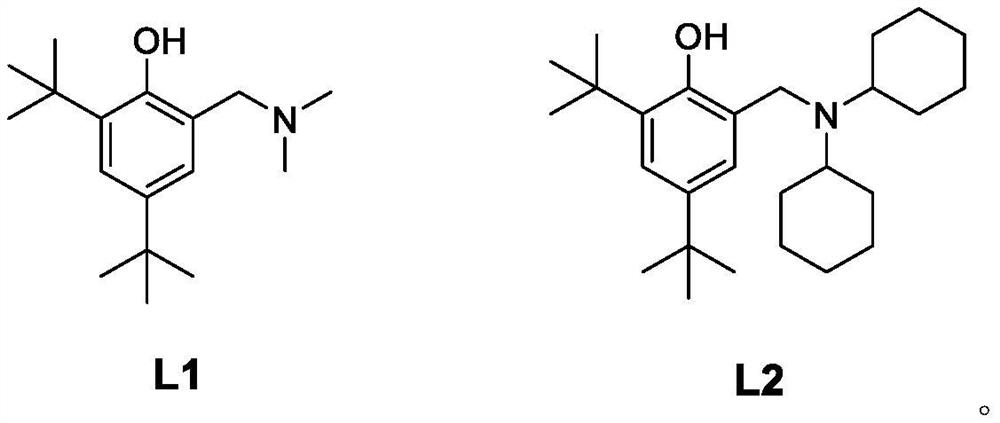
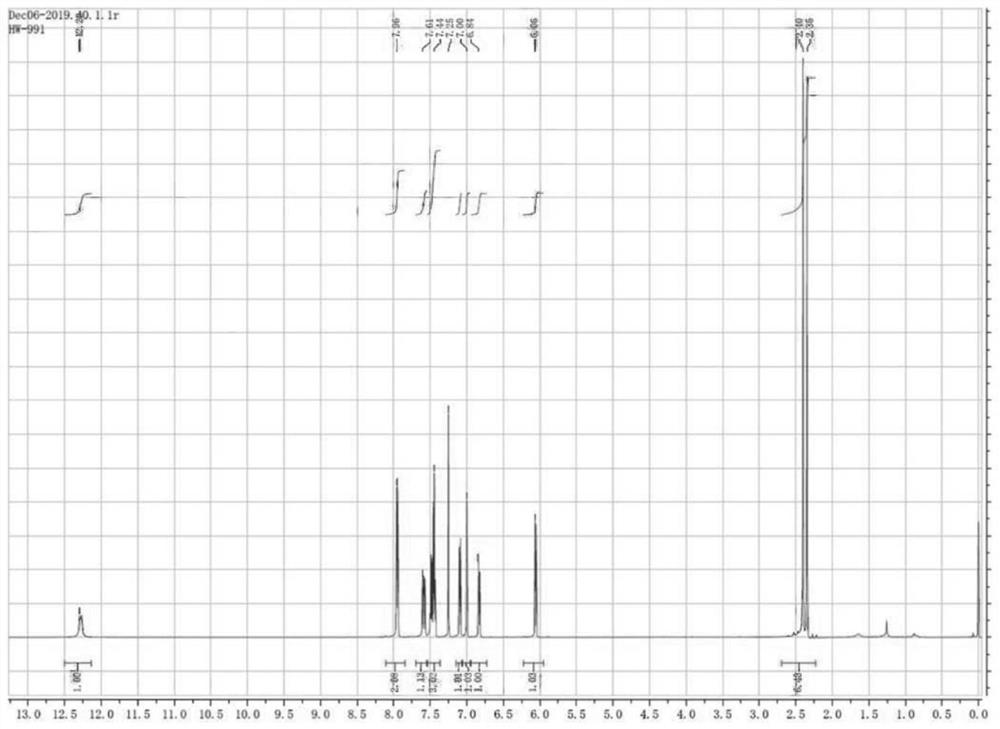
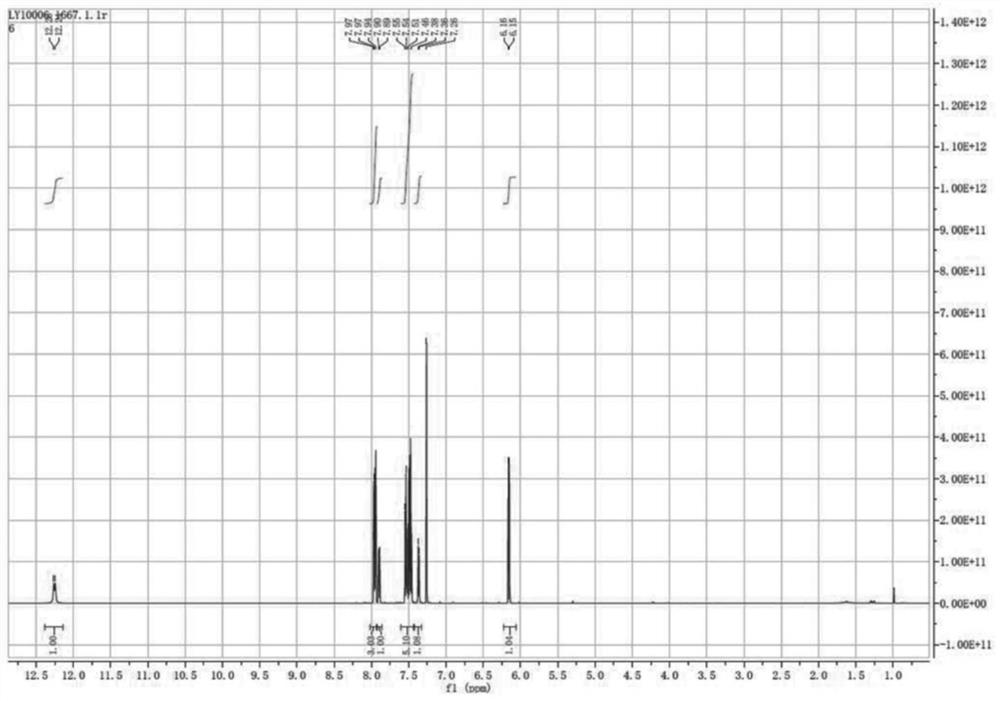
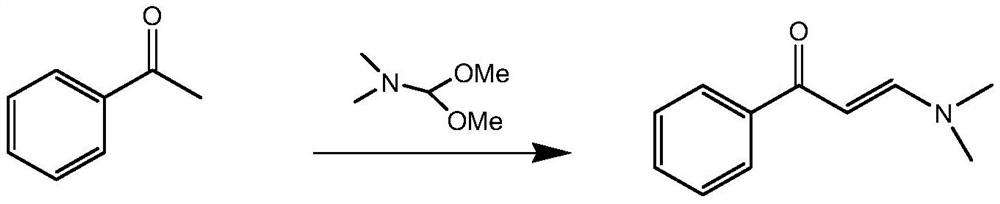
Patents
Literature
32results about How to "Simple post-processing purification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
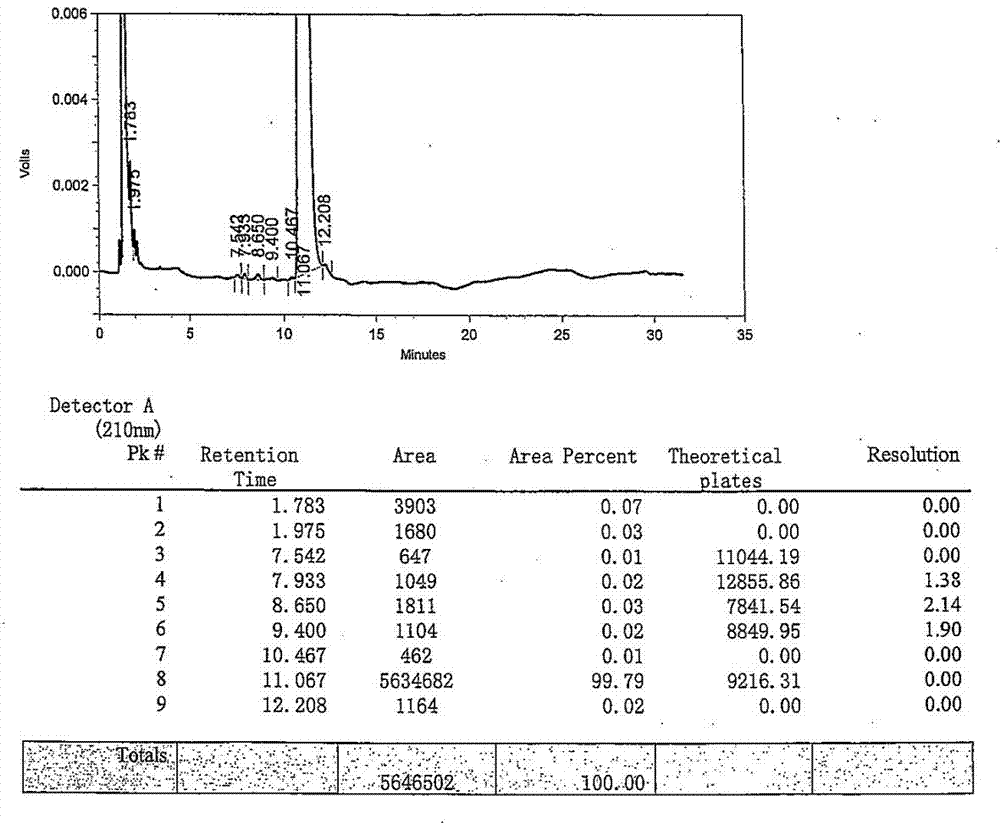
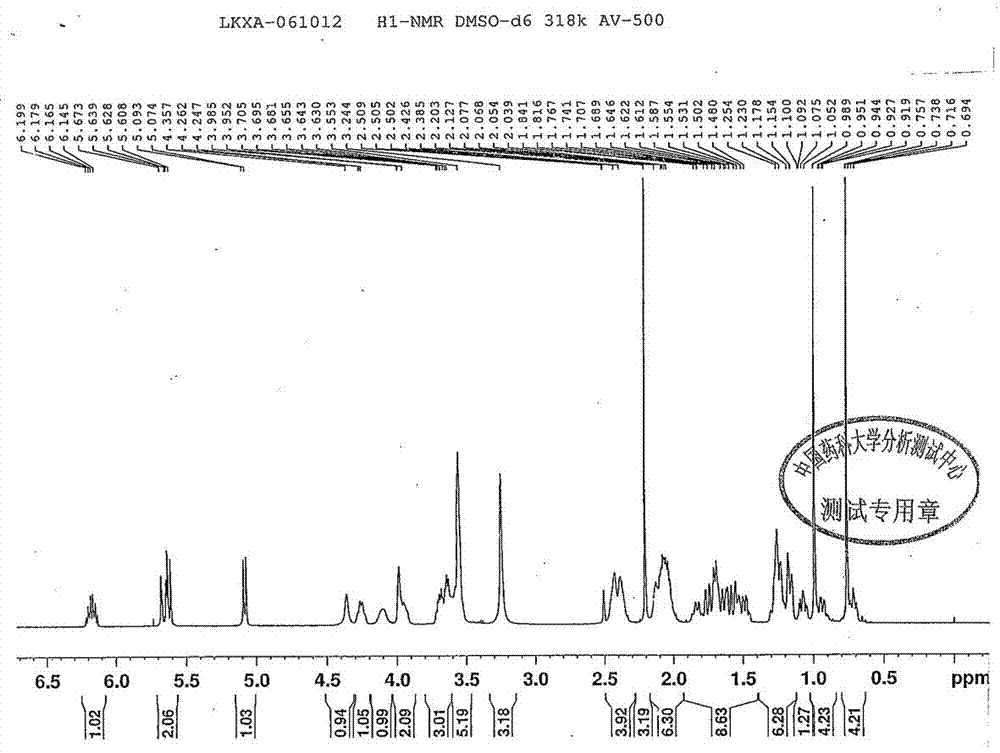
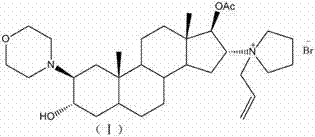
Novel method for preparing rocuronium bromide
The invention relates to a novel method for preparing rocuronium bromide 1-[17beta-acetoxyl-3alpha-hydroxyl-2beta-(4-morpholinyl)-androstane-16beta-yl]-1-(2-propenyl) pyrrole bromide, the problem of chemoselectivity of pyrrolidine open epoxy in an original line is solved, generation of byproducts is avoided, reaction yield is greatly improved, the production cost is reduced, column chromatography separation is avoided, and aftertreatment purification is implemented easily.
Owner:JIANGSU QINGJIANG PHARMA
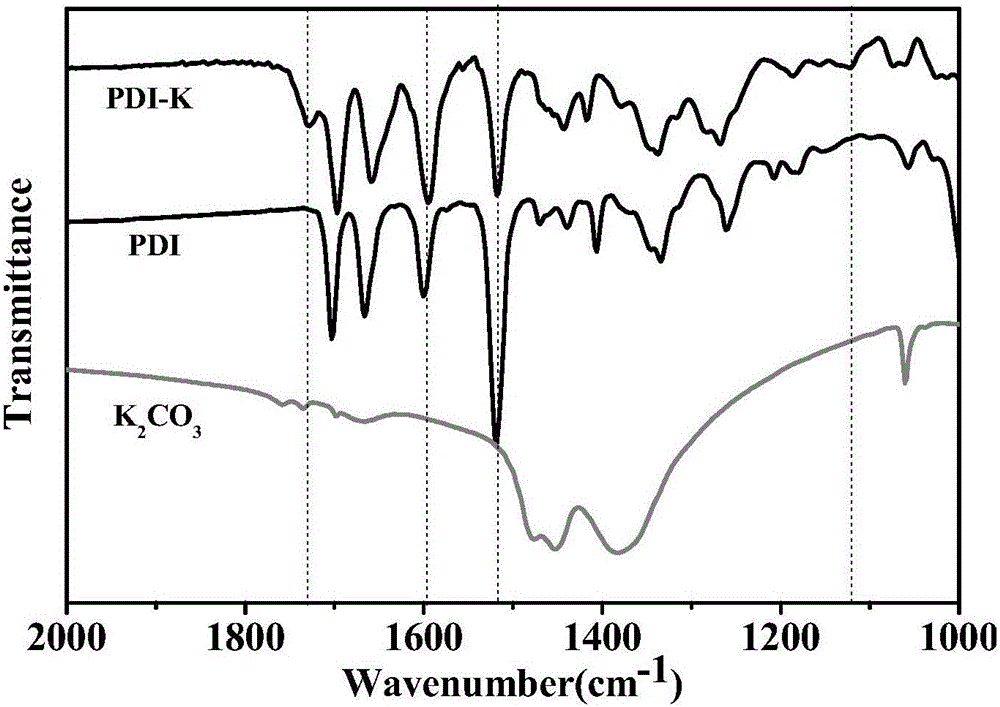
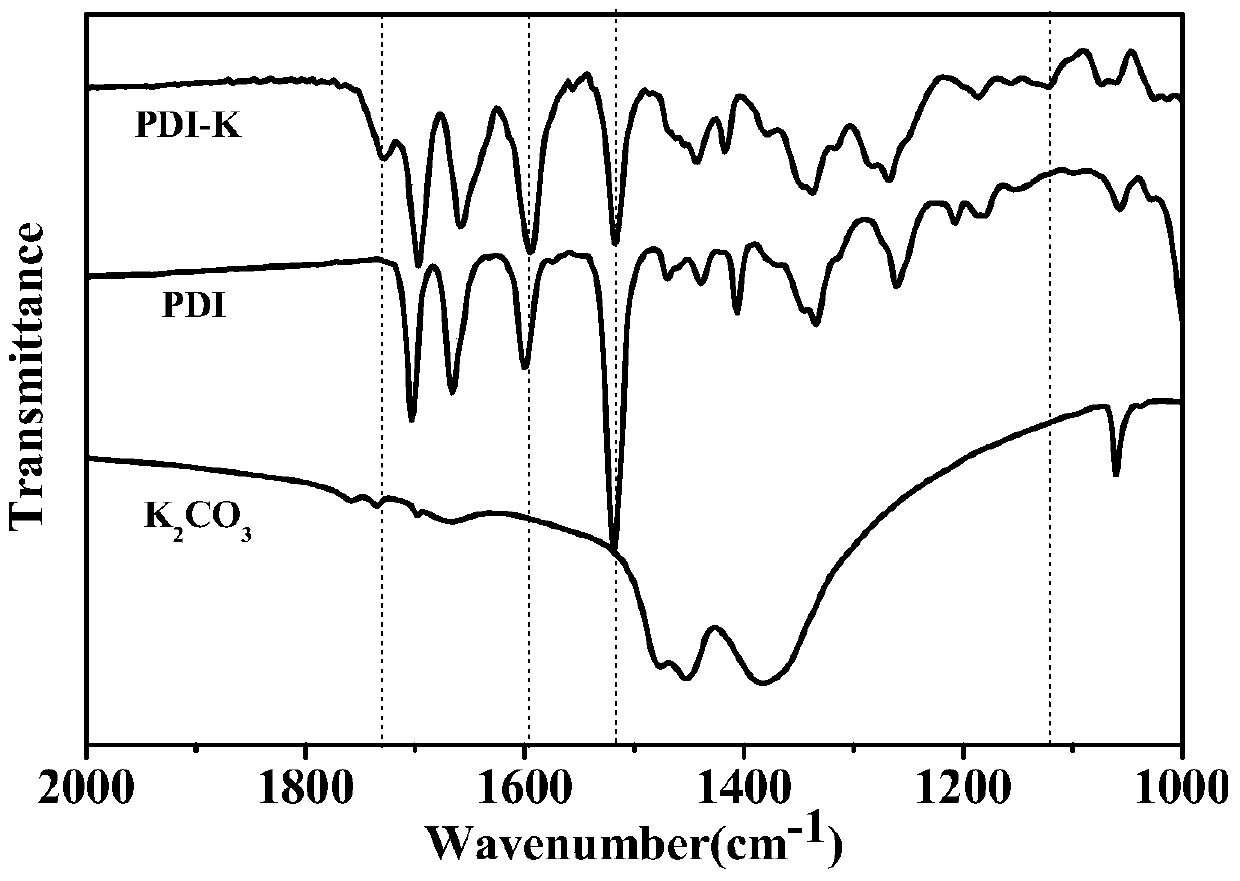
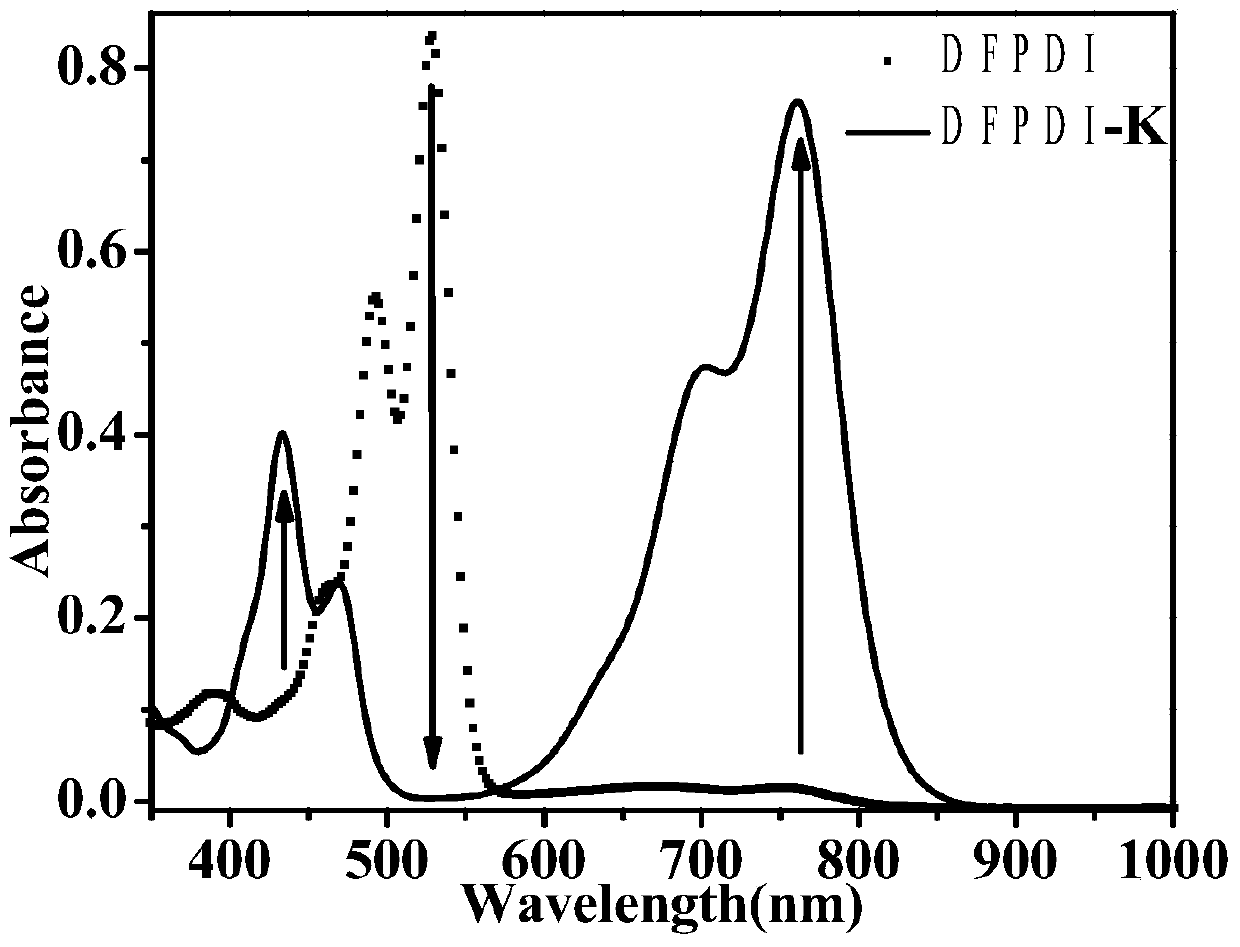
Preparation method of reduction-state ionic salt of perylene bisimide and derivative thereof
ActiveCN105732623AImprove thermal stabilityGood chemical stabilityOrganic chemistryType transformationOrganic base
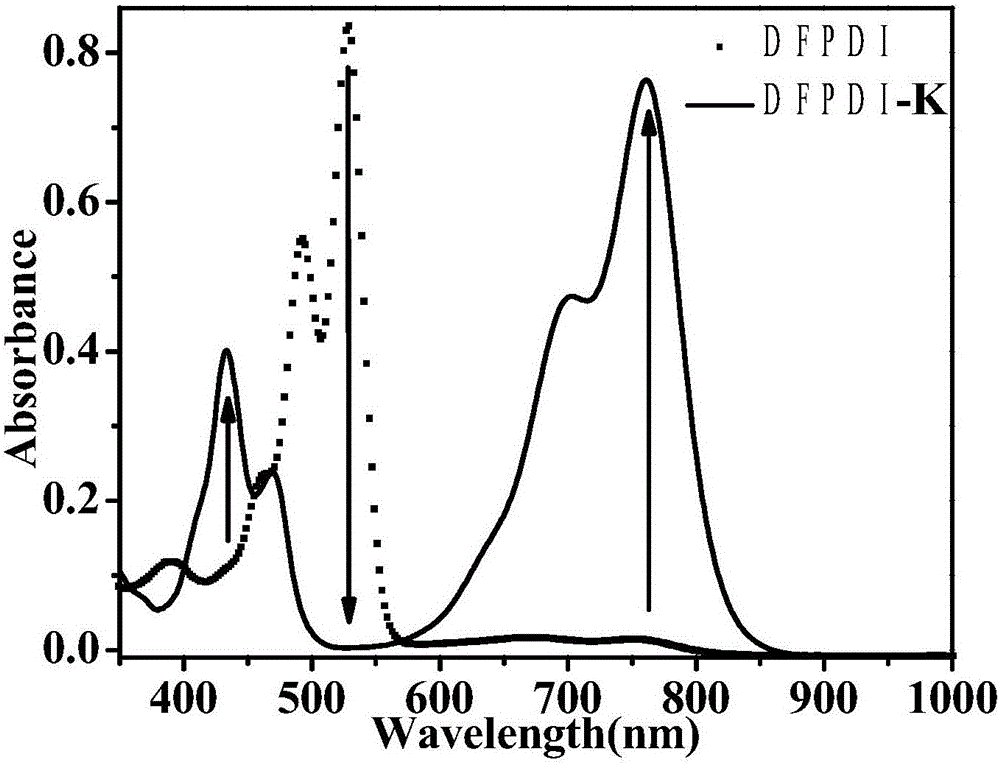
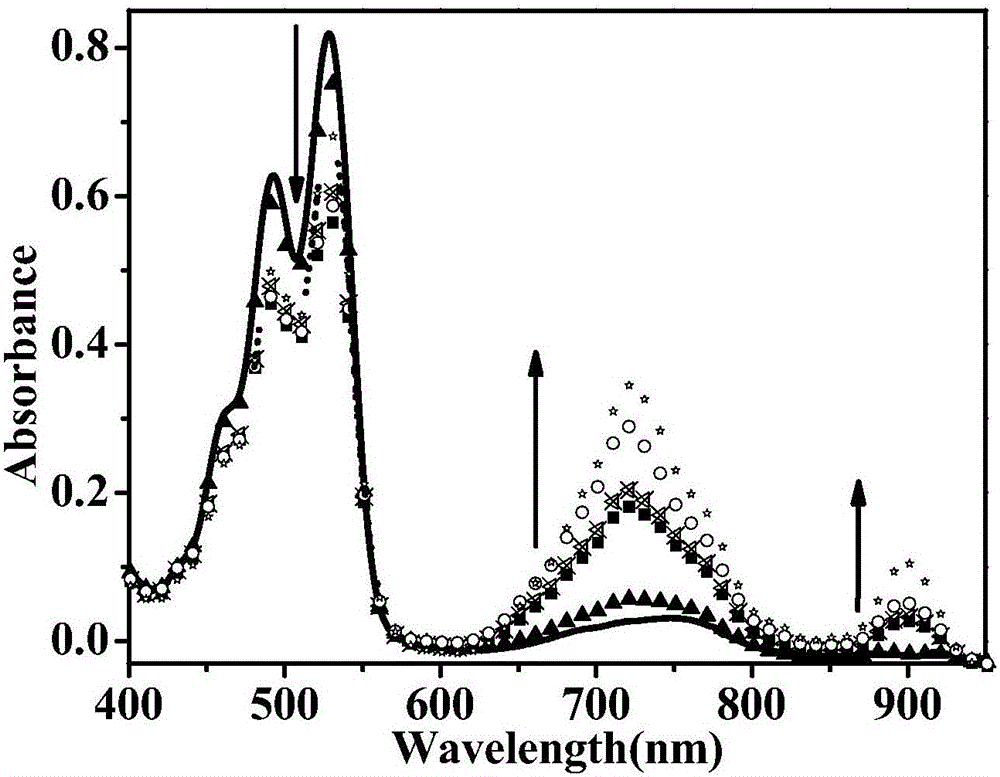
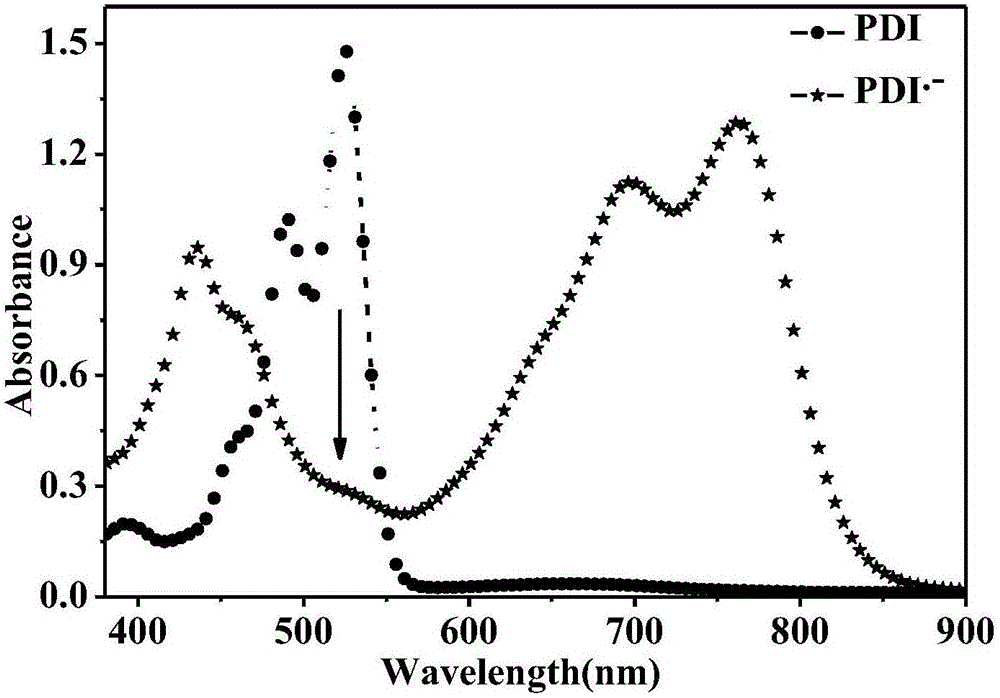
The invention discloses the reduction-state ionic salt of perylene bisimide and a derivative thereof as well as a preparation method. The preparation method comprises the following steps: with perylene-3,4,9,10-tetracarboxylic acid dianhydride as a raw material, synthesizing a perylene bisimide derivative with low LUMO energy level and excellent photoelectric performance through a bromination reaction, an amidation reaction and a nucleophilic substitution reaction; and synthesizing the perylene bisimide monovalent ionic salt with stable environment relatively easily according to the benzoquinone type transformation mechanism in an alkaline condition (organic base such as triethylamine, and inorganic base such as potassium carbonate). The method for synthesizing the perylene bisimide ionic salt is novel, unique and simple and realizes high yield; industrialization can be implemented while the effect is good; and moreover, the material in a reduction state responds to metal ions and acids different in oxidability.
Owner:YANSHAN UNIV
Preparation method and application of naphthylamine compounds and salts thereof
ActiveCN111559991APrevent invasionInhibit transferOrganic active ingredientsOrganic chemistryChemical compoundP phosphate
