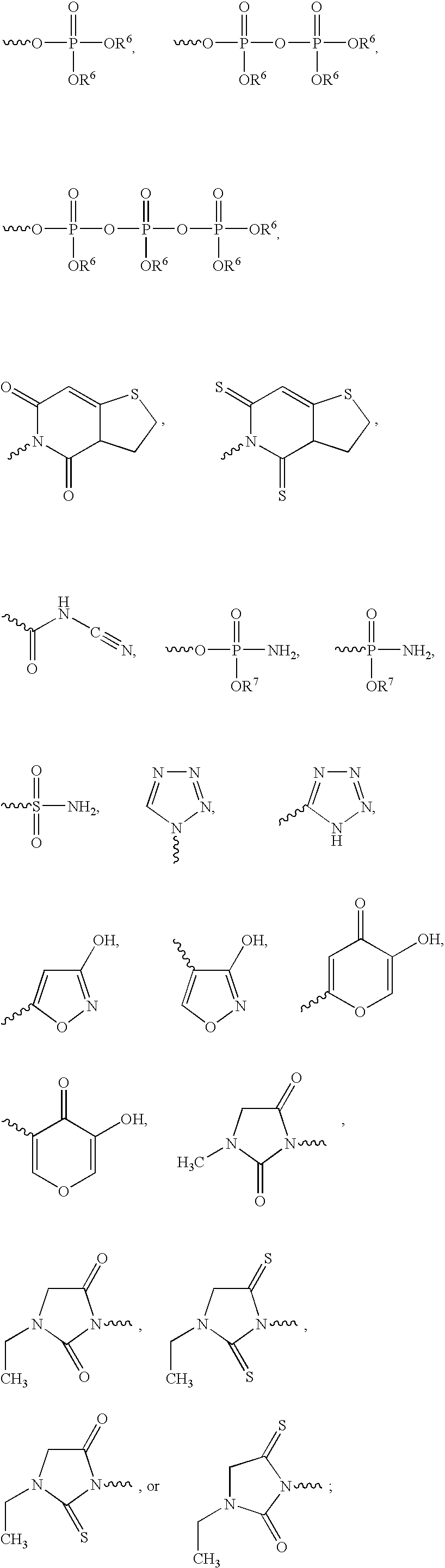
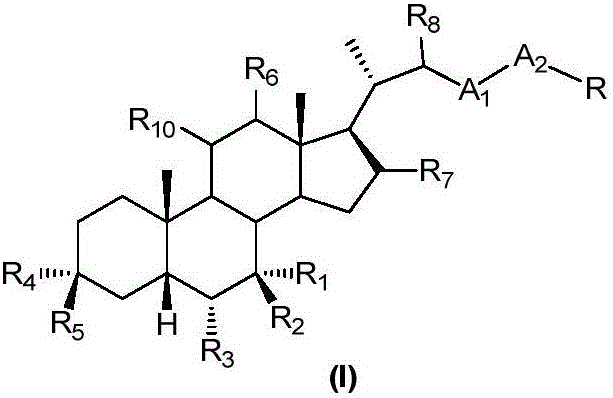
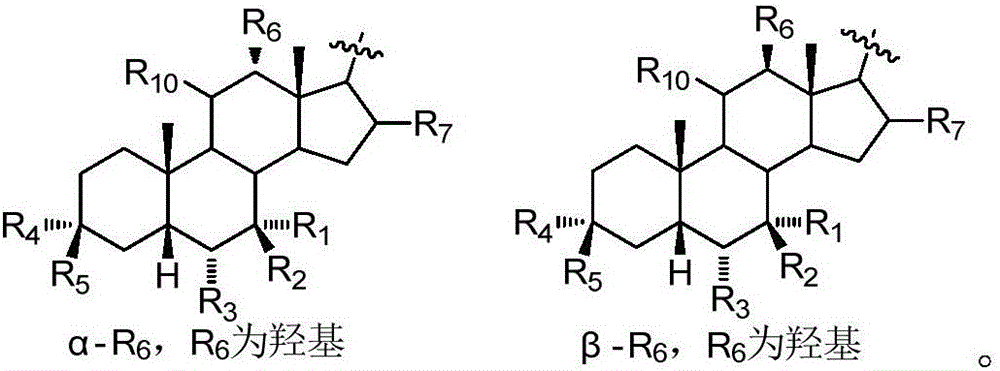
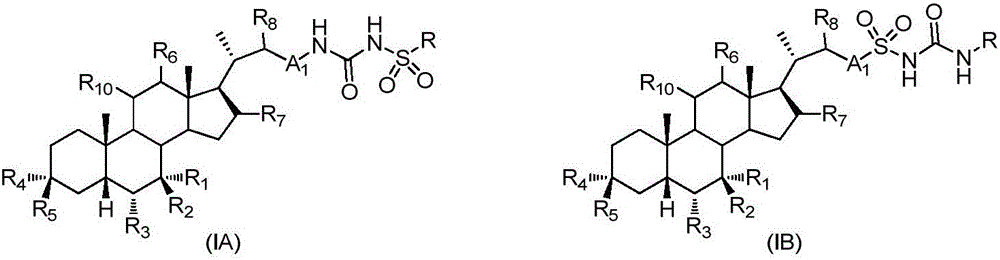
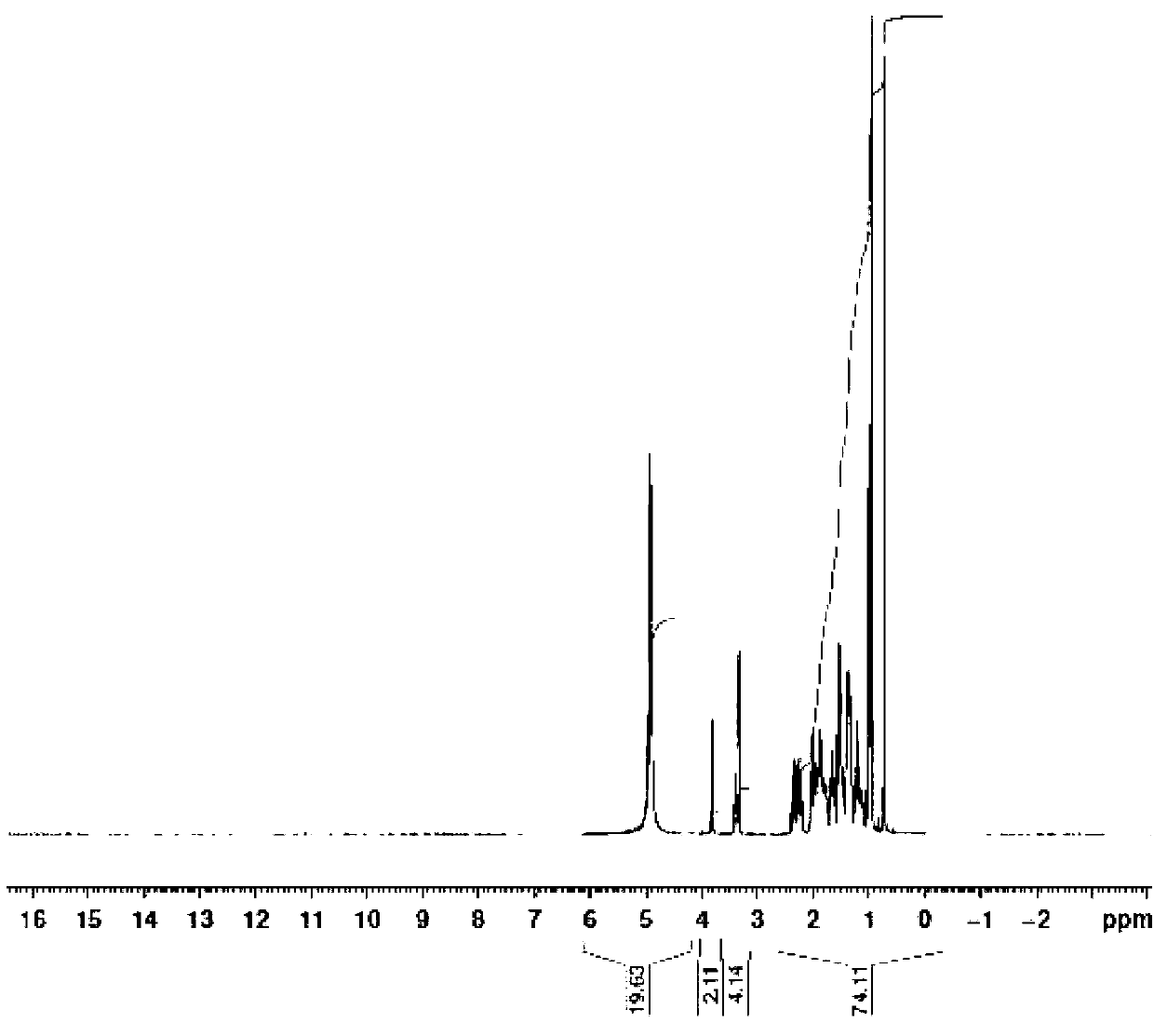
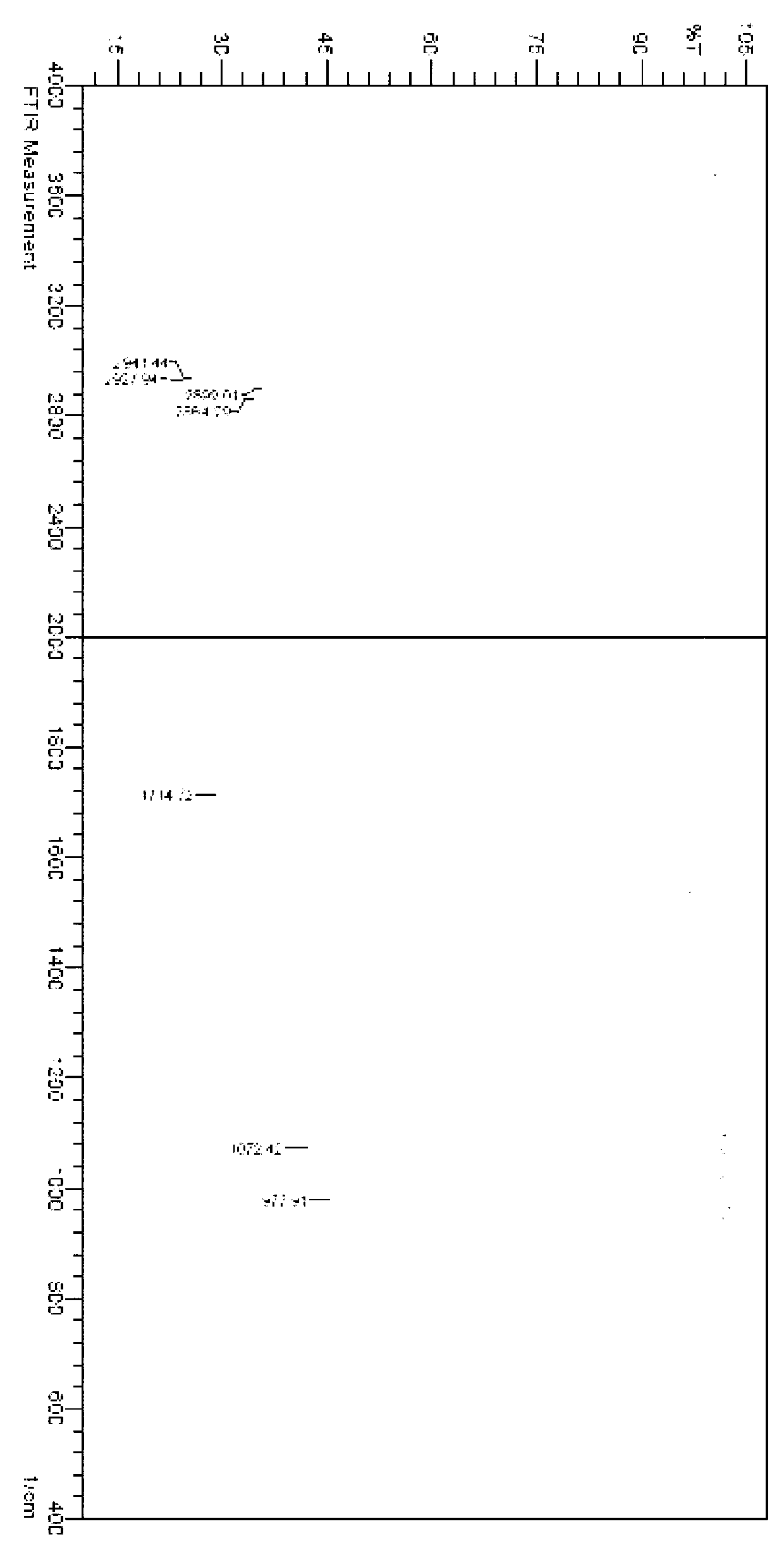
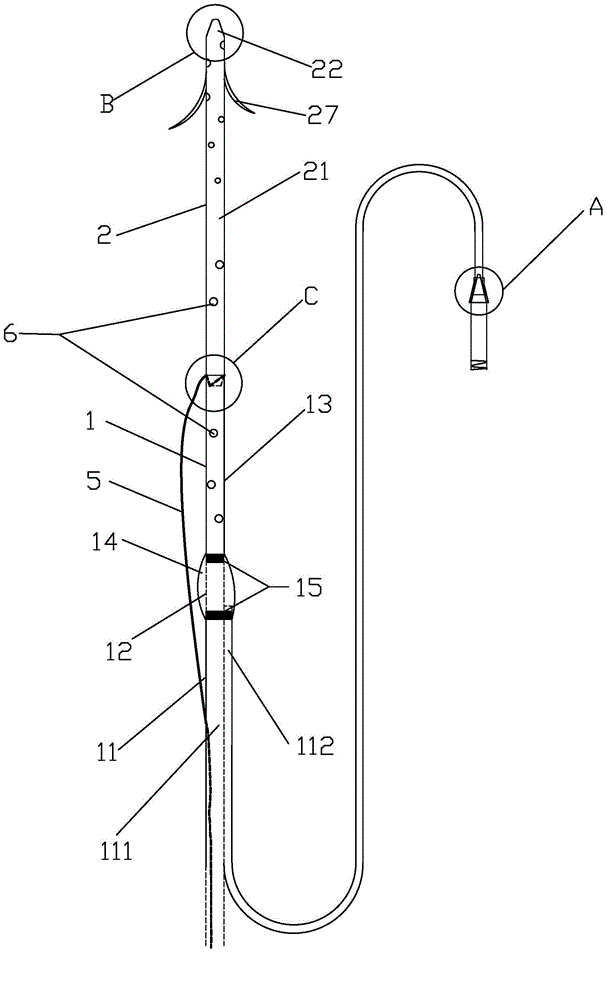
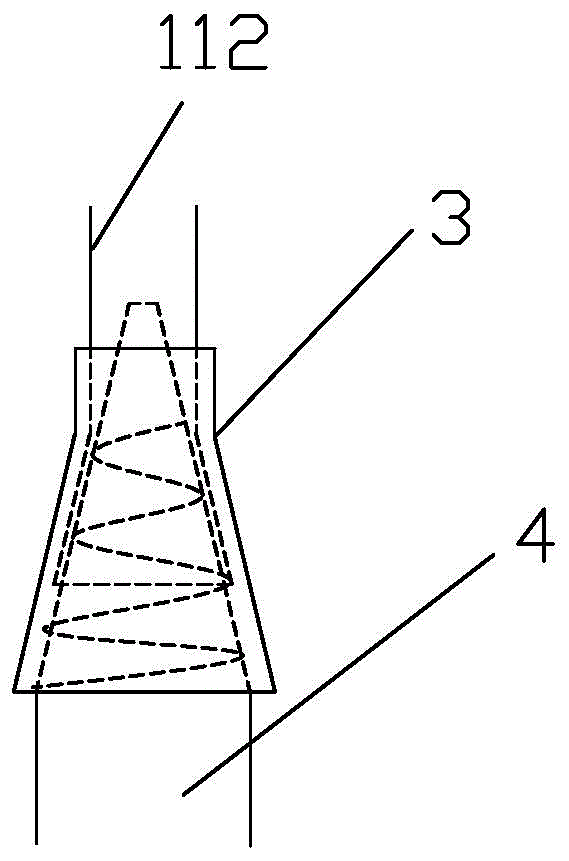
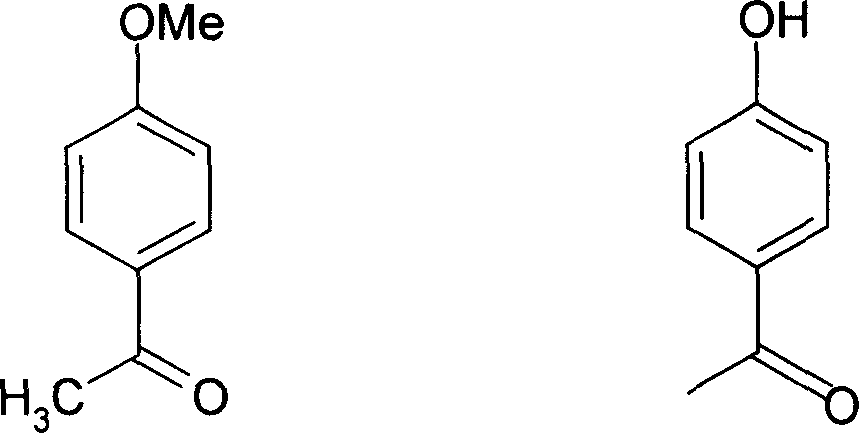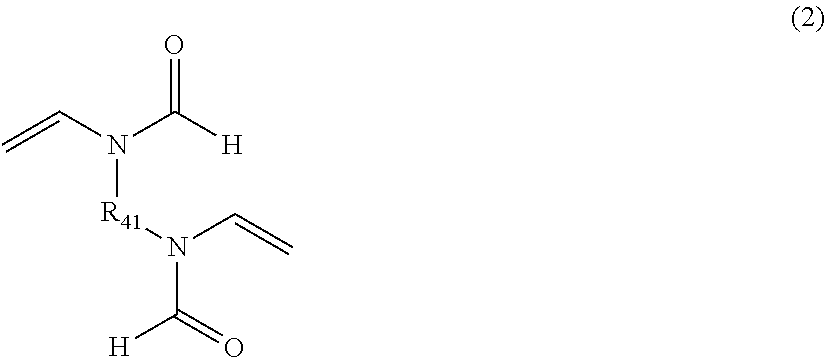Patents
Literature
415 results about "Bile Juice" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bile Juice is a dark green to yellowish brown fluid, produced by the liver of most vertebrates, that aids the digestion of lipids (fat) in the small intestine. In humans, bile is produced continuously by the liver (liver bile), and stored and concentrated in the gallbladder (gallbladder bile).
