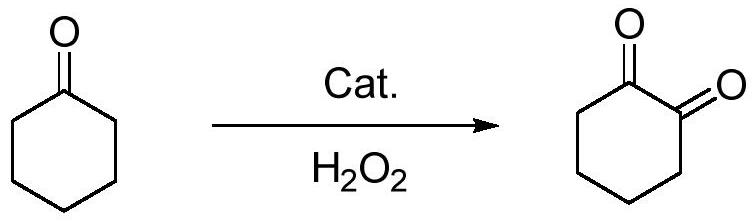
A kind of preparation method of 1,2-cyclohexanedione
A technology of cyclohexanedione and cyclohexanone, applied in the field of chemistry, can solve the problems of high price, low reaction yield, and many waste water, and achieves enhanced power supply and steric hindrance, simple post-processing purification, and green and clean process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under nitrogen protection, 198.0g cyclohexanone (99%, 2.0mol), 3.9g cuprous iodide (99%, 0.02mol), 6.2g ligand L1 (99%, 0.02mmol) were added in a 500mL reaction flask, The structural formula of ligand L1 is as follows:
[0034]
[0035] After feeding, start stirring with a stirring speed of 600rpm, add 38.9g concentration of 35% hydrogen peroxide (35%, 0.4mol) dropwise at room temperature, dropwise time 2hr, after dropwise addition, heat up to 60°C, keep warm for 3hr, after the reaction, Washing and liquid separation, the organic layer was rectified under reduced pressure to recover 156.2g (99.1%, 1.58mol) of cyclohexanone and 44.0g (99.3%, 0.39mol) of 1,2-cyclohexanedione, with a yield of 92.9% (based on cyclohexanone Hexanone meter).
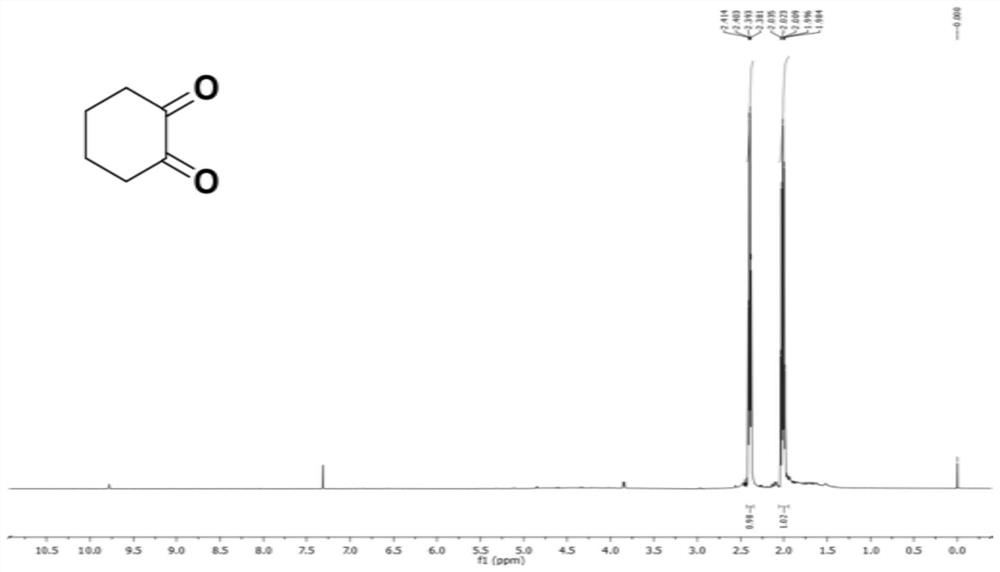
[0036] Such as figure 1 Shown is the present embodiment 1,2-cyclohexanedione 1 H NMR spectrum: 1 H NMR (500MHz, CDCl 3 ): δ2.38-2.41 (t, J=5.5Hz, 6.0Hz, 4H), 1.98-2.04 (m, 4H).
Embodiment 2
[0038] Under nitrogen protection, 237.6g cyclohexanone (99%, 2.4mol), 11.6g cuprous bromide (99%, 0.08mol), 31.2g ligand L2 (99%, 0.08mmol) were added in a 500mL reaction flask, The structural formula of ligand L2 is as follows:
[0039]
[0040] After feeding, start stirring at a stirring speed of 600rpm. Add 54.4g of 50% hydrogen peroxide (50%, 0.8mol) dropwise at room temperature for 1.5hr. , washed with water and separated, and the organic layer was rectified under reduced pressure to obtain 169.9g (99.2%, 1.72mol) of cyclohexanone and 73.5g (99.1%, 0.65mol) of 1,2-cyclohexanedione, with a yield of 95.6% (based on Cyclohexanone meter).
[0041] It should be noted that cuprous chloride is similar in structure to cuprous bromide in this embodiment, so the catalyst in this embodiment can also be replaced by cuprous chloride.
Embodiment 3
[0043] Under nitrogen protection, add 297.0g cyclohexanone (99%, 3.0mol), 0.43g cuprous oxide (99%, 0.003mol), 0.76g ligand L3 (99%, 0.003mol) in the 500mL reaction bottle, formulate The structural formula of body L3 is as follows:
[0044]
[0045] After feeding, start stirring with a stirring speed of 600rpm. Add 40.8g of 25% hydrogen peroxide (25%, 0.3mol) dropwise at room temperature for 1hr. After the dropwise addition, heat up to 40°C and keep the temperature for 6hr. After the reaction, Washing and liquid separation, the organic layer was rectified under reduced pressure to recover 278.2g (99.3%, 2.81mol) of cyclohexanone, 13.6g (99.0%, 0.12mol) of 1,2-cyclohexanedione, and the yield was 63.2% (based on cyclohexanone Hexanone meter).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com