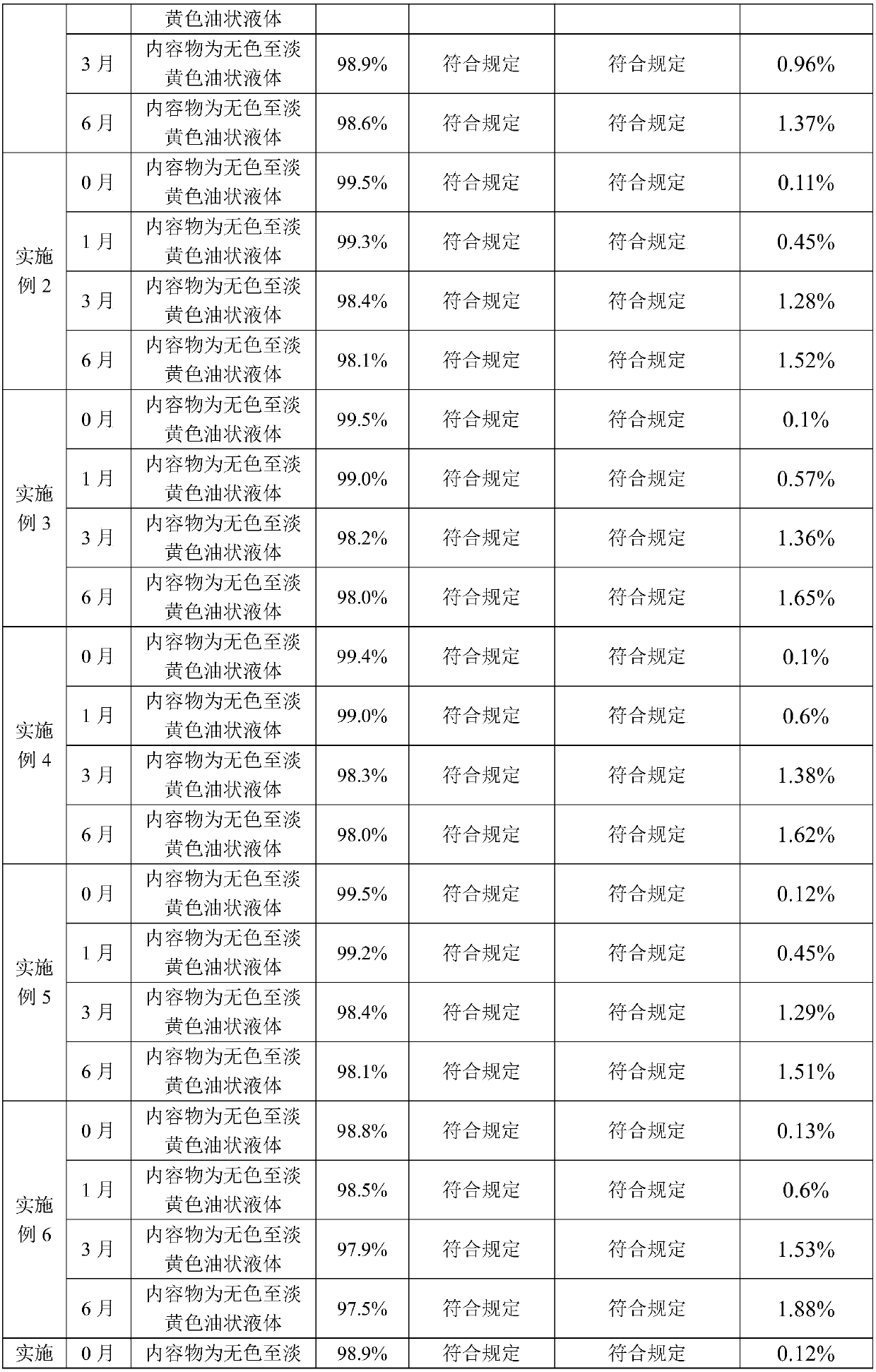
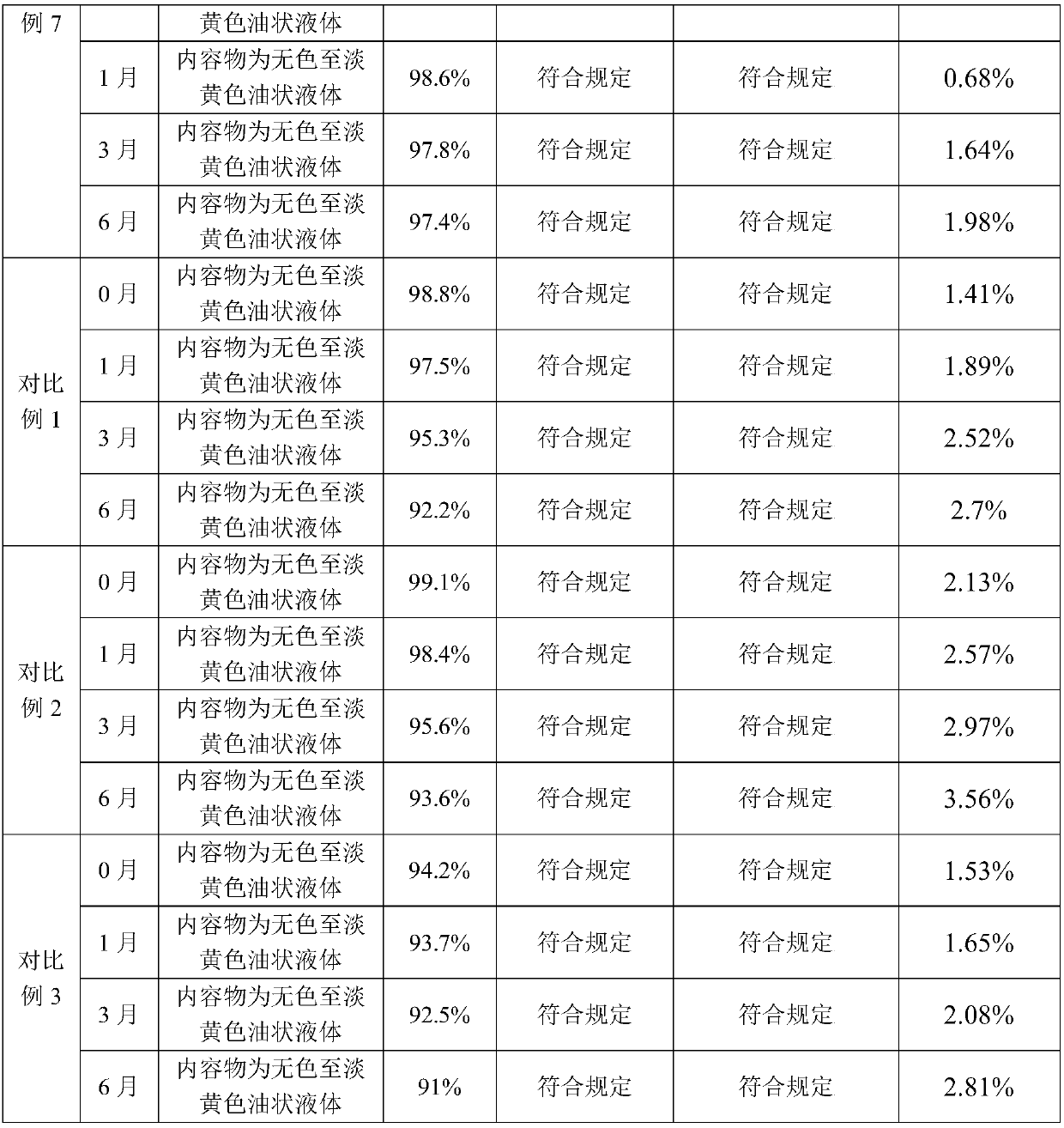
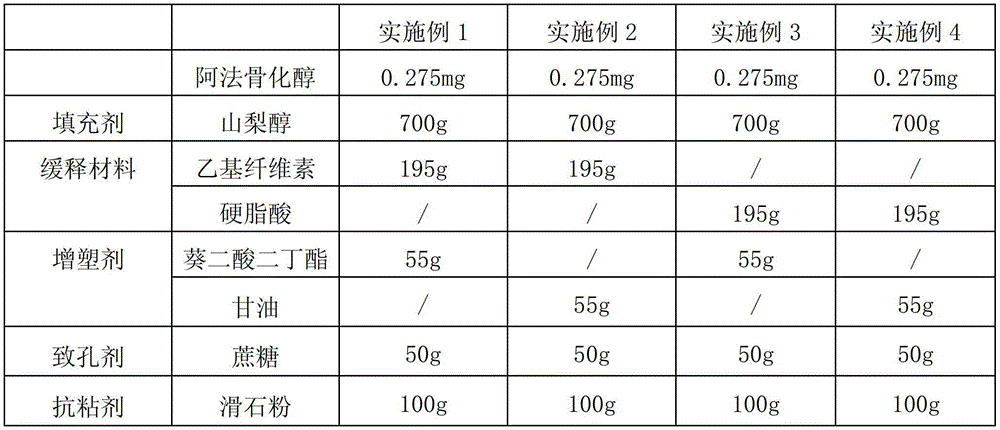
Alfacalcidol dispersible tablet and preparation method thereof
ActiveCN103110598APromote dissolutionFast absorptionOrganic active ingredientsMetabolism disorderFlavouring agentMedicine
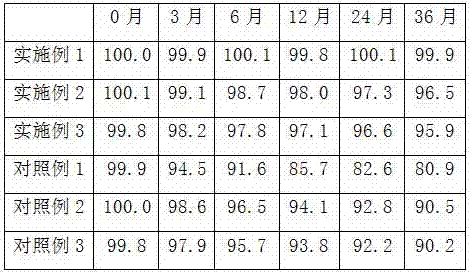
The invention discloses an alfacalcidol dispersible tablet and a preparation method of the alfacalcidol dispersible tablet. The alfacalcidol dispersible tablet is prepared from an alfacalcidol clathrate compound and a preparation supplementary material, wherein the preparation supplementary material comprises a filling agent, a disintegrating agent, a diluting agent, a lubricating agent, a flow aid and a flavouring agent; and the alfacalcidol clathrate compound is prepared by carrying out clathration on alfacalcidol and 2,6-dimethyl-Beta-cyclodextrin based on the molar ratio of 4: 1 to 0.5: 3. The alfacalcidol dispersible tablet has the advantages of being quick to dissolve out, fast to absorb, high in bioavailability, excellent in stability, convenient to take, etc.
Owner:CP PHARMA QINGDAO CO LTD
Alfacalcidol capsules and preparation method thereof
ActiveCN103110606APromote dissolutionFast absorptionOrganic active ingredientsMetabolism disorderMedicineDissolution
The invention discloses alfacalcidol capsules and a preparation method of the alfacalcidol capsules. The alfacalcidol capsules comprise alfacalcidol and formulation excipients, wherein the formulation excipients comprise a filler, a disintegrant, a surfactant, a diluent, a lubricant, a glidant and a flavoring agent. The alfacalcidol capsules disclosed by the invention have the advantages of quickness in dissolution, fastness in absorption, high bioavailability, good stability and convenience in taking.
Owner:CP PHARMA QINGDAO CO LTD
Preparation method for alfacalcidol
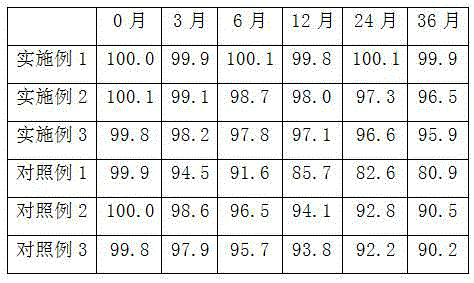
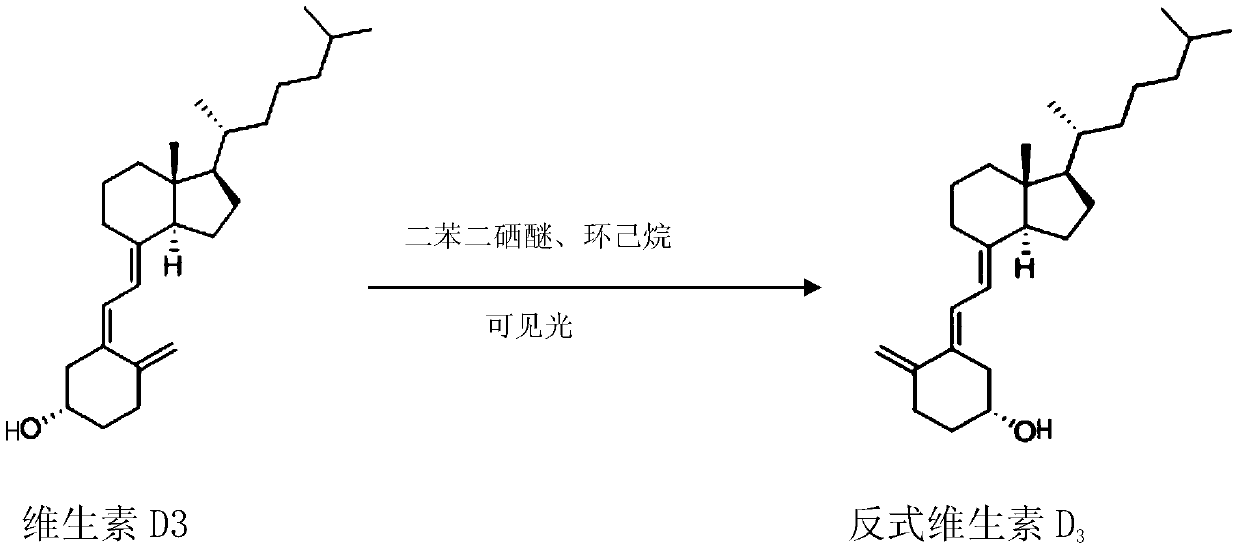
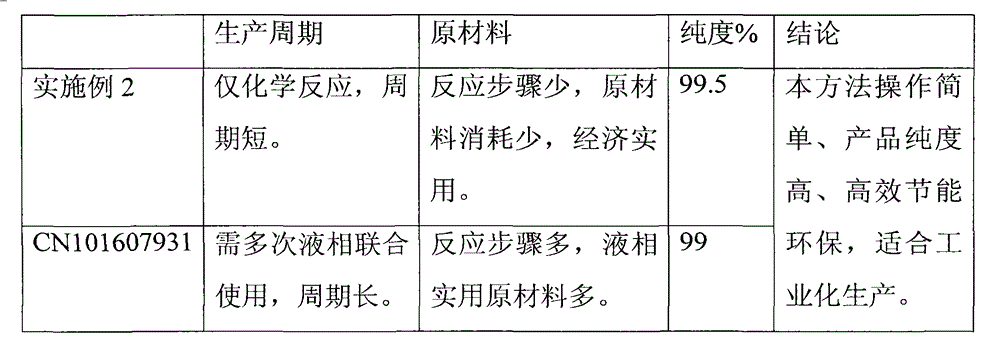
The invention relates to a preparation method for a compound, in particular to the preparation method for separating and purifying alfacalcidol. Aiming at the deficiencies of the conventional preparation technology, the invention provides a novel method for preparing alfacalcidol with good yield and high purity. The preparation method comprises the steps as follows: taking vitamin D3 as a raw material; removing most of trans-isomer impurities generated after chemical reaction by utilizing Diels-Alder reaction; and refining and purifying by preparative high pressure liquid chromatography to prepare high-purity alfacalcidol. According to the preparation method, the reaction conditions are moderate, the yield is high, and the purity of alfacalcidol reaches up to 99.5%.