Aerosol drug formulations containing hydrofluoroalkanes and alkyl saccharides
InactiveUS6932962B1Function increaseGood dispersionPowder deliveryDispersion deliveryAerosol drugsActive agent
Aerosol formulations suitable for use in pressurised metered dose inhalers comprise a hydrofluoroalkane propellant, an medicament for inhalation and a surfactant which is a a C8–C16 fatty acid or salt thereof, a bile salt, a phospholipid, or an alkyl saccharide.
Owner:ASTRAZENECA AB
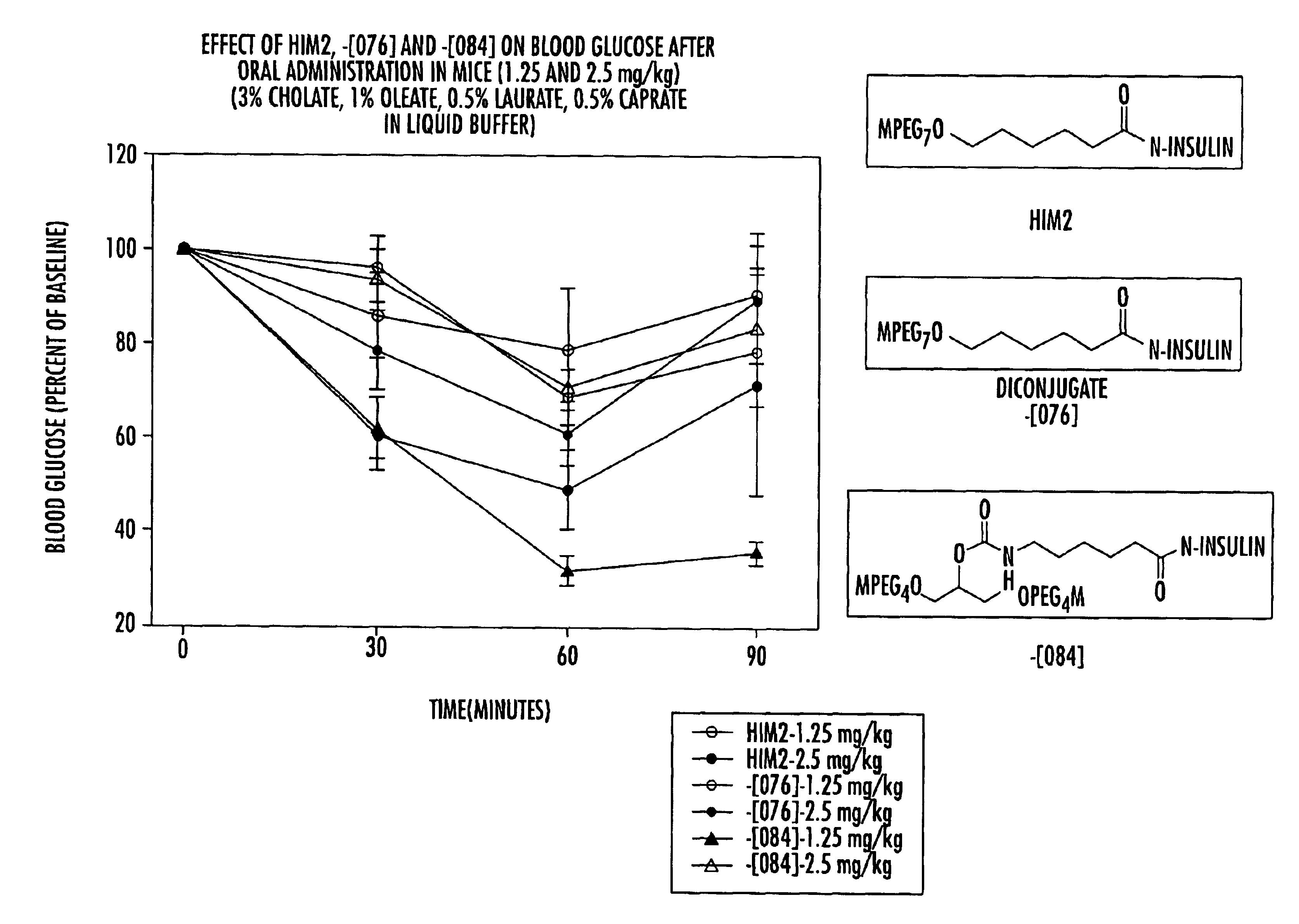
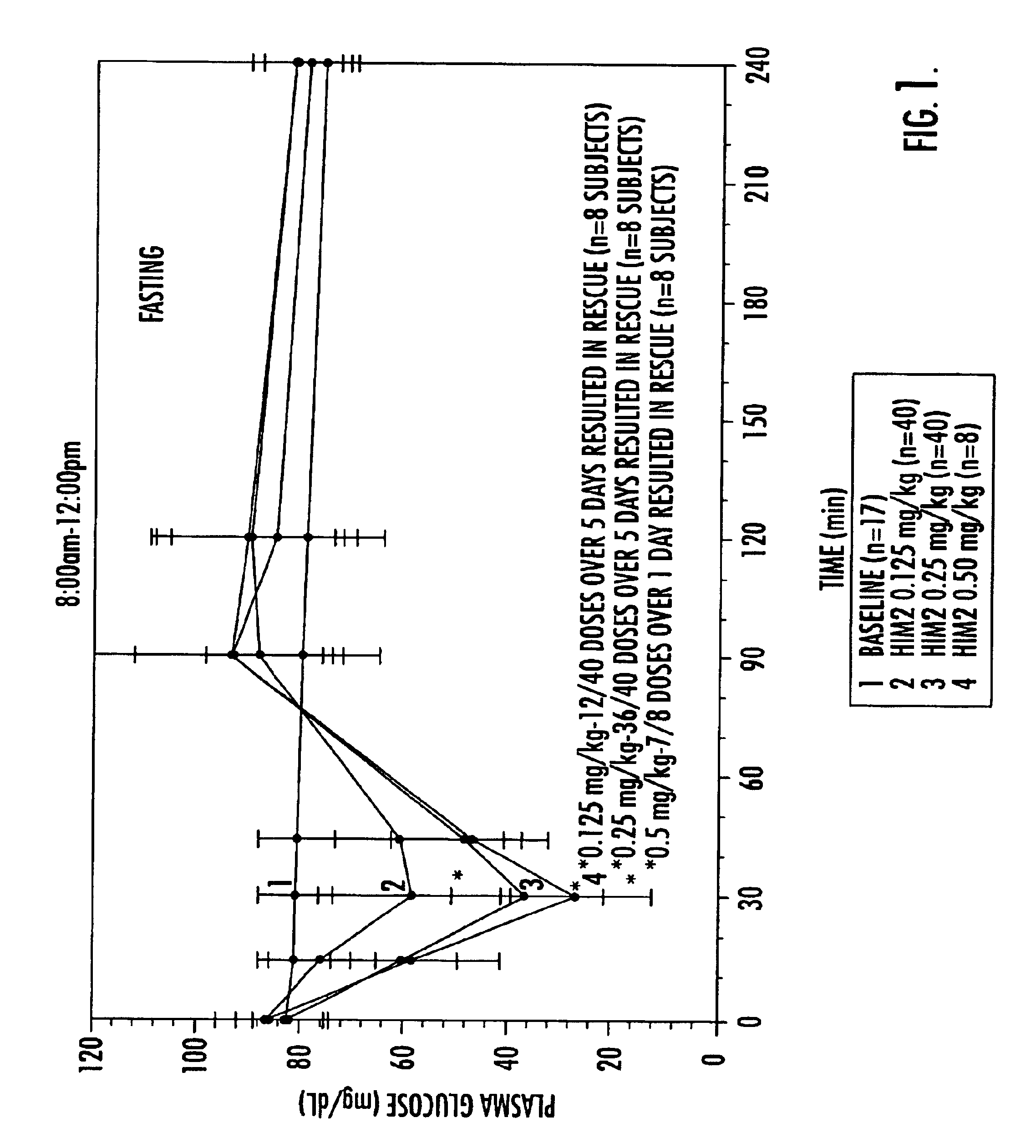
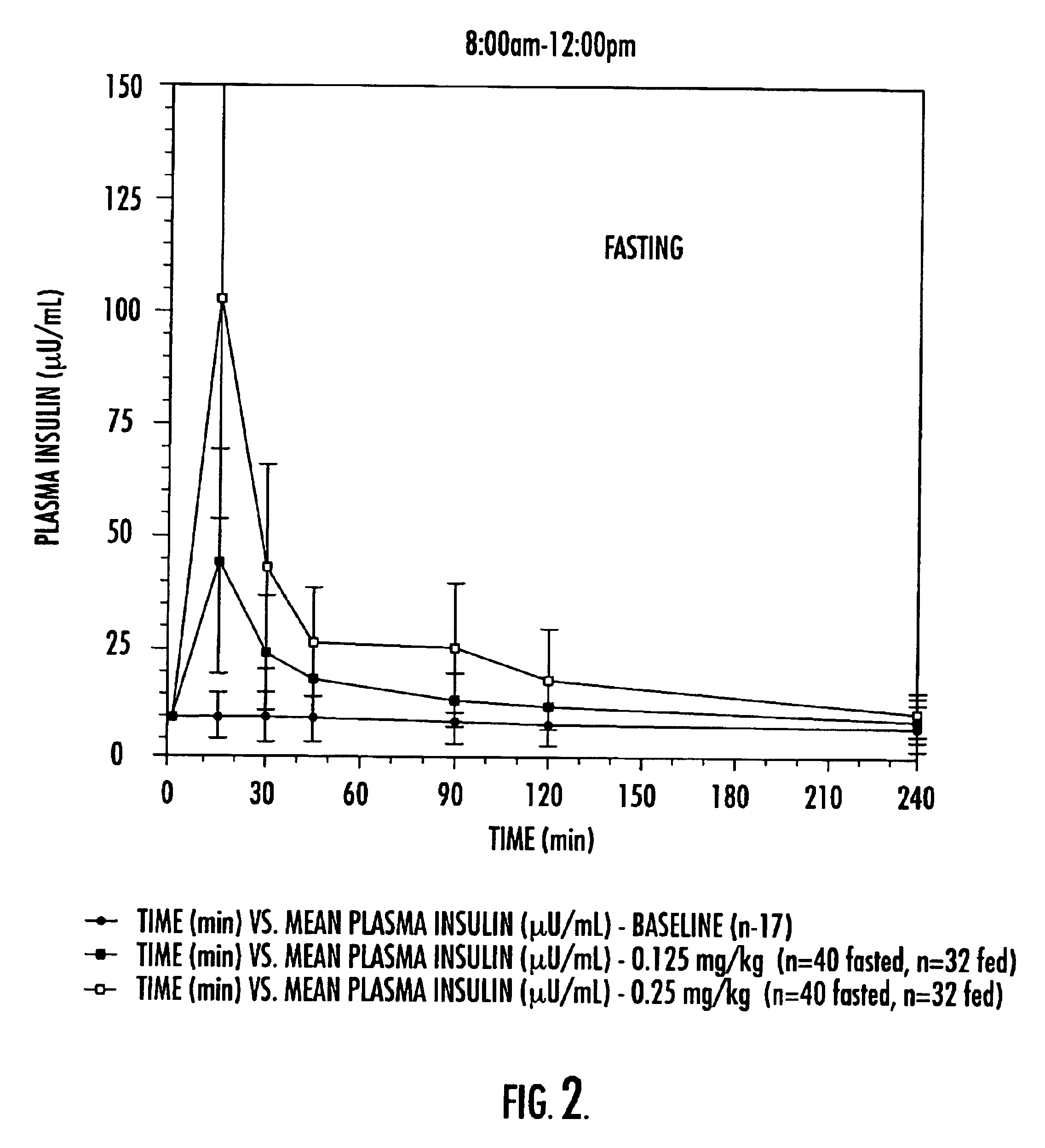
Pharmaceutical compositions of insulin drug-oligomer conjugates and methods of treating diseases therewith
InactiveUS6867183B2Lower precipitation pointReadily re-solubilizesOrganic active ingredientsBiocideBile JuiceOligomer
Pharmaceutical compositions that include an insulin drug-oligomer conjugate, a fatty acid component, and a bile salt component are described. The insulin drug is covalently coupled to an oligomeric moiety. The fatty acid component and the bile salt component are present in a weight-to-weight ratio of between 1:5 and 5:1. Methods of treating an insulin deficiency in a subject in need of such treatment using such pharmaceutical compositions are also provided, as are methods of providing such pharmaceutical compositions.