The invention discloses a preparation method and application of naphthylamine compounds and salts thereof, concretely provides a method for synthesizing the naphthylamine compounds by taking 4-formylphenol as a substrate, and also provides a preparation method of hydrochloride and phosphate of the naphthylamine compounds. The preparation method has the characteristics of short synthesis route, cheap and easily available raw materials, simple post-treatment and purification, relatively high total yield and the like. The method has important significance for expanding the variety of naphthylamine derivatives and researching the naphthylamine derivatives in the aspects of biology and medicine. Meanwhile, the antitumor effect and mechanism of the prepared naphthylamine compounds and hydrochloride and phosphate thereof are further studied, and the result shows that the naphthylamine compounds and hydrochloride and phosphate thereof have the following advantages: (1) cancer cell proliferation, growth or migration is inhibited; and (2) cancer cell apoptosis is promoted or cancer cell migration capability or invasion capability is reduced, and meanwhile, the compounds can also be used as an inhibitor for tyrosine phosphorylation of STAT3.
Owner:HENAN RADIOMEDICAL SCI & TECH CO LTD
Reduced ionic salt of perylene bisimide and derivative of perylene bisimide and preparation method
InactiveCN106521543AImprove air stabilityImprove stabilityElectrolysis componentsElectrolytic organic productionPeryleneElectrochemistry
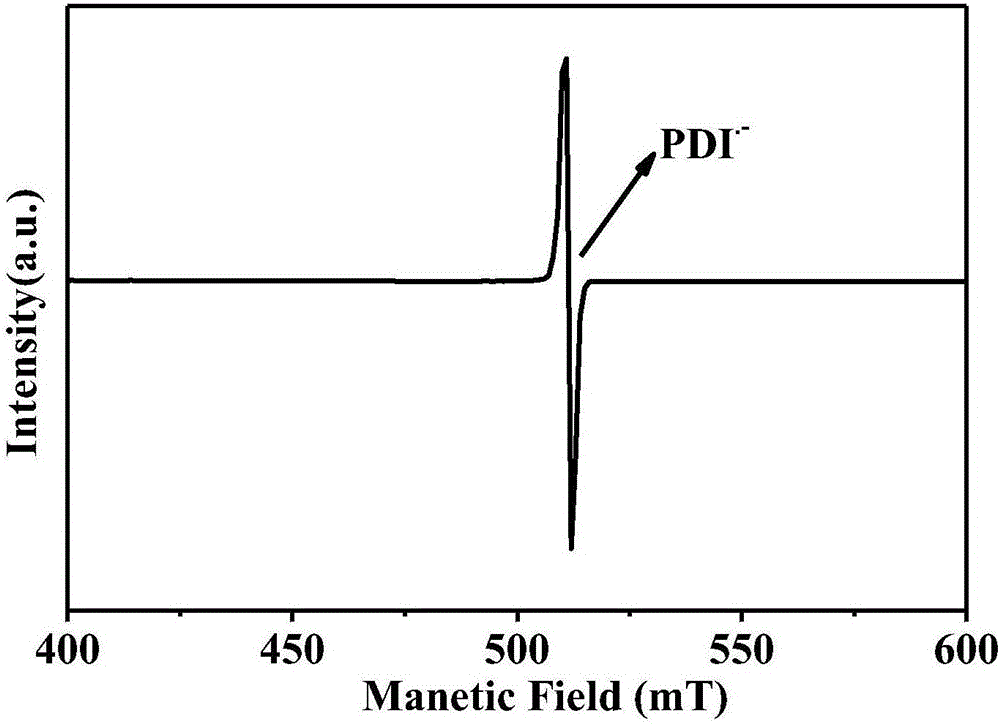
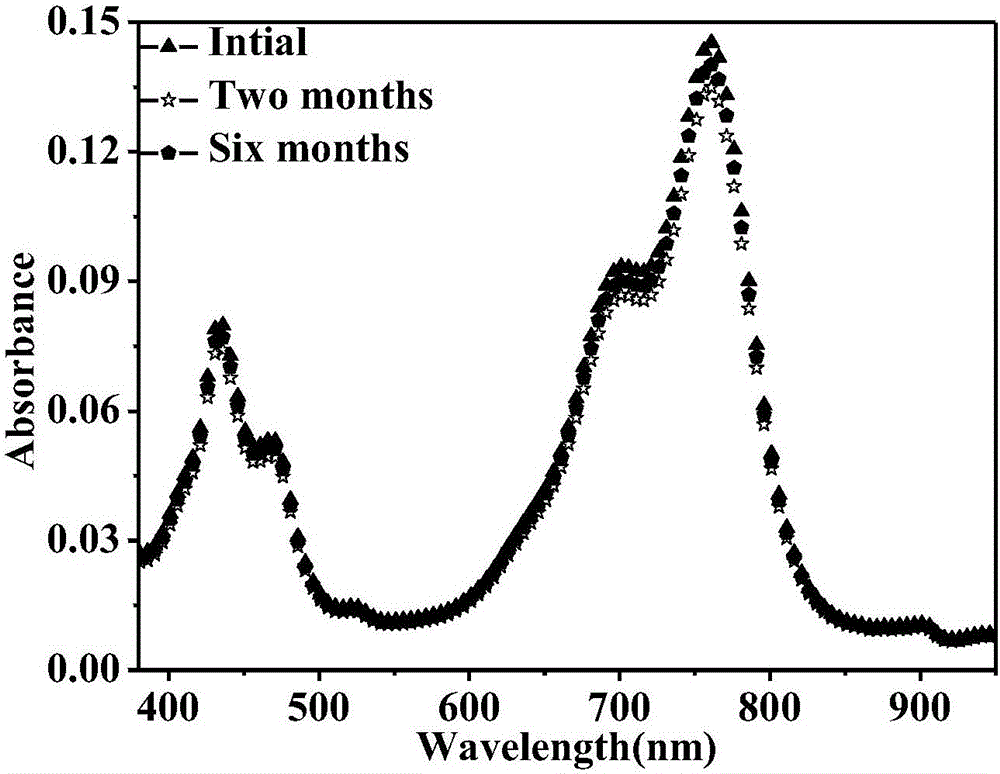
The invention relates to a reduction state of perylene bisimide and a derivative of the perylene bisimide and a preparation method. Perylene-3,4,9,10-tetracarboxylic acid dianhydride is adopted as a raw material, and the perylene bisimide derivative with the low LUMO energy level and excellent photoelectric property is obtained through a bromination reaction, an amidation reaction and a nucleophilic substitution reaction in a synthesis manner; and then under the condition of the normal temperature, a first reduction potential is applied through an electrochemical method to easily obtain an air stable perylene bisimide radical anion through synthesis. The method for preparing perylene bisimide ionic salt is novel, unique, simple and high in yield and can achieve industrialization, and the effect is good; and materials of this kind have high stability on air in the reduction state, the science difficult problems are solved, and practical application is facilitated.
