Method for preparing indolone derivative
A derivative, indolinone technology, applied in the field of medicinal chemistry synthesis, can solve the problems of unsuitability for scale-up production, low yield, reduced production efficiency, etc., and achieve the effects of low toxicity, high total yield and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
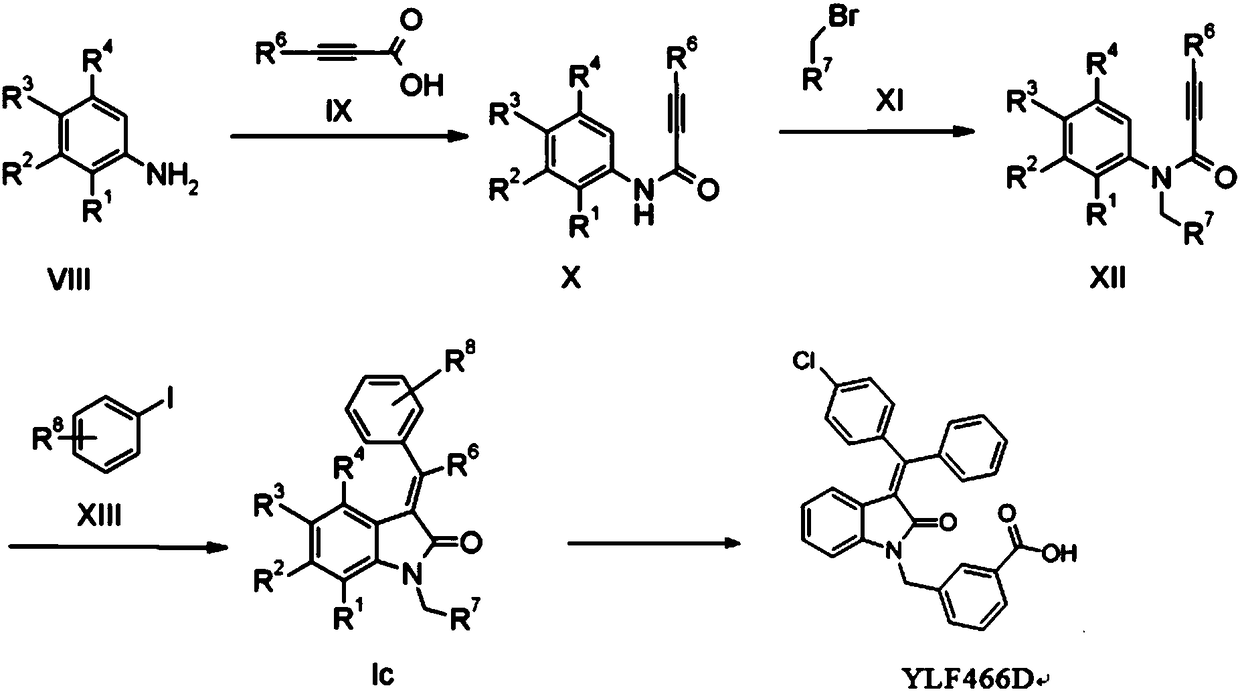
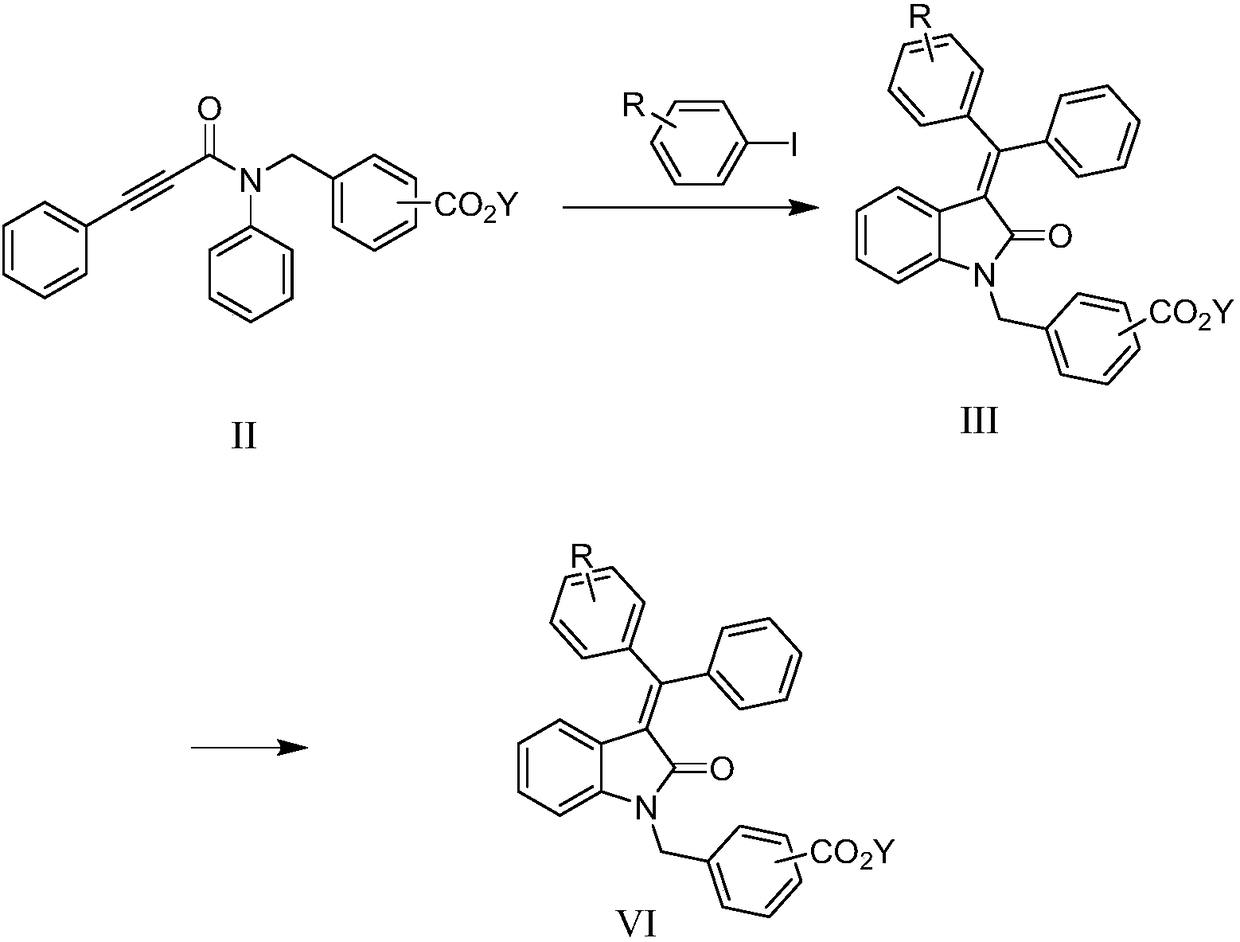
[0042] In a preferred embodiment, the preparation method of YLF466D provided by the invention comprises the following steps:
[0043]
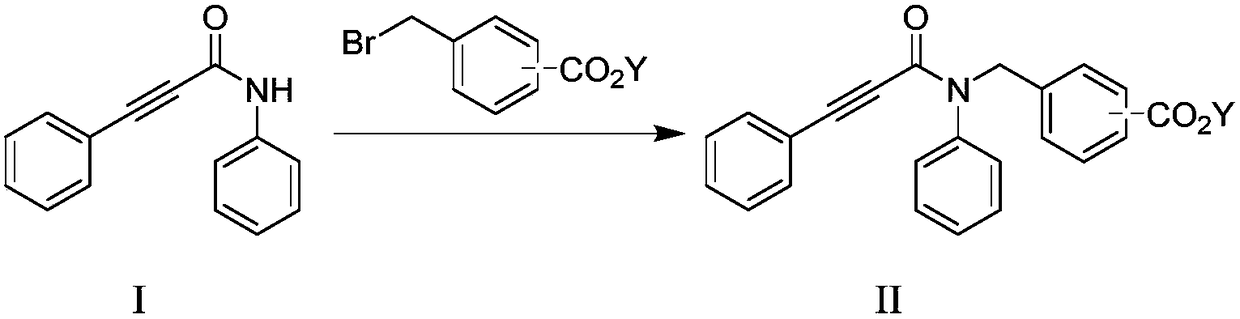
[0044] (1) Phenylpropiolic acid and aniline undergo a condensation reaction under the action of a condensing agent to obtain an amide intermediate I (compound of formula I). Phenylpropiolic acid and aniline react in a suitable solvent under the action of a condensing agent to obtain the amide intermediate I conveniently. The condensing agent used is a condensing agent such as DCC, EDC, DIC or BDDC.
[0045] (2) At an appropriate temperature, the intermediate I obtained in step (1) and methyl 3-bromomethylbenzoate carry out a nucleophilic substitution reaction in an appropriate solvent under the action of a base to obtain intermediate II (formula II compound).
[0046] Specifically, the temperature of the substitution reaction is 30°C to 120°C, preferably 40°C to 60°C; the base can be sodium hydride, K 2 CO 3 、Na 2 CO 3 , Li 2 CO 3 , ...
Embodiment 11
[0059] The preparation of formula I compound
[0060]
[0061] Phenylpropiolic acid (2000g, 13.7mol) and aniline (1911g, 20.5mol) were dissolved in dichloromethane (15L), then EDCI (5252.6g, 27.4mol) was added in batches, and the reaction temperature was controlled below 30°C. After the addition is complete, the reaction takes about 20 hours at room temperature.