Owner:CP PHARMA QINGDAO CO LTD
Alfacalcidol solution and preparation method thereof
InactiveCN104800156AIncrease contentReduce dosageOrganic active ingredientsSkeletal disorderDistilled waterHypromellose
The invention relates to an alfacalcidol solution and a preparation method thereof. The solution is prepared from the following ingredients by weight percent: 0.0005 percent of alfacalcidol, 0.9-1.2 percent of polyvinylpyrrolidone K30, 27-30 percent of hydroxypropyl methylcellulose water solution, 12-14 percent of lauryl sodium sulfate, 0.2 percent of an antioxygen, 0.5 percent of a corrigent, and the balance of distilled water. Compared with the prior art, the content of alfacalcidol in the solution is remarkably increased; the medicine dose is reduced; the stability of alfacalcidol for light and air is improved; the bioavailability of alfacalcidol is remarkably improved as well.
Owner:CP PHARMA QINGDAO CO LTD
Oral solution containing alfacalcidol
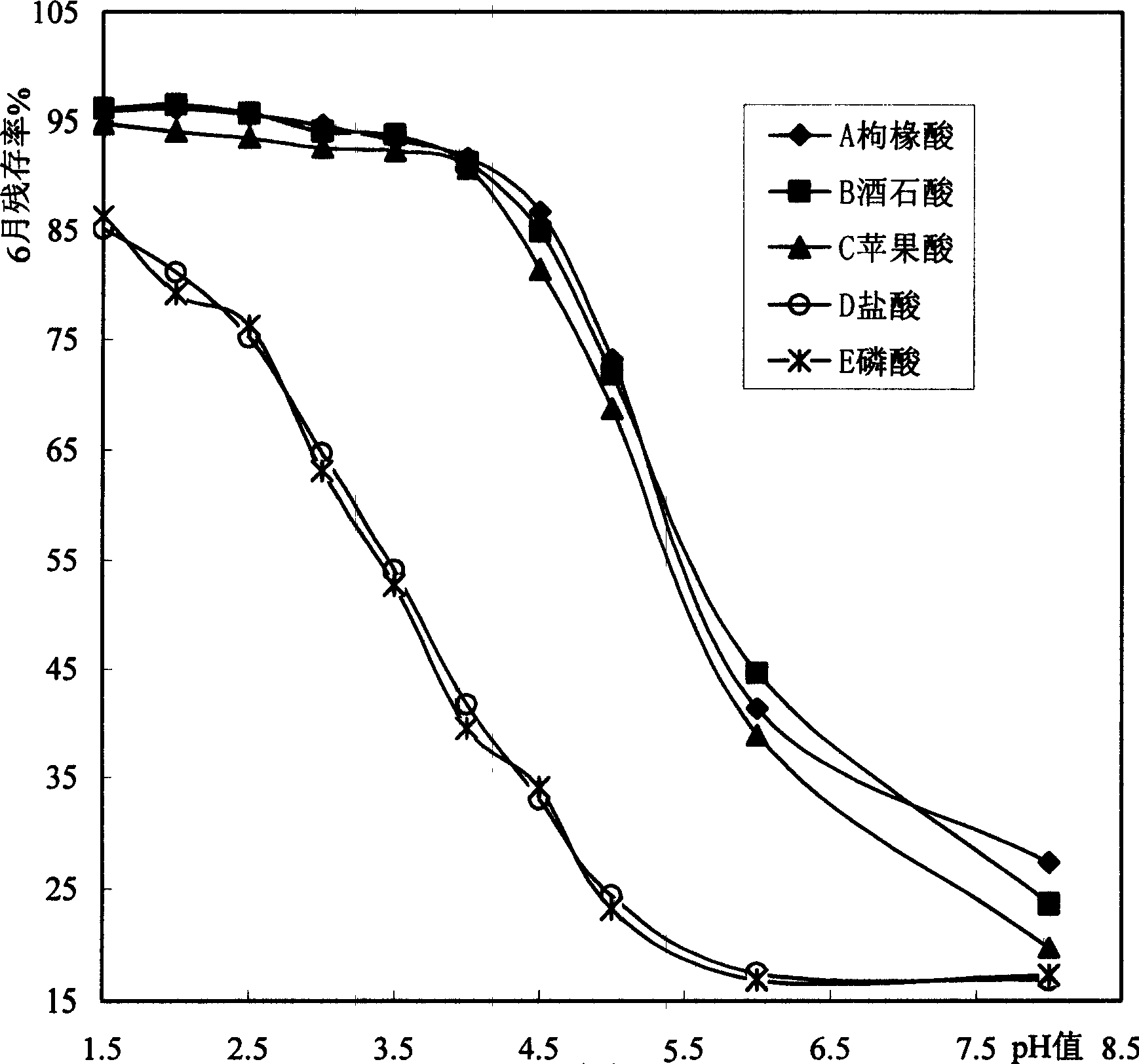
The present invention relates to medicine technology, and is especially one kind of oral solution containing alfacalcidol. The oral solution contains: alfacalcidol in therapeutically effective amount, therapeutically acceptable organic acid supplementary material in such amount as to regulate the pH value of the or al solution to 2.0-4.0, and water. The oral solution containing alfacalcidol has high stability.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Alfacalcidol injection and preparation method thereof
InactiveCN104800155AIncrease contentReduce dosageOrganic active ingredientsPharmaceutical delivery mechanismMedicineHypromellose
The invention relates to an alfacalcidol injection and a preparation method thereof. The injection is prepared from the following ingredients by weight percent: 0.0005 percent of alfacalcidol, 1.2-1.6 percent of polyvinylpyrrolidone K30, 18-24 percent of hydroxypropyl cellulose aqueous solution, 1.2-1.7 percent of sodium dodecyl sulfate, 0.1 percent of an antioxygen, a moderate amount of an isoosmotic adjusting agent, and the balance of water for injection. The content of alfacalcidol in the preparation is remarkably increased; the medicine dose is decreased; the stability of alfacalcidol for light and air is improved; the bioavailability of alfacalcidol is remarkably improved as well.
Owner:CP PHARMA QINGDAO CO LTD
Medicament for treating degenerative osteopathy and preparation method thereof
InactiveCN101703764ADefinite curative effectSafe to takeOrganic active ingredientsPeptide/protein ingredientsVitamin CMagnesium stearate
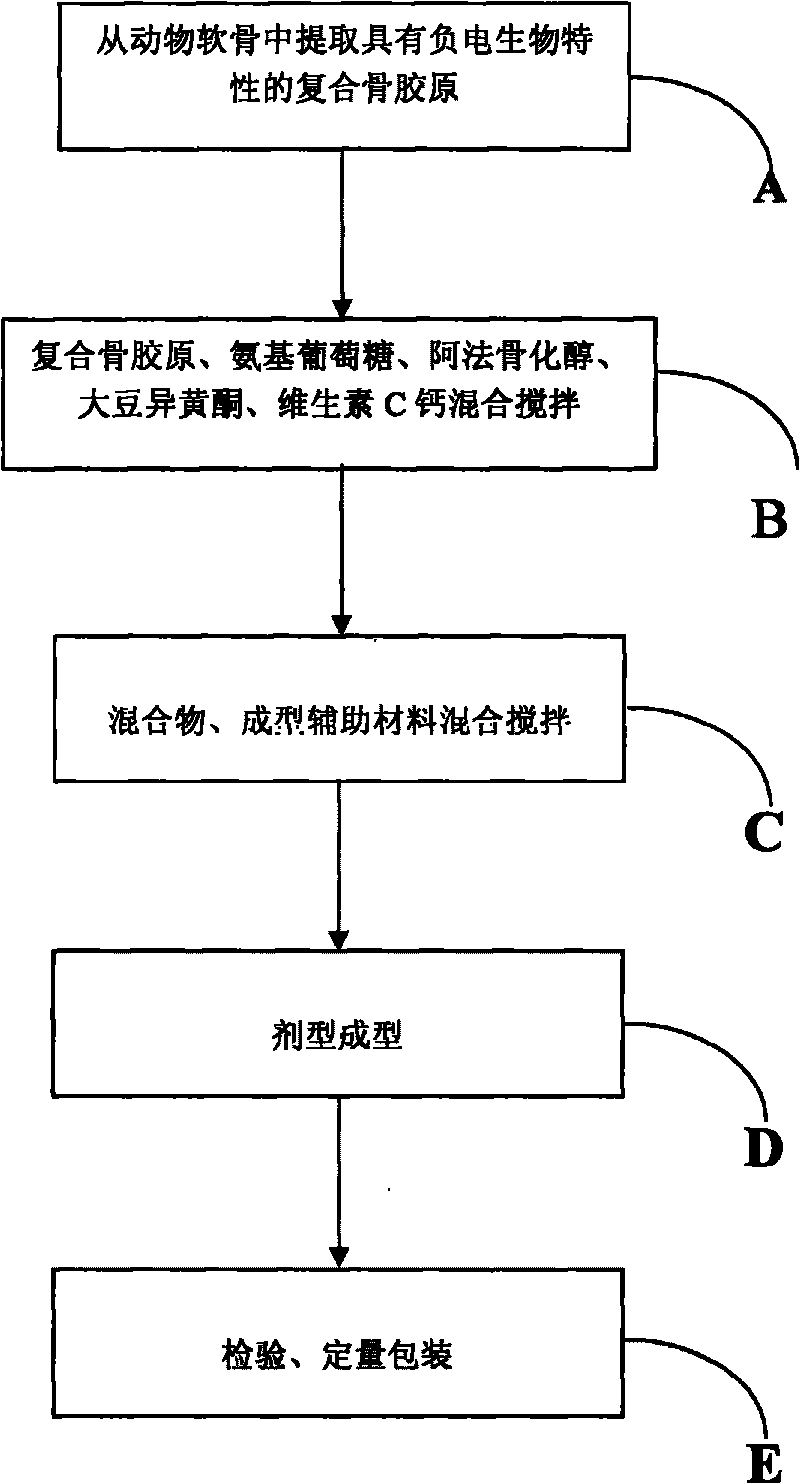
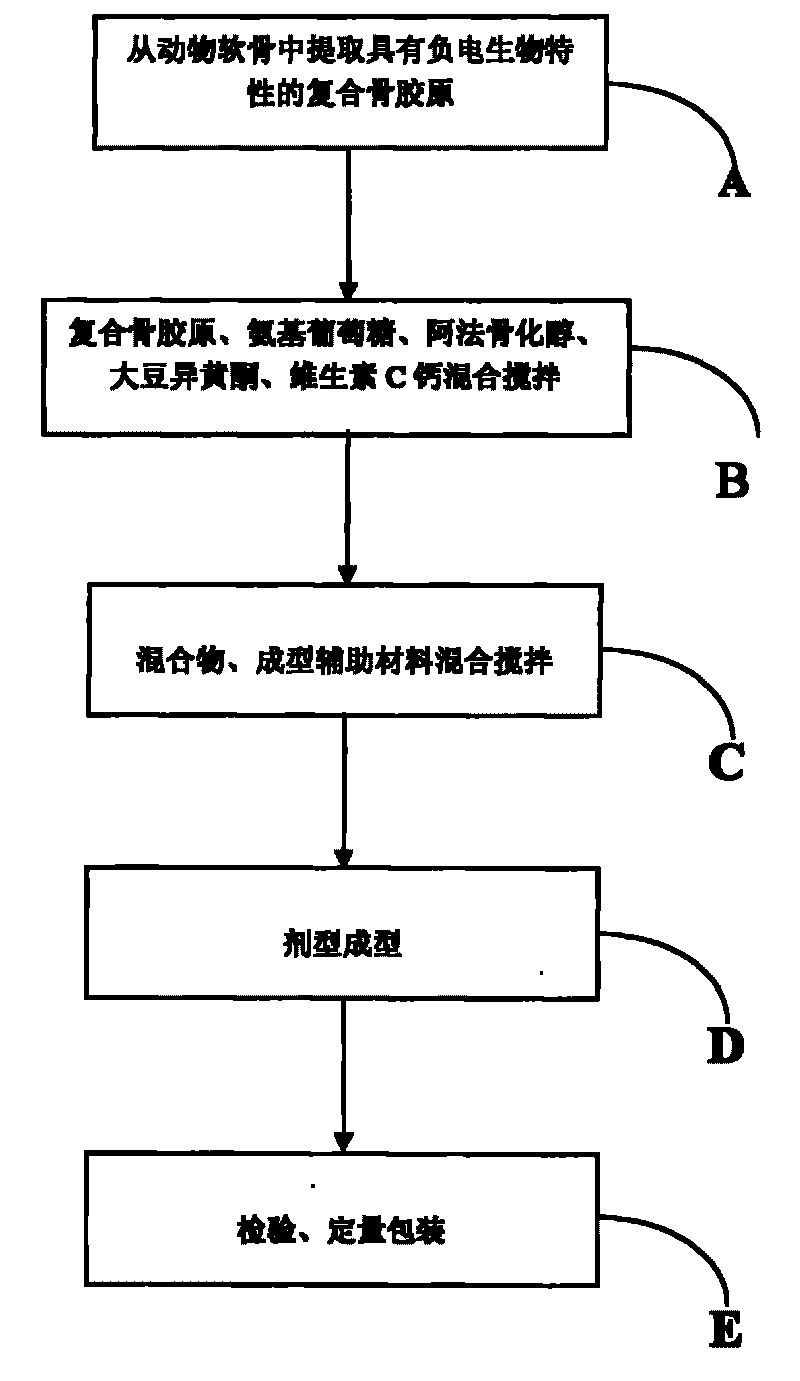
The invention relates to a medicament for treating degenerative osteopathy, which mainly comprises the following raw materials by mass: 20 to 60 kilograms of compound bone collagen, 20 to 60 kilograms of glucosamine, 0.05 to 1.0 kilogram of Alfacalcidol, 3 to 30 kilograms of soy isoflavones, 3 to 30 kilograms of vitamin C calcium, 10 to 60 kilograms of cane sugar, 1 to 20 kilograms of dextrin, 1 to 20 kilograms of high-expansion type carboxyrnethyl starch sodium, 0.5 to 10 kilograms of magnesium stearate and 5 to 50 kilograms of silicon dioxide. The invention also provides a method for preparing the medicament.
