Fluorinated silicone lube oil synthetic method
A synthesis method and technology of lubricating oil, applied in lubricating compositions, petroleum industry, additives, etc., can solve the problems affecting product quality control and production process, affecting the storage stability of silicone oil products, affecting the catalytic efficiency of catalysts, etc., and achieve excellent comprehensive The effect of performance, short response time and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
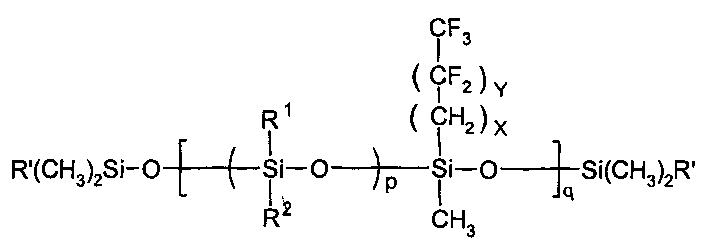
[0029] The three-necked reaction flask is equipped with electromagnetic stirring, reflux condenser, thermometer and water bath, and the fluorine-containing mixed cyclosiloxane (R 1 , R 2 Methyl, X=2, Y=6) 16g, end-capping agent divinyltetramethyldisiloxane 2.4g (15% of mixed cyclosiloxane weight) and 95% sulfuric acid 1.92g were added to the reaction flask in sequence After stirring for 30 minutes, the temperature was raised to 30°C; after 40 hours of reaction, it was cooled to room temperature, 20ml of n-hexane was added, washed with water until neutral, and then the solvent and low fraction were evaporated to obtain 13.5g of fluorine-containing polysiloxane with a yield of 73.5% . The product is a colorless transparent liquid with a viscosity (-40°C) of 1536.6mm 2 / s, the fluorine content is 44.3%.
Embodiment 2
[0031] The three-necked reaction flask is equipped with electromagnetic stirring, reflux condenser, thermometer and water bath, and the fluorine-containing mixed cyclosiloxane (R 1 is methyl, R 2 Ethyl, X=2, Y=0) 16g, end-capping agent divinyltetramethyldisiloxane 3.2g (20% of the weight of the mixed cyclosiloxane) and 0.8g perchloric acid were added to the reaction flask in sequence After stirring for 30 minutes, the temperature was raised to 30°C; after 30 hours of reaction, it was cooled to room temperature, 20ml of n-hexane was added, washed with water until neutral, and then the solvent and low fraction were evaporated to obtain 15.6g of fluorine-containing polysiloxane with a yield of 80.9% . Product viscosity (-40°C) is 605.9mm 2 / s, the fluorine content is 20.7%.
Embodiment 3
[0033] The three-necked reaction flask is equipped with electromagnetic stirring, reflux condenser, thermometer and water bath, and the fluorine-containing mixed cyclosiloxane (R 1 , R 2 is methyl, X=2, Y=10) 20g, end-capping agent divinyltetramethyldisiloxane 3g and 0.8g95%H 2 SO 4 Add it to the reaction flask in turn, stir for 30 minutes and then heat up to 30°C; react for 30 hours and cool to room temperature, add 20ml of n-hexane, wash with water until neutral, then distill off the solvent and low fractions to obtain 17g of fluorine-containing polysiloxane solid. The rate is 73.5%. The fluorine content of the product was 56.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com