Patents
Literature
115 results about "Trifluoroacetic anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride. Like many acid anhydrides, it may be used to introduce the corresponding trifluoroacetyl group. The corresponding acyl chloride, trifluoroacetyl chloride, is a gas, making it inconvenient to work with. Trifluoroacetic anhydride is the recommended desiccant for trifluoroacetic acid.
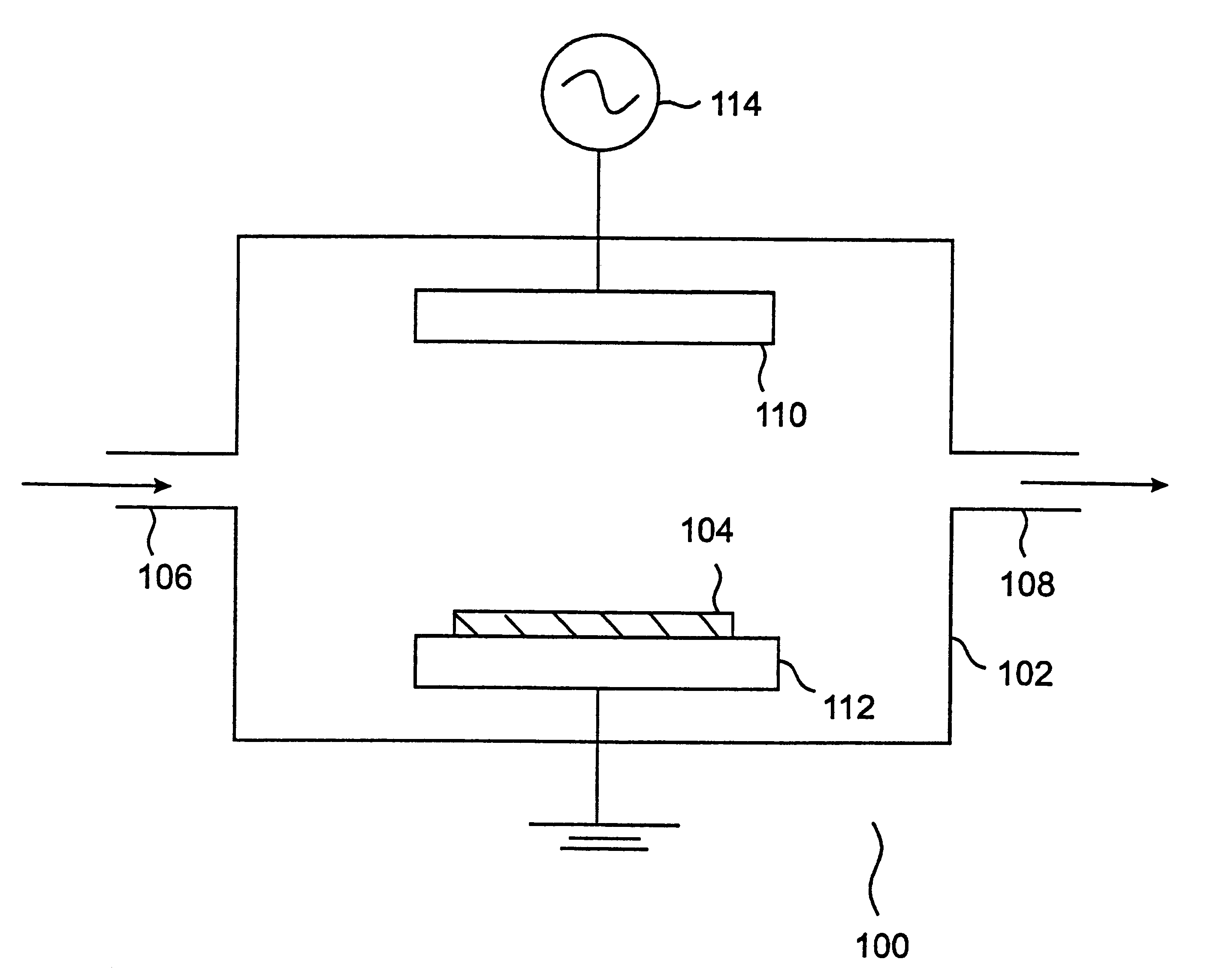
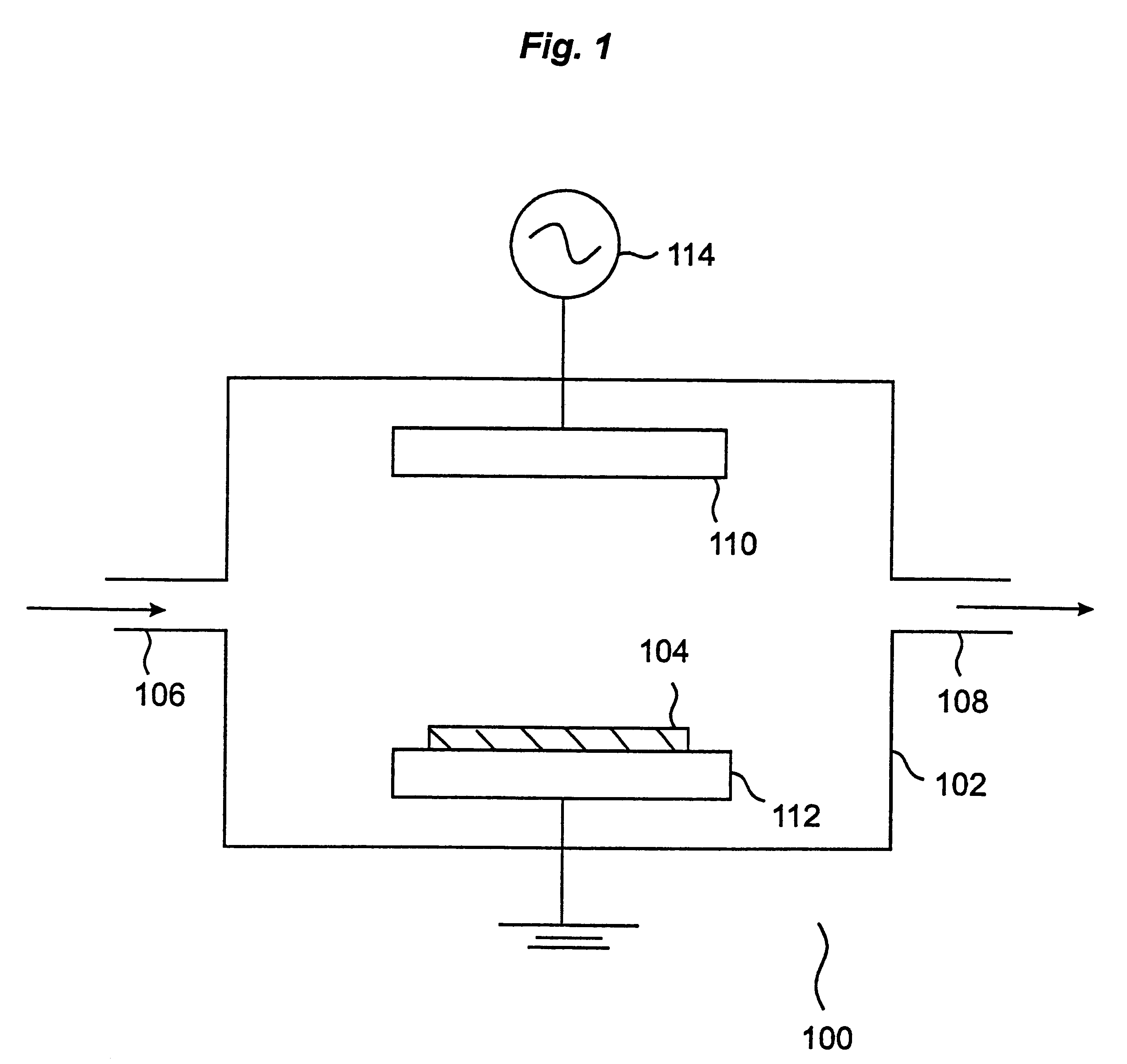
Plasma cleaning and etching methods using non-global-warming compounds
InactiveUS6242359B1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingTrifluoroacetic anhydrideChemical vapor deposition
Provided is a novel method of cleaning a chemical vapor deposition processing chamber having deposits on an inner surface thereof is provided. The process involves forming a plasma from one or more gases comprising a fluorine-containing but otherwise halogen-free non-global-warming compound, and contacting active species generated in the plasma with the inner surface of the chamber, with the proviso that the non-global-warming compound is not trifluoroacetic anhydride. Also provided is a method of etching a layer on a silicon wafer. The method involves the steps of: (a) introducing a silicon wafer into a processing chamber, the silicon wafer comprising a layer to be etched; and (b) forming a plasma from one or more gases comprising a fluorine-containing but otherwise halogen-free non-global-warming compound. Active species generated in the plasma are contacted with the silicon wafer, thereby etching the layer, with the proviso that the non-global-warming compound is not trifluoroacetic anhydride. The chemistries in accordance with the invention provide environmentally benign alternatives to the conventionally used global-warming chemistries for chamber cleaning and semiconductor etching processes.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
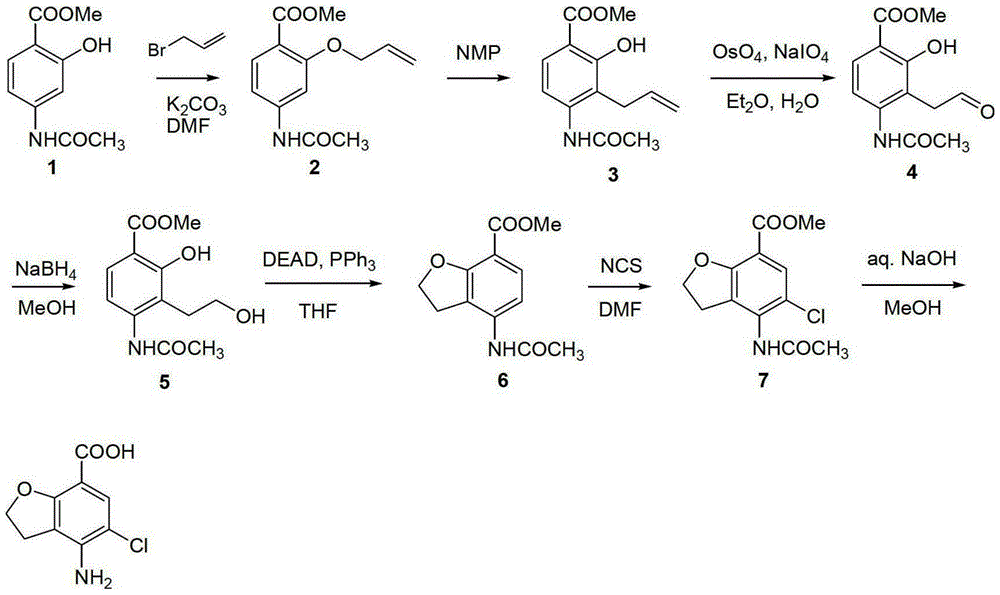
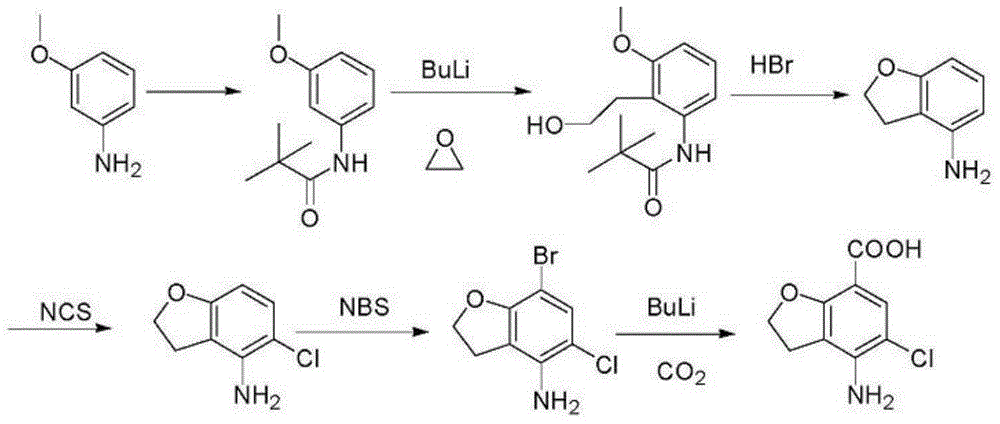
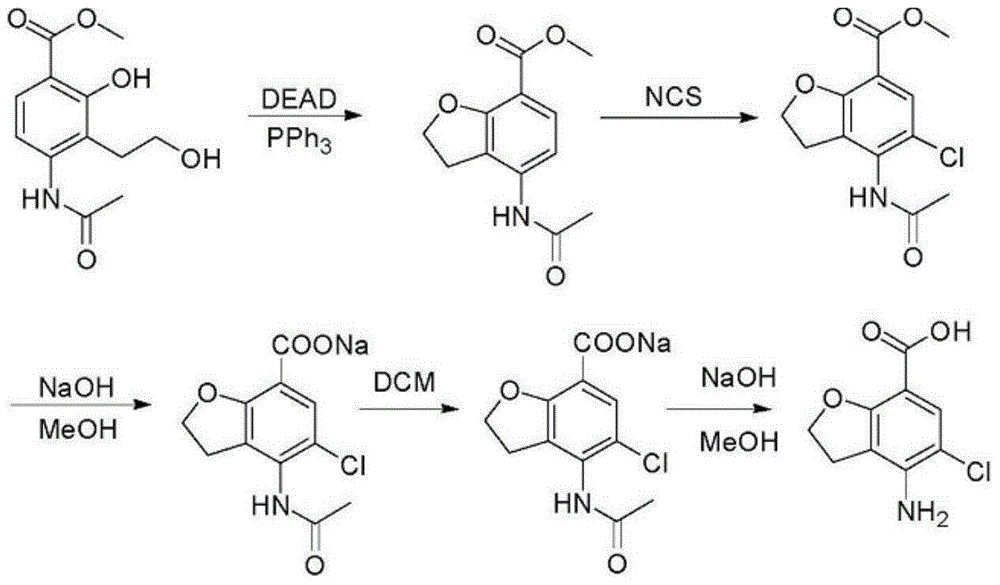
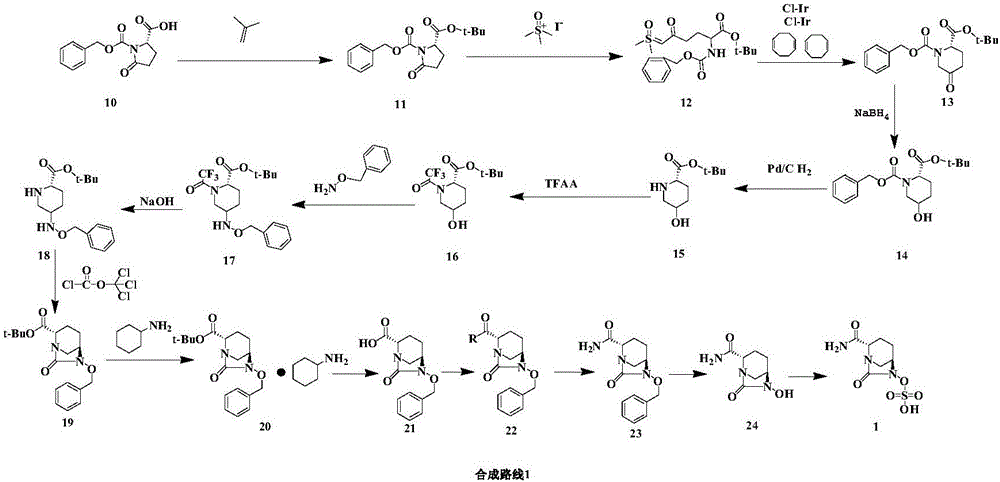
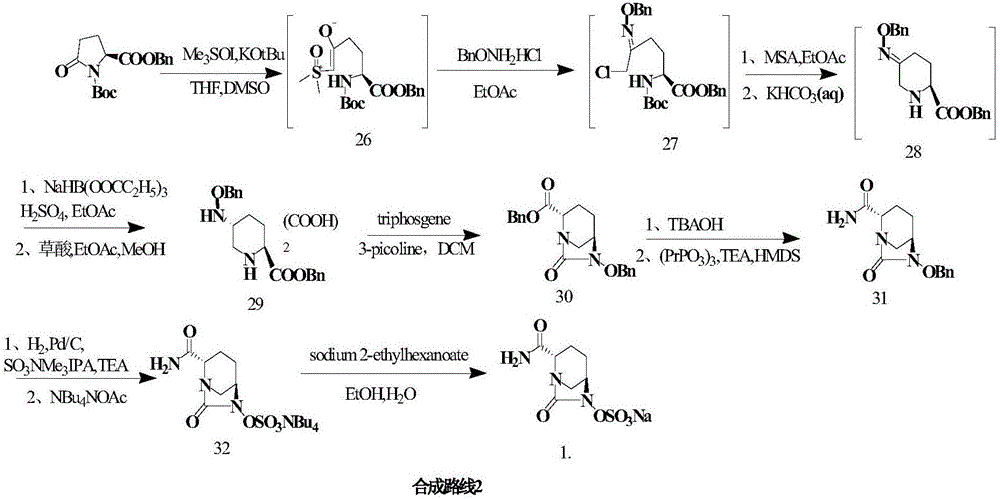
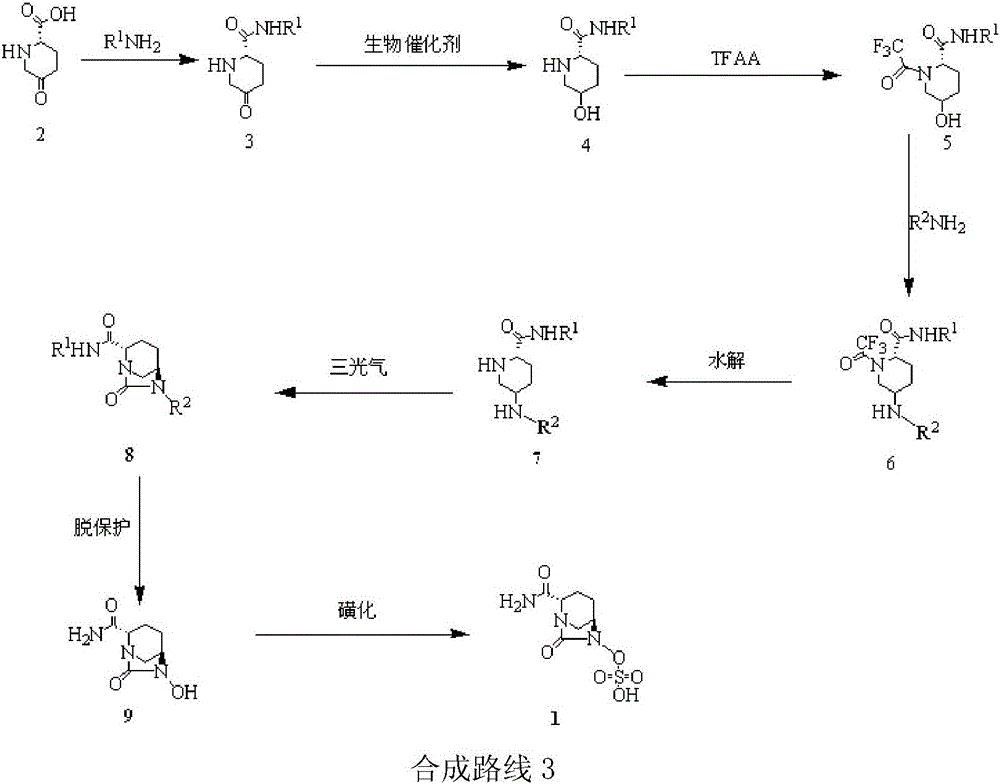