Owner:YANSHAN UNIV
Preparation method of isomer impurities of vildagliptin
InactiveCN107311907AQuality improvementHigh purityOrganic chemistryChemical industryTrifluoroacetic anhydride
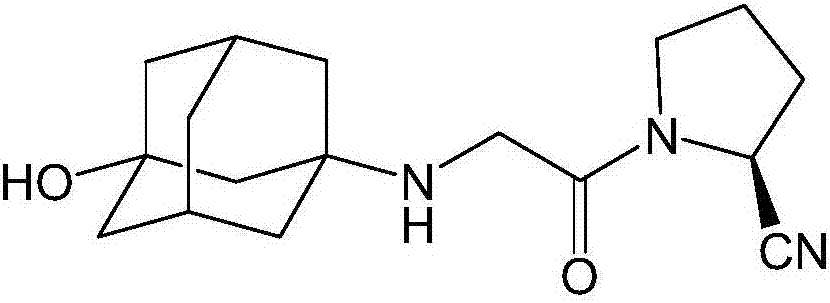
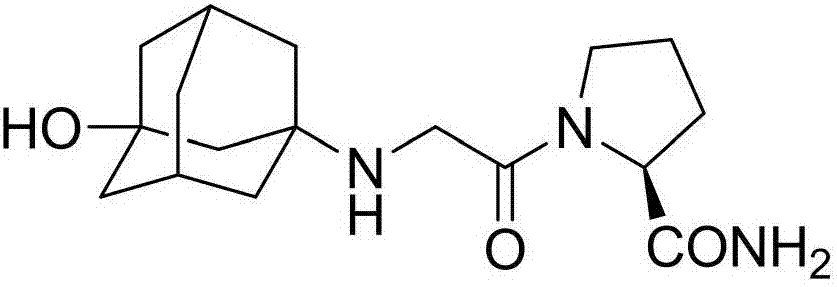
The invention discloses a preparation method of isomer impurities of vildagliptin, and relates to the technical fields of medicine and chemical industry. The preparation method comprises the steps: S1: enabling D-prolinamide (I) serving as a raw material to react with chloroacetyl chloride to obtain a reaction product (II), and then enabling the reaction product (II) to react with trifluoroacetic anhydride to obtain a reaction product (III); S2: enabling the reaction product (III) to react with 3-amino-1-adamantanol to generate a target product (IV). The preparation method disclosed by the invention has the advantages that the preparation process is simple, the operation is simple and convenient, reaction time is short, and the post-treatment purification is simple and effective, which is beneficial to industrialized production. The purity of manufactured impurities of the vildagliptin is high and is as high as 99.1% through HPLC (high-performance liquid chromatography) detection. By further studying the isomer impurities of the vildagliptin, the quality of the vildagliptin can be better controlled, and the drug safety is improved.
Owner:合肥创新医药技术有限公司
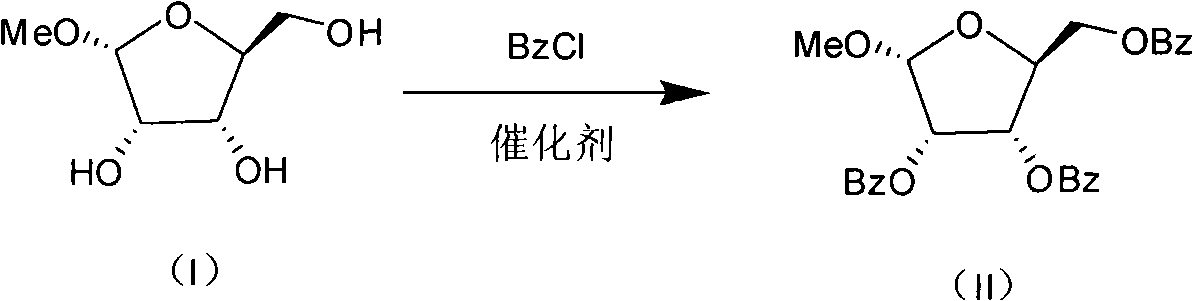
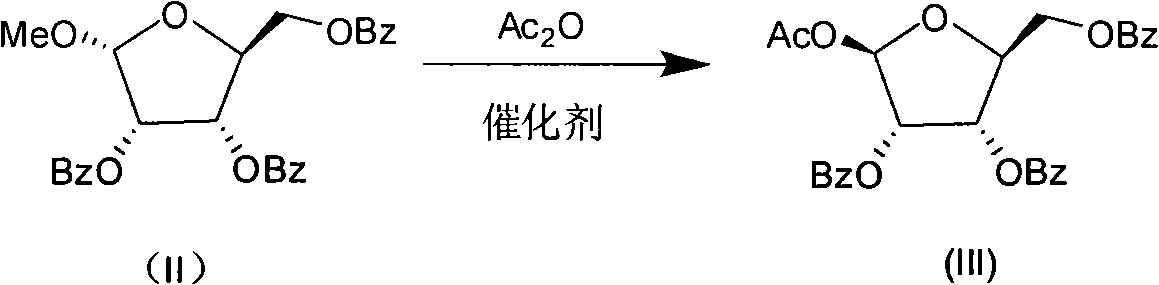
Production process for preparing 1-oxy-acetyl-2,3,5-3-benzoyl-beta-Lribofuranose
InactiveCN101274950AHigh yieldSimple post-processing purificationEsterified saccharide compoundsSugar derivativesAcetic acidBenzoyl chloride
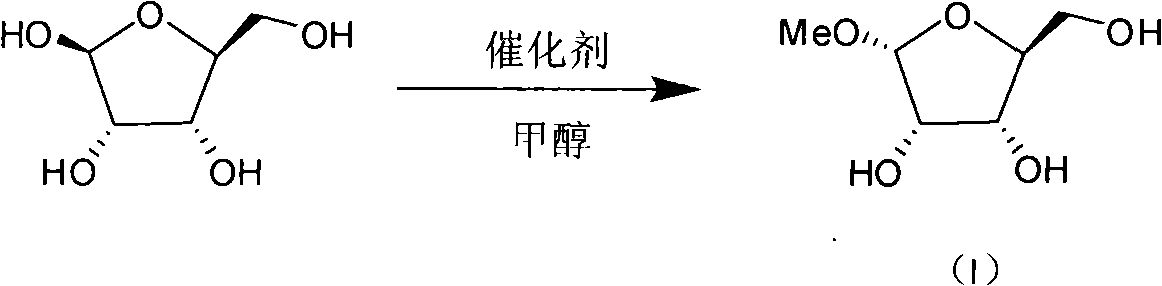
The method discloses a production technique for preparing 1-oxo-acetyl-2, 3, 5-tri-oxo-benzoyl-beta-L-ribofuranose, which has the advantages of high yield, simple post treatment and purification, etc., and is suitable for industrial production. The production technique comprises the following three steps that: step 1: L-ribose, methanol and hydrogen chloride are adopted as raw materials which are methylated and synthesized into 1-oxo-methyl-L-ribofuranose (I); step 2: 1-oxo-methyl-L-ribofuranose (I) and benzoyl chloride are adopted as raw materials which are esterified and systhesized into 1-oxo-methyl-2, 3, 5-tri-oxo-benzoyl-L-ribofuranose (II); step 3: the 1-oxo-methyl-2, 3, 5-tri-oxo-benzoyl-L-ribofuranose (II) and anhydride acetic acid are adopted as raw materials which are acetylated into 1-oxo-acetyl-2, 3, 5-tri-oxo-benzoyl-beta-L-ribofuranose (III).
Owner:CHENGDA PHARM CO LTD
Method for preparing carboxyl polymeric copper phthalocyanine nanoparticles
The invention relates to a method for preparing carboxyl polymeric copper phthalocyanine nanoparticles, and belongs to the field of high polymer nanomaterials. At present, the method for preparing the carboxyl polymeric copper phthalocyanine nanoparticles is not provided. The method comprises the following steps of: adding dilute alkali solution of carboxyl polymeric copper phthalocyanine into acid solution of a surfactant to obtain colloidal solution; and demulsifying, standing, precipitating, washing, centrifuging and freeze-drying the colloidal solution to obtain the carboxyl polymeric copper phthalocyanine nanoparticles. The method has the advantages of simple and convenient operation and safety.
Owner:BEIJING UNIV OF CHEM TECH
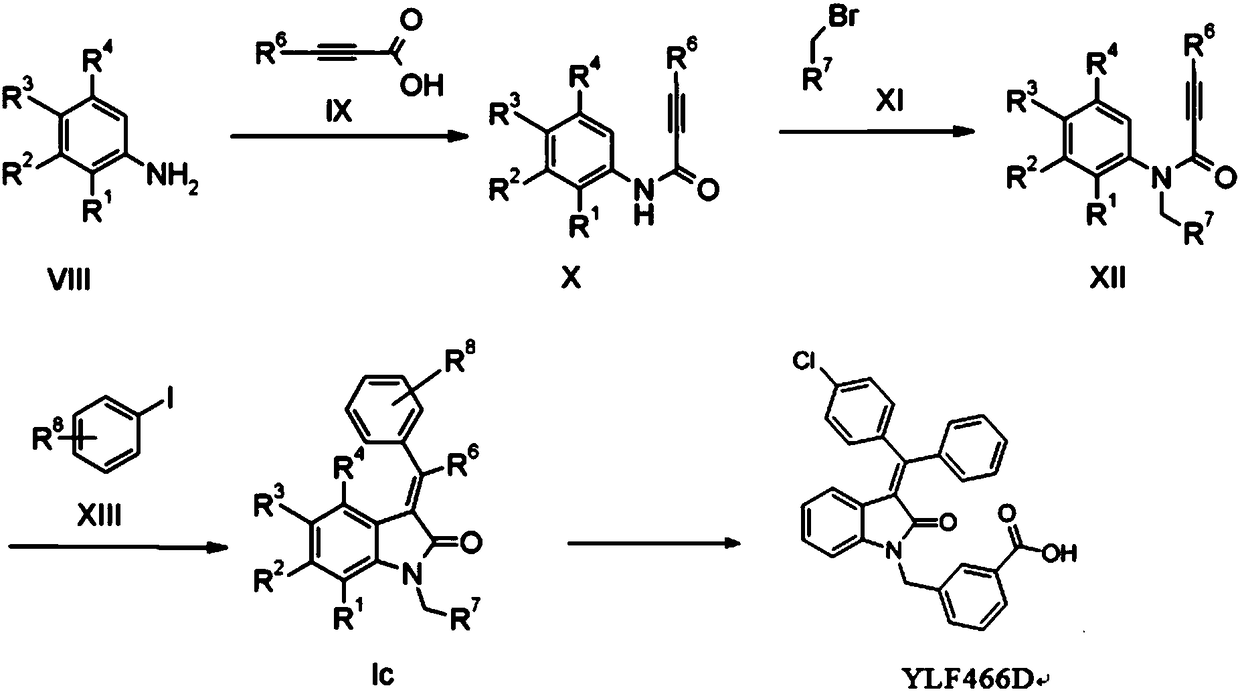
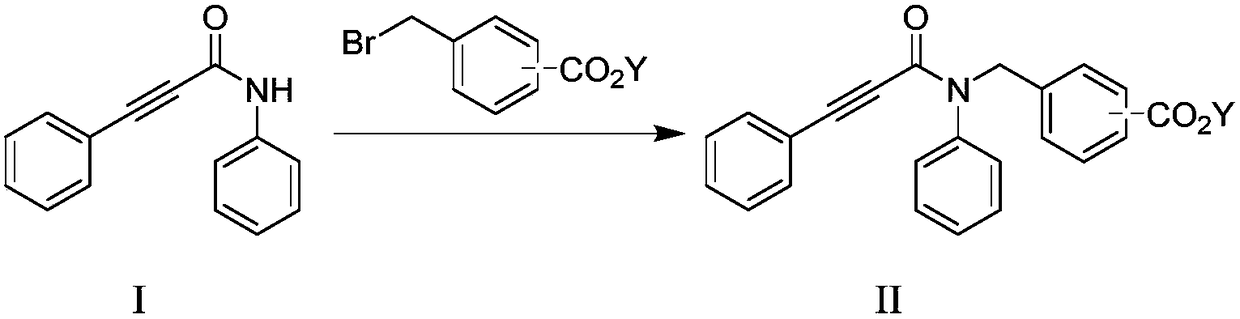
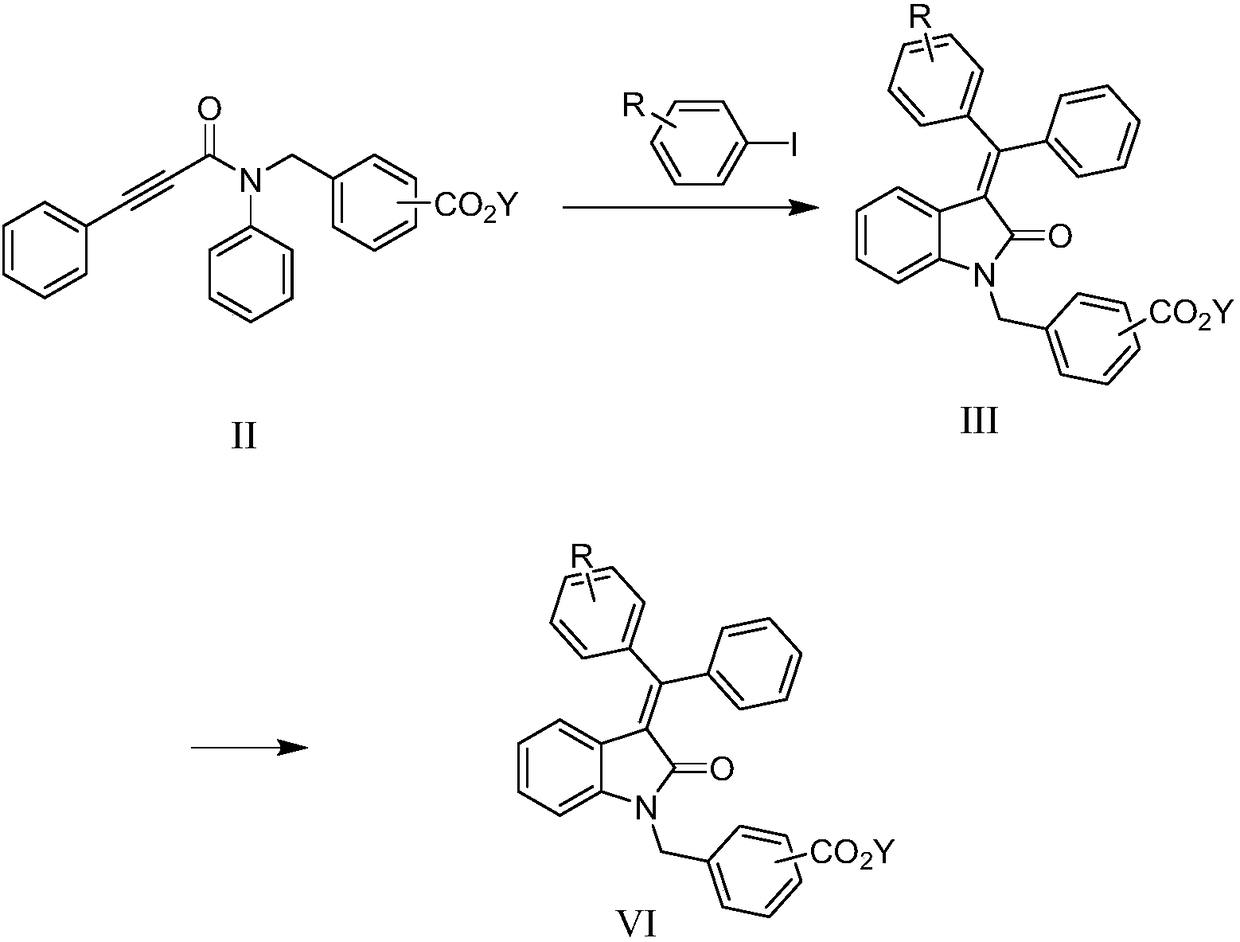
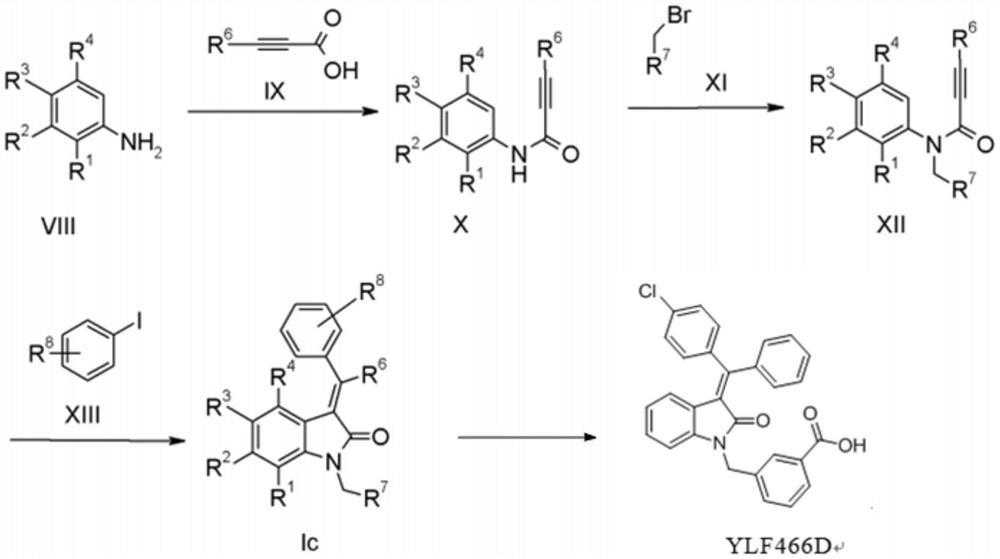
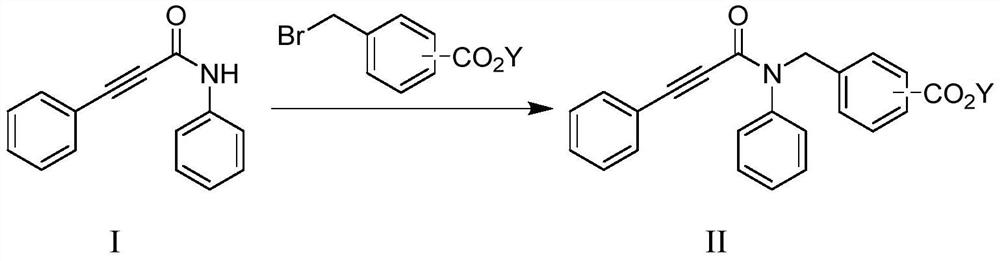
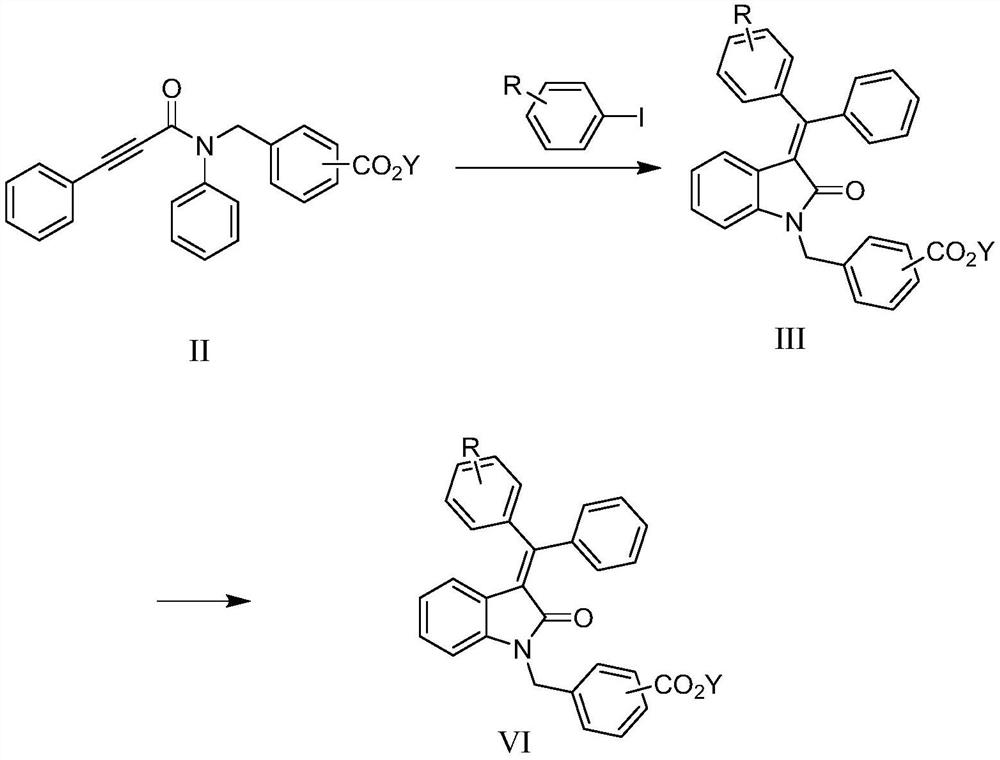
Method for preparing indolone derivative
ActiveCN108658834ALow toxicityHigh yieldOrganic compound preparationCarboxylic acid amides preparationAnilineNucleophilic substitution
The invention discloses a method for preparing an indolone derivative. The method disclosed by the invention comprises the step of subjecting phenylpropiolic acid and aniline, which serve as startingraw materials, to four steps, i.e., condensation, nucleophilic substitution, palladium catalyzing coupling and hydrolyzing sequentially, thereby obtaining the indolone derivative. According to the method disclosed by the invention, the raw materials are readily available, the operation is simple and convenient, the production efficiency is high, enlarged production is facilitated, and the productis high in total yield and easy to purify.
