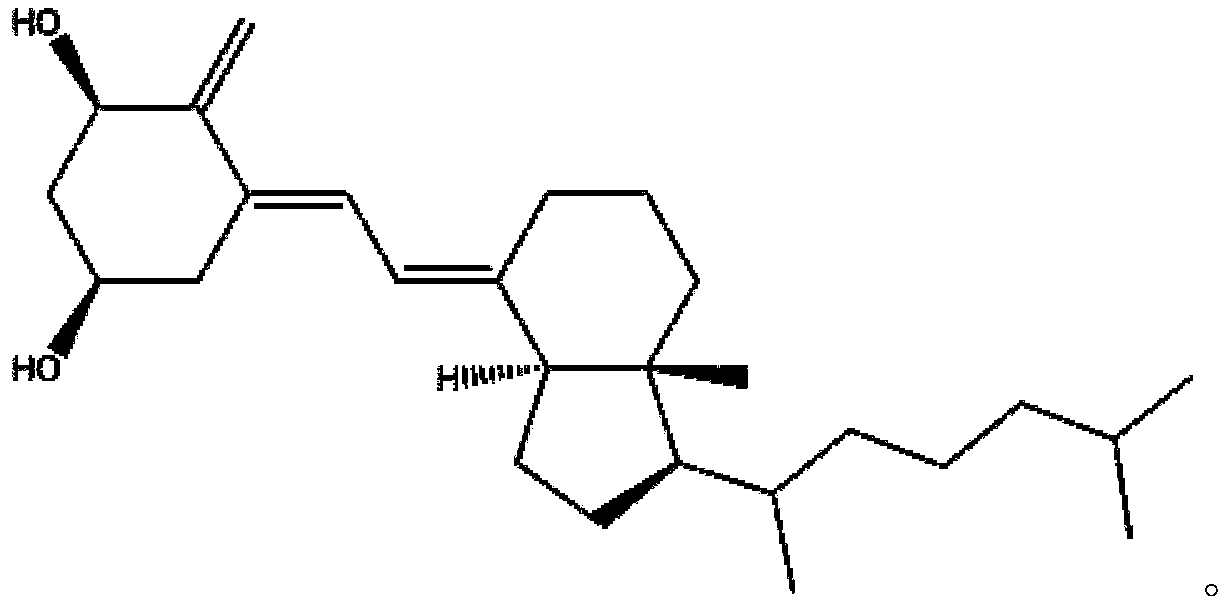
Alfacalcidol oral liposome drug and preparation method and application thereof
An alfacalcidol and liposome technology, applied in liposome delivery, drug combination, drug delivery, etc., can solve many adverse reactions, poor stability of oral alfacalcidol, poor bioavailability, etc. To improve the therapeutic effect, increase oral absorption and bioavailability, and improve storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
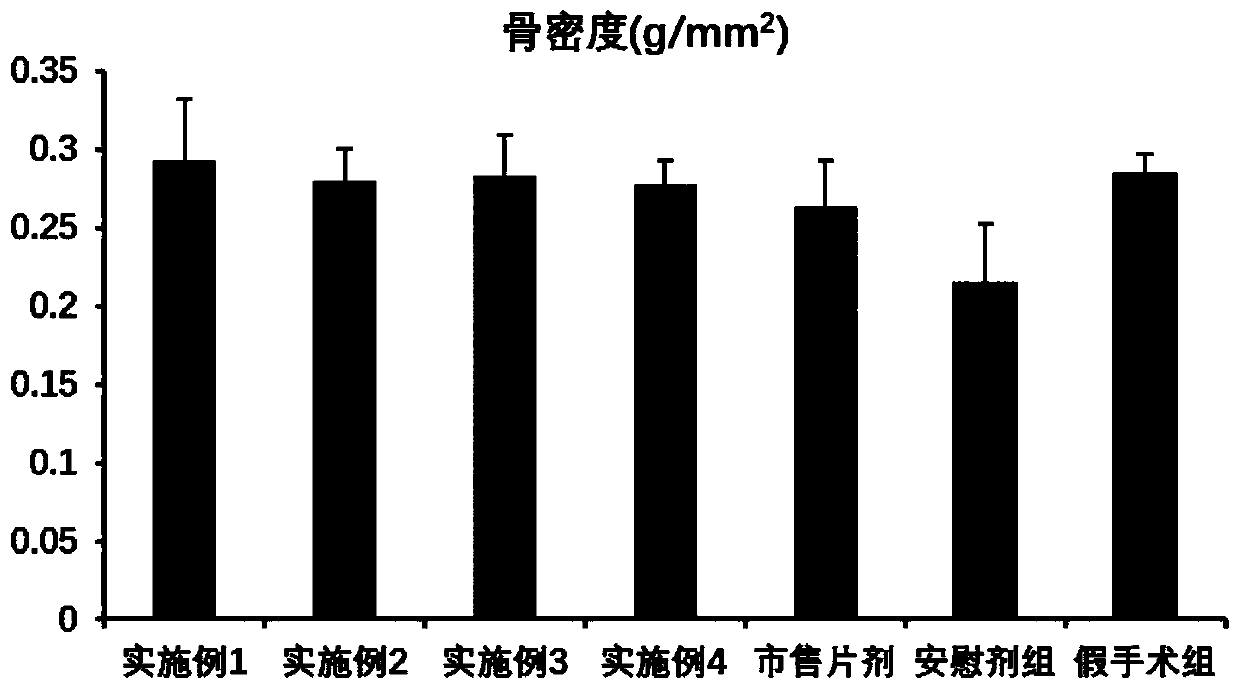
Examples
Embodiment 1
[0030] Weigh 1 mg of alfacalcidol, 20 mg of soybean lecithin, 5 mg of cholesterol, and 2 mg of vitamin E in a round-bottomed flask, add 20 ml of dichloromethane and ultrasonically dissolve it in a short-term water bath. The round bottom flask was placed on a rotary evaporator, and dichloromethane was evaporated under reduced pressure at 40°C until a lipid film was formed on the inner wall of the round bottom flask, and the round bottom flask was placed in a desiccator to vacuum for 24 hours. Weigh 2mg of vitamin C and 1mg of EDTA-2Na and dissolve them in 20ml of 0.01M pH7.4 phosphate buffer, add the buffer into a round bottom flask to hydrate and elute the lipid film, and ultrasonically disperse it in a water bath for 30min to prepare lipid Coarse suspension of plastids. Subsequently, the liposome coarse suspension probe was sonicated at 200w for 30s to obtain a liposome suspension with a smaller particle size and uniform dispersion. 10 mg of trehalose is added to the liposom...
Embodiment 2