Method for synthesizing beta-lactamase inhibitor Avibactam
The invention relates to a method for synthesizing a beta-lactamase inhibitor Avibactam and belongs to the technical field of preparation of beta-lactamase inhibitors. The method disclosed by the invention comprises the following steps: (1) taking a compound 2 as a raw material, and reacting with R<1>NH2 so as to produce a compound 3; (2) enabling the compound 3 and a biocatalyst to produce a compound 4 in an organic solvent in the presence of glucose or sucrose; (3) enabling the compound 4 to react with trifluoroacetic anhydride so as to obtain a compound 5; (4) enabling the compound 5 to react with R<2>ONH2 so as to produce a compound 6; (5) hydrolyzing the compound 6 under alkaline conditions so as to produce a compound 7; (6) enabling the compound 7 to react with triphosgene so as to produce a compound 8; (7) enabling the compound 8 to react with ammonium formate in the presence of a catalyst so as to produce a compound 9 in the organic solvent, wherein the catalyst is a Pd / C system; and (8) enabling the compound 9 to react with a sulfonating agent, thereby obtaining the product 1. The method disclosed by the invention is stable in process, high in yield, simple and safe in operation and low in production cost.
Owner:YIYUAN XINQUAN CHEM
Synthesizing method of vinorelbine tartrate
InactiveCN101037446ASave raw materialsReduce manufacturing costOrganic chemistrySynthesis methodsTrifluoroacetic acid
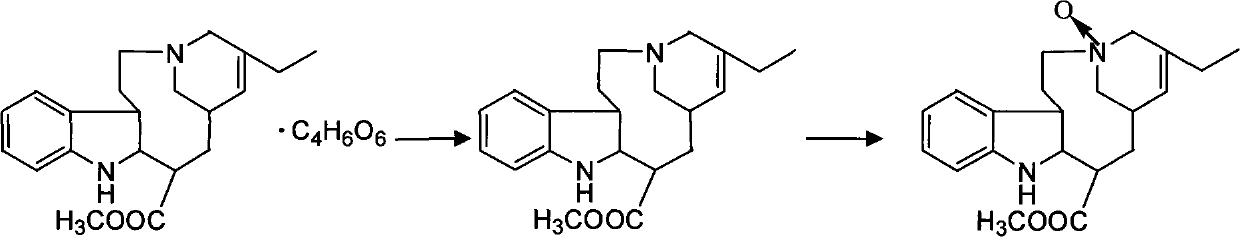
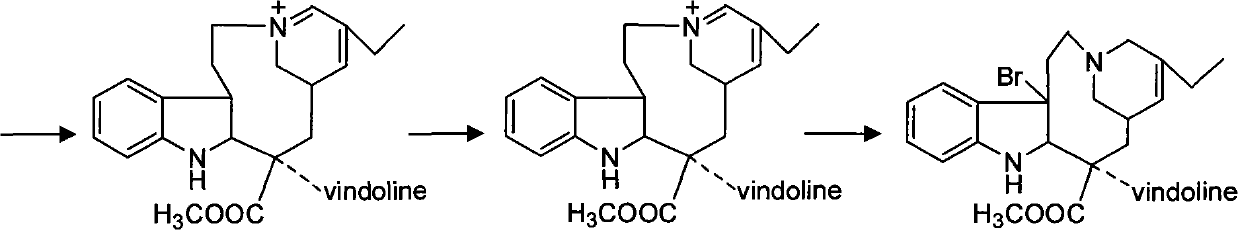
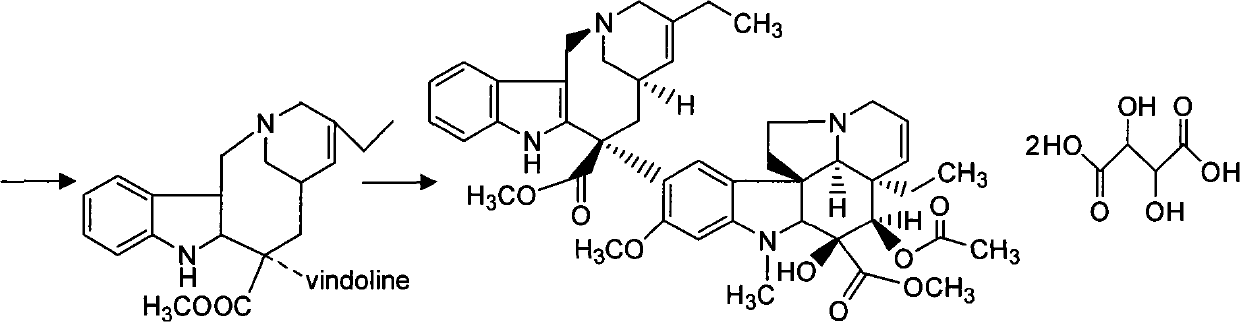
The invention discloses a synthesis method of vinorelbine bitartrate which is characterized in that the method includes following steps: (1) having the catharanthine tartrate and vindoline as initial material, reacting with ferric trichloride and hydrochloric acid; (2) reacting the above product with the sldium borohydride to get dehydrated vinblastine; (3) ring-opening the dehydrated vinblastine by trifluoroacetic anhydride and rearranging to obtain the vinorelbine bitartrate. The method has a low production cost and a high yield and has a good application future.
