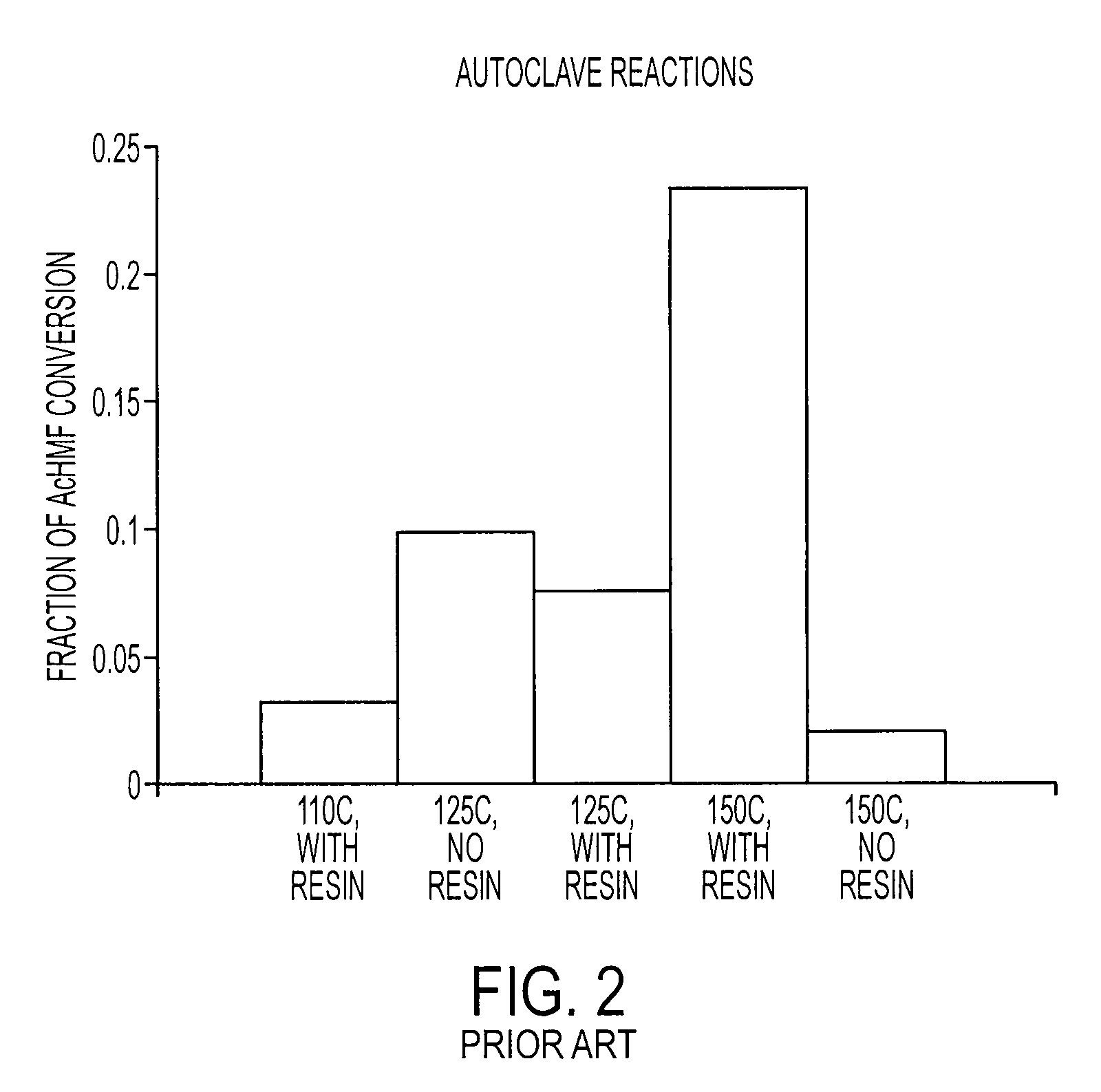
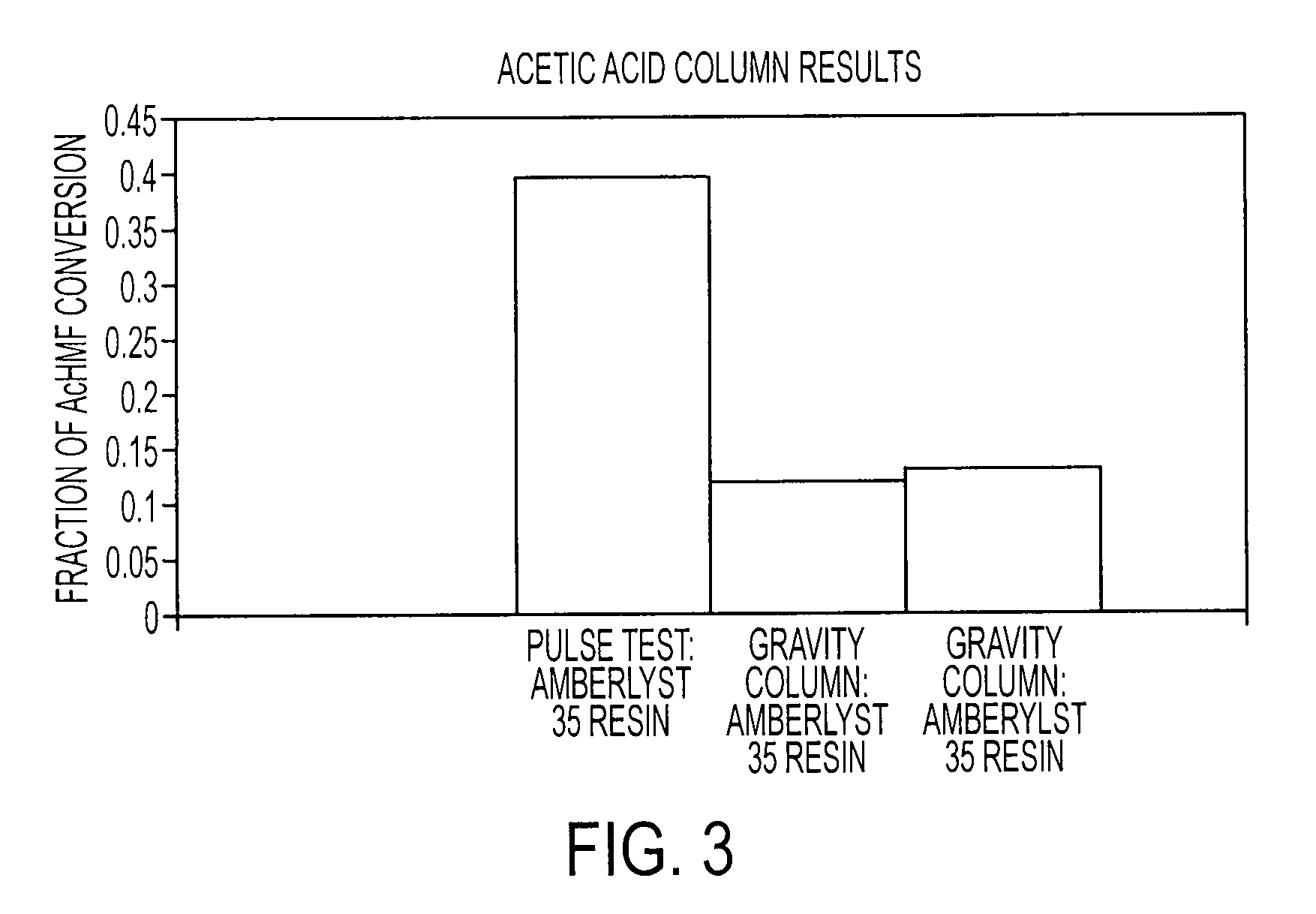
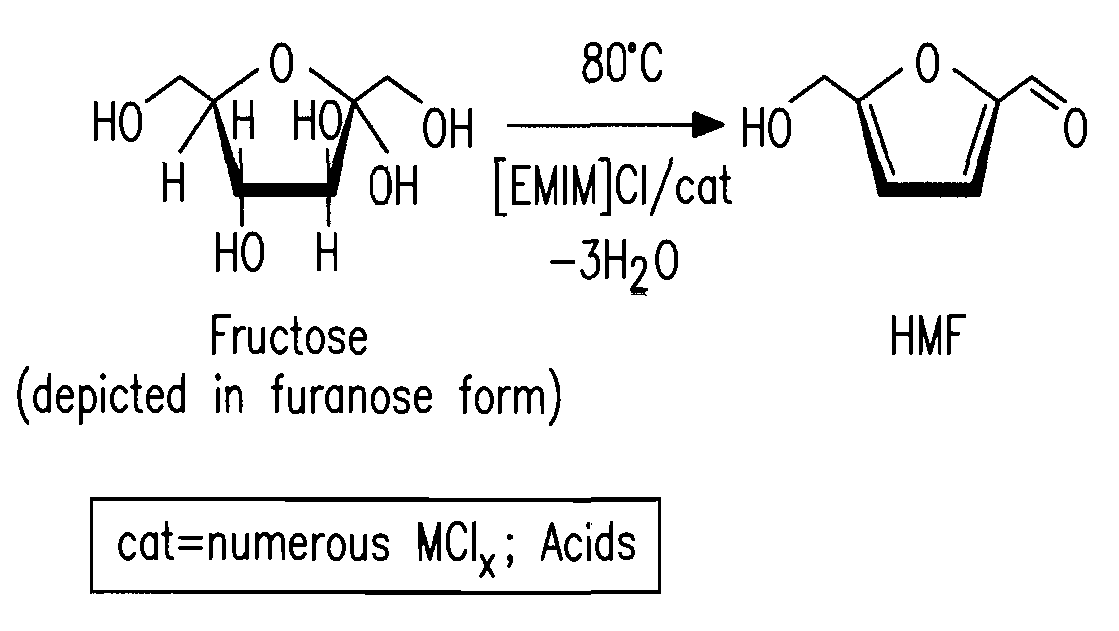
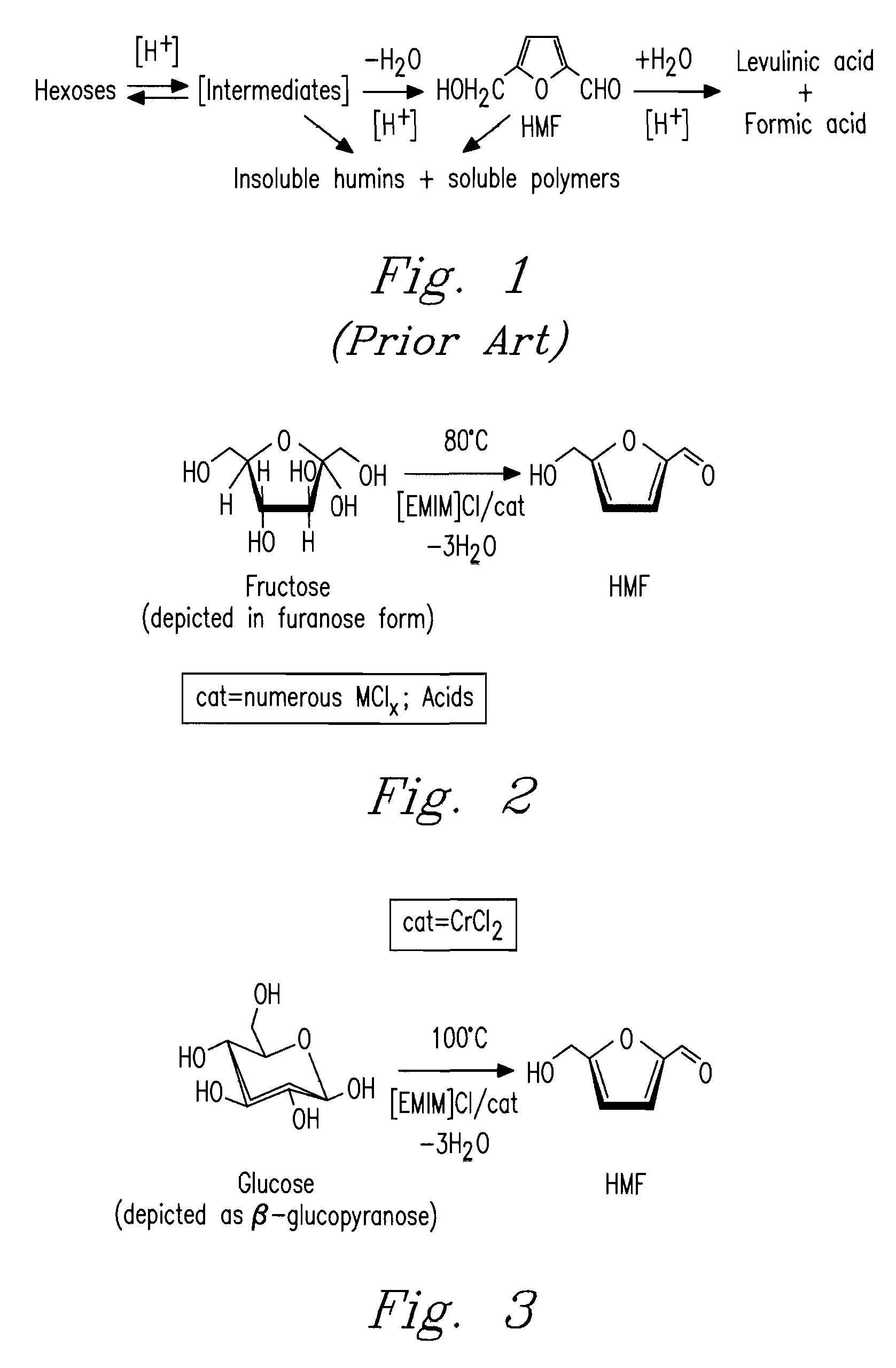
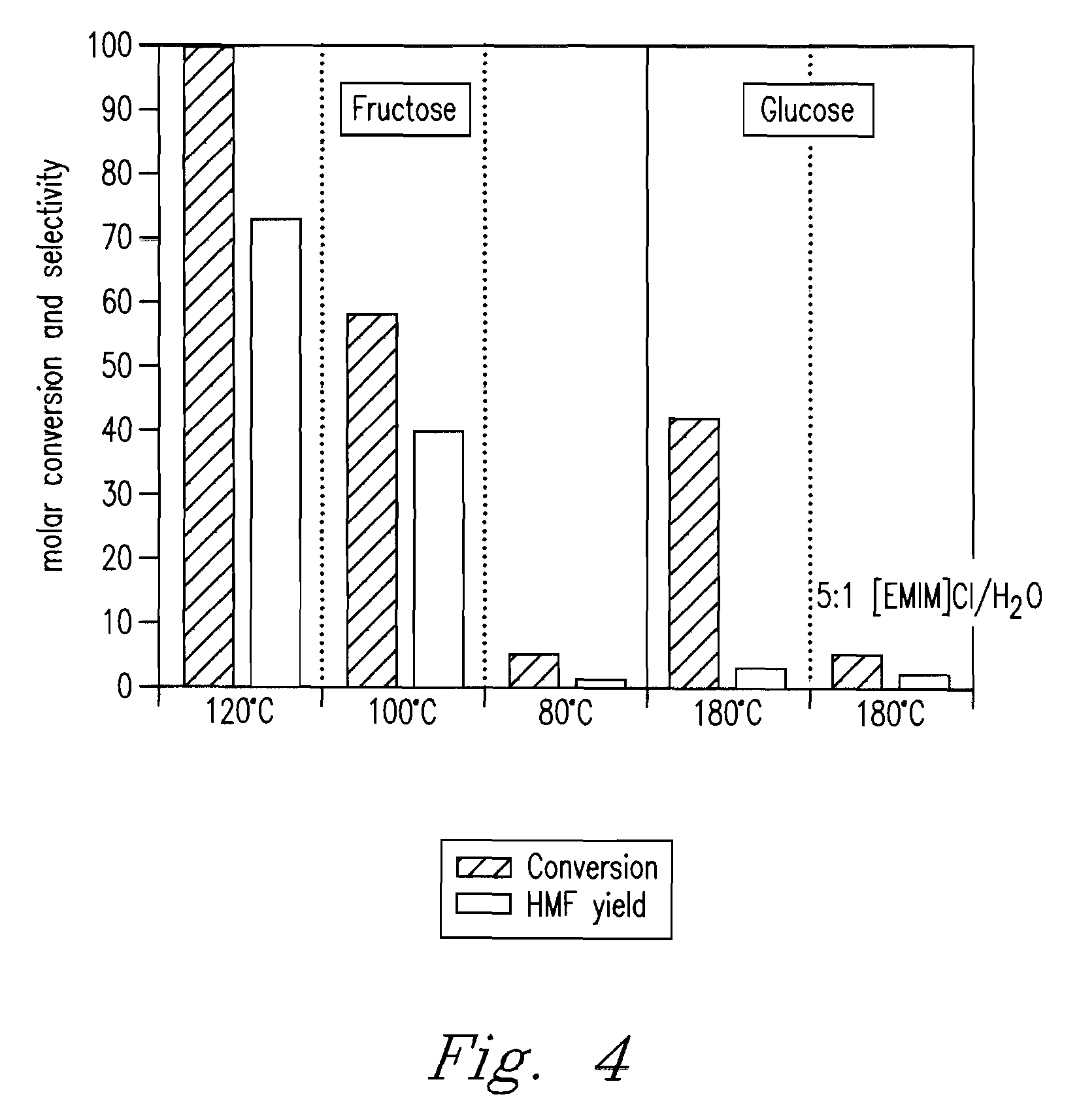
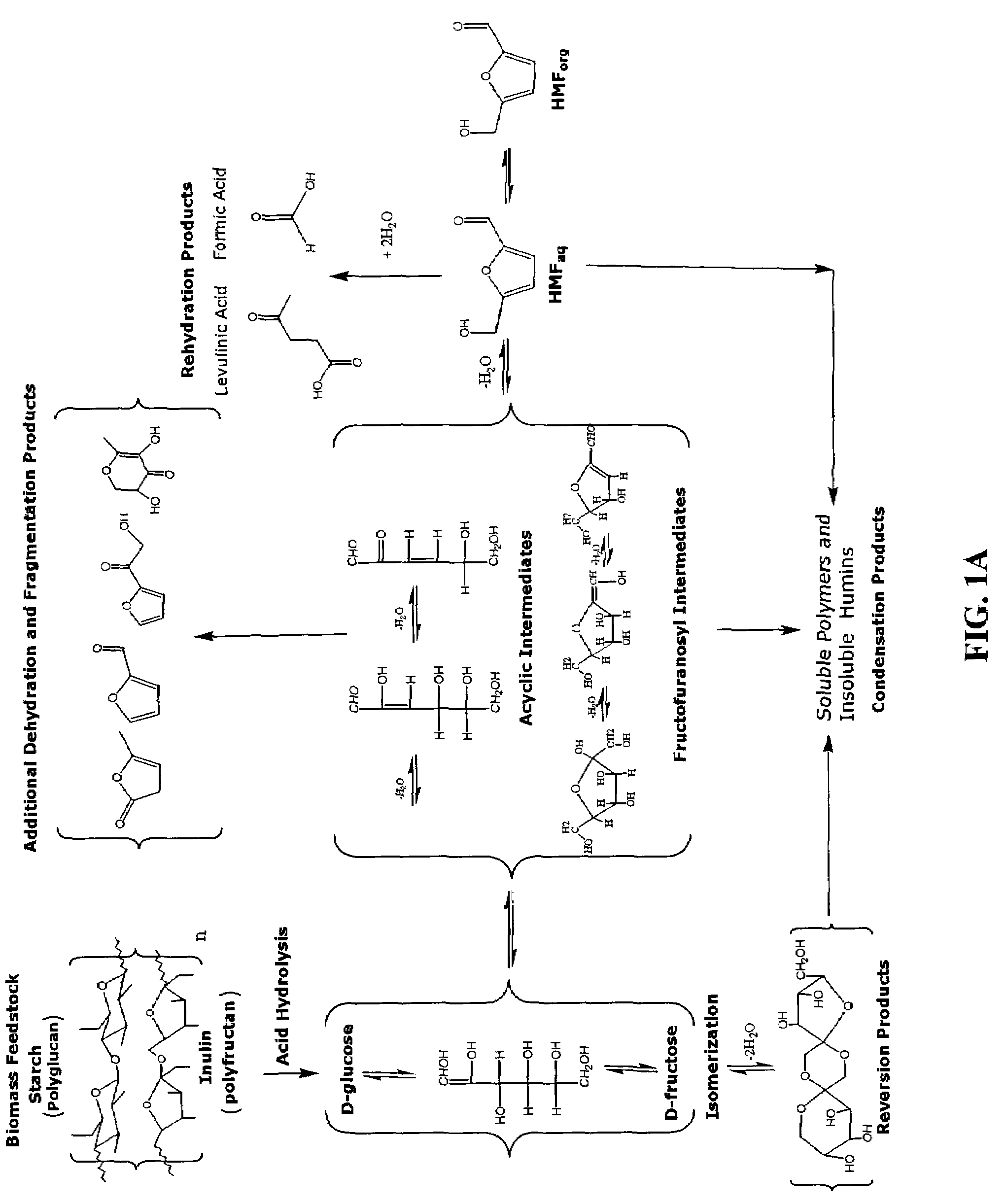
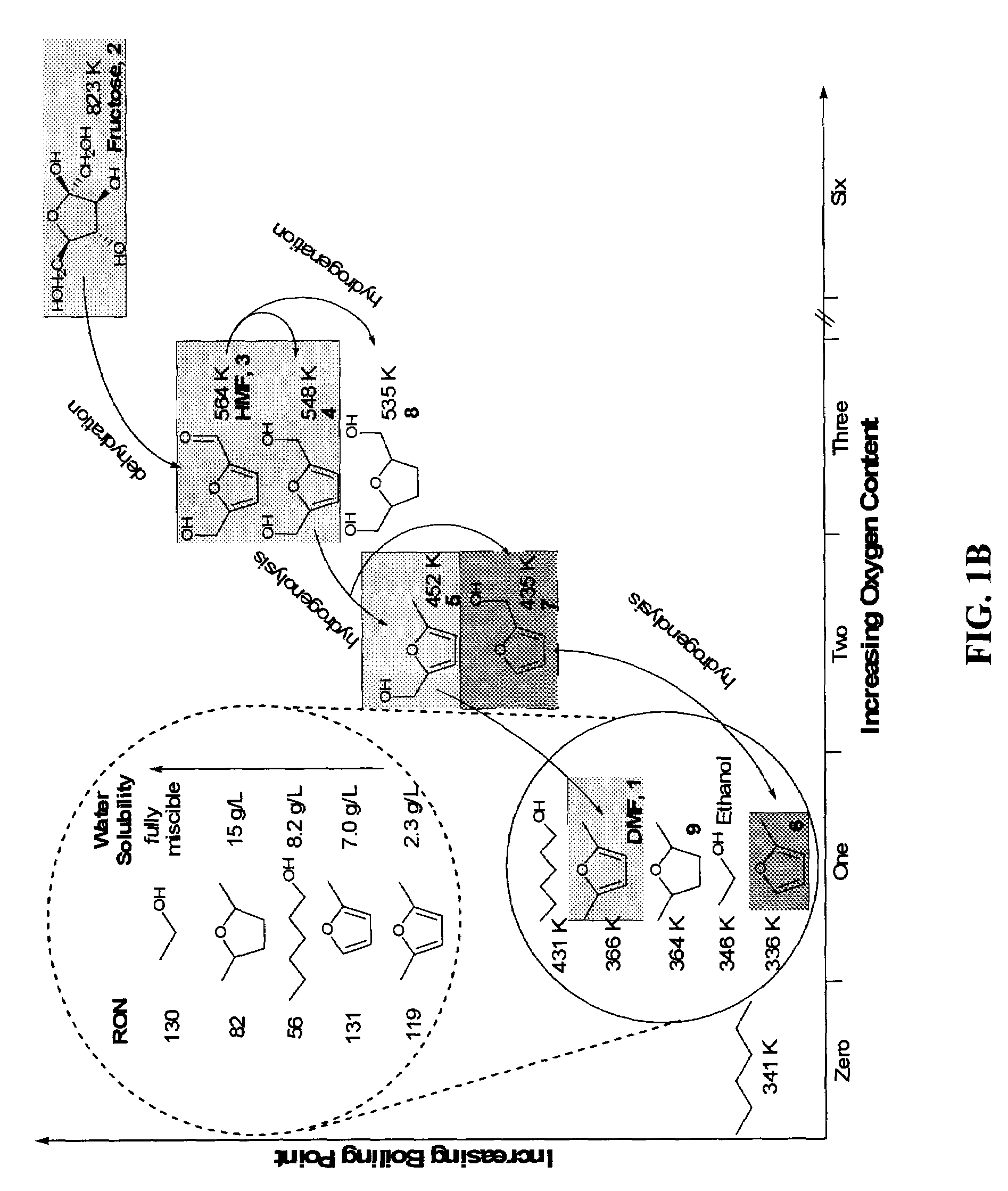
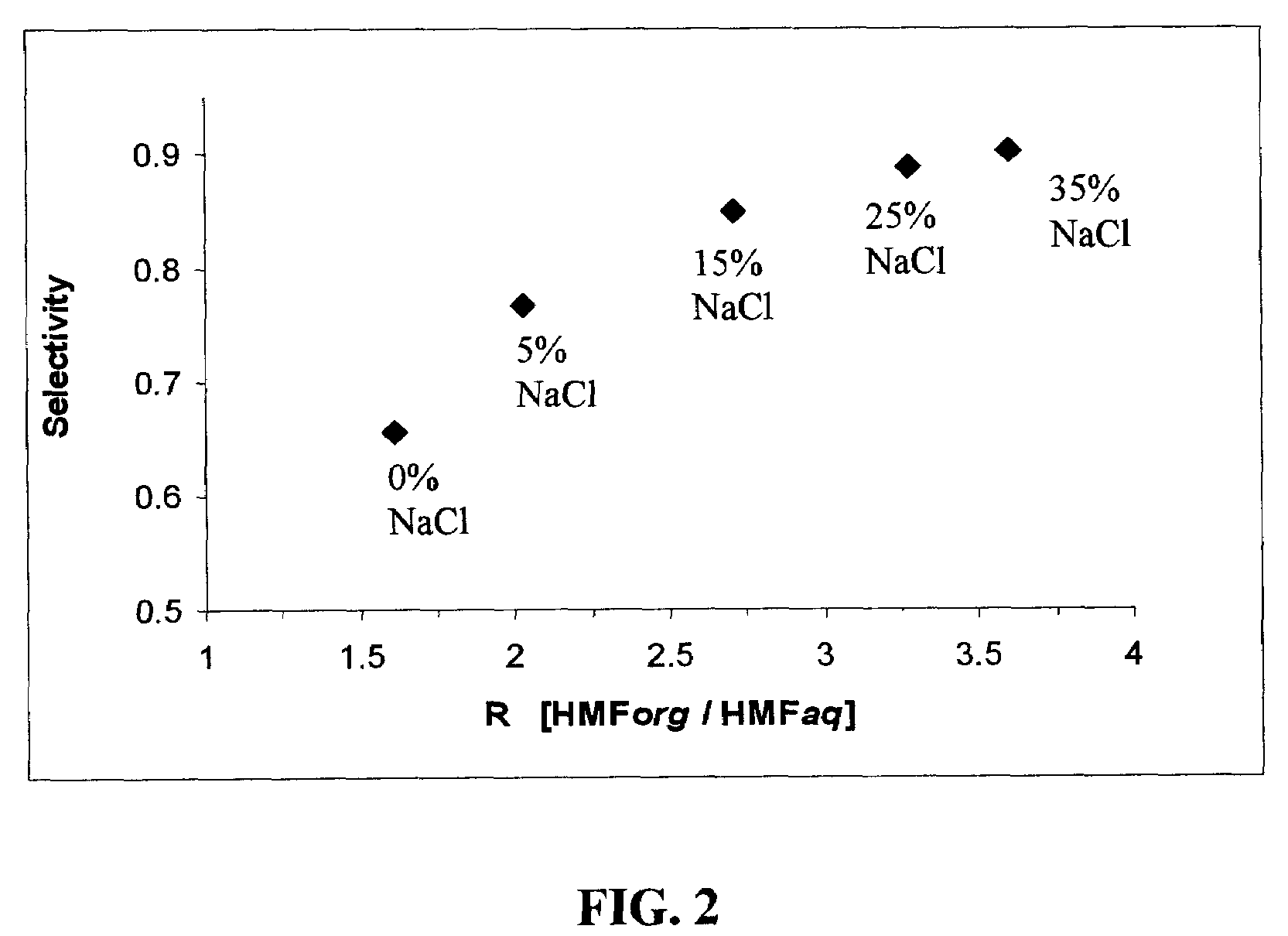
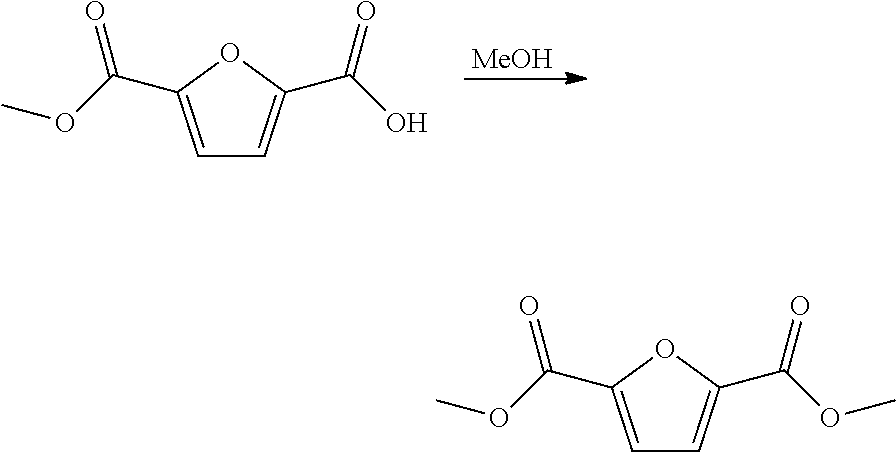
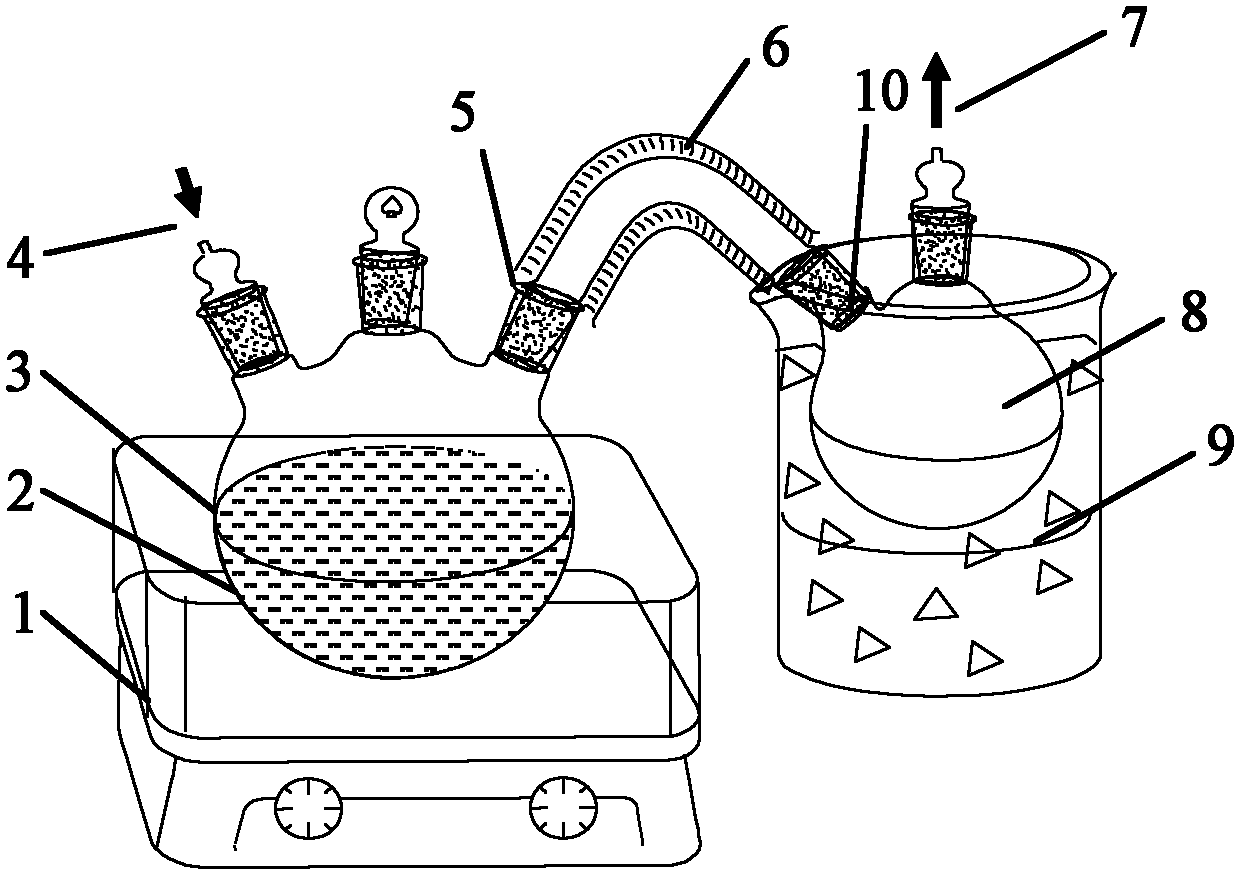
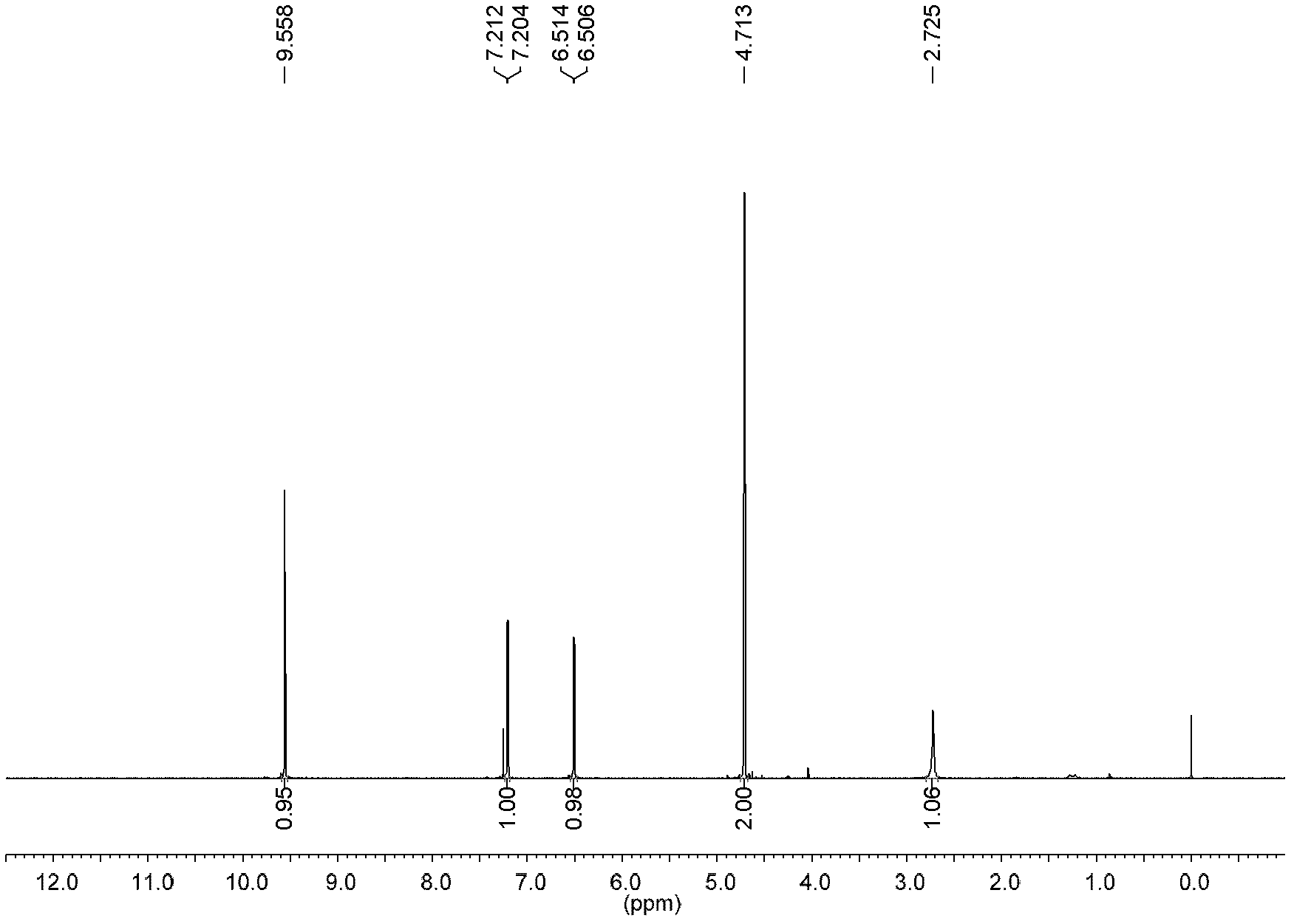
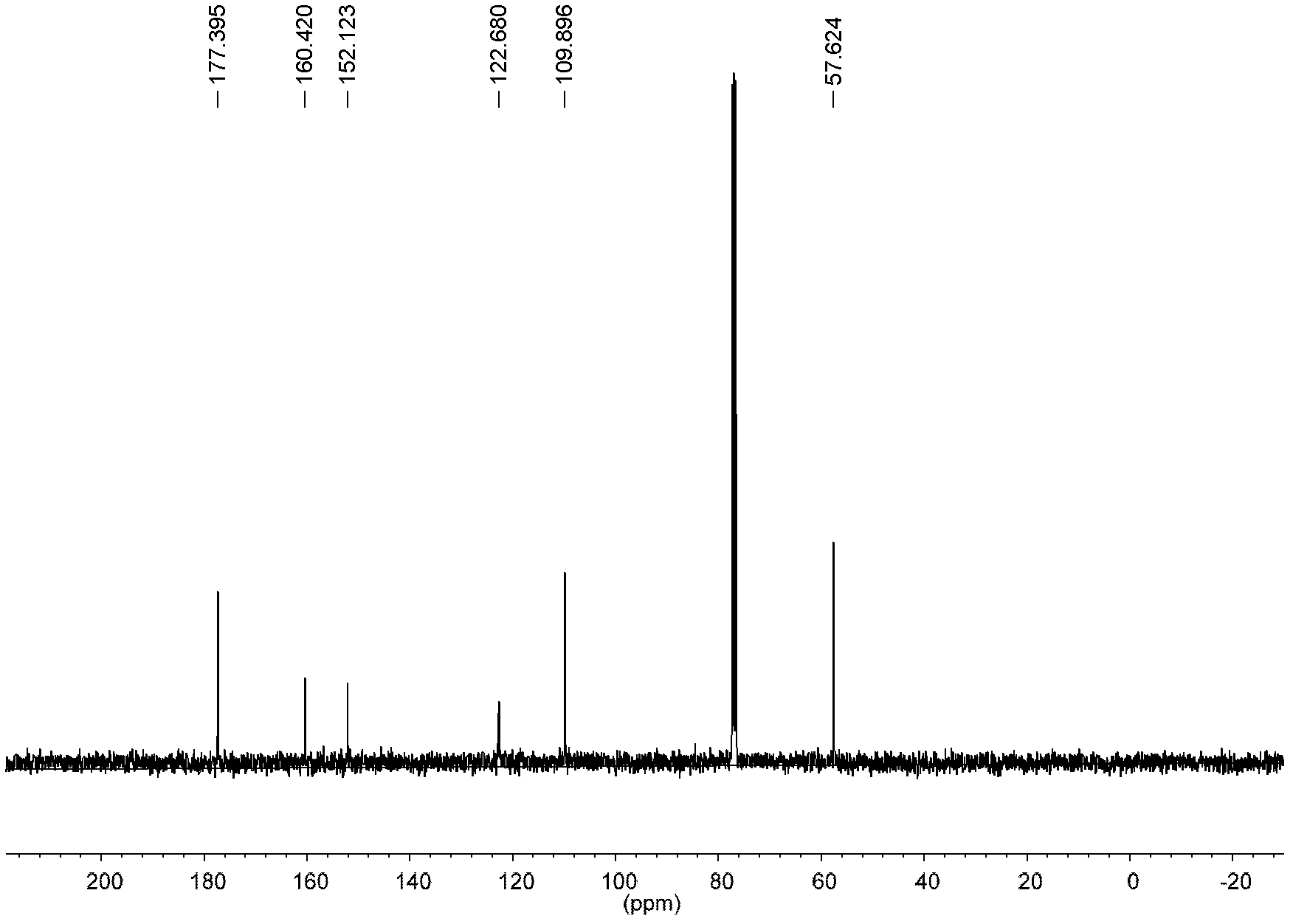