Owner:BIOCON LTD
Polyhydroxylated bile acids for treatment of biliary disorders
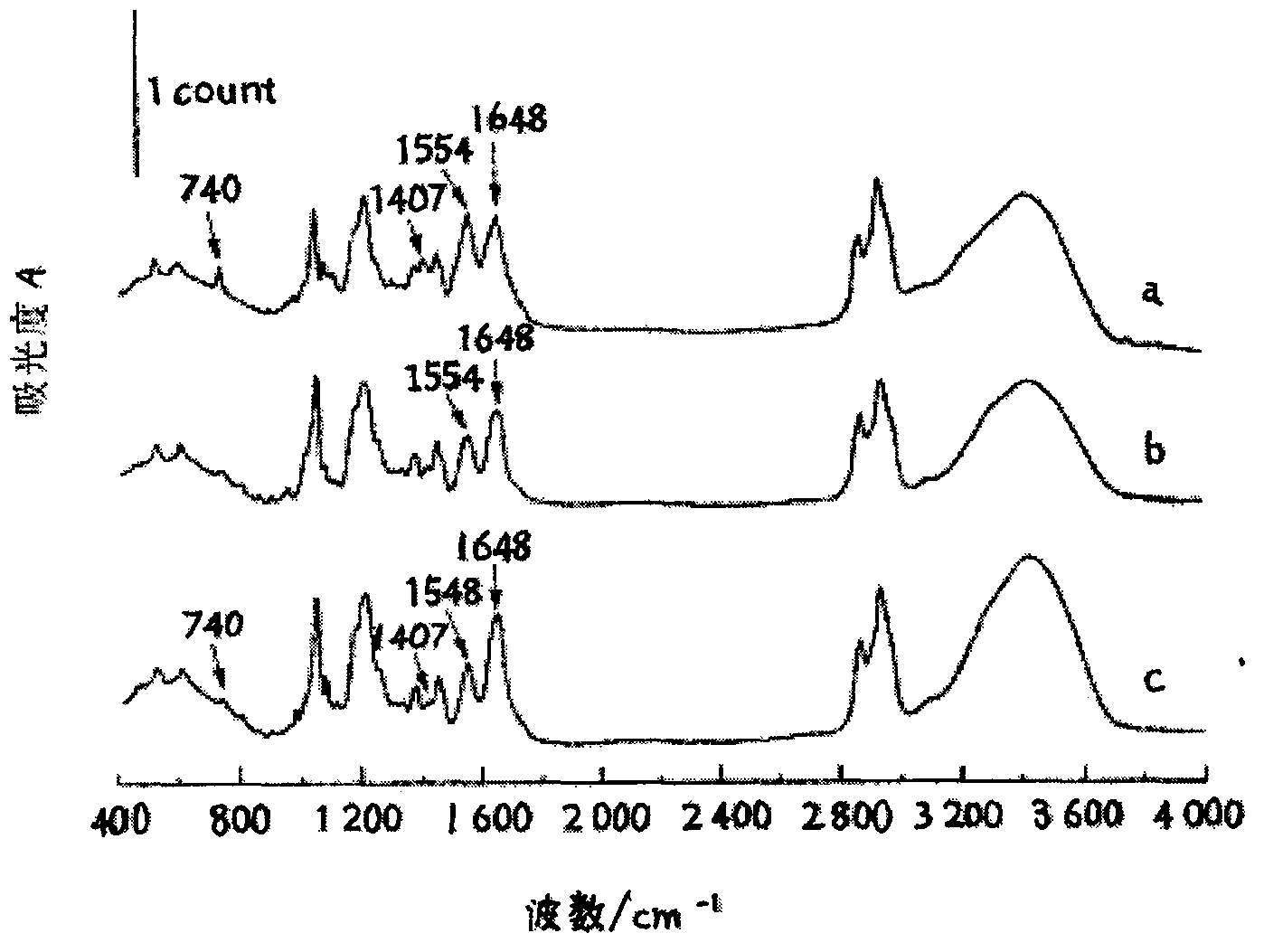
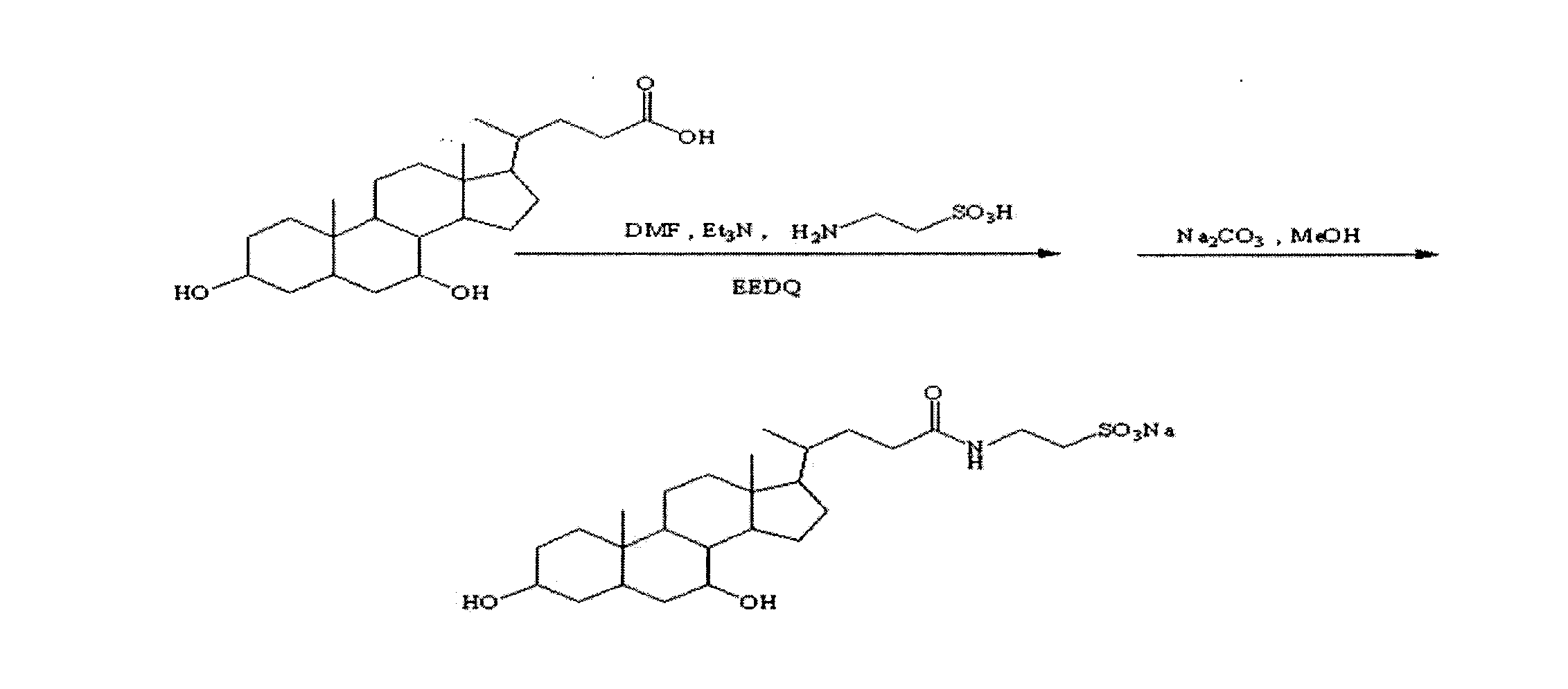
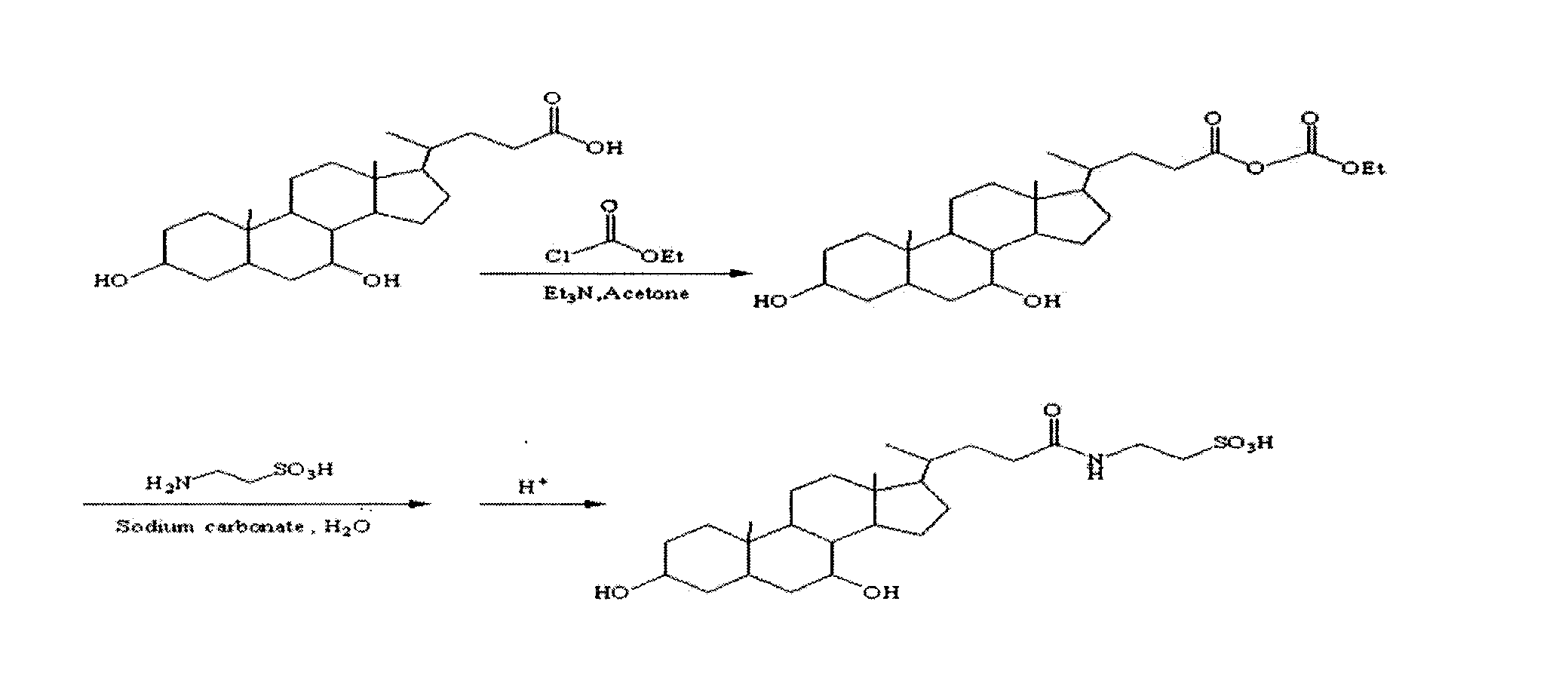
The invention provides, in part, polyhydroxylated bile acids for treating biliary disorders, for example, biliary disorders arising out of cholestasis of portal hypertension. The invention also provides, in part, polyhydroxylated bile acids for stimulating bile flow. New compounds 2α,3α,7α,12α-tetrahydroxy-5β-cholanoic acid and 3α.4α,7α,12α-tetrahydroxy-5β-cholanoic acid are disclosed, uses thereof and synthesis thereof.
Owner:QING BILE THERAPEUTICS
Dihydroxyl compounds and compositions for cholesterol management and related uses
The present invention relates to novel dihydroxyl compounds, compositions comprising hydroxyl compounds, and methods useful for treating and preventing a variety of diseases and conditions such as, but not limited to aging, Alzheimer's Disease, cancer, cardiovascular disease, diabetic nephropathy, diabetic retinopathy, a disorder of glucose metabolism, dyslipidemia, dyslipoproteinemia, hypertension, impotence, inflammation, insulin resistance, lipid elimination in bile, obesity, oxysterol elimination in bile, pancreatitis, Parkinson's disease, a peroxisome proliferator activated receptor-associated disorder, phospholipid elimination in bile, renal disease, septicemia, metabolic syndrome disorders (e.g., Syndrome X), thrombotic disorder. Compounds and methods of the invention can also be used to modulate C reactive protein or enhance bile production in a patient. In certain embodiments, the compounds, compositions, and methods of the invention are useful in combination therapy with other therapeutics, such as hypocholesterolemic and hypoglycemic agents.