Owner:烟台药物研究所
Succinimide esters and methods of making, treating and detecting same
The invention provides succinimide ester as well as a preparation method, a treatment method and a detection method thereof. The preparation method of the succinimide ester comprises the following step: in the presence of triethylamine, carrying out condensation reaction on a compound A and TSTU in a solvent to obtain the succinimide ester. According to the preparation method, generation of impurities can be inhibited, the purity and the yield of the product are improved, and the difficulty of post-treatment purification is reduced; the product can be kept stable in the post-treatment process, so that the reaction yield is increased; in the detection method disclosed by the invention, a derivation method is adopted, and a detection sample is converted into a relatively stable amide or ester compound, so that the stability and accuracy of detection are ensured.
Owner:湖北华大基因研究院
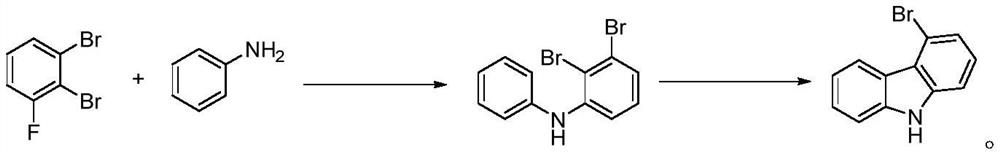
A kind of preparation method of 4-bromocarbazole
ActiveCN111960989BAvoid the reverting ring stepReduce manufacturing costOrganic chemistryPtru catalystCarbazole
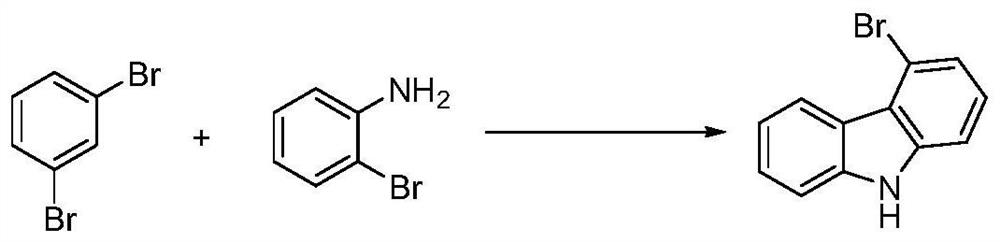
The invention discloses a preparation method of 4-bromocarbazole, which belongs to the technical field of chemistry. A kind of preparation method of 4-bromocarbazole, under the action of catalyst, ligand and alkali, o-bromoaniline and m-dibromobenzene undergo C-N coupling and C-C coupling series reaction, one-pot method and one-step synthesis 4-bromocarbazole, wherein, the m-dibromobenzene is both a reactant and simultaneously dibromobenzene is also used as a reaction solvent, and the remaining m-dibromobenzene can be reclaimed after the reaction finishes. The preparation method of the present invention uses cheap and easy-to-obtain o-bromoaniline and m-dibromobenzene as raw materials, and obtains the product 4-bromocarbazole in one step through a one-pot series reaction. Compared with the prior art, it avoids the reduction of the nitro group into a ring steps, that is, the reaction steps are shortened, which has the advantages of low production cost and environmental friendliness.
Owner:SINOSTEEL NANJING NEW MATERIALS RES INST CO LTD
A kind of method for preparing cyclocarbonate
ActiveCN109265489BHigh reaction catalytic efficiencyMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationSimple Organic CompoundsPtru catalyst
The invention discloses a method for preparing cyclocarbonate, which belongs to the technical field of organic compound preparation. The method of the invention can realize the synthesis of cyclocarbonate compounds from carbon dioxide and epoxy compounds under mild conditions. The method has mild reaction conditions, can be realized at normal temperature and pressure, has high catalyst efficiency and wide substrate universality. The technical scheme of the present invention is as follows: in the presence of a quaternary ammonium salt, carbon dioxide and an epoxy compound are used to synthesize a cyclocarbonate compound through the action of a diglycolamine-bridged bisaryloxy rare earth-zinc heterobimetallic complex catalyst.
Owner:SUZHOU UNIV
Preparation method of reduced ionic salt of peryleneimide and its derivatives
ActiveCN105732623BImprove thermal stabilityGood chemical stabilityOrganic chemistryType transformationOrganic base
The invention discloses the reduction-state ionic salt of perylene bisimide and a derivative thereof as well as a preparation method. The preparation method comprises the following steps: with perylene-3,4,9,10-tetracarboxylic acid dianhydride as a raw material, synthesizing a perylene bisimide derivative with low LUMO energy level and excellent photoelectric performance through a bromination reaction, an amidation reaction and a nucleophilic substitution reaction; and synthesizing the perylene bisimide monovalent ionic salt with stable environment relatively easily according to the benzoquinone type transformation mechanism in an alkaline condition (organic base such as triethylamine, and inorganic base such as potassium carbonate). The method for synthesizing the perylene bisimide ionic salt is novel, unique and simple and realizes high yield; industrialization can be implemented while the effect is good; and moreover, the material in a reduction state responds to metal ions and acids different in oxidability.
Owner:YANSHAN UNIV
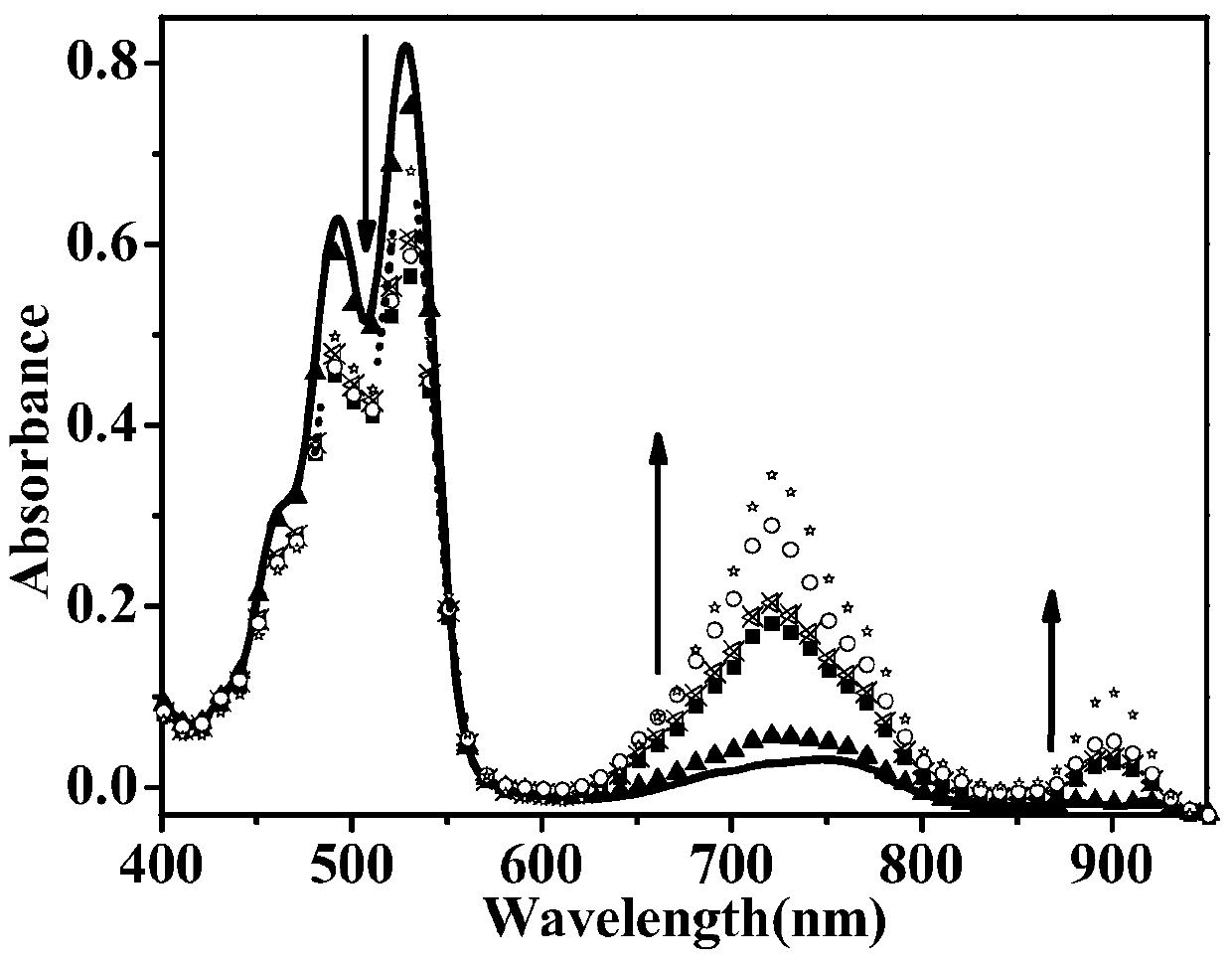
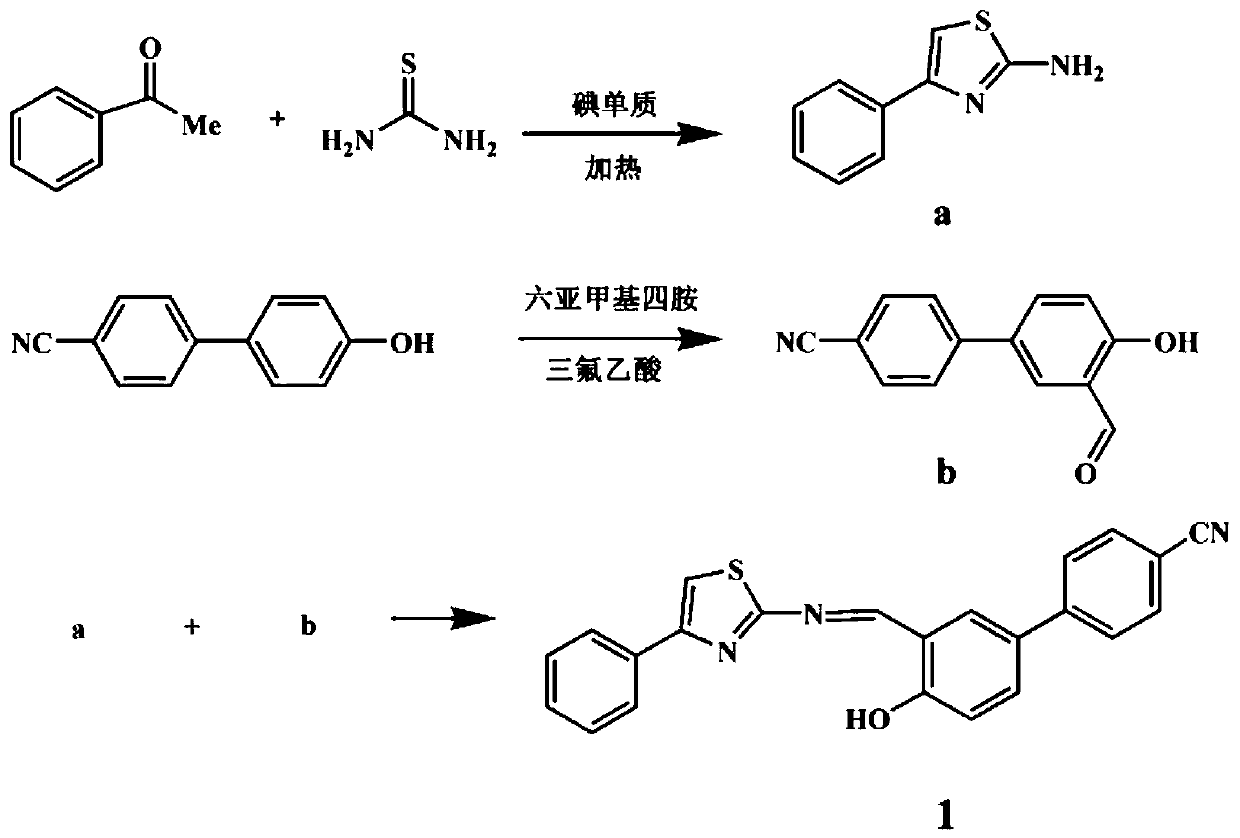
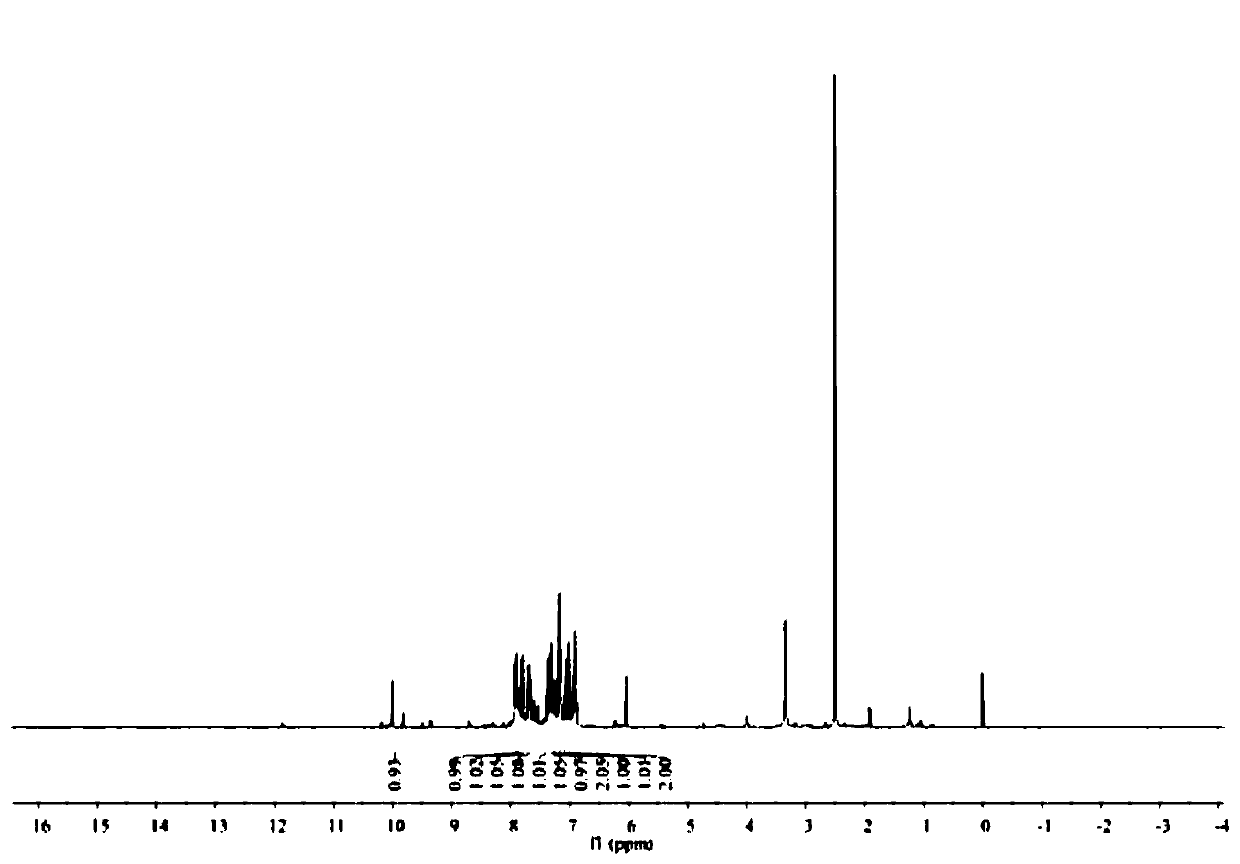
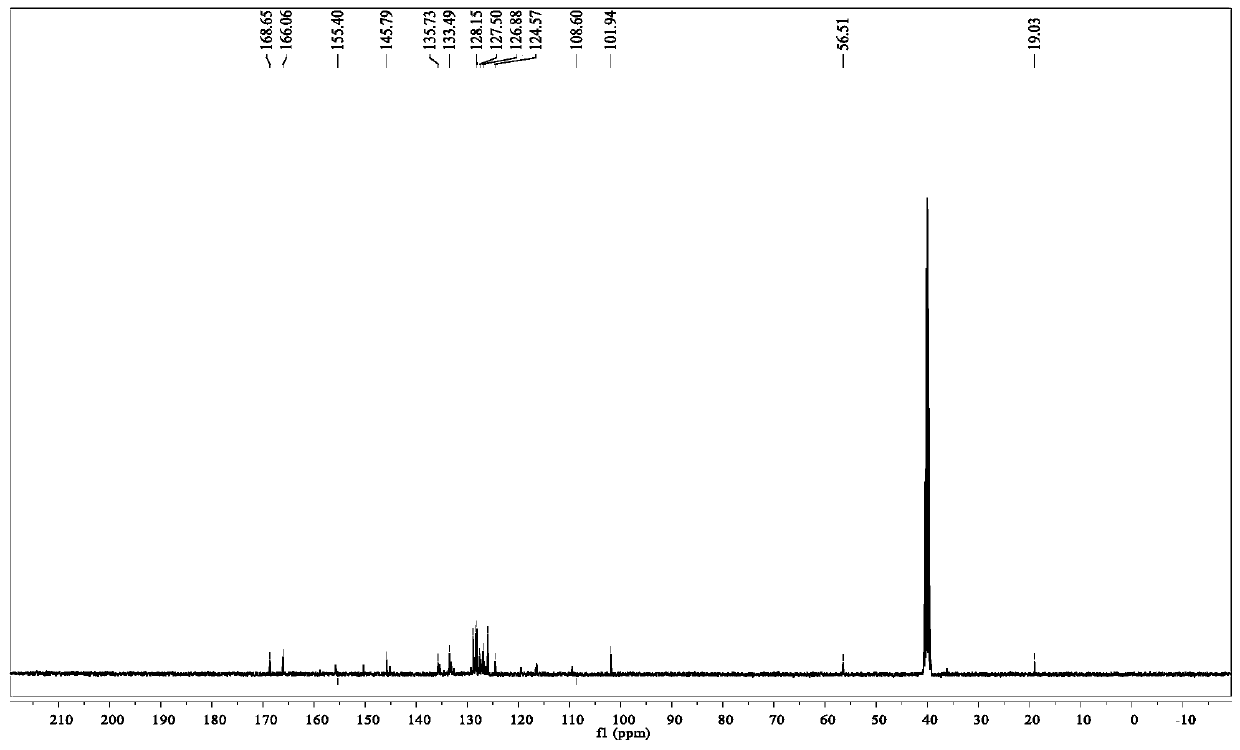
A fluorescent sensing material based on phenylthiazole and p-cyanobiphenol, its preparation method and application
ActiveCN107382901BStable structureSynthetic conditions are mildOrganic chemistryFluorescence/phosphorescencePtru catalystMeth-
The invention relates to a fluorescence sensing material based on phenylthiazole and p-cyanodiphenol, as well as a preparation method and application of the fluorescence sensing material, and belongs to the technical field of a chemical fluorescence sensing material. The preparation method comprises the following steps: taking 2,6-dihydroxymethyl p-methylphenol and thiosemicarbazide as basic raw materials, performing reaction on the premise of taking acetophenone and thiourea as basic raw materials and taking an iodine elementary substance as a catalyst to prepare 2-amino-4-phenylthiazole; preparing 3-formyl-4-hydroxybenzyl cyanide by taking p-cyano-phenol as a basic raw material and under the existence of hexamethylenetetramine; performing nucleophilic reaction to combine the 2-amino-4-phenylthiazole and the 3-formyl-4-hydroxybenzyl cyanide to obtain the fluorescence sensing material. Due to the own conjugation structure, the fluorescence sensing material prepared by the method emits orange red fluorescence, has sensitive selective identification performance on heavy metal Fe<3+> and shows fluorescence quenching, the response time is short, the change of a fluorescence signal is visible to naked eyes under an ultraviolet lamp, and the interference of other common metal ions is small.
Owner:JIANGSU UNIV
A kind of preparation method and application of naphthylamine compound and its salt
ActiveCN111559991BPrevent invasionInhibit transferOrganic active ingredientsOrganic chemistryA-NaphthylamineChemical compound