Owner:刘全胜
Preparation method for vinorelbine tartrate
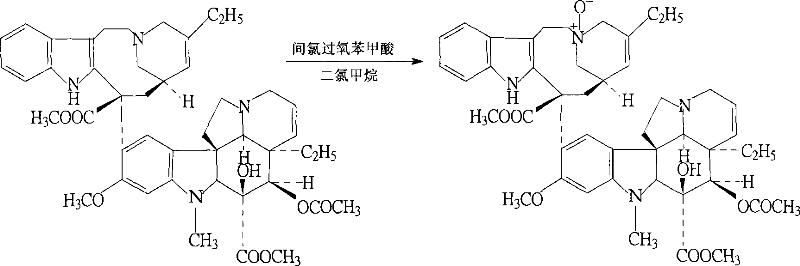
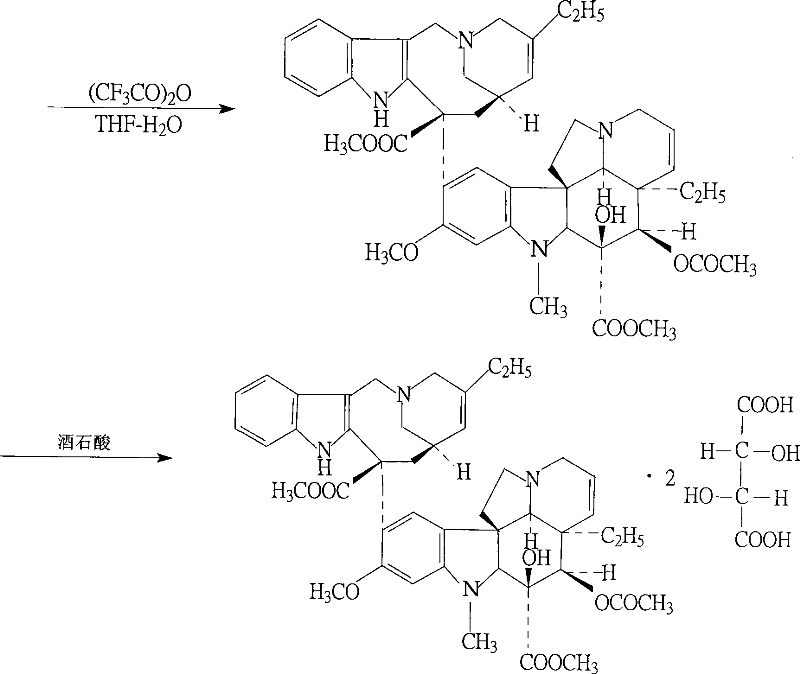
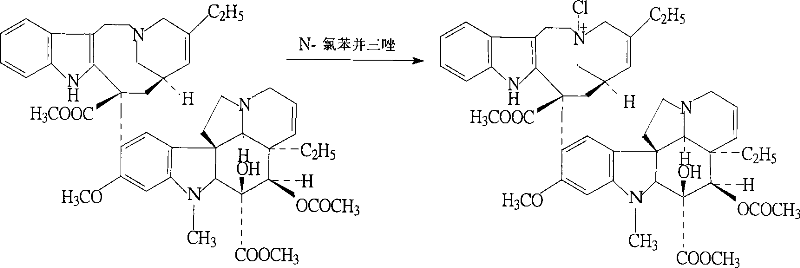
ActiveCN102199165AReduce manufacturing costLow impurity contentCarboxylic acid salt preparationTrifluoroacetic anhydrideSodium borohydride
The invention discloses a preparation method for vinorelbine tartrate; the preparation method comprises the following steps of: taking tartrate vinblastine as a starting material; using ammonia water for dissociating the starting material, reacting the dissociated starting material with the metachloroperbenzoic acid so as to obtain vinblastine-N oxide, then reacting the vinblastine-N oxide with vindoline and trifluoroacetic anhydride, carrying out the reduction through sodium borohydride so as to obtain dehydrated vinorelbine, treating the dehydrated vinorelbine so as to obtain vinorelbine, and finally salifying the vinorelbine with tartaric acid so as to obtain the vinorelbine tartrate. The preparation method has the advantages of low cost, few side reaction, easily controlled operation process, higher yield and product purity and wide industrialized application prospect.
Owner:HUBEI HONCH PHARMA
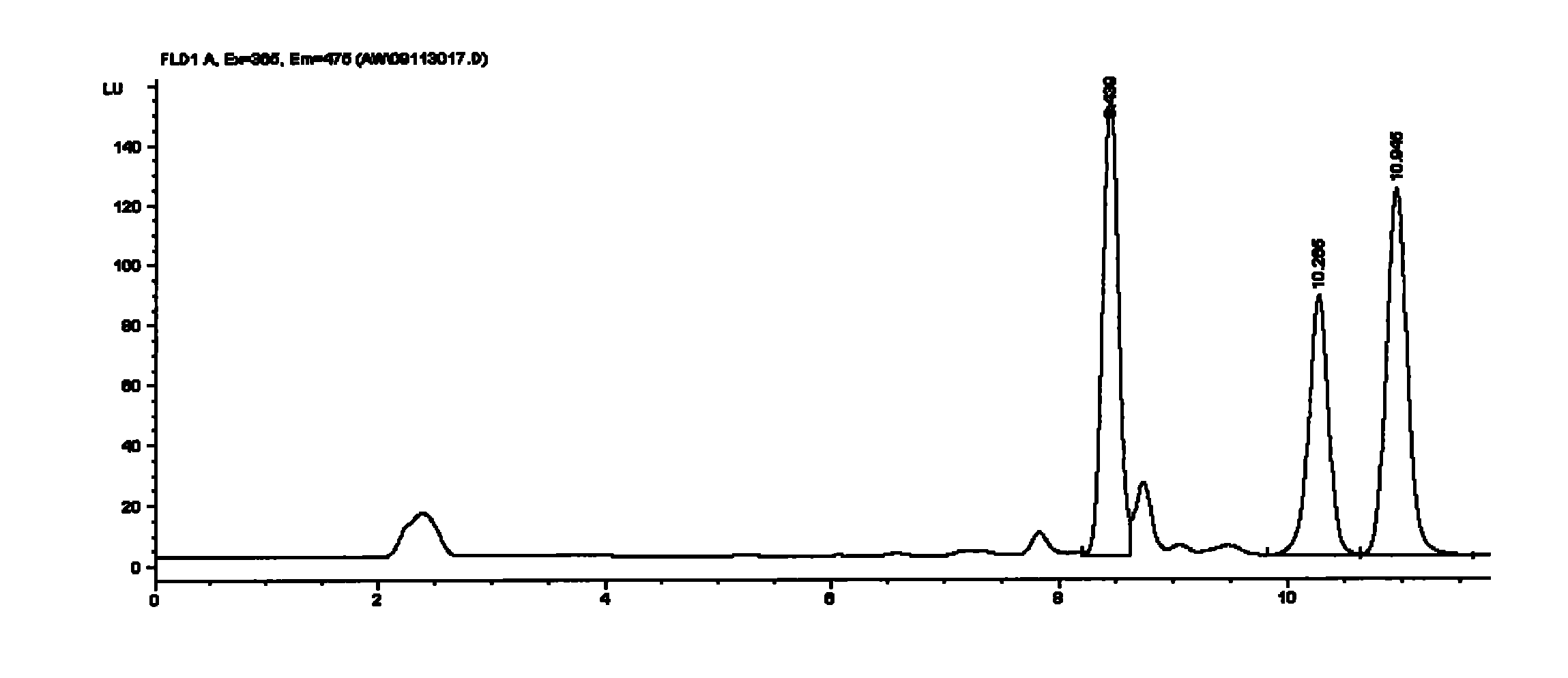
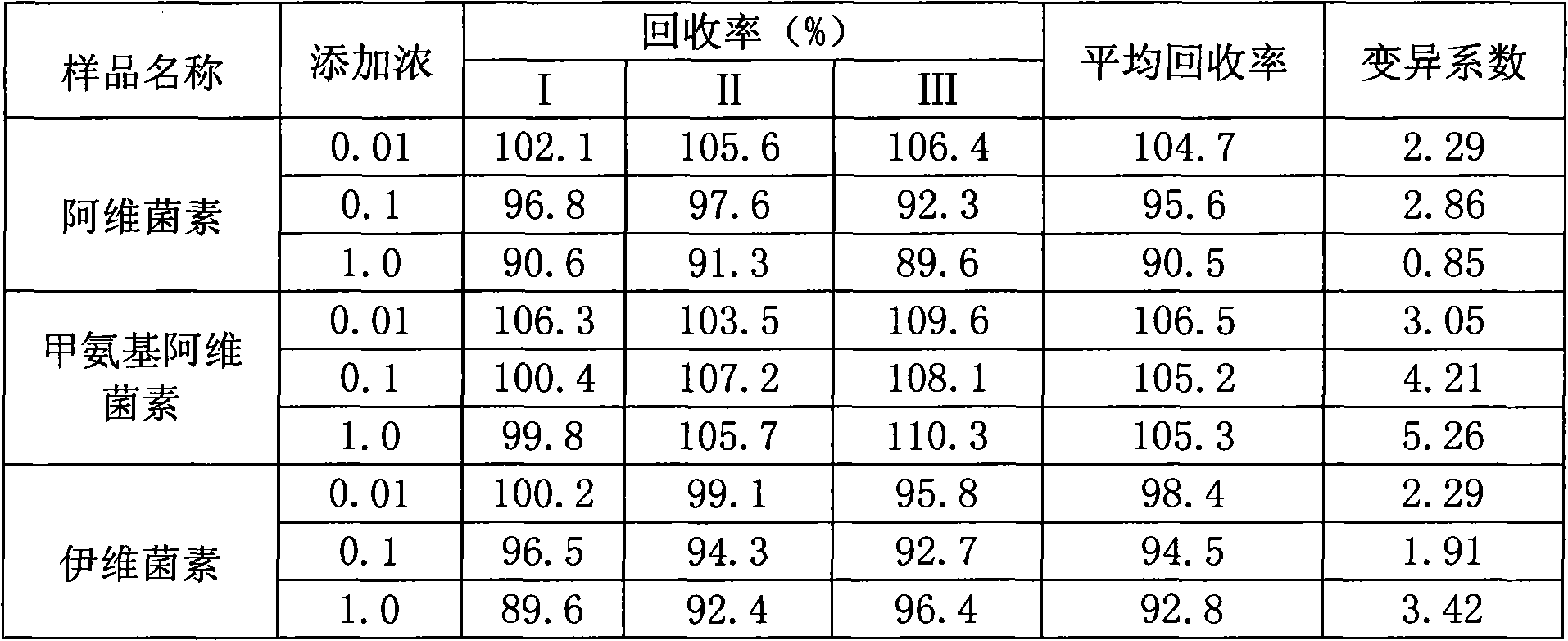
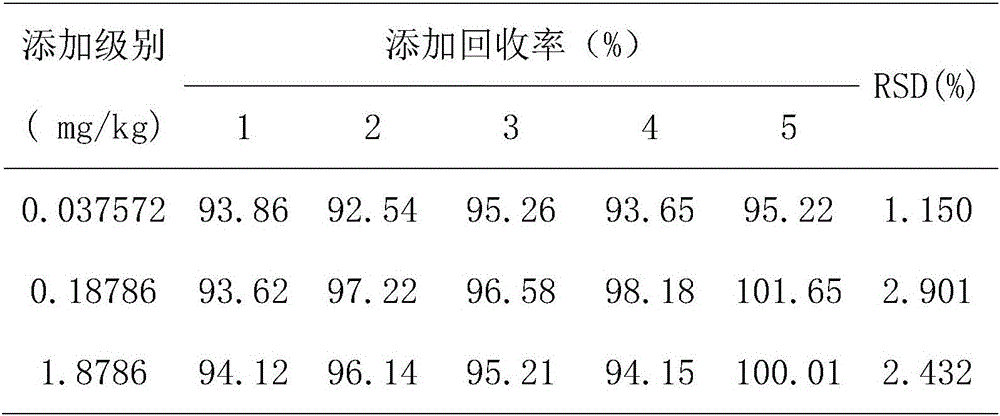
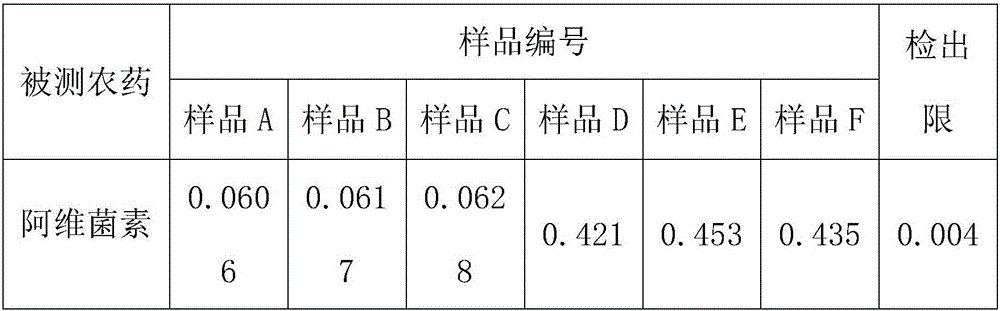
Method for detecting avermectins pesticide multi-residues in cereal agricultural products
InactiveCN101893570AEfficient extractionSimple and time-saving handlingComponent separationFluorescence/phosphorescenceAcetic anhydrideFluorescence
The invention discloses a method for detecting avermectins pesticide multi-residues in cereal agricultural products, which comprises the following steps of: extracting the avermectins pesticide residues from the cereal agricultural products with acetonitrile, separating an organic phase containing avermectins pesticides from a water phase under the action of salting-out, performing fluorescence derivatization on the extracted avermectins pesticides by a trifluoro acetic anhydride (TFAA)-N-methylimidazole (NMIM)-acetonitrile (ACN) method in an anhydrous state, and realizing the trace residue analysis of the pesticides by adopting a high performance liquid chromatography-fluorescence detection method. The method of the invention has the advantages of performing trace analysis and detection on the residues of avermectin, emamectin benzoate and ivermectin in a plurality of cereal agricultural products, such as rice, wheat, corn and the like, along with simplicity, convenience, time saving, low cost and the like.