Owner:ESPERION THERAPEUTICS
Sulfonylurea derivative and pharmaceutical composition and application thereof
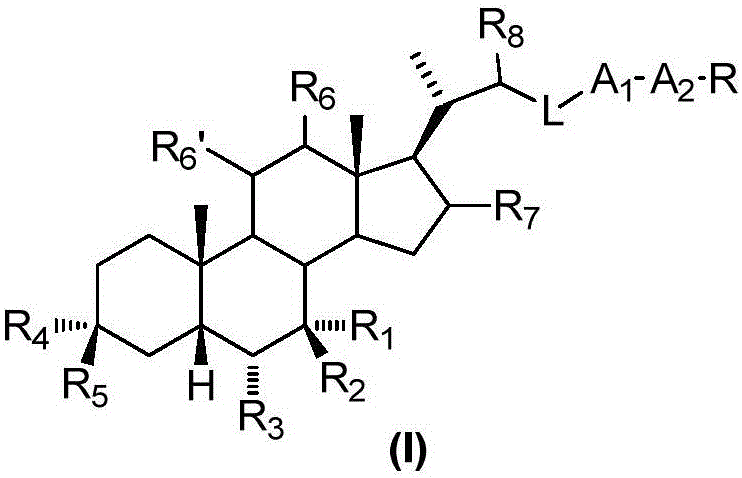
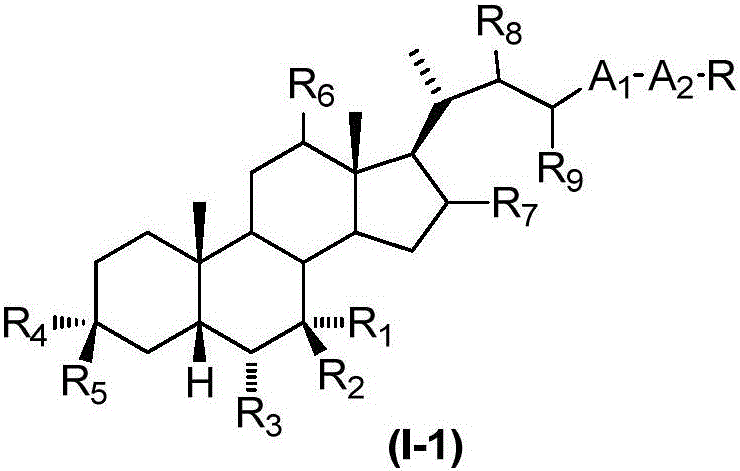
The invention relates to a preparation method and application of a sulfonylurea compound and a composition containing the same component as FXR and / or TGR5 agonist, the FXR and / or TGR5 agonist is a compound shown as a formula (I), or a pharmaceutically acceptable salt, a solvate, a prodrug, an isomer and a stable isotope derivative thereof. The compounds can be used for treatment of FXR and / or TGR5 mediated diseases including primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity and other field.
Owner:SHANGHAI DE NOVO PHARMA
Duodenal internal covering membrane made of degradable biocompatible material and application thereof
ActiveCN102626330ANo allergic reactionNo toxic reactionSurgeryNon-surgical orthopedic devicesBile JuiceMedical equipment
The invention provides a duodenal internal covering membrane, which is made of degradable shape memory biocompatible material and relates to degradable medical equipment internally installed in a digestive tract. After the internal covering membrane is implanted into a duodenum, chyme can be split from bile and pancreatic juice, so stomach exudates are prevented from being directly digested, absorbed and metabolized in the duodenum, and the histocompatibility is good; after being implanted into a human body, the duodenal internal covering membrane is steady, is not easy to slide off to be incarcerated and can be gradually degraded in the human body after 2 months to 5 years, so complicated operation and the injury to organs and tissue, which are caused when the duodenal internal covering membrane is removed from the human body in the future, are avoided, a rebound effect achieved after the original barrier is instantaneously and thoroughly dismounted can be slowed down, and the duodenal internal covering membrane can be prepared into medical equipment which is used for curing obesity and diabetes mellitus and does not need to be removed from the human body in the future; and tube-shaped part sinking induced by gastrointestinal motility and jejunum content backflow caused by the decrease of a gap between a tube-shaped part of the internal covering membrane and the inner wall of the duodenum can be avoided. The prepared medical equipment for curing the obesity and the diabetes mellitus can achieve the effects of dropping prevention, removal exemption, rebound inhibition and injury reduction.
Owner:万平
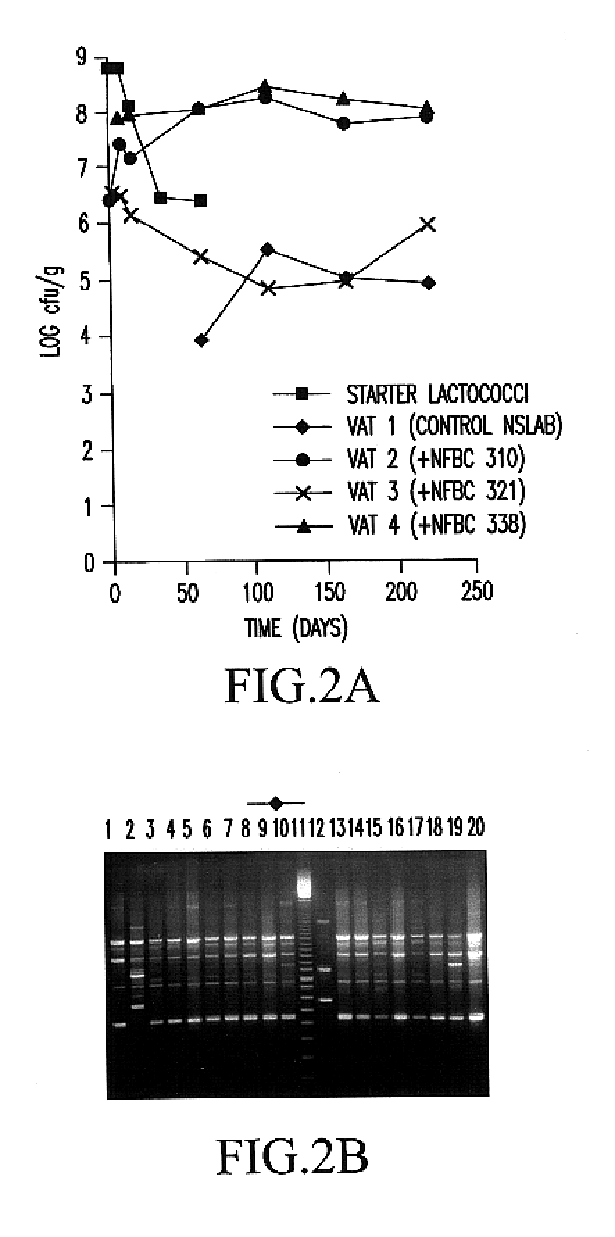
Method for preparing probiotic brown lactobacillus beverage product with large amount of bacteria
ActiveCN101731330AStrong toleranceImprove the detection rateMilk preparationBiotechnologyMaillard reaction
The invention discloses a method for preparing a probiotic brown lactobacillus beverage product with large amount of bacteria, which comprises the steps of Maillard reaction, probiotic fermentation and post treatment, wherein the probiotic fermentation step comprises: hydrating and sterilizing defatted milk powder or defatted fresh milk, reacting the defatted milk powder or the defatted fresh milk for 2.5 hours at the temperature of between 95 and 98 DEG C till the milky yellow becomes brown at the end point of the reaction, cooling the reaction product to between 37 and 38 DEG C, inoculating the reaction product to a Lactobacillus casei strain, statically fermenting the strain for 48 to 72 hours at the temperature of between 37 and 38 DEG C till the pH reaches 3.8 to 4.0, and cooling the fermentation product to between 2 and 6 DEG C; and the post treatment steps comprises: preparing base solution by using sugar, high fructose corn syrup, pectin and purified water, wherein the weight ratio of the base solution to the probiotic fermentation solution is 1 to 3; and the pH is adjusted to between 3.5 and 3.6; homogenizing the base solution, cooling the base solution to between 2 and 6 DEG C, canning products after passing detection, maturing the products for 4 to 8 hours at the temperature of between 2 and 6 DEG C, and delivery the products from a storage. The number of the living bacteria in the shelf life of the product can reach 1*108 to 1*1,010cfu / ml; and the product has zero fat and super clear mouthfeel, has no viscosity, has high probiotic detection rate, and can effectively keep or recover the intestinal microbial balance.
Owner:SHANDONG DEYI DAIRY IND
High efficiency technique for extracting bilirubin and bile acid by using animal bile as raw material
The present invention relates to process of extracting cholic acid, deoxycholic acid and bilirubin from bile of pig, ox and sheep. Bile of pig, ox and sheep consists of water in about 97 %, bile acid in about 2.5 % and bilirubin in about 0.4 %; and contains also phospholipid, cholesterol, Na, K, Ca, phosphate, carbonate, small amount of protein, and other components. Fresh bile is treated through cooling, filtering to defat, basic hydrolysis, acidification and organic solvent extracting to obtain bilirubin; the rest solution is further treated through deep saponification, acidification and organic solvent precipitation to obtain the mixture of cholic acid and deoxycholic acid; and the mixture is re-crystallization separated to obtain high purity cholic acid and deoxycholic acid.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Separation purification preparation method of chenodeoxycholic acid in pig's bile
InactiveCN1869044ASimple and fast operationLow costUnknown materialsSteroidsCholic acidChenodeoxycholic acid
A process for separating the chenodeoxycholic acid from pig's gall and purifying it includes such steps as preparing general cholic acid from the mother liquid generated by extracting the cholerythrin from pig's gall, saponifying, regulating pH value to obtain crude chenodeoxycholic acid, decoloring, defatting, preparing the deposit of barium chenodeoxycholate, reacting on potassium carbonate to remove Ba, regulating pH value, and purifying by silicon gel column.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for producing high-purity chenodeoxy cholic acid from poultry and livestock bile
InactiveCN1775798ASimple extraction processSimple production processAntiinfectivesSteroids preparationCholic acidBile Juice
The invention discloses a method to manufacturing high purity chemocholic acid from fowl bile that belongs to refinement chemical and medicine field. It includes the following steps: a. saponification reaction: mixing the fowl bile and sodium hydroxide and reacting for 20-30 hours under the certain temperature; b. neutral reaction: adding dilute hydrochloric acid after saponification reaction and gaining raw cholic acid after filtering; c. column chromatography: adding dilute sodium hydroxide into raw cholic acid and injecting into chromatography column, uniting the eluent; super filtering: taking super filtering by super filtering film; e. gaining chemocholic acid that the purity is over 96% after adding dilute acid, filtering and drying. The invention has simple technology, high purity, low cost and environment protection.
Owner:SHANDONG BOERDE BIOLOGICAL SCI & TECH
Method for preparing chenodeoxycholic acid
The invention relates to a method of distilling the chenodeoxychoilc acid from the offcuts which be gained by distilling the bilirubin from the bile of the pig. With the offcuts of the alkali saponification, the deposit is treated with the oxidation treatment by means of the hydrogen peroxide, the filtrate is treated by the dilute sulfuric acid and decolored by means of the active carbon and solved, crystaled using the ethyl acetate again and again; the coordinate production of the hyodeoxycholic acid is purified, the mother liquor is concentrated to the cream and melted using the alkali and have the salifying treatment with the chloride of barium, so the cholate is formed, the cholate is treated to take off barium when the cholate suspending the aqueous solution containing the sodium carbonate, then it is acidificated with the dilute sulfuric acid and is separated ,deposited and dried to gain the crude chenodeoxycholic acid which is decolored using the active carbon and is melted, rimed and dried using the ethyl acetate. Then the finished production of the chenodeoxycholic acid form.The whole technics has some merits of the abundant material, the amity of the environment, the safety and innocuity, producing many outputs from the single stuff and easy to the mass production. The invention is used for distilling the chenodeoxycholic acid.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Method for separating and purifying chenodeoxycholic acid from duck gall
The invention provides a method for separating and purifying chenodeoxycholic acid from duck gall, and concretely comprises to the following steps: dissolving and extracting a duck gall paste crude product, extracting ethyl acetate, and ultrafiltering and removing impurities. The method has the following advantages that 1) the raw material source of chenodeoxycholic acid can be enlarged, the method for separating and purifying chenodeoxycholic acid from the duck gall is provided; and 2) the content of chenodeoxycholic acid extracted from the duck gall by a traditional method is low with about 60%. The HPLC content of chenodeoxycholic acid obtained by using the method of the invention can reach more than 96%, and the extraction efficiency of chenodeoxycholic acid is enhanced.