The invention discloses a preparation method and application of a naphthylamine compound and its salt, a method for synthesizing a naphthylamine compound with 4-formylphenol as a substrate, and simultaneously provides its hydrochloride and phosphate salts. The preparation method has the characteristics of short synthesis route, cheap and easy-to-obtain raw materials, simple post-treatment and purification, and high overall yield. It is of great significance to expand the types of naphthylamine derivatives and to study them in biology and medicine. At the same time, this application has further studied the anti-tumor effect and mechanism of the obtained naphthylamine compounds and their hydrochloride and phosphate salts. The results show that the naphthylamine compounds and their hydrochloride and phosphate salts have: (1) inhibition Proliferation, growth or migration of cancer cells; (2) Promote apoptosis of cancer cells or reduce migration or invasion of cancer cells, and also act as an inhibitor of STAT3 tyrosine phosphorylation.
Owner:HENAN RADIOMEDICAL SCI & TECH CO LTD
A kind of preparation method of (s)-glycidyl phthalimide
ActiveCN110885325BSimple post-processing purificationHigh yieldOrganic chemistry methodsPolymer sciencePtru catalyst
Owner:XINFA PHARMA
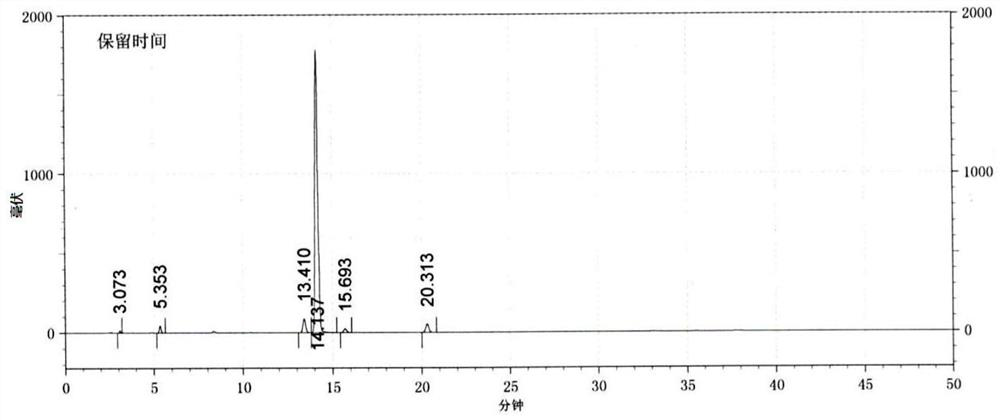
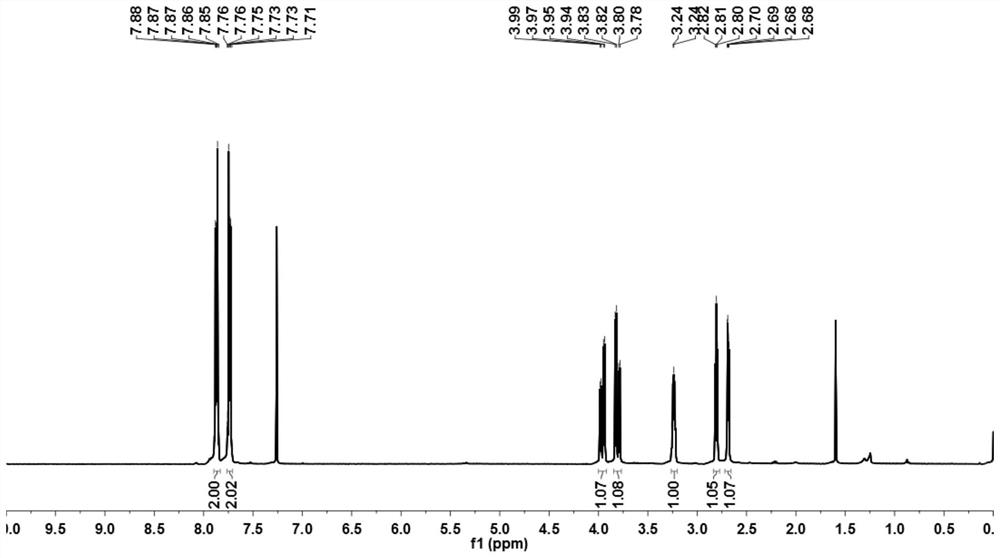
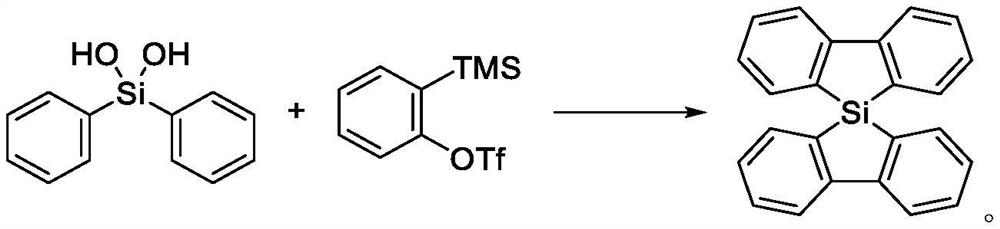
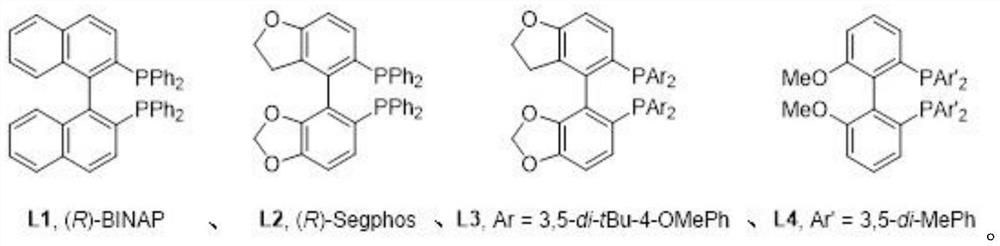
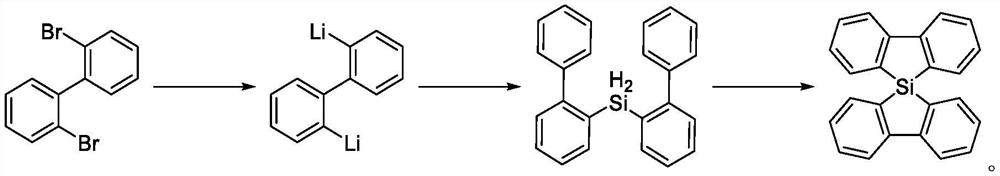
Method for synthesizing 5, 5-spirosilafluorene through C-H arylation cyclization reaction
PendingCN113956278AImprove stabilityLower activation energySilicon organic compoundsChemical recyclingPtru catalystPhenyl group
The invention discloses a method for synthesizing 5, 5-spirosilafluorene. The method comprises the following steps: taking 2-(trimethylsilyl)phenyl trifluoromethanesulfonate and diphenyldihydroxysilane as initial raw materials, in the presence of a solvent and under the action of a catalyst, a ligand and an alkali, carrying out C-H arylation cyclization reaction, and synthesizing the 5, 5-spirosilafluorene by a one-pot method. According to the synthesis method, the adopted ligand is an aryl phosphine derivative, the aryl phosphine derivative and rhodium salt can generate a rhodium intermediate, the reaction efficiency of the catalyst is effectively improved, continuous and rapid reaction is achieved, the reaction condition is mild, a one-pot two-step coupling cyclization mode is adopted, and through the C-H arylation cyclization process, a spirosilafluorene structure containing two five-membered silicon heterocycles is constructed, reaction steps are shortened, expensive 2-bromodiphenyl or 2, 2 '-dibromodiphenyl is prevented from being used as a reaction raw material, and a new synthesis route is provided for 5, 5-spirosilafluorene.
Owner:SINOSTEEL NANJING NEW MATERIALS RES INST CO LTD
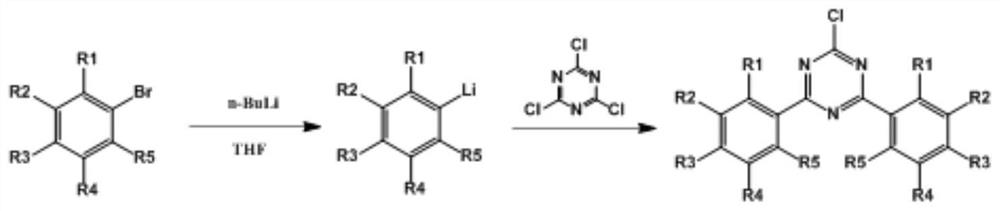
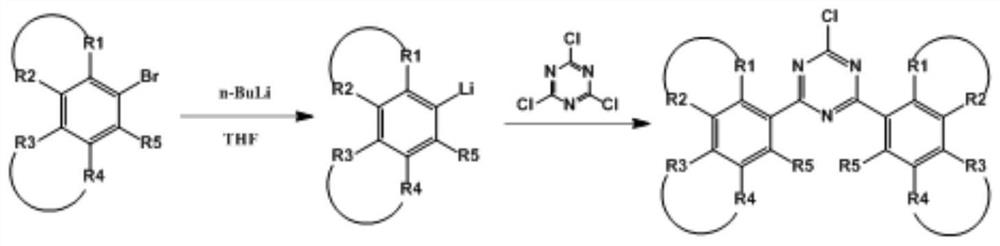
Triazine compound and preparation method thereof
PendingCN113024477AEasy to operateLess side effectsOrganic chemistryChemical synthesisBiochemical engineering
The invention belongs to the technical field of organic chemical synthesis, and particularly relates to a triazine compound and a preparation method thereof. Most of existing triazine compounds and derivatives thereof are prepared through ring closing or an aromatic halogenated compound Grignard method, the reaction process is complex, and manpower and material resources are wasted. The invention provides the triazine compound. The general formula of the compound is shown in the specification, or reaction raw materials are cheap and easy to obtain, the reaction operation is simple, side reactions are few, and the yield is high.
Owner:陕西维世诺新材料有限公司
Preparation method of (S)-glycidyl phthalimide
ActiveCN110885325ASimple post-processing purificationHigh yieldOrganic chemistry methodsPolymer sciencePtru catalyst
The invention provides a preparation method of (S)-glycidyl phthalimide (II). Phthalimide (III) and (S)-1-substituted epoxypropane (IV) which are used as raw materials react under the action of a catalyst to generate 2-((S)-3-substituted-2-hydroxypropyl)isoindoline-1,3-dione (V), and the 2-((S)-3-substituted-2-hydroxypropyl)isoindoline-1,3-dione (V) is subjected to a cyclization reaction to obtainthe (S)-glycidyl phthalimide (II). The method has the advantages of cheap and easily available raw materials, easiness in realization of reaction conditions, low cost, short reaction route, simplicity in operation, simplicity in post-treatment, few side reactions, high yield and high purity of the target product, and suitableness for industrial production.
Owner:XINFA PHARMA
Synthesis method of 3-n-butyl-1-(3H)-isobenzofuranone
The invention discloses a method for synthesizing 3-n-butyl-1-(3H)-isobenzofuranone. The method comprises the following steps: synthesizing 3-n-butyl-1-(3H)-isobenzofuranone; mixing a Grignard reagentwith Lewis acid; adding o-formylbenzoic acid into the reaction system, adjusting the pH value to be acidic by an acidic reagent, extracting the reaction product by using an organic solvent, and concentrating the reaction product to remove the organic solvent to obtain 3-n-butyl-1-(3H)-isobenzofuranone. The method is simple to operate, does not need a complicated post-treatment process, and has the advantages of high product purity, low cost and certain industrial prospects.
Owner:JIANGSU SIMCERE PHARMA
Preparation method for health product resveratrol
InactiveCN107337584AGood cycle repeatabilitySimple post-processing purificationOrganic compound preparationGroup 5/15 element organic compoundsImpurityPollution
The present invention provides a preparation method of health care product resveratrol. 1. The deprotection method used in the present invention is green, environmentally friendly and pollution-free. economic value. 2. The catalyst used in the present invention has good cycle repeatability, which can reach more than 50 times. 3. The method involved in the present invention requires simple operation, mild reaction conditions, strong specificity, simple post-treatment and purification of the product, and is suitable for industrial production. 4. The resveratrol prepared by the method involved in the present invention has no impurities and extremely high purity.
