Synthetic process of homodimer of FKBP ligand
A technology of homodimer and synthesis process, which is applied in the direction of organic chemistry, can solve the problem of high R&D cost, and achieve the effect of simple post-processing, low R&D cost and favorable separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be described in detail below in conjunction with the accompanying drawings and specific embodiments.
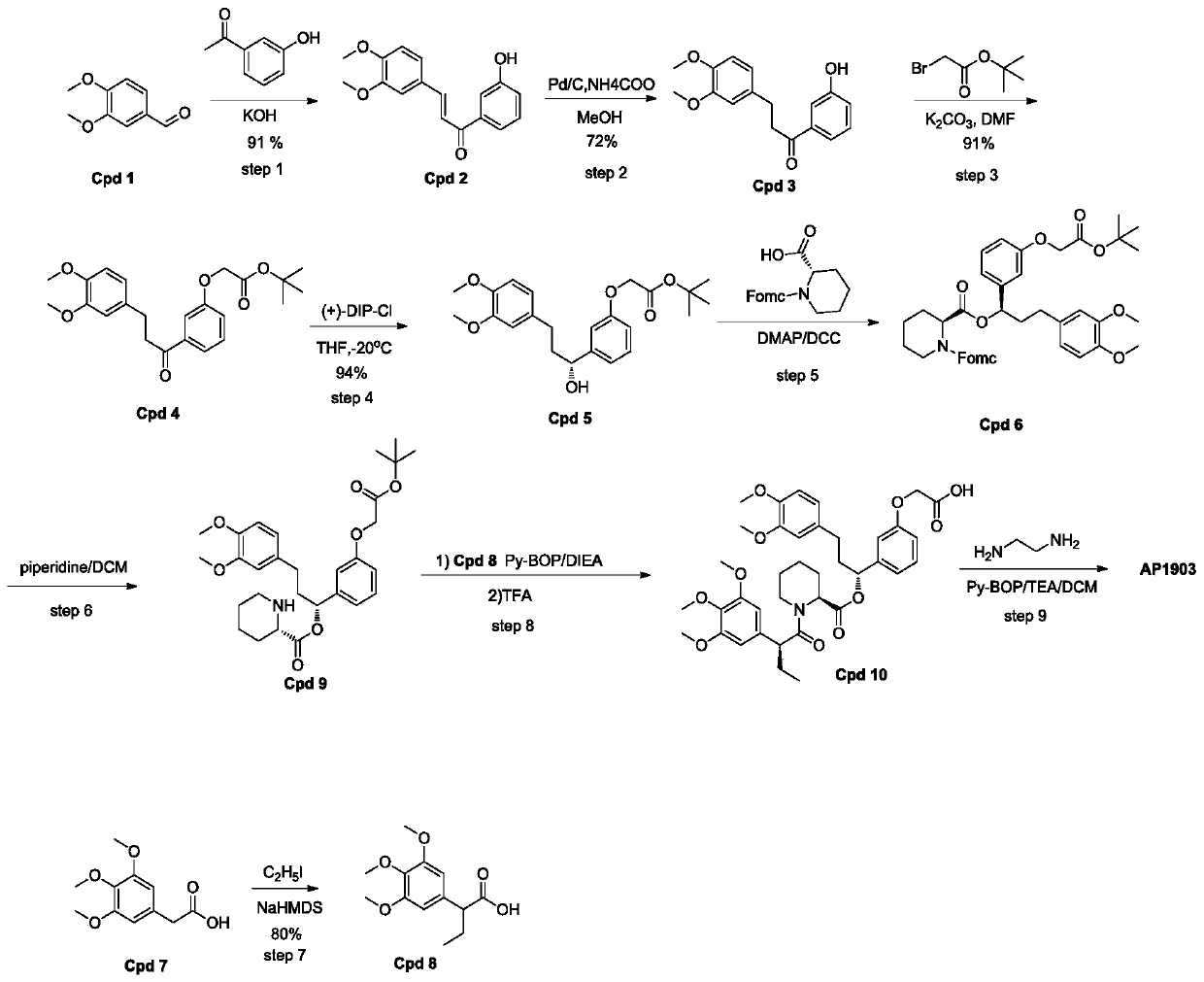
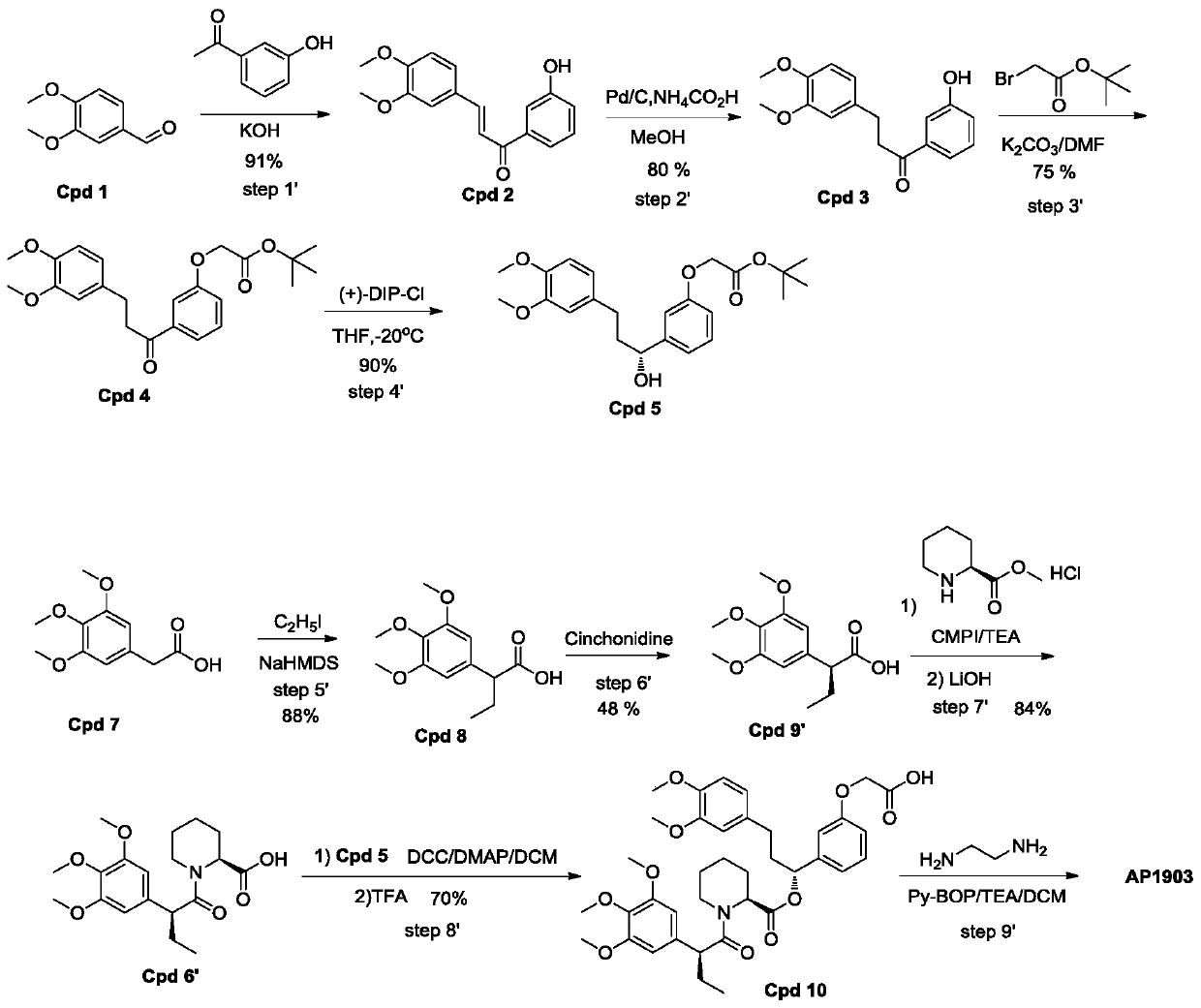
[0032] Such as figure 2 As shown, a homodimer synthesis process of FKBP ligand, the homodimer of FKBP ligand is AP1903, and the specific operation steps for synthesizing AP1903 are:
[0033] Step1':
[0034] Add 3,4-dimethoxybenzaldehyde (400g, 2.41mol) and 3'-hydroxyacetophenone into a 5L round bottom flask, pour 3.2L ethanol to dissolve into a clear solution, and then inject 30% of Potassium hydroxide solution was 1.2L. After the injection was completed, the reaction system was stirred at room temperature for 16 hours. After the reaction was completed, 3.4L of a mixed solvent of ethanol / water=1 / 1 was added to the reactant, and then 720mL of concentrated HCL was added to neutralize to pH= 6. After acidification, 4L of water was added to the reaction system, and a yellow solid was precipitated, left to stand for 10 hours, and suction filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com