Method for preparing 2,5-furandicarboxylic acid through catalytic oxidation
A furandicarboxylic acid and catalytic oxidation technology, applied in the direction of organic chemistry, can solve the problems of reduced activity and poor catalyst reusability, and achieve the effects of low cost, improved catalytic performance and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
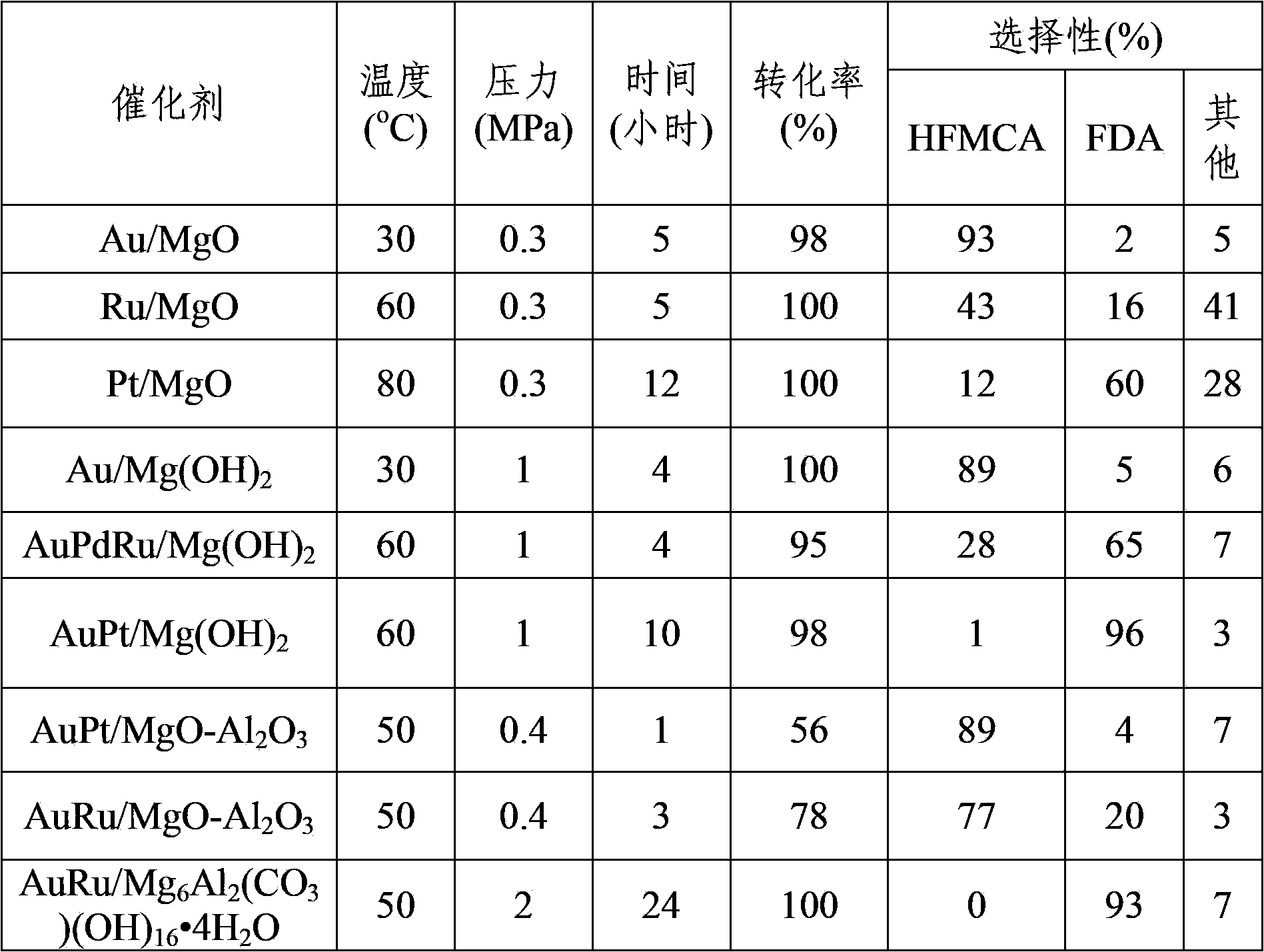
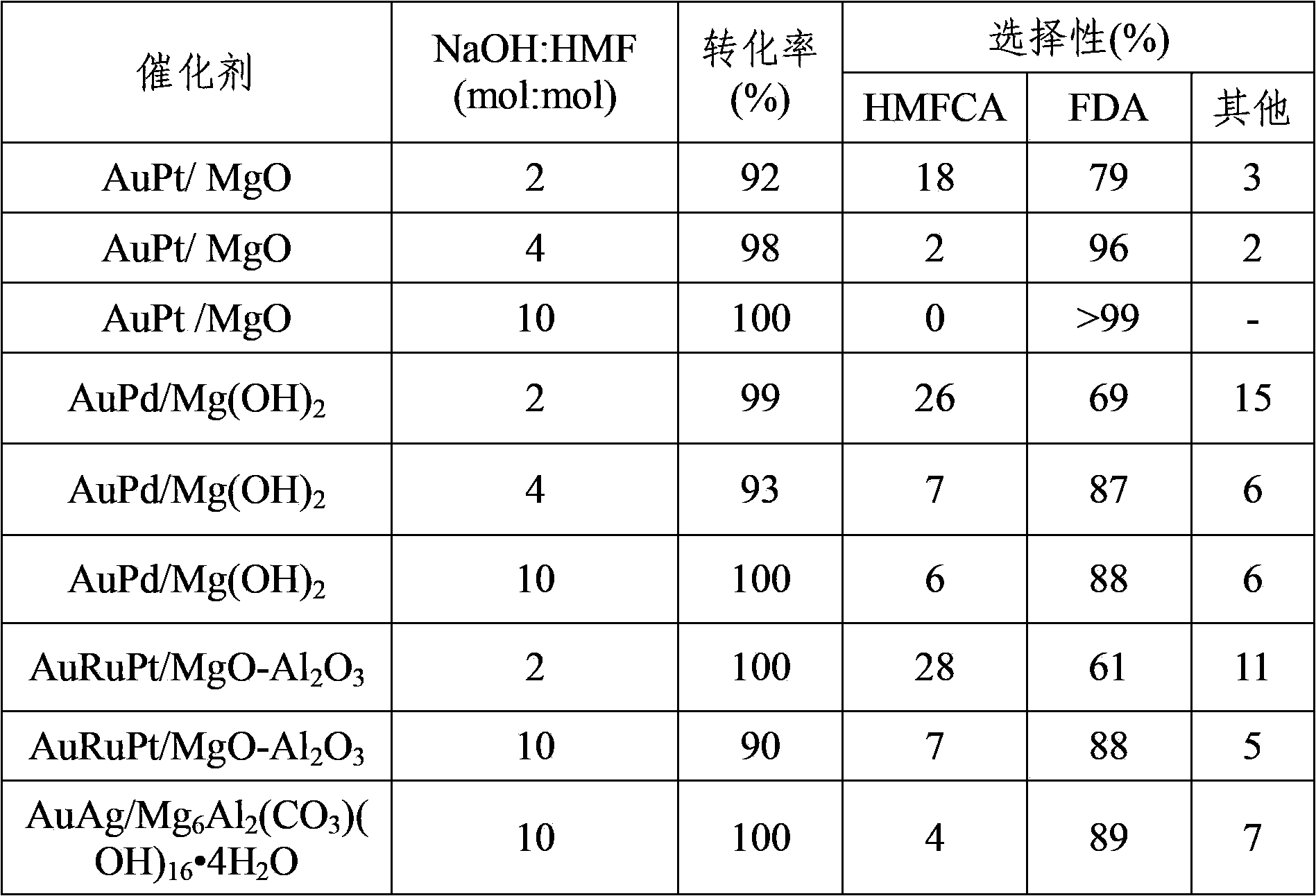
[0019] Add Au / MgO (Au 0.5wt%) catalyst, 1mmol 5-hydroxymethylfurfural, NaOH, and 10ml water into a stainless steel autoclave with polytetrafluoroethylene lining inside, in which metal: 5-hydroxymethylfurfural: NaOH=0.015:1:4 (mol:mol:mol). Adopt automatic temperature controller to heat up to reaction temperature 30 ℃, add 0.3MPa oxygen, react for 5 hours, keep pressure constant in the reaction process. The reaction product was analyzed by HPLC, and the reaction results are shown in Table 1.
Embodiment 2
[0021] Add Ru / MgO (Ru 5%) catalyst, 1 mmol 5-hydroxymethylfurfural, NaOH and 10 mL water into a stainless steel autoclave with a polytetrafluoroethylene liner inside, where metal: 5-hydroxymethylfurfural: NaOH =0.015:1:4 (mol:mol:mol). Adopt automatic temperature controller program to heat up to reaction temperature 60 ℃, add 0.3MPa oxygen, react for 5 hours, keep pressure constant in the reaction process. The reaction product was analyzed by HPLC, and the reaction results are shown in Table 1.
Embodiment 3
[0023] Pt / MgO (Pt 2wt%) catalyst, 2mmol 5-hydroxymethylfurfural, Na 2 CO 3 Add 10 ml of water to a stainless steel autoclave with a polytetrafluoroethylene liner inside, where metal: 5-hydroxymethylfurfural: Na 2 CO 3 =0.015:1:4 (mol:mol:mol). Adopt automatic temperature controller to heat up to reaction temperature 80 DEG C, add 0.3MPa oxygen, react for 12 hours, keep pressure constant in the reaction process. The reaction product was analyzed by HPLC, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com