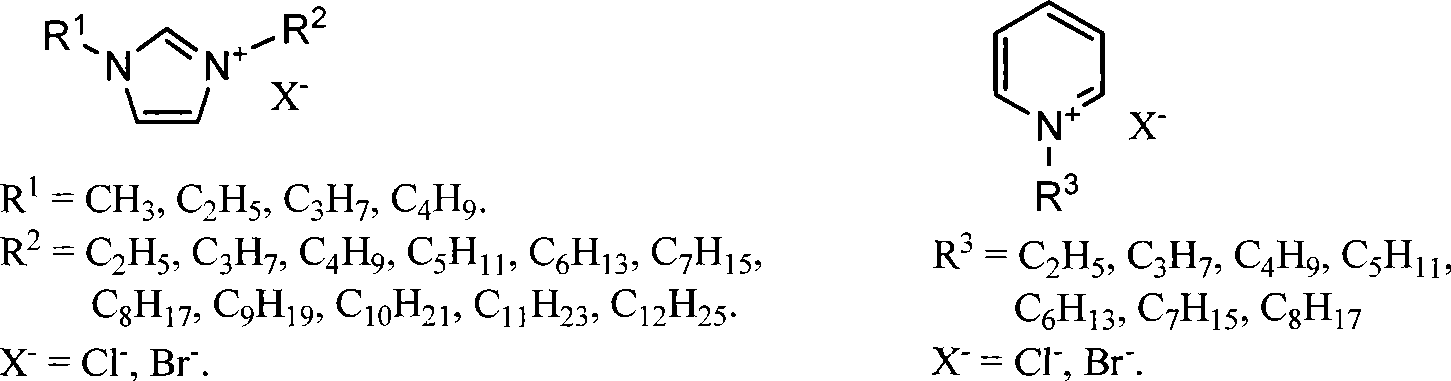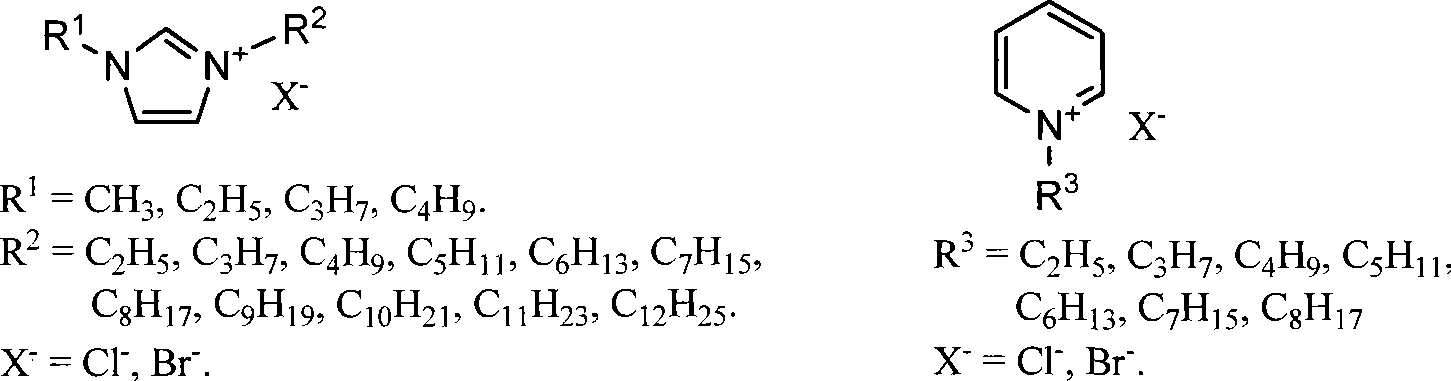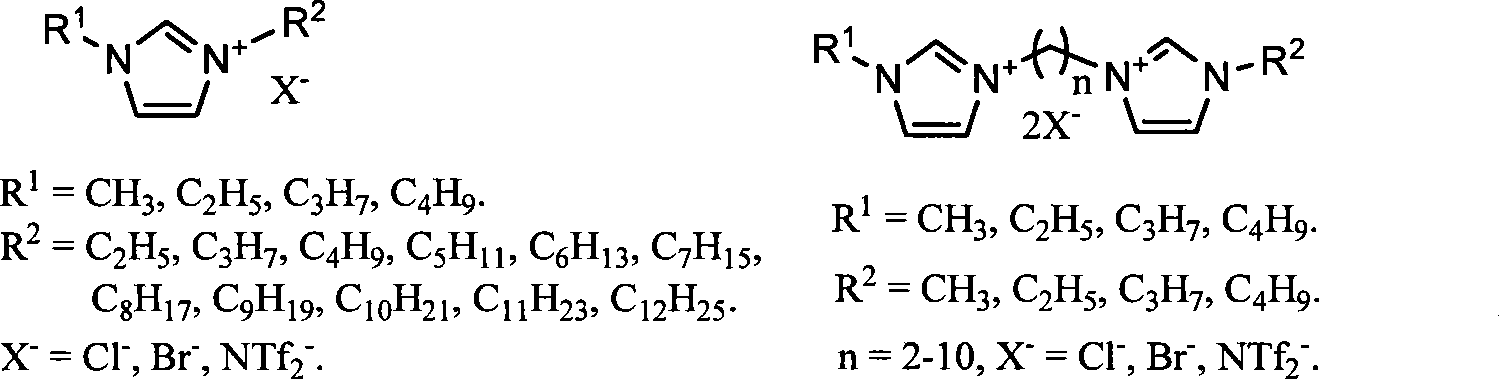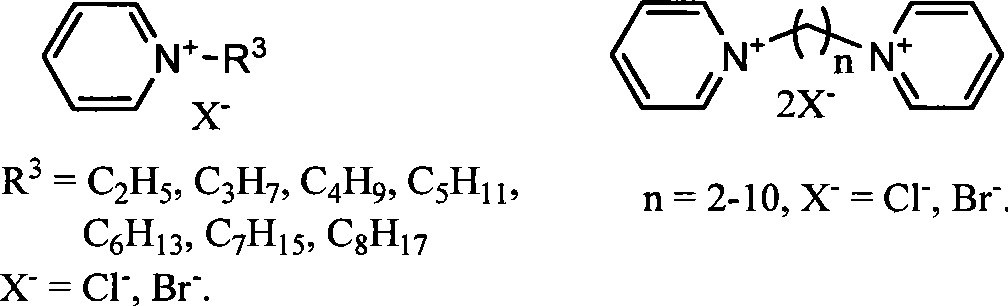Patents
Literature
1327 results about "Hydroxymethylfurfural" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
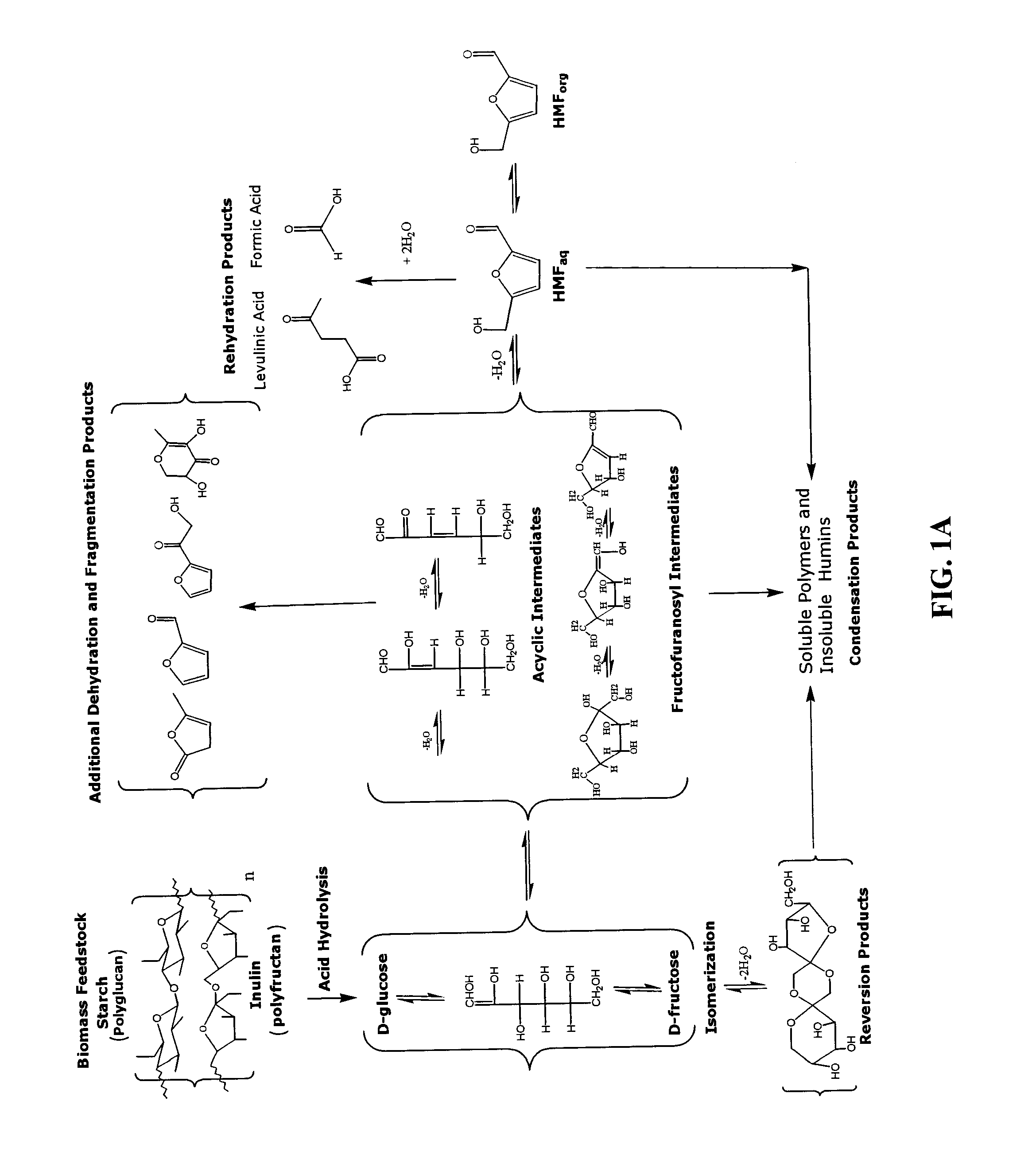
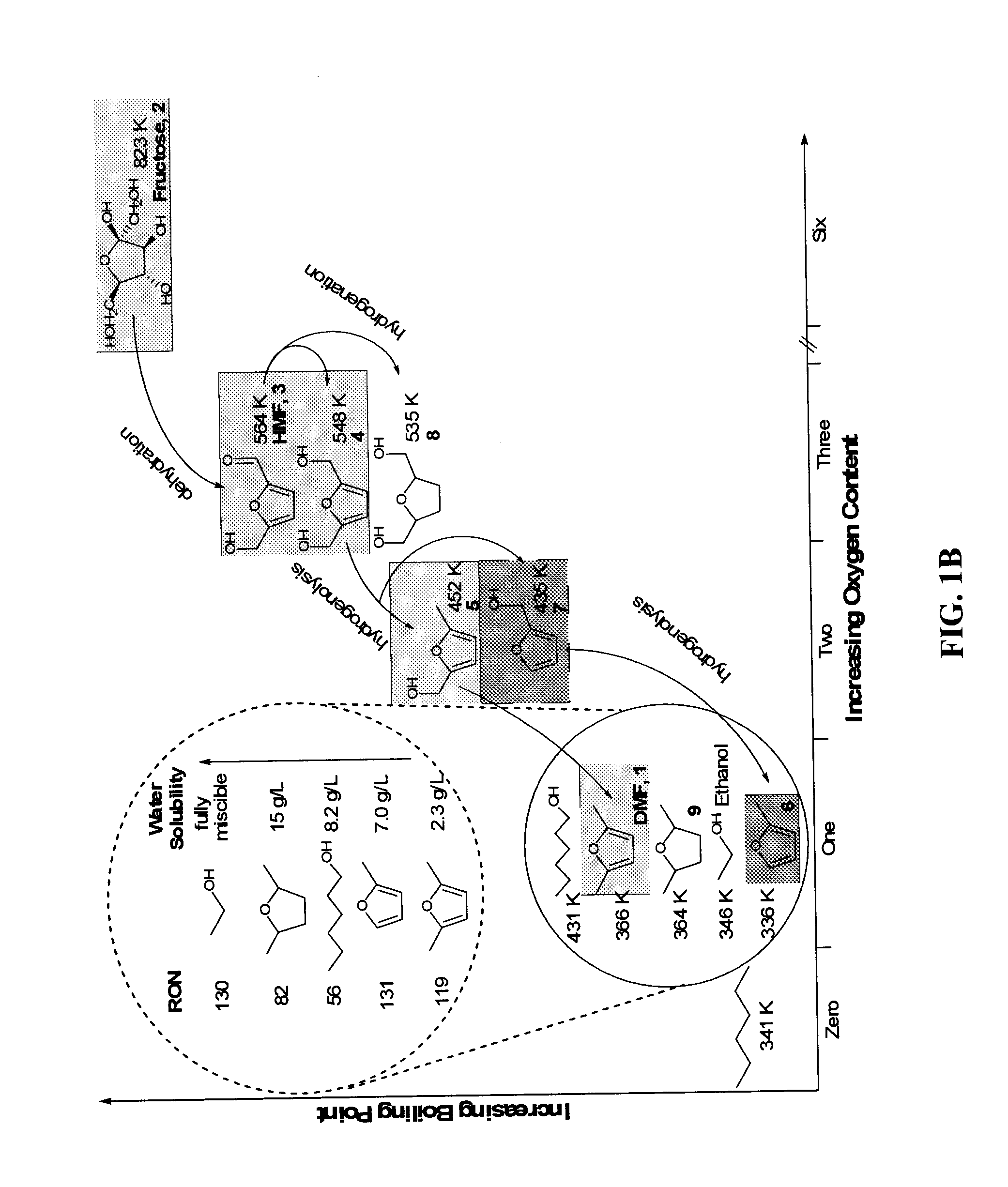
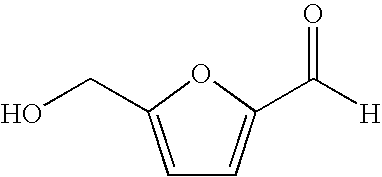
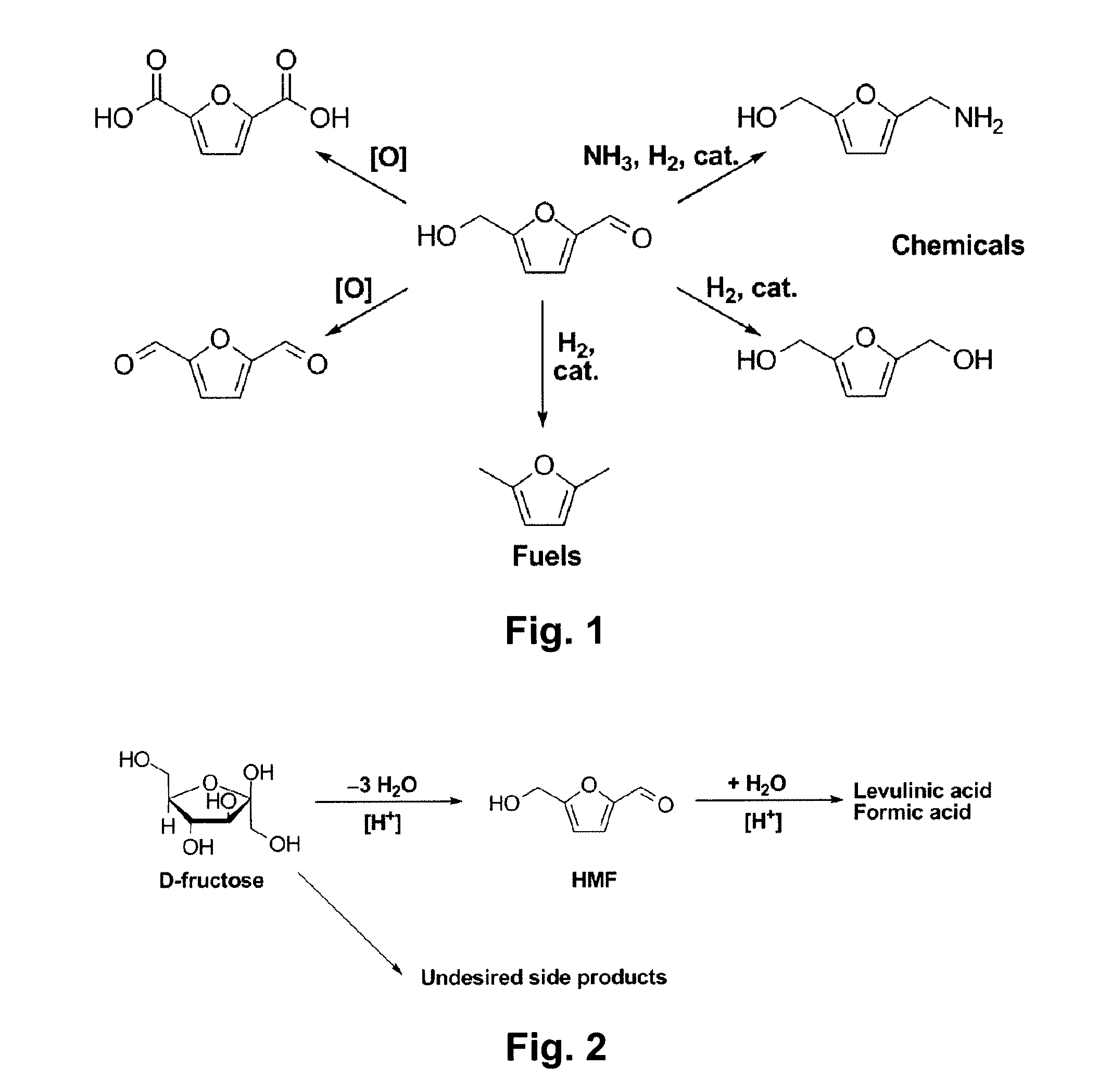
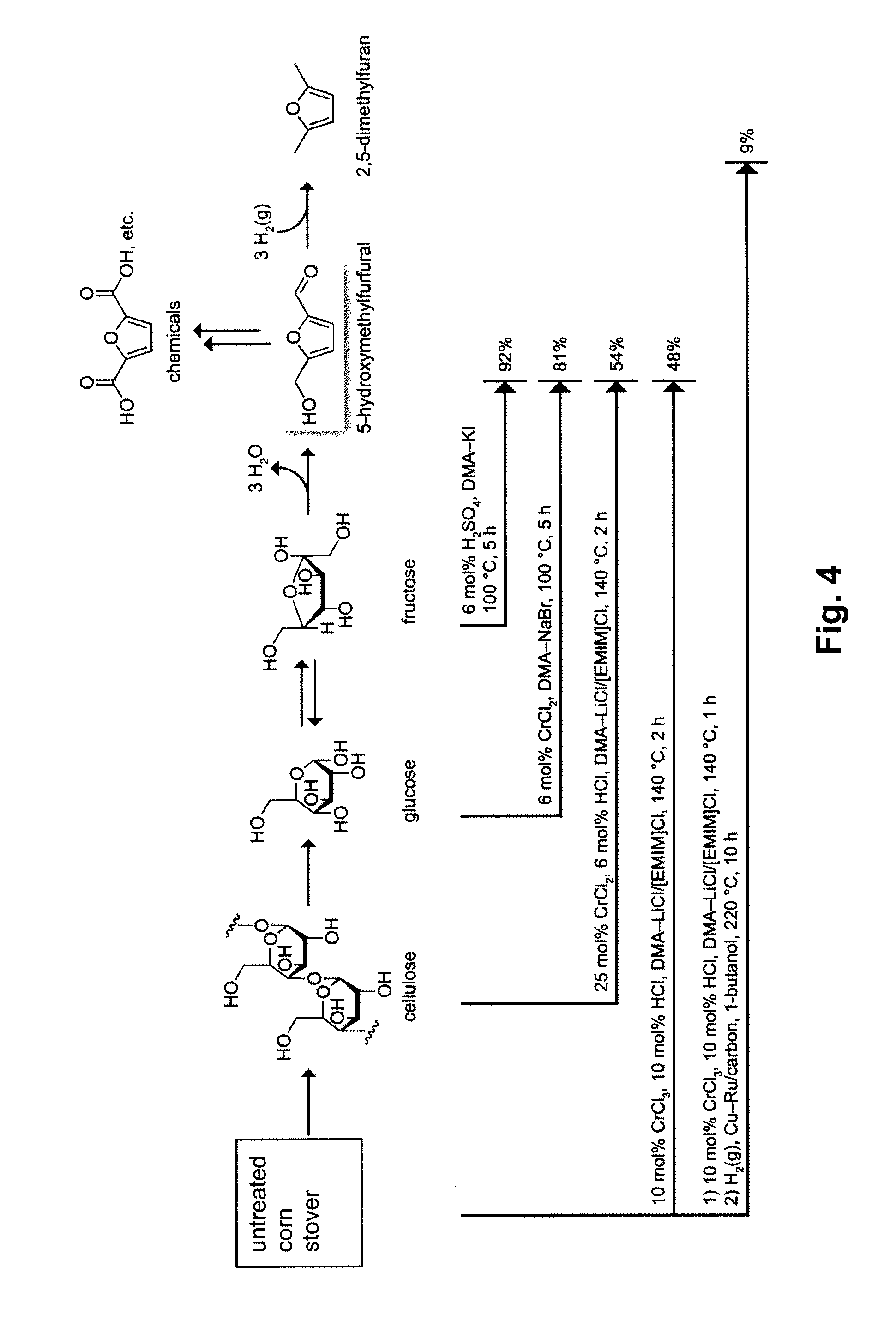
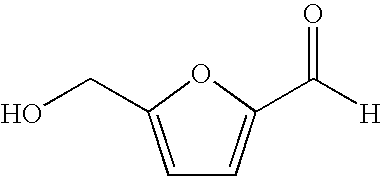