[0062] The reaction solution was washed with 8L of 2.5mol / L dilute hydrochloric acid and 5L of 1mol / L dilute hydrochloric acid, respectively. The organic phase was washed once with 8L saturated sodium bicarbonate and once with 8L saturated brine, and methylene chloride was recovered to obtain a yellow-white solid powder, which was dissolved in 6600mL of anhydrous methanol, and 1 volume (10000ml) of purified water was added dropwise for crystallization. After filtering, the solid was washed with purified water, and air-dried at 40°C to obtain 2885.4 g of the compound of formula I, which was an off-white solid powd...
Embodiment 12
[0065] The preparation of formula I compound
[0066] Phenylpropiolic acid (20.0g, 0.14mol) and aniline (19.11g, 0.21mol) were dissolved in dichloromethane (150mL), then DIC (1,3-diisopropylcarbodiimide , 34.0g, 0.27mol), the reaction temperature is controlled below 30°C. After the addition is complete, the reaction takes about 20 hours at room temperature. The reaction solution was washed with 80 mL of 2.5 mol / L dilute hydrochloric acid and 50 mL of 1 mol / L dilute hydrochloric acid, respectively. The organic phase was washed once with 80mL of saturated sodium bicarbonate and once with 80mL of saturated brine, and dichloromethane was recovered to obtain a yellow-white solid powder, which was dissolved in 67mL of anhydrous methanol, and 100mL of purified water was added dropwise for crystallization, filtered, and the solid was purified with After washing with water, air-drying at 40°C gave 26.6 g of the compound of formula I, off-white solid powder. Yield: 86.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com