Owner:武汉德晶尔丽生物科技股份有限公司
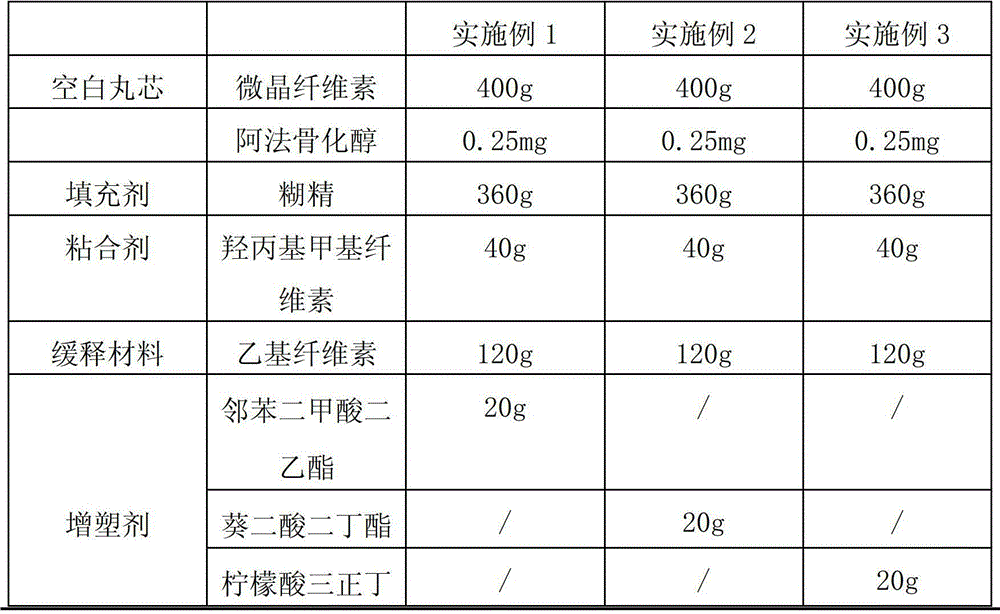
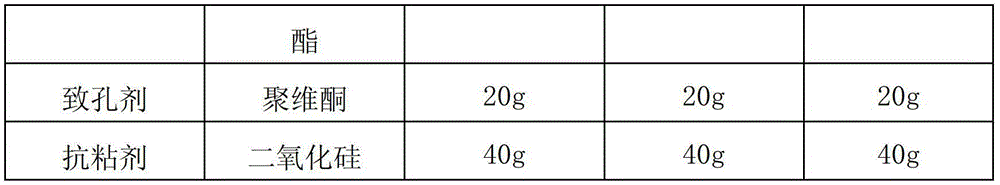
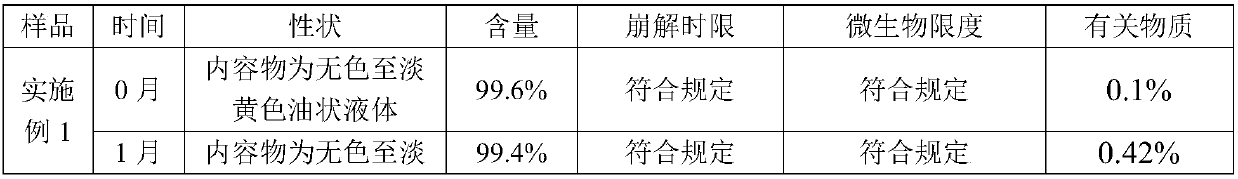
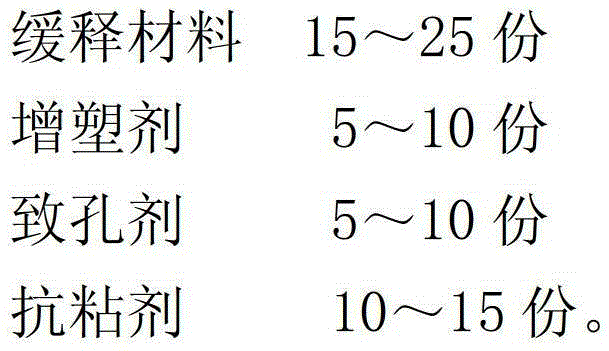
Alfacalcidol sustained-release capsule and preparation method thereof
ActiveCN103142555ASmooth releaseLittle side effectsOrganic active ingredientsMetabolism disorderSustained release pelletsSide effect
The invention discloses an alfacalcidol sustained-release capsule and a preparation method thereof. The alfacalcidol sustained-release capsule disclosed by the invention is prepared through filling an alfacalcidol sustained-release pellet in a capsule shell; and during preparation, the alfacalcidol sustained-release pellet is obtained through coating a drug-containing layer and a coating layer outside a blank pellet core and then filled in the capsule shell, thereby obtaining the alfacalcidol sustained-release capsule. According to the alfacalcidol sustained-release capsule and the preparation method of the alfacalcidol sustained-release capsule, the drug release is uniform, the aims of long acting and curative effect improving can be reached, and the dosage can be reduced while the same drug action is maintained, so that side effects to patients caused by drug taking are reduced; and the preparation method is simple, the quality of obtained products is stable, and thus, the method is applicable to large-scale production application.
Owner:CP PHARMA QINGDAO CO LTD
Alfacalcidol soft capsule and preparation method thereof
InactiveCN109568287AEffective protectionImprove stabilityOrganic active ingredientsMetabolism disorderAntioxidantShielding gas
The invention relates to an alfacalcidol soft capsule and a preparation method thereof. The contents of the alfacalcidol soft capsule comprise the following components in parts by weight: 85-125 partsof oily substrate, 0.01-0.2 part of antioxidant and 0.000001-0.000015 part of alfacalcidol, wherein the oily substrate is midchain triglyceride fatty acid. The preparation method comprises the following steps: (1) mixing antioxidant with a part of oily substrate to obtain a mixture; (2) pouring the rest oily substrate into a liquid preparation tank, and then adding the mixture acquired in step (1) into the liquid preparation tank, stirring and uniformly mixing; (3) placing the tank in a dark place, adding alfacalcidol into the liquid preparation tank, and uniformly stirring and mixing under the atmosphere of protective gas to obtain contents of the alfacalcidol soft capsule; (4) preparing the contents into the soft capsule in a dark place. The alfacalcidol soft capsule is high in stability.
Owner:GUANGZHOU XINGQUN PHARMA
Soft alfacalcidol capsule and preparation method thereof
ActiveCN104800187AIncrease contentReduce dosageOrganic active ingredientsMetabolism disorderSoftgelPolythylene glycol
The invention relates to a soft alfacalcidol capsule and a preparation method thereof. The content of the soft capsule is prepared from the following ingredients in parts by weight: 35-50 parts of alfacalcidol, 15-20 parts of glycerinum, 20-40 parts of polyethylene glycol 4000, 10-20 parts of polyethylene glycol 6000, 10-20 parts of an accelerant, 45-57 parts of a thickener and 30-60 parts of an antioxygen. The preparation method improves the content of active ingredients in the preparation and reduces medicine dose; by adding specific ingredients including the glycerinum, polyethylene glycol 4000 and polyethylene glycol 6000, the stability and bioavailability of the soft capsule are effectively improved; the preparation formulation of conventional alfacalcidol preparations is successfully changed, so that alfacalcidol can be better taken and absorbed by patients.