Owner:安徽世华化工有限公司
A kind of preparation method of indolinone derivative
ActiveCN108658834BRaw material stabilityEasy to operateOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryAniline
Owner:烟台药物研究所
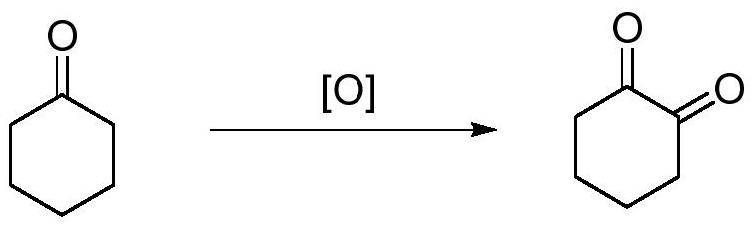
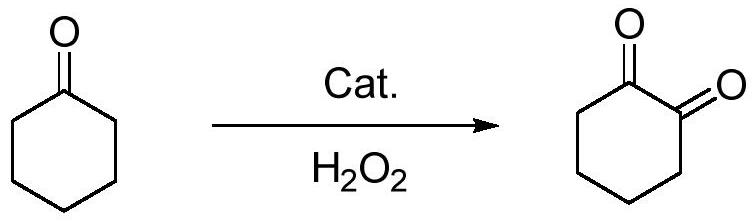
A kind of preparation method of 1,2-cyclohexanedione
ActiveCN112441891BGreen Cleaning ProcessMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsCyclohexanonePtru catalyst
The invention discloses a preparation method of 1,2-cyclohexanedione, which belongs to the technical field of chemistry. The preparation method is: under the protection of nitrogen, stir cyclohexanone, catalyst and ligand evenly, add hydrogen peroxide dropwise at room temperature, and the dropwise addition time is 1-2h. ~6h, after the reaction is finished, wash and separate the liquids, and rectify the organic layer under reduced pressure to obtain 1,2-cyclohexanedione, wherein the catalyst is a copper salt catalyst, and the ligand is a β-diketoimine ligand body. Compared with the reported methods, this method uses cheap and easily available hydrogen peroxide as an oxidant, the by-product is water, the reaction system is green and clean, cyclohexanone is used as both a reactant and a solvent during the reaction, the reaction conditions are mild, and the operation is convenient. The method has high yield, green and clean process, and provides a new method for the synthesis of 1,2-cyclohexanedione.
Owner:SINOSTEEL NANJING NEW MATERIALS RES INST CO LTD
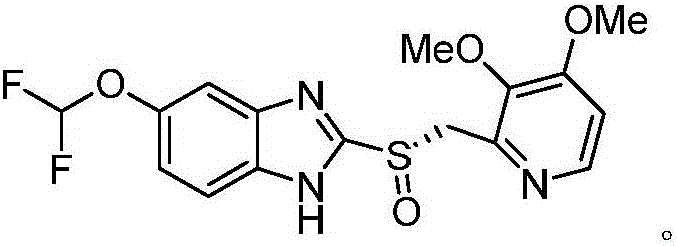
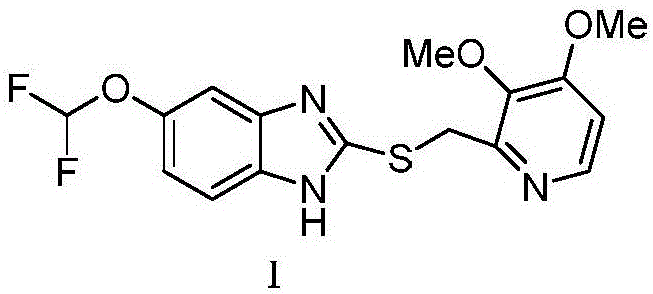
Synthesis process of (L)-pantoprazole
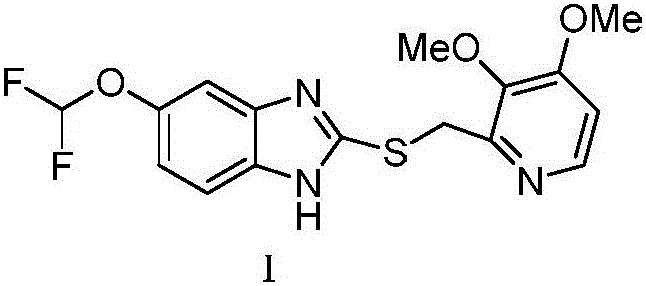
The invention discloses a synthesis process of (L)-pantoprazole. The synthesis process comprises the following steps: 1) in the presence of iodine and alkali, 5-(difluoromethoxy)-2-mercapto-1H-benzimidazole and 2-(chloromethyl)-3,4-dimethoxypyridine hydrochloride are subjected to stirring reaction to obtain the pantoprazole thioether as shown in the formula I, and the pantoprazole thioether represented by the formula I shown in the specification and hydrogen peroxide are subjected to oxidation reaction in the presence of (R)-(-)-binaphthol phosphate to obtain the (L)-pantoprazole. The synthesis process is good in stereo selectivity, high in the product yield, mild in the conditions, simple in the steps and easier in after-treatment and purification.
Owner:QINGDAO YUNTIAN BIOTECH
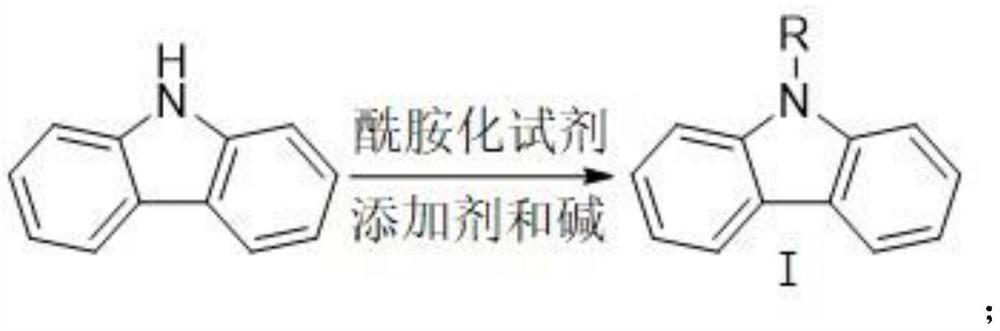
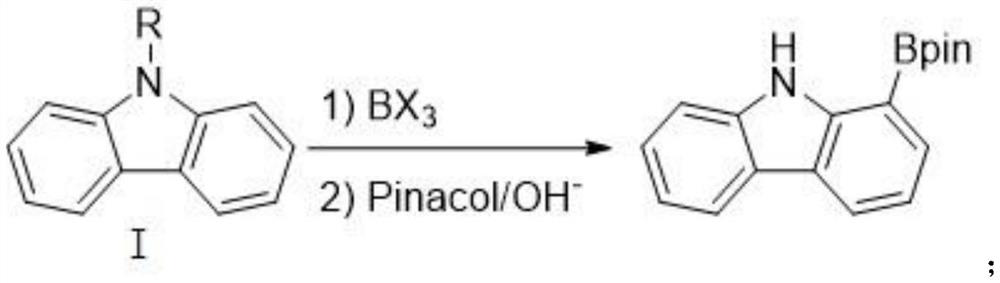
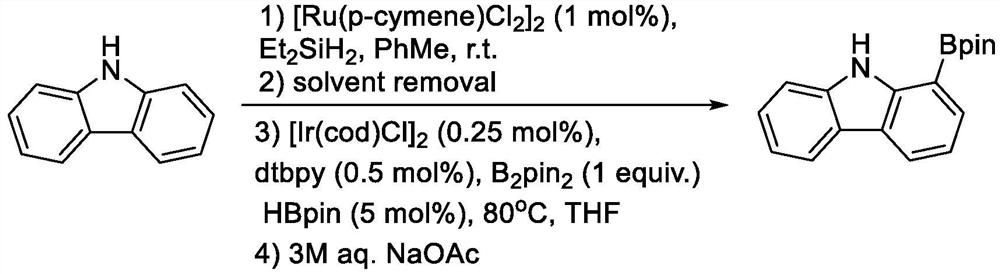
Method for synthesizing 1-carbazole-boronic acid pinacol ester through ortho-oriented boronation of amide
PendingCN113831360AReduce the temperatureReduce usageGroup 3/13 element organic compoundsPtru catalystOrtho position
The invention discloses a method for synthesizing 1-carbazole-boronic acid pinacol ester through ortho-oriented boronation of amide. The method comprises the following steps: (1) carrying out amidation reaction on carbazole and an amidation reagent under the action of a catalyst and alkali to obtain an N-acyl carbazole intermediate I; and (2) adding boron trihalide (BX3) and an organic solvent, carrying out boronation reaction, then adding pinacol and an alkali, carrying out esterification and deprotection reaction to obtain the 1-carbazole-boronic acid pinacol ester. According to the synthesis method disclosed by the invention, carbazole which is cheap and easy to obtain is used as a raw material, and the 1-carbazole-boronic acid pinacol ester is prepared by creatively adopting a carbazole 9-site N-acylation and oriented ortho-boronation method, so that not only is the influence of carbazole N-H on a catalytic reaction avoided, but also a strong oriented effect is achieved, the selectivity of oriented electrophilic boronation of a carbazole C1 site is increased, a guiding group is easy to remove in situ, a lithium reagent and transition noble metal iridium and palladium catalysts are not used, the reaction conditions are mild, and post-treatment purification is simple.
Owner:SINOSTEEL NANJING NEW MATERIALS RES INST CO LTD
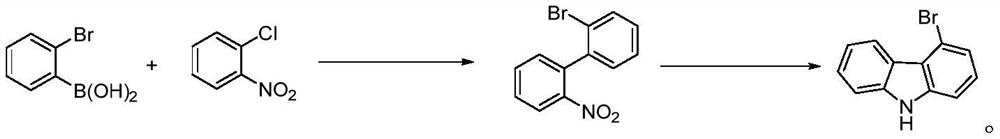
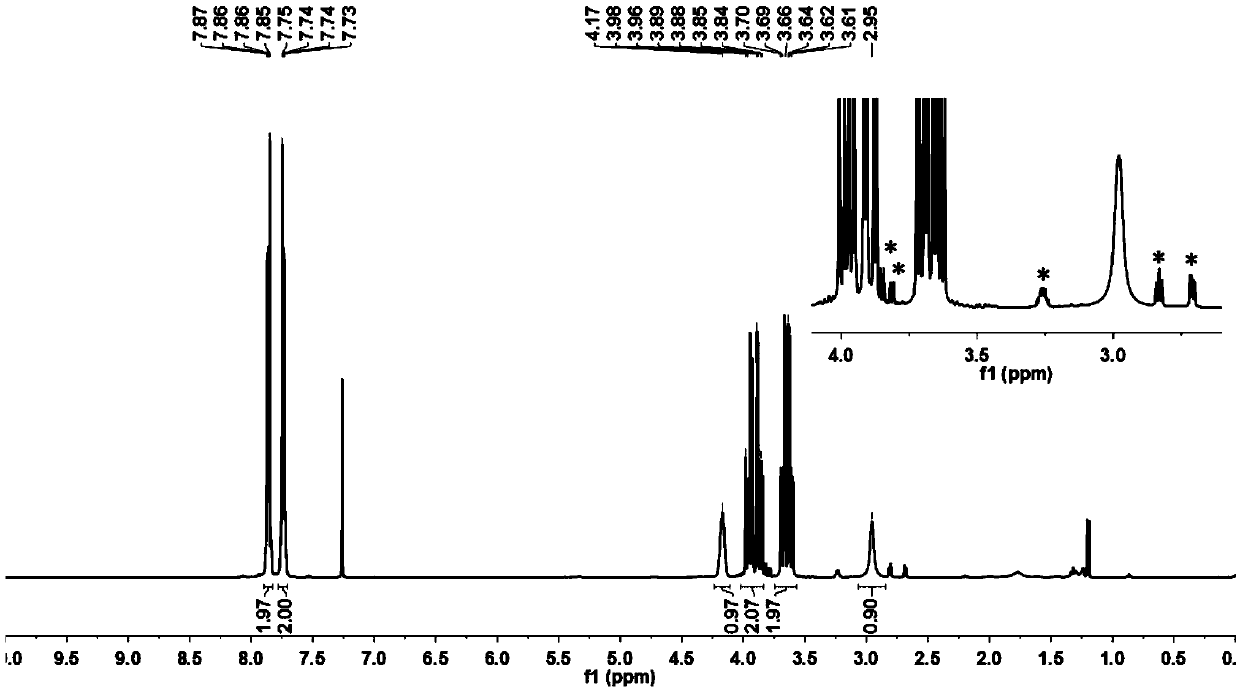
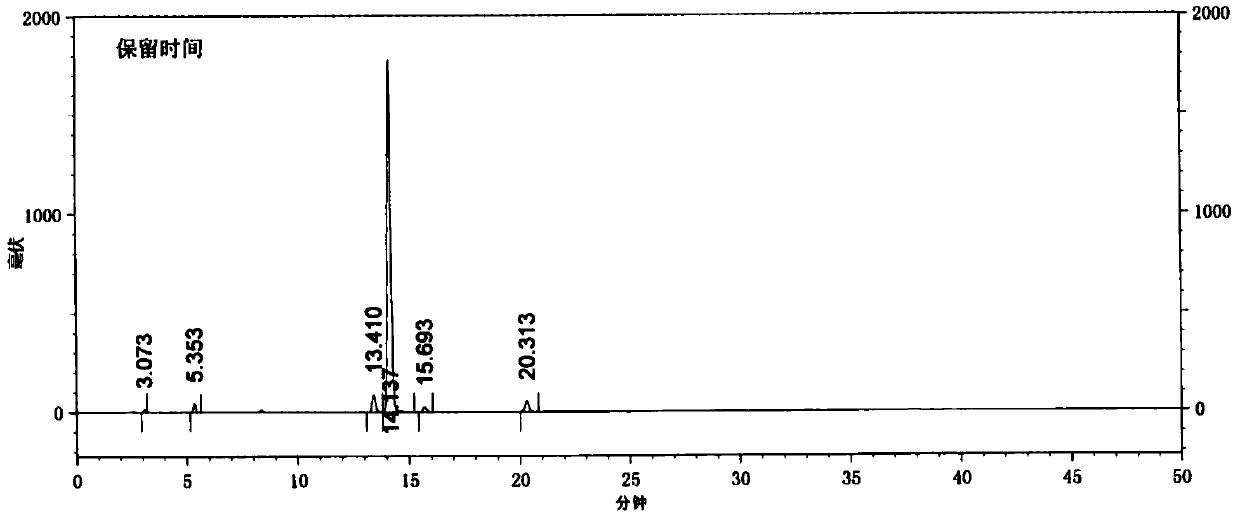
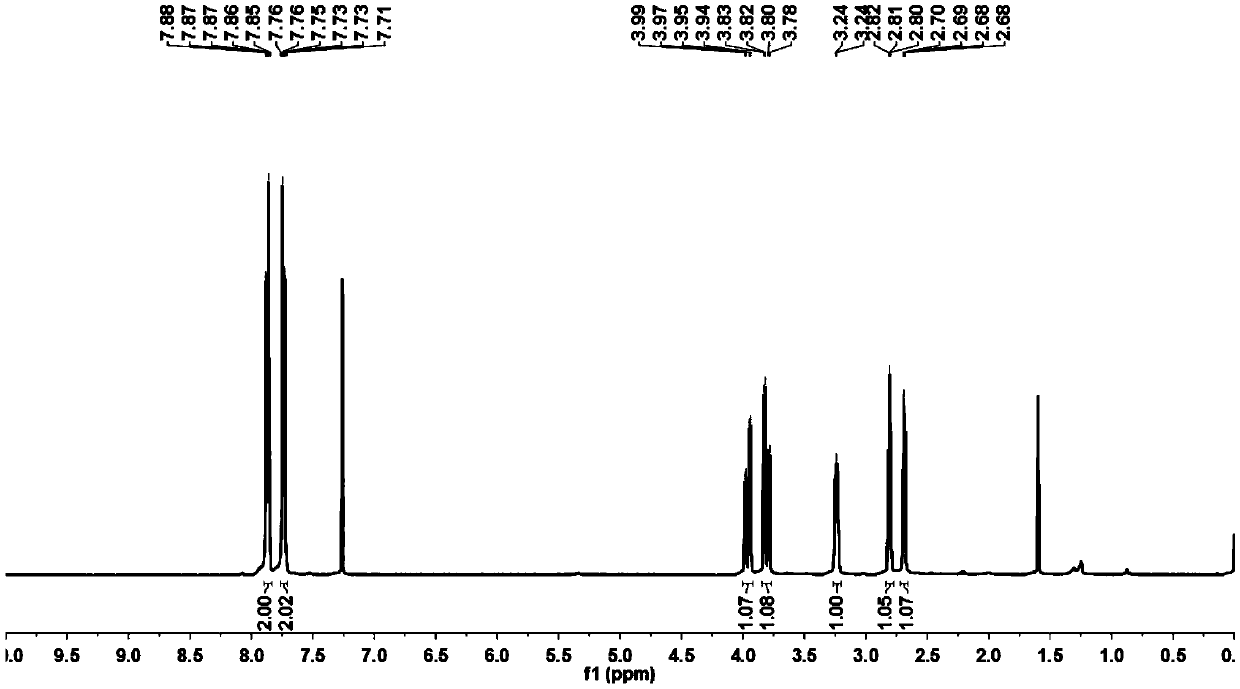
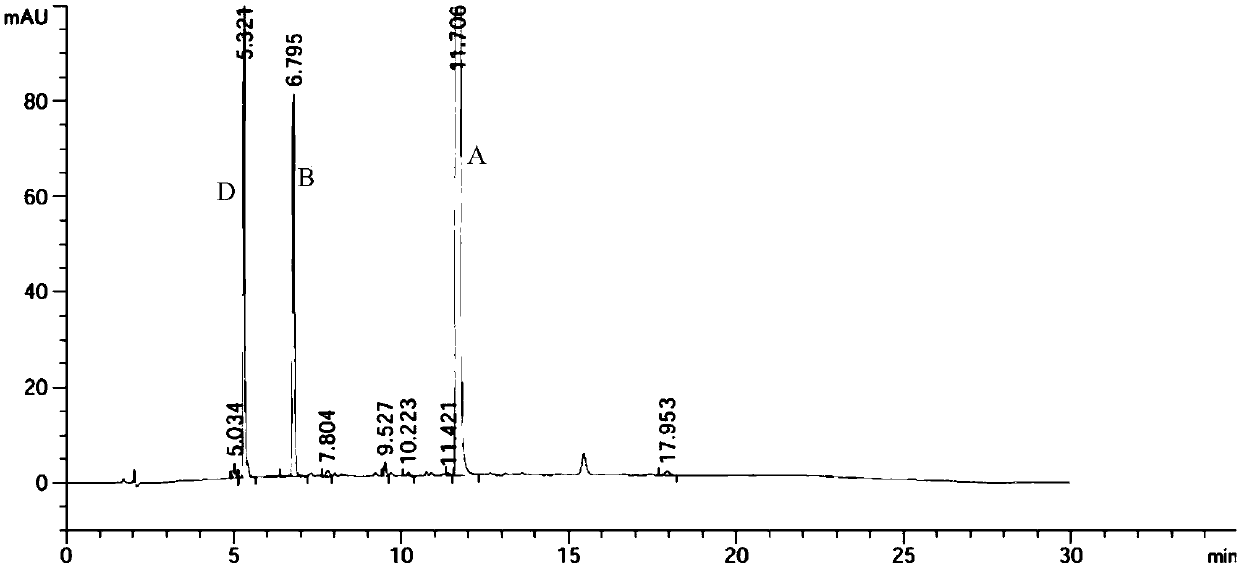
A kind of preparation method of indolo[2,3-a]carbazole
ActiveCN112250685BRaw materials are easy to getShort reaction stepsOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystOrganic base
The invention discloses a preparation method of indolo[2,3-A]carbazole, which belongs to the technical field of chemistry. The preparation method is as follows: under the action of catalyst, ligand and base, o-dibromobenzene and o-phenylenediamine undergo C-N coupling and C-C coupling series reaction to obtain indolo[2,3-A ] carbazole; Catalyst is palladium salt catalyst and copper salt catalyst, and wherein, palladium salt catalyst comprises palladium chloride, palladium acetate, four (triphenylphosphine) palladium, palladium trifluoroacetate, palladium acetylacetonate or three (dibenzylidene Acetone) dipalladium, copper salt catalyst includes cuprous iodide, cuprous chloride, cuprous bromide, cuprous oxide, copper sulfate, cupric chloride, copper acetate or copper trifluoroacetate; ligand is phosphine ligand ; The base is an organic base or an inorganic base. The invention uses cheap and easy-to-obtain o-phenylenediamine and o-dibromobenzene as raw materials, and compared with the prior art, the reaction steps are short, the raw materials are easy to obtain, the production cost is low, the environment is friendly, and the like.