Owner:上海市农业技术推广服务中心
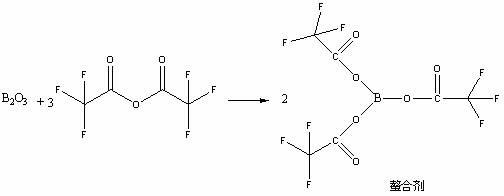
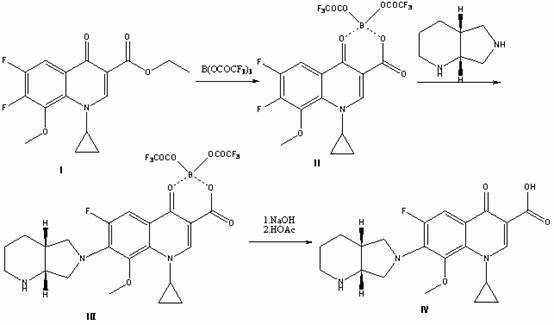
High selectivity method for synthesizing moxifloxacin
ActiveCN102351858AAvoid it happening againSimple processing methodOrganic chemistryAcetic anhydrideIce water
The invention discloses a high selectivity method for synthesizing moxifloxacin. The method comprises the following steps of: reacting boric anhydride with trifluoro acetic anhydride to obtain a chelant; reacting 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester with the chelant, cooling to room temperature, adding ice water, performing suction filtration, and washing a filter cake with water until neutrality to obtain a 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate; and reacting the 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate with (S,S)-2,8-diazabicyclo[4,3,0]nonane to obtain a 1-cyclopropyl-6-fluoro-7-([S,S]-2,8-diazabicyclo[4,3,0]nonane-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid ethyl ester trifluoroacetic anhydride boronized chelate, recycling a solvent under reduced pressure, adding alkali, refluxing, discoloring, filtering, freezing, performing suction filtration, and drying a filter cake. The method is simple, mild in conditions, and high in selectivity, avoids difficultly separated impurities, is high in reaction yield and product purity, and is suitable for industrial production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Sulfonic acid analog anion surfactants rapid checking method
InactiveCN101308121APromote innovationShort reaction timeComponent separationPreparing sample for investigationGas phaseTrifluoroacetic anhydride
The invention discloses a fast detection method for detecting anionic surface active agent of sulfuric acids, which comprises: a potassium iodide is mixed with a sulfuric acid negative ion surface active agent by a molar ratio of 1:1-10:1, then N and N-dimethyl formamide having the amount of 5 to 20ml / mol of potassium iodide are added into the mixture, the mixture is agitated to reach complete dissolution, then trifluoroacetic anhydride with the molar ratio one to ten times the amount of potassium iodide is added into the solution, the solution turns into red and is taken for implementing the conventional gas phase chromatography-mass spectrum detection and then data processing analysis when the color stops darkening, thereby obtaining the detecting result. The detection method has advantages of fast and simple reaction process and easy control of conditions, and makes up defects that the traditional detection method has false positive easily and can not ensure detection results and has high requirements on sample pre-processing, and the method is particularly suitable for qualitative and quantitative analysis of all components in complex compounds such as anionic surface active agent.
Owner:袁慧娟
[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof
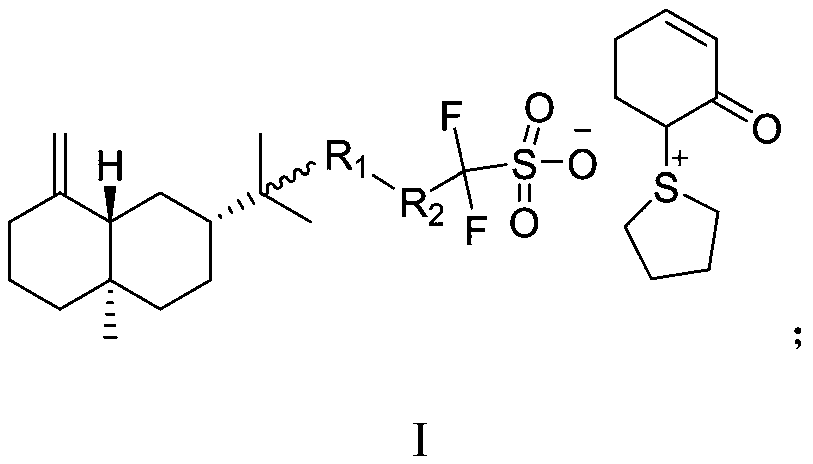
The invention relates to [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances and provides a structure, a preparation method and application of a novel artemisinine 10-locus derivative. The structural formula of the [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substance is shown in a formula I. The invention also relates to pharmaceutically-acceptable slats, a solvate, an optical isomer or a polymorphic substance of the [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances and a medicinal composition by taking the compounds as active components. As novel antimalarial agents, anti-tumor agents and antifungal agents, the compounds can be used for treating or preventing malaria, mycotic infection, malignant tumor and the like. The compounds can be prepared by reacting dihydroartemisinine as an initial raw material with trifluoroacetic anhydride / triethylamine to obtain 10(R)-trifluoro-acetoxyl-9,10-dihydroartemisinine, directly reacting the 10(R)-trifluoro-acetoxyl-9,10-dihydroartemisinine with hydroxy benzaldehyde without separation to obtain (10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde, and reacting the (10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde with the substituted amino (sulfur) urea compounds in acid catalysts and alcohol solvents to obtain target compounds.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of thioether compound and product of preparation method
InactiveCN106748927AEliminate problems such as metal residueImprove biological activitySteroidsSulfide preparationTrifluoromethanesulfonic anhydrideAcetonitrile
The invention discloses a preparation method of a thioether compound and a product of the sulfoether compound. The method comprises the following steps: adding a sulfoxide compound into acetonitrile or dichloromethane under the protection of gas which does not react with a substrate, cooling the mixture, then adding trifluoromethanesulfonic anhydride or trifluoroacetic anhydride, adding an arylphenol compound, keeping the temperature and stirring for 2 to 4 hours, adding potassium phosphate, heating to 65 to 75 DEG C, stirring for 20 to 28 hours, and carrying out extraction and concentration chromatography to obtain a target product. The method for preparing the sulfoether compound has the characteristics of simplicity, efficiency, mildness and economy.
Owner:NANJING FORESTRY UNIV
Preparation method of isomer impurities of vildagliptin
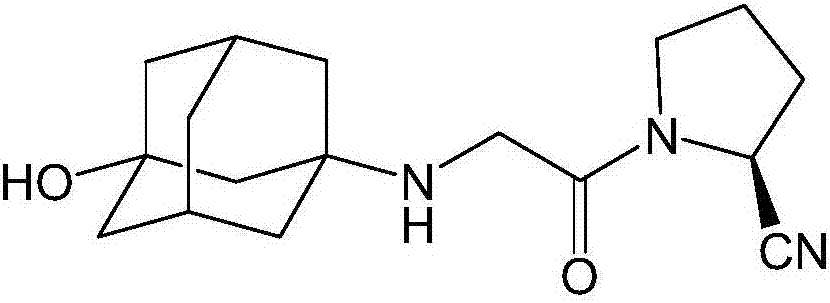
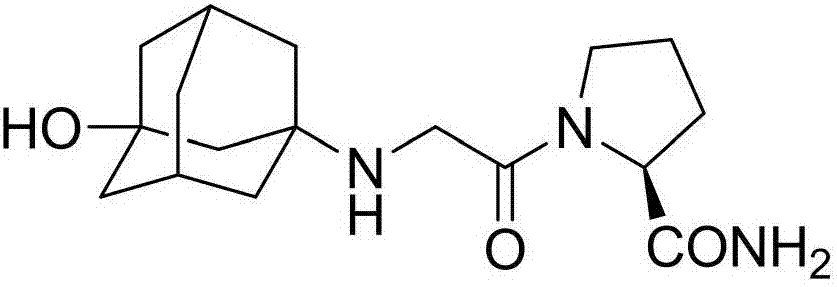
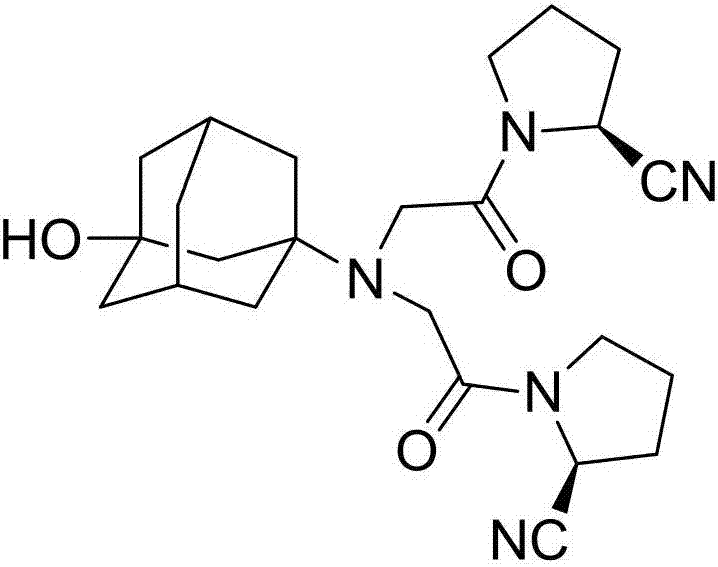
InactiveCN107311907AQuality improvementHigh purityOrganic chemistryChemical industryTrifluoroacetic anhydride
The invention discloses a preparation method of isomer impurities of vildagliptin, and relates to the technical fields of medicine and chemical industry. The preparation method comprises the steps: S1: enabling D-prolinamide (I) serving as a raw material to react with chloroacetyl chloride to obtain a reaction product (II), and then enabling the reaction product (II) to react with trifluoroacetic anhydride to obtain a reaction product (III); S2: enabling the reaction product (III) to react with 3-amino-1-adamantanol to generate a target product (IV). The preparation method disclosed by the invention has the advantages that the preparation process is simple, the operation is simple and convenient, reaction time is short, and the post-treatment purification is simple and effective, which is beneficial to industrialized production. The purity of manufactured impurities of the vildagliptin is high and is as high as 99.1% through HPLC (high-performance liquid chromatography) detection. By further studying the isomer impurities of the vildagliptin, the quality of the vildagliptin can be better controlled, and the drug safety is improved.
Owner:合肥创新医药技术有限公司
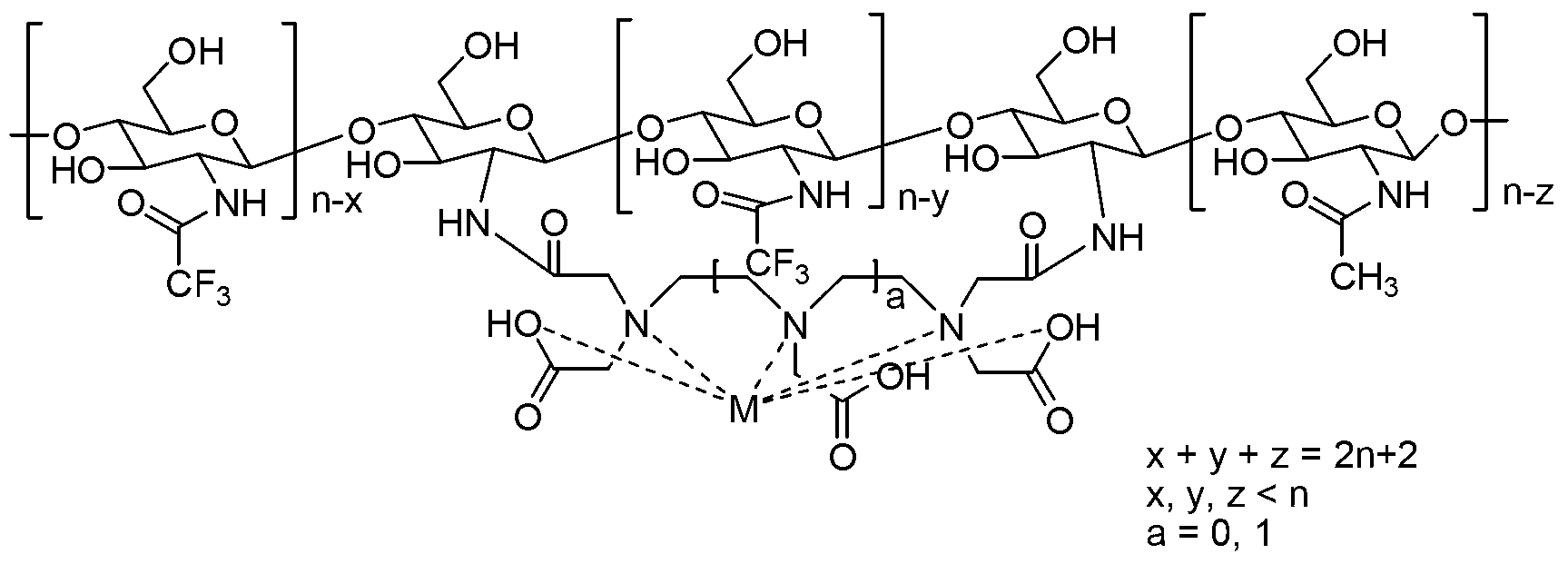
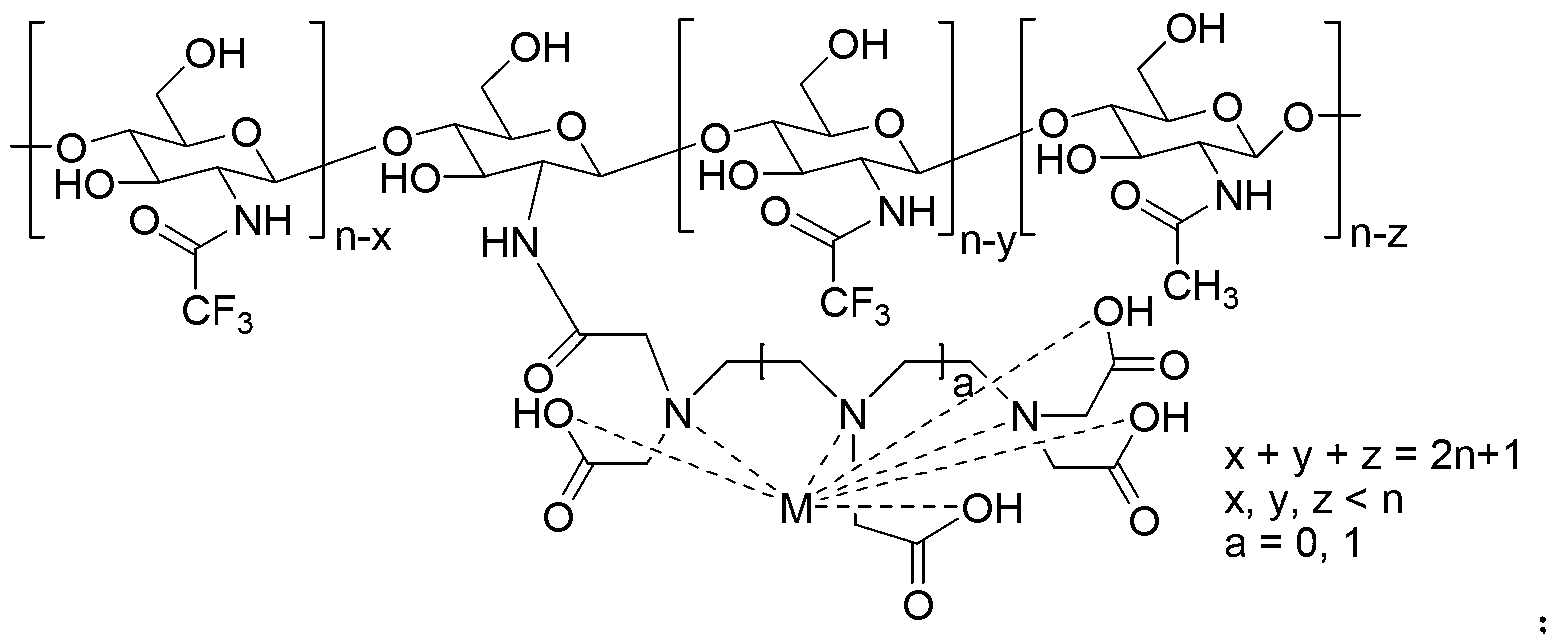
Fluorine-containing potential dual-function probe based on chitosan structure and preparation method thereof
InactiveCN103316362AGood water solubilityWith F-probe functionRadioactive preparation carriersSolubilityOrganic base
The invention discloses a fluorine-containing potential dual-function probe based on a chitosan structure and a preparation method thereof and relates to the fields of magnetic resonance imaging and fluorine chemistry. The preparation method comprises the following steps of: adding oligomeric or polymeric chitosan chained with a diethylenetriamine pentaacetic acid (DTPA) or ethylenediaminetetraacetic acid (EDTA) structure and an organic base into a dry organic solvent, slowly adding trifluoroacetic anhydride, performing complete reaction at room temperature, then adding distilled water and a metal inorganic salt into a system, continuously reacting at the room temperature till the reaction is complete, performing vacuum concentration and dehydration, adding acetone or anhydrous ethanol, standing, and then filtering to get a target product, namely a fluorine-containing magnetic resonance contrast medium based on the chitosan structure. The fluorine-containing magnetic resonance contrast medium based on the chitosan structure, disclosed by the invention, has good water solubility, the relaxivity is 10-14L.mmol<-1>.s<-1>, which is 1-3 times of that of an existing common MRI (magnetic resonance imaging) contrast medium, namely magnevist (Gd-DTPA (gadopentetate dimeglumine)); and the fluorine-containing magnetic resonance contrast medium simultaneously has a potential 19F probe function due to the containing of a plurality of trifluoromethyl groups.
