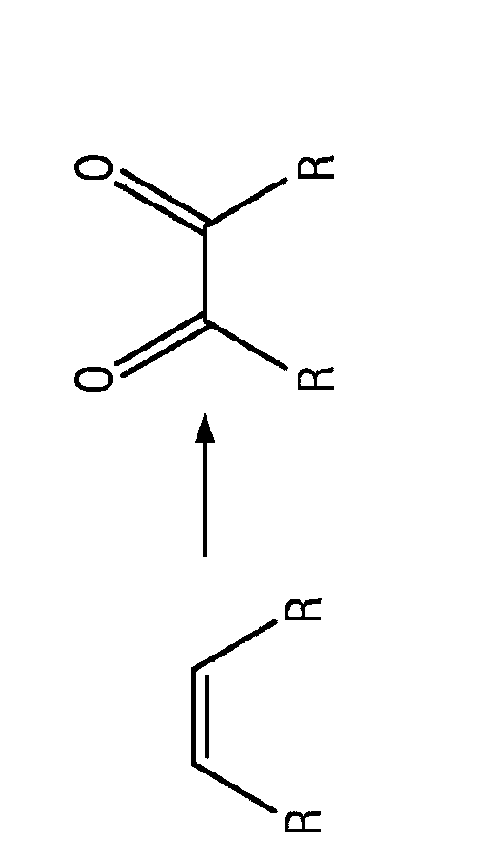
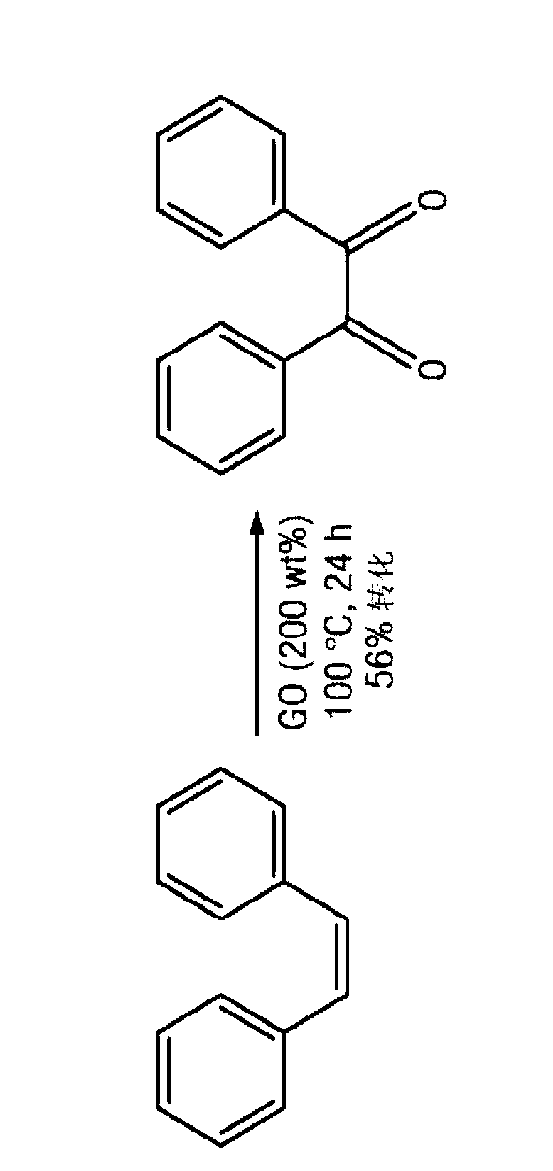
Patents
Literature
1855results about "Carbonyl compound preparation by oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Light-Emitting Device Material and Light-Emitting Device
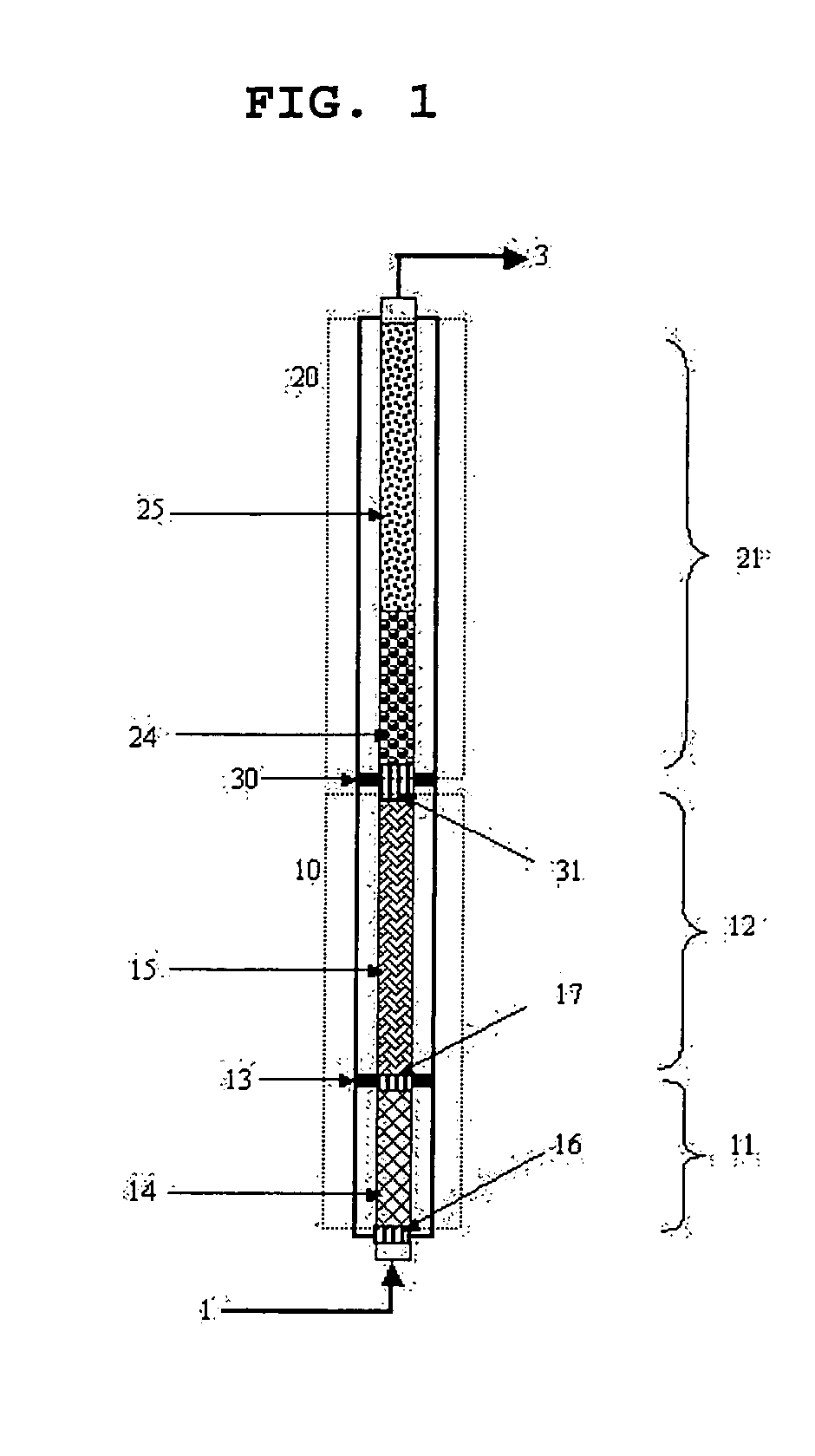
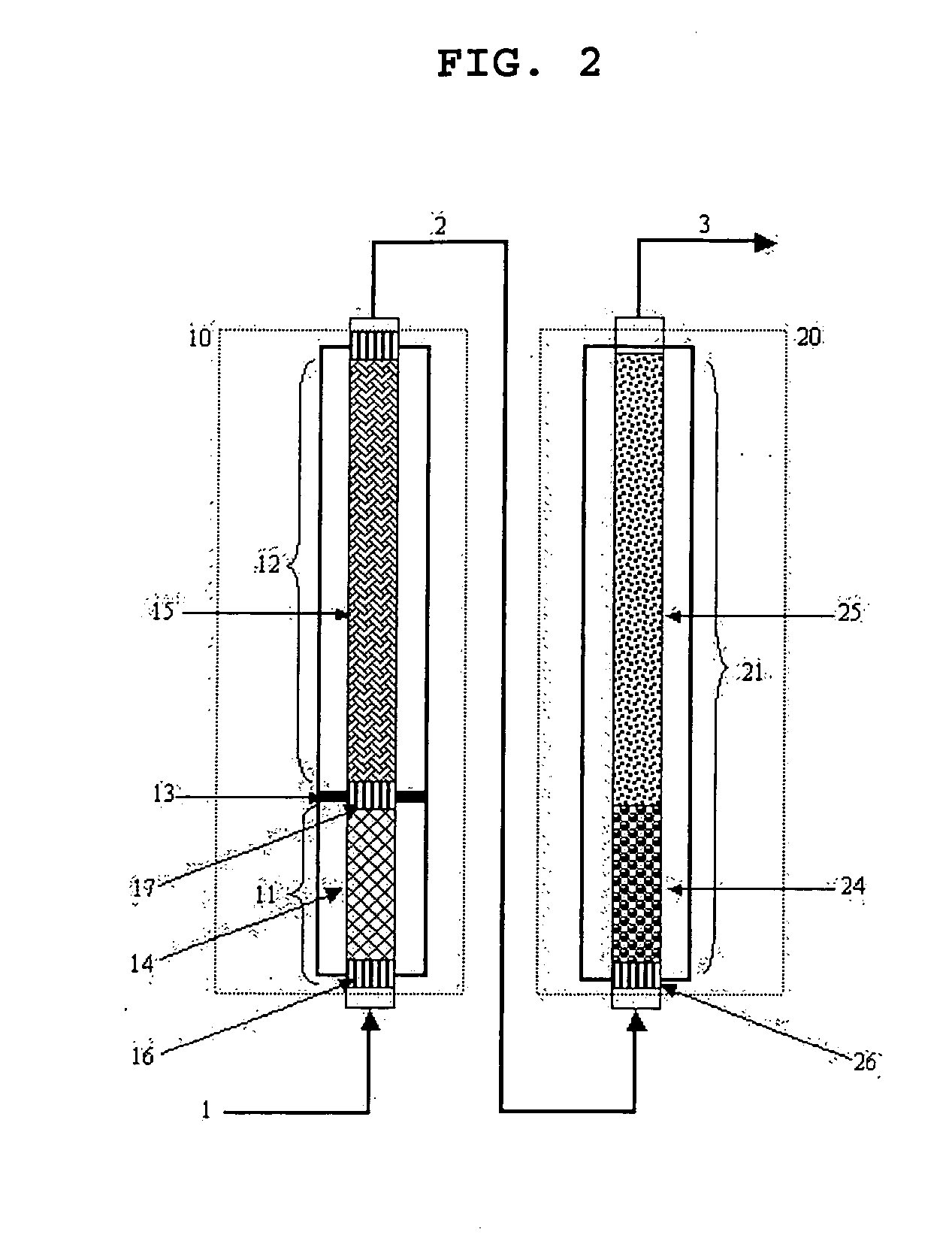
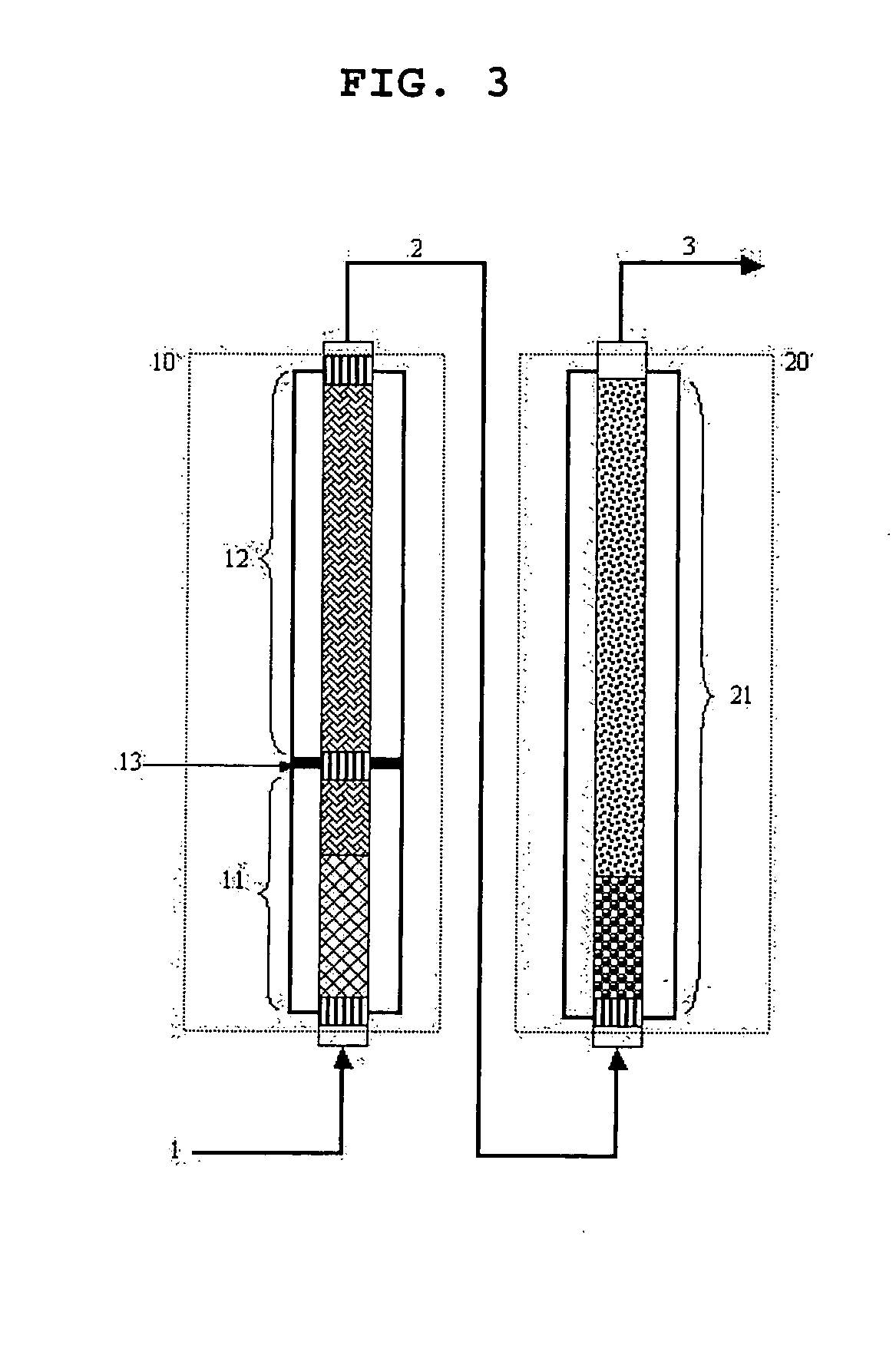
ActiveUS20070247063A1High film stabilitySolve low luminous efficiencyDischarge tube luminescnet screensOrganic compound preparationAnthraceneHydrogen atom
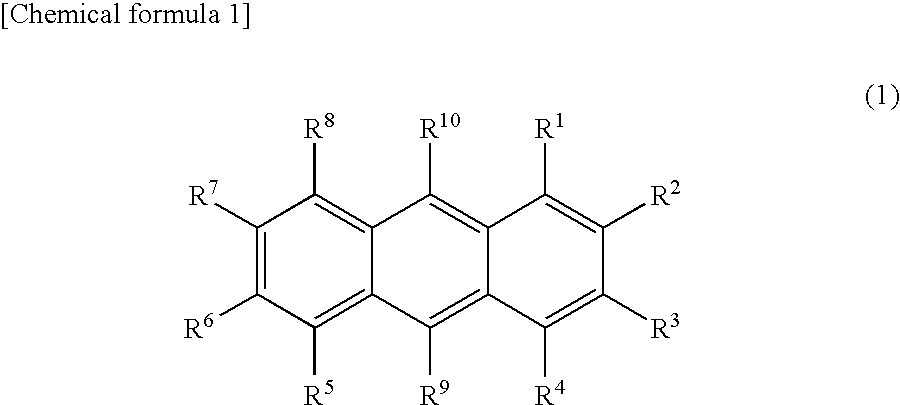
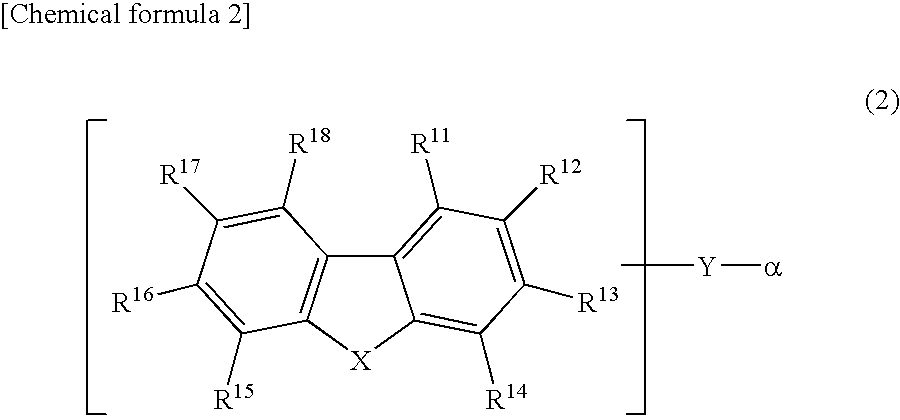
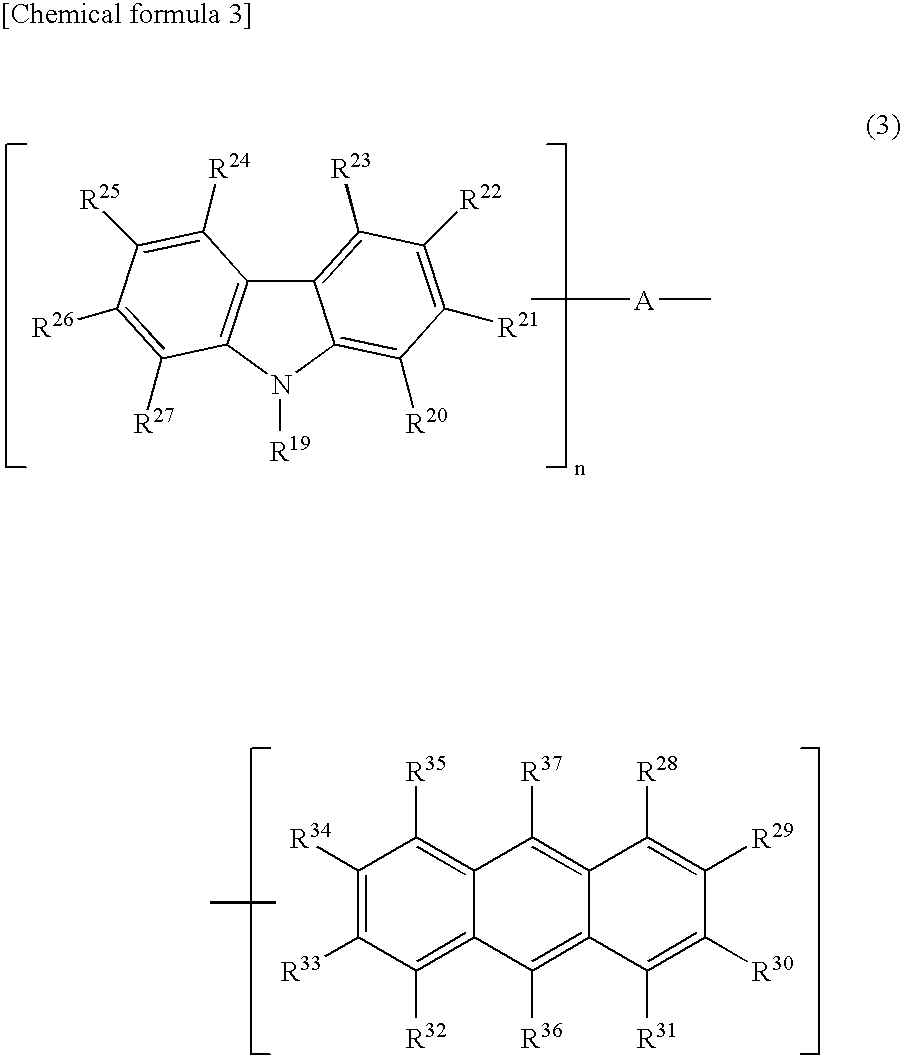
The present invention is a light emitting device material characterized by containing an anthracene compound represented by the following general formula (1) or general formula (3), and the present invention allows a light emitting device having high luminous efficiency and excellent durability. (R1 to R10 are a hydrogen atom, alkyl group, cycloalkyl group, heterocyclic group or the like. At least one of the R1 to R10 is a substituent represented by the following general formula (2).) (R11 to R18 are a hydrogen atom, alkyl group and cycloalkyl group. X is an oxygen atom or sulfur atom, and Y is a single bond; arylene group or heteroarylene group. Any one of the R11 to R18 is used for linking with Y, and