Owner:SINOSTEEL NANJING NEW MATERIALS RES INST CO LTD
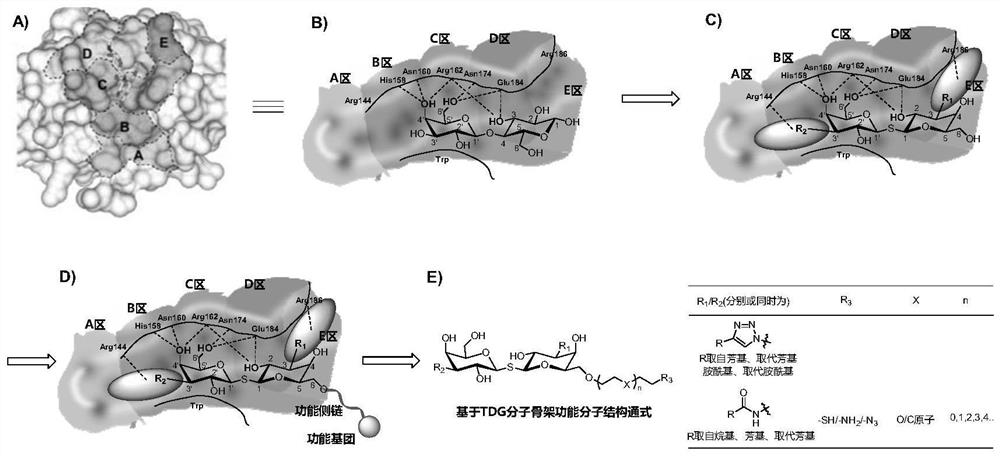
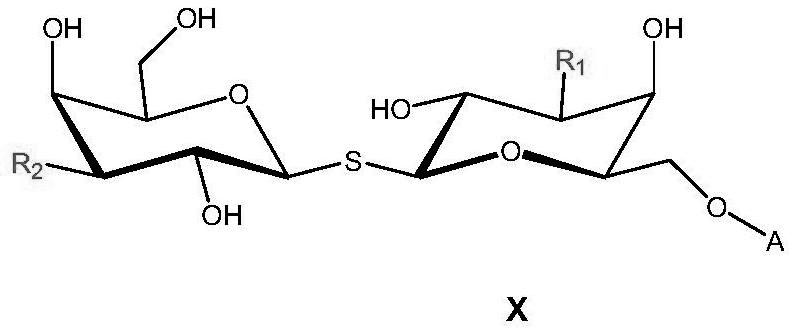
A kind of functional carbohydrate molecule based on tdg molecular skeleton and preparation method thereof
ActiveCN112745372BEfficient constructionDegenerate reaction stepsSugar derivativesAntipyreticCarboxyl radicalProtein target
The present invention designs a saccharide compound whose structure is shown in formula X, wherein R 1 , R 2 independently selected from substituted amido, substituted triazolyl, substituted amino; R 3 Selected from sulfhydryl, azide, amino, carboxyl; A is or -C m H 2m R 3 , X is selected from oxygen atoms; n is selected from 0, 1, 2, 3, 4, 5, 6, 7; m is selected from 2, 3, 4, 5, 6, 7, 8, 9, 10. The present invention adopts the synthesis strategy of "first side chain derivatization, then glycosylation coupling", realizes the side chain derivatization modification of the TDG molecular skeleton, and realizes the efficient synthesis of compounds. The invention utilizes the recognition and binding of TDG sugar ligand molecules to the target protein, and plays the role of the target head that recognizes and binds to the target protein, and uses it as the target head molecule to construct functional targeting molecules, which has wide application in the fields of tumor detection, tumor immunity and the like prospect.
Owner:SHENZHEN INST OF ADVANCED TECH
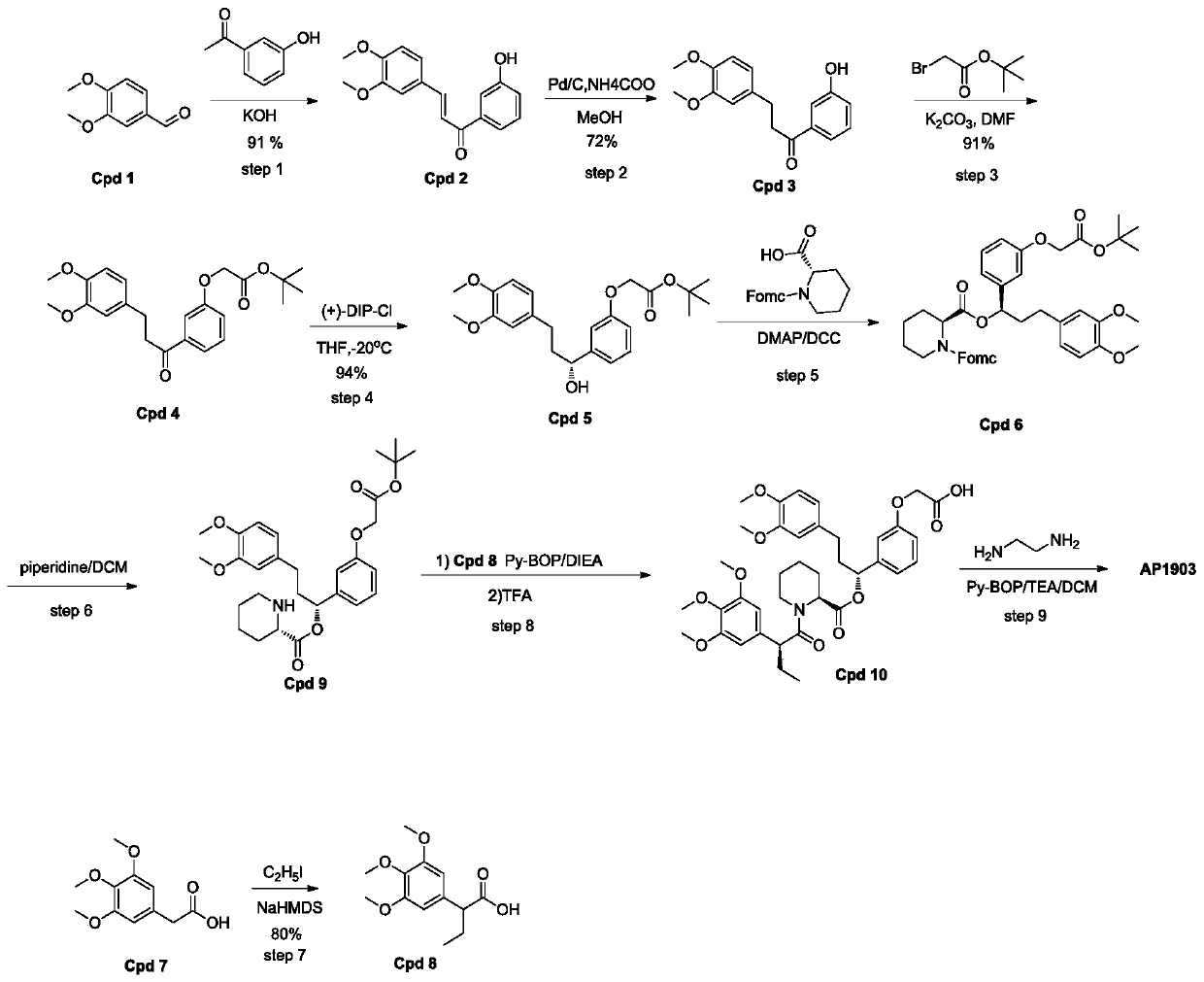
Synthetic process of homodimer of FKBP ligand
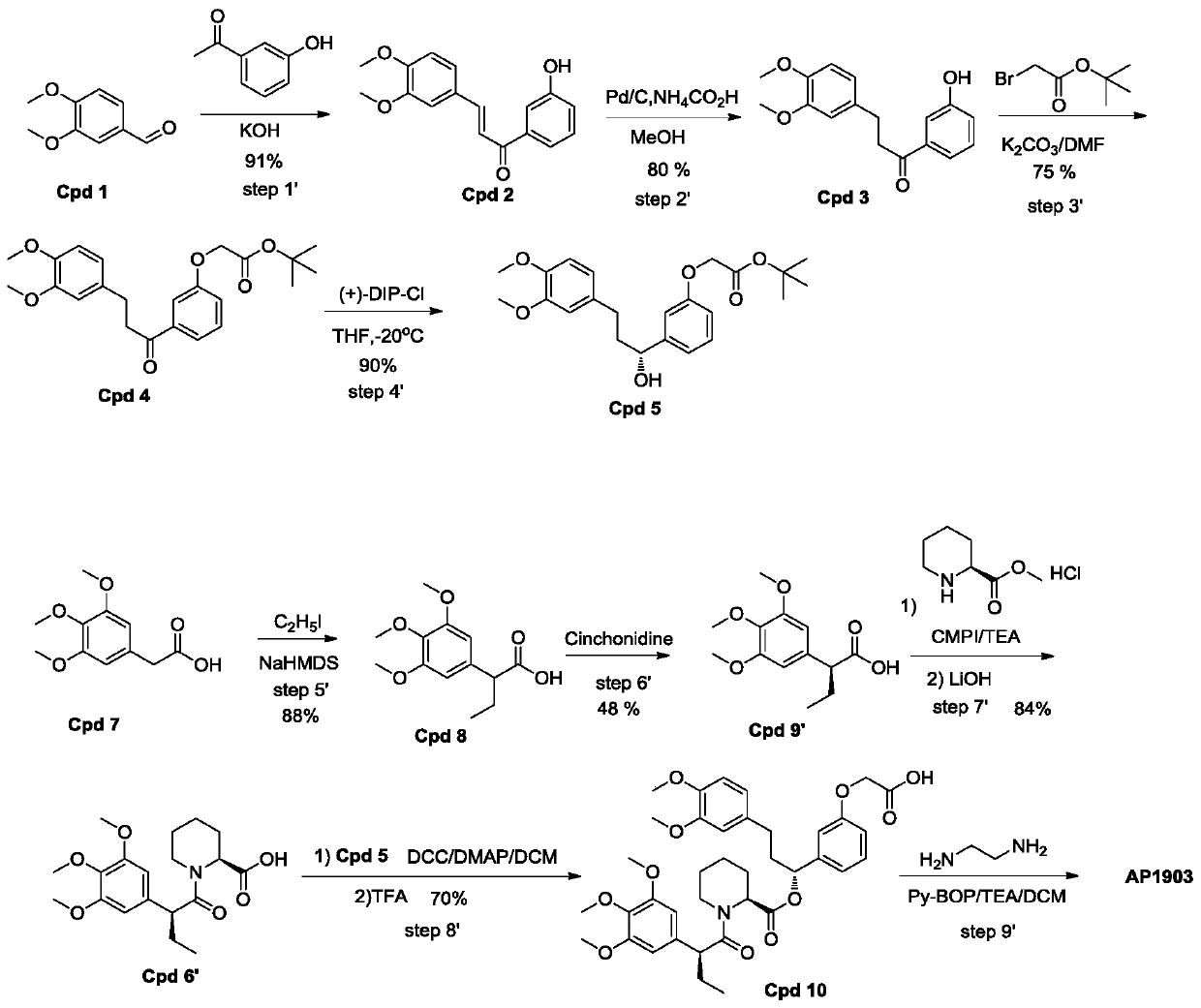
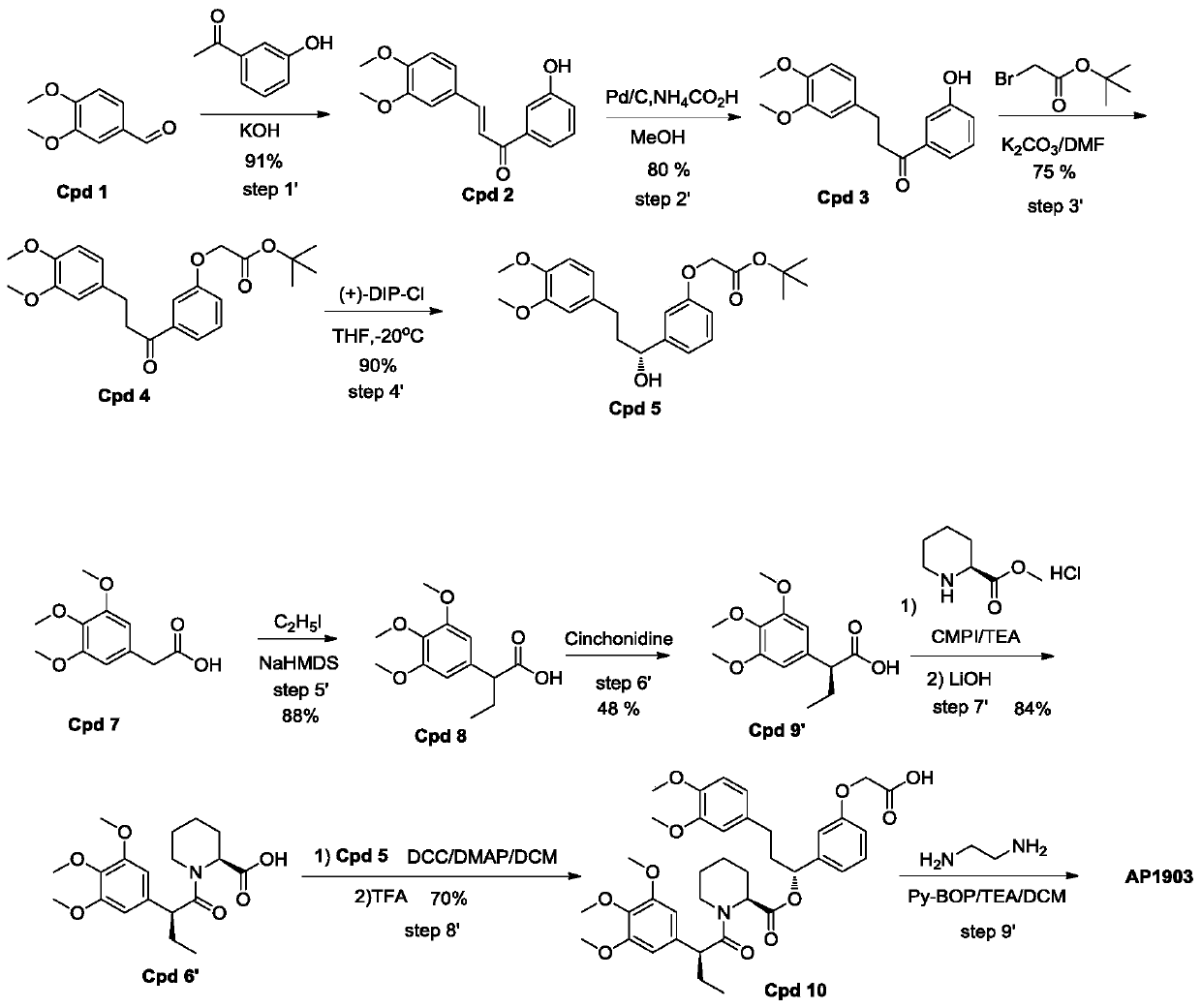
ActiveCN109734655APost-processing is simpleFacilitate separation and purificationOrganic chemistryMethyl formateChemistry
The invention discloses a synthesis process of homodimer of FKBP ligand and belongs to an improved synthesis process of AP1903, wherein the homodimer of FKBP ligand refers to AP1903. According to theoptimized route of the synthesis process chooses, (S)-piperidine-2-methyl formate is connected to the compound Cpd9' as selected, an acid Cpd6' obtained after ester hydrolysis is easier to be purified by post-treatment purification, and precipitation or extraction treatment can be chosen by the post-treatment purification. Although the total reaction steps of the synthetic route in the prior literature are the same as the optimized route of the invention, the synthetic route in the prior literature belongs to the vertical route, and the post-treatment of the fifth, sixth and eighth steps requires column chromatography, thereby leading to a higher research and development cost. The optimized AP1903 synthetic route belongs to the parallel route, and the post-treatment is simpler and more advantageous for separation and purification, so that the cost of research and development is relatively low and the economic benefit is obviously superior to that of the synthetic route in the prior art.