Owner:CP PHARMA QINGDAO CO LTD
Inspection method for alfacalcidol tablet related substances
ActiveCN108663442AReliable impurity profile referenceHigh probability of degradationComponent separationSilica gelImpurity
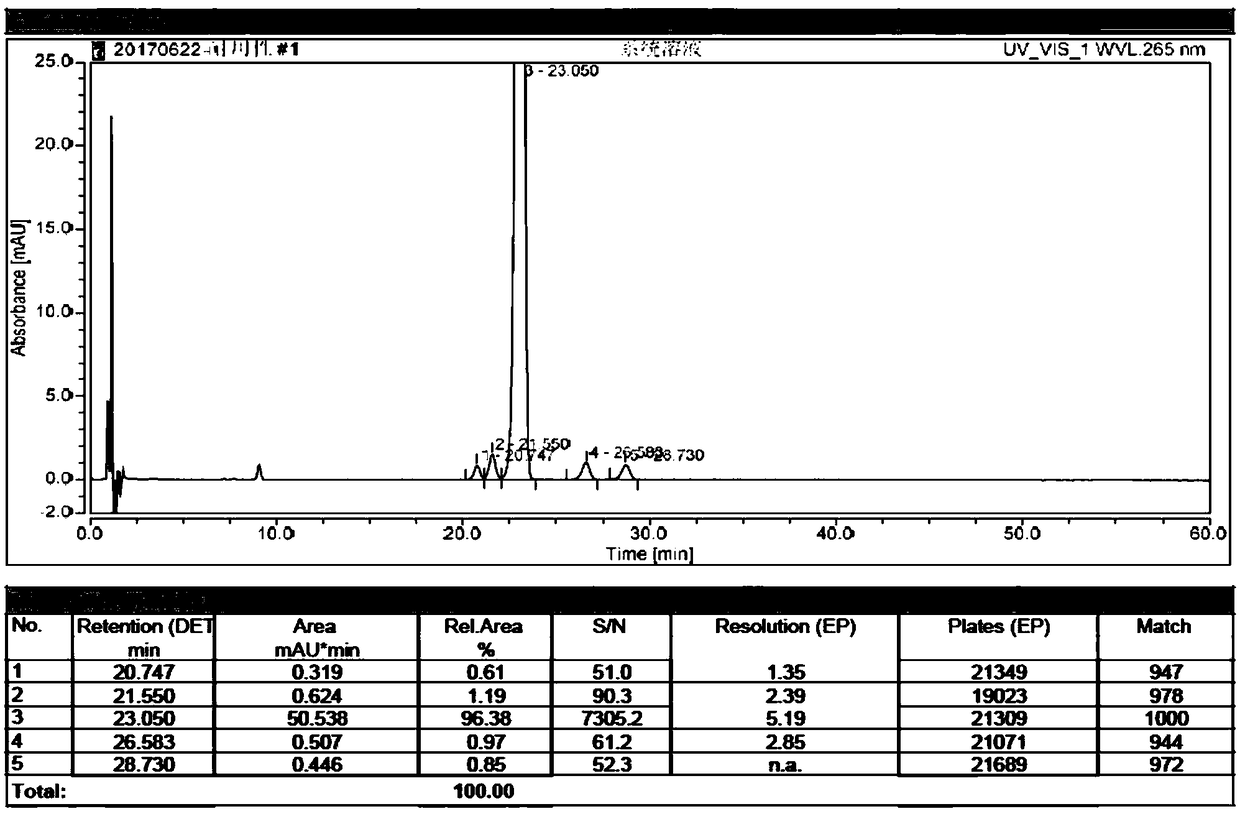
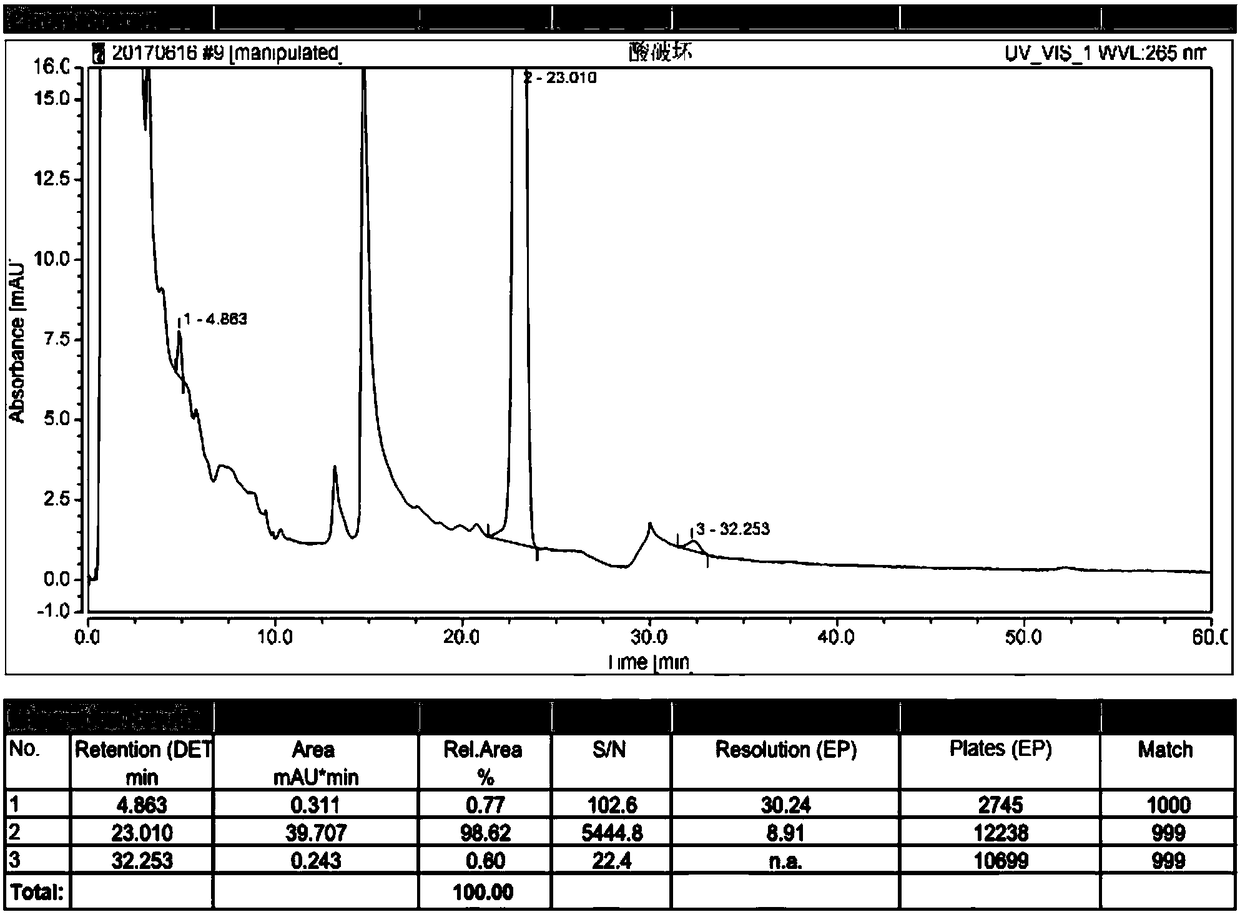
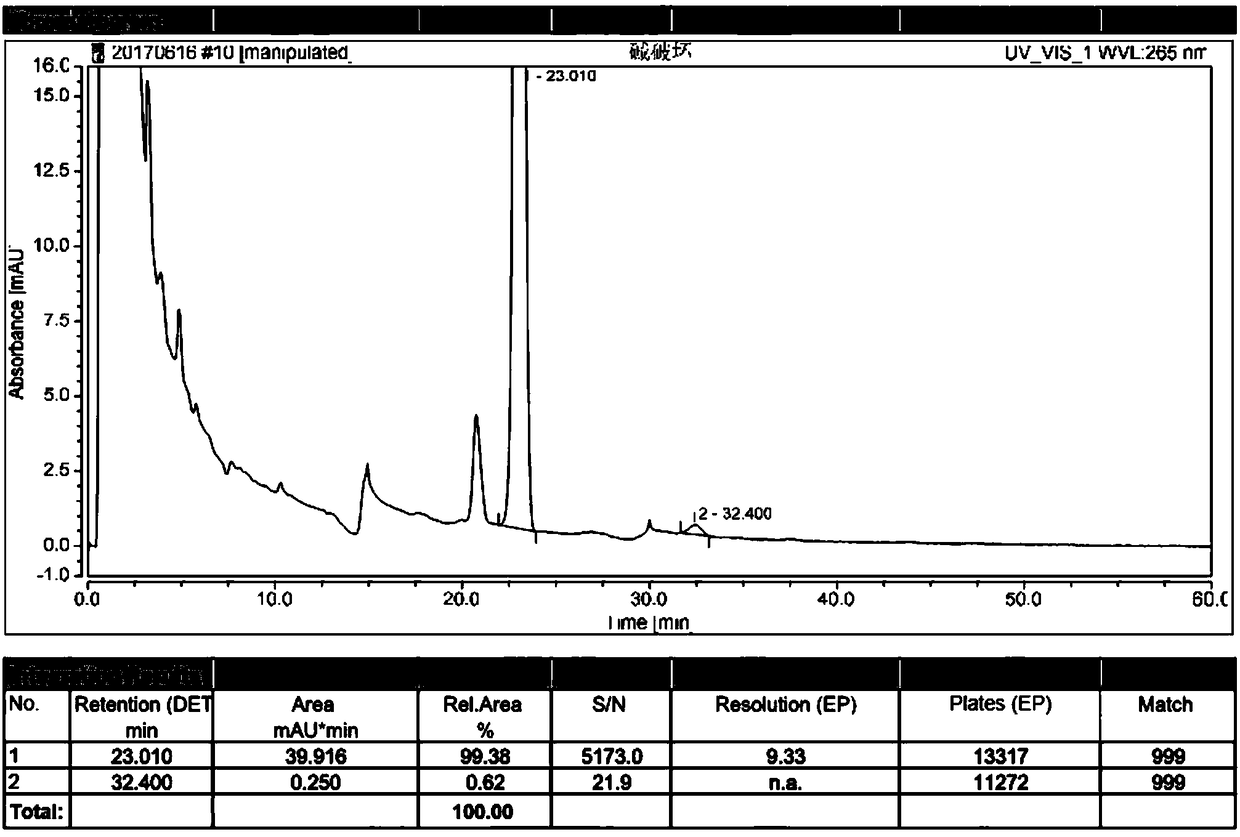
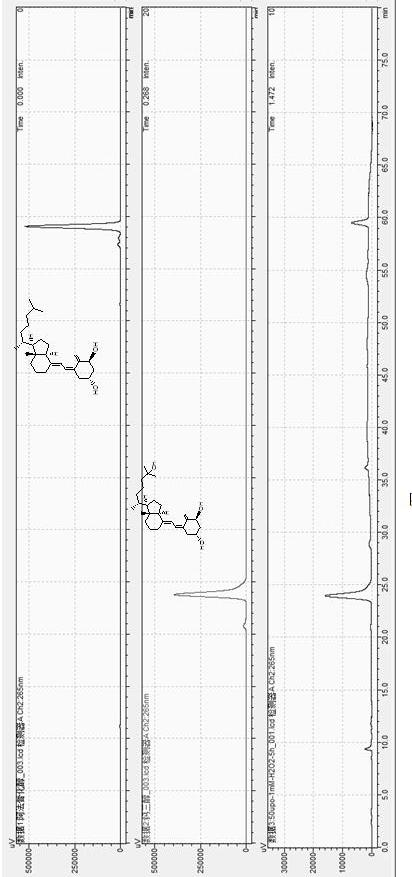
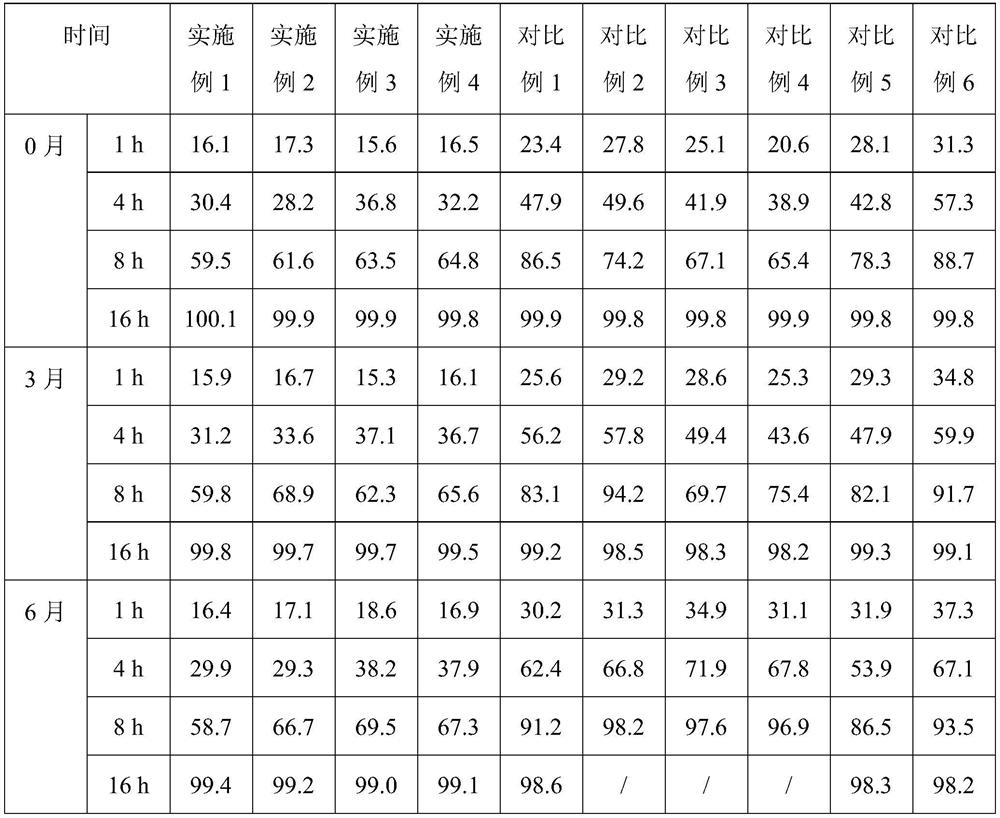
The invention discloses a high performance liquid chromatography method for alfacalcidol tablet related substances. A sample solution and a control solution are obtained by adopting octadecylsilane bonded silica as a filler and an acetonitrile-water mixed solution as a mobile phase, adopting an ultraviolet detector for a wavelength of 265 nm and especially adopting a vortex-extraction-concentration way, so the detection of an extremely low dose of a drug in the above tablet and related substances thereof is greatly ensured. The method be used to simultaneously analyze all known impurities in the alfacalcidol tablet, and allows the content of each known impurity to be effectively controlled by a main component self-contrasted technology with a correction factor, the resolution among all impurity peaks and the resolution between a main peak and the adjacent impurity peak are respectively greater than 1.5, and the peak purities of the main peak and all the impurity peaks are respectivelymore than 1.0. The high performance liquid chromatography method is a simple and reliable analysis method for the quality control of the alfacalcidol tablet.
Owner:NANJING HERON PHARM CO LTD
Medicament for treating osteoporosis
InactiveCN103239460AMake up for the shortcomings of the drug aloneFull effectOrganic active ingredientsSkeletal disorderMechanism of actionPharmaceutical drug
The invention relates to a medicament for treating postmenopausal osteoporosis of women. The medicament is characterized by containing alfacalcidol and alendronate sodium. The alfacalcidol and the alendronate sodium have the same point and the different points on the mechanism of action. By utilizing the alfacalcidol and the alendronate sodium, complementation of the mechanism of action can be realized, the defect of single mechanism is remedied, and treatment to face osteoporosis is better realized.
Owner:CP PHARMA QINGDAO CO LTD
Alfacalcidol powder and preparation method thereof
ActiveCN104800166AIncrease contentImprove bioavailabilityPowder deliveryOrganic active ingredientsIcing sugarPyrrolidinones
The invention discloses an alfacalcidol powder and a preparation method thereof. The alfacalcidol powder is prepared from the following ingredients in parts by weight: 5-8 parts of alfacalcidol, 15-20 parts of polyvinylpyrrolidone, 60-70 parts of starch, 60-70 parts of powdered sugar, 10-15 parts of microcrystalline cellulose and 5-8 parts of aerosil. The preparation method has the benefits that the content of alfacalcidol in the preparation is increased and the medicine dose for patients is decreased; with the addition of the polyvinylpyrrolidone, the bioavailability and stability of the alfacalcidol powder are increased and the clinical effect is remarkable.