α is used for linking with the anthracene skeleton.) (R19 to R37 are a hydrogen atom, alkyl group, cycloalkyl group, heterocyclic group or the like. n is 1 or 2. A is a heteroarylene group or arylene group. Any one of the R19 to R27 and any one of the R28 to R37 are used for linking with A.)
Owner:IDEMITSU KOSAN CO LTD
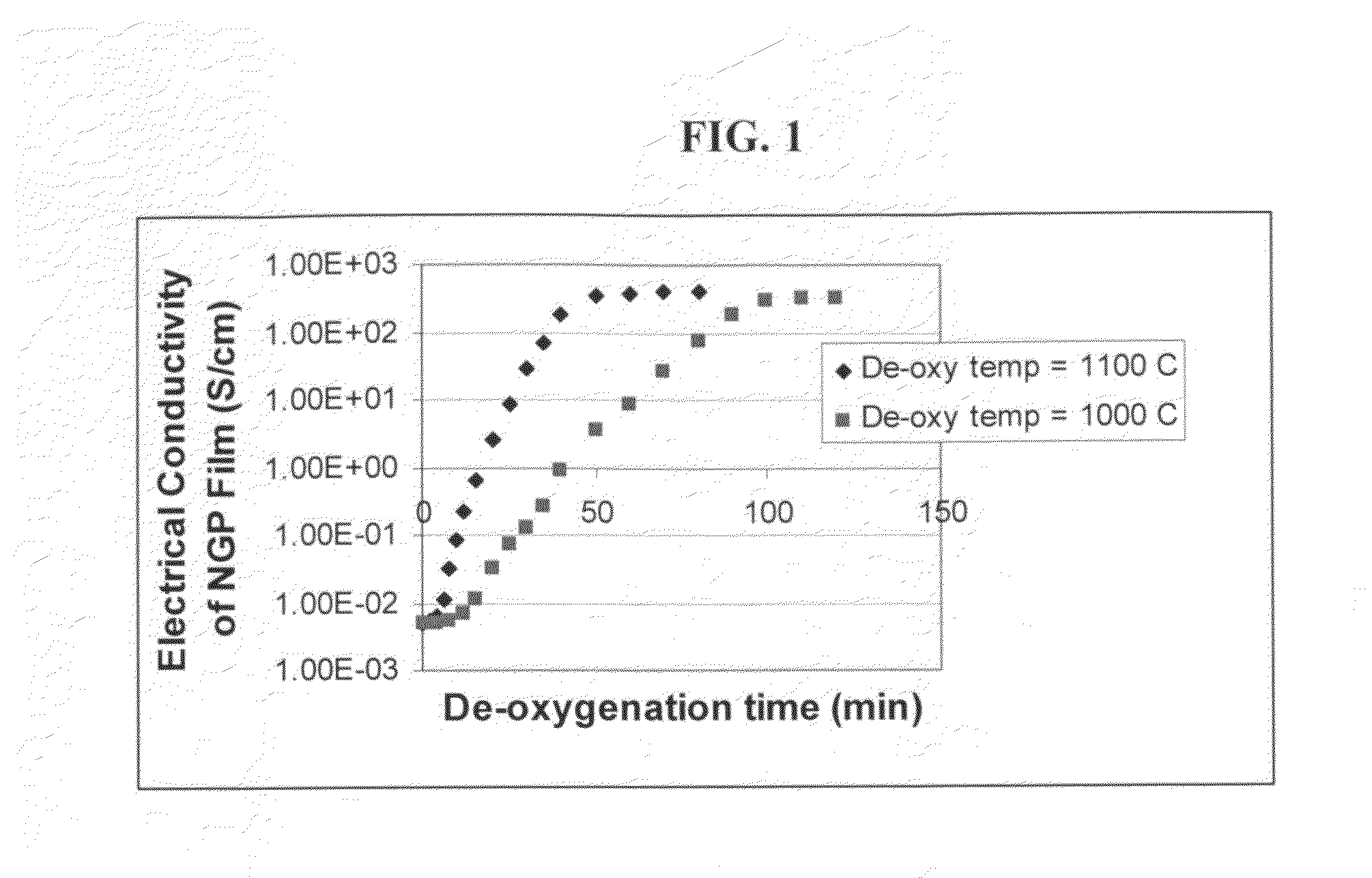
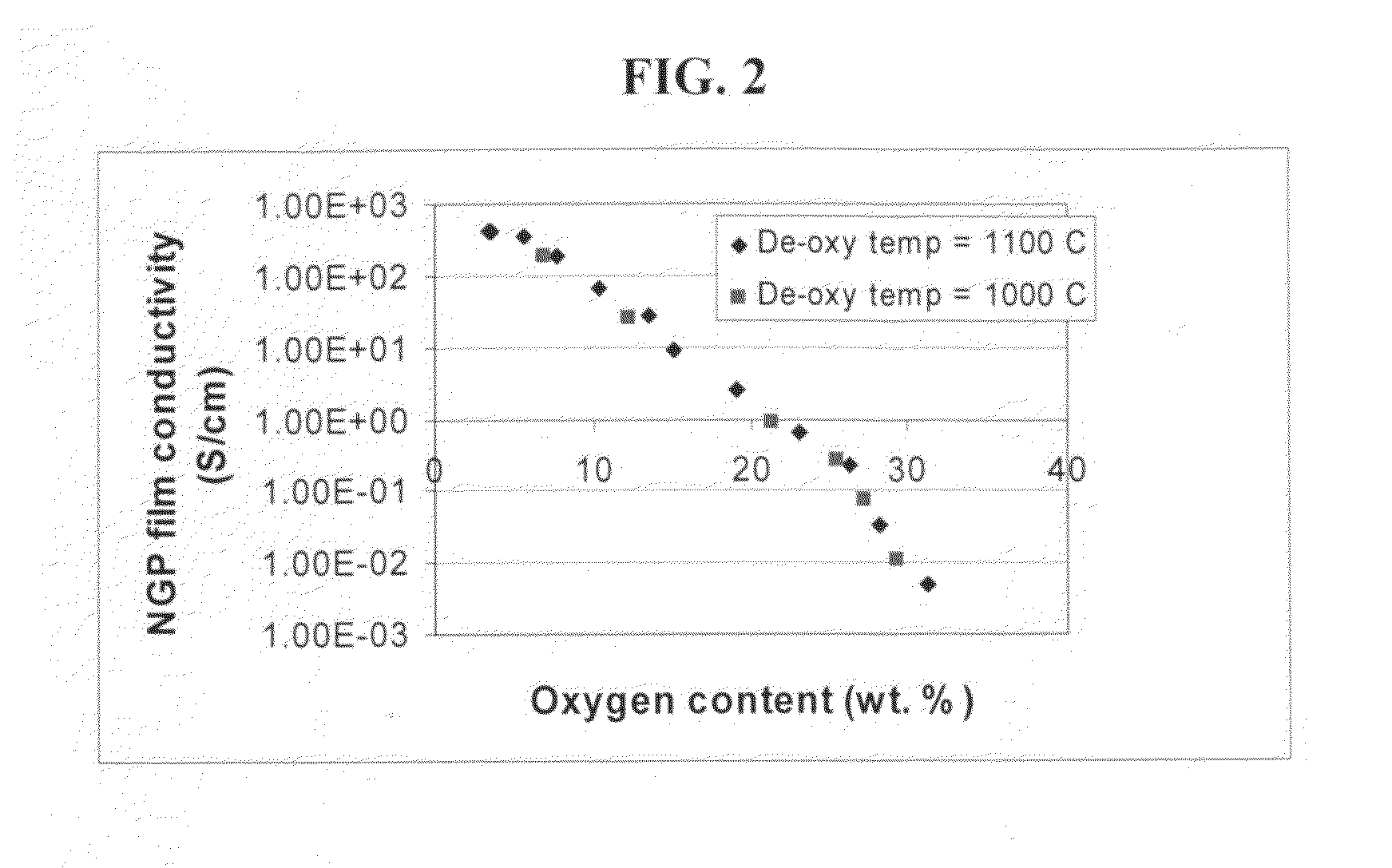
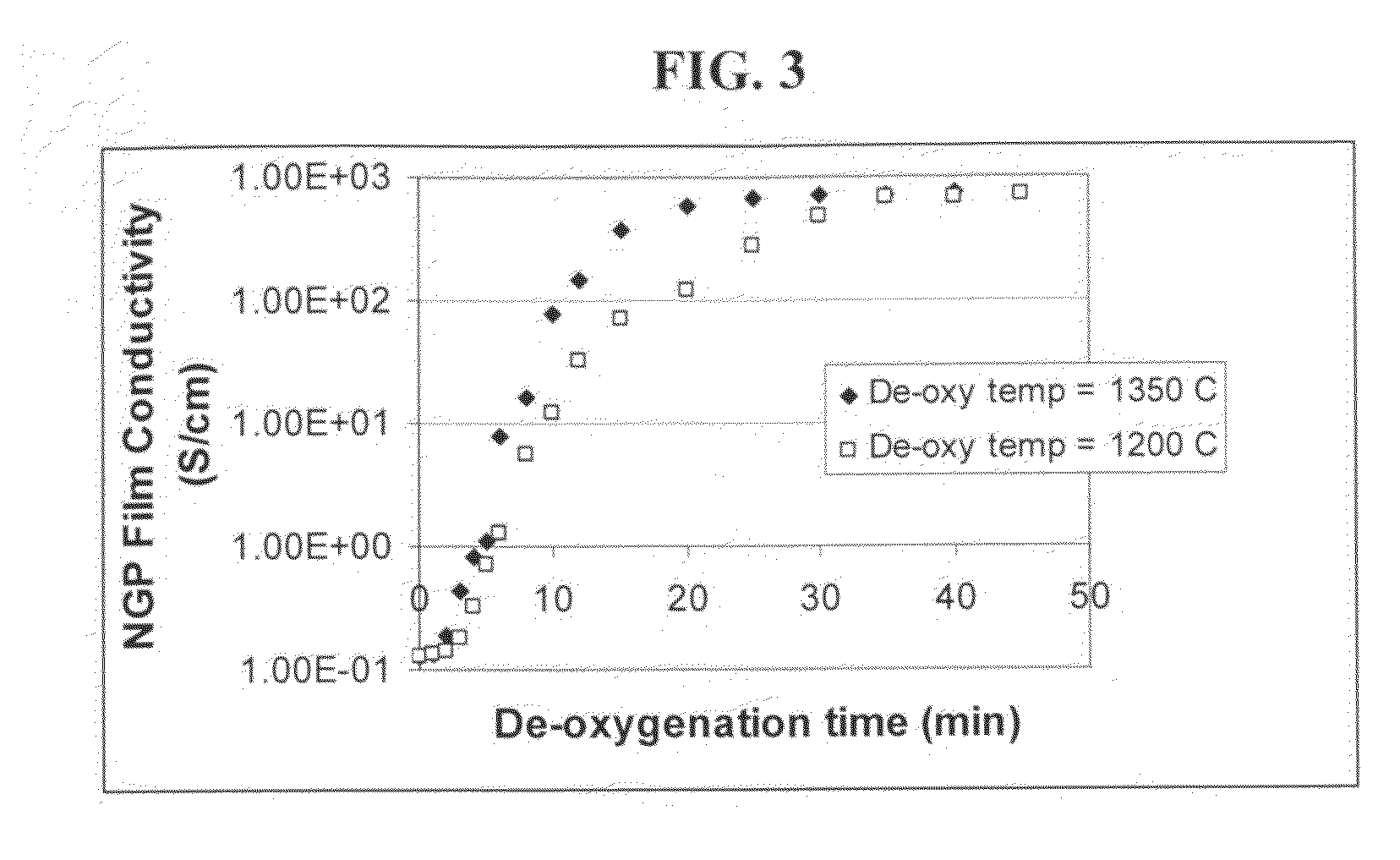
Process for producing dispersible and conductive Nano Graphene Platelets from non-oxidized graphitic materials
ActiveUS20100056819A1Impart dispersibilityImpart solubilityMaterial nanotechnologyPigmenting treatmentDisplay deviceSolar cell
The present invention provides a process for producing nano graphene platelets (NGPs) that are both dispersible and electrically conducting. The process comprises: (a) preparing a pristine NGP material from a graphitic material; and (b) subjecting the pristine NGP material to an oxidation treatment to obtain the dispersible NGP material, wherein the NGP material has an oxygen content no greater than 25% by weight. Conductive NGPs can find applications in transparent electrodes for solar cells or flat panel displays, additives for battery and supercapacitor electrodes, conductive nanocomposite for electromagnetic wave interference (EMI) shielding and static charge dissipation, etc.