Owner:苏州天绿生物制药有限公司 +1
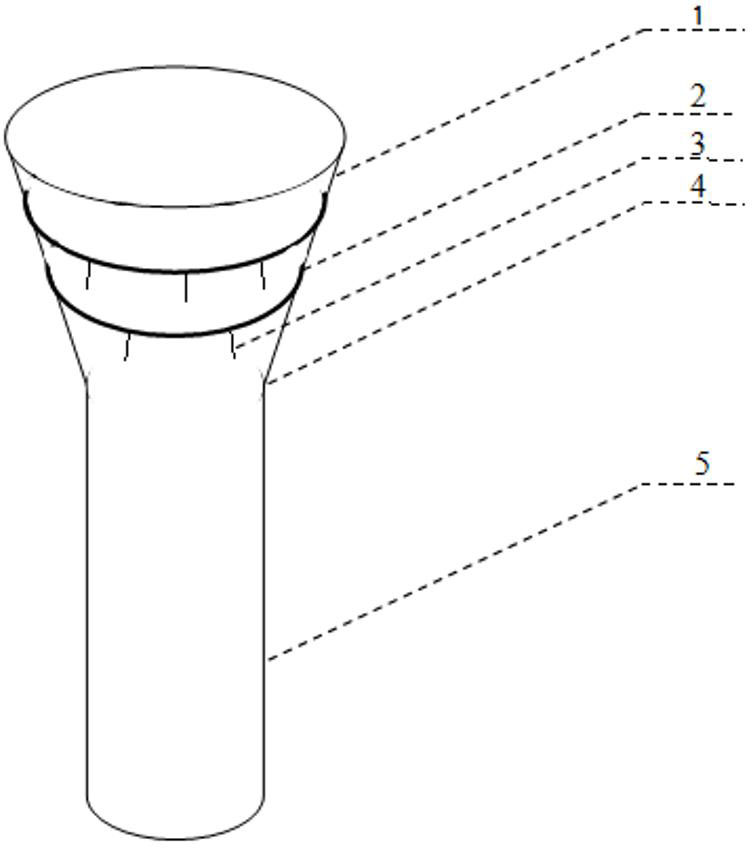
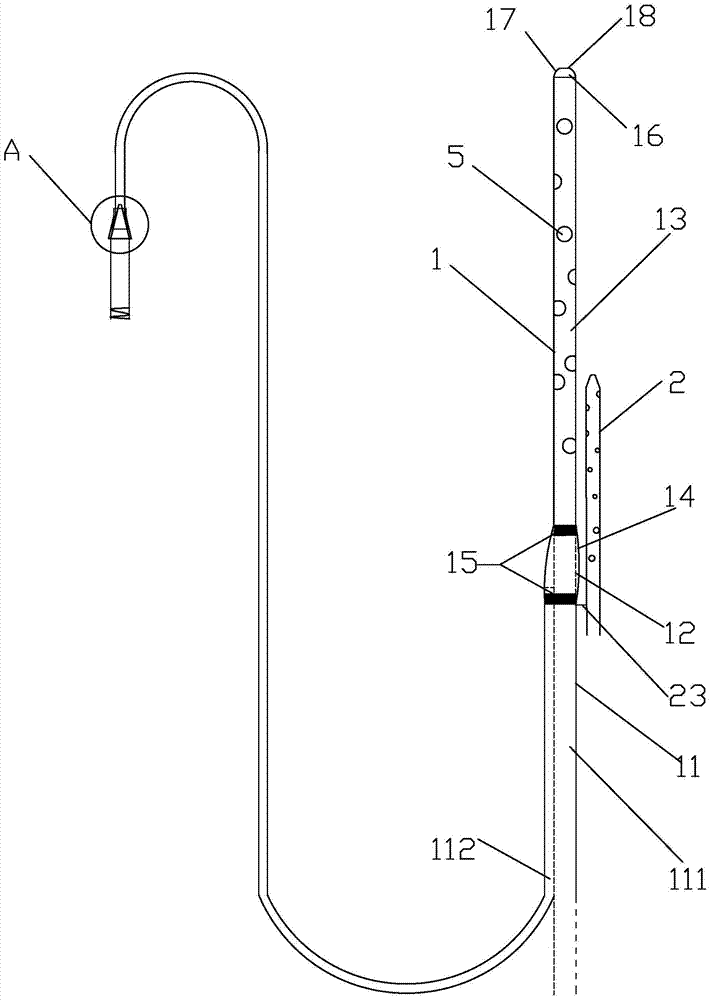
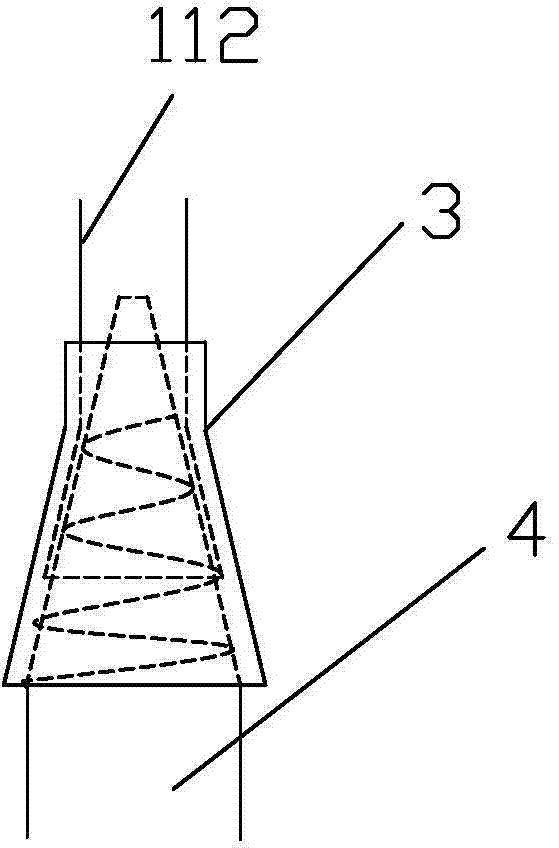
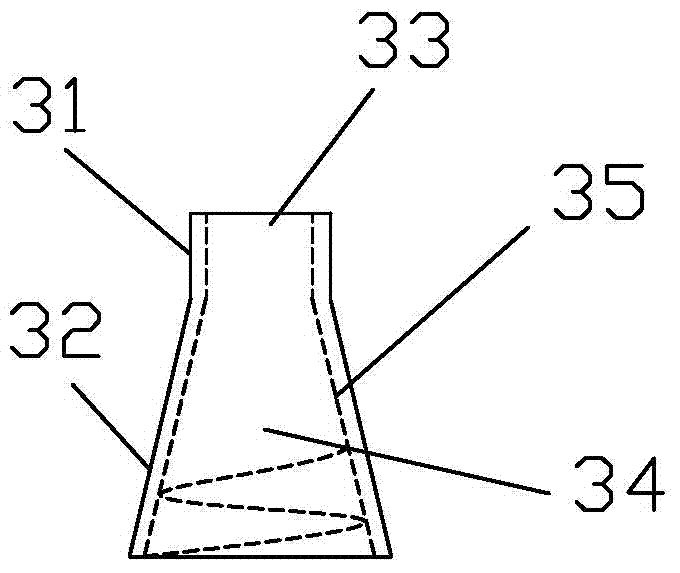
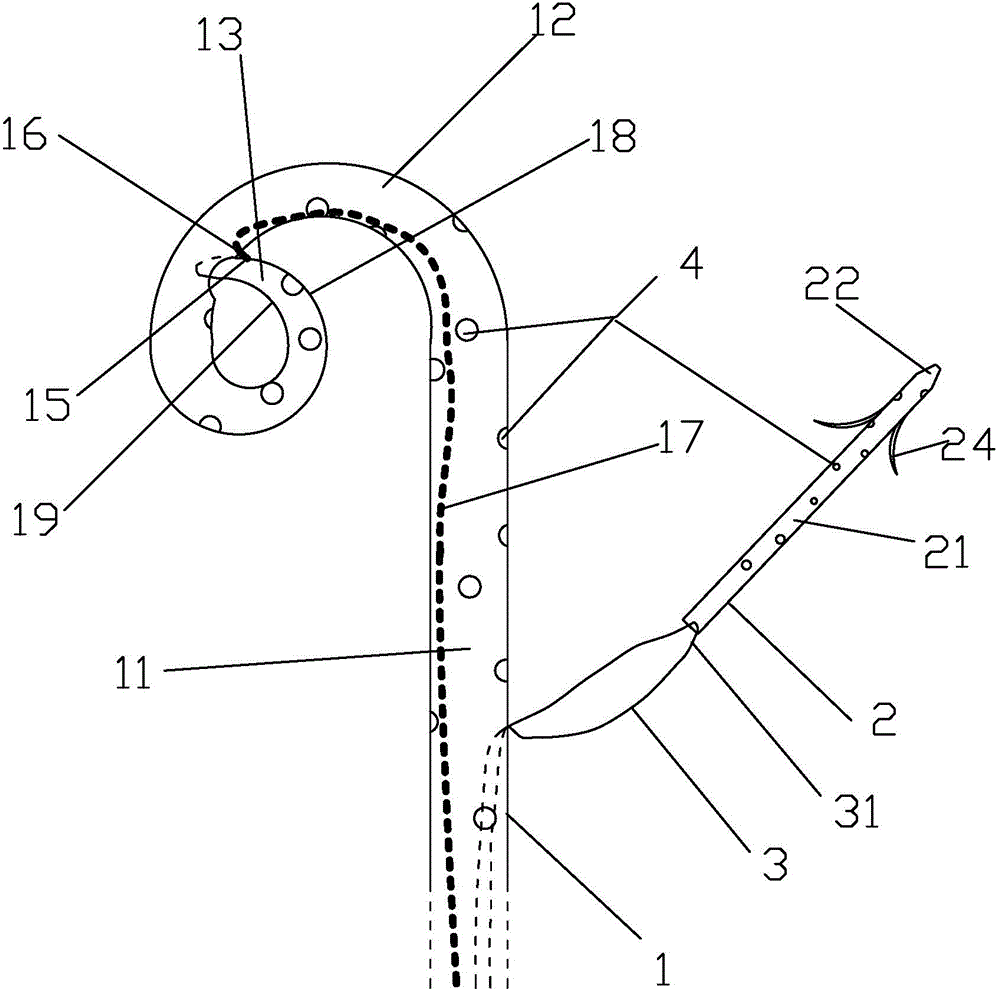
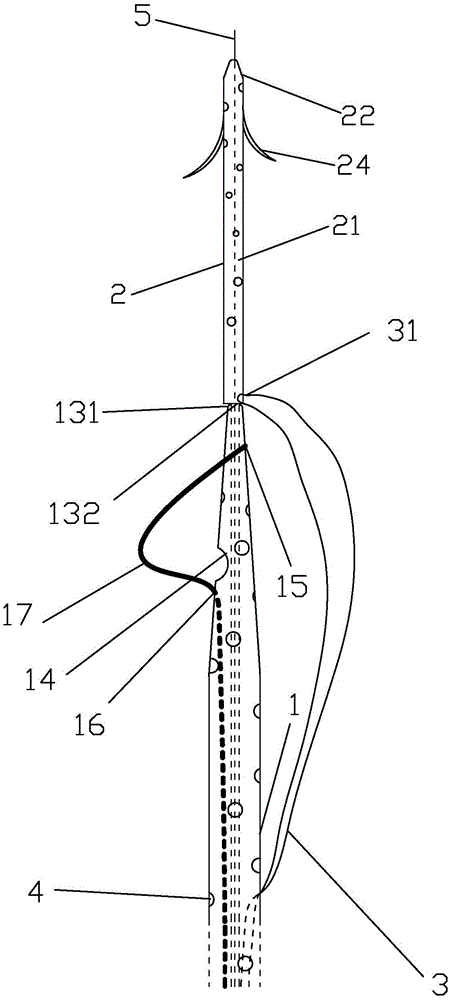
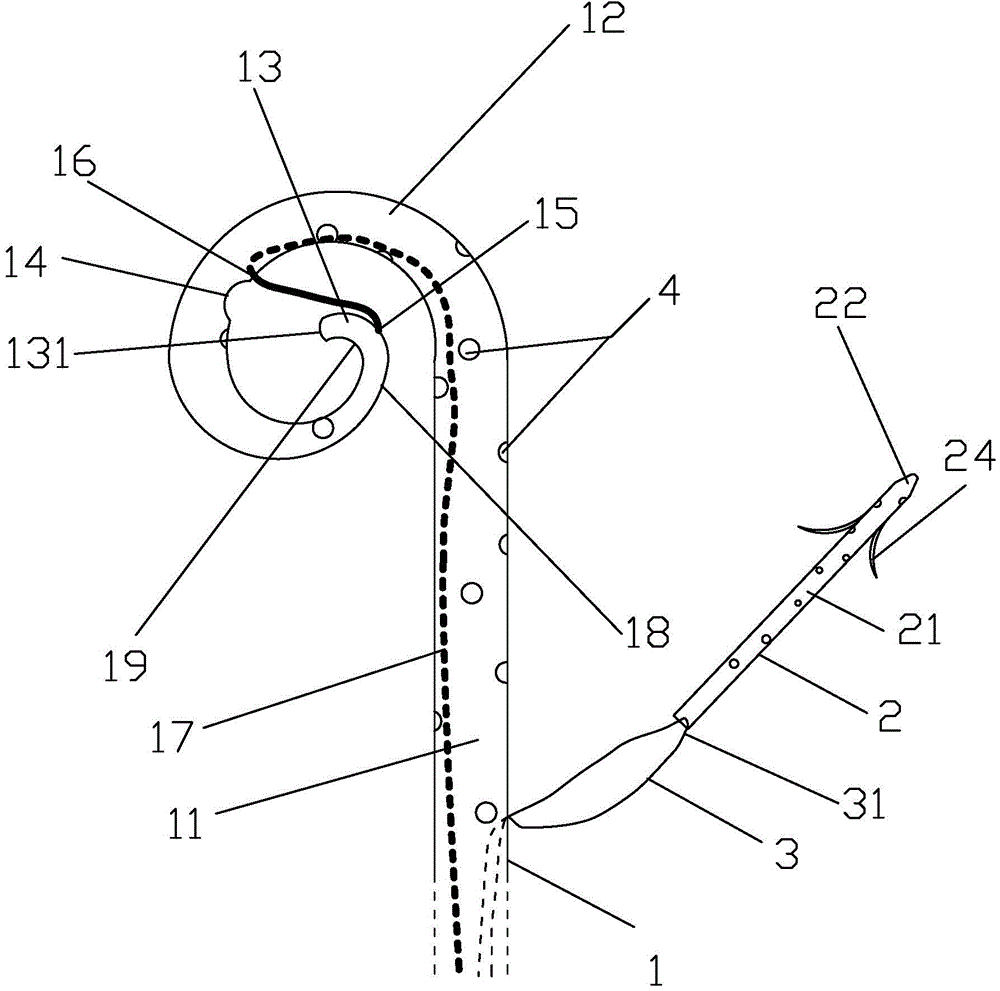
Integrated bile and pancreatic juice drainage pipe
The invention discloses an integrated bile and pancreatic juice drainage pipe which comprises a water sac type bile outer drainage pipe and a pancreatic juice inner drainage pipe. The water sac type bile outer drainage pipe comprises a drainage pipe section I, a drainage pipe section II and a drainage pipe section III which are communicated in sequence. The outer wall of the drainage pipe section II is sleeved with a balloon thin film. The pancreatic juice inner drainage pipe comprises a drainage pipe body, a pipe cap and a drainage pipe tail portion. The end, far away from the drainage pipe section II, of the drainage pipe section III is matched with the drainage pipe tail portion. The integrated bile and pancreatic juice drainage pipe has strong tension force, can have the function of avoiding disengagement of the water sac type bile outer drainage pipe, is suitable for bile ducts of various diameters, has no damage to bile duct mucosa, duodenal papillae and upper gastrointestinal tract mucosa, and cannot cause obstruction of biliary tracts while avoiding displacement of the water sac type bile outer drainage pipe. The pancreatic juice inner drainage pipe is connected with the water sac type bile outer drainage pipe through a line, and the function of avoiding displacement or disengagement of the pancreatic juice inner drainage pipe is achieved.