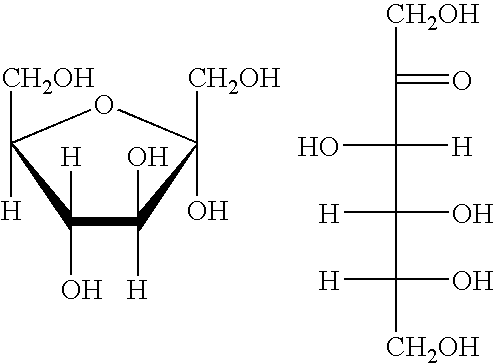
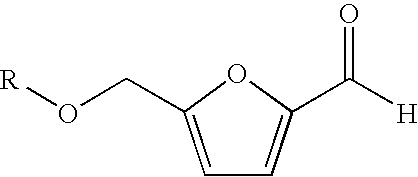
Hydroxymethylfurfural (HMF), also 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of certain sugars. It is a white low-melting solid (although commercial samples are often yellow) which is highly soluble in both water and organic solvents. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups.
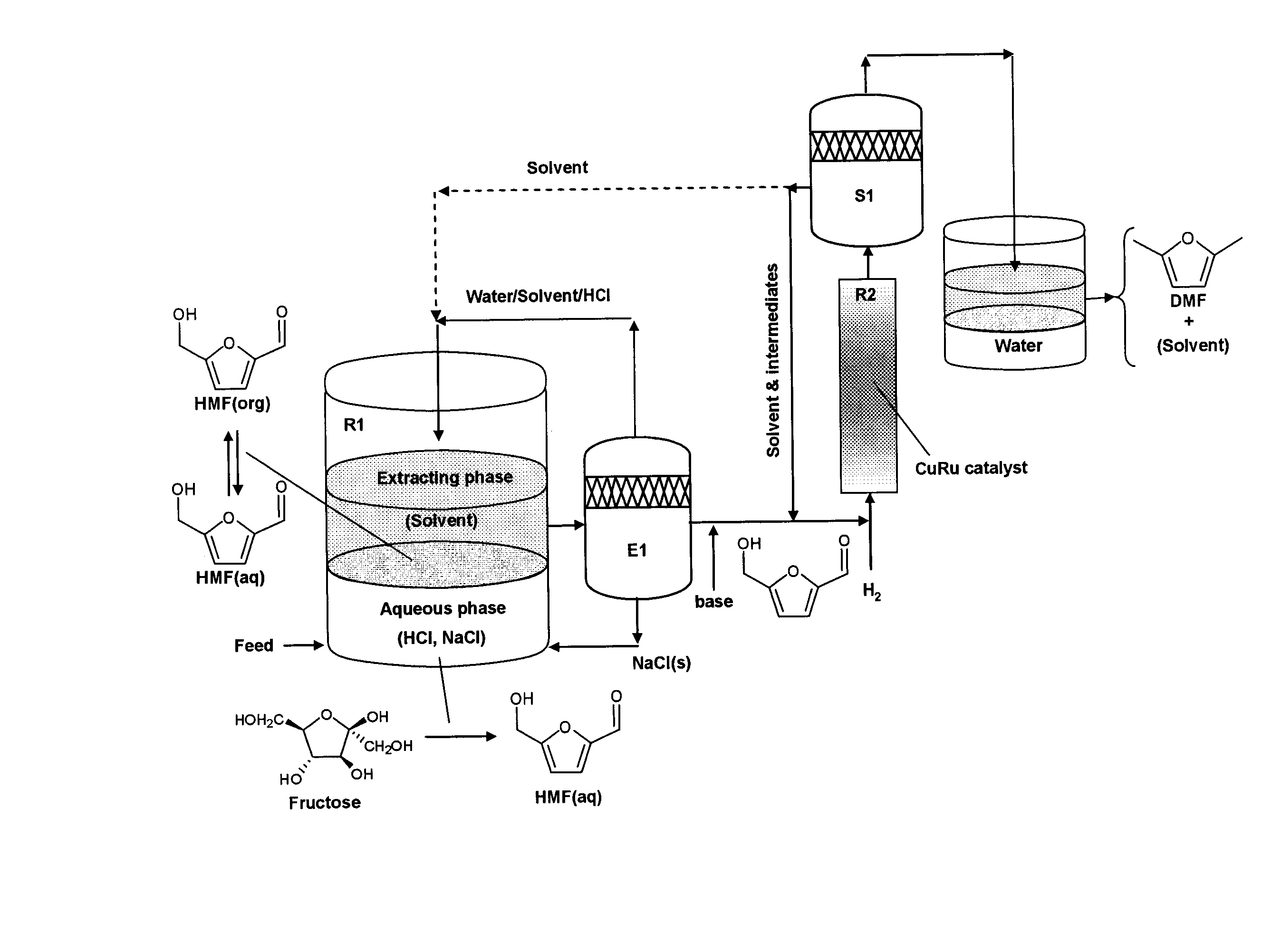
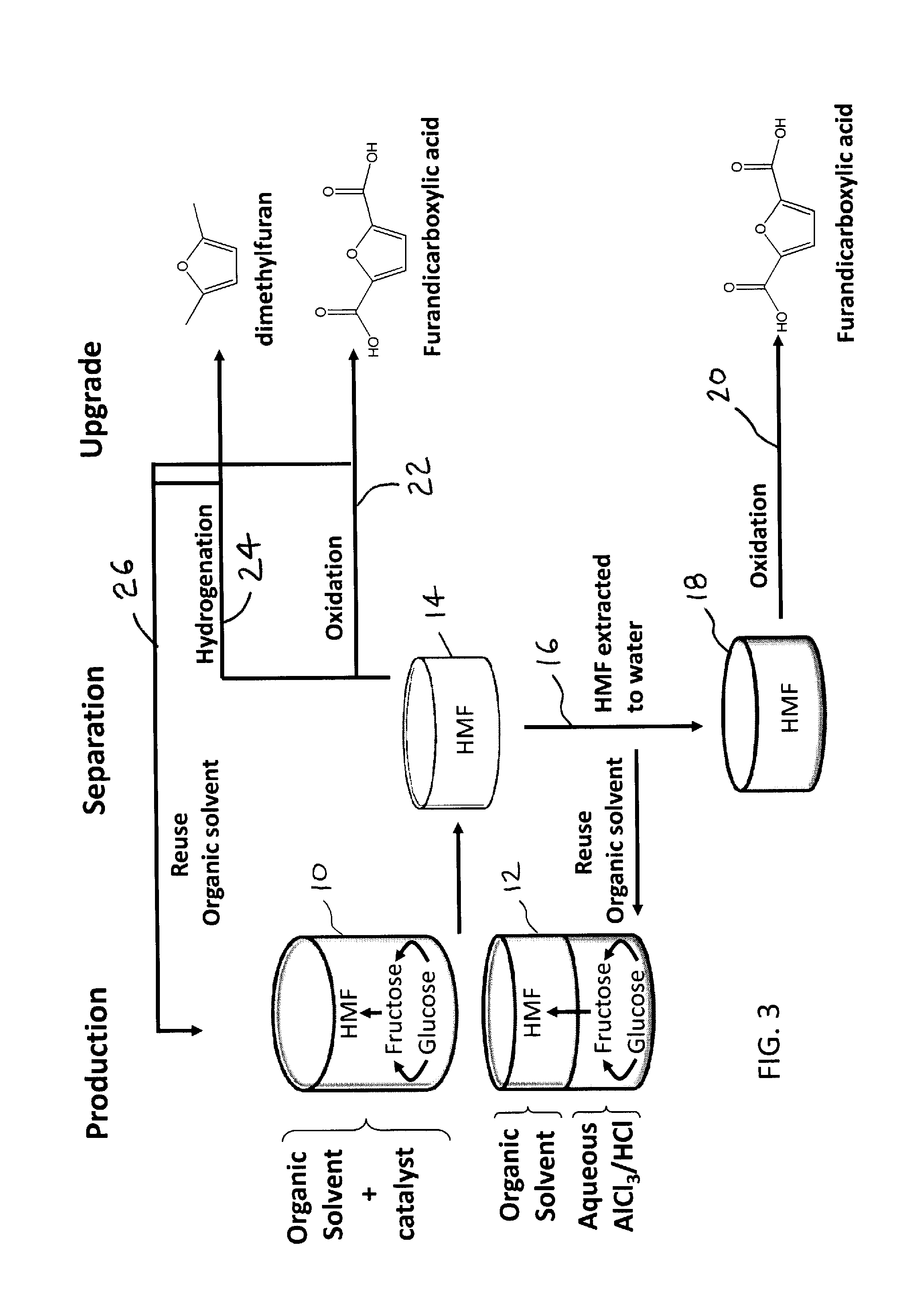
Catalytic process for producing furan derivatives in a biphasic reactor
Described is a catalytic process for converting sugars to furan derivatives (e.g. 5-hydroxymethylfurfural, furfural, dimethylfuran, etc.) using a biphasic reactor containing a reactive aqueous phase and an organic extracting phase. The process provides a cost-effective route for producing di-substituted furan derivatives. The furan derivatives are useful as value-added intermediates to produce polymers, as precursors to diesel fuel, and as fuel additives.