Owner:JIANGSU UNIV
High performance liquid chromatography detection method for abamectin content in edible vegetable oil
InactiveCN106226444AEfficient extractionLow costComponent separationLiquid Chromatography-FluorescencePretreatment method
The invention discloses a high performance liquid chromatography detection method for the abamectin content in edible vegetable oil. The method comprises the steps that a sample is subjected to vortex oscillation extraction through methanol, purified with an ODS C18 solid-phase extraction column, subjected to derivatization through N-methylimidazole and trifluoroacetic anhydride and lastly determined through a liquid chromatography-fluorescence method. According to the method, few reagents are adopted, and the cost is reduced; a sample pretreatment method is simple, the purification effect is good, maintenance of instruments in the using process is reduced, and the technical problems that when a liquid chromatography-ultraviolet detector is used, the sensitivity is too low, and matrix interference is serious are solved; a determined result is accurate and good in repeatability, the detection specificity and sensitivity are high, and a reference is provided for detection of the abamectin content in the edible vegetable oil.
Owner:FOSHAN HAIYUE ZHIDA TECH CO LTD
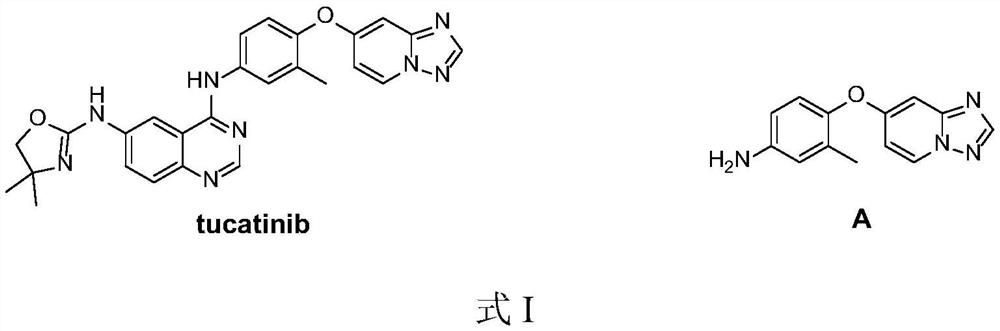
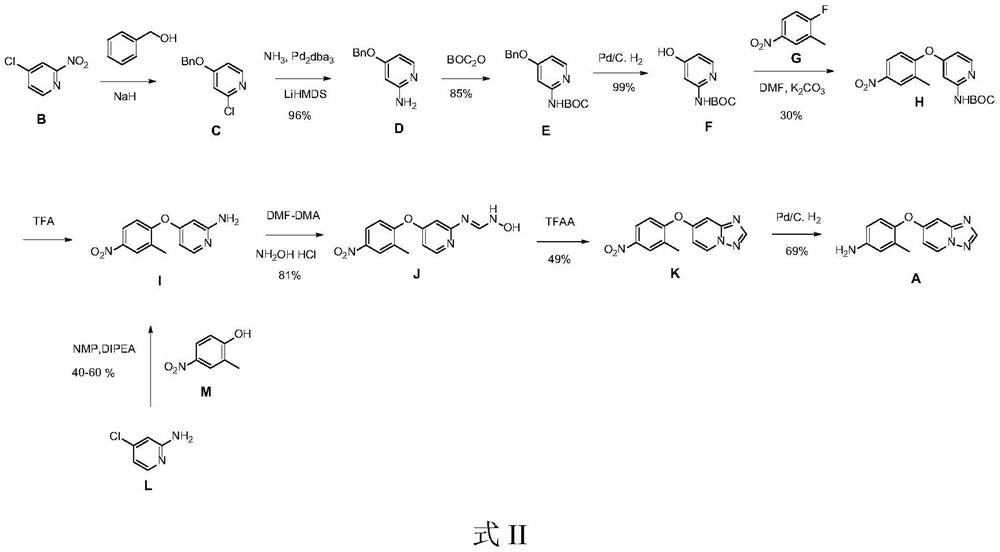
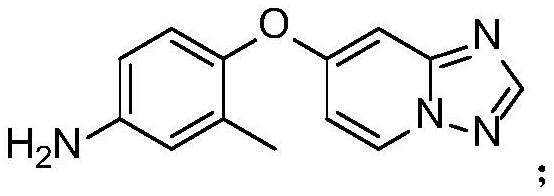
Preparation method of Tucatinib intermediate
PendingCN112898298ARaw materials are easy to getReduce manufacturing costOrganic chemistryMethylanilineHydroxylamine
The invention discloses a preparation method of a tucatinib intermediate, which comprises the following steps: firstly, reacting 4-chloropyrido-2-amine with N,N-dimethylformamide dimethyl acetal, and then adding hydroxylamine hydrochloride for reaction to obtain a product I; reacting the product I with trifluoroacetic anhydride to obtain a product II; finally, carrying out heating reaction on the product II and 4-amino-2-methylphenol in a DMF / potassium carbonate system to prepare the tucatinib intermediate 4-([1,2,4]triazolo[1,5-a]pyridine-7-yloxy)-3-methylaniline. According to the preparation method of the tucatinib intermediate, the raw materials are easy to obtain, and the production cost can be remarkably reduced; the whole process is simple, the whole time consumption is short, the production efficiency is high, the yield is high, the reaction condition is mild, the post-treatment is simple and convenient, and the method is suitable for large-scale preparation and has a great application prospect.
Owner:SHANGHAI FAMO BIOTECH CO LTD
Hexafluoro dianhydride preparation method
ActiveCN104529965AHigh molecular weightImprove mechanical propertiesOrganic chemistryAcetic anhydrideFlexible circuits
The invention discloses a hexafluoro dianhydride preparation method comprising the following steps: (1) hexafluoro tetraacid is added into anhydrous acetic anhydride; the mixture is stirred and heated to reflux, such that a first ring-closure dehydration reaction is carried out; when the reaction is finished, a treatment is carried out, such that a hexafluoro dianhydride crude product with a ring-closure rate no higher than 98.0% is obtained; (2) the hexafluoro dianhydride crude product obtained in the step (1) is added into anhydrous trifluoroacetic anhydride; the mixture is stirred and heated to reflux, such that a second ring-closure dehydration reaction is carried out; when the reaction is finished, a treatment is carried out, such that a hexafluoro dianhydride finished product with a ring-closure rate no lower than 99.5% is obtained. Polyimide synthesized with the high-ring-closure-rate hexafluoro dianhydride prepared with the method provided by the invention has relatively high molecular weight, good mechanical performance, and good thermal properties. The polyimide can be widely applied in high-tech fields such as optical communication, colorless transparent flexible circuit board, solar cell substrate, organic flexible transparent conductive film substrate, and the like.
Owner:大连新阳光材料科技有限公司
Sulfonium sulfonate salt photoacid generator synthesized from patchouli alcohol and synthesis method for sulfonium sulfonate salt photoacid generator
PendingCN111138405AReduce spreadHigh molecular weightOrganic chemistry methodsSulfonic acids salts preparationChemical synthesisSilanes
The invention discloses a sulfonium sulfonate salt photoacid generator synthesized from patchouli alcohol and a synthesis method for the sulfonium sulfonate salt photoacid generator, belonging to thefields of chemical synthesis and photoetching materials. The photoacid generator has a structural general formula which is described in the specification. In the structural general formula, R1 is oneselected from the group consisting of groups which are described in the specification; and R2 is one selected from the group consisting of an alkyl group, a cycloalkyl group, a heteroalkyl group, a heterocycloalkyl group, an ester group-containing alkyl group and a fluorine-containing alkyl group. The synthesis method for the photoacid generator comprises the following steps: allowing the patchouli alcohol to react with a sulfonate compound so as to obtain an intermediate; allowing the intermediate to react with (cyclohexyl-1,5-dienyloxy)-trimethyl-silane, tetramethylene sulfoxide and trifluoroacetic anhydride so as to obtain the sulfonium sulfonate salt photoacid generator. According to the invention, a raw material, namely the patchouli alcohol adopted in the method provided by the invention has large molecular weight, so the photoacid generator formed by the patchouli alcohol also has large molecular weight; diffusion of the photoacid generator can be reduced; and improvement of theedge roughness, reduction of the line width roughness and improvement of the resolution ratio are facilitated.
Owner:上海博栋化学科技有限公司
Synthetic method of 3,5-2-fluoro-(trifluoromethyl)benzophenone
InactiveCN102964233ALow costSimple process conditionsCarbonyl compound preparation by condensationIce waterGrignard reagent
The invention discloses a synthetic method of a compound, which belongs to the technical field of organic synthesis. A synthetic method of 3,5-2-fluoro-(trifluoromethyl)benzophenone comprises the following steps: putting solvents and iodine into a four-port reaction kettle, and under the protection of nitrogen, putting magnesium chips and a small amount of bromoethane into the reaction kettle to carry out initiation reaction, wherein the reaction time is controlled in 15min; dropwise adding 3,5-difluorobromobenzene into the reaction kettle at 30-40 DEG C; after the adding step is completed, stirring the obtained mixture for 2h at 35 DEG C so as to obtain a Grignard reagent; slowly dropwise adding the obtained Grignard reagent into a trifluoroacetic anhydride solvent solution at 20-30 DEG C; and after the adding step is completed, carrying out thermal reaction for 1-1.5h at 25 DEG C; slowly adding the obtained reaction liquid into ice water, separating out an organic layer, extracting a water layer by using ethyl acetate, combining with the organic layer, carrying out water washing, drying and solvent recovery on the organic layer so as to obtain a coarse product, and carrying out rectification on the coarse product so as to obtain a pure product. The synthetic method disclosed by the invention is low in raw material cost, simple in process condition, easy to operate, moderate to the environment, and high in product yield.
Owner:大连九港生物科技有限公司
Sulfonium sulfonate salt photoacid generator synthesized from guaiacol and synthesis method for sulfonium sulfonate salt photoacid generator
PendingCN111138406AReduce spreadHigh molecular weightSulfonic acids salts preparationPhotosensitive materials for photomechanical apparatusChemical synthesisSilanes
The invention discloses a sulfonium sulfonate salt photoacid generator synthesized from guaiacol and a synthesis method for the sulfonium sulfonate salt photoacid generator, belonging to the fields ofchemical synthesis and photoetching materials. The photoacid generator has a structural general formula which is described in the specification. In the structural general formula, R1 is one selectedfrom the group consisting of groups which are described in the specification; and R2 is one selected from the group consisting of a covalent bond, an alkyl group, a cycloalkyl group, an ester group containing alkyl group and a fluorine-containing alkyl group. The synthesis method for the photoacid generator comprises the following steps: allowing the guaiacol to react with a sulfonate compound soas to obtain an intermediate; and allowing the intermediate to react with (cyclohexyl-1,5-dienyloxy)-trimethyl-silane, tetramethylene sulfoxide and trifluoroacetic anhydride so as to obtain the sulfonium sulfonate salt photoacid generator is obtained. According to the invention, a raw material, namely the guaiacol adopted in the method provided by the invention has large molecular weight, so the photoacid generator formed by the guaiacol also has large molecular weight; diffusion of the photoacid generator can be reduced; and improvement of the edge roughness, reduction of the line width roughness and improvement of the resolution ratio are facilitated.