Owner:DALIAN UNIV
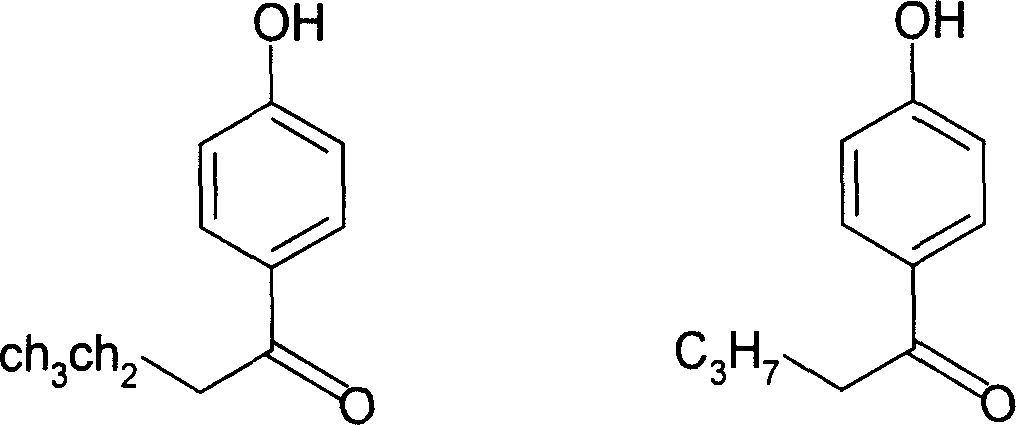
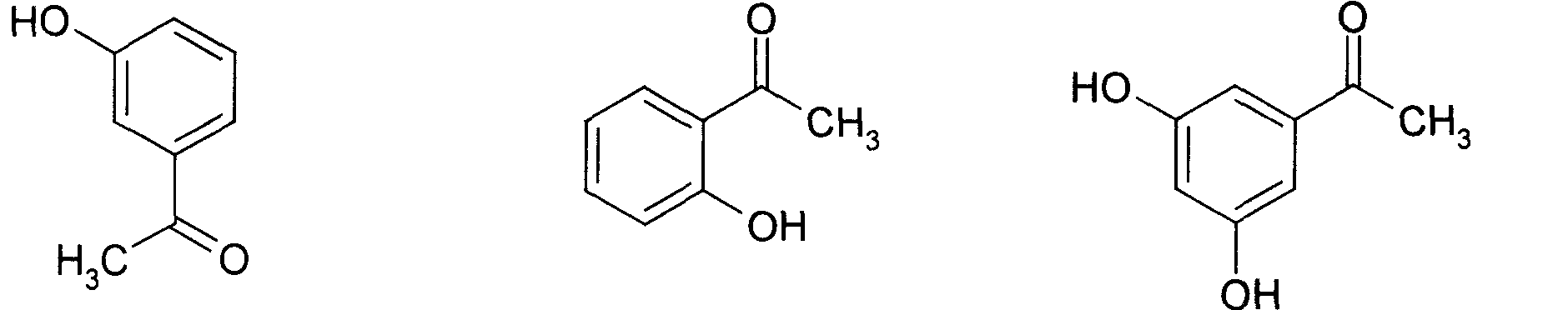
Application of p-hydroxybenzene ethanone and its derivant in preparation of agentia treating gallstone disease
InactiveCN101108173AEasy to prepareFunction doesDigestive systemKetone active ingredientsDiseaseBile Juice
The invention discloses an application of hydroxyacetophenone and its derivatives in preparing drugs for curing cholelithiasis, which can enhance the excretion of bile and lower down the content of cholesterin and bilirubin in the bile, thus realizing the dual effects of bile expelling and lithiasis resolving. Therefore, the invention can be applied to treat lithiasis.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
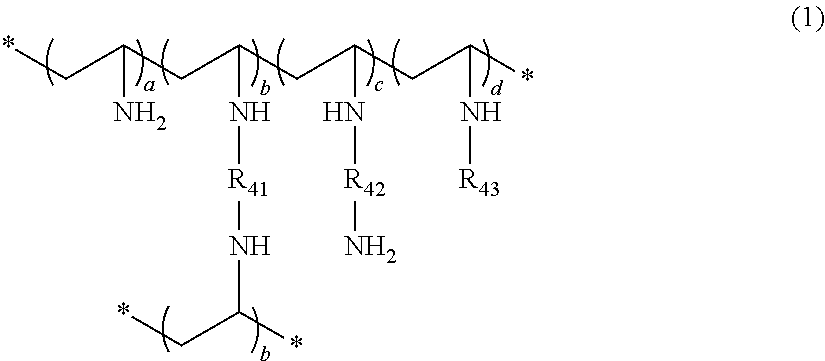
Crosslinked polyvinylamine, polyallylamine, and polyethyleneimine for use as bile acid sequestrants
ActiveUS20130022570A1Efficient removalOrganic active ingredientsNervous disorderCross-linked polyethyleneBile acid binding
The present invention provides a crosslinked amine and amide polymers effective for binding and removing bile salts from the gastrointestinal tract. These bile acid binding polymers or pharmaceutical compositions thereof can be administered to subjects to treat various conditions, including hypercholesteremia, diabetes, pruritis, irritable bowel syndrome-diarrhea (IBS-D), bile acid malabsorption, and the like.
Owner:RELYPSA INC
Process for the manufacture of probiotic cheese
InactiveUS6872411B1Improve the level ofLow costMilk preparationBacteriaMicrobiologyLactobacillus paracasei
A process for the manufacture of a probiotic cheese, such as Cheddar cheese, comprises adding a 0.0-5.5% inoculum of a strain of Lactobacillus paracasei, which is non-pathogenic, acid and bile tolerant and adherent to human epithelial cells, as a starter adjunct to cheese milk, said L. paracasei strain being capable of growing during the ripening phase to a level of 107 cfu / g or greater. The L. paracasei strains are found to grow and proliferate to high cell numbers (in excess of 108 cfu / g) in the cheese over eight months of ripening, even when added at a relatively low inoculum. The presence of the L. paracasei strains is found to have negligible effects on cheese composition, flavor and aroma.
Owner:ENTERPRISE IRELAND +1
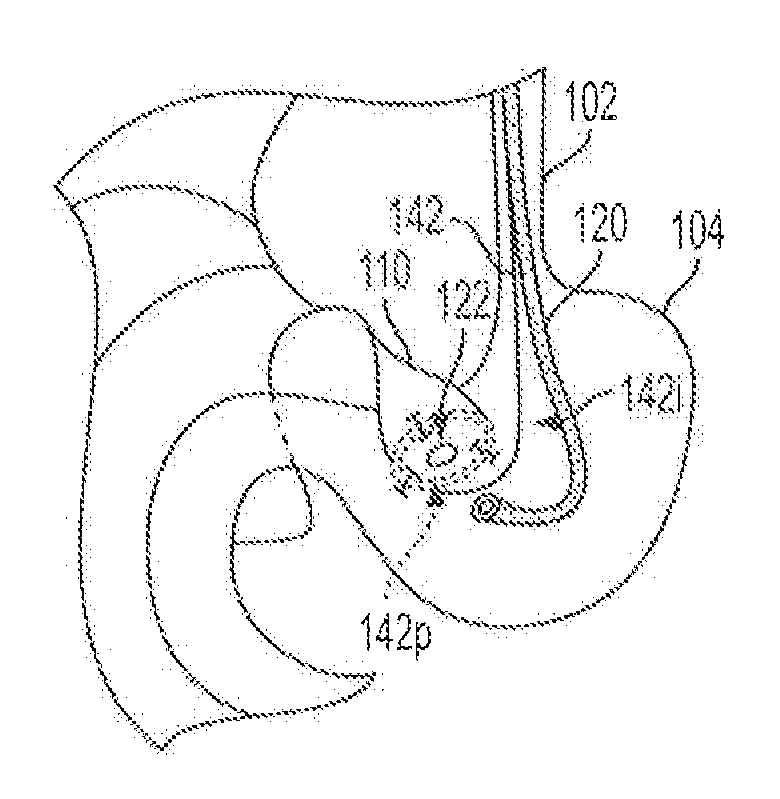
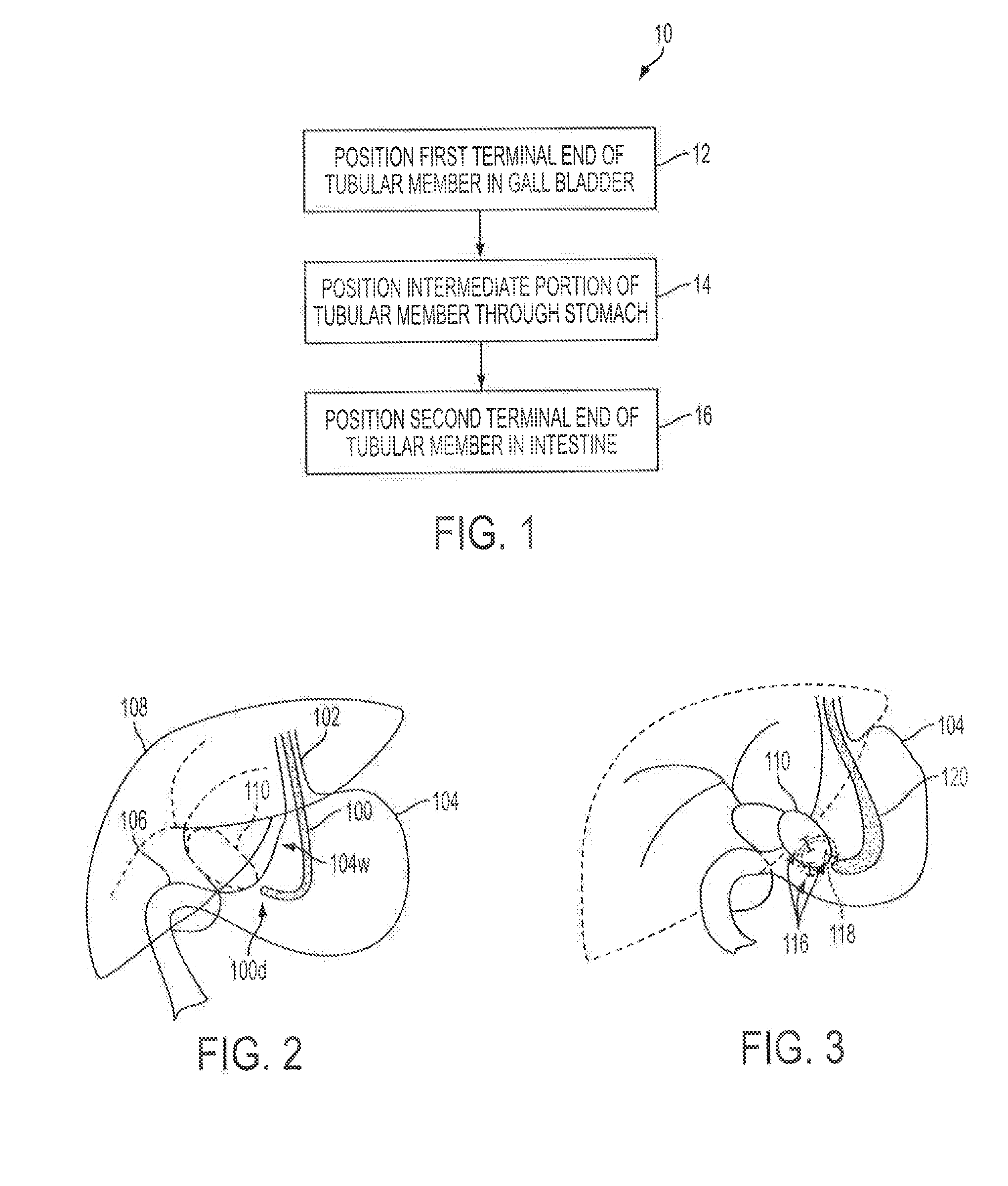
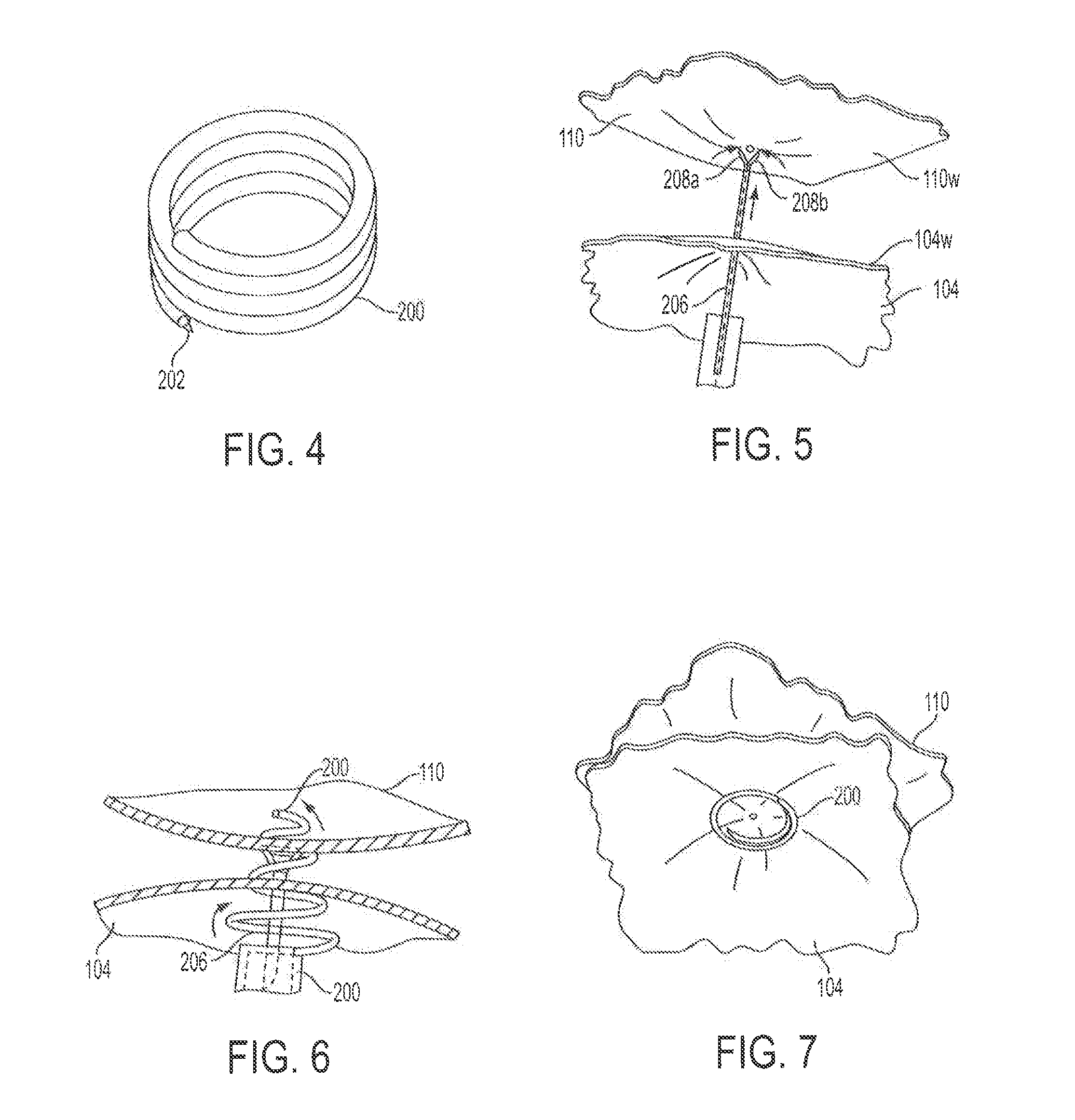
Methods for biliary diversion
Methods are provided for biliary diversion. In one embodiment, a tubular member can be implanted within a patient by positioning a proximal end of the tubular member in the patient's gall bladder, positioning a distal end of the tubular member in the patient's intestine, and positioning a length of the tubular member extending between the proximal and ends thereof within the patient's stomach. Bile can therefore be allowed to pass from the gall bladder into the tubular member's proximal end, flow through the tubular member, and exit through the tubular member's distal end to enter the patient's gastrointestinal tract at the intestine.