Owner:CP PHARMA QINGDAO CO LTD
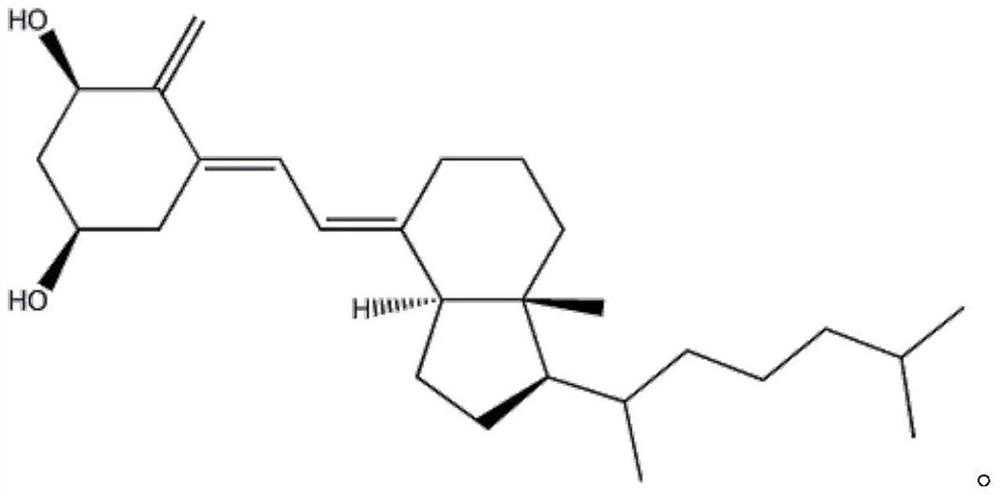
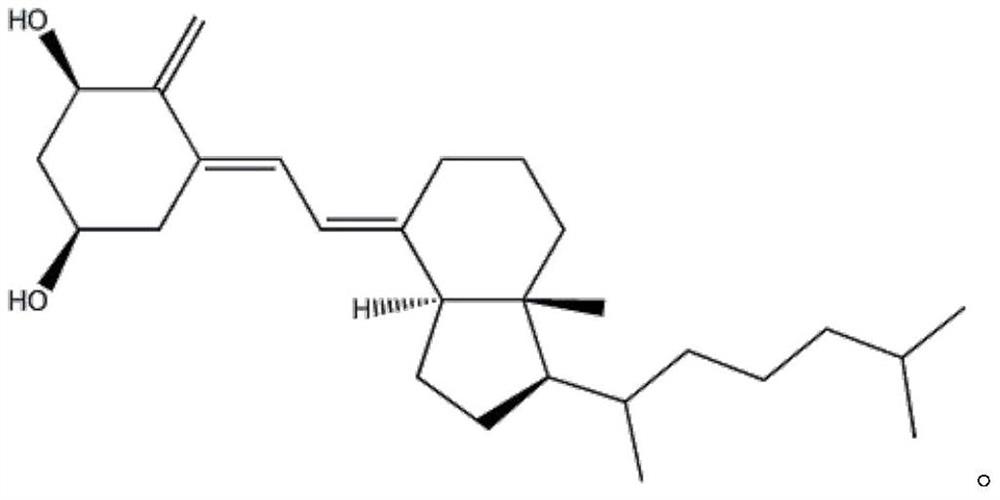
Alfacalcidol derivative and preparation method thereof
InactiveCN109516938AConvenient for clinical operationGood water solubilityOrganic chemistrySolubilityWater soluble
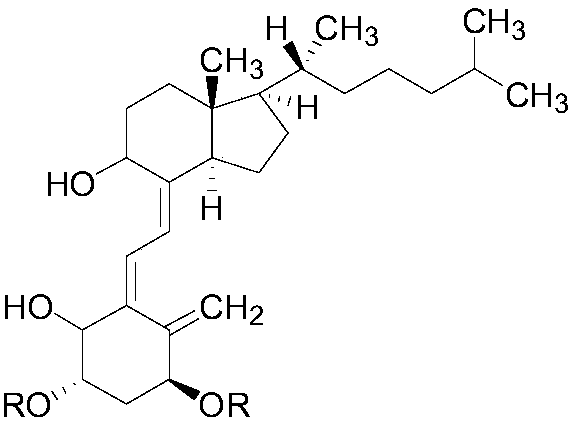
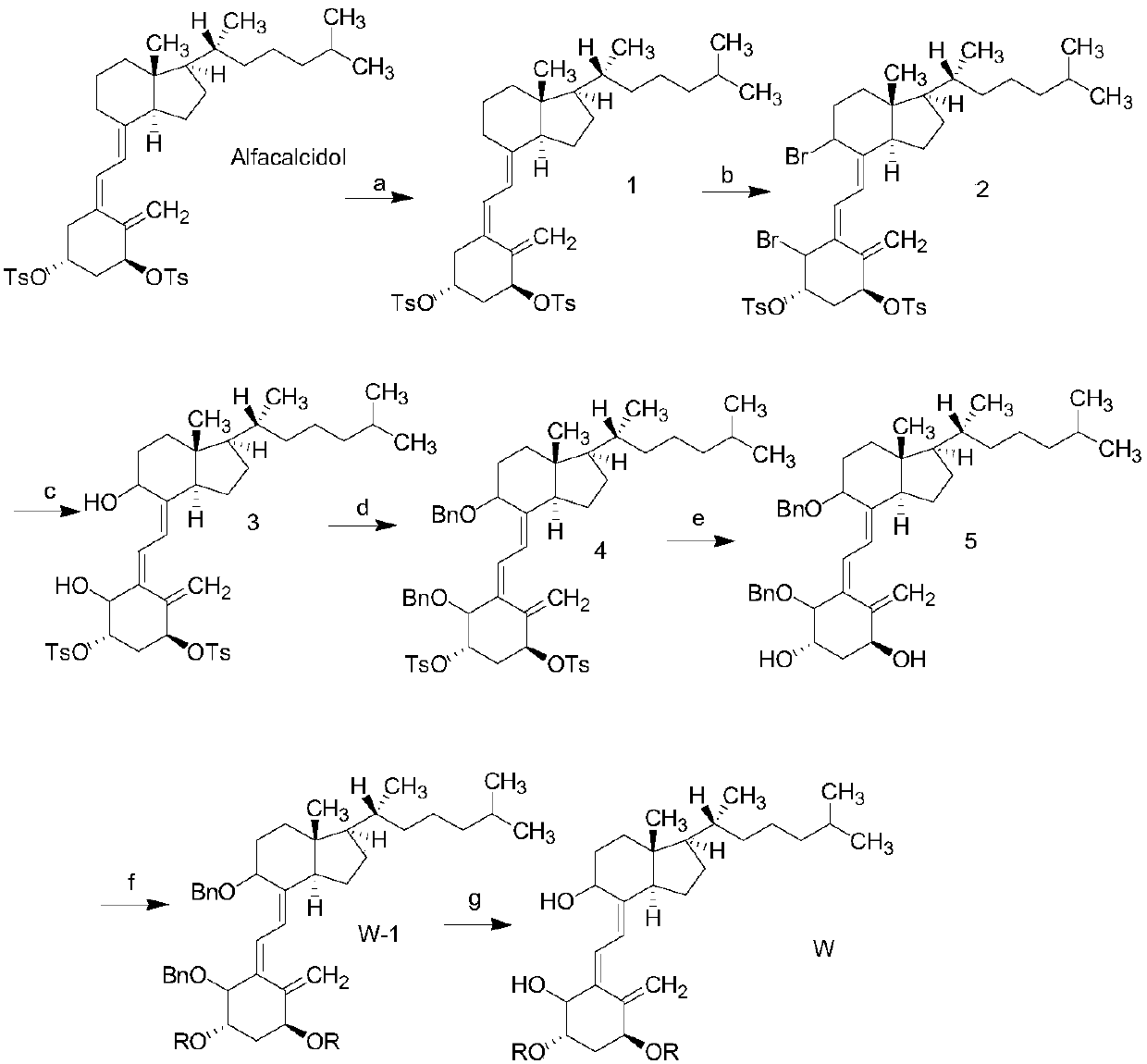
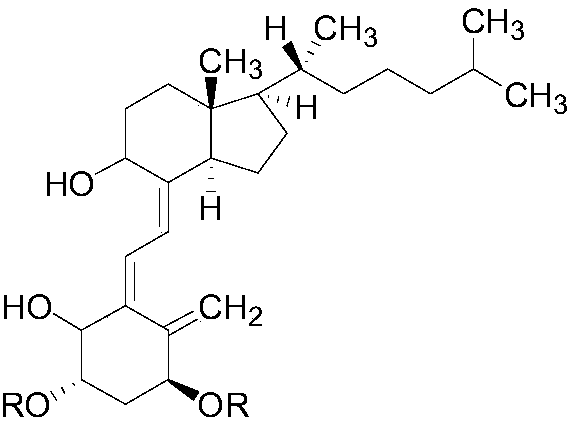
The invention provides an alfacalcidol derivative and a preparation method thereof. The molecular structural formula is as follows, wherein R is selected from the follows. According to the alfacalcidol derivative and the preparation method thereof provided by the invention, vitamin D3 is taken as an initiator and is subjected to 7-step reaction, thereby acquiring a target product. Compared with alfacalcidol, the alfacalcidol derivative has higher water solubility and is more beneficial to clinic application of drugs.
Owner:ZHUOHE PHARM GRP CO LTD
Medicine preparation for treating postmenopausal osteoporosis
InactiveCN103070872AEasy to takeMake up for the shortcomings of the drugOrganic active ingredientsSkeletal disorderOsteopetrosisBioavailability
The invention provides a medicine preparation for treating postmenopausal osteoporosis and belongs to the technical field of medicines. The medicine preparation contains alfacalcidol, tibolone and necessary pharmaceutical excipients. The medicine preparation for treating postmenopausal osteoporosis, provided by the invention, has the advantages of good disintegration, good absorbing degree, high bioavailability, quick curing effect and convenience for taking. Pharmacological actions of the alfacalcidol and the tibolone can be taken to a maximum extent, the defect of single preparation in efficacy is compensated, and the medicine preparation is an excellent medicine preparation for treating the postmenopausal osteoporosis.
Owner:CP PHARMA QINGDAO CO LTD
Alfacalcidol soft capsule and preparation method thereof
ActiveCN111214453AImprove stabilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderSoftgelPolyethylene glycol
The invention belongs to the technical field of medicine preparation, and particularly relates to an alfacalcidol soft capsule and a preparation method thereof. The capsule is prepared from a capsuleshell solution and capsule contents, wherein the capsule contents comprise alfacalcidol, medium-chain triglyceride, ascorbyl palmitate, gliadin, polyethylene glycol, phospholipid and glycerol monolaurate; and the capsule shell solution comprises gelatin, glycerol, water, ethylparaben, sodium alginate and chitosan. The preparation method of the alfacalcidol soft capsule is simple, and the preparedalfacalcidol soft capsule is good in stability and bioavailability and suitable for promotion and application.
Owner:GUANGZHOU XINGQUN PHARMA
Alfacalcidol dropping pill and preparation method thereof
The invention discloses an alfacalcidol dropping pill and a preparation method thereof. The alfacalcidol dropping pill comprises an alfacalcidol clathrate compound, a matrix and a stabilizing agent. The alfacalcidol clathrate compound comprises active ingredients and clathration material. The clathration material is alpha-cyclodextrin, beta-cyclodextrin or hydroxy beta-cyclodextrin, and the matrix is a mixer formed by more than two of polyethylene glycol 6000, polyethylene glycol 4000, polyoxyethylene monostearate, sodium stearate, glycerin gelatin, poloxamer, stearic acid and the like. The weight ratio of the active ingredients to the clathration material is 4:2-2:3. The alfacalcidol dropping pill is good in rounding degree, high in medicine dissolving-out speed and high in biological availability.
Owner:CP PHARMA QINGDAO CO LTD
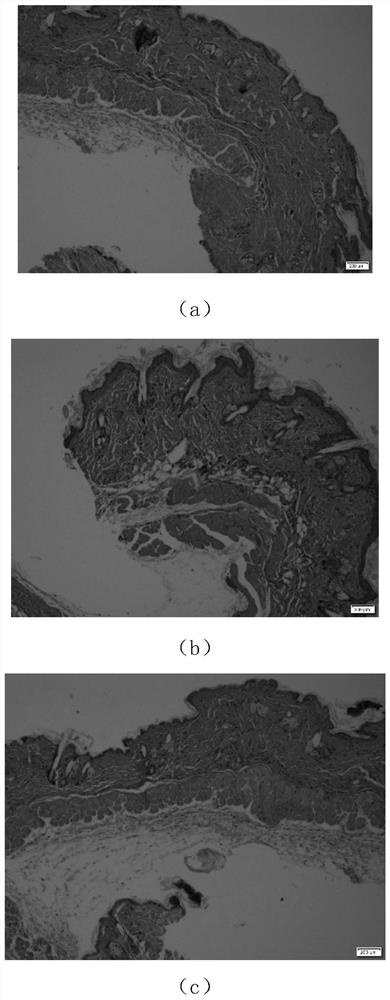
Application of alfacalcidol in preparing external preparation for intervening in skin light injury
InactiveCN111743906AIncrease proliferative activityInhibit expressionOrganic active ingredientsPharmaceutical delivery mechanismCell-Extracellular MatrixFibroblast
The invention discloses an application of alfacalcidol in preparing an external medicine for preventing and / or treating skin light injury. The alfacalcidol in the invention can improve the proliferation activity of fibroblasts; the expression of MMP-1 and MMP-3 can be inhibited, and the degradation of an extracellular matrix is reduced; the skin light injury symptom can be obviously relieved; thecompound can be used for preparing external medicines for intervening in skin light injury, and has a wide clinical application prospect.