Owner:WISCONSIN ALUMNI RES FOUND
Conversion of carbohydrates to hydroxymethylfurfural (HMF) and derivatives
InactiveUS20090156841A1Increase conversion rateStable formOrganic compound preparationCarboxylic compound preparationMANGANESE ACETATEFuran
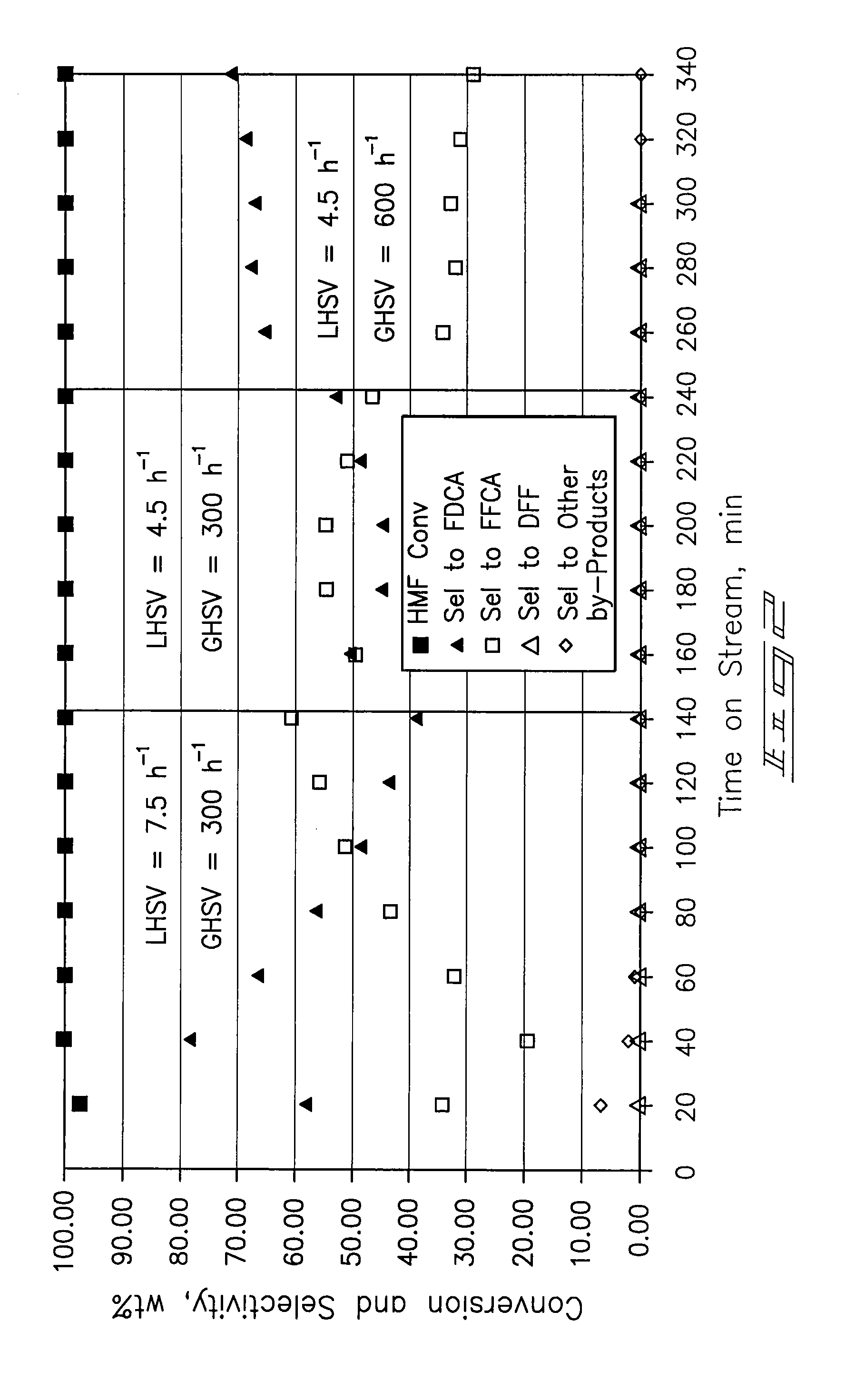
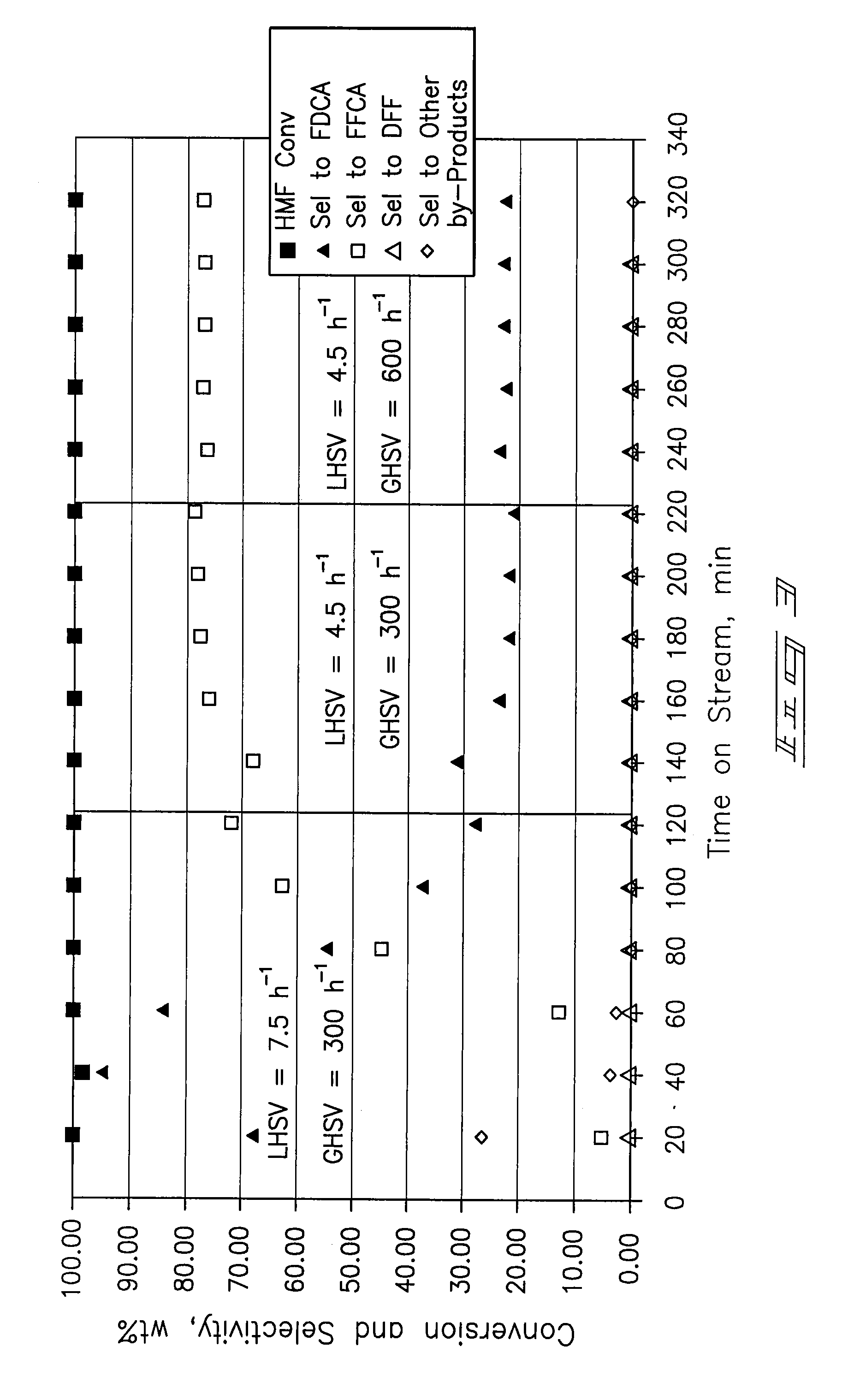
A method of producing substantially pure HMF, HMF esters and other derivatives from a carbohydrate source by contacting the carbohydrate source with a solid phase catalyst. A carbohydrate starting material is heated in a solvent in a column and continuously flowed through a solid phase catalyst in the presence of an organic acid, or heated with the organic acid and a solid catalyst in solution to form a HMF ester. Heating without organic acid forms HMF. The resulting product is purified by filtration to remove the unreacted starting materials and catalyst. The HMF ester or a mixture of HMF and HMF ester may then be oxidized to 2,5-furandicarboxylic acid (FDCA) by combining the HMF ester with an organic acid, cobalt acetate, manganese acetate and sodium bromide under pressure. Alternatively, the HMF ester may be reduced to form a furan or tetrahydrofuran diol.
Owner:ARCHER DANIELS MIDLAND CO
Methods for conversion of carbohydrates in ionic liquids to value-added chemicals
Methods are described for converting carbohydrates including, e.g., monosaccharides, disaccharides, and polysaccharides in ionic liquids to value-added chemicals including furans, useful as chemical intermediates and / or feedstocks. Fructose is converted to 5-hydroxylmethylfurfural (HMF) in the presence of metal halide and acid catalysts. Glucose is effectively converted to HMF in the presence of chromium chloride catalysts. Yields of up to about 70% are achieved with low levels of impurities such as levulinic acid.
Owner:BATTELLE MEMORIAL INST
Catalytic process for producing furan derivatives in a biphasic reactor
Owner:WISCONSIN ALUMNI RES FOUND
Hydroxymethylfurfural Reduction Methods and Methods of Producing Furandimethanol
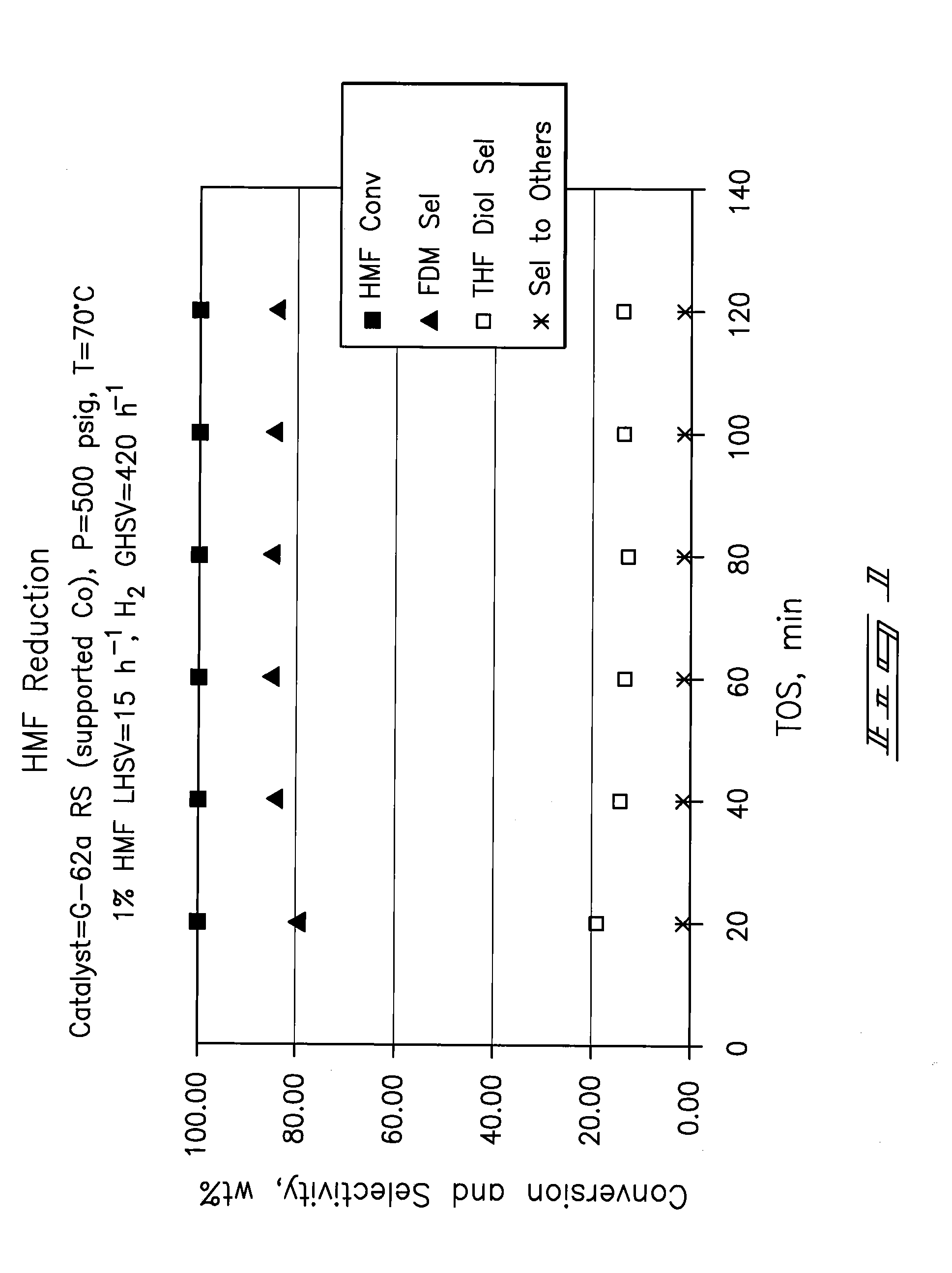
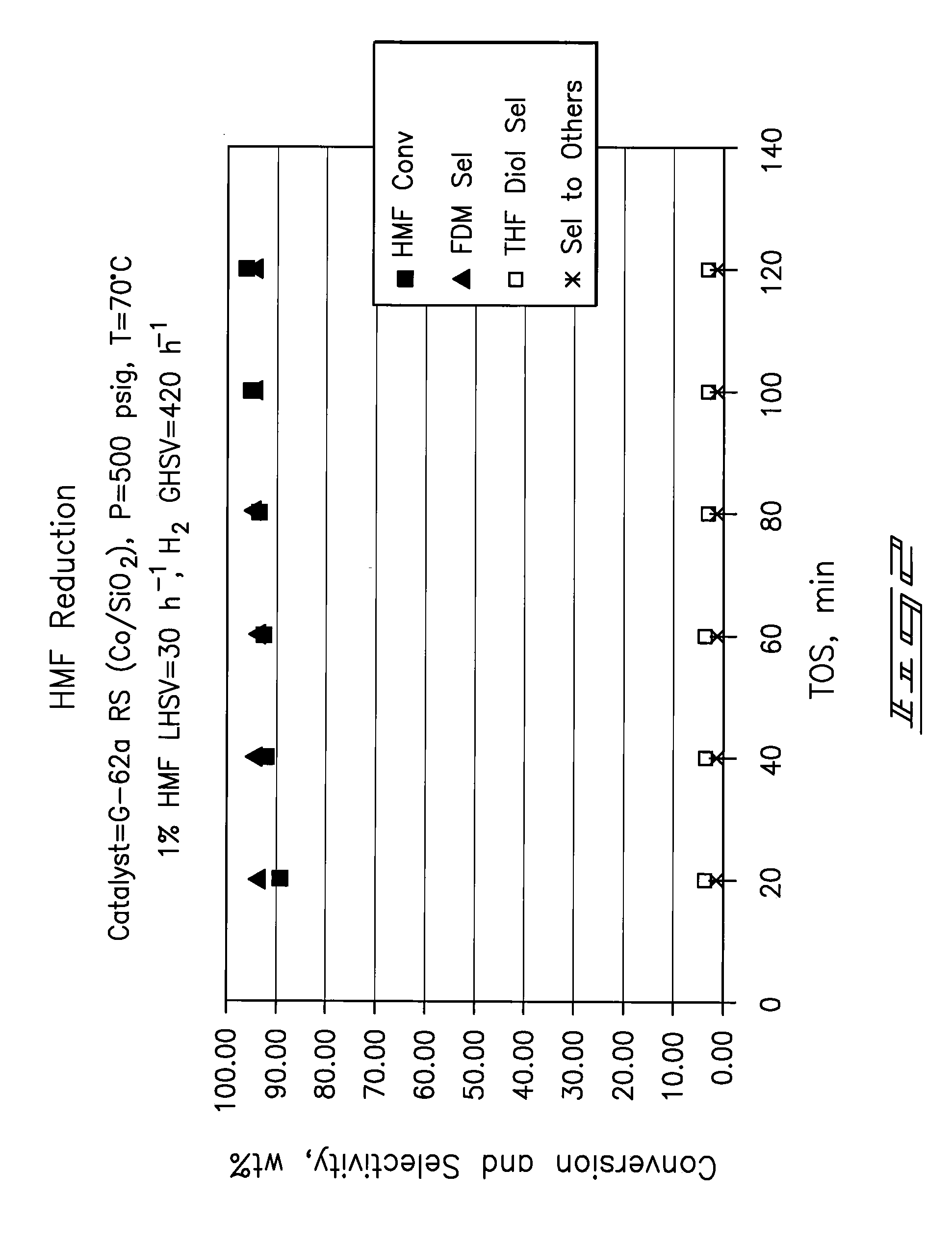
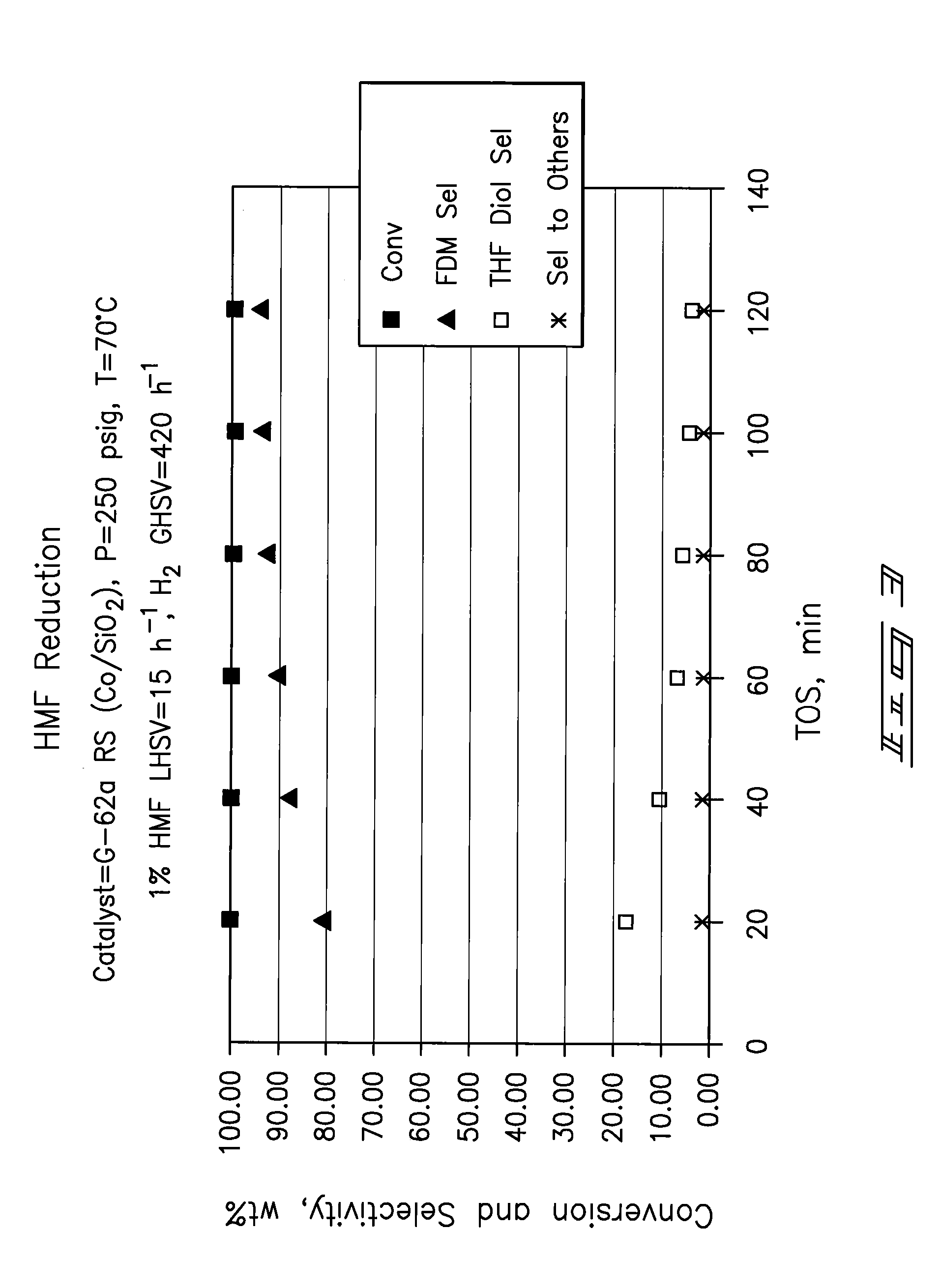
A method of reducing hydroxymethylfurfural (HMF) where a starting material containing HMF in a solvent comprising water is provided. H2 is provided into the reactor and the starting material is contacted with a catalyst containing at least one metal selected from Ni, Co, Cu, Pd, Pt, Ru, Ir, Re and Rh, at a temperature of less than or equal to 250° C. A method of hydrogenating HMF includes providing an aqueous solution containing HMF and fructose. H2 and a hydrogenation catalyst are provided. The HMF is selectively hydrogenated relative to the fructose at a temperature at or above 30° C. A method of producing tetrahydrofuran dimethanol (THFDM) includes providing a continuous flow reactor having first and second catalysts and providing a feed comprising HMF into the reactor. The feed is contacted with the first catalyst to produce furan dimethanol (FDM) which is contacted with the second catalyst to produce THFDM.
