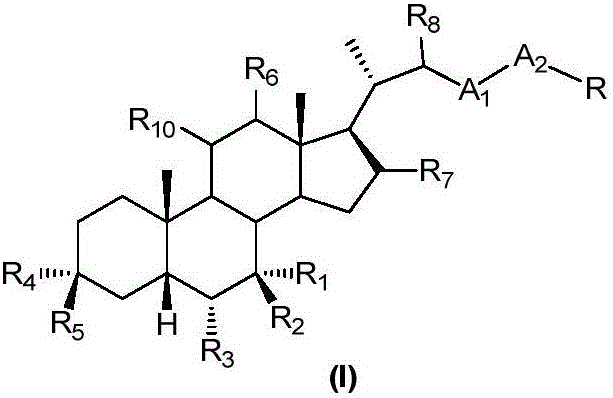
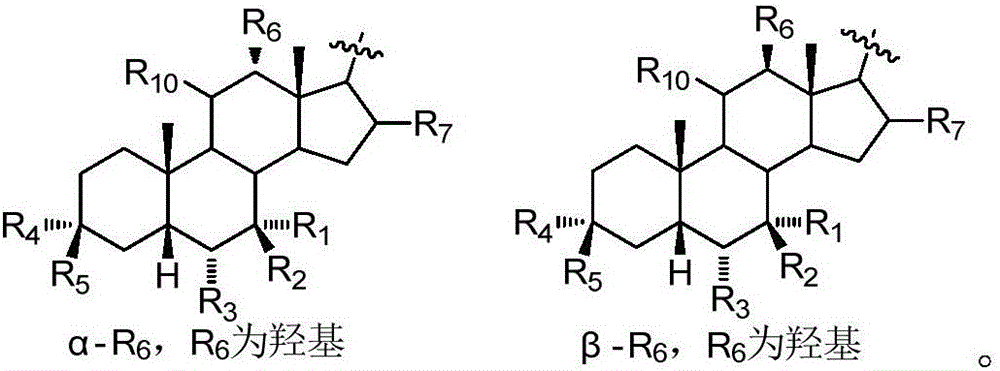
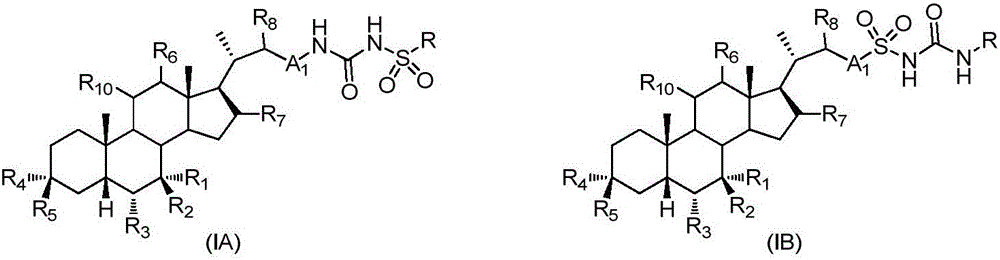
Sulfonylurea derivative and pharmaceutical composition and application thereof
A technology of derivatives and compounds, applied in the field of isotope derivatives, can solve problems such as listing and no drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Embodiment 1: the synthesis of compound 1.7
[0139]
[0140] Step 1: Synthesis of Compound 1.1
[0141] In a 5L four-neck round bottom flask, add chenodeoxycholic acid (190g, 484mmol), sodium bromide (2.5g, 24mmol), tetrabutylammonium bromide (0.5g, 1.55mmol), methanol (616mL), acetic acid (200 mL), water (49 mL), and ethyl acetate (1.33 L). The reaction solution was stirred at room temperature for 15 minutes until the solution was clear, and cooled to 0°C. Sodium hypochlorite (~5%, 756g, 508mmol) was slowly added dropwise to the reaction system, and the internal temperature was controlled at 1-2°C (exothermic). After the dropwise addition, stirring was continued for 30 minutes, and the temperature was slowly raised to 5°C. Stir at this temperature for 3 hours until the starting material disappears by TLC. The reaction was quenched by adding saturated sodium bisulfite (3.3%, 83 g, 26 mmol) until the starch potassium iodide was free of peroxide. Water (2 L) was a...
Embodiment 2
[0165] Embodiment 2: the synthesis of compound 2.5
[0166]
[0167] Step 1: Synthesis of Compound 2.1
[0168] Compound 1.7 (10g, 24mmol) and 4-dimethylaminopyridine (DMAP) (300mg, 2.4mmol) were dissolved in a mixed solution of acetic anhydride (50mL) and toluene (100mL), and the reaction system was reacted at 110°C for 2 Cool to room temperature one day later, remove the solvent by rotary evaporation under reduced pressure, dissolve the residue in ethyl acetate (200mL), wash the organic phase with cold water (3×50mL) and saturated brine (100mL), separate the organic phase and wash with anhydrous sodium sulfate After drying and concentration, the residue was purified by Flash column chromatography (ethyl acetate / petroleum ether=0-50%) to obtain compound 2.1 (10.4 g, yield: 86%) as a white solid.
[0169] m / z:[M-H] – 503
[0170] Step 2: Synthesis of compound 2.2
[0171] Under ice-bath conditions, dissolve compound 2.1 (1.0 g, 2.0 mmol) in dry tetrahydrofuran (30 mL), ...
Embodiment 3
[0182] Embodiment 3: the synthesis of compound 3.2
[0183]
[0184] Step 1: Synthesis of Compound 3.1
[0185] Under ice-bath conditions, compound 2.1 (3.65g, 7.24mmol) was added to trifluoroacetic acid (6.6mL) and trifluoroacetic anhydride (11.4g, 54.3mmol), stirred until dissolved, and sodium nitrite (1.5 g, 21.7mmol), the addition was completed, and the reaction system was stirred under ice bath for 1 hour, and then stirred at 40°C for 2 hours. The reaction system was neutralized with saturated aqueous sodium bicarbonate solution, then extracted with ethyl acetate (3×50 mL), the organic phases were combined, washed with saturated brine, the organic phase was separated and dried with anhydrous sodium sulfate, concentrated, and the residue After purification by Flash column chromatography (ethyl acetate / petroleum ether=0-20%), compound 3.1 (2.4 g, yield: 70%) was obtained as a pale yellow oil.
[0186] 1 H NMR (400MHz, CDCl 3 ):δ5.11(s,1H),4.54-4.62(m,1H),2.22-2.38(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com