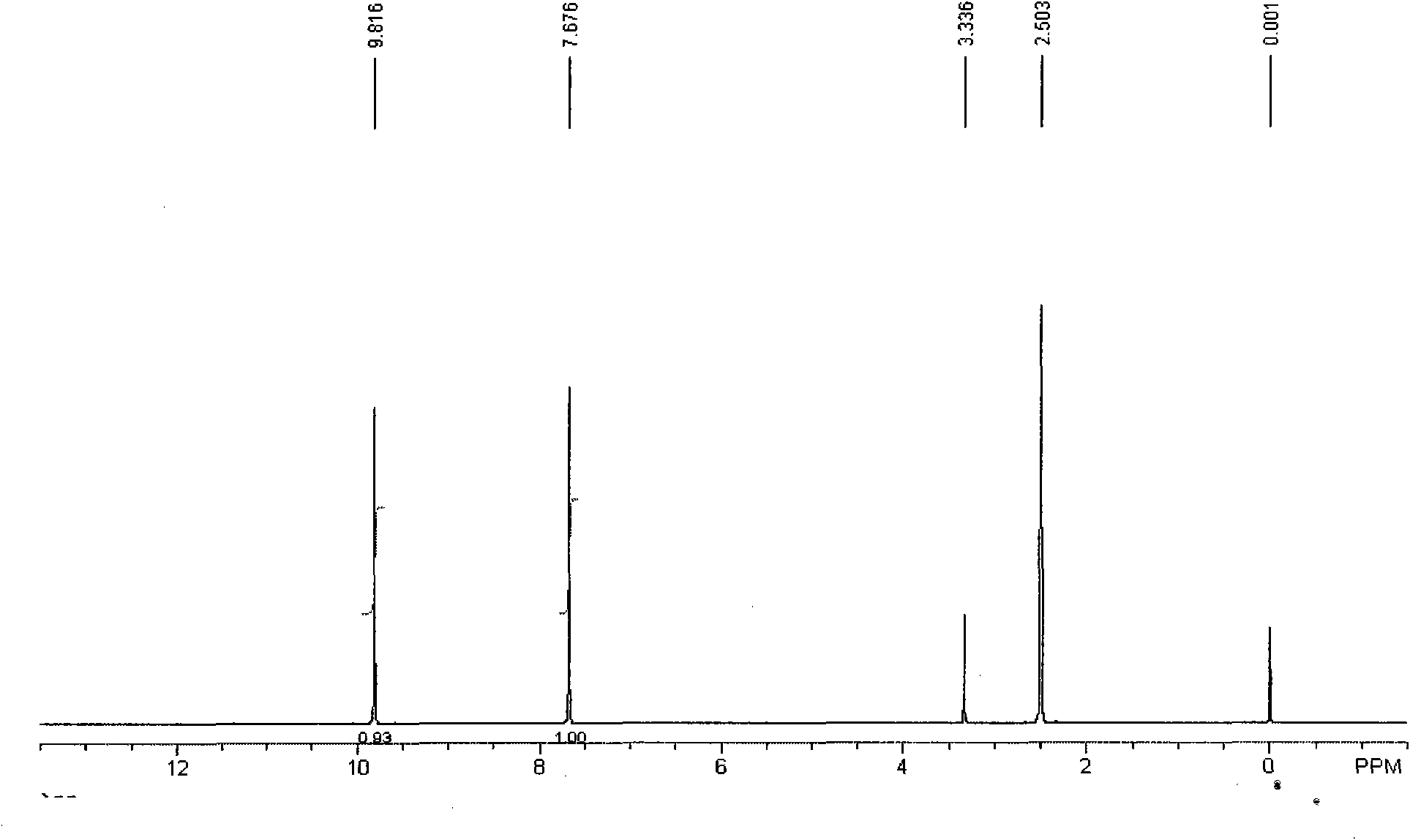
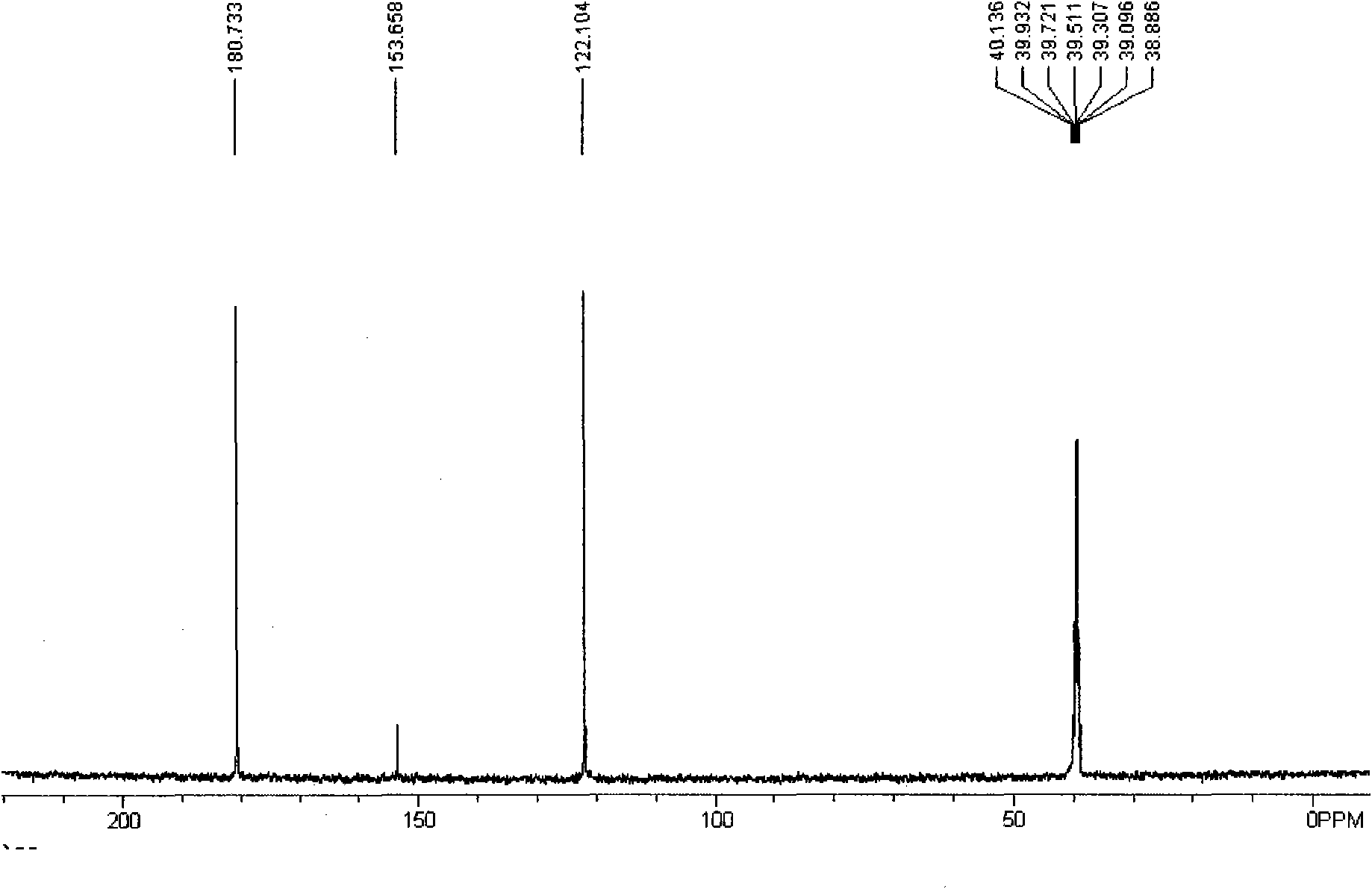
Method for preparing 2,5-diformylfuran by oxidizing 5-hydroxymethylfurfural
A technology of diformylfuran and hydroxymethylfurfural, which is applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of easy corrosion and toxicity, low product yield, unsatisfactory results, etc. Inexpensive, simple catalyst system, low catalyst consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Add 0.1mol of 5-hydroxymethylfurfural, 0.001mol of vanadyl phosphate, and 0.001mol of copper sulfate into a 100mL reaction kettle, add 5mL of acetonitrile, close the kettle, fill it with oxygen at a pressure of 0.5MPa, and then raise the temperature while stirring To 80 ℃, and keep 3h, if the oxygen partial pressure drops, add oxygen. After the reaction reached a predetermined time, the reacted mixture was cooled to room temperature. Samples were taken and analyzed by gas chromatography, and the conversion rate of raw materials was greater than 99%, and the selectivity was greater than 99%. The solvent was separated by distillation, the solid was washed with water, and a white solid was obtained by filtration. Dry in vacuo to give a white solid. The product purity reaches more than 99%. The isolated yield is 98%.
Embodiment 2