Owner:BATTELLE MEMORIAL INST
Method of producing 2,5-furandicarboxylic acid
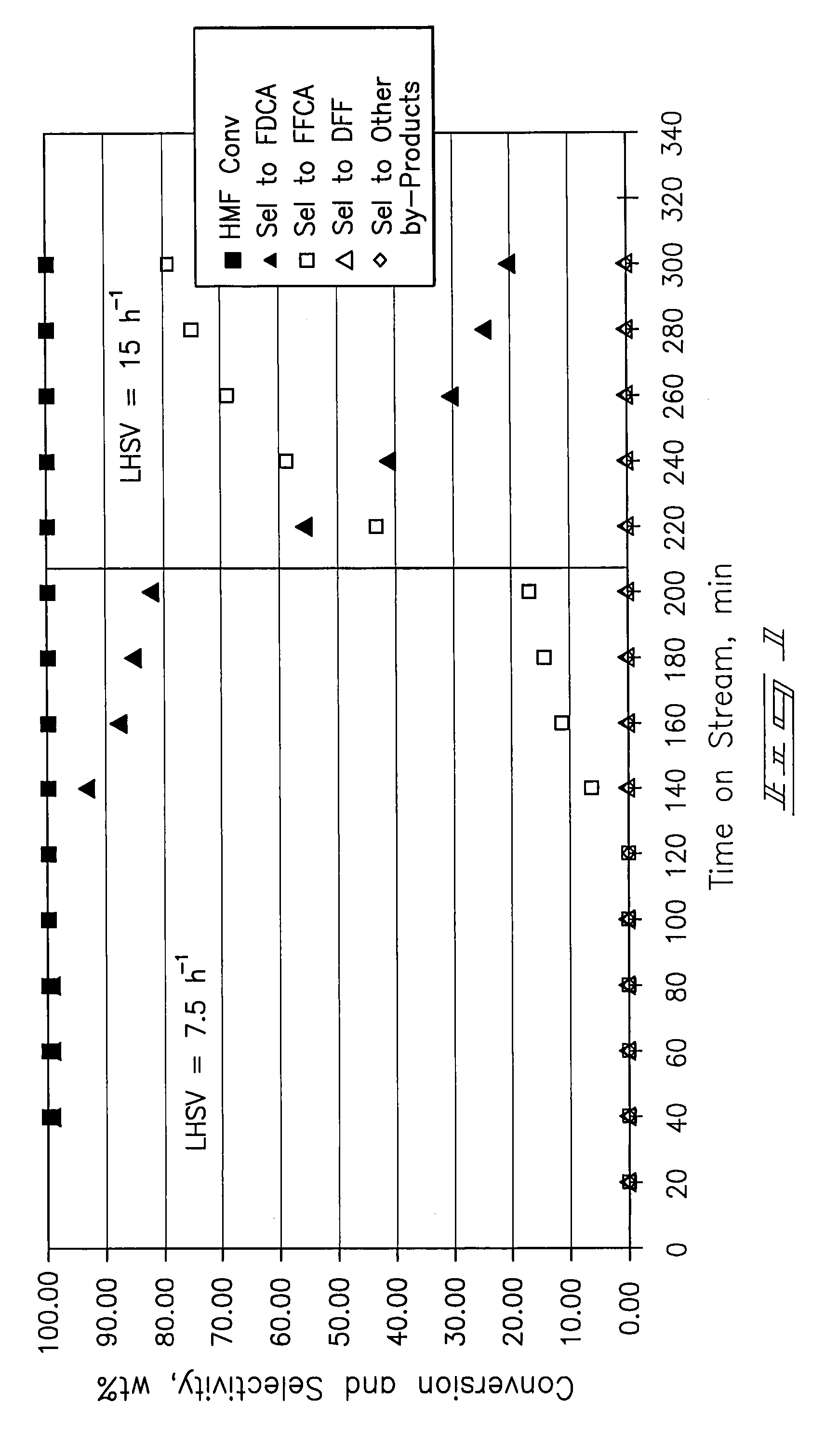
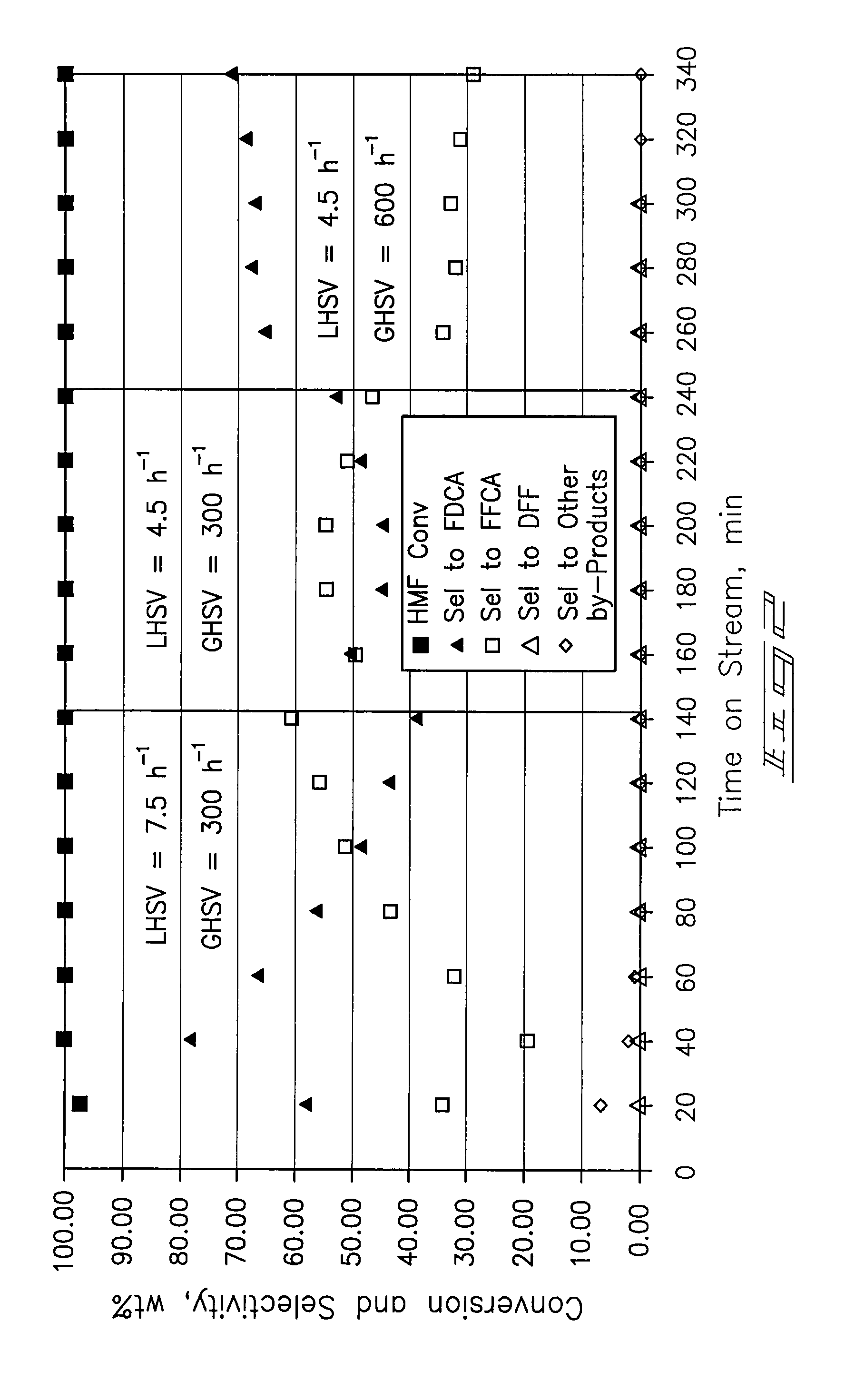
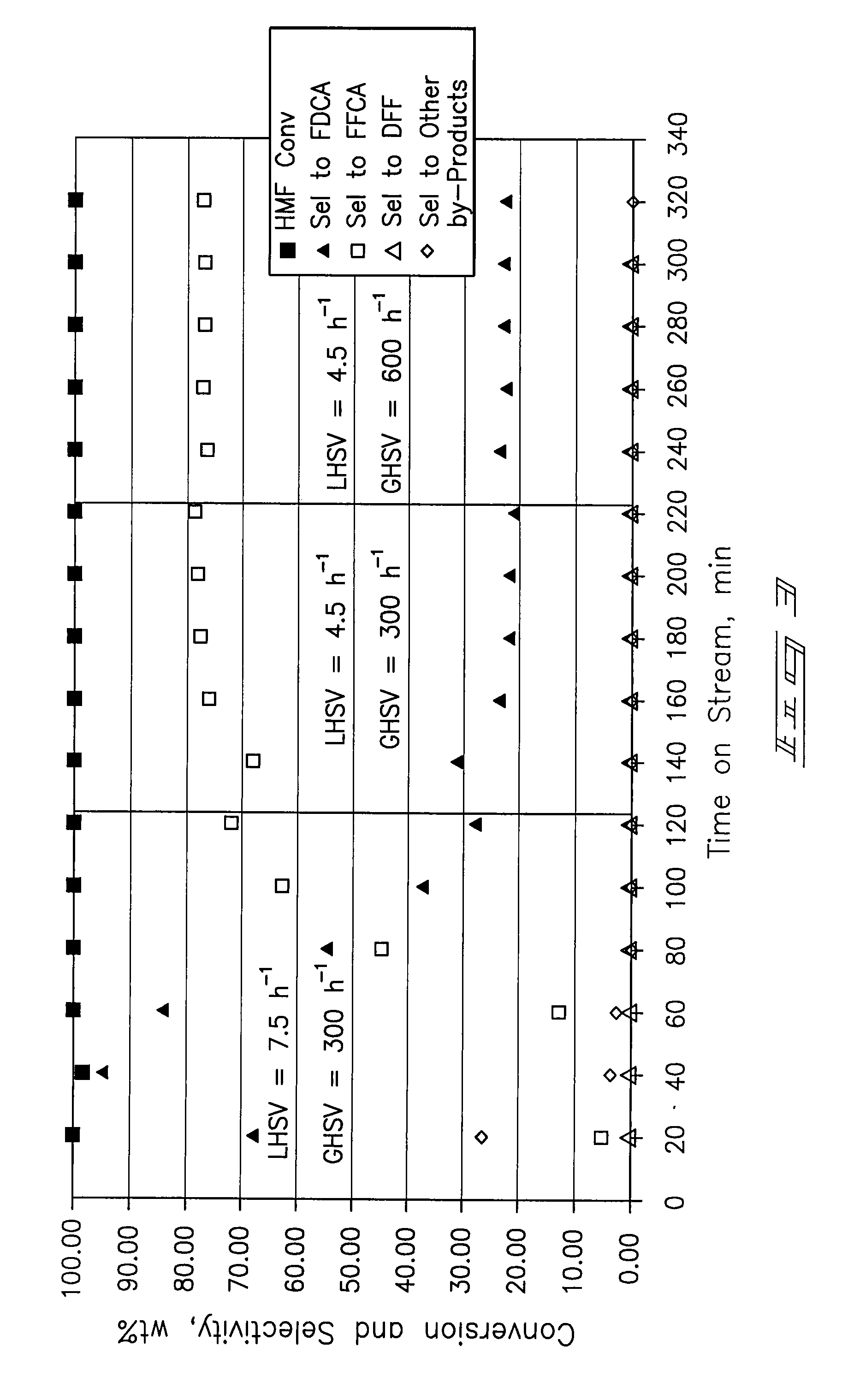
Provided is a method of producing FDCA by which high-purity FDCA can be produced in high yield. 2,5-furandicarboxylic acid is produced by: bringing 5-hydroxymethylfurfural into contact with an oxidant in an organic acid solvent in the presence of bromine and a metal catalyst; and allowing 5-hydroxymethylfurfural and the oxidant to react with each other while removing water produced by the reaction.
Owner:CANON KK
Processes for the preparation and purification of hydroxymethylfuraldehyde and derivatives
A method for utilizing an industrially convenient fructose source for a dehydration reaction converting a carbohydrate to a furan derivative is provided. Recovery methods also are provided. Embodiments of the methods improve upon the known methods of producing furan derivatives.
Owner:ARCHER DANIELS MIDLAND CO
Hydroxymethyl Furfural Oxidation Methods
InactiveUS20080103318A1Organic chemistryMetal/metal-oxides/metal-hydroxide catalystsPlatinumHydrogen
A method of oxidizing hydroxymethylfurfural (HMF) includes providing a starting material which includes HMF in a solvent comprising water into a reactor. At least one of air and O2 is provided into the reactor. The starting material is contacted with the catalyst comprising Pt on a support material where the contacting is conducted at a reactor temperature of from about 50° C. to about 200° C. A method of producing an oxidation catalyst where ZrO2 is provided and is calcined. The ZrO2 is mixed with platinum (II) acetylacetonate to form a mixture. The mixture is subjected to rotary evaporation to form a product. The product is calcined and reduced under hydrogen to form an activated product. The activated product is passivated under a flow of 2% O2.
Owner:BATTELLE MEMORIAL INST
Biomass refining by selective chemical reactions
InactiveUS20110071306A1High substrate loadingShort reaction timeSugar derivativesSugar derivatives preparationChemical reactionPropanoic acid
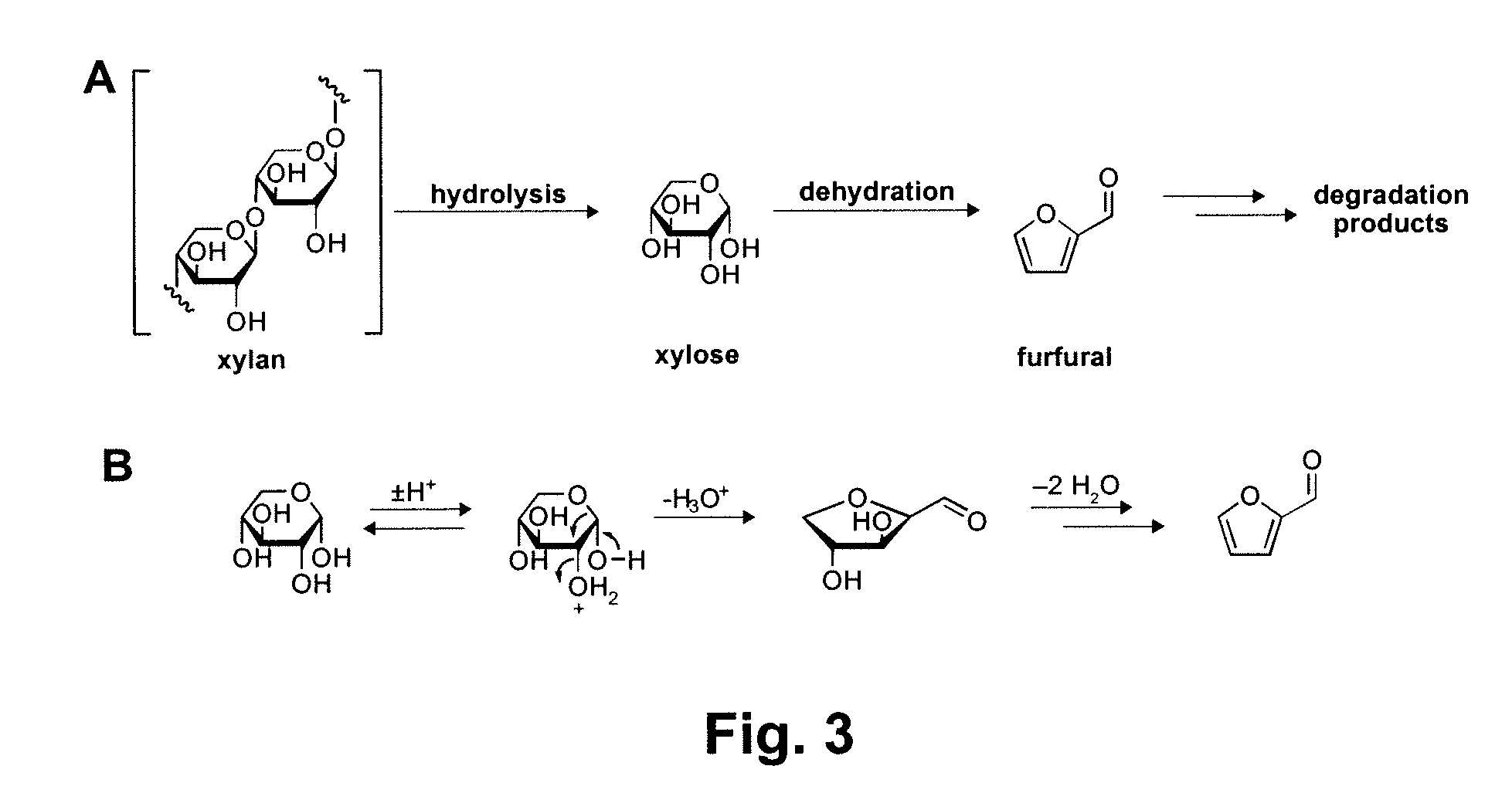
A method is disclosed for the acid hydrolysis of carbohydrates in or from biomass, using a solvent system including an aqueous ether, where the ether form a majority of the system, which affords high yields to the platform chemicals such as 2-furfural and 5-hydroxymethylfurfural (5-HMF). The later can also undergo a domino reaction to chemicals including levulinic acid, particularly with oxygenated anions and greater water content. A total dissolution and reaction of biomass occurs under a range of relatively mild conditions (combined Severity range ˜2.2-2.6). Lignin and lignin derived products can be easily separated by precipitation.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chemical Transformation of Lignocellulosic Biomass into Fuels and Chemicals
A method for converting a carbohydrate to a furan in a polar aprotic solvent in the presence of a chloride, bromide, or iodide salt or a mixture thereof and optionally in the presence of an acid catalyst, a metal halide catalyst and / or an ionic liquid (up to 40 wt %). The method can be employed in particular to produce furfural or 5-hydroxymethylfurfural.