Owner:南通华山药业有限公司
Synthesis method of vitamin D analogue intermediate
ActiveCN110204469AShorten the production cycleHigh yieldOrganic chemistrySynthesis methodsActive Vitamin D
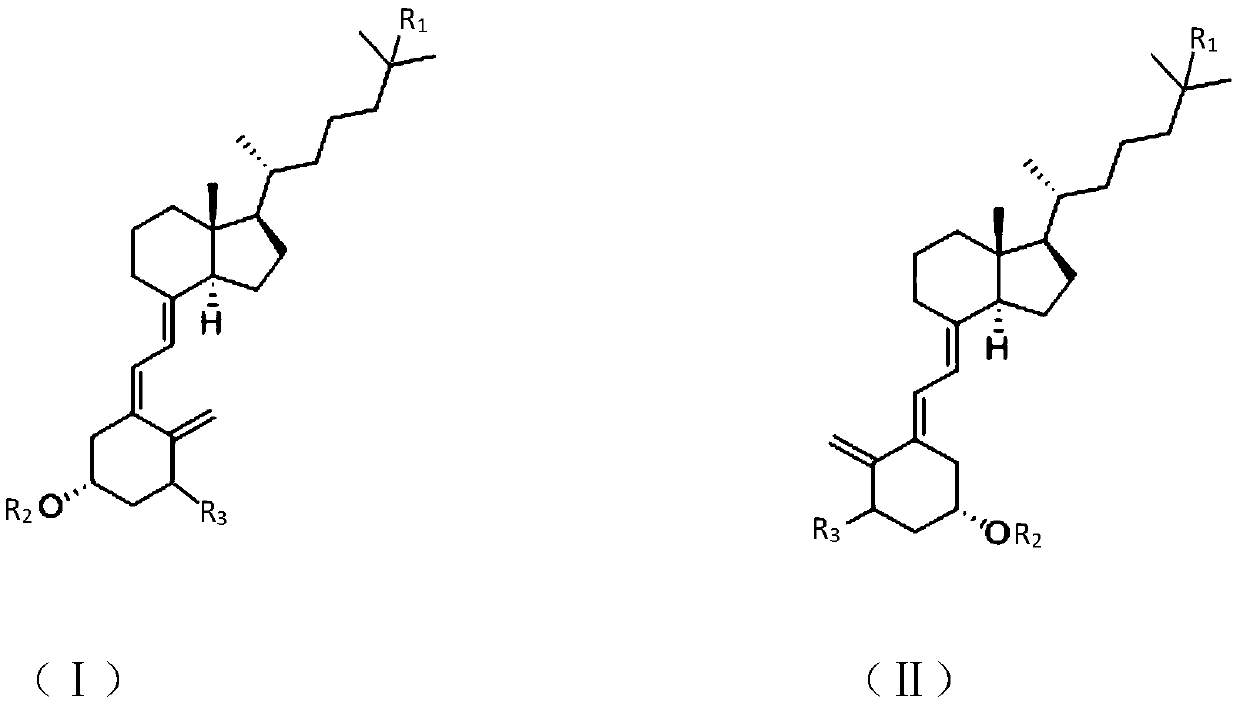
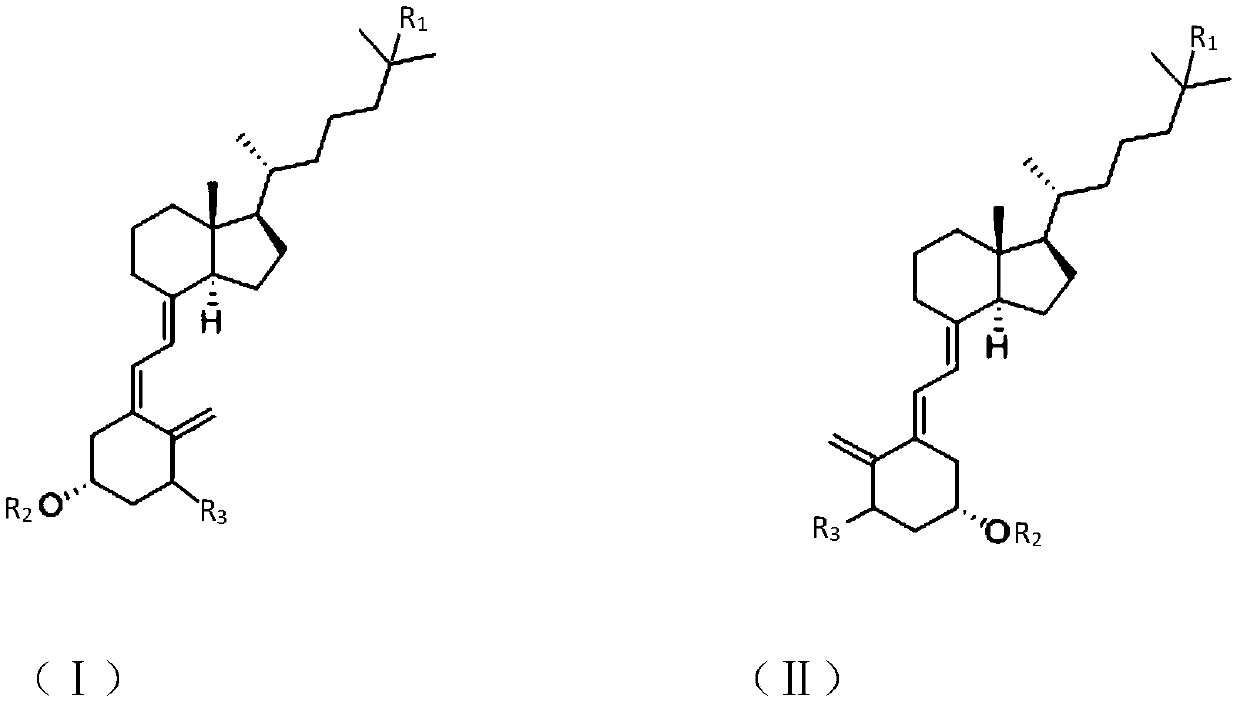
The invention discloses a synthesis method of a vitamin D analogue intermediate, and discloses a photochemical reaction method for preparing a revere intermediate (II) from a cis-form initiator (I). The intermediate prepared through the method can serve as the intermediate for synthesizing an active vitamin D analogue (such as alfacalcidol and calcitriol), the production cycle of active substancescan be shortened, and the yield is improved.
Owner:南通华山药业有限公司
Alfacalcidol sustained-release granule and preparation method thereof
ActiveCN103142503ASmooth releaseLittle side effectsOrganic active ingredientsMetabolism disorderSide effectPlasticizer
The invention discloses an alfacalcidol sustained-release granule and a preparation method thereof. The alfacalcidol sustained-release granule disclosed by the invention consists of a quick-release granule core and a sustained-release coating layer, wherein the quick-release granule core consists of alfacalcidol and filler, and the sustained-release coating layer consists of a sustained-release material, a plasticizer, a pore-forming agent and an antisticking agent; and during preparation, the alfacalcidol sustained-release granule is obtained through coating the sustained-release coating layer outside the quick-release granule core. According to the alfacalcidol sustained-release granule and the preparation method of the alfacalcidol sustained-release granule, the drug is convenient in taking and slow in release, the aims of long acting and curative effect improving can be reached, and the dosage can be reduced while the same drug action is maintained, so that side effects to patients caused by drug taking are reduced; and the preparation method is simple, the quality of obtained products is stable, and thus, the method is applicable to large-scale production application.
Owner:CP PHARMA QINGDAO CO LTD
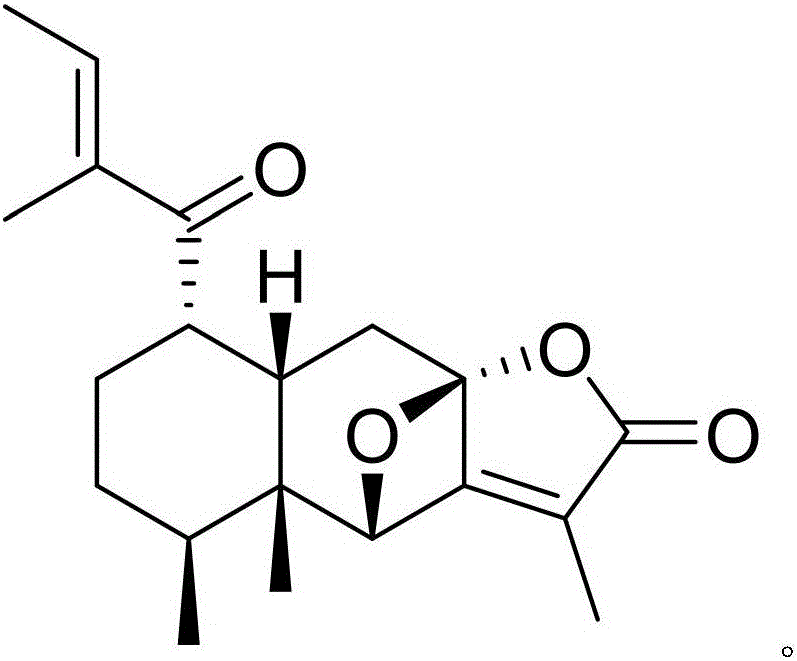
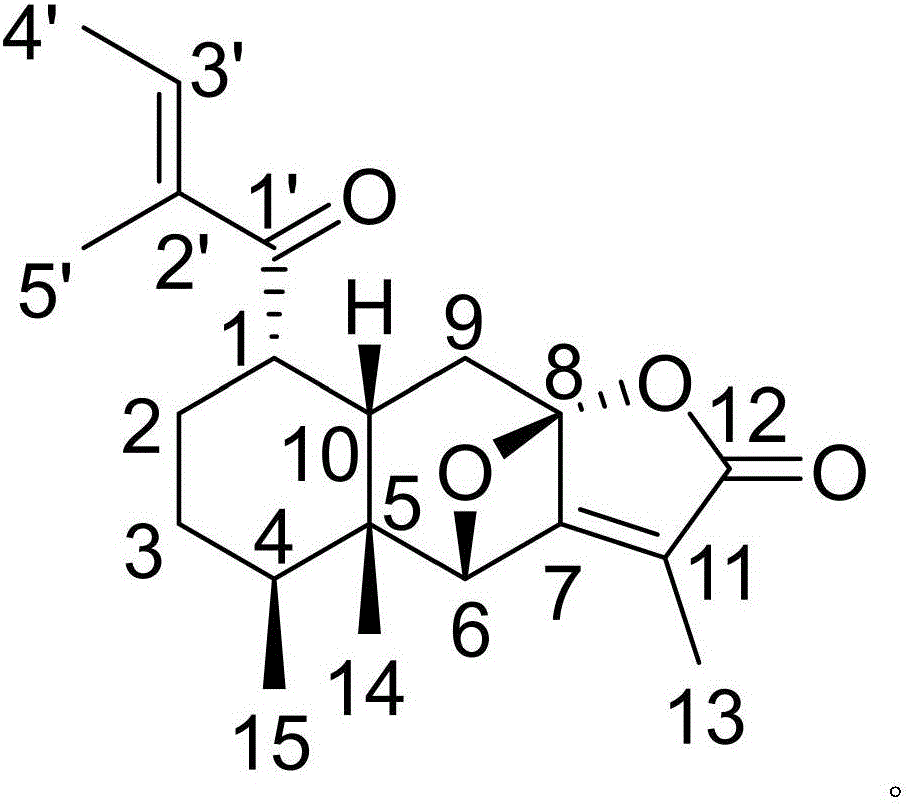
Pharmaceutical composition of alfacalcidol and medical application of pharmaceutical composition
InactiveCN105837591AGood treatment effectNovel structureOrganic active ingredientsOrganic chemistryNatural productPlant Tubers
The invention discloses a pharmaceutical composition of alfacalcidol and its medical application. The pharmaceutical composition of alfacalcidol provided by the invention contains alfacalcidol and a kind of The natural product compound (Ⅰ) with a novel structure, when alfacalcidol and compound (Ⅰ) act alone, can interfere with myocardial fibrosis in rats with renovascular hypertension by reducing the concentration of collagen; When pyridoxol and compound (I) act in combination, the intervention effect is better, and it can be developed into a drug for preventing and treating myocardial fibrosis in patients with renovascular hypertension, which has outstanding substantive features and significant progress compared with the prior art.
Owner:陈斌
Eczema treatment with vitamin D and analogs thereof method, composition and cream
InactiveUS20100081637A1Quick reliefGood moisturizing effectBiocideOrganic active ingredientsVitamin d 3Ergocalciferol
An eczema treatment method comprises application of a therapeutically effective composition in the form of an aqueous topical cream to an affected area of an exterior skin region of a patient, the aqueous topical cream comprising water, a water-soluble organic liquid, a surface active agent, and vitamin D3 compound or another claimed analog thereof, the vitamin D3 compound or the analog constituting at least approximately 10 international units per gram (IU / gram), and, most preferably at least approximately 400 IU / gram. An eczema treatment composition comprises a therapeutically effective formulation comprising at least approximately 50%-90% water, between approximately 1-5% PEG-40 stearate, between approximately 1-5% steareth-2, and cholecalciferal at a concentration of at least approximately 50 international units per gram (IU / g) of composition, and, most preferably at least approximately 400 IU / g. A topical vitamin D cream for therapeutic treatment of eczema includes vitamin D or an analog thereof chosen from the group consisting of cholecalciferol, alfacalcidol, calcifedol, and ergocalciferol; water; a water-soluble organic liquid; and a surface active agent, the vitamin D or analog being in a concentration of at least approximately 50 international units per gram (IU / g) of cream. Remarkably, relief from eczema conditions occurs immediately or in less than 3 days.
Owner:INNOVIA SKINCARE CORP
Alfacalcidol oral liposome drug and preparation method and application thereof
PendingCN111588696AGood storage stabilityPromote absorptionOrganic active ingredientsInorganic non-active ingredientsBile JuiceCholesterol
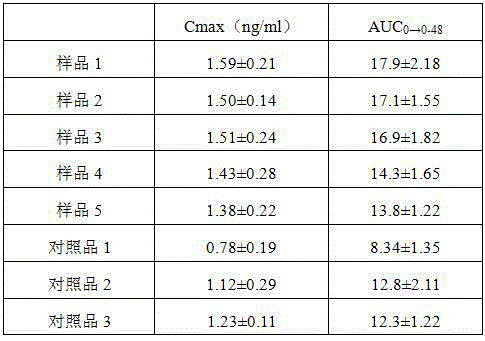
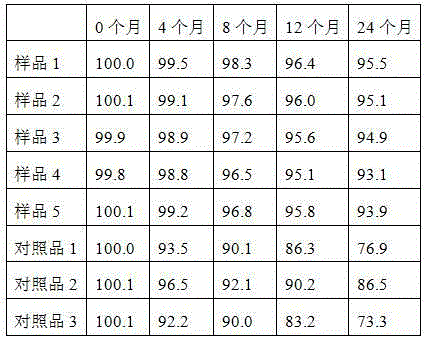
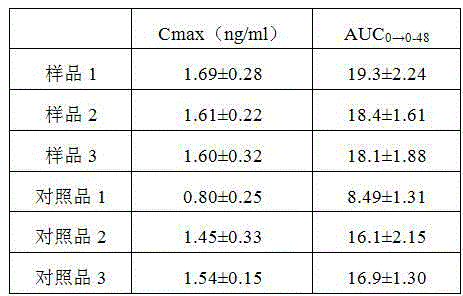
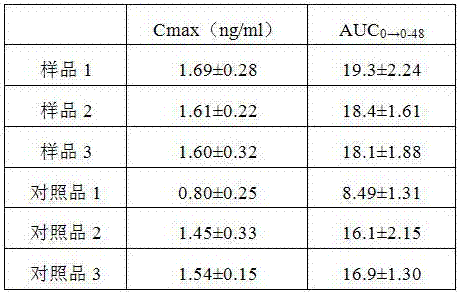
The invention discloses an alfacalcidol oral liposome drug and a preparation method and application thereof. The alfacalcidol oral liposome drug is prepared from the following raw materials in parts by weight: 1 part of alfacalcidol, 5-50 parts of phospholipid, 1-10 parts of cholesterol and 1-5 parts of an antioxidant. Alfacalcidol is encapsulated by liposome so that the chemical stability of alfacalcidol can be improved, the alfacalcidol can be protected from being damaged by gastric juice, bile, digestive enzymes and other substances through liposome, and the stability of the alfacalcidol ingastrointestinal tracts can be improved, so that absorption and bioavailability of the alfacalcidol in small intestines are improved; and compared with commercially available alfacalcidol tablets, the oral alfacalcidol lipidosome drug has high blood drug level and improved bioavailability, and the treatment effect on osteoporosis is improved.