[0019] Example 2: 10-fold magnification experiment: Add 1mol 5-hydroxymethylfurfural, 0.01mol vanadyl phosphate, and 0.01mol copper sulfate to a 200mL reaction kettle, add 50mL acetonitrile, close the kettle, and fill it with oxygen at a pressure of 0.5MPa. Then the temperature was raised to 80°C under stirring, and kept for 3h, and oxygen was supplemented if the partial pressure of oxygen decreased. After the reaction reached the predetermined time, according to the method described in Example 1, the reacted mixture was cooled to room temperature for sampling analysis. The raw material conversion rate is greater than 99%, and the selectivity is greater than 99%. The isolated yield is greater than 97%.
Embodiment 3
[0020] Embodiment 3: 0.1mol 5-hydroxymethylfurfural, 0.003mol vanadyl sulfate, 0.003mol cupric chloride, 0.005mol potassium nitrite are added in the 100mL reactor, add 5mL toluene, close the still, fill in oxygen pressure is 0.1MPa, then raise the temperature to 120°C with stirring, and keep it for 12h. If the partial pressure of oxygen drops, supplement oxygen. After the reaction reaches the predetermined time, according to the method described in Example 1, cooling and sampling analysis, the conversion rate of raw materials is greater than 99%, and the selectivity is greater than 99%. The separation yield is greater than 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com