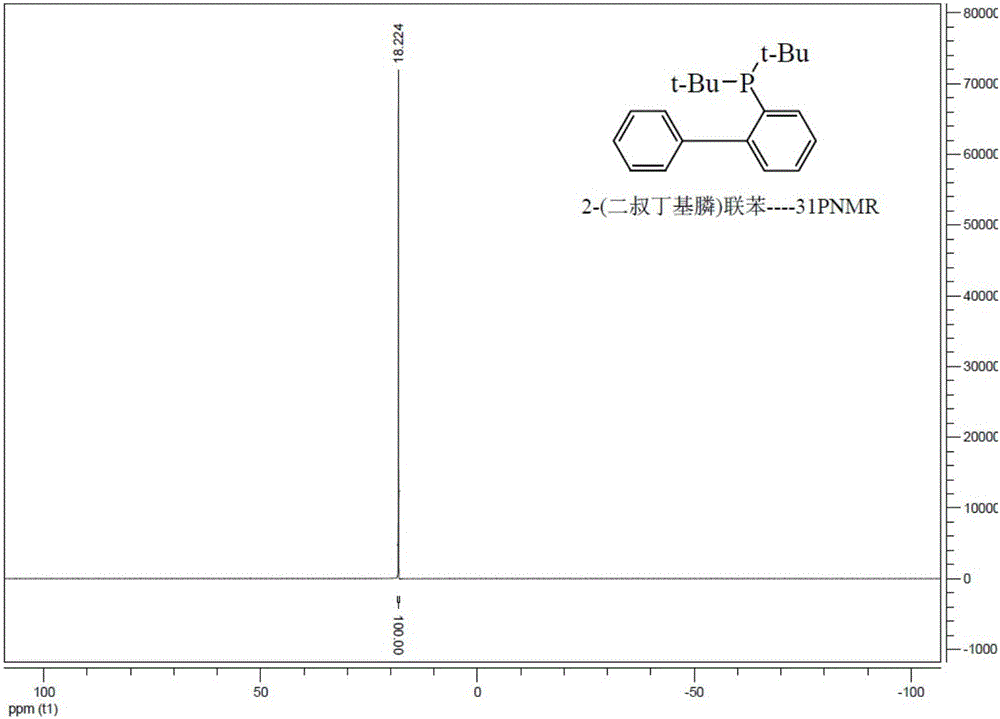Preparation method of phosphinobenzene compound
A compound and chlorine compound technology, applied in the field of preparation of phosphine and benzene compounds, can solve problems affecting product purity and quality, inconvenient scale-up production operation, troublesome post-processing, etc., to achieve guaranteed purity and quality, high yield, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the present invention comprises:
[0044]
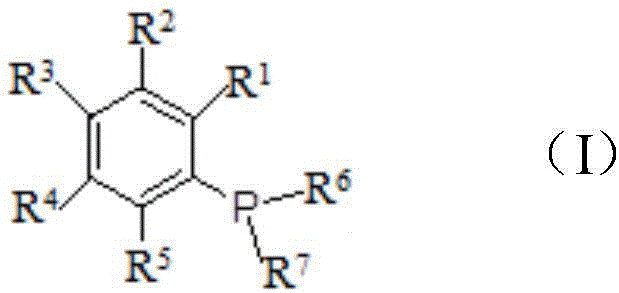
[0045] Under the protection of inert gas such as nitrogen, the bromobenzene compound of general formula (III), wherein R 1 , R 2 , R 3 , R 4 and R 5 The definition is as before, reacts with magnesium chips and organic solvents such as anhydrous tetrahydrofuran (THF) or anhydrous methyl tetrahydrofuran to make the bromobenzene compound Grignard reagent of general formula (II), wherein R 1 , R 2 , R 3 , R 4 and R 5 As defined above, reflux for 2-10 hours; Add tetrakis(triphenylphosphine) palladium at room temperature, stir for 10 minutes to 3 hours, add dropwise the phosphine chloride compound of general formula (IV) at room temperature, wherein R 6 and R 7 The definition is as before, reflux reaction 1-10 hour; Under ice-water bath, dropwise add saturated weak acid and weak base salt solution such as saturated ammonium chloride aqueous solution to the reaction solution to quench the reactio...