Owner:GLOBAL GRAPHENE GRP INC
Cationic lipids and uses thereof
InactiveUS20090285881A1Reduce tumor volumeBiocideFatty acid chemical modificationLipid formationDisease
Cationic lipids, cationic lipid based drug delivery systems, ways to make them and methods of treating diseases using them are disclosed.
Owner:ABBOTT LAB INC
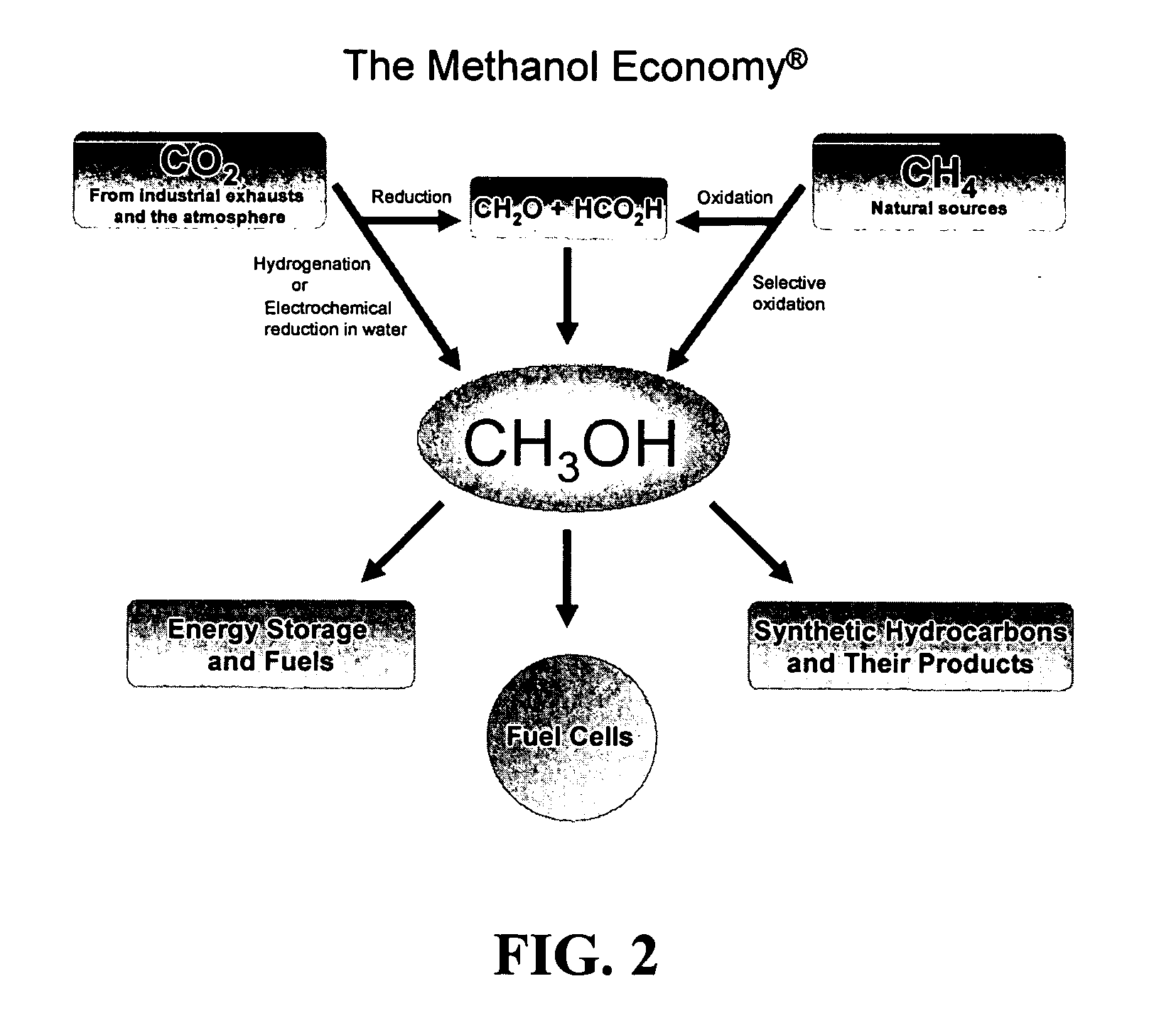
Selective oxidative conversion of methane to methanol, dimethyl ether and derived products
ActiveUS20060235088A1Minimize or eliminate the disadvantages or dangers inherentPreparation by oxidation reactionsElectrolysis componentsFormate EstersDimethyl ether
The present invention relates to a method of producing methanol from a methane source by oxidizing methane under conditions sufficient to a mixture of methanol and formaldehyde while minimizing the formation of formic acid and carbon dioxide. The oxidation step is followed by treatment step in which formaldehyde is converted into methanol and formic acid which itself can further be converted into methanol via catalytic hydrogenation of intermediately formed methyl formate.
Owner:UNIV OF SOUTHERN CALIFORNIA
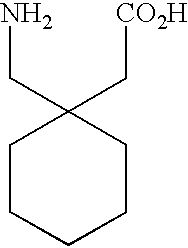
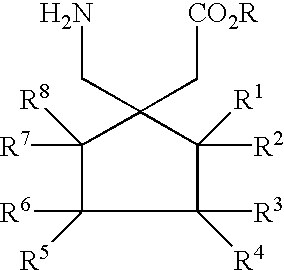
Fused bicyclic or tricyclic amino acids
The compounds of the instant invention are bicyclic or tricyclic amino acids useful in the treatment of epilepsy, faintness attacks, hypokinesia, cranial disorders, neurodegenerative disorders, depression, anxiety, panic, pain, arthritis, neuropathological disorders, sleep disorders, visceral pain disorders, and gastrointestinal disorders. Processes for the preparation of the final products and intermediates useful in the process are included. Pharmaceutical compositions containing one or more of the compounds are also included.
Owner:PFIZER INC
Polyketone Plasticizers
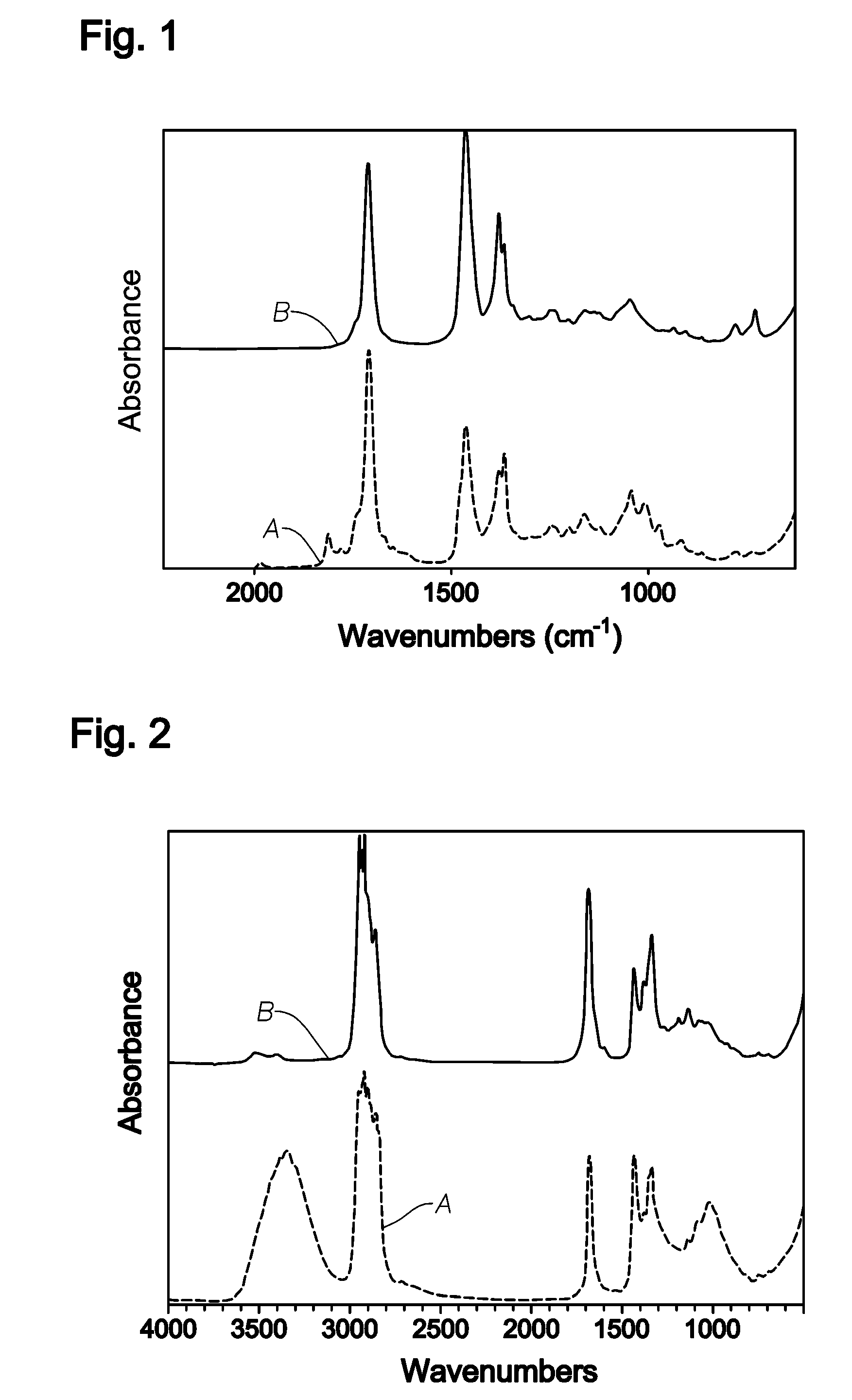
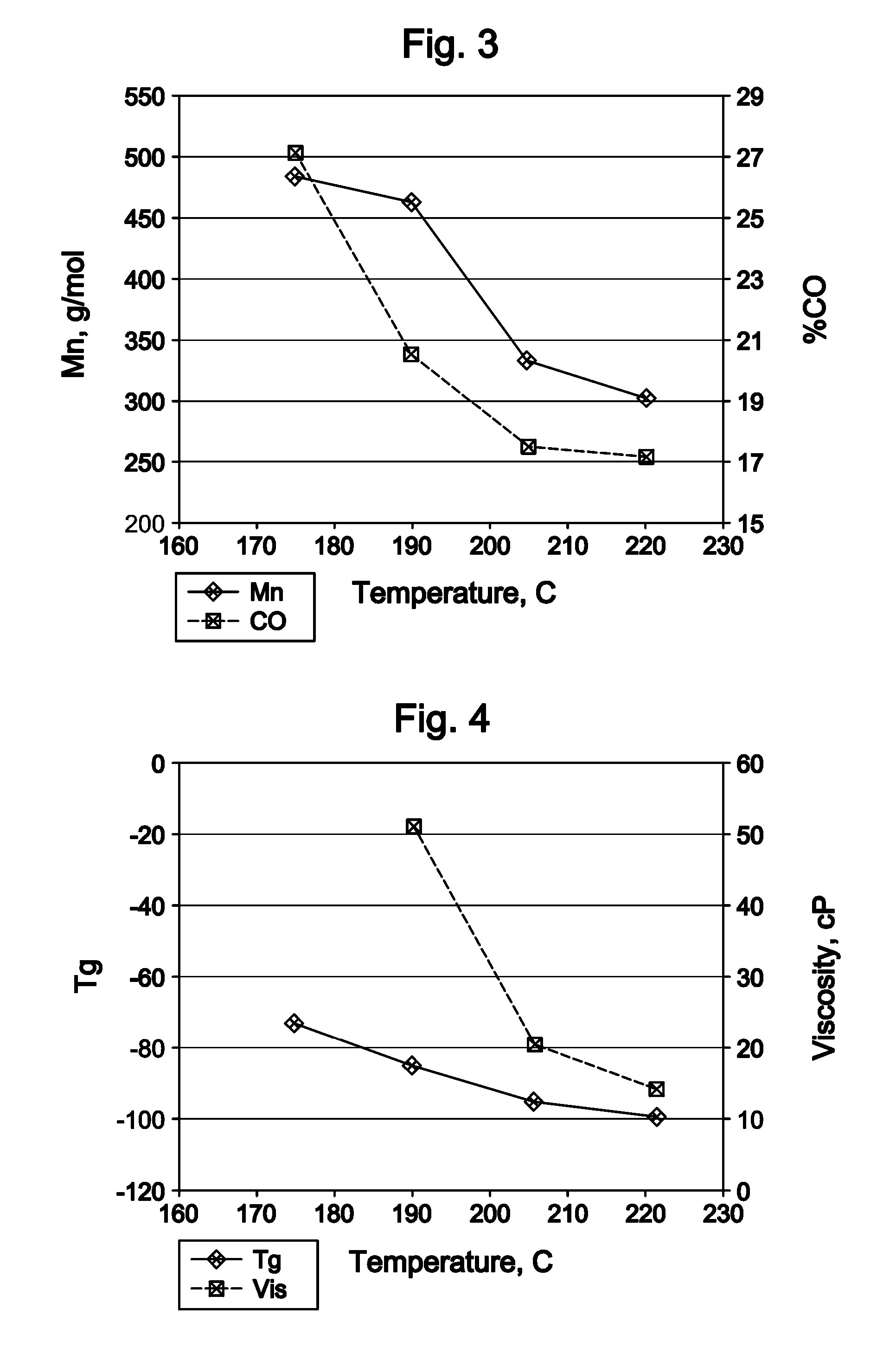
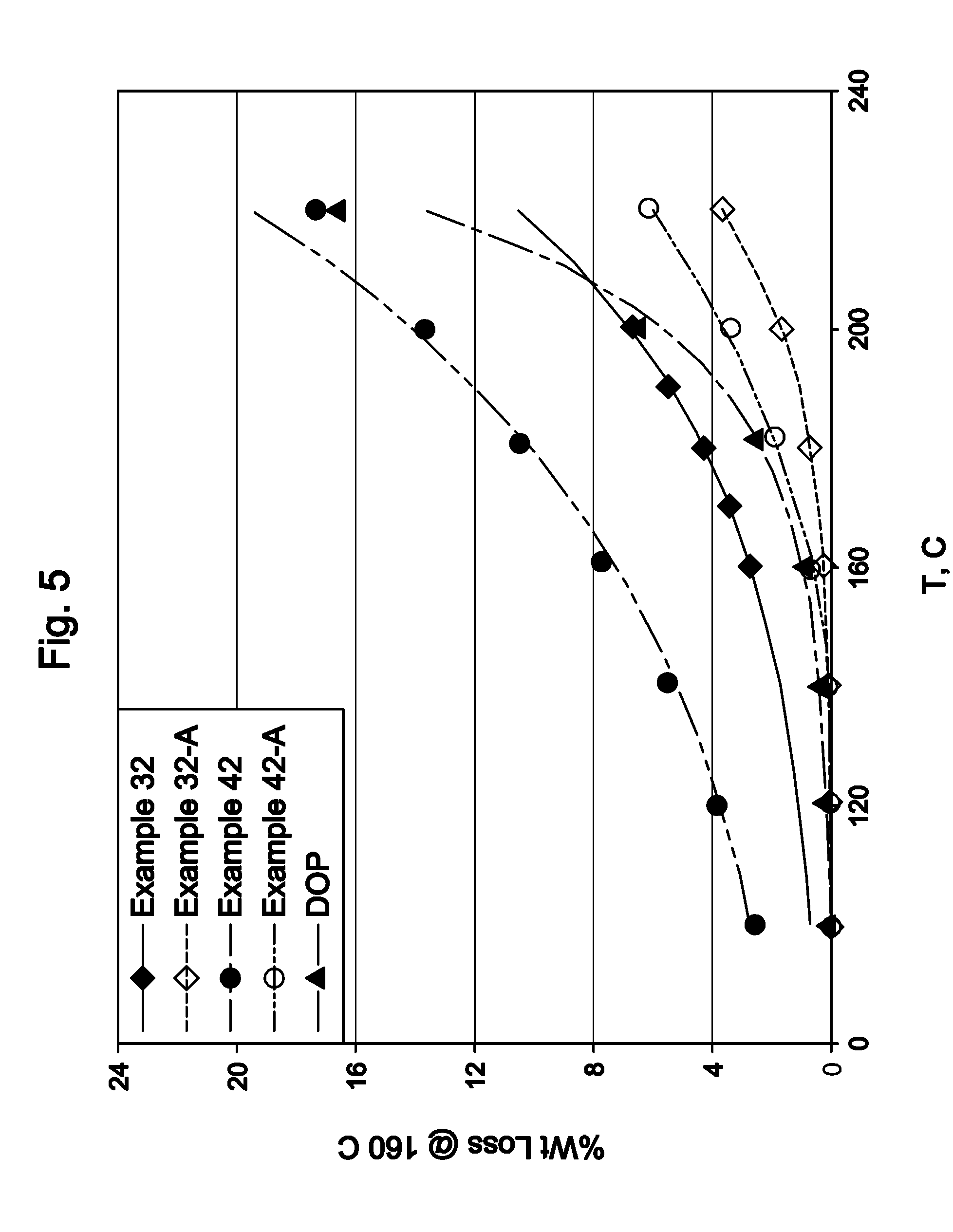
InactiveUS20080242895A1Remove colorEasy to separateOrganic compound preparationCarbonyl compound preparation by oxidationPolymer sciencePlasticizer
The invention relates to polyketone compounds and the at least partially hydrogenated products thereof, the use of said polyketone compounds and / or the at least partially hydrogenated products thereof as plasticizers, processes of making polyketone compounds and the at least partially hydrogenated products thereof, compositions comprising the polyketone compounds and / or the at least partially hydrogenated products thereof, and to articles formed from products of the invention.