Owner:ETHICON ENDO SURGERY INC
Sulphonylaminocarbonyl derivatives, and pharmaceutical compositions and use thereof
The invention relates to preparation methods of sulphonylaminocarbonyl derivatives and compositions containing the same component and a use of the sulphonylaminocarbonyl derivatives as FXR and / or TGR5 agonists; the agonists are the sulphonylaminocarbonyl derivatives represented by the formula I, or pharmaceutically acceptable salts, prodrugs, solvates, hydrates, polymorphs, isomers, and stable isotope derivatives thereof. The compounds can be used for treatment of diseases and symptoms mediated by FXR and / or TGR5 and other therapeutic fields, wherein the diseases and symptoms include primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity.
Owner:SHANGHAI DE NOVO PHARMA
Y-type bile pancreatic juice drainage tube with water bag
The invention discloses a Y-type bile pancreatic juice drainage tube with a water bag. The Y-type bile pancreatic juice drainage tube comprises a water bag type bile outer drainage tube and a pancreatic juice inner drainage tube. The water bag type bile outer drainage tube comprises a drainage tube section I, a drainage tube section II and a drainage tube section III. The drainage tube section II is sleeved with a balloon film, and the pancreatic juice inner drainage tube comprises a drainage tube body and a tube cap I located at the upper end of the drainage tube body. The side wall of the drainage tube body is connected with the side wall of the drainage tube section I through a connecting part. The Y-type bile pancreatic juice drainage tube has the effects of preventing the water bag type bile outer drainage tube from disengaging and preventing the pancreatic juice inner drainage tube from shifting or disengaging, is suitable for bile ducts of various diameters and has no injury to the bile duct mucous membrane, the duodenal papilla and the upper digestive duct mucosa in the placing-in and taking-out processes, bile duct obstruction will not be caused while the water bag type bile outer drainage tube is prevented form shifting, and while the water bag type bile outer drainage tube is taken out, the pancreatic juice inner drainage tube can be taken out.
Owner:DALIAN UNIV
Chinese medicinal herb pills for treating cholecystitis and gallstone
InactiveCN102526616AAlleviate inflammatory lesionsEasy to shrinkDigestive systemPill deliveryMedicinal herbsBile Juice
The invention relates to Chinese medicinal herb pills for treating cholecystitis and gallstone. The Chinese medicinal herb pills are prepared from 22 gouts of Chinese medicinal herbs, namely coptis root, baikal skullcap root, phellodendron, corydalis tuber, curcuma, costus root, loosestrife herb, Japanese climbing fern spore, dark plum, white peony root, fine-leaf schizonepeta herb, green tangerine peel, immature bitter orange, turmeric, polygonum cuspidatum, Szechwan chinaberry fruit, gentian, bupleurum, oriental wormwood, chicken's gizzard-membrane, compound of glauber-salt and liquorice and slaked lime. The pills can lighten the pathological change of cholecystitis, promote gallbladder contraction and promote gallstone excretion and biliary excretion; by the pills, biliary excretion components are radically changed, the metabolism requirement of the human body is met, calculi are gradually disintegrated and deposited, the micro environment in the gall bladder is recovered when the calculi are discharged, and the aim of thoroughly discharging the calculi is fulfilled. The Chinese medicinal herb pills have the advantages of quick response, short treatment course, high healing rate, low recurrence rate, treatment for both symptoms and root causes, no toxic or side effects and the like.
Owner:马金桥
Bile and pancreatic juice drainage tube
The invention discloses a bile and pancreatic juice drainage tube which comprises a loop type bile external drainage tube and a pancreatic juice internal drainage tube. The loop type bile external drainage tube comprises a straight tube, an elastic loop pipe I and an elastic loop pipe II which are connected in sequence, wherein the elastic loop pipe I and the elastic loop pipe II are in the shapes of Archimedean curves one the whole. The pancreatic juice internal drainage tube comprises a drainage tube body and a tube cap located at the upper end of the drainage tube body, and a line II is arranged on the drainage tube body and penetrates through the side wall of the straight tube and extends in a tube cavity of the straight tube. The bile and pancreatic juice drainage tube reduces the probability of slipping of the loop type bile external drainage tube, does not stimulate bile duct mucosae, duodenal papilla, gastrointestinal mucosae and throat mucosae, and has an effect of preventing the pancreatic juice internal drainage tube from dislocating or escaping.
Owner:DALIAN UNIV
Composition for dispelling effects of alcohol and protecting liver and preparation method of composition
InactiveCN105126016ASimple and fast operationEasy to implementDigestive systemAntinoxious agentsCholagoguesBile Juice
An embodiment of the invention belongs to the technical field of healthcare products and discloses a composition for dispelling effects of alcohol and protecting the liver and a preparation method of the composition. The composition for dispelling the effects of alcohol and protecting the liver is prepared from, by weight, 50-150 parts of rhizoma curcumae longae, 50-150 parts of fructus phyllanthi, 50-150 parts of medicine terminalia fruits and 50-150 parts of olives, wherein the rhizoma curcumae longae has an anti-inflammatory function, a liver protection function and a cholagogue effect; the fructus phyllanthi and the medicine terminalia fruits have liver protection effects and are capable of realizing reduction of liver injuries and realizing an anti-liver fibrosis function; polyphenols in the olives have functions of blood purifying, liver soothing, stomach harmonizing and qi regulating and are capable of effectively alleviating symptoms such as headache, dizziness, stomachache, nausea, poor mental state and the like caused by alcohol consumption. Choleresis increasing and gallbladder contract promoting functions of the rhizoma curcumae longae and lipid metabolism promotion and gastrointestinal peristalsis promotion functions of the olives promote liver protection functions of the fructus phyllanthi and the medicine terminalia fruits, and remarkable healthcare effects of dispelling the effects of alcohol and protecting the liver are achieved owing to synergistic interaction of the four ingredients. The preparation method of the composition is suitable for industrial popularization and application.
Owner:北京世纪合辉医药科技股份有限公司
Probiotic composite composition containing bifidobacterium and lactobacillus acidophilus and application
InactiveCN106860483AStrong acid and bile resistanceImprove constipationOrganic active ingredientsDigestive systemNutrientLactobacillus acidophilus
The invention provides a probiotic composite composition containing bifidobacterium and lactobacillus acidophilus and application of the probiotic composite composition. The probiotic composite composition is prepared from the following components in parts by weight: 0.2 to 0.8 part by weight of probiotics, 0.1 to 0.7 part by weight of inulin and 1.5 to 2.7 parts by weight of soluble dietary fiber. The probiotic composite composition provided by the invention is a composite intestinal health nutrient product; the number of viable bacteria is not attenuated along with time; a flora has good acid resistance and bile resistance; the probiotic composite composition has clinically-verified health efficacy; for a 28-unit type probiotic composite preparation, each unit contains the probiotic composite composition and a description; the probiotic composite composition is used for crowds with constipation, ozostomia and skin problem and crowds taking antibiotics; according to the probiotic composite composition, constipation and ozostomia symptoms of the crowds can be improved, the skin type is improved, side effects of the antibiotics are reduced, and the life quality of the crowds is improved.
Owner:家家乐购(北京)科技有限公司
Oral Chinese medicinal composition as well as preparing, taking and quality inspecting methods and use thereof
ActiveCN101433641AGood curative effectEasy to takeAntipyreticComponent separationDiseaseTreatment effect