Owner:WISCONSIN ALUMNI RES FOUND
Method for preparing 5-hydroxymethylfurfural by solid acid catalysis
InactiveCN102399201AAccelerated precipitationEasy to separateCatalyst carriersOrganic chemistrySolid acidCarbonization
The invention discloses a method for preparing 5-hydroxymethylfurfural by solid acid catalysis. The method comprises the following steps of taking lignin residue hydrolyzed by biomass as carrier raw material of solid acid; synthesizing the raw material into solid acid through a two-step way of carbonization and sulfonation, so that the waste material is recycled and the problem of disposal of pollutants is solved. In the dehydration reaction of lignin-based solid acid catalyzed fructose and glucose, the yield of 5-hydroxymethylfurfural can be respectively 84% and 68%; after the dehydration reaction, the lignin-based solid acid is easy to separate and reusable, the acid density of the solid acid can be reduced after being reused five times, and the yield of the 5-hydroxymethylfurfural is still higher than 75%. The method provided by the invention can be used for realizing emission without pollution, and is helpful for realizing environment-friendly producing process of 5-hydroxymethylfurfural.
Owner:XISHUANGBANNA TROPICAL BOTANICAL GARDEN CHINESE ACAD OF SCI
Injectio for activating pulse and its preparing method
ActiveCN1628793AImprove securityEnhance pharmacological effectsUnknown materialsCardiovascular disorderMedicinal herbsHydroxymethylfurfural
Disclosed is an injection for activating pulse and its preparing method, wherein the injection is prepared from the effective parts extracted from Chinese medicinal herbs including gen-seng, lilyturf root, schisandra chinensis. The prepared injection preparation can increase gen-seng glycosides extraction rate.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Method for cogeneration of 5-hydroxymethyl-furfural, acetylpropionic acid and formic acid by high temperature catalytic dehydration of glucose in formic acid
The invention discloses a method for coproduction of 5-hydroxymethyl furfural, an acetylpropionic acid and a formic acid through high-temperature catalysis and dehydration of the formic acid of glucose. The method specifically comprises the following steps: firstly, establishment of a formic acid reaction system, namely the glucose is added into the formic acid solution, and the weight ratio of the glucose to the formic acid in the reaction system is 0.05-0.2 to 1; and the mixture is reacted for 2 to 6 hours in the presence of the catalyst at a temperature of between 120 and 220 DEG C which is higher than the boiling temperature of the formic acid, and the reaction system is single-phase reaction or biphase reaction; and secondly, separation of products after reaction by a fractionating tower device, namely graded separation of 5-HMF, LA and the formic acid. The method can convert the glucose into the products, namely the 5-HMF, the LA and the formic acid with high added values through effective acid catalysis and dehydration, and has high conversion of reactant during the reaction process and obvious economic benefit; and the 5-HMF, the LA and the formic acid can be directly taken as chemical products to be further converted, and are good raw materials for synthesizing other chemical products.
Owner:SOUTH CHINA UNIV OF TECH
Hydroxymethyl furfural oxidation methods
Owner:BATTELLE MEMORIAL INST
Method for preparing furfural compounds from biomass
The invention relates to a method for preparing furfural compounds from biomass. The method specifically comprises the preparation of a solid acid catalyst and a reaction process of the solid acid catalyst. The method disclosed by the invention can also be applied to biomass derivatives. The method comprises the steps of adding biomass or biomass derivatives and the solid acid catalyst to a reactor, filling with protective gas, and adopting an organic solvent / saturated inorganic salt aqueous-solution two-phase reaction system, thereby obtaining furfural and 5-hydroxymethylfurfural in a high yield manner under mild conditions. The method has the advantages that after the reaction is completed, the produced furfural and 5-hydroxymethylfurfural are efficiently extracted into an upper-layer organic phase, the solid acid catalyst and an unreacted substrate are retained in a bottom-layer water phase, the furfural, the 5-hydroxymethylfurfural or a mixture of the furfural and the 5-hydroxymethylfurfural can be obtained through simple separation, and fine chemicals and liquid fuels can be prepared in a manner that the furfural, the 5-hydroxymethylfurfural or the mixture of the furfural and the 5-hydroxymethylfurfural serves as a reaction intermediate; and the used catalyst is pollution-free and can be recovered and reused, so that the method has good industrial application prospects.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing 2,5-furandicarboxylic acid through catalytic oxidation
ActiveCN103724303AImprove catalytic performanceLow costOrganic chemistryCatalytic oxidationReusability
The invention discloses a method for synthesizing 2,5-furandicarboxylic acid through catalytic oxidation by using 5-hydroxymethylfurfural as a raw material. 5-hydroxymethylfurfural can be oxidized by using oxygen or air as an oxidant in the presence of a catalyst prepared by loading an alkaline carrier with noble metal with high efficiency and high selectivity to synthesize 2,5-furandicarboxylic acid. A catalytic reaction is easy to operate and mild in conditions; when 5-hydroxymethylfurfural is fully converted, the selectivity of the product-2,5-furandicarboxylic acid can reach more than 99%; the catalyst is good in reusability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Methods of making alkyl lactates and alkyl levulinates from saccharides
InactiveUS20150045576A1Organic compound preparationPreparation by carbon monoxide or formate reactionLevulinic acidAlcohol
Unique methods have been developed to convert saccharides into value-added products such as alkyl lactates, lactic acid, alkyl levulinates, levulinic acid, and optionally alkyl formate esters and / or hydroxymethylfurfural (HMF). Useful catalysts include Lewis acid catalysts and Brønsted acid catalysts including mineral acids, metal halides, immobilized heterogeneous catalysts functionalized with a Brønsted acid group or a Lewis acid group, or combinations thereof. The saccharides are contacted with the catalyst in the presence of various alcohols.
Owner:BATTELLE MEMORIAL INST
Method to convert monosaccharides to 5-(hydroxymethyl) furfural (HMF) using biomass-derived solvents
Described is a process to produce hydroxymethyl furfural (HMF) from biomass-derived sugars. The process includes the steps of reacting a C5 and / or C6 sugar-containing reactant derived from biomass in a monophasic or biphasic reaction solution comprising water and a co-solvent. The co-solvent can be beta-, gamma-, and / or delta-lactones derived from biomass, tetrahydrofuran (THF) derived from biomass, and / or methyltetrahydrofuran (MTHF) derived from biomass. The reaction takes place in the presence of an acid catalyst and a dehydration catalyst for a time and under conditions such that at least a portion of glucose or fructose present in the reactant is converted to HMF.
Owner:WISCONSIN ALUMNI RES FOUND
Method for preparing 5-hydroxymethyl-furfural
InactiveCN101386611ALow costCorrosion resistance requirements are not highOrganic chemistryAlternative fuelsSolvent
The invention relates to a method for transforming biomass sugar source into 5-hydroxymethyl-furfural, in particular to a method for preparing the 5-hydroxymethyl-furfural. The method comprises the following steps: using ionic liquid as a solvent, hexose or hexose source biomass as raw material substrates, and 0.5 to 50 percent (relative to the mass of the biomass sugar source) of acidic ionic liquid, inorganic acid or organic acid as a catalyst, and performing the reaction of materials for 1.5 minutes to 23 hours at normal pressure and at a temperature of between 80 DEG C and 100 DEG C to efficiently generate HMF. The method has the advantages of high HMF selectivity, less acid consumption, moderate operating conditions, fast reaction, reusable ionic liquid, simple process, environment protection and the like, and opens up a new approach for preparing commodity chemicals and replacing fuels starting from renewable biological resources.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
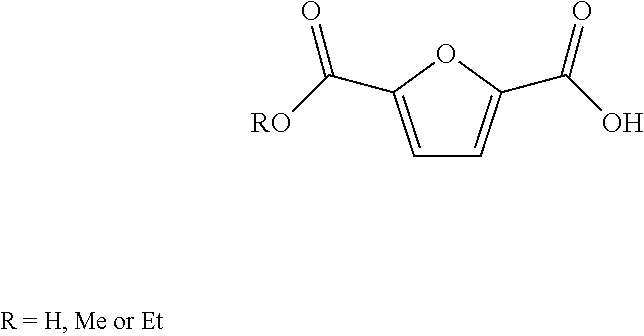
Method for the preparation of 2,5-furandicarboxylic acid and esters thereof
ActiveUS8519167B2Organic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranHydroxymethylfurfural
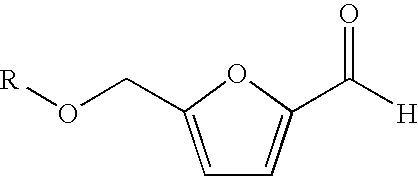
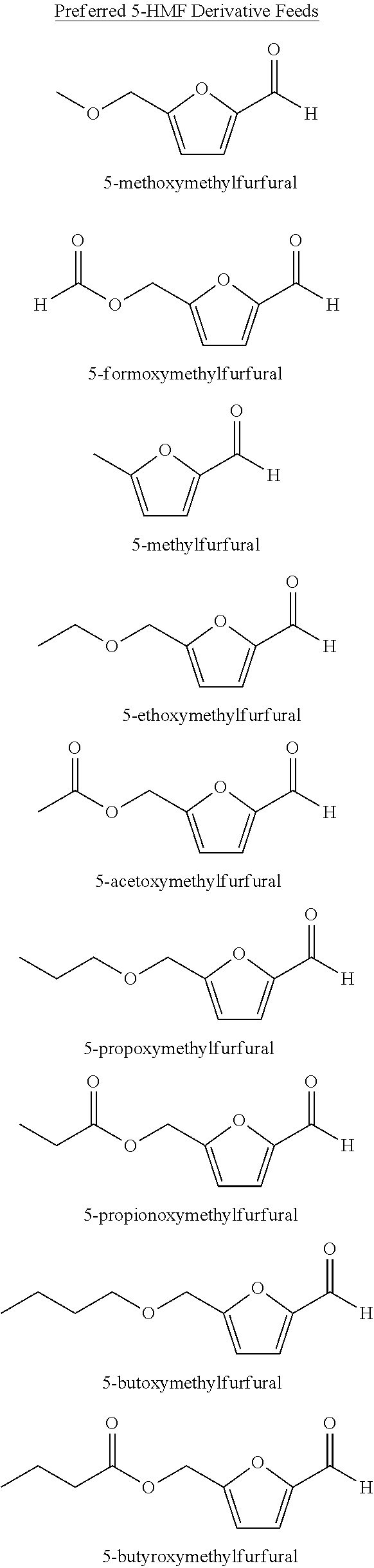
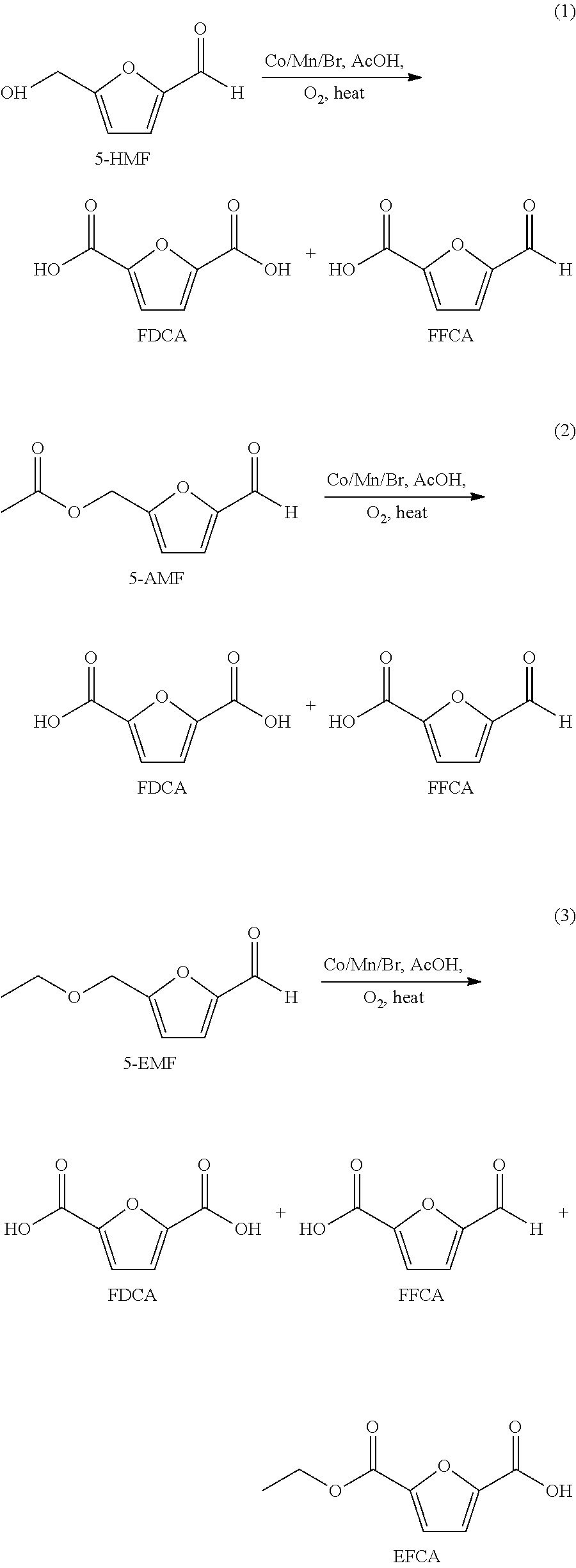
A method for the preparation of 2,5-furandicarboxylic acid (“FDCA”) and / or an alkyl ester of FDCA includes the step of contacting a feed comprising a starting material selected from 5-alkoxymethylfurfural, 2,5-di(alkoxymethyl)furan and a mixture thereof with an oxidant in the presence of an oxidation catalyst. The feed may also comprise 5-hydroxymethylfurfural as a further starting material.
Owner:FURANIX TECH BV
Method to produce, recover and convert furan derivatives from aqueous solutions using alkylphenol extraction
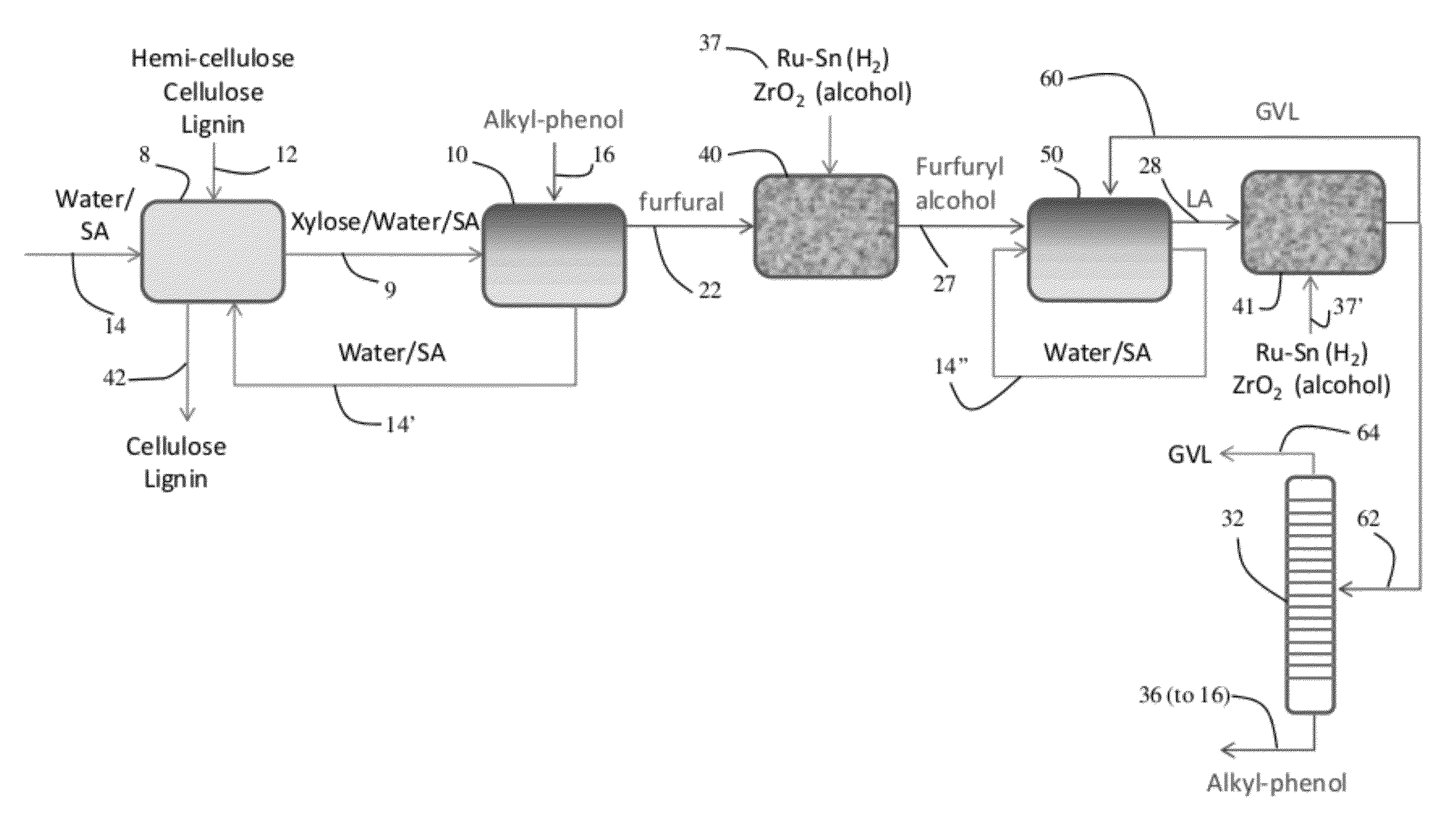
ActiveUS20120302765A1Cost-effectiveLow production costOrganic compound preparationCarboxylic acid esters preparationFuranAlkylphenol
Described is a catalytic process for converting biomass to furan derivatives (e.g., furfural, furfuryl alcohol, etc.) using a biphasic reactor containing a reactive aqueous phase and an organic extracting phase containing an alkylphenol. The process provides a cost-effective route for producing furfural, furfuryl alcohol, levulinic acid hydroxymethylfurfural, γ-valerolactone, and the like. The products formed are useful as value-added intermediates to produce polymers, as precursors to diesel fuel, and as fuel additives.