Owner:南通华山药业有限公司
Method for separating chiral compound by using molecular sieves
ActiveCN102942431AEfficient separationSimple methodOptically-active compound separationOrganic racemisationMolecular sieveEsomeprazole Sodium
The invention relates to a method for separating chiral compounds by using molecular sieves, in particular to a separation method of calcitriol, alfacalcidol and esomeprazole sodium. Aiming at overcoming the defects of the existing separation technique, the invention provides a new method for separating the chiral compounds, which has the advantages of high yield and higher purity. By using different molecular sieves, chiral isomer impurities which are produced during preparation can be separated and removed. The method for separating chiral compounds by using molecular sieves has the advantages that the simple is simple and feasible, the pollution is small and the method is suitable for mass production.
Owner:CP PHARMA QINGDAO CO LTD
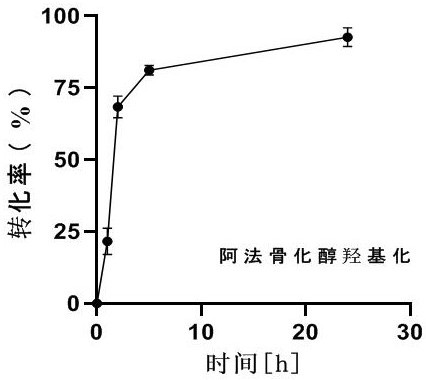
Method for synthesizing calcitriol through oxidase hydroxylation
ActiveCN114717271ASimple preparation processHigh yieldFermentationEnzymatic synthesisOxidative enzyme
The invention discloses a non-coenzyme-dependent enzymatic synthesis method for synthesizing calcitriol through oxidase hydroxylation, and 1, 25-dihydroxy vitamin D3 (calcitriol) which can be used as an intermediate or a raw material medicine of various medicines is directly prepared from alpha calcitriol through a one-pot one-step method. The preparation process is simple and convenient, the yield is high, the downstream treatment process is simple, and no other solvent or cofactor is used except water and acetone (or other organic cosolvents) in the preparation process. The method is a green biosynthesis method and has relatively high atom economy and step economy.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
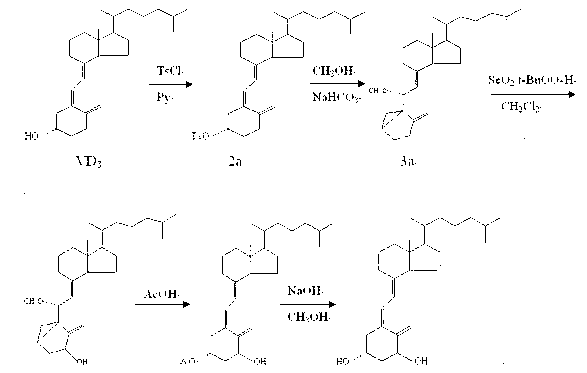
Improved preparation method of alfacalcidol
InactiveCN111072540AReduce generationAvoid hydrolysisOrganic chemistry methodsCombinatorial chemistryVitamin
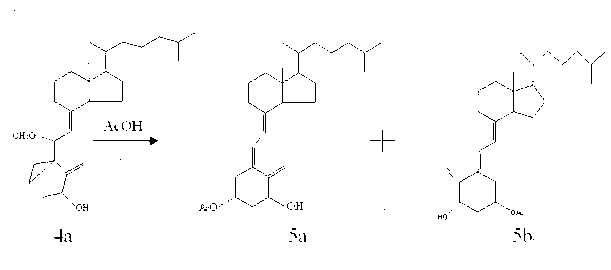
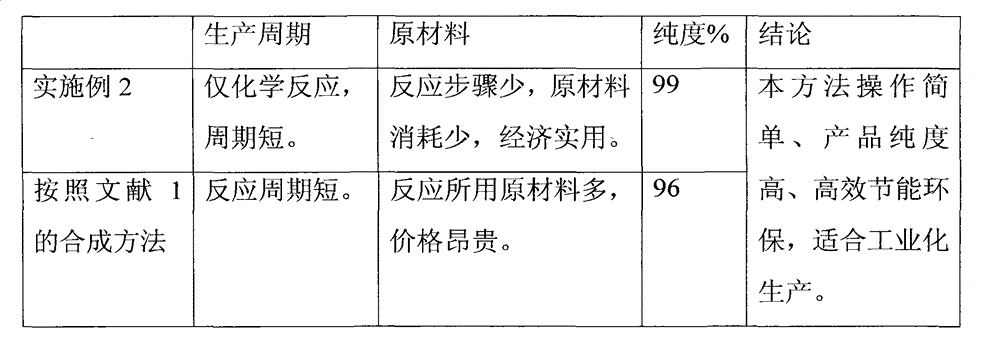
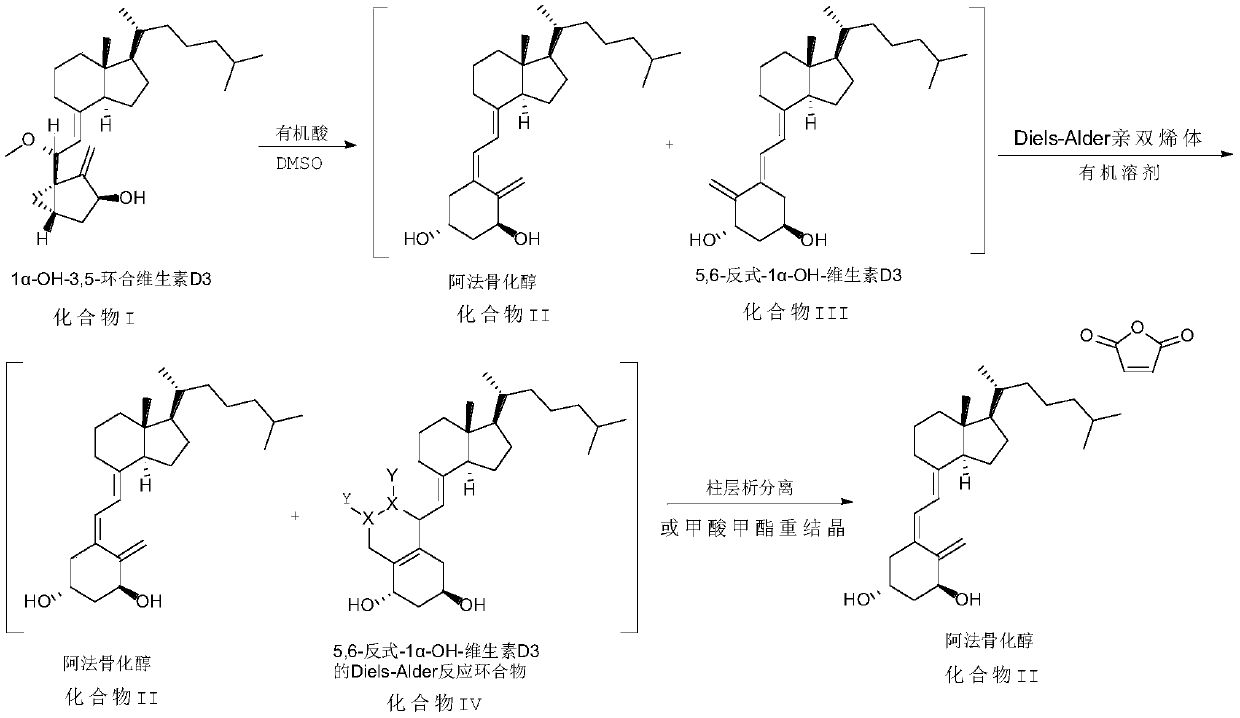
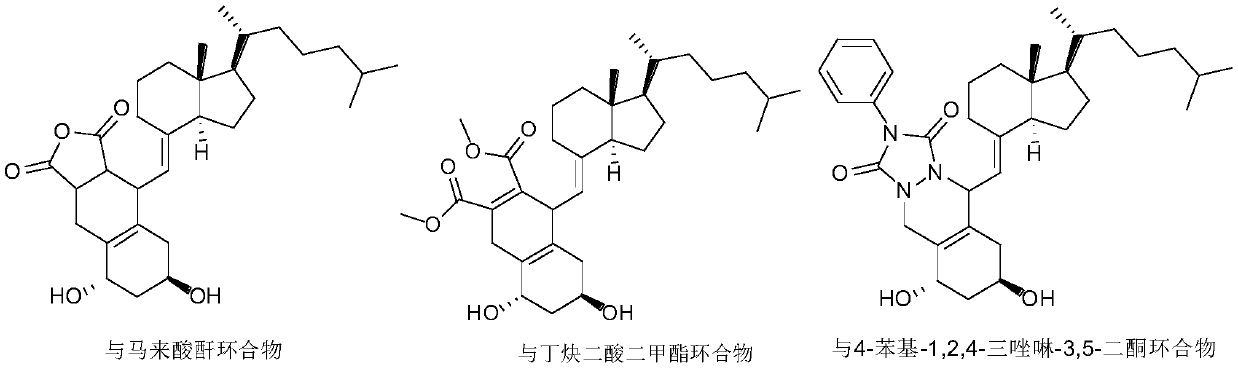
The invention discloses an improved preparation method of alfacalcidol. The preparation method is characterized by comprising the following steps: starting from 1 alpha-OH-3,5-cyclized vitamin D3, directly carrying out ring-opening hydrolysis without an acetate intermediate to obtain alfacalcidol and a trans-isomer thereof, carrying out a Diels-Alder reaction, selectively reacting with the trans-isomer, and carrying out column chromatography separation or methyl formate recrystallization to obtain a pure alfacalcidol product. The method is simple and convenient to operate, mild in reaction condition and high in total yield, and is suitable for large-scale synthesis of products.