Owner:上海博栋化学科技有限公司
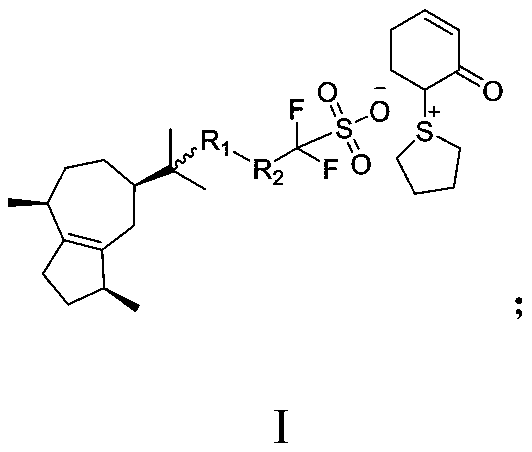
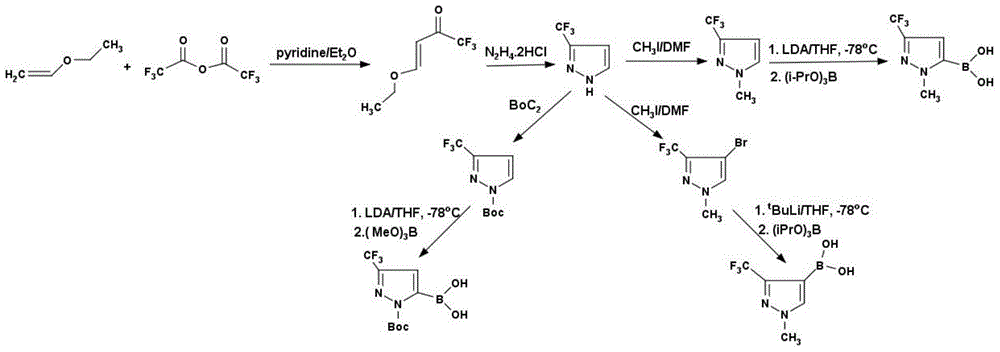
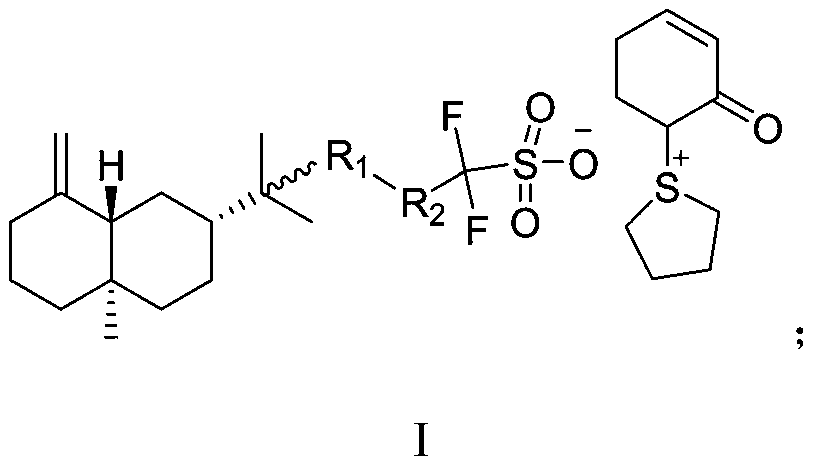
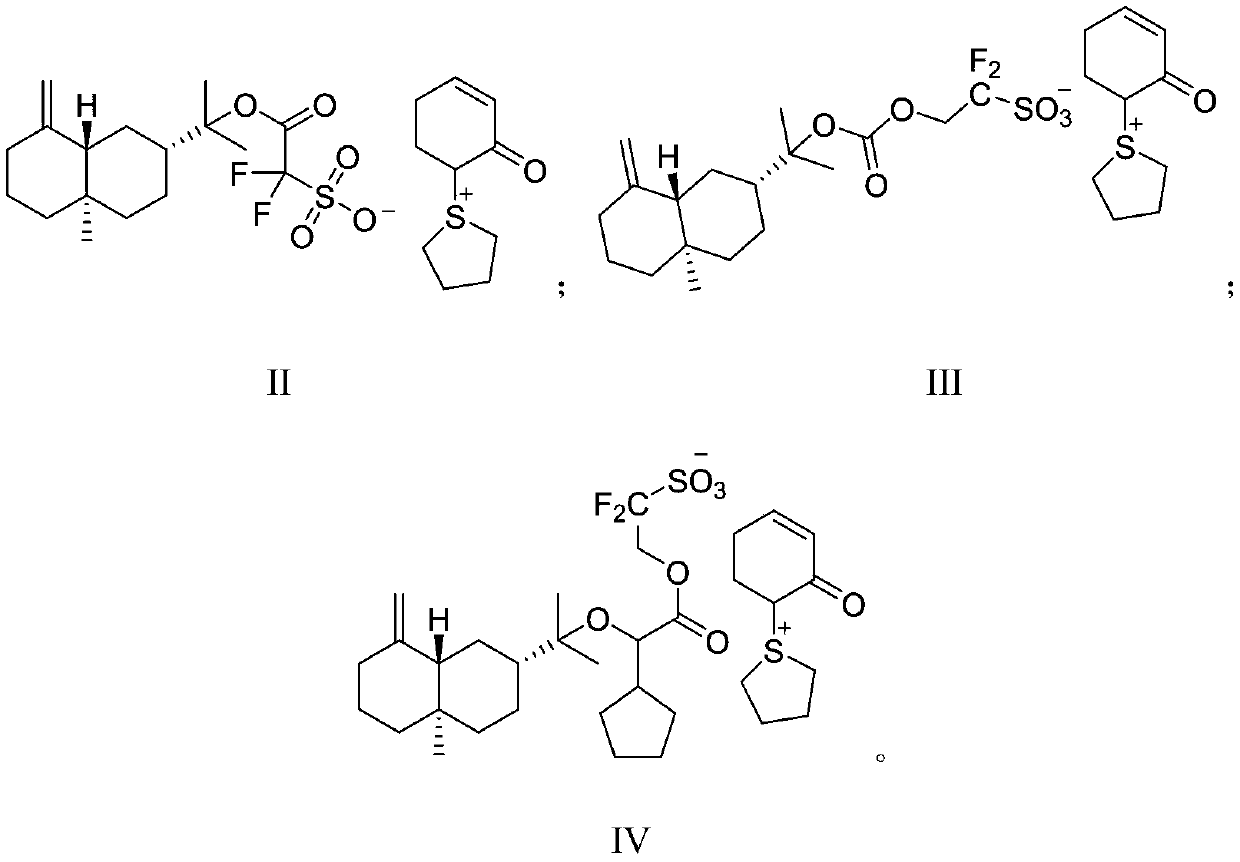
Method for preparing 3-trifluoromethyl pyrrole boric acid
InactiveCN104478911AHigh yieldHigh purityGroup 3/13 element organic compoundsChemical synthesisKetone
The invention discloses a method for preparing 3-trifluoromethyl pyrrole boric acid, belonging to the technical field of organic chemical synthesis. The method is characterized by comprising the following steps: (I) with trifluoroacetic anhydride as a start raw material, generating 4-ethoxy-3-alkenyl trifluoroacetyl ketone through reaction between the trifluoroacetic anhydride and ethyl vinyl ether; (II) performing heating reflux of 4-ethoxy-3-alkenyl trifluoroacetyl ketone and hydrazine dihydrochloride in an ethanol solution to obtain 3-trifluoromethyl pyrrole; and (III) performing functional group conversion of 3-trifluoromethyl pyrrole to generate 1-methyl-3-trifluoromethyl-5-pyrrole boric acid, N-tert-butyl acyl-3-trifluoromethyl-5-pyrrole boric acid and 1-methyl-3-trifluoromethyl-4-pyrrole boric acid respectively. The method has the beneficial effects that the generated target compound has good purity and stable properties, the method is simple and easy to operate, and the preparation scale is easy to enlarge to realize industrial production.
Owner:成都安斯利生物医药有限公司
Sulfonium sulfonate salt photoacid generator synthesized from beta-cineole, and synthesis method thereof
PendingCN111116546AHigh molecular weightReduce spreadSulfonic acids salts preparationPhotosensitive materials for photomechanical apparatusChemical synthesisSilanes
The invention discloses a sulfonium sulfonate salt photoacid generator synthesized from beta-cineole, and a synthesis method thereof, and belongs to the field of chemical synthesis and photoetching materials. The structural general formula of the photoacid generator is shown in the description; and in the formula, R1 is one of three formulas also shown in the description, and R2 is one of an alkylgroup, a naphthenic base, an ester group-containing alkyl group and a fluorine-containing alkyl group. The synthesis method of the photoacid generator comprises the following steps: reacting beta-cineole with a sulfonate compound to obtain an intermediate; and reacting the intermediate with (cyclohexyl-1,5-dienyloxy)-trimethyl-silane, tetramethylene sulfoxide and trifluoroacetic anhydride to obtain the sulfonium sulfonate photoacid generator. The beta-cineole used as the raw material has a high molecular weight, and the photoacid generator formed by the beta-cineole also has a high molecularweight, so that the diffusion of the photoacid generator can be reduced, and the improvement of the edge roughness, the reduction of the line width roughness and the improvement of the resolution ratio are facilitated.
Owner:上海博栋化学科技有限公司
Method and device for preparing heptafluoroisobutyronitrile
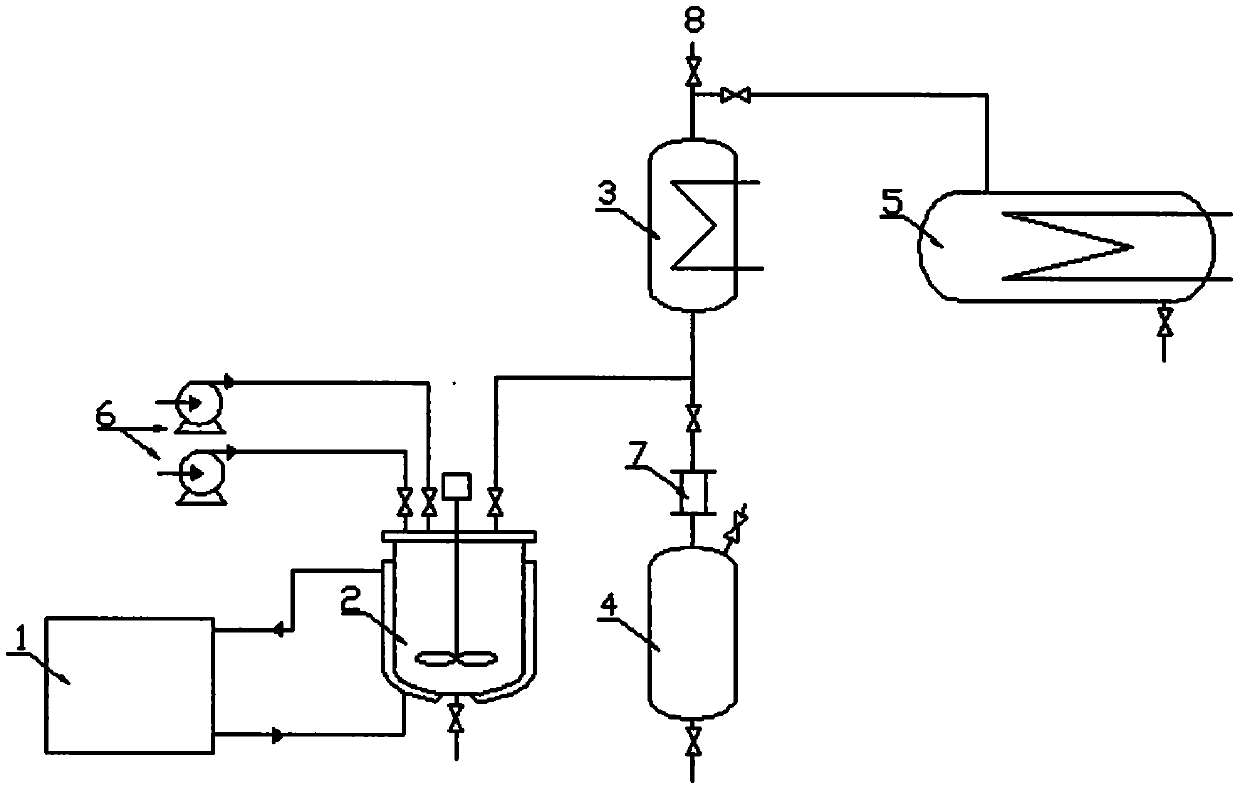
PendingCN111205200AReduce investmentEasy to operateOrganic compound preparationCarboxylic acid amides preparationPerfluoroacetic AcidReaction temperature
The invention discloses a method and device for preparing heptafluoroisobutyronitrile, wherein the method comprises the steps: adding methyl heptafluoroisobutyrate into a reaction kettle, adding an alcoholic solution of ammonia, and controlling the reaction temperature to be lower than 0 DEG C; carrying out a reaction for 2-5 h, heating to 60 DEG C, finishing the reaction when the content of methyl heptafluoroisobutyrate in a reaction solution is less than 0.1%, removing a methanol solvent by vacuum distillation, and removing the vacuum when the content of methanol in the reaction solution inthe reaction kettle is less than 1%; and adding a dimethylformamide / pyridine solvent into the reaction system, controlling the reaction temperature to be lower than 0 DEG C, adding a dehydrating agenttrifluoroacetic anhydride, and collecting heptafluoroisobutyronitrile gas. According to the method, an intermediate heptafluoroisobutyrylamide synthesis step and a step device for preparing heptafluoroisobutyronitrile by dehydrating heptafluoroisobutyrylamide are integrated; transfer of an intermediate heptafluoroisobutyramide is not needed and water washing, extraction, recrystallization and other operations are avoided, so the heptafluoroisobutyronitrile is directly synthesized, the equipment investment is greatly reduced, the operation process is simplified, and the method is suitable forindustrial production.