Embodiment 1
[0059] Preparation of di-tert-butylphenylphosphine
[0060]
[0061] Under the protection of nitrogen, in a 1L three-necked flask, a Grignard reagent was made from 27g of bromobenzene, 5g of magnesium chips and 400mL of anhydrous THF, refluxed for 2 hours, cooled to room temperature, added 2g of tetrakis(triphenylphosphine) palladium, After stirring for 10 minutes, 33 g of di-tert-butylphosphine chloride was added dropwise at room temperature, and the mixture was refluxed for 3 hours. In an ice-water bath, 200 mL of saturated ammonium chloride aqueous solution was added dropwise to the reaction solution to quench, the liquid was separated, the organic phase was desolvated, crystallized by adding methanol, and filtered to obtain 36 g of white di-tert-butylphenylphosphine, with a yield of 94.7%.
Embodiment 2
[0063] Preparation of 2-(di-tert-butylphosphine)biphenyl
[0064]
[0065] Under nitrogen protection, in a 1L three-neck flask, Grignard reagent was made from 40g of 2-bromobiphenyl, 5g of magnesium chips and 400mL of anhydrous THF, refluxed for 2 hours, cooled to room temperature, and 2g of tetrakis(triphenylphosphine ) palladium, stirred for 30 minutes, added dropwise the di-tert-butylphosphine chloride of 33g at room temperature, and refluxed for 2 hours. Add dropwise 200mL of saturated ammonium chloride aqueous solution to the reaction solution under an ice-water bath to quench, separate the liquids, desolventize the organic phase, add methanol to crystallize, and filter to obtain 49g of white 2-(di-tert-butylphosphine)biphenyl. 95.7%.
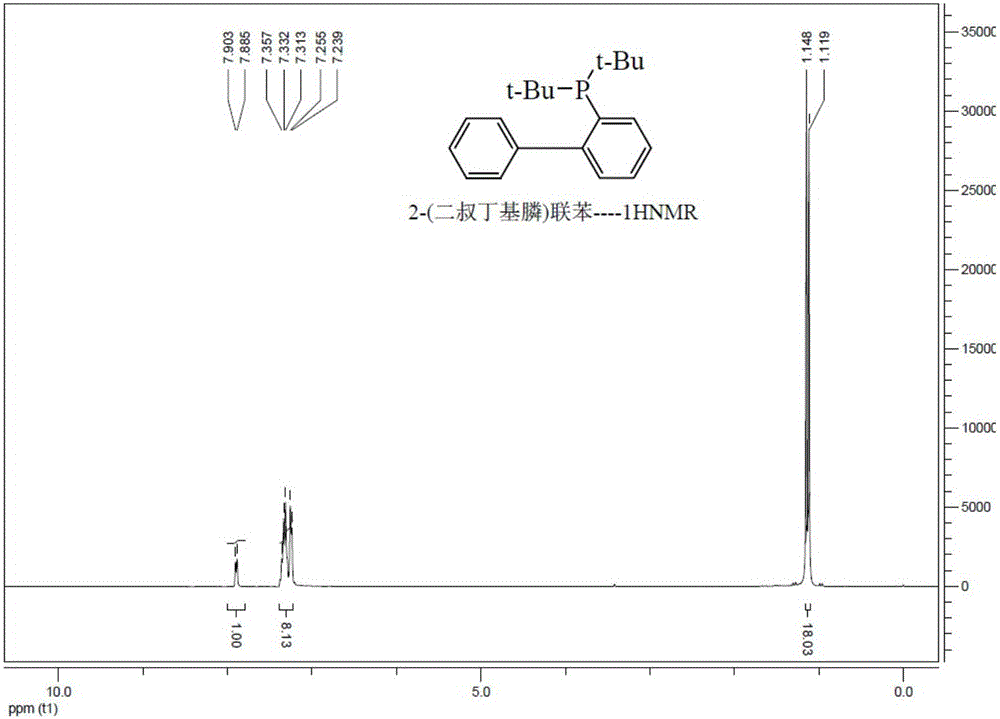
[0066] figure 1 and figure 2 The prepared 2-(di-tert-butylphosphine)biphenyl 1 H-NMR spectrum and 31 P-NMR spectrum, the characterization results are as follows:
[0067] 400MHz- 1 H-NMR (CDCl3): δ=1.1-1.2 (m, 18H); 7.8-7.9 (s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com