Owner:WISCONSIN ALUMNI RES FOUND
Method for preparing 5-hydroxymethyl-furfural
InactiveCN101456850ALow costCorrosion resistance requirements are not highOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAlternative fuelsSolvent
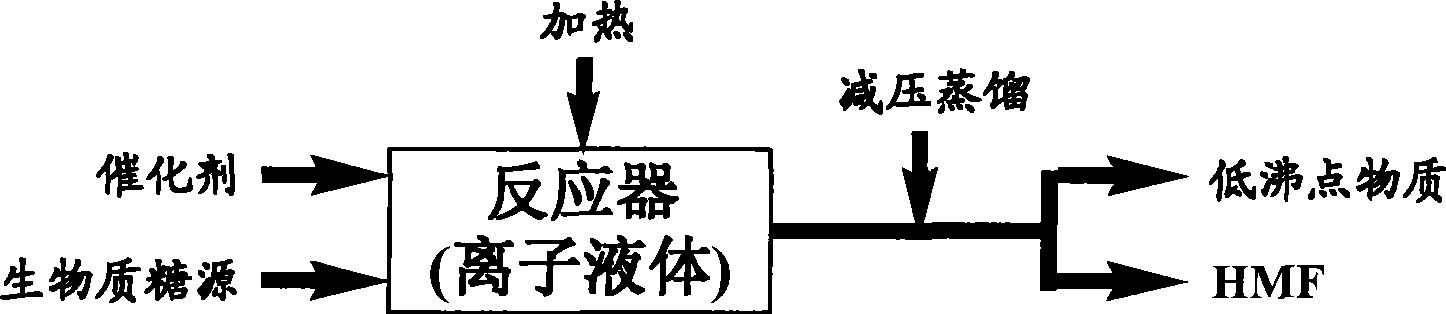
The invention relates to a method for preparing 5-hydroxymethylfurfural (HMF) by transforming a biomass saccharine source. The method comprises the following concrete steps: using an ionic liquid as a solvent, hexose or hexose source biomass as a raw material substrate, and acid as a catalyst, heating the mixture to react for 5 minutes to 20 hours at normal pressure, and then depressurizing and distilling the mixture under the condition that an operation temperature is not higher than 180 DEG C to obtain the HMF after the reaction is ended; and after the distilled remainder is cooled, directly adding the remainder into the biomass saccharine source, and repeating the processes of reaction and separation to obtain the HMF, so as to realize recycling of an ionic liquid catalyst system and semi-continuous preparation of the HMF. By using the method, once through yield of the HMF reaches as high as 94 percent. The method has the advantages of high selectivity, low acid consumption, mild condition, quick reaction, reusability of the ionic liquid, low cost, simple process, environmental protection, semi-continuous production and the like, and provides new technology for industrialized production of the HMF by transforming the biomass saccharine source, so as to develop a new path for preparing common chemicals in a large scale by using biomass resources and replacing fuels.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing 2,5-diformylfuran by oxidizing 5-hydroxymethylfurfural
ActiveCN101987839AReduce dosageLow pricePhysical/chemical process catalystsOrganic chemistryOxygenHydroxymethylfurfural
The invention discloses a method for preparing 2,5-diformylfuran by selectively oxidizing 5-hydroxymethylfurfural in the presence of a catalyst. In the method, oxygen or air is used as an oxygen source, the catalyst is a composite catalyst system a composite catalyst system consisting of vanadium oxide and an auxiliary agent, and at the temperature of between 20 and 120 DEG C, the 5-hydroxymethylfurfural is selectively oxidized into the 2,5-diformylfuran. The method has high oxidation efficiency and high product yield; the catalyst is cheap and readily available; the reaction conditions are mild; the product is easy to separate and purify; and the purity of the product is over 99 percent. Therefore, the method has bright application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
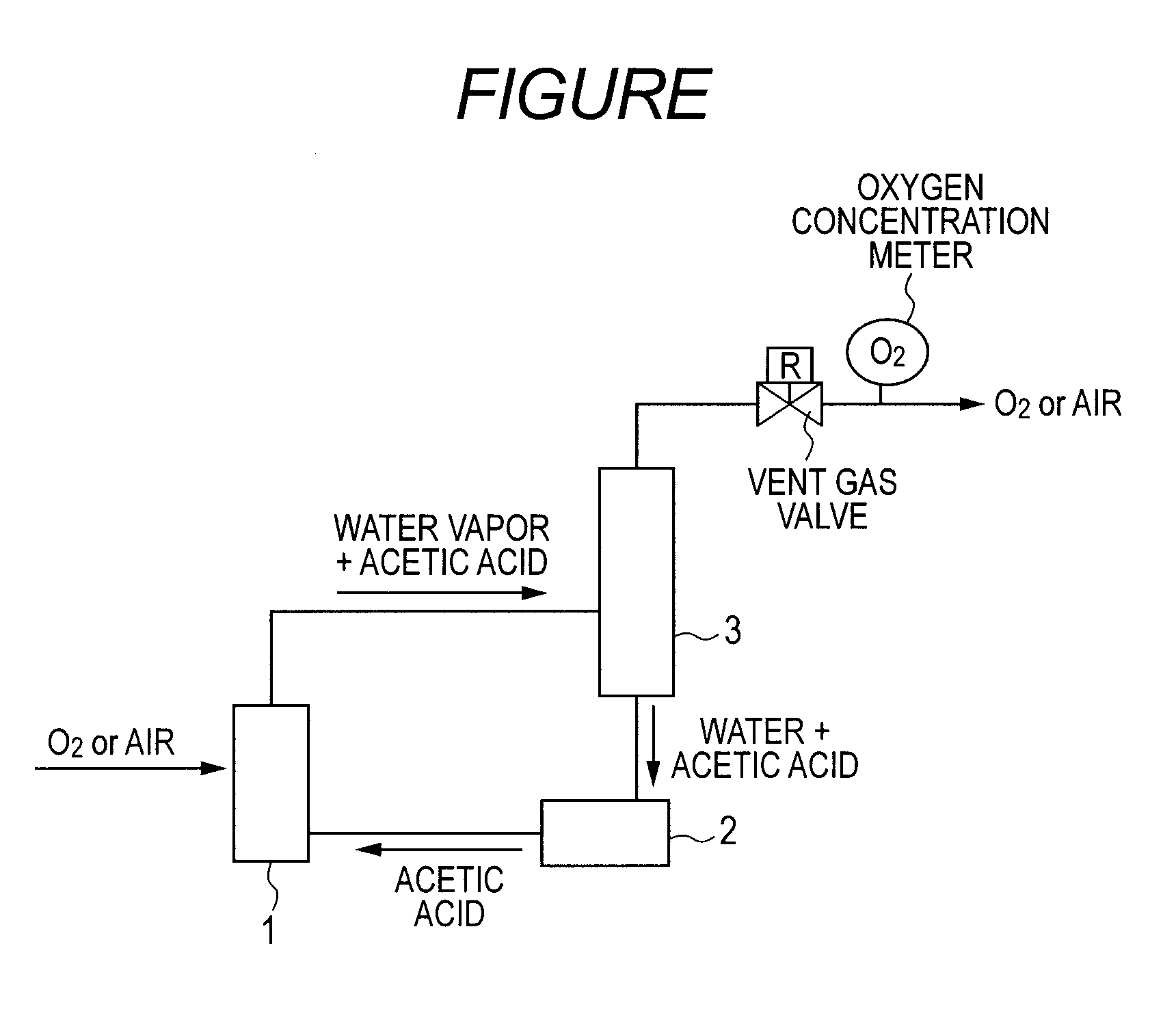
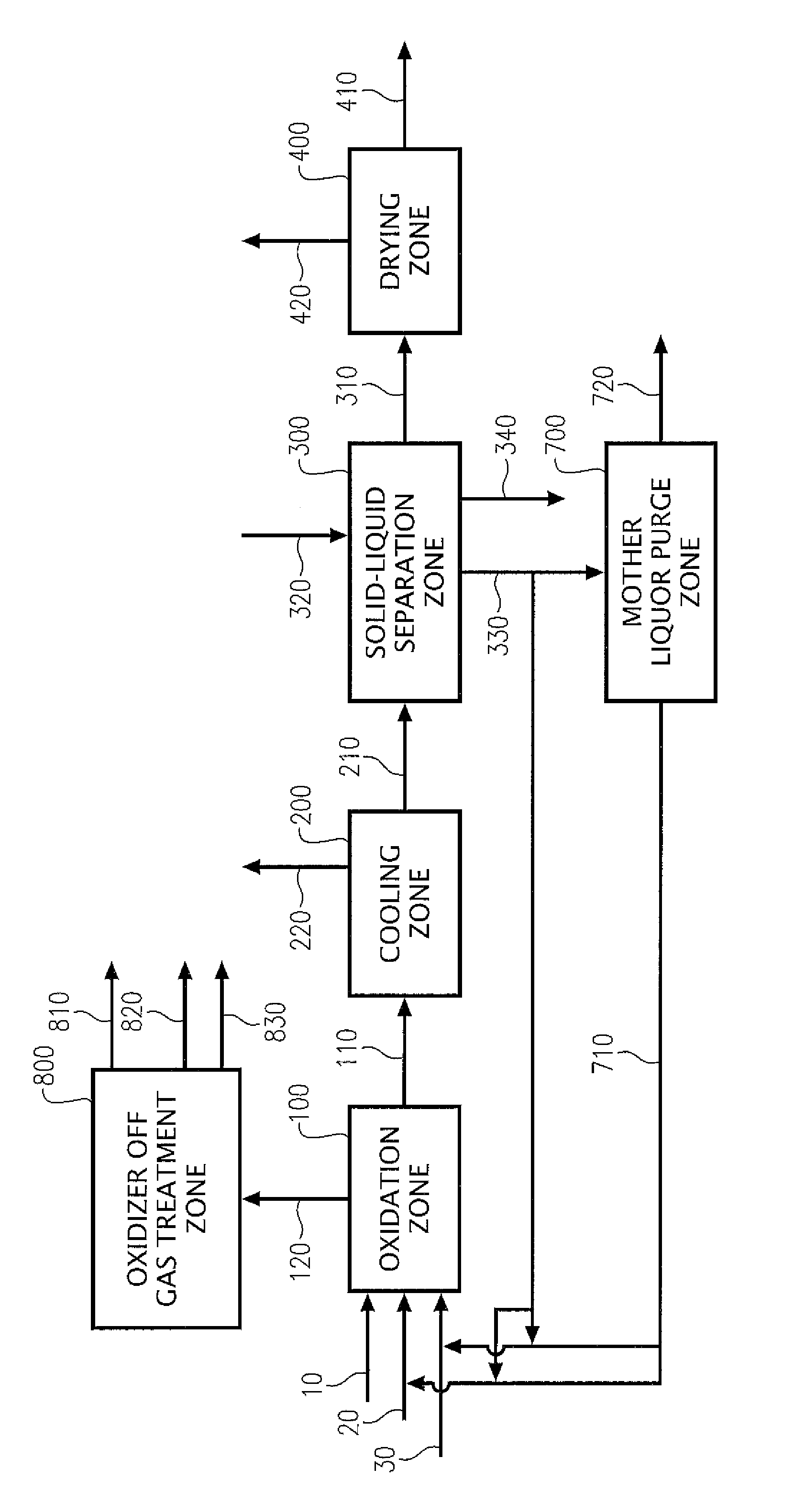
Oxidation process to produce a crude dry carboxylic acid product
Disclosed is a process to produce a dry purified carboxylic acid product comprising furan-2,5-dicarboxylic acid (FDCA). The process comprises oxidizing at least one oxidizable compound selected from the following group: 5-(hydroxymethyl)furfural (5-HMF), 5-HMF esters (5-R(CO)OCH2-furfural where R=alkyl, cycloalkyl and aryl), 5-HMF ethers (5-R′OCH2-furfural, where R′=alkyl, cycloalkyl and aryl), 5-alkyl furfurals (5-R″-furfural, where R″=alkyl, cycloalkyl and aryl), mixed feed-stocks of 5-HMF and 5-HMF esters and mixed feed-stocks of 5-HMF and 5-HMF ethers and mixed feed-stocks of 5-HMF and 5-alkyl furfurals to generate a crude carboxylic acid slurry comprising FDCA, cooling a crude carboxylic acid slurry in cooling zone to form a cooled slurry stream. The cooled slurry stream is routed to a solid-liquid separation zone to generate a crude wet cake stream comprising FDCA that is dried in a drying zone to generate a dry carboxylic acid product stream comprising crude FDCA (cFDCA).
Owner:EASTMAN CHEM CO
Method for preparing 5-hydroxymethylfurfural by degrading carbonhydrate through ionic liquid
InactiveCN102399203AAvoid separationShort reaction timeOrganic chemistryHydroxymethylfurfuralReaction system
The invention discloses a method for preparing 5-hydroxymethylfurfural (HMF) by degrading carbonhydrate through ionic liquid. The method comprises the following steps of: adding the carbonhydrate, the ionic liquid and a cocatalyst into a reactor, introducing an entrainer, continuously vacuumizing a reaction system to make the vacuum degree kept between 100 and 500Pa, and performing catalysis reaction at the temperature of between 120 and 180DEG C for 10 to 60 minutes; and during reaction, putting a separator connected with a product outlet of the reactor into an ice bath for collecting a product, wherein the separator is communicated with the product outlet of the reactor through a heat insulating connecting tube, and the separator has the same vacuum degree as the reactor. The catalysis reaction and product separation are coupled, and high vacuum distillation is adopted and the entrainer is introduced in the reaction process to make the generated water and 5-HMF leave the reaction system, so that the aim of enhancing the generation of the 5-HMF and the product separation is fulfilled and a problem that the 5-HMF is hard to separate is effectively solved.
Owner:ZHEJIANG UNIV
Process for preparing 5-hydroxymethyl-furfural
The invention relates to a preparation method for 5-hydroxymethyl furfural, which belongs to production technology of chemicals, in particular to a method for synthesizing 5-hydroxymethyl furfural (HMF) from carbohydrates by adopting a catalytic way. The method for preparing the 5-hydroxymethyl furfural uses heteropolyacid or heteropolyacid salt as a catalyst, and the 5-hydroxymethyl furfural with high purity can be obtained at a high yield by modulating the catalyst activity, choosing proper solvents, adding additives, optimizing the reaction temperature and time and improving the post treatment technologies, and the corrosion to devices and the environmental pollution can be avoided when using liquid super acids. In the system of saturated sodium chloride solution-positive butanol, the yield of the HMF can reach 70 percent under the catalysis reaction of phosphotungstic acid for 4 minutes at 175 DEG C.
Owner:DALIAN UNIV OF TECH
Method for purifying, reclaiming and condensing sugar in lignocellulose prehydrolysis liquid
ActiveCN101787398AEasy to operateEasy for industrialized continuous productionGlucose productionCelluloseFiltration membrane
The invention relates to a method for reclaiming and condensing sugar and removing toxicity inhibitors in lignocellulose prehydrolysis liquid by the applied nanofiltration technology. The method is realized by the following scheme: the pH value of the lignocellulose prehydrolysis liquid is firstly adjusted to 2.0-5.0, and the filtration pretreatment is carried out to remove suspended impurities; and then a filtration membrane is used for condensing sugar and remove inhibitors, the sugar of glucose, xylose and the like is trapped by the nanofiltration membrane, the weakly acidic inhibitors (formic acid, acetic acid, levulinic acid and the like) and the furfural inhibitors (furfural, 5-hydroxymethyl furfural and the like) continually penetrate through the nanofiltration membrane, the various inhibitors in the prehydrolysis liquid are removed, and the sugar in the prehydrolysis liquid is condensed to realize the purification, reclaiming and condensation of the sugar in the prehydrolysis liquid, so that the fermentability of the sugar is improved. The invention has the advantages of simple and safe operation, high efficiency and energy saving, and facilitates the continuous production; the nanofiltration has effects of purification, reclaiming and condensation; and the method can realize the industrial production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Processes for the preparation and purification of hydroxymethylfuraldehyde and derivatives
ActiveUS20060142599A1Easy to dehydrateOrganic compound preparationCarboxylic compound preparationRecovery methodFuran
A method for utilizing an industrially convenient fructose source for a dehydration reaction converting a carbohydrate to a furan derivative is provided. Recovery methods also are provided. Embodiments of the methods improve upon the known methods of producing furan derivatives.
Owner:ARCHER DANIELS MIDLAND CO
Method for catalytic preparation of 5-hydroxymethyl furfural from carbohydrates
InactiveCN102101851ALow costGood choiceOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsNiobiumSolid acid
The invention relates to a niobium-containing compound catalyst for efficiently converting carbohydrates into 5-hydroxymethyl furfural, which has high activity and selectivity for preparing hydroxymethyl furfural (HMF) by dehydrolyzing saccharides and is an ideal catalyst for preparing HMF. When the reaction is performed under moderate conditions, the target product HMF can have a high yield. The catalyst is environment-friendly, is easy to separate and recycle, can be reused, is convenient and practical to operate, and can not corrode the equipment, thereby being an ideal solid acid catalyst for preparing HMF, and having significant industrial application meanings.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing hydroxymethyl-furfural from glucide under low temperature and normal pressure
InactiveCN101475543AReduce equipment costsReduce manufacturing costOrganic chemistryOrganic solventDistillation
The invention provides a method for preparing hydroxymethylfurfural by saccharide at low temperature and normal pressure. The method comprises the following steps: mixing a saccharide raw material and a reaction solvent in a mass ratio of 1:3-50; heating the mixed solvent to a temperature of between 100 and 180 DEG C; adding a catalyst which is 0.01 to 2 percent of the mass of the solvent; reacting in an atmosphere of inert gas protection for 30 minutes to 10 hours; cooling a reaction system; extracting the hydroxymethylfurfural by an organic solvent, and generating the hydroxymethylfurfural by reaction; drying the hydroxymethylfurfural by distillation; and crystallizing the hydroxymethylfurfural to obtain the product. The method has the advantages of high selectivity, high yield, and low cost.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com