Owner:EXXONMOBIL CHEM PAT INC
Cationic lipids and uses thereof
Cationic lipids, cationic lipid based drug delivery systems, ways to make them and methods of treating diseases using them are disclosed.
Owner:ABBOTT LAB INC
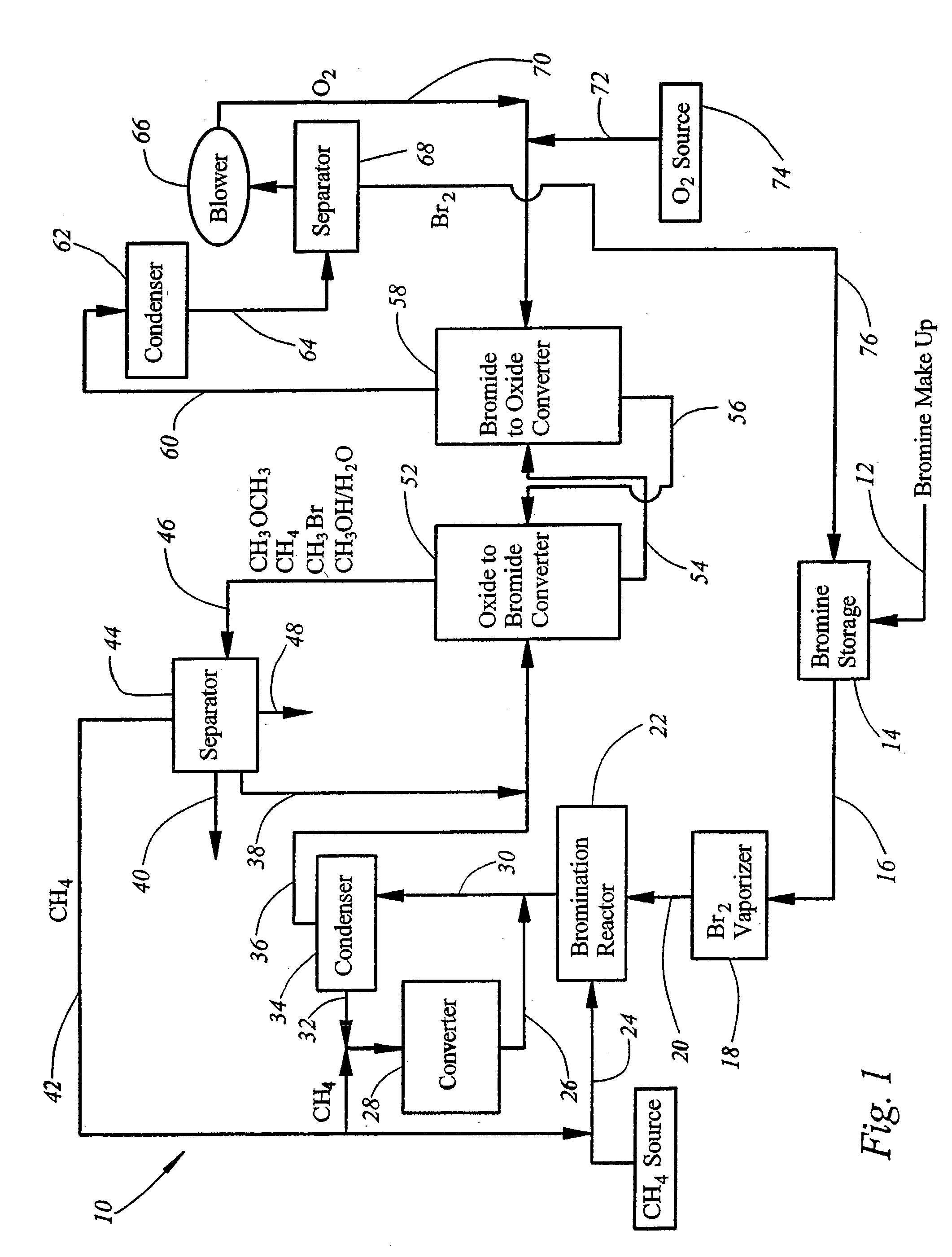
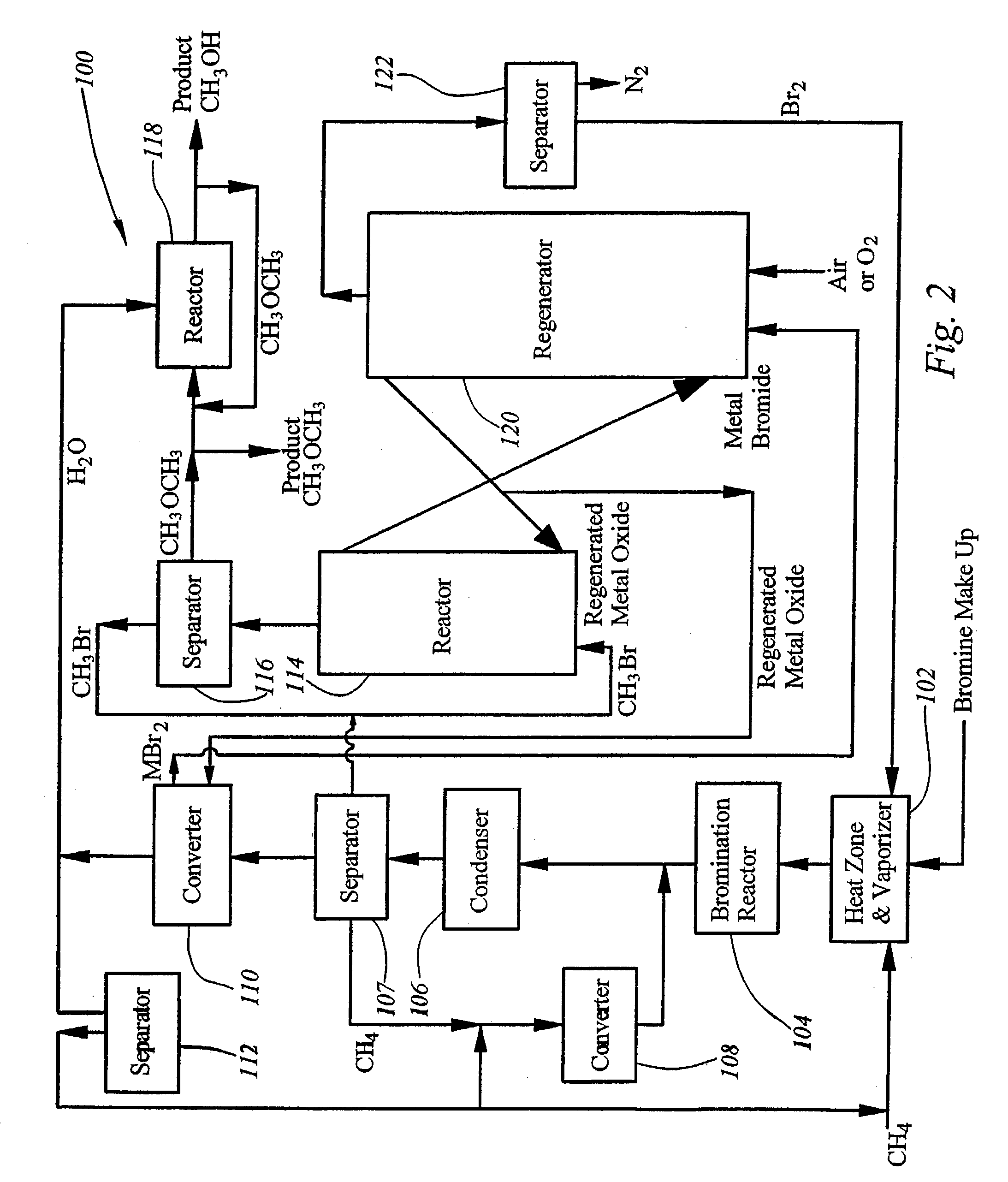
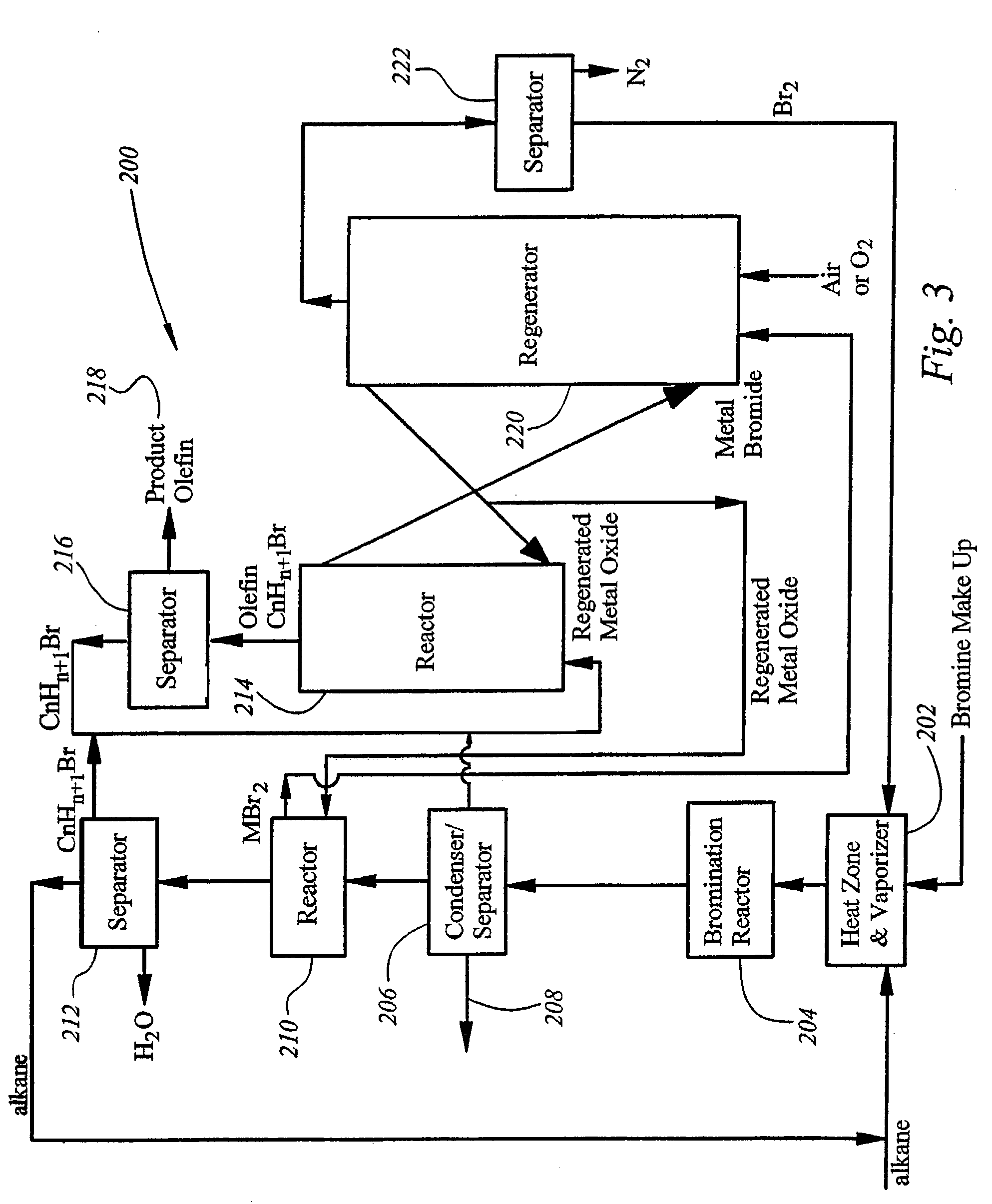
Integrated process for synthesizing alcohols, ethers, aldehydes, and olefins from alkanes
InactiveUS7148390B2Less corrosiveImprove securityOrganic compound preparationChemical industryHydrogen halideAlkane
Alcohols, ethers, aldehydes, and olefins are manufactured from alkanes by mixing an alkane and a halogen selected from the group including chlorine, bromine, and iodine in a reactor to form alkyl halide and hydrogen halide. The alkyl halide only or the alkyl halide and the hydrogen halide are directed into contact with metal oxide to form an alcohol and / or an ether, or an olefin and metal halide. The metal halide is oxidized to form original metal oxide and halogen, both of which are recycled.
Owner:REACTION 35 LLC
Vapor phase oxidation of propylene to acrolein
InactiveUS6437193B1Organic compound preparationCarbonyl compound preparation by oxidationChemistryOxygent
An improved method for the selective vapor phase oxidation of propylene to acrolein in a recirculating solids reactor system using a bismuth molybdate multimetal oxide involving specific reactant concentrations (preferably 5 mol % to 30 mol % propylene, 0 to 20 mol % oxygen, and the remainder inert gas), particle size (1 to 300 micrometers), temperature (250 to 450.degree. C.) and gas (1 to 15 seconds) and solids (2 to 60 seconds) residence times. Such a process leads to improved selectivity and propylene conversion.
