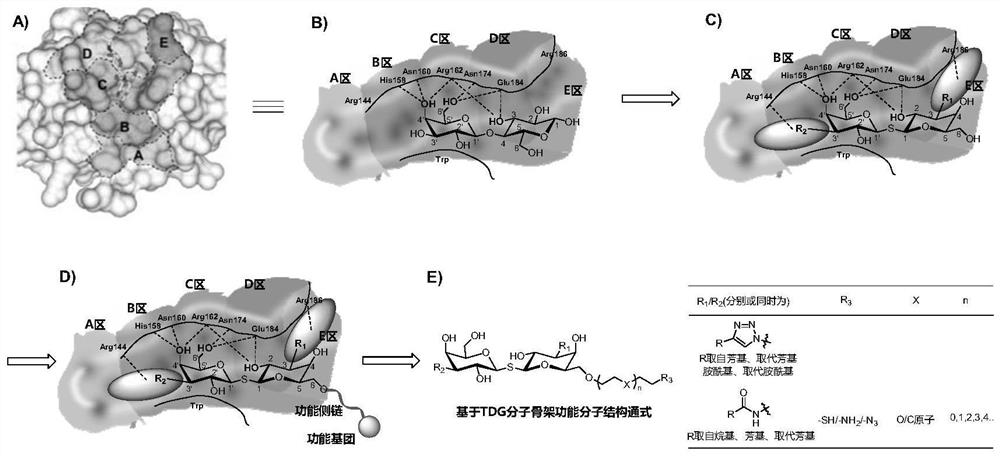
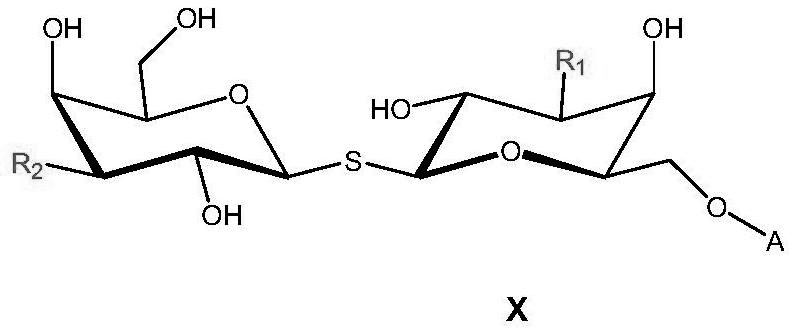
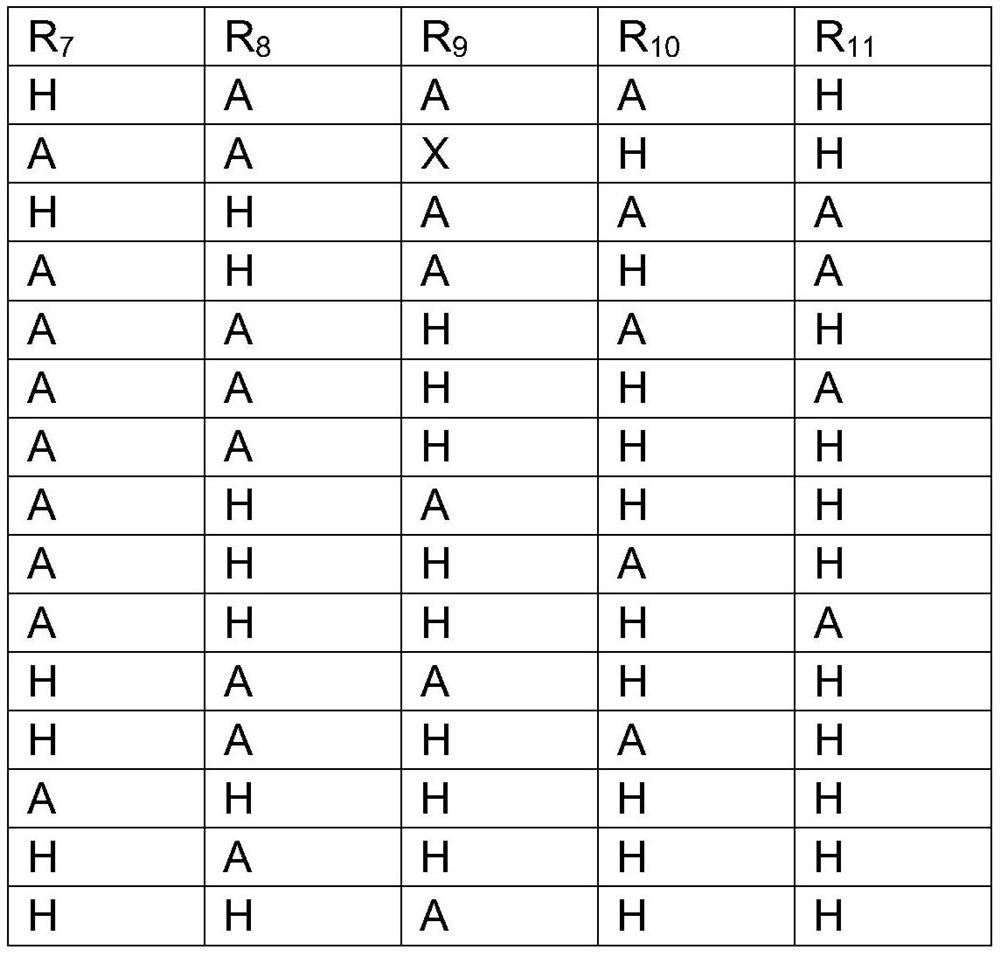
A kind of functional carbohydrate molecule based on tdg molecular skeleton and preparation method thereof
A sugar and aryl technology, applied in the field of functional sugar molecules based on the TDG molecular skeleton and its preparation, can solve the problems of unreported, blocking signal channels, and insignificant biological effects, etc., and achieve a wide range of applications, The effect of stable response and obvious application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109]
[0110] Diacetone glucose (40 g, 153.7 mmol) dissolved in Ac 2 O / DMSO (v / v=1:2, 150mL) mixed solution, stirred at room temperature overnight, TLC (PE:EA=1:1) detected the reaction was complete, the reaction solution was diluted with dichloromethane DCM, washed with water 3 times, The organic phases were combined, washed with 30% hydrogen peroxide, dried over anhydrous sodium sulfate, and concentrated to obtain a brown syrup intermediate, which was directly used in the next step.
[0111] The brown syrup intermediate was dissolved in pyridine (50 mL), acetic anhydride (20 mL) was added dropwise, and the reaction was placed in an oil bath at 90°C and refluxed overnight. TLC (PE:EA=1:1) detected that the reaction was complete, and the reaction solution was cooled to room temperature. After concentration, it was purified by column chromatography (isocratic elution, PE:EA=3:1) to obtain colorless syrup I-1 (39.5 g, 85% in 2steps). 1 H NMR (400M, CDCl 3 )δ6.04(d,J=5.5Hz...
Embodiment 2
[0113]