Owner:SHANDONG HUBBLE KISEN BIOLOGICAL TECH CO LTD
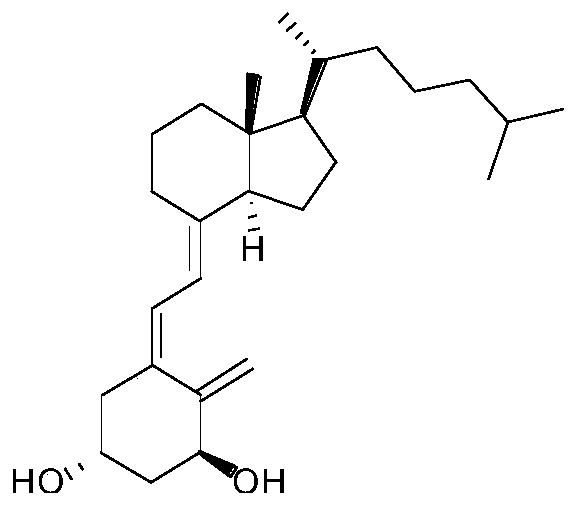
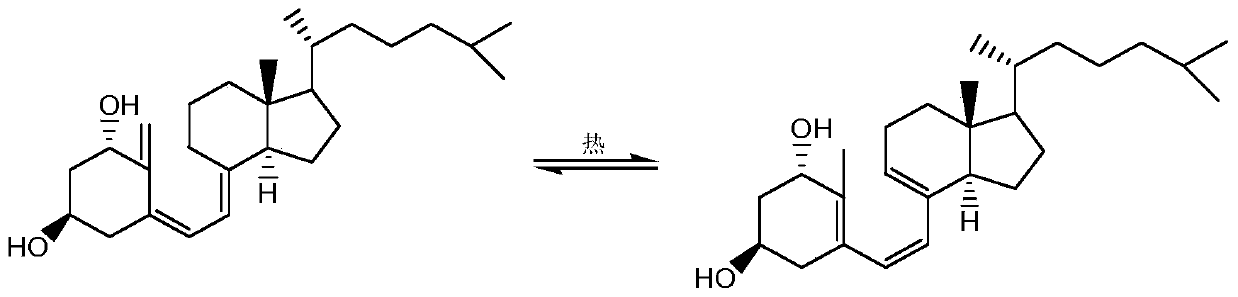
Alfacalcidol precursor preparation method
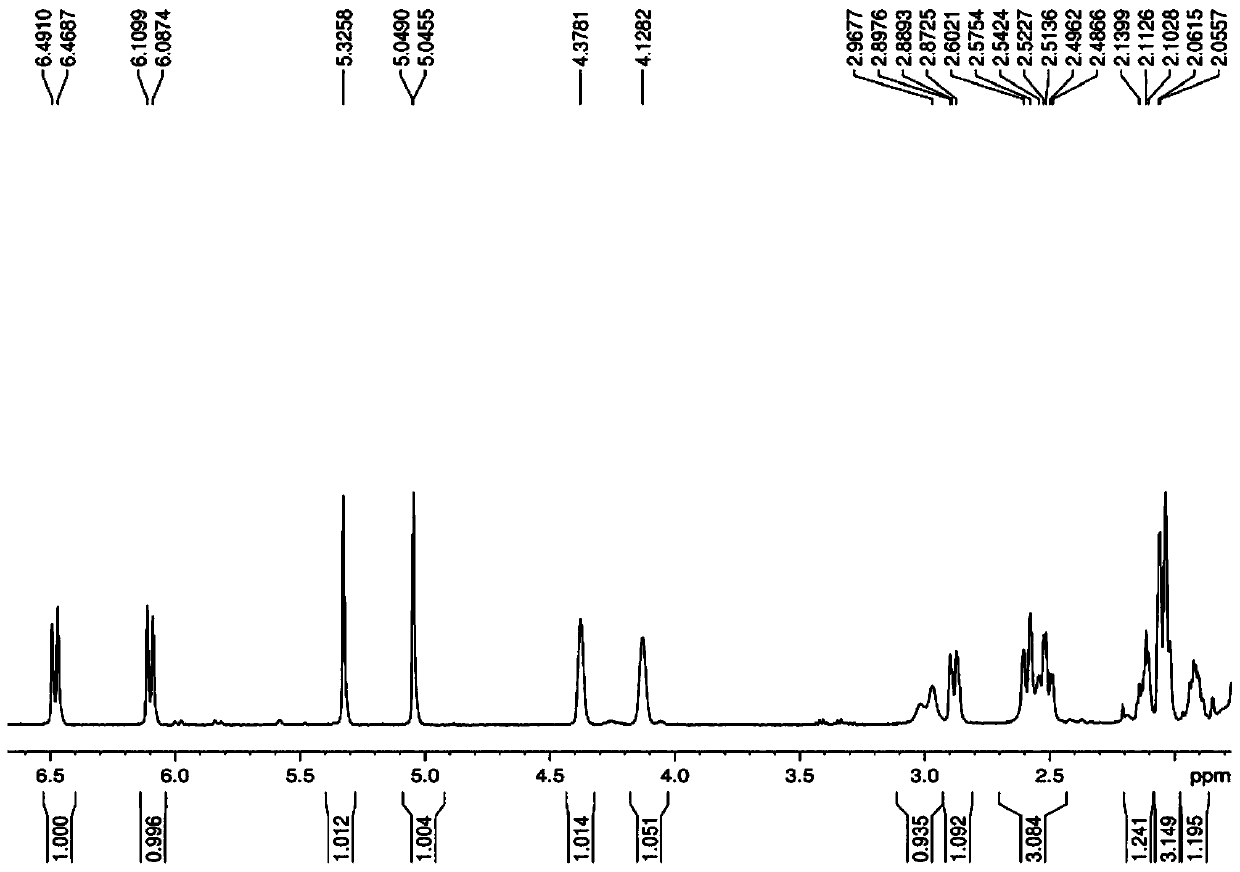
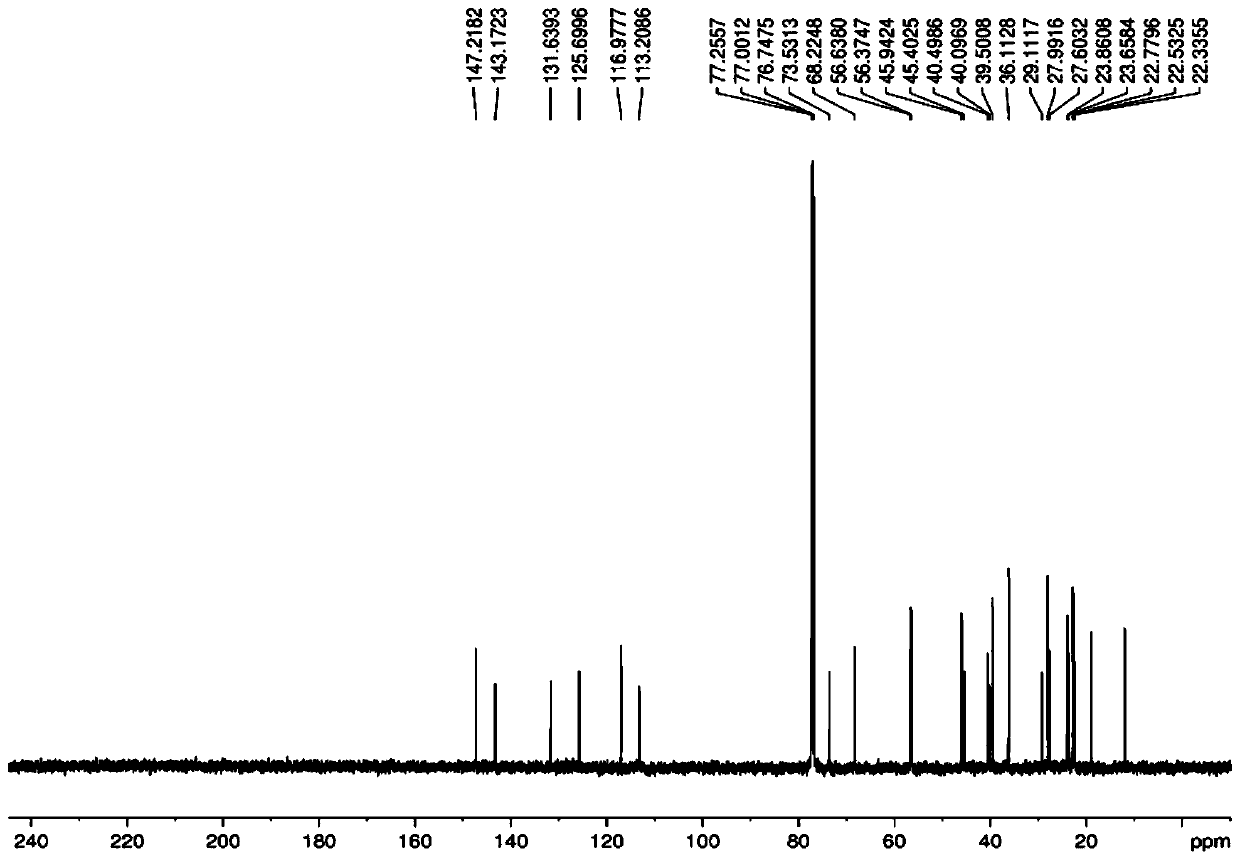
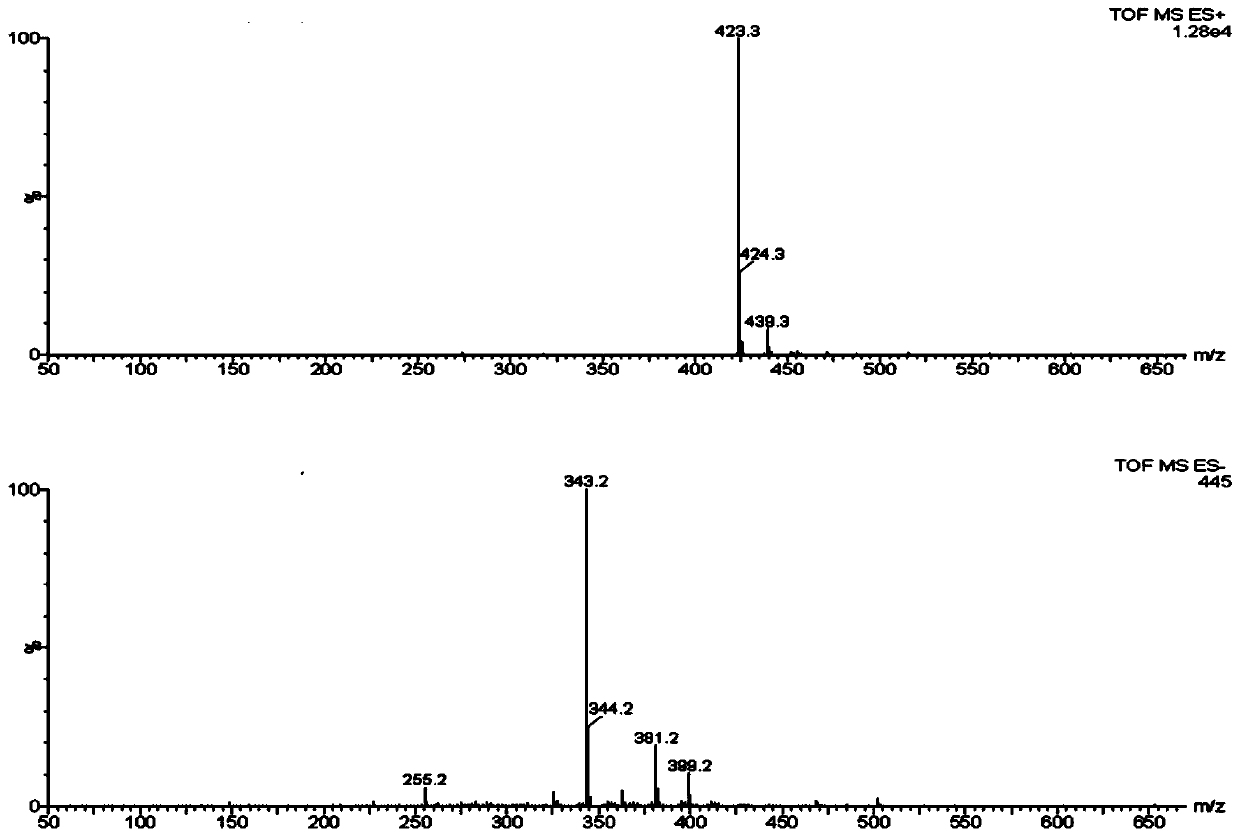
The invention provides an alfacalcidol precursor preparation method. According to the method, an alfacalcidol precursor is prepared through high-temperature water bath reflux, and is separated and purified through high performance liquid chromatography, the structure of the alfacalcidol precursor is identified through HRMS and NMR, and the result shows that the obtained compound is the alfacalcidol precursor. According to the invention, the method is simple in process, short in period, low in cost, high in yield and capable of achieving cyclic preparation.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
A kind of alfacalcidol soft capsule and preparation method thereof
ActiveCN111214453BImprove stabilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderSoftgelPolyethylene glycol
The invention belongs to the technical field of medicine preparation, and in particular relates to an alfacalcidol soft capsule and a preparation method thereof. Prepared from capsule shell liquid and capsule contents; wherein, the capsule contents include: alfacalcidol, medium-chain triglycerides, ascorbyl palmitate, gliadin, polyethylene glycol, phospholipids , monoglyceride laurate; the components of the capsule shell liquid include: gelatin, glycerin, water, ethylparaben, sodium alginate, and chitosan. The preparation method is simple, and the prepared alfacalcidol soft capsules have good stability and bioavailability, and are suitable for popularization and application.
Owner:GUANGZHOU XINGQUN PHARMA
A kind of alfacalcidol powder and preparation method thereof
ActiveCN104800166BWell mixedIncrease contentPowder deliveryOrganic active ingredientsIcing sugarMedicine
The invention discloses an alfacalcidol powder and a preparation method thereof. The alfacalcidol powder is prepared from the following ingredients in parts by weight: 5-8 parts of alfacalcidol, 15-20 parts of polyvinylpyrrolidone, 60-70 parts of starch, 60-70 parts of powdered sugar, 10-15 parts of microcrystalline cellulose and 5-8 parts of aerosil. The preparation method has the benefits that the content of alfacalcidol in the preparation is increased and the medicine dose for patients is decreased; with the addition of the polyvinylpyrrolidone, the bioavailability and stability of the alfacalcidol powder are increased and the clinical effect is remarkable.
Owner:CP PHARMA QINGDAO CO LTD
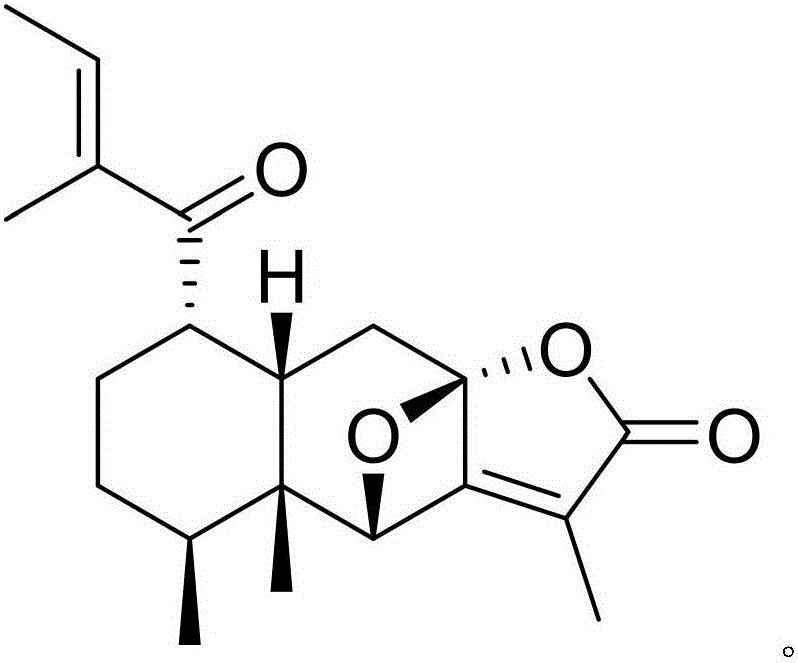
A kind of directional synthesis method and application of isomer impurity of alfacalcidol
ActiveCN108218750BProcess conditions are easy to controlEasy to routeOrganic chemistryComponent separationSynthesis methodsDiol
The invention discloses an isomer impurity PY2, namely, (5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-1 beta,3 beta-diol, of alfacalcidol and a preparation method of the isomer impurity and belongs to the technical field of chemical pharmacy. The high-purity related impurity PY2 of alfacalcidol can be used as an impurity standard substance in detection and analysis of an alfacalcidol product, so that accurate positioning and quality determination of the impurity in the detection and analysis of the alfacalcidol product are improved, control on the impurity can be strengthened, and the quality ofthe alfacalcidol product can be improved. The method is simple to operate and good in repeatability, raw materials are cheap and easy to obtain, and the HPLC purity is higher than or equal to 99.5%.
Owner:NANJING HERON PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com