Owner:昊华气体有限公司
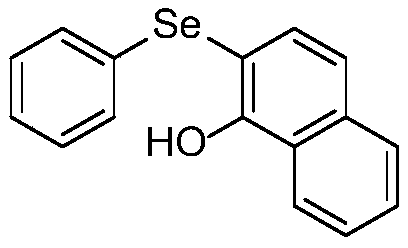
Synthetic method for diaryl selenide
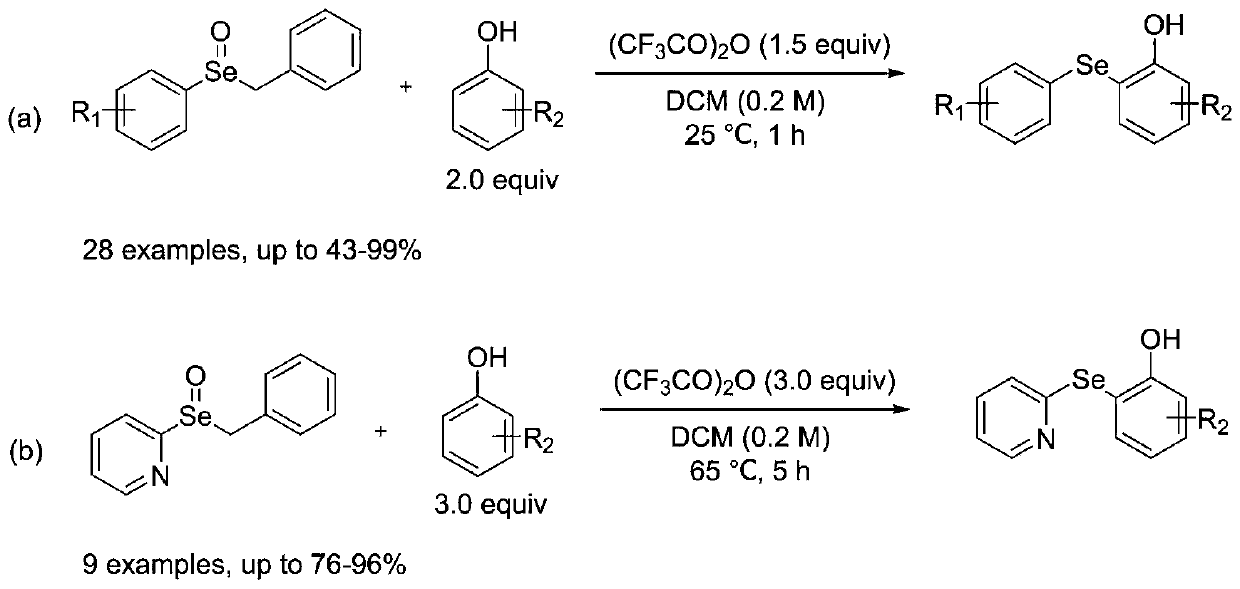
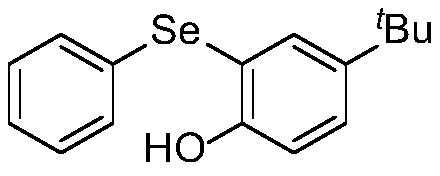
ActiveCN110272366AImprove compatibilityMild reaction conditionsOrganic chemistryOrganic synthesisFiltration
The invention discloses a synthetic method for diaryl selenide, and belongs to the technical field of organic synthesis. According to the method, aryl benzyl selenium oxide or 2-pyridyl benzyl selenium oxide and a phenolic compound are dissolved in anhydrous dichloromethane under a nitrogen atmosphere, and an activator trifluoroacetic anhydride is added at room temperature to obtain a reaction system; the reaction system is sealed in a nitrogen atmosphere, a reaction is performed under a stirring condition, a saturated sodium hydrogen carbonate solution is added for quenching the reaction, dichloromethane is adopted for washing, then a saturated salt solution is adopted for washing, phase separation is carried out, an organic phase is subjected to drying, filtration and reduced pressure distillation to remove a solvent, and through slica column chromatography, the diaryl selenide is obtained. The method has the advantages that no transition metal catalysis exists, the reaction conditions are mild, the functional group compatibility is excellent, the yield is generally high, and environmental friendliness is achieved.
Owner:YUNNAN UNIV
Synthetic method for trifluoromethyl methylation arene
InactiveCN104098432ALow priceLower requirementOrganic compound preparationHalogenated hydrocarbon preparationTrifluoromethylationTrifluoroacetic acid
The invention provides a synthetic method for a trifluoromethyl methylation arene. The trifluoromethyl methylation arene is prepared by reacting a halogenated arene, copper powder, elemental iodine and trifluoroacetic anhydride in an organic solvent under an inert atmosphere protection state. The method has the advantages of being simple and efficient, cheap in raw materials, low in synthetic cost, high in universality and repeatability, and the like, and can be popularized and applied to the fields of the synthesis of trifluoromethyl methylation aromatic derivatives.
Owner:FUQING BRANCH OF FUJIAN NORMAL UNIV
Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof
The invention discloses a diarylo[d,f]oxacycloheptane-3-ketone compound and a synthesis method thereof, relating to an oxacycloheptane-3-ketone compound and a synthesis method thereof. The invention aims to solve the problems that in the prior art, the diarylo[d,f]oxacycloheptane-3-ketone compound is difficult to synthesize, the pollution is serious and the industrial production can not be realized. The structural formula of the diarylo[d,f]oxacycloheptane-3-ketone compound is shown in the specification. The method takes 2-phenylphenoxyacetic acid derivatives or 2-naphthphenoxyacetic acid derivatives as raw materials, and comprises the following steps of: carrying out reaction between trifluoroacetic anhydride and lewis acid as catalysts in an organic solvent; pouring ice water and layering; adjusting the pH value of the organic layer to 8-9 by the aqueous solution of sodium carbonate; washing the organic layer without a water layer by saturated salt water; and concentrating to obtain a solid crude product, and recrystallizing to obtain the diarylo[d,f]oxacycloheptane-3-ketone compound. The method disclosed by the invention is mainly used for synthesizing the diarylo[d,f]oxacycloheptane-3-ketone compound.
Owner:HEILONGJIANG UNIV
Preparation method of prucalopride intermediate
InactiveCN104529960AReduce usageStarting materials are cheap and readily availableOrganic chemistryBulk chemical productionSodium acetateAluminium chloride
The invention discloses a preparation method of a prucalopride intermediate. The preparation method comprises the following steps: step a, protecting t amino group of a compound I by using trifluoroacetic anhydride to obtain a compound II; step b, under the protection of nitrogen gas, enabling the compound II, chloroacetyl chloride, anhydrous aluminium chloride, nitrobenzene and a halocarbon solvent to react to obtain a compound III; step c, enabling the compound III, an alcohol solvent and sodium acetate to have a reflux reaction to obtain a compound IV; step d, enabling the compound IV, the alcohol solvent, raney nickel and nitrogen with the pressure of 3.0Mpa to react to obtain a compound V; step e, enabling the compound V and N-chloro-succinimide to have a substitution reaction to obtain a compound VI; step f, removing an amino protective group from the compound VI to obtain a compound VII. The method disclosed by the invention has the advantages that initial raw materials are easily available, is simple and convenient to operate, is relatively mild in reaction condition, is capable of avoiding the use of heavy metal reagents and high-toxicity reagents, is high in yield and is quite suitable for large-scale industrial production.
Owner:CHENGDU BAIYU PHARMA CO LTD
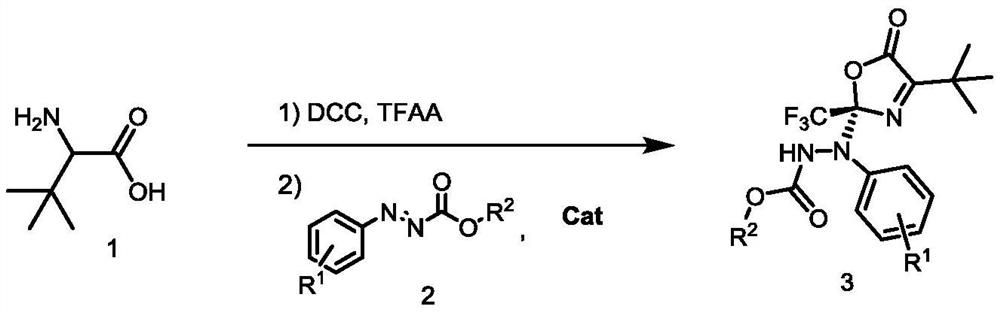
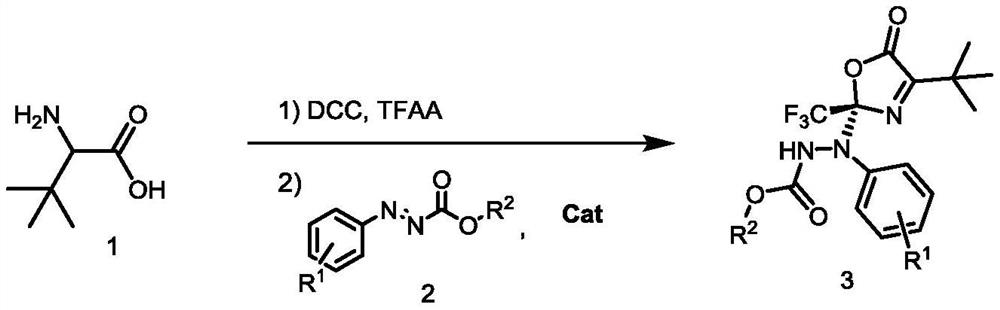
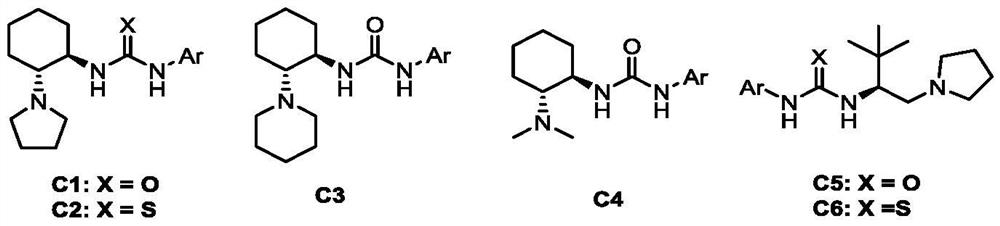
Method for synthesizing optically active benzo carboxylic acid ester compound through asymmetric addition reaction
ActiveCN113200933AHigh yieldHigh enantioselectivityOrganic chemistry methodsPtru catalystTrifluoroacetic acid
The invention discloses a method for synthesizing an optically active benzo carboxylic acid ester compound through an asymmetric addition reaction, and belongs to the technical field of asymmetric synthesis in organic chemistry. Tert-leucine is used as an initial raw material and reacts with trifluoroacetic anhydride to generate a 2-trifluoroalkyl oxazole-5 (2H) ketone intermediate product, and then in the presence of a chiral bifunctional tertiary amine urea catalyst, the optical benzo carboxylic acid ester compound is synthesized through asymmetric addition reaction and one-pot two-step synthesis. The method has the advantages that the reaction raw materials are easy to obtain, the catalyst is simple in structure, the catalytic efficiency is high, the reaction conditions are mild, post-treatment is simple, and the high-optical-activity benzo carboxylic acid ester compound is obtained.