Owner:都创(上海)医药科技股份有限公司
A homodimer synthesis process of fkbp ligand
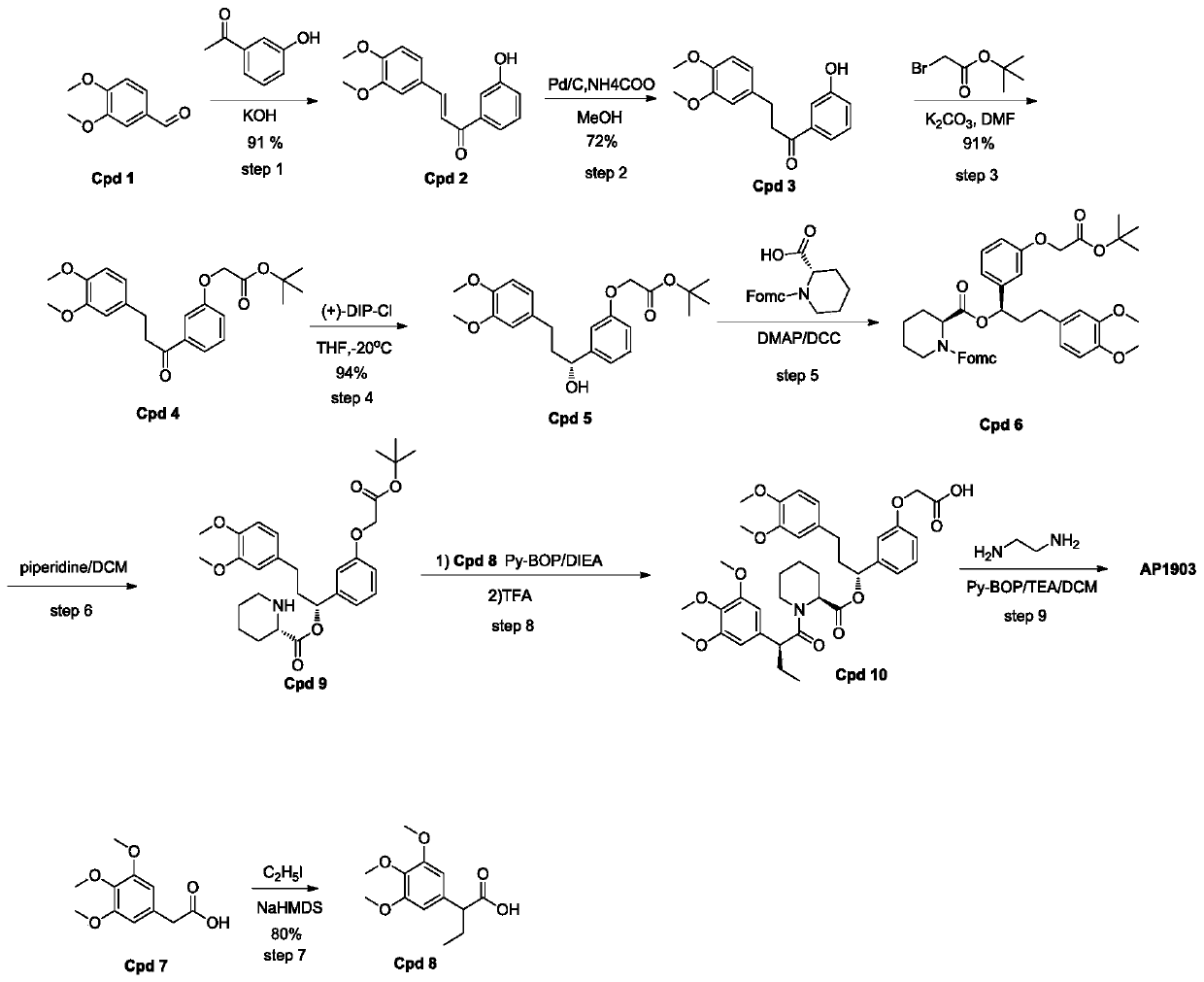
ActiveCN109734655BSimple post-processing purificationR & D costs are highOrganic chemistryDimerChemical compound
The invention discloses a synthesis process of homodimer of FKBP ligand and belongs to an improved synthesis process of AP1903, wherein the homodimer of FKBP ligand refers to AP1903. According to theoptimized route of the synthesis process chooses, (S)-piperidine-2-methyl formate is connected to the compound Cpd9' as selected, an acid Cpd6' obtained after ester hydrolysis is easier to be purified by post-treatment purification, and precipitation or extraction treatment can be chosen by the post-treatment purification. Although the total reaction steps of the synthetic route in the prior literature are the same as the optimized route of the invention, the synthetic route in the prior literature belongs to the vertical route, and the post-treatment of the fifth, sixth and eighth steps requires column chromatography, thereby leading to a higher research and development cost. The optimized AP1903 synthetic route belongs to the parallel route, and the post-treatment is simpler and more advantageous for separation and purification, so that the cost of research and development is relatively low and the economic benefit is obviously superior to that of the synthetic route in the prior art.
Owner:BIRDO (SHANGHAI) PHARMATECH CO LTD
Synthesis method of 4, 4 '-diaminodiphenyl ether
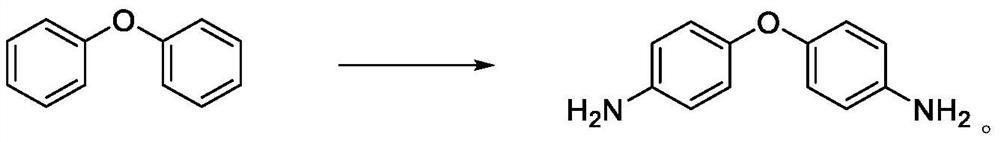
PendingCN114085158AImprove power supply capacityLarge steric hindranceOrganic compound preparationAmino-hyroxy compound preparationDiphenyl etherDiaminodiphenyl ether
The invention discloses a synthesis method of 4, 4 '-diaminodiphenyl ether, the synthesis method comprises the following step: taking diphenyl ether, hydrogen peroxide, ammonia water and bromide as initial raw materials, synthesizing the 4, 4'-diaminodiphenyl ether through one-step reaction in the presence of a solvent, a catalyst and a ligand. The technical problems that high temperature and high pressure are needed for reaction conditions, the selectivity is poor and the yield is low in an existing 4, 4 '-diaminodiphenyl ether synthesis process are solved; the adopted Mannich alkali ligand has strong power supply property and large steric hindrance, and is coordinated with the copper salt, so that the catalytic effect of the copper salt can be remarkably improved; the adopted brominated salt enables diphenyl ether to be subjected to an oxidative bromination reaction to generate the 4, 4 '-dibromodiphenyl ether intermediate. The synthesis process can be completed at the room temperature, the reaction condition is mild, selectivity is good, aftertreatment purification is simple, the yield is high, and the product quality is good.
Owner:SINOSTEEL NANJING NEW MATERIALS RES INST CO LTD
Preparation method and application of N-alpha-beta-containing unsaturated ketone compound
PendingCN112552193ALow raw material costSimple post-processing purificationOrganic chemistryOrganic compound preparationUnsaturated ketoneMethyl palmoxirate
The invention relates to a preparation method of an N-alpha-beta-containing unsaturated ketone compound. The preparation method comprises the steps of (1) mixing the components, and heating to reflux;(2) heating and refluxing until the reaction system turns yellow, cooling, and separating out a solid; and (3) pulping by using methyl tert-butyl ether to obtain a yellow solid product. According tothe preparation method of the N-alpha-beta-containing unsaturated ketone compound, a reaction solvent, a catalyst and an oxidant are not needed, so that the raw material cost is reduced, the post-treatment purification is simple, the yield is high, the operation is simple, and the preparation cost is reduced.
Owner:烟台共进医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
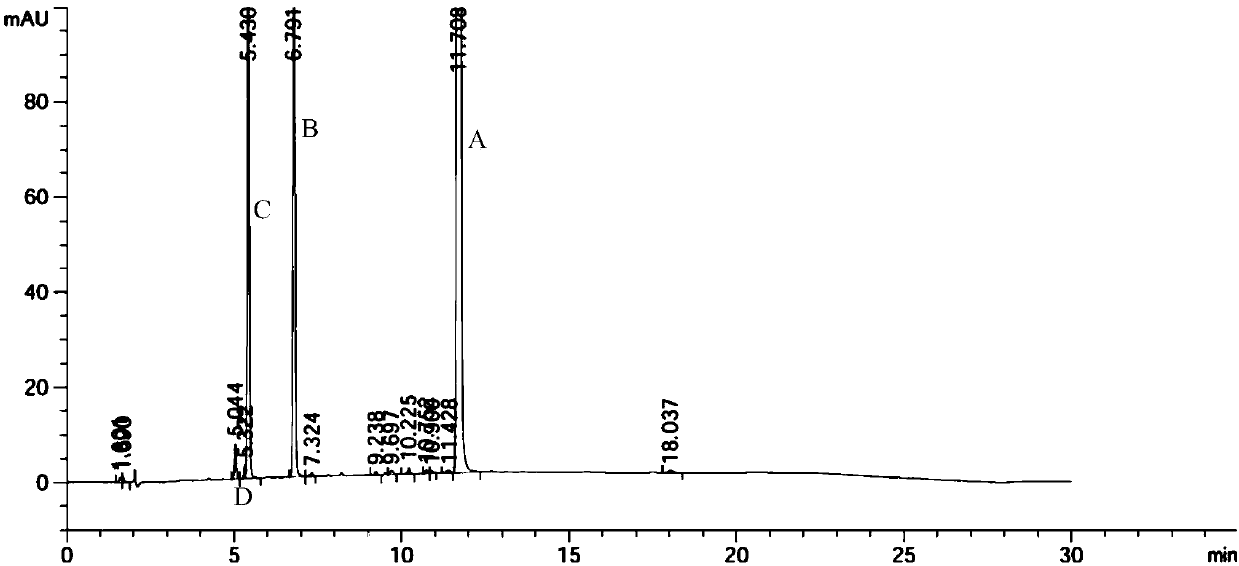
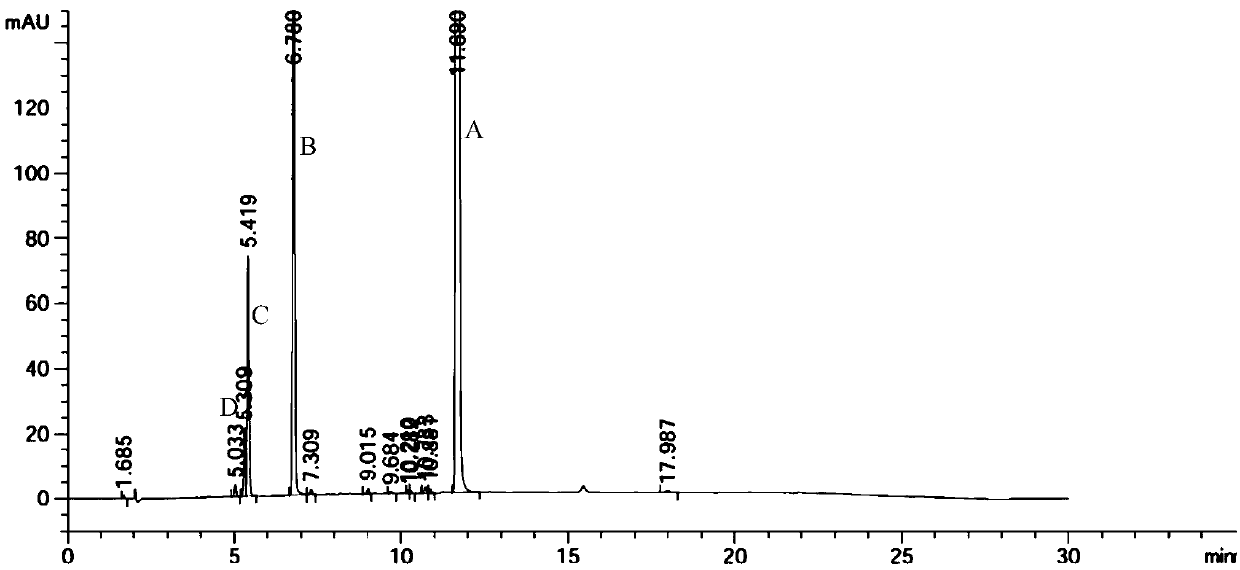
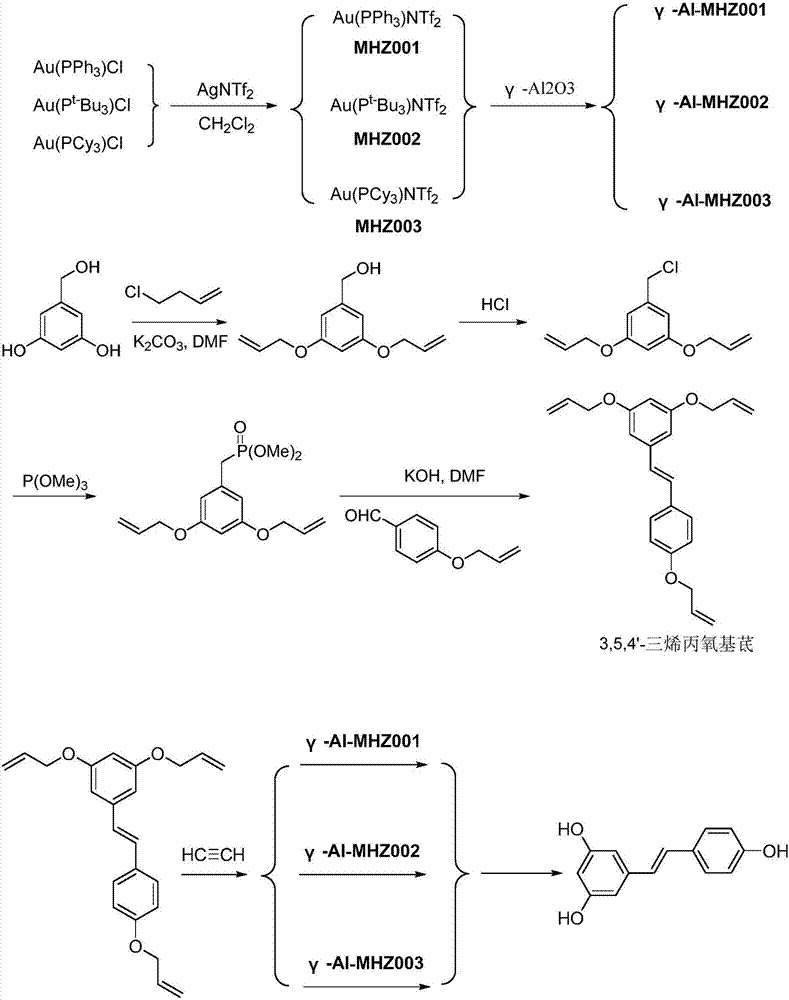
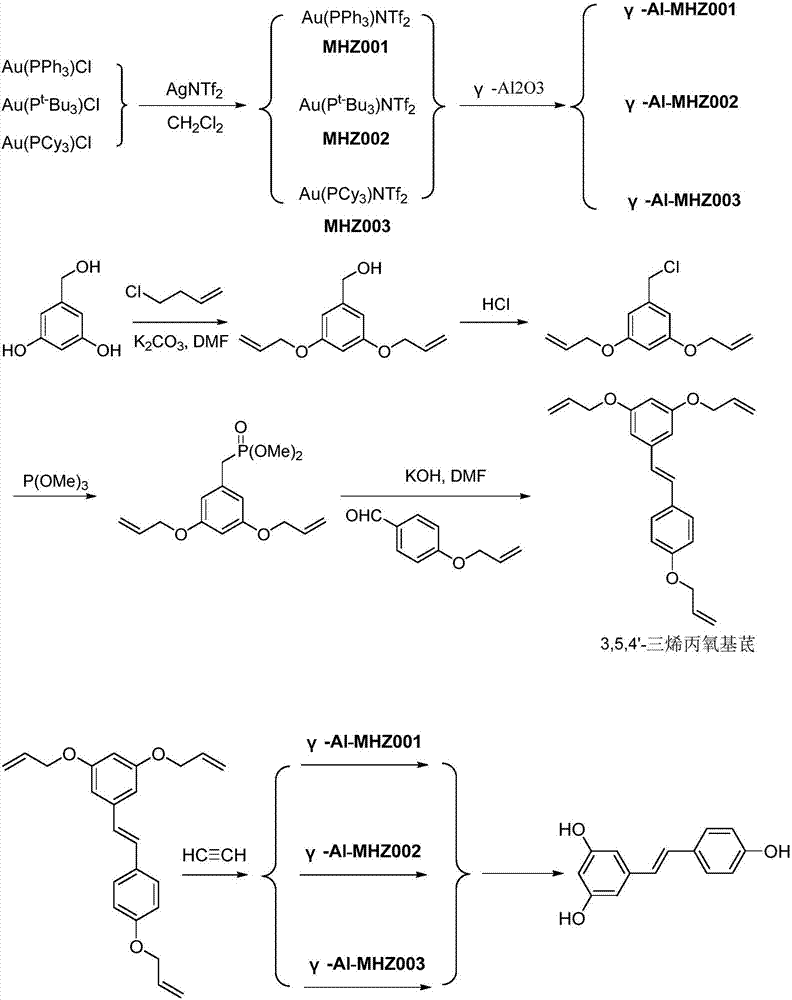
![A kind of preparation method of indolo[2,3-a]carbazole A kind of preparation method of indolo[2,3-a]carbazole](https://images-eureka.patsnap.com/patent_img/da44a711-e547-4490-ac82-9d14133800ac/HDA0002797569190000011.png)
![A kind of preparation method of indolo[2,3-a]carbazole A kind of preparation method of indolo[2,3-a]carbazole](https://images-eureka.patsnap.com/patent_img/da44a711-e547-4490-ac82-9d14133800ac/BDA0002797569180000011.png)
![A kind of preparation method of indolo[2,3-a]carbazole A kind of preparation method of indolo[2,3-a]carbazole](https://images-eureka.patsnap.com/patent_img/da44a711-e547-4490-ac82-9d14133800ac/BDA0002797569180000012.png)