The invention discloses an orally-administered traditional Chinese medicine composition. The composition is prepared from traditional Chinese medicine raw materials such as desmodium, rhubarb, Herba Artemisiae Capillaris, achyranthes, Alisma orientale, tuckahoe, an animal gall product and so on, has functions of clearing heat and promoting diuresis, soothing liver and benefiting gall, regulating qi and dredging stranguria, promoting urination, promoting the secretion of bile, resisting inflammation, killing pain, relieving fever, resisting bacteria and so on, is used for treating diseases such as cholelithiasis and urinary calculus or hepatobiliary lithiasis caused by damp-heat of liver and gallbladder as well as symptoms such as right epigastric pain, upper abdomen fullness and distention, nausea, anorexia to greasiness, jaundice, yellow and greasy fur or osphyalgia, hematuria and so on, and has definite treatment effect and no apparent adverse reaction. The invention also relates to a preparation method, an administration method and a quality checking method for the orally-administered traditional Chinese medicine composition.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
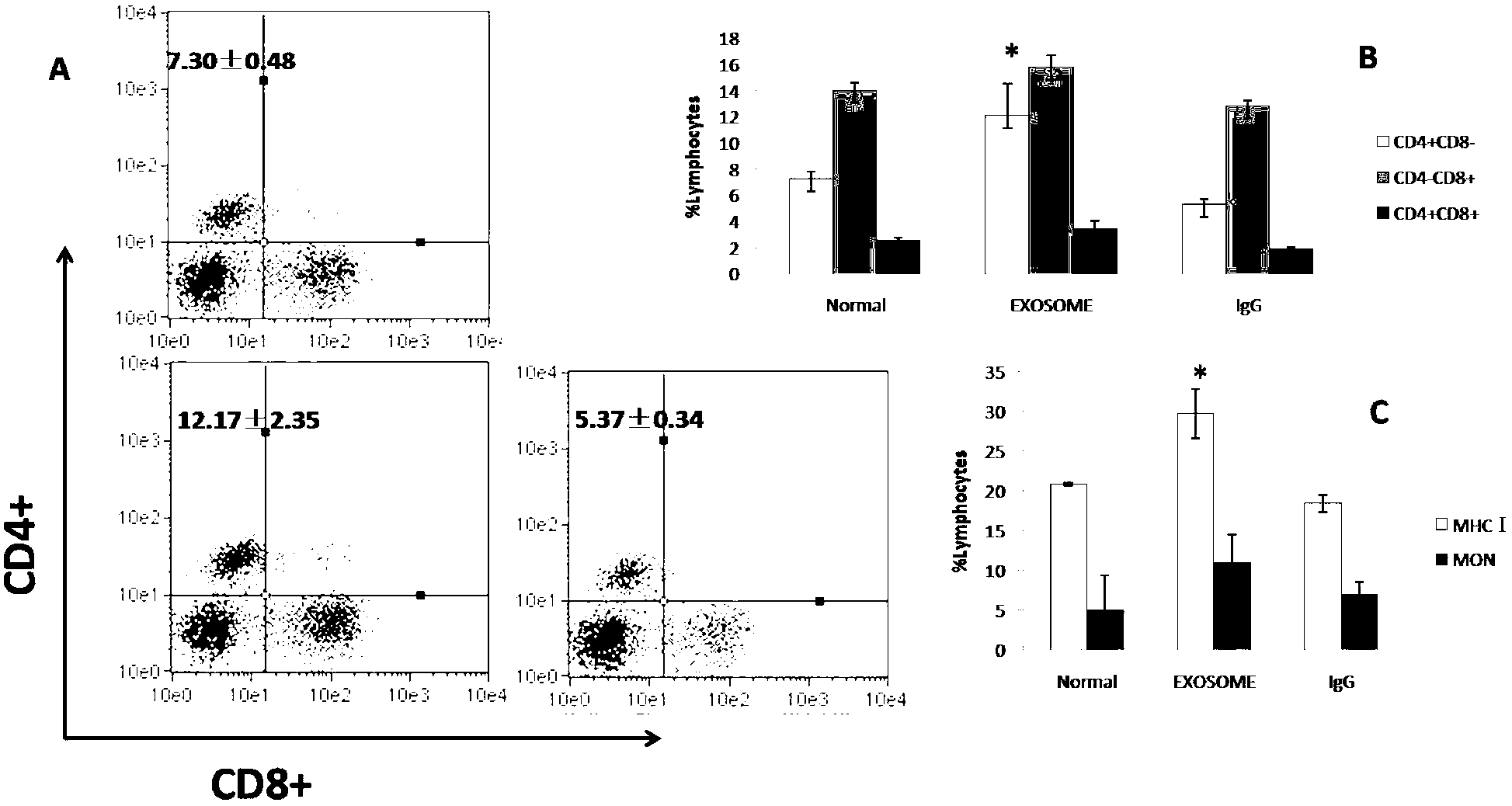
Method for extracting exosome from chicken bile and application of exosome in immunology
InactiveCN102697812AEasy to maintain biological activityExtraction time is shortDigestive systemAntibody ingredientsDiseaseIntestinal structure
The invention belongs to the field of molecular immunology, and in particular relates to an exosome secreted and released from the epidermal cells of a chicken liver, gall bladder and bile duct, and a preparation method of the exosome. The exosome contains hepatogenic proteins, such as C-reactive protein, aspartate aminotransferase, and vitronectin, epithelium-derived mucin and proteins related to an immune reaction, such as immunoglobulin, and can be taken as a biological indicator for studying liver diseases and as an adjuvant for immune reaction for immune-regulation, and can also be takenas a carrier for treating the diseases of livers, gall bladders and intestines.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
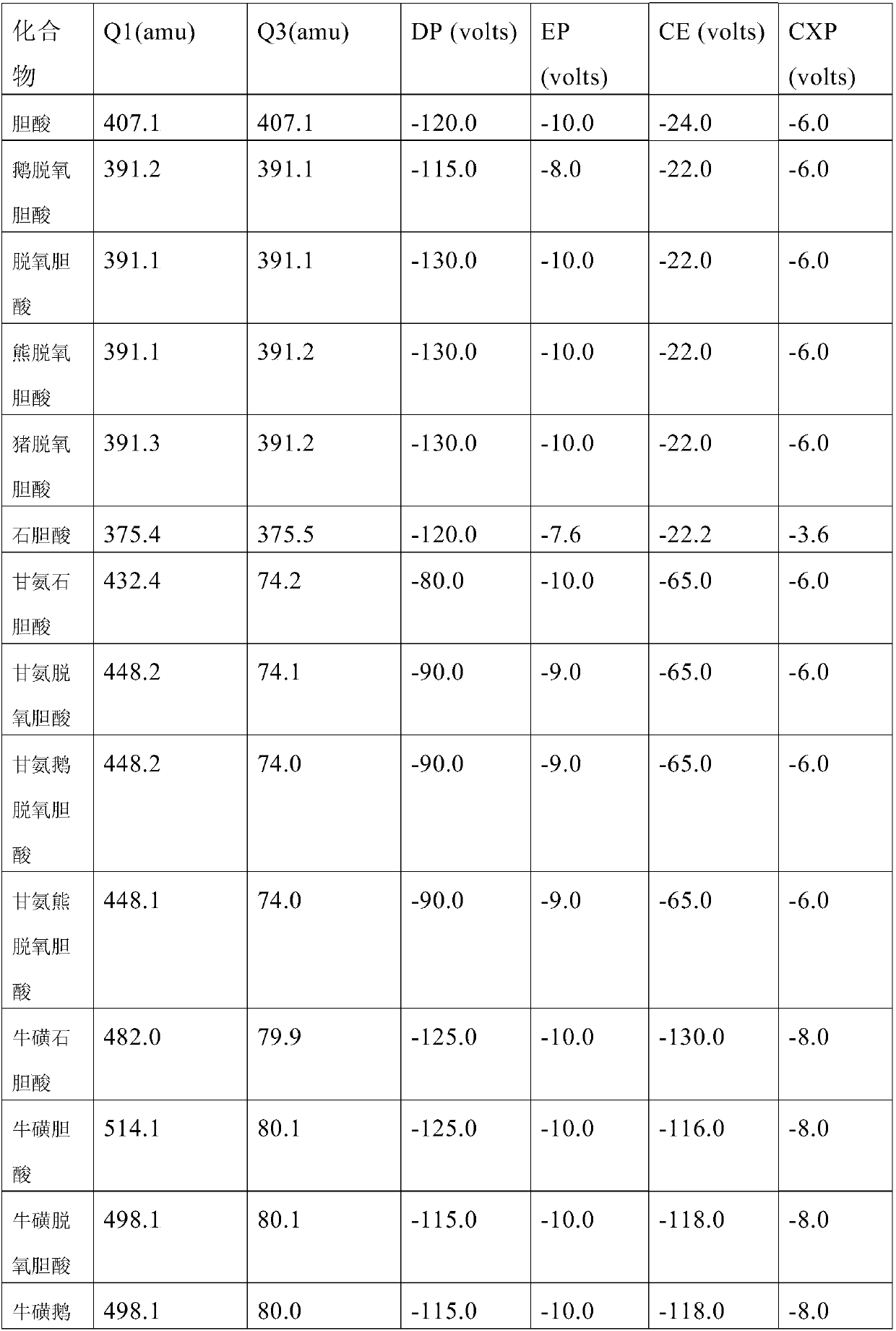
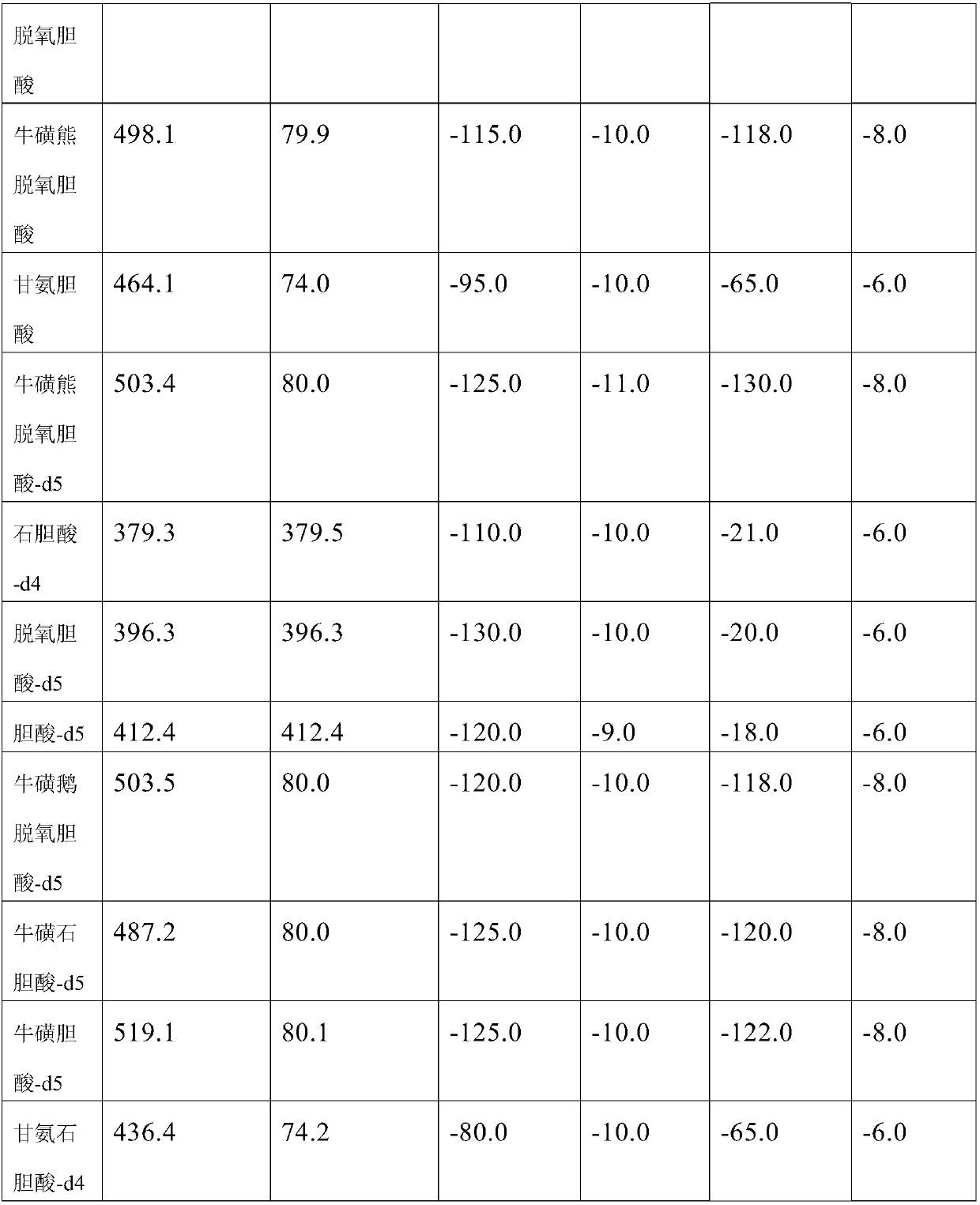
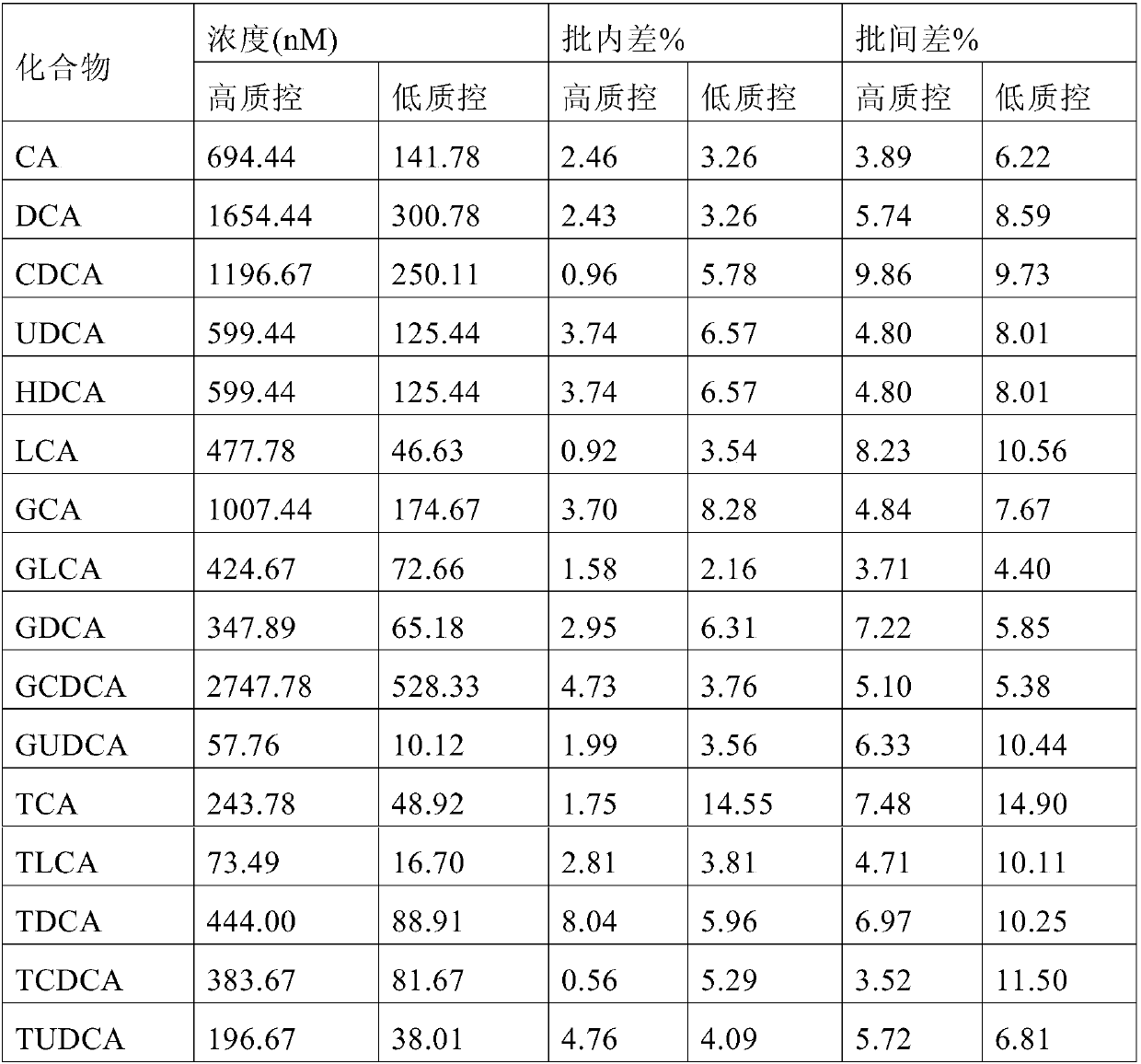
Quantification of 16 bile acids in bile and serum based on LC-MS/MS
The invention discloses quantification of 16 bile acids in the bile and serum based on LC-MS / MS. Specifically, the invention provides a detection kit for bile acids through a high performance liquid chromatography and mass spectrometry method. The kit comprises a quality control substance, an isotope internal standard extracted liquid, a diluent, a conversion liquid, a reconstitution fluid, a mobile phase additive A and a mobile phase additive B. The kit can be used for detecting 16 bile acids, respectively, and is less in time consumption and high in detection efficiency.
Owner:上海可力梅塔生物医药科技有限公司 +3
Artificial bear bile
ActiveCN102836175AFormula SafetyLow toxicityOrganic active ingredientsSenses disorderFormularyBile Juice
The invention provides an artificial bear bile. The formula of the artificial bear bile comprises the following ingredients by mass percent: 35-48 of tauroursodeoxycholic acid, 50-63 of mixed choline, 1-3 of amino acid and 0.5-1 of trace element, wherein the mixed choline is an avian bile or a mixture of a plurality of avian biles and one or a mixture of a plurality of ox bile, sheep bile, rabbit bile and dog bile. The whole pharmacological tests prove that the artificial bear bile powder has the pharmacological functions which are similar to natural bear bile in the aspects of analgesia, sedation, convulsion prevention, inflammatory prevention, liver and gallbladder protection and the like, and can be used for completely substituting for the natural bear bile, the industrial production can be realized; and moreover, the price of the artificial bear bile is lower than that of the natural (drainage) bear bile, so the artificial bear bile is easily accepted. In addition, the molecular pharmacology also proves that the artificial bear bile has the functions of inhibiting MGc80-3 cancer cell growth and proliferation.
Owner:HANGZHOU BAOJI BIO TECH
Pharmaceutical compositions for combination therapy
The present invention relates to a pharmaceutical composition comprising a combination of an FXR agonist and at least one lipid lowering agent (e.g., PPAR-alpha agonist, PPAR-delta agonist, PPAR-alpha and delta dual agonist, and / or statin). Also disclosed is use of the combination for the treatment or prevention of a FXR mediated disease or condition, such as primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), portal hypertension, bile acid diarrhea, NAFLD (nonalcoholic fatty liver disease), NASH (non-alcohol-induced steatohepatitis), and other chronic liver diseases. The combination of the present invention is useful for the treatment or prevention of conditions related to elevated lipid and liver enzyme levels. The present invention also relates to packs or kits including the pharmaceutical combination.
Owner:INTERCEPT PHARMA INC
A kind of snake gallbladder health-preserving tea and preparation method thereof
ActiveCN103609780BEliminate eye diseasesHeat-clearing and detoxifyingSenses disorderNervous disorderBile JuiceDigestion
The invention relates to the technical field of health-care foods, and discloses snake gall health tea and a preparation method thereof. The health tea disclosed by the invention is prepared from the following raw materials in parts by weight: 80-120 parts of tea, 2-10 parts of chrysanthemum, 0.5-5 parts of medlar, 2-10 parts of liquorice, 5-15 parts of dandelion, 0.5-5 parts of mint, 0.5-5 parts of rhizoma zingiberis and 0.5-5 parts of snake bile. The method comprises the following steps: firstly, decocting the chrysanthemum, the medlar, the liquorice, the dandelion and the mint to prepare traditional Chinese medicine juice; soaking the rhizoma zingiberis and the snake bile by alcohol, so as to prepare the snake bile; evenly mixing the traditional Chinese medicine juice, the snake bile and the tea respectively; and drying, inspecting and split charging, so as to obtain the snake gall health tea. According to the snake gall health tea disclosed by the invention, the traditional Chinese medicines are carefully selected to be combined with the health tea, so that the efficacy of the snake gall in the tea is developed to the maximal extent; the health tea is processed by adopting a modern scientific method, so that the prepared tea reserves the original ecology shape; the tea is limpid green, fresh and heavy in color and luster after being brewed, mellow in smell, long in aftertaste after being drunk, and beneficial to eye health after being drunk for a long period of time, has the effects of energizing and reinforcing intelligence, preventing phlegm from forming, stopping coughing, invigorating spleen to promote digestion and removing fatigue, and is a natural health-care treasure which is sweet in taste.
Owner:山东菊福堂生物科技股份有限公司
Preparation method of enzyme induced bezoar
The invention relates to a method for preparing enzyme-natured bezoar. In the method, colon bacillus is natured in vitro; colon bacillus beta-GA enzyme is extracted and implanted to the gall of animals, such as cattle, sheep, pig, etc; the effective ingredients of gall bezoar are catalyzed and totally precipitated, then the bezoar is prepared. Namely: the colon bacillus is natured in 3.6-4.0% of albumen brothculture at 35-38 DEG C of constant temperature for 24 hours and centrifugally precipitated, then colon bacillus precipitation is gained; then 10-15g of the colon bacillus precipitation is added into 400mlpH6.8 of trihydroxymethyl aminomethane-hydrochloric acid buffer solution, vibrated in an ultrasonic groove for 15-20 minutes and centrifuged, then the colon bacillus beta-GA enzyme solution is gained; 28-32ml of the solution is added into 3,000ml of the gall of the animals, such as cattle, sheep, pig, etc, and 20-30g of borax is added, enzymatic reaction is carried out on the mixture at 35-38 DEG C of constant temperature for 24 hours, the bezoar of the invention is gained. The invention is characterized in that the raw materials are abundant, the technique is simple, the equipment is less, the investment is less, the cost is low, the benefit is high; the therapeutic effect of the enzyme-natured bezoar is the same as or better than that of the natural bezoar; and the enzyme-natured bezoar can be prepared industrially.
Owner:刘化侠
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com