Owner:EI DU PONT DE NEMOURS & CO +1
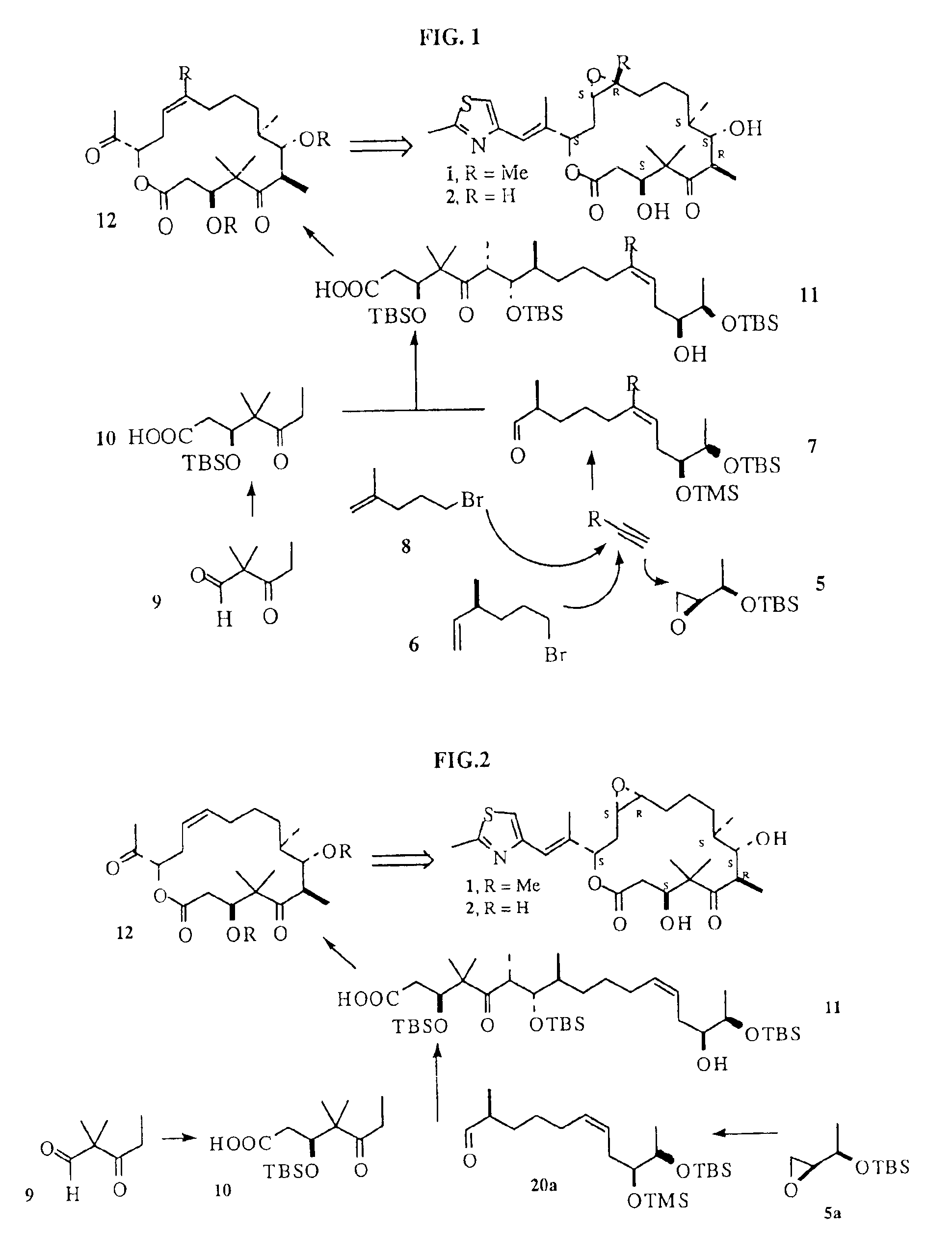
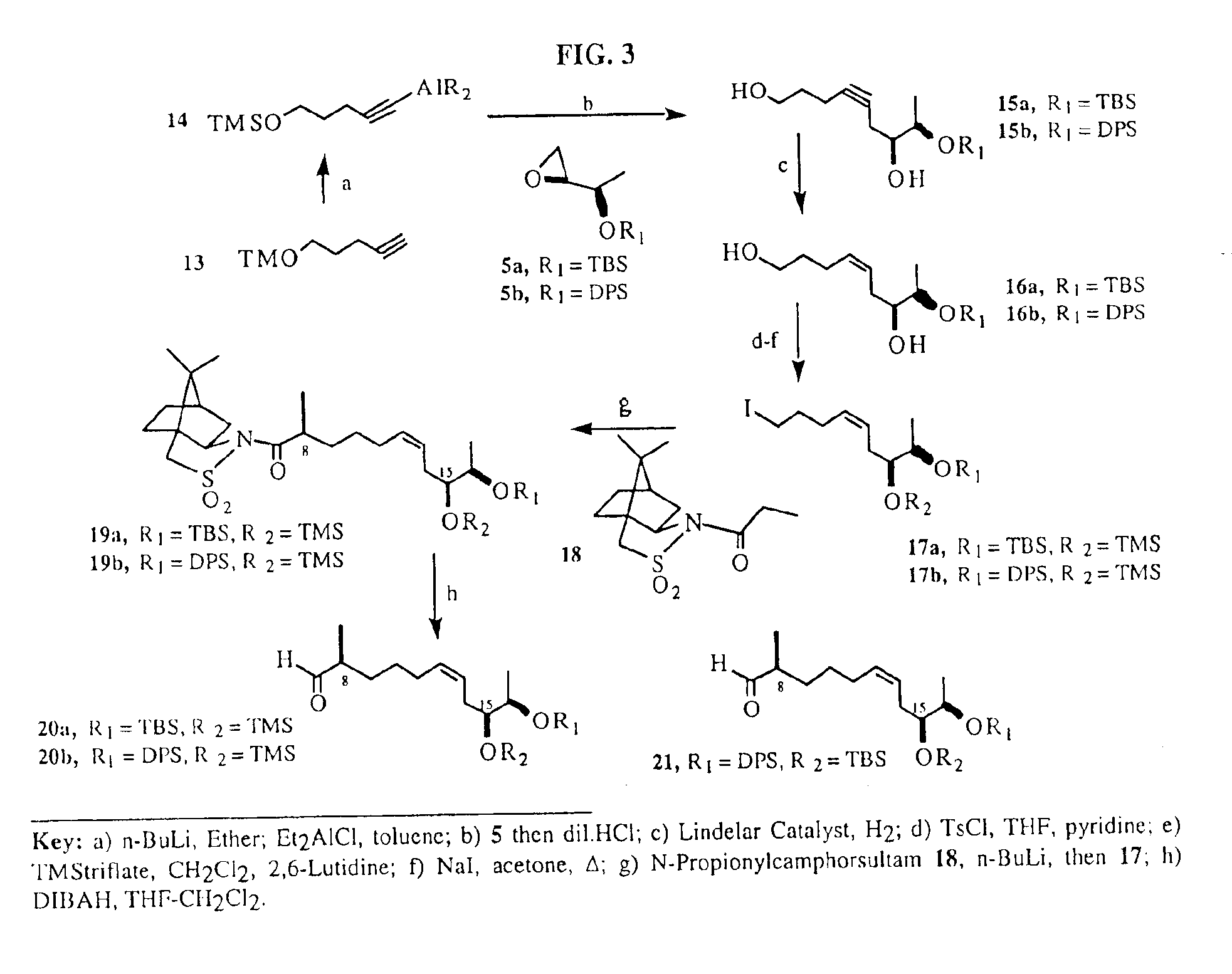
Synthesis of epothilones and related analogs
InactiveUS6989450B2Easy to understandOrganic active ingredientsGroup 4/14 element organic compoundsEpothiloneStereoisomerism
The present invention relates to methods for use in producing epothilones and analogs and derivatives thereof. A general method according to the present invention broadly comprises performing an aldol condensation of a first compound with a second compound thereby to form a third compound selected from the formulas: and stereoisomers thereof, and performing a macrolactonization of the third compound. The present invention also provides chemical compounds, and methods for producing such chemical compounds, that are useful in producing epothilones and analogs and derivatives thereof.
Owner:UNIVERSITY OF MISSISSIPPI
Method for atmospheric catalytic oxidation of cyclohexane by metalloporphyrin
InactiveCN1405131APreparation by oxidation reactionsOxygen compounds preparation by hydrocarbon oxidationCyclohexanoneReaction temperature
The invention relates to a new process in which under the catalytic action of metal porphyrin the cyclohexane can be selectively oxdated by air and made into cyclohexanol and cyclohexanone. Under the condition of 2-10 atm air, reaction temp. is 125-150 deg.C, it selects and uses micro-oxo double metal porphyrin and single metal porphyrin or their solid carrier as main catalyst, and uses transition metal salt or oxide as co-catalyst, the metal porphyrin can high-effectively and high-selectively catalyze oxidation of cyclohexane by using air in the biological concentratino as biological enzyme. The dose of metal porphyrin is small and said metal porphyrin can be repeatedly used, and said catalyst dose also is small, its catalystic effect is good, said catalyst can be used for making homogeneous catalysis, and after it is solid-carried, it also can be used for making heterogeneous catalysis.
Owner:HUNAN UNIV
Technology for preparing organic fuel through directly converting carbon dioxide by using sunlight and photothermal catalyst
InactiveCN104016825AReduce energy consumptionHigh reactivityHydrocarbon from carbon oxidesOrganic compound preparationSynthesis methodsUltraviolet lights
The invention discloses a technology for preparing organic fuel through directly converting carbon dioxide by using sunlight and a photothermal catalyst. Sunlight is utilized to supply light and heat for the synthesis and catalytic process of the photothermal catalyst, and the photothermal catalyst can simultaneously absorb and utilize ultraviolet light, visible light and infrared light parts in sunlight, so that a phtothermal catalytic reaction is induced to prepare the organic fuel through reducing carbon dioxide by using hydrogen. The photothermal catalyst comprises the following components: an active component which is a 2-30 nano-scale non-stoichiometric oxide belonging to a VIII-family element in a transition family and a carrier material which is an oxide or carbon material with the specific surface area of 30-1000cm<2> / g, alkaline resistance, high heat conductivity or photocatalytic activity. A steeping and in-situ sintering method or photodepositing and in-situ sintering method is used as a synthesis method so that the energy consumption is low, and the photothermal catalyst has high activity and long service life by using a solar-assisted in-situ sintering technology. The technology for preparing organic fuel through directly converting carbon dioxide by using sunlight and the photothermal catalyst is low in energy consumption in the catalytic process, high in organic fuel production efficiency and stable in catalyst activity.
Owner:TIANJIN UNIV
Oxidation of methanol and/or dimethyl ether using supported molybdenum-containing heteropolyacid catalysts
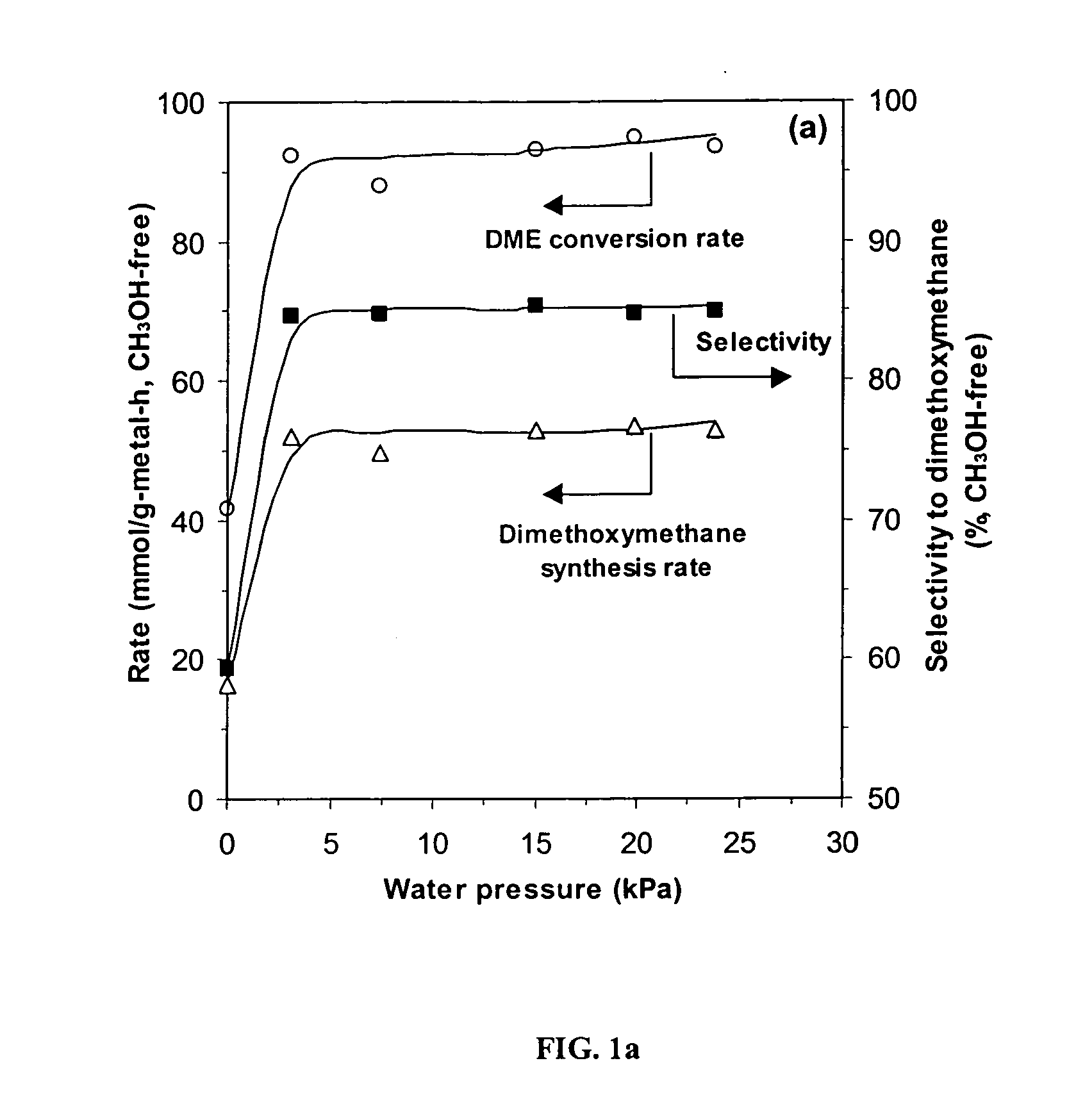
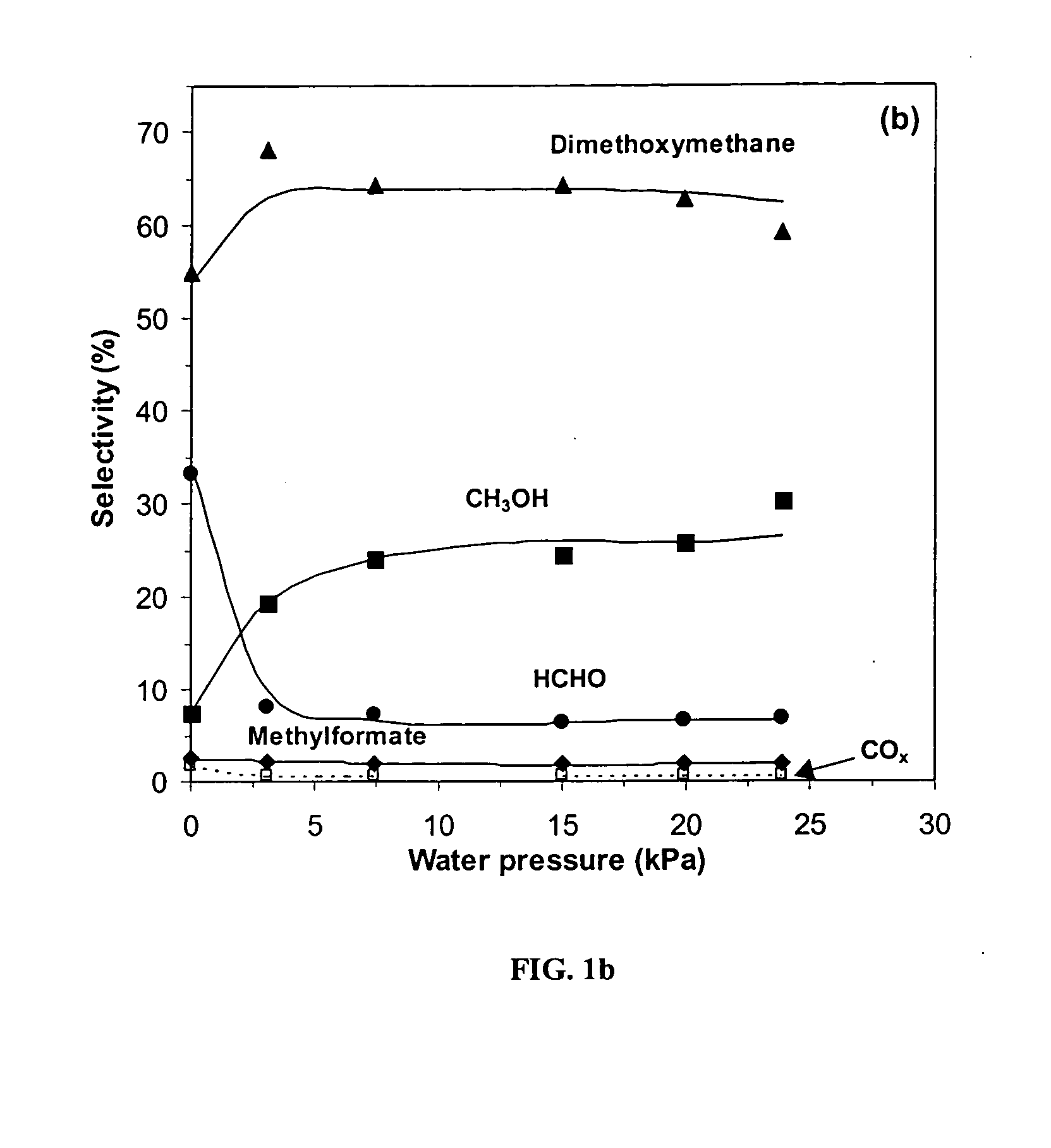
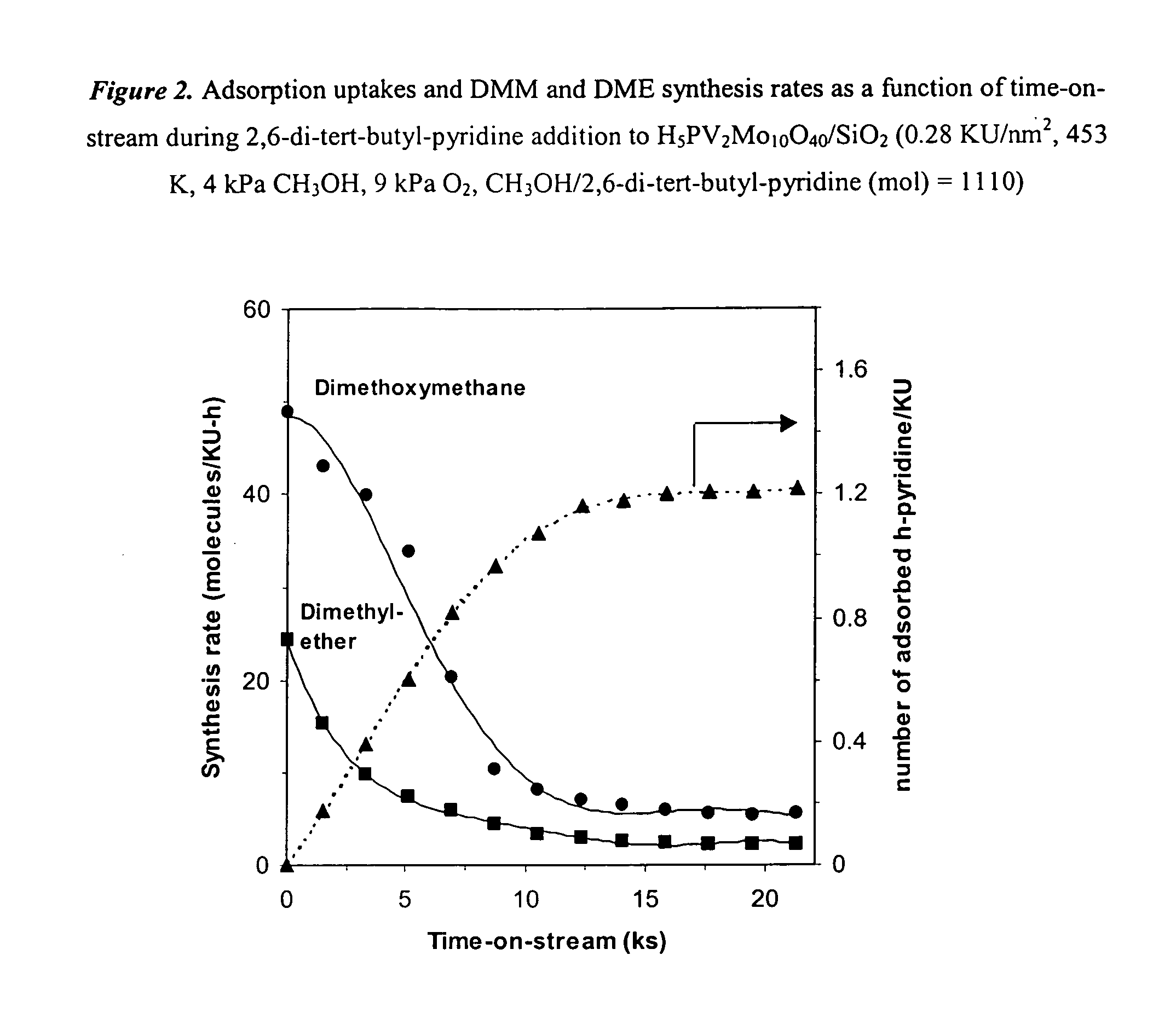
InactiveUS20050154226A1Reduce productionPromote conversionOrganic compound preparationPreparation by carbon monoxide or formate reactionOxygenDimethoxymethane
Feeds comprising methanol, dimethyl ether or a mixture of the two are oxidized by contacting the feed with an oxygen-containing gas and a supported heteropolyacid catalyst containing molybdenum or molybdenum and vanadium. The primary products are methylal (dimethoxymethane) and methyl formate. Production of dimethyl ether from methanol can be inhibited by partial deactivation of acid sites on the Keggin catalyst.
Owner:REGENTS OF CALIFORNIA UNIV OF THE +1
Preparation of titanosilicate zeolite TS-1
InactiveUS20070059237A1Reduce processing stepsRetain its shapeAluminium compoundsMolecular sieve catalystsCrystallizationExternal Liquid
A method is disclosed for preparing crystalline titanosilicate zeolite TS-1 from a reaction mixture containing only sufficient water to produce zeolite TS-1. In one embodiment, the reaction mixture is self-supporting and may be shaped if desired. In the method, the reaction mixture is heated at crystallization conditions and in the absence of an added external liquid phase, so that excess liquid need not be removed from the crystallized product.
Owner:CHEVROU USA INC
Selective oxidation of alkylbenzenes