[0032] Weigh 1 mg of alfacalcidol, 50 mg of egg yolk lecithin, 10 mg of cholesterol, and 1 mg of dibutyl hydroxytoluene into a round bottom flask, add 20 ml of dichloromethane and ultrasonically dissolve it in a short-term water bath. The round bottom flask was placed on a rotary evaporator, and dichloromethane was evaporated under reduced pressure at 40°C until a lipid film was formed on the inner wall of the round bottom flask, and the round bottom flask was placed in a desiccator to vacuum for 24 hours. Weigh 2mg of sodium sulfite and 1mg of EDTA-2Na and dissolve them in 20ml of 0.01M pH7.4 phosphate buffer, add the buffer into a round bottom flask to hydrate and elute the lipid film, and ultrasonically disperse it in a water bath for 30 minutes to prepare lipid body suspension. Subsequently, the liposome coarse suspension probe was sonicated at 200w for 30s to obtain a liposome suspension with a smaller particle size and uniform dispersion. 10 mg of mannose is added into ...
Embodiment 3
[0034] Weigh 1 mg of alfacalcidol, 10 mg of hydrogenated soybean lecithin, 1 mg of cholesterol, and 1 mg of tert-butyl-4-hydroxyanisole into a round bottom flask, add 20 ml of dichloromethane and ultrasonically dissolve it in a water bath for a short time. The round bottom flask was placed on a rotary evaporator, and dichloromethane was evaporated under reduced pressure at 40°C until a lipid film was formed on the inner wall of the round bottom flask, and the round bottom flask was placed in a desiccator to vacuum for 24 hours. Weigh 2mg of sodium thiosulfate and 1mg of EDTA-2Na and dissolve in 20ml of 0.01M pH7.4 phosphate buffer, add the buffer into a round bottom flask to hydrate and elute the lipid film, and ultrasonically disperse it in a water bath for 30min to prepare A coarse suspension of liposomes was obtained. Subsequently, the liposome coarse suspension probe was sonicated at 200w for 30s to obtain a liposome suspension with a smaller particle size and uniform disp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com