[0114] Compound 1-1 (39.5 g, 131.5 mmol) was dissolved in ethyl acetate (100 mL), Pd / C powder (2.0 g) was added, and after stirring, it was placed in a hydrogen (40 psi) atmosphere and stirred for 3 hours. TLC (PE: EA=1:1) Check that the reaction is complete, filter out the Pd / C powder, and concentrate the filtrate to obtain a pale yellow syrup intermediate (36.6g, 92%), 1 H NMR (400MHz, CDCl3) δ 5.82 (d, J=4.1Hz, 1H), 5.10-5.06 (m, 1H), 4.81 (dd, J=5.6, 4.1Hz, 1H), 4.14-4.06 (m, 2H), 3.57-3.51(m, 1H), 2.14(s, 3H), 1.59(s, 3H), 1.45(s, 3H), 1.39(s, 3H), 1.36(s, 3H). 13 C NMR (101MHz, CDCl3)δ 169.7, 114.6, 109.4, 105.1, 81.5, 78.6, 75.3, 71.9, 66.5, 26.9, 26.8, 25.4, 20.7.
[0115] The light yellow syrup obtained in the previous step was dissolved in methanol (100 mL), sodium methoxide was added to adjust the pH of the reaction solution to 8-10, stirred at room temperature for 30 min, TLC (PE:EA=1:1) showed that the reaction was complete, and 732 type H was added. ...
Embodiment 3
[0117]
[0118] Compound I (29.9 g, 114.88 mmol) was dissolved in dichloromethane (100 mL), pyridine (27.81 mL, 344.6 mmol) was added, and after cooling to ice bath temperature, trifluoromethanesulfonic anhydride (38.6 mL, 229.7 mmol) was added dropwise, Stir under ice bath for 30min, TLC (PE:EA=1:1) showed that the reaction was complete, the reaction solution was successively passed through 1N HCl a.q. , sat. NaHCO 3a.q.Washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain a brown syrup intermediate, which was directly used in the next reaction;
[0119] Azidotrimethylsilane (52.9 mL, 402.1 mmol) and sodium fluoride (15.9 g, 379.1 mmol) were mixed with DMF (150 mL), refluxed in an oil bath at 100 °C for 1 hour, cooled to room temperature, and added with The DMF solution of the brown syrup intermediate obtained in the first step, the mixture was placed in a 50 ° C oil bath to reflux for overnight reaction, TLC (PE:EA=1:1) detected tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com