Owner:HENAN NORMAL UNIV
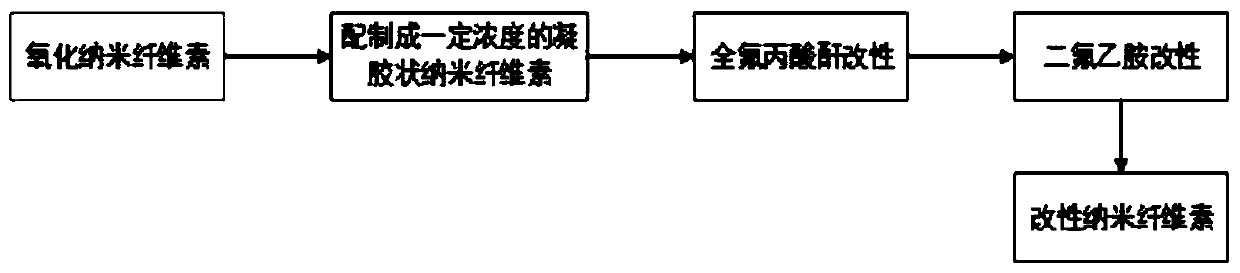
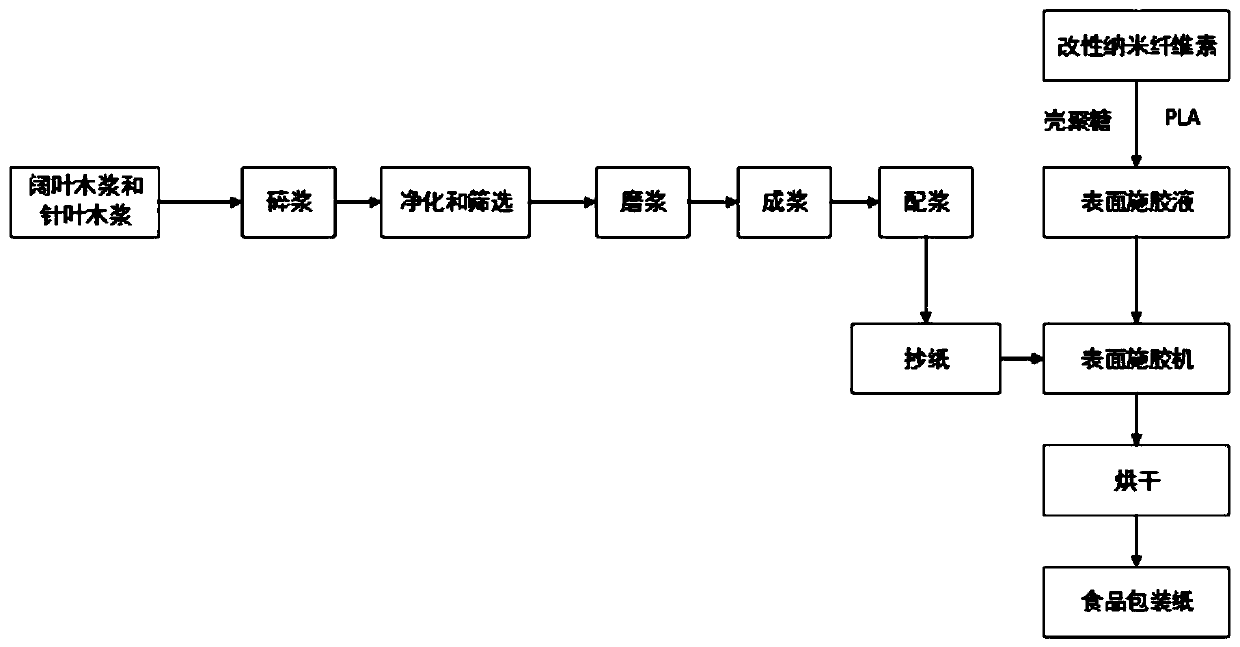
Fluorine-contained hydrophobic oil-proof modified nanocellulose for food packaging paper and preparation method of nanocellulose
ActiveCN110804105AExtended shelf lifeLarge specific surface areaFlexible coversWrappersFiberTrifluoroacetic anhydride
The invention discloses fluorine-contained hydrophobic oil-proof modified nanocellulose for food packaging paper and a preparation method of the nanocellulose and belongs to the technical field of functional materials. According to the preparation method, firstly, oxidized nanocellulose is diluted into a nanocellulose water suspension with the concentration of 1-1.5%, then evaporation is performedto obtain gel nanocellulose, then trifluoroacetic anhydride and 2,2-difluoroethylamine are sequentially used for modifying the nanocellulose, and the fluorine-contained hydrophobic oil-proof modifiednanocellulose is obtained. The fluorine-contained hydrophobic oil-proof modified nanocellulose has good water resistance and film-forming performance; after the nanocellulose is spread on the surfaceof the food packaging paper, a uniform film can be formed to effectively prevent permeation of water vapor and oxygen, and therefore the shelf life of food is prolonged; and besides, since the nanocellulose has a great specific surface area and contains abundant hydroxide radicals, the nanocellulose can be tightly combined with fibers, the binding force between the fibers and the strength of thepaper are improved, and the reliability of the food packaging paper in use is improved.
Owner:浙江恒川新材料有限公司
Detection method for in-situ measuring content of phenyllactic acid in fermentation samples
The invention relates to a detection method for in-situ measuring content of phenyllactic acid in fermentation samples, wherein a sample is measured by gas chromatography after the sample is pre-processed; the pre-processing of the sample comprises the steps of: evenly mixing a liquid fermentation sample or a smashed solid fermentation sample with deionized water; heating up the sample for evaporating the sample to dryness; adding the deionized water into the sample and oscillating, and centrifuging the solution; heating up the supernatant for removing water; adding 0.2-0.5mL of 3.0-3.4 mol / L concentrated hydrochloric acid-normal butanol mixed liquor; maintaining solution at 65-72 DEG C for 12-15 minutes; drying the solution by nitrogen; adding 0.2-0.5mL of a mixed solution of trifluoroacetic anhydride and ethyl acetate mixed solution at a ratio of 0.15-0.3:1; maintaining the temperature of the solution at 78-85 DEG C for 12-15 minutes; drying the solution by nitrogen; dissolving by ethyl acetate; and centrifuging and taking the supernatant for detection. The detection sensitivity of the method is high; the lowest detection limit of phenyllactic acid is 4.2-7g / mL.
Owner:HENAN BUSINESS SCI RES INST
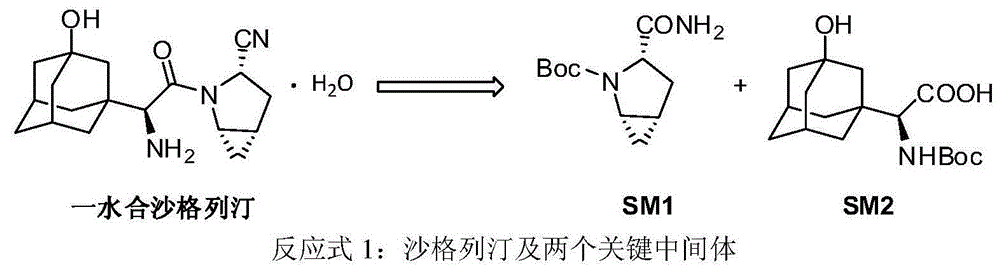
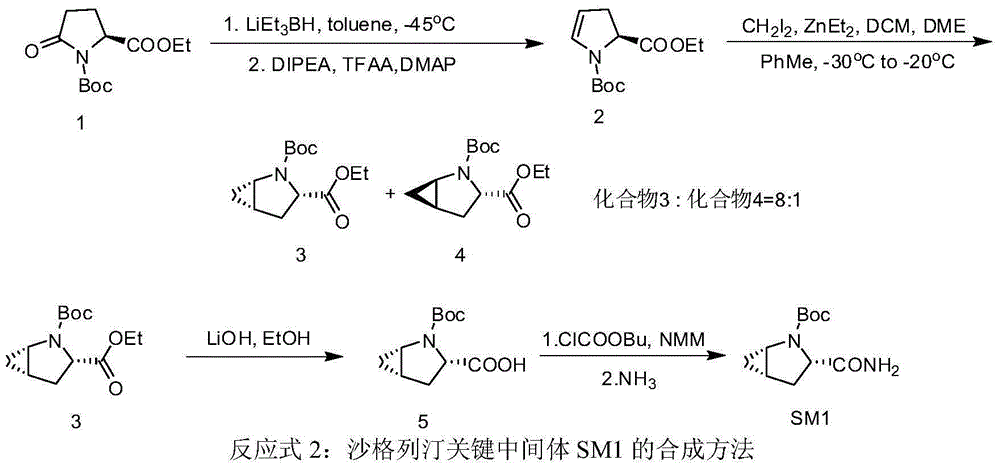
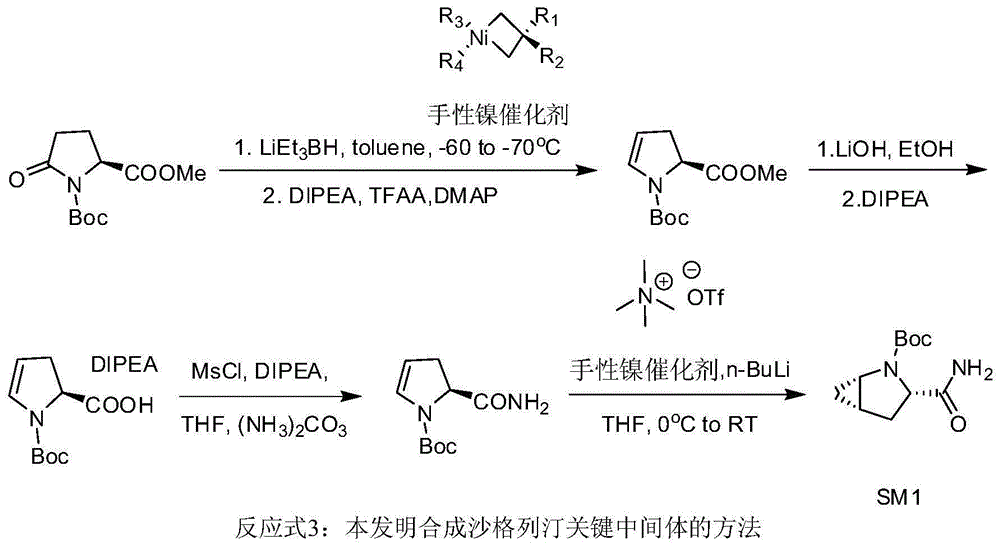
Preparation method of key intermediate of saxagliptin
InactiveCN106554301AImprove conversion rateReduce manufacturing costOrganic chemistryTert-Butyloxycarbonyl protecting groupTrifluoroacetic anhydride
The invention relates to a preparation method of (1S, 3S, 5S)-3-(amidogen carbonyl)-2- azabicyalo [3.1.0] hexane-2-tert-butyl formate. The preparation method comprises the following steps that (1), Boc-L-pyroglutamic acid methyl ester is restored through lithium triethylborohydride and then dewatered through trifluoroacetic anhydride to obtain (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole ethyl formate; (2), DIPEA is added in the hydrolysis reaction of (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole ethyl formate under a alkaline condition, then salifying is conducted, and (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formic acid N, N-diisopropyl ethylamine salt is obtained; (3), (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formic acid N and N-diisopropyl ethylamine salt obtained in the step (2) are subjected to amidating, and (S)-1-N- tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formamide is obtained; and (4), (S)-1-N-tert-butyloxycarbonyl-2,3-dihydro-2-pyrrole formamide is catalyzed through a chirality nickel catalyst and subjected to a ciprofloxacin reaction, and a target material with single configuration, namely (1S, 3S, 5S)-3-(amidogen carbonyl)-2-azabicyalo [3.1.0] hexane-2-tert-butyl formate (SM1), is obtained.
Owner:HYBIO PHARMA
Preparation methods for Suc-Ile-Glu(gamma-Pip)-Gly-Arg-pNA.HCl
ActiveCN107474108ASuitable for mass productionHigh yieldPeptide preparation methodsBulk chemical productionArginineP-Nitroaniline
The invention belongs to the field of peptide synthesis, and specifically relates to a preparation method for Suc-Ile-Glu(gamma-Pip)-Gly-Arg-pNA.HCl. The method comprises the following steps: by adopting bis-alloc (bis-Alloc), protecting a guanidino group of arginine from being influenced by acid and alkali; and connecting p-phenylenediamine onto amino acid, and carrying out oxidation by using hydrogen peroxide and trifluoroacetic anhydride so as to improve the yield, the safety and the environment-friendliness of condensation of paranitroaniline and amino acid, wherein proper protecting groups are adopted for various amino acids, thereby improving the stability of the reaction process. The method provided by the invention has advantages like environment-friendly process, safety, high yield and relatively low preparation cost, and is applicable to large-scale preparation.
Owner:SHANGHAI SUNBIO TECH
Nitroreductase-responsive diagnosis and treatment integrated probe and preparation method and application thereof
ActiveCN113292541AAvoid background fluorescence interferenceAvoid damageOrganic chemistryPhotodynamic therapyNitroreductaseTrifluoroacetic anhydride
The invention belongs to the field of biological medicine, and discloses a nitroreductase-responsive diagnosis and treatment integrated probe and a preparation method and application thereof. The structural general formula of the diagnosis and treatment integrated probe is described in the specification. The specific preparation method comprises the following steps: reacting hydrogen peroxide with trifluoroacetic anhydride in an organic solvent, then adding a near-infrared fluorescent dye for continuous reaction, and after the reaction is finished, separating and purifying to obtain the diagnosis and treatment integrated probe. The diagnosis and treatment integrated probe can show fluorescence enhanced response to nitroreductase in a tumor microenvironment, and is used for nitroreductase and living tumor imaging; meanwhile, the diagnosis and treatment integrated probe generates active oxygen under laser irradiation and is used for photodynamic therapy of tumors. The diagnosis and treatment integrated probe has high biological safety, the preparation method is easy and convenient to operate, low in cost and easy to popularize, and the diagnosis and treatment integrated probe has potential application value in the aspects of early diagnosis and treatment of malignant tumors.
Owner:SHANXI MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/eb64c4a8-e8b1-40f0-8f34-a589ab94d906/DSA00000298513800011.png)
![[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/eb64c4a8-e8b1-40f0-8f34-a589ab94d906/FSA00000298513900011.png)
![[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/eb64c4a8-e8b1-40f0-8f34-a589ab94d906/BSA00000298514000011.png)


![Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/a8fd5aaa-3050-4717-8dcc-739ac7722b10/HDA00001906852900011.png)
![Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/a8fd5aaa-3050-4717-8dcc-739ac7722b10/HDA00001906852900021.png)
![Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/a8fd5aaa-3050-4717-8dcc-739ac7722b10/HDA00001906852900031.png)