Preparation method of isomer impurities of vildagliptin
A technology for isomers and impurities, which is applied in the field of preparation of vildagliptin isomer impurities, to achieve the effects of improved safety, simple preparation process and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
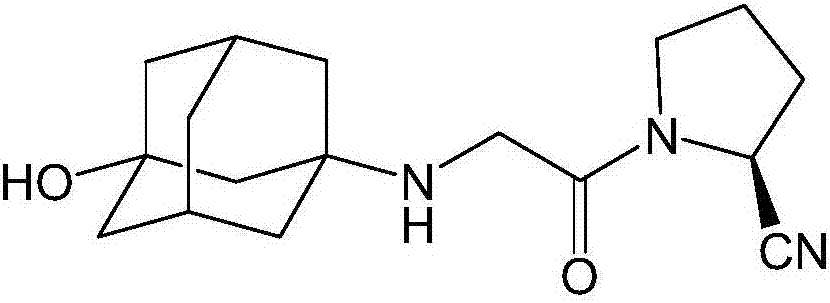
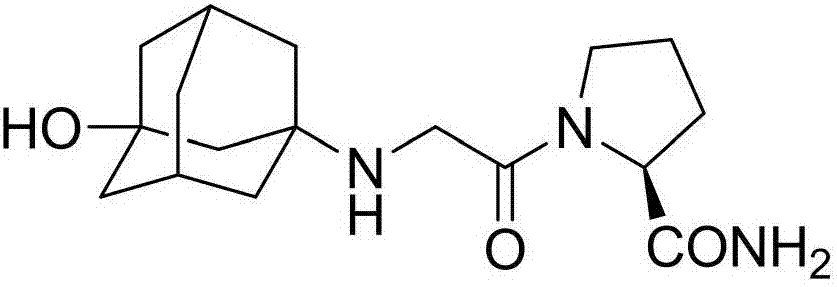
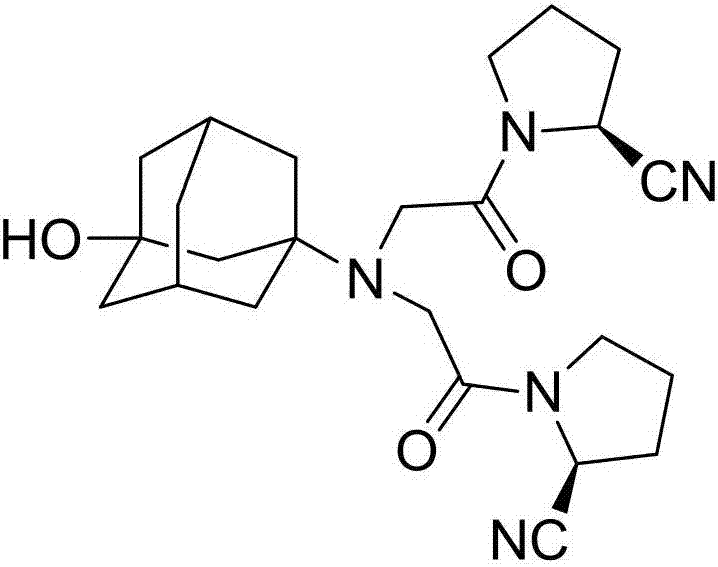
[0039] The present invention provides a kind of preparation method of vildagliptin isomer impurity, the steps are as follows:
[0040] S1. Dissolve potassium carbonate and chloroacetyl chloride in tetrahydrofuran to obtain a chloroacetyl chloride solution; dissolve D-prolinamide (I) in tetrahydrofuran, add dropwise to the chloroacetyl chloride solution, and react at 20°C for 1.5h ; The reaction product (II) was obtained, filtered, the filtrate was taken, dried, trifluoroacetic anhydride was added to the filtrate, reacted at 20°C for 0.5h, and the solvent was spin-dried to obtain a crude product of the reaction product (Ⅲ), which was purified;
[0041] S2. Potassium carbonate and 3-amino-amantadinol were dissolved in tetrahydrofuran, catalyzed by potassium iodide, reacted with the reaction product (Ⅲ), kept at 50°C for 4.5 hours to cool down, filtered, and the filtrate was spin-dried to obtain the target Product (IV) crude, purified.
Embodiment 2
[0043] The present invention provides a kind of preparation method of vildagliptin isomer impurity, the steps are as follows:
[0044] S1. Dissolve potassium carbonate and chloroacetyl chloride in tetrahydrofuran, and the molar ratio of potassium carbonate and chloroacetyl chloride is 1.5:1 to obtain a chloroacetyl chloride solution; dissolve D-prolineamide (I) in tetrahydrofuran and add dropwise Into the chloroacetyl chloride solution, the molar ratio of D-prolineamide and chloroacetyl chloride is 1:1, react at 25°C for 1.5h; obtain the reaction product (II), filter, take the filtrate, dry, add to the filtrate Trifluoroacetic anhydride, the molar ratio of D-prolineamide and trifluoroacetic anhydride is 1:1.2, react at 25°C for 0.5h, and spin the solvent to obtain the crude product of reaction product (Ⅲ), which is purified;
[0045] S2. Potassium carbonate and 3-amino-amantadinol are dissolved in tetrahydrofuran, the molar ratio of potassium carbonate and 3-amino-adamantanel ...
Embodiment 3
[0047] The present invention provides a kind of preparation method of vildagliptin isomer impurity, the steps are as follows:
[0048] S1. Dissolve potassium carbonate and chloroacetyl chloride in tetrahydrofuran, and the molar ratio of potassium carbonate and chloroacetyl chloride is 2.5:1 to obtain a chloroacetyl chloride solution; dissolve D-prolineamide (I) in tetrahydrofuran and add dropwise Into the chloroacetyl chloride solution, the molar ratio of D-prolineamide and chloroacetyl chloride is 1:1.1, react at 25°C for 2.5h; get the reaction product (II), filter, take the filtrate, dry it, and add to the filtrate Trifluoroacetic anhydride, the molar ratio of D-proline amide and trifluoroacetic anhydride is 1:1.5, react at 25°C for 1.5h, and spin the solvent to obtain the crude product of reaction product (Ⅲ), and the crude product of reaction product (Ⅲ) Wash with saturated sodium bicarbonate solution to pH = 7-8, add ethyl acetate for extraction, collect the organic layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com