InactiveUS20070265476A1Organic compound preparationOrganic chemistry methodsAlkaline earth metalStructural isomer
The present invention relates to a process for producing phenol and a ketone of general formula R1COCH2R2 (I), in which R1 and R2 each independently represent an alkyl group having from 1 to 4 carbon atoms, said process comprising: (a) providing an alkylbenzene feedstock comprising (i) an alkylbenzene of general formula (II) in which R1 and R2 have the same meaning as in formula (I) and (ii) at least one structural isomer of said alkylbenzene of formula (II) in an amount of at least 0.7% of the weight of alkylbenzene of formula (II), (b) submitting the alkylbenzene feedstock to oxidation conditions in the presence of oxygen and in the presence of a cyclic imide of formula (III): in which X represents a carbonyl (CO) group or a sulfonyl (SO2) group, n is 0, 1, 2, 3 or 4, R3 is one or several groups selected from a hydrogen atom, a halogen atom, an alkyl group, an alkoxy group, an amino group and R4 is a hydrogen atom, an alkaline metal cation or an alkaline earth metal cation, or in the presence of N,N′,N″-trihydroxyisocyanuric acid (THICA), to produce a product comprising a hydroperoxide of general formula (IV) in which R1 and R2 have the same meaning as in formula (I), and (c) converting the hydroperoxide of formula (IV) into phenol and a ketone of formula (I).
Owner:EXXONMOBIL CHEM PAT INC
Methanol oxidation over bulk metal vanadate catalysts
InactiveUS20050038299A1High selectivityIncrease flow rateOrganic compound preparationHeterogenous catalyst chemical elementsSufficient timeVanadate
A method wherein a methanol-containing gas stream is passed in contact with a catalyst comprising a supported or unsupported bulk vanadate catalyst in the presence of an oxidizing agent for a time sufficient to convert at least a portion of the methanol to formaldehyde (CH2O).
Owner:LEHIGH UNIVERSITY
Catalyst for synthesizing benzaldehyde and benzyl alcohol from toluol, the preparation process and application thereof
InactiveCN1485131AEasy to separateEase of usePreparation by oxidation reactionsCarbonyl compound preparation by oxidationAlkaline earth metalBenzaldehyde
A catalyst of synthesizing benzaldehyde and benzyl alcohol by methylbenzene, its manufacturing method and application. The active component is zirconium, or transit metals, alkaline metal, alkaline-earth metals, metals of IIIAíóIVA and VA Group in the Periodic Table. The method comprises solving a zirconium compound or a zirconium compound and a compound of an element as the active component, adding a precipitator; drying, burning, pulverizing to particles. In reactions, a gas containing oxygen is the oxygen source, such as oxygen and air, an organic solution wouldn't be used. The catalyzing reaction is 180-195 degree C, the pressure is 0.8-1.2Mpa. when the conversion rate of methylbenzene is 13.0úÑ, the total selectivity of benzaldehyde and benzyl alcohol is 86.6úÑ. In the method, the catalyst is easy to be separated and could be recyclable, the reaction condition is mild.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Metal-organic framework supported heteropoly acid catalyst for synthesizing glutaraldehyde and production method of metal-organic framework supported heteropoly acid catalyst
InactiveCN104437645AGood dispersionGood effectOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationCyclopentenePtru catalyst
The invention relates to a metal-organic framework supported heteropoly acid catalyst for synthesizing glutaraldehyde and a production method of the metal-organic framework supported heteropoly acid catalyst. The catalyst is prepared by a one-step synthesis method, namely a heteropoly acid component with catalytic oxidation activity is introduced into a duct of a metal-organic framework material UiO-66 in the process of synthesizing the metal-organic framework material UiO-66. The catalyst has the characteristics that the catalyst has a crystal framework structure, the active component is highly dispersed, and the heteropoly acid is over-high in load. The catalyst is applied to catalytic selective oxidation reaction of cyclopentene, so that the selectivity and the yield of the glutaraldehyde is greatly improved; the numerical value is much higher than the reported homogeneous catalysis level; and the metal-organic framework supported heteropoly acid catalyst has important industrial application value.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
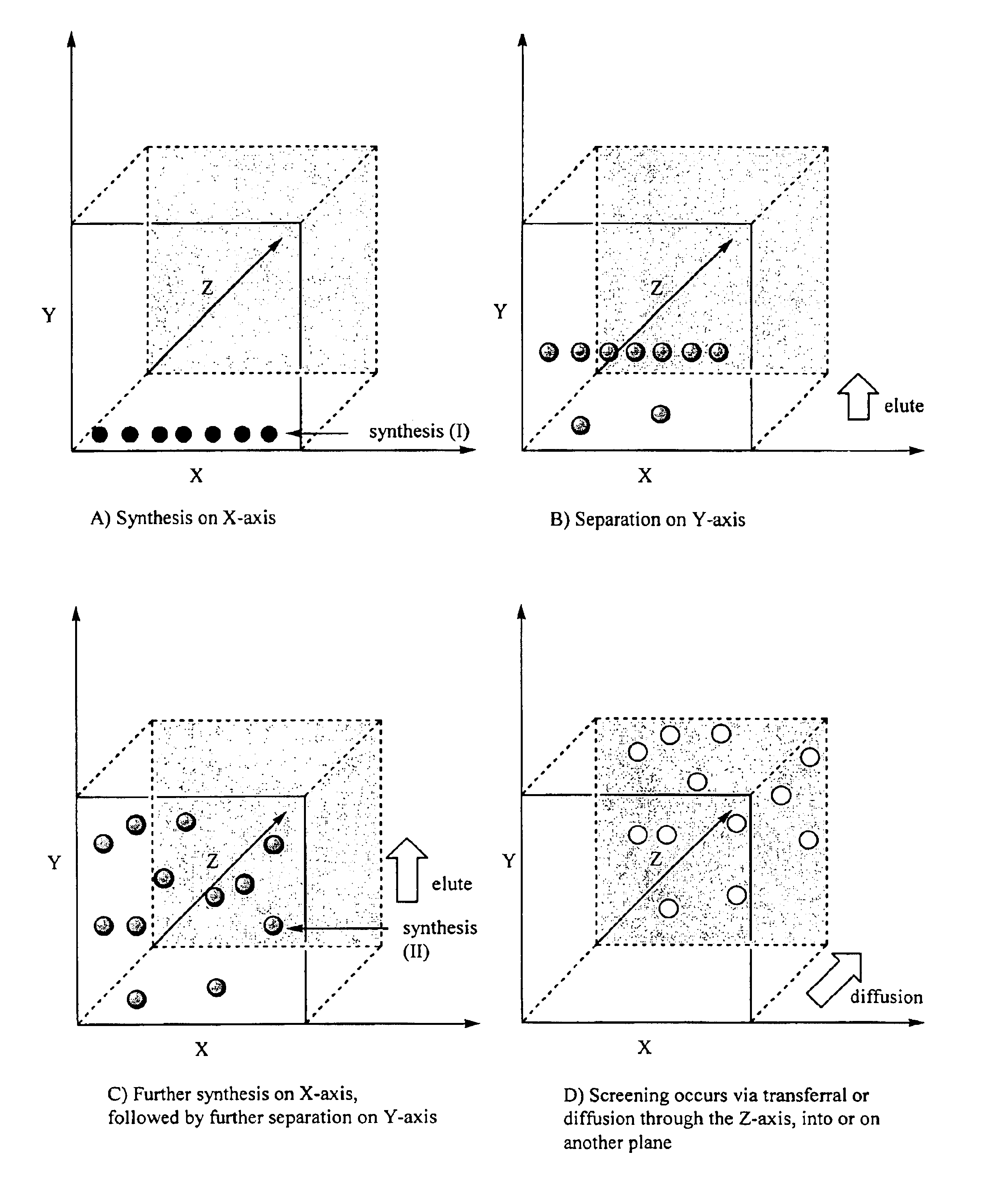
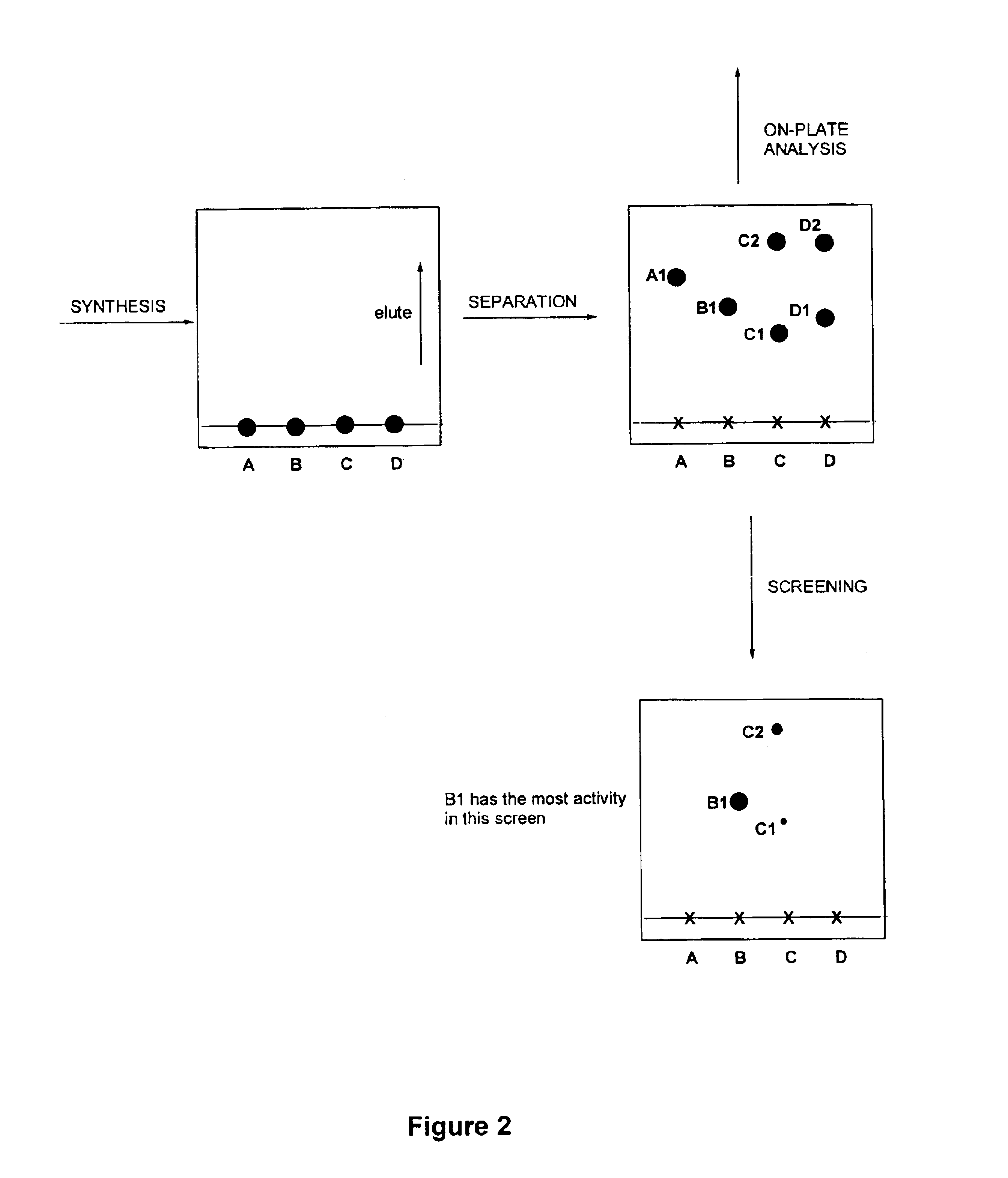
Method for synthesis, separation and screening of a plurality of compounds in the same bulk of a stationary phase
InactiveUS6858434B1Less time-consumingSequential/parallel process reactionsComponent separationCelluloseStationary phase
A method for sequentially performing a synthesis, separation and screening of chemical entities, especially a combinatorial library, is described. The method utilises a bulk of a stationary phase (e.g. silica gel, aluminium oxide, cellulose, etc. for example arranged on a backing) for the performance of the synthesis, separation and screening. The technique described enables a rapid route from synthesis to the testing of chemical compounds. Screening can be performed without need for reaction work-up. Preferred screening methods are those used to determine the biological activity of the compounds.
Owner:SINVENT AS
Preparation method and application of load type nano-gold catalyst
InactiveCN101829567AImprove loading efficiencyEasy to operatePreparation by oxidation reactionsCarbonyl compound preparation by oxidationHalloysiteGold particles
The invention discloses a preparation method and an application of a load type nano-gold catalyst. The preparation method comprises the following steps of: (1) adding 2.0g of halloysite nanotube carrier, 2.05 to 6.15mL of chlorogold acid solution with a concentration of 10g / L and 40 to 120mL of deionized water to a 250mL flask with three necks; (2) putting the flask into an oil bath with the temperature of 60 DEG C; adjusting the pH of the solution to 8 to 12 by using 4.0M ammonia water; then carrying out stirring reflux at 95 DEG C-105 DEG C for 1 hour; filtering; rinsing for 5 minutes by using 10 to 20mL of 4.0M ammonia water; washing twice with 15 to 20mL of hot water; drying for 1 to 2 hours at 100 DEG C; and finally roasting for 3 to 4 hours in air at 300 DEG C to obtain the load type nano-gold catalyst. The invention has the advantages of simple and convenient preparation method, uniformly dispersed gold particles and high loading efficiency. The catalyst provided by the invention can be used for cyclohexene oxidation of cyclonene and cyclohexenol, with the advantages of mild reaction condition, good activity and selectivity and less catalyst utilization quantity.
Owner:ZHEJIANG UNIV
Method of producing unsaturated aldehyde and unsaturated acid in fixed-bed catalytic partial oxidation reactor with enhanced heat control system
ActiveUS20050049435A1Improve performanceUnified performanceOrganic compound preparationCarbonyl compound preparation by oxidationExothermic reactionChemistry
The present invention provides a process of producing unsaturated aldehydes and unsaturated acids from olefins by fixed-bed catalytic partial oxidation in a shell-and-tube heat exchanger-type reactor. In this process, the reactor comprises a first-step reaction zone of mainly producing the unsaturated aldehydes, a second-step reaction zone of mainly producing the unsaturated acids, or both the two zones. The first-step reaction zone is divided into two or more zones by a partition. Each of the divided shell spaces is filled with a heat transfer medium, and the heat transfer medium in each shell space is maintained at isothermal temperature or a temperature difference of 0-5 ° C. The temperatures of the heat transfer media in each of the divided shell spaces are set to increase in the moving direction of reactants. In order to facilitate the removal of heat generation at a location where the partition is placed, a reaction inhibition layer is disposed in the first-step reaction zone. Also, in order to protect the catalyst layer from a highly exothermic reaction, the process is performed at a limited temperature difference between the temperature in a hot spot and the temperature of a molten salt. If the improved heat control system according to the present invention is used, the heat stability of the catalyst layer will be secured and the yields of intermediate and final products can be increased.
Owner:LG CHEM LTD
Novel fragrance and methods for production of 5-epi-beta-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one, and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one
The present invention is directed to novel methods for production of 5-epi-β-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one, which are useful for their fragrant qualities. In one embodiment the present invention describes a method for production of 5-epi-β-vetivone by the use of premnaspirodiene as starting material. In another embodiment the present invention describes a method for production of 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one by the use of premnaspirodiene as starting material. In yet another embodiment the present invention describes a novel method for production of premnaspirodiene from a terpene substrate. Use of the fragrant components or any composition containing the component can be advantageously employed in the perfumery industry.
Owner:EVOLVA INC
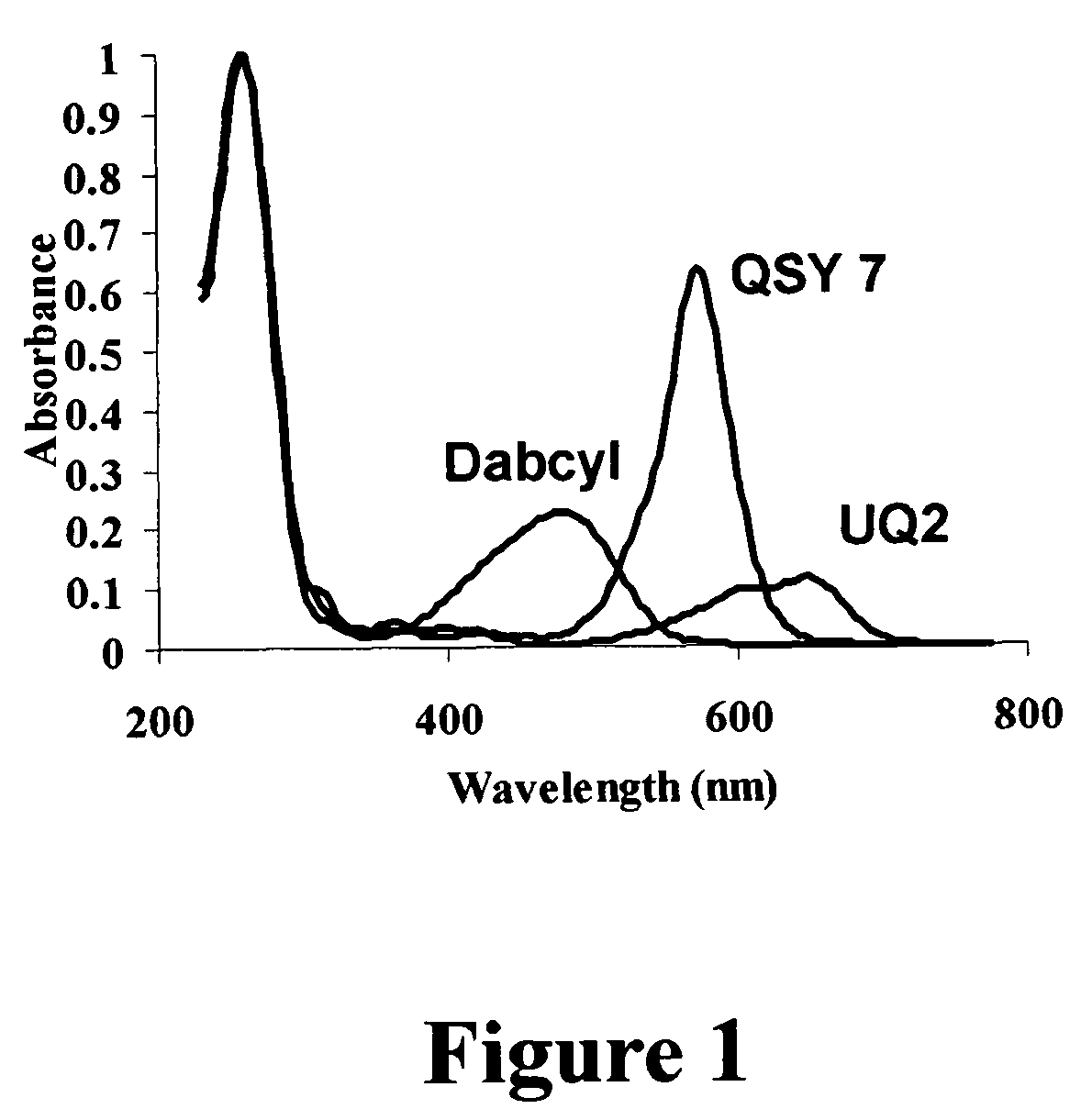
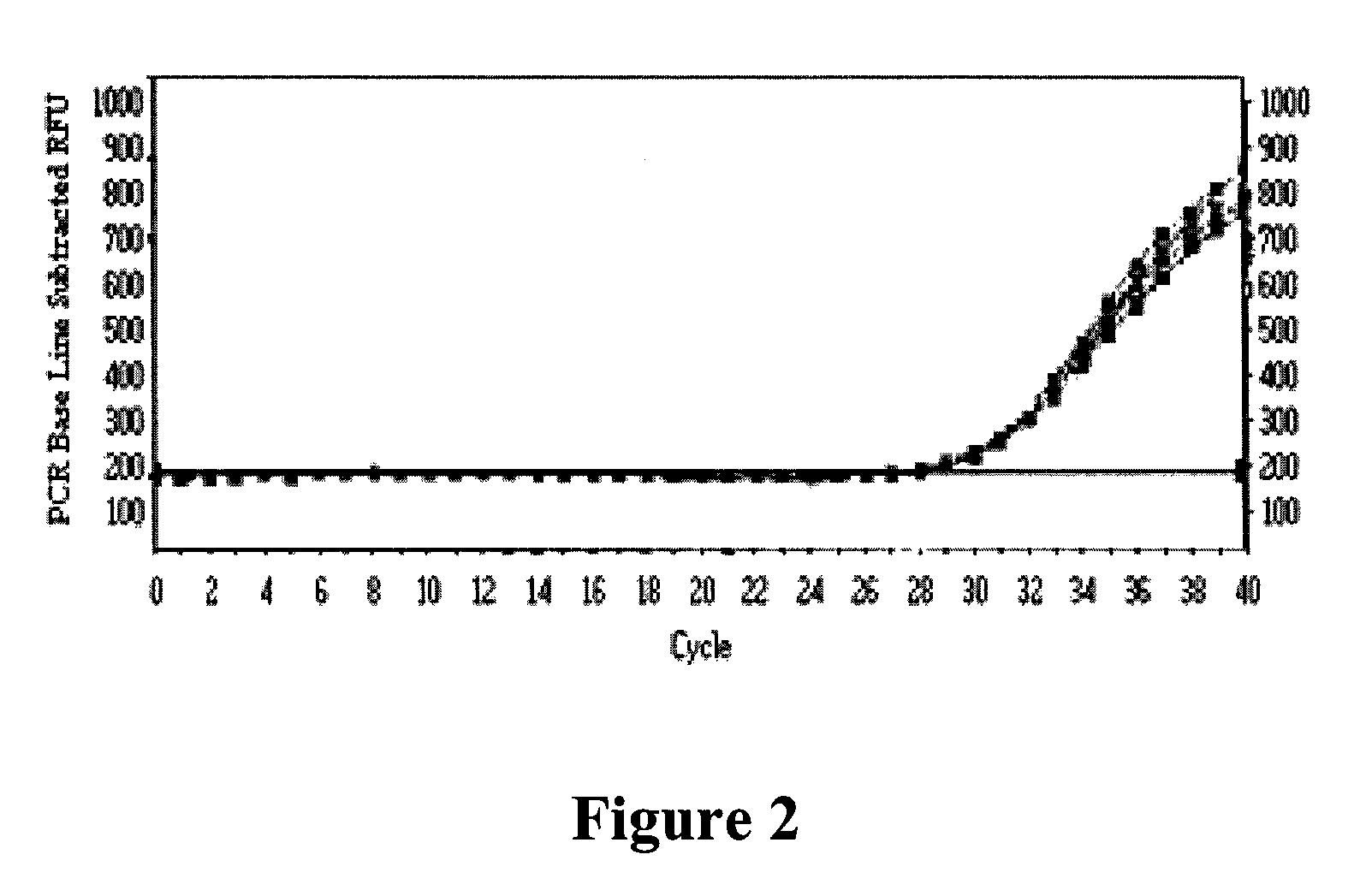
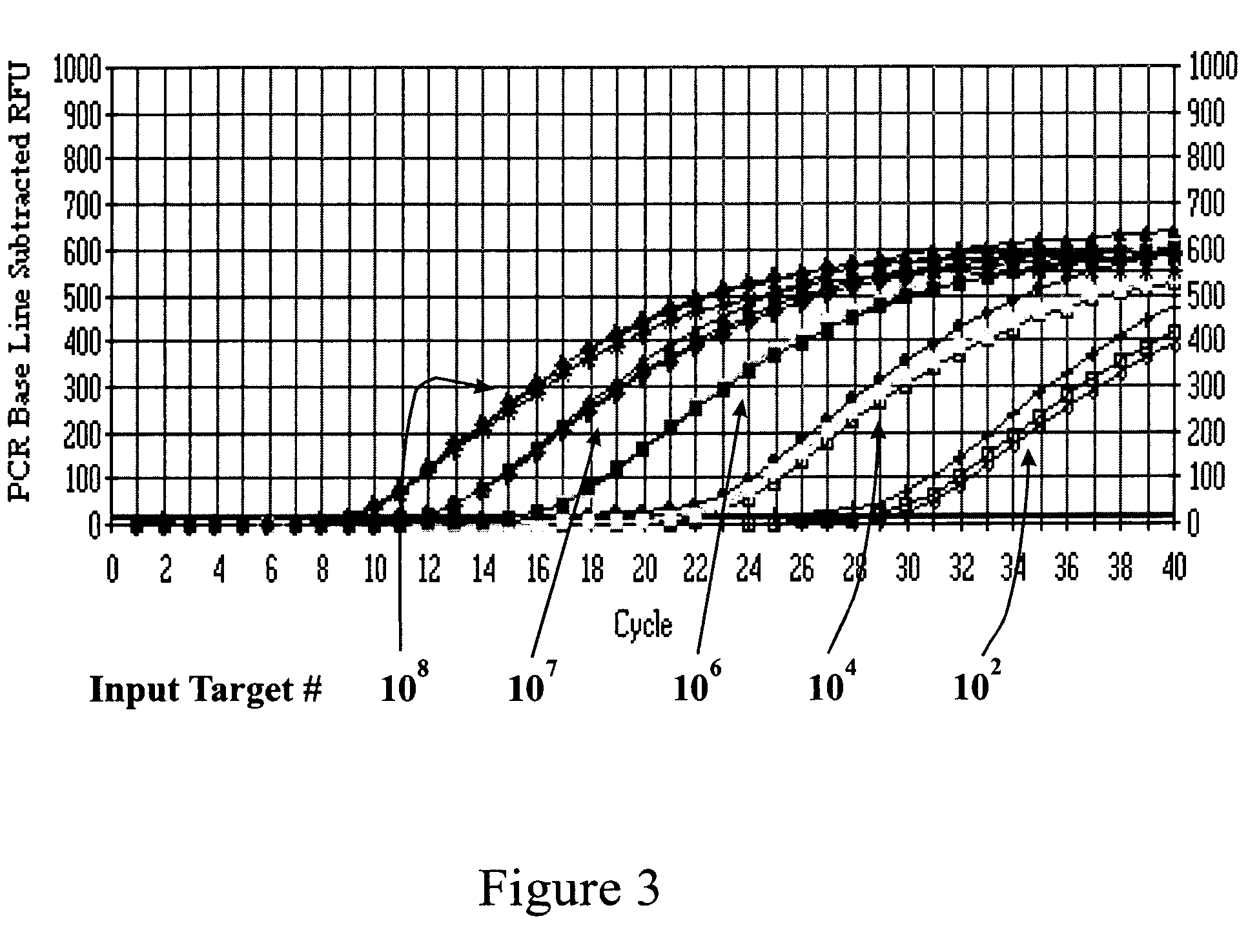
Methods of detecting fluorescence with anthraquinone quencher dyes
The invention provides novel anthraquinone compositions that are useful as broad-spectrum quenchers of fluorescence and provides methods for making and using them. The anthraquinone quenchers can be conjugated to a variety of biologically relevant compounds, including lipids, nucleic acids, polypeptides, and more specifically antigens, steroids, vitamins, drugs, haptens, metabolites, toxins, environmental pollutants, amino acids, peptides, proteins, nucleotides, oligonucleotides, polynucleotides, carbohydrates, and their analogs. The invention also provides kits comprising, in one or more containers, at least one anthraquinone quencher dye composition of the present invention, and instructions for using that composition.
Owner:INTEGRATED DNA TECHNOLOGIES
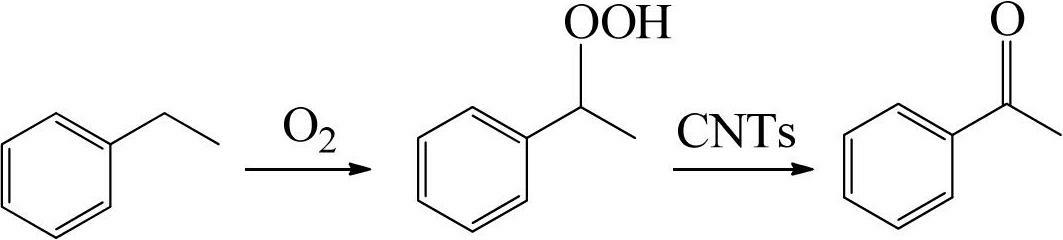
Method for producing acetophenone through catalytic oxidation of ethylbenzene
InactiveCN102675072ARich sourcesHigh selectivityCarbonyl compound preparation by oxidationCatalytic oxidationOxygen
The invention discloses a method for producing acetophenone through catalytic oxidation of ethylbenzene. The method comprises the following steps of: (1) adding the ethylbenzene, a solvent, an initiating agent and a carbon material catalyst into a reactor, and mixing to form mixed suspension liquid; (2) raising the temperature of the mixed suspension liquid obtained in the step (1) to 50-250 DEG C, introducing sufficient oxygen or air which serve as an oxidizing agent, keeping the pressure of 0.1 to 5MPa, and reacting for 0.1 to 20 hours; (3) separating the reaction mixture obtained in the step (2), and thus obtaining a solid catalyst and a liquid phase mixture; and (4) separating the liquid phase mixture obtained in the step (3), and thus obtaining the acetophenone. By the method, the corrosion to equipment and the pollution to the environment due to the usage of acetic acid and a metal catalyst are prevented; the problems that a homogeneous catalyst is difficult to recover and is inactivated in the ethylbenzene oxidation process can be solved; and the method is high in reaction selectivity and high in activity. The catalyst used in the method is high in stability and can be recovered and reused, so that the cost is reduced, and the efficiency is improved.
Owner:SOUTH CHINA UNIV OF TECH
Method for producing monocyclic ketones C7-C20
InactiveUS20050203316A1Improve efficiencyOrganic compound preparationCarbonyl compound preparation by oxidationKetoneNitrous oxide
The invention relates to a method for producing monocyclic ketones C7-C20. The method of patent protection sought is based on the oxidation reaction of monocyclic alkenes C7-C20 into corresponding monocyclic ketones with nitrous oxide or a mixture thereof with inert gas. The process proceeds at a temperature ranging from 20 to 350° C. and a pressure of the nitrous oxide ranging from 0.01 to 100 atm. The process provides a high selectivity involving the target product, blast-resistant work operations and is promising for industrial use.
Owner:INST KATALIZA IMENI GK BORESKOVA SIBIRSKOGO OTDELENIA ROSSIISKOI ACAD NAUK
Benzyl alcohol selective oxidation catalyst, preparation method and application thereof
InactiveCN101564692AEasy to prepareHigh catalytic activityOrganic compound preparationCarbonyl compound preparation by oxidationPore diameterBENZYL ALCOHOL/WATER
The invention discloses a benzyl alcohol selective oxidation catalyst, which consists of a carrier and metal components, wherein the carrier is MgAl2O4, the metal components are selected from one or more metallic oxides of V, Mo, W, Ag, Cu, Zn, Co, Ni, Fe or Sn, and in terms of the total weight of the catalyst, the catalyst comprises 80.0 to 99.9 percent of the MgAl2O4 and 0.1 to 20.0 percent of the metallic oxides. The specific surface areas of the prepared carrier MgAl2O4 and the MeOx / MgAl2O4 catalyst are between 200 and 400 m / g, the most probable pore diameter is between 3 and 6 nanometers, and the particle size is less than 100 nanometers.
Owner:NORTHWEST UNIV(CN)
Double-metal MOF (Metal-Organic Framework) catalyst as well as preparation method and application thereof
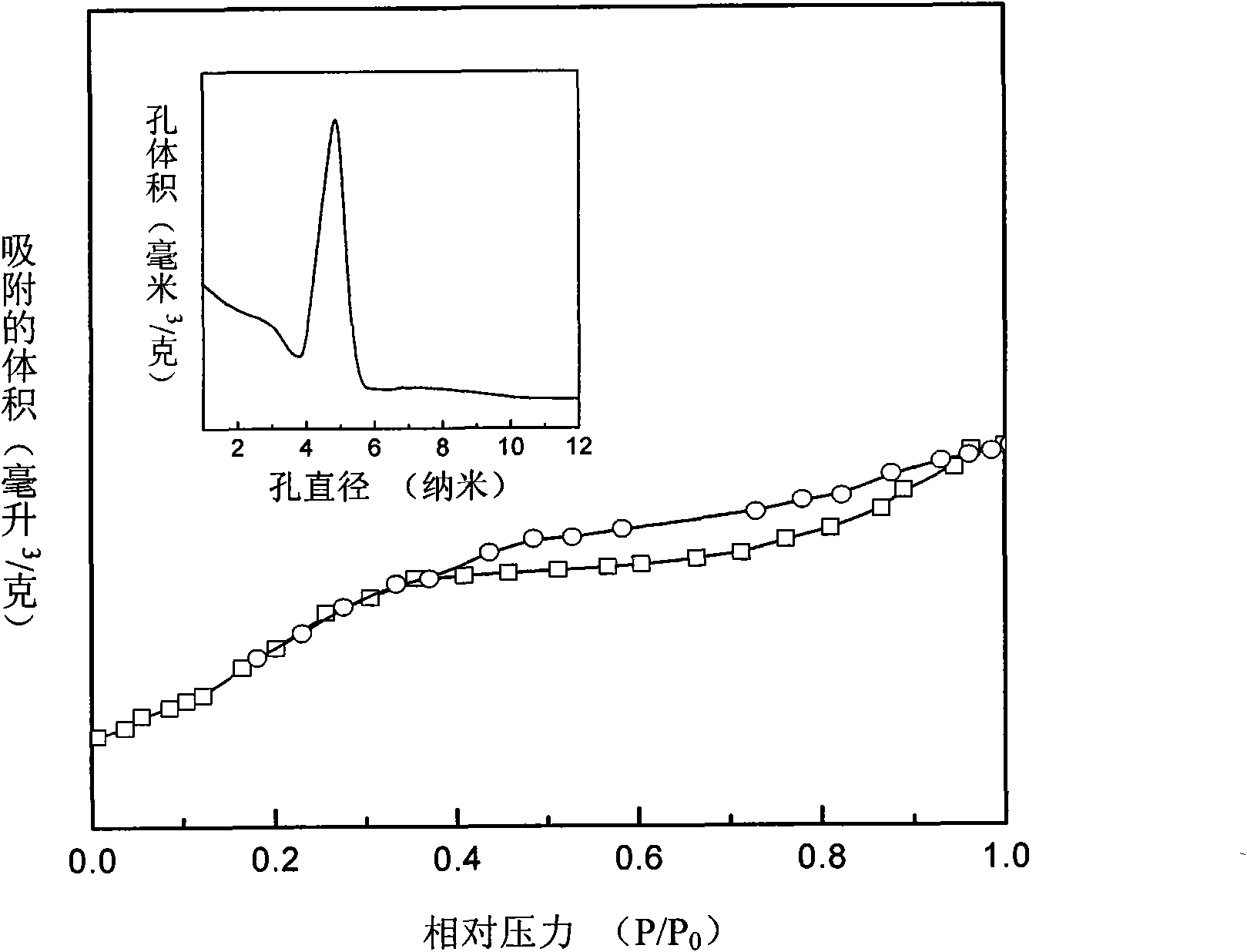
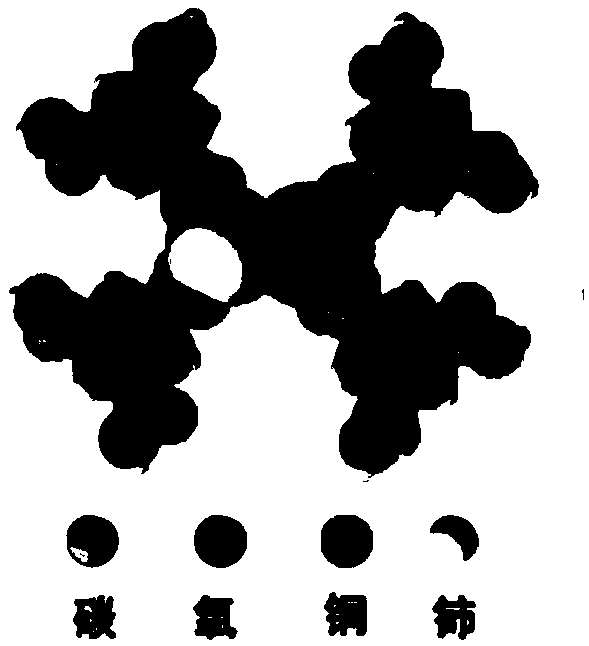
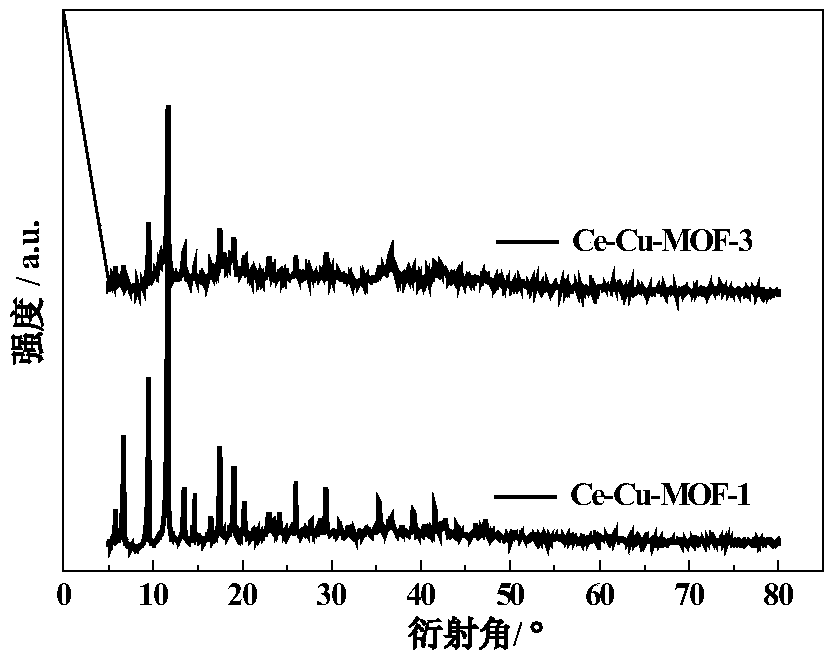
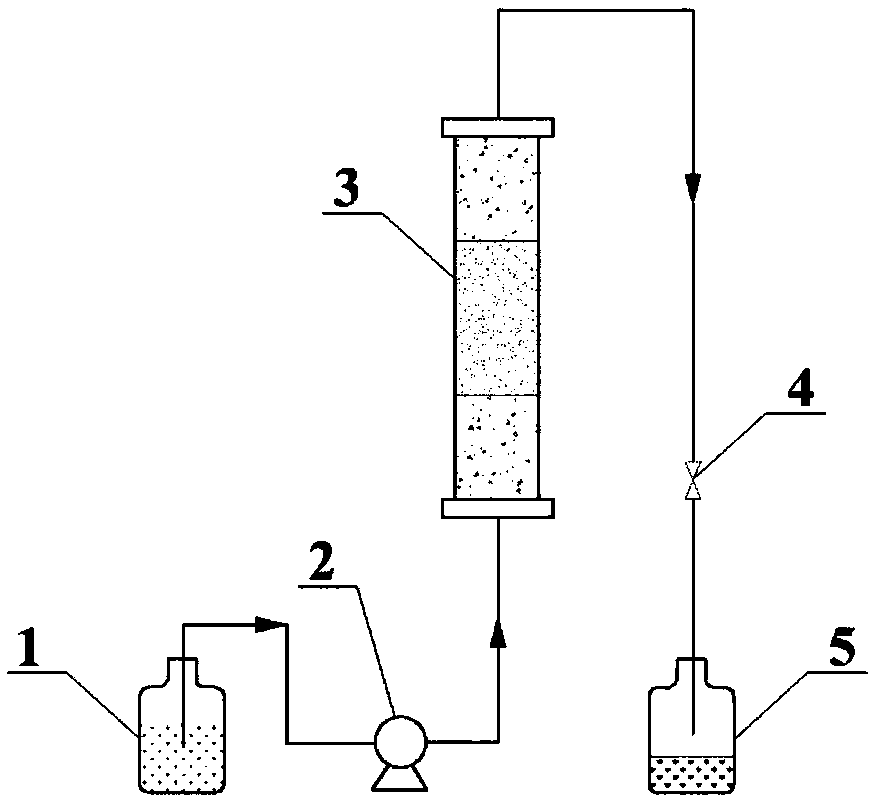
InactiveCN108772103AEasy to prepareEasy to separate and recycleOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsCeriumFixed bed
The invention relates to a double-metal MOF (Metal-Organic Framework) catalyst. Two inorganic metal centers and an organic ligand are self-assembled through a coordination bond to form a three-dimensional cage-shaped structure; the specific surface area is 170 to 1,145m<2> / g, the pore capacity is 0.18 to 0.48cm<3> / g and the average pore diameter is 1.34 to 3.55nm; any two types of nitrate of metalcopper, nickel, cobalt and cerium are used as metal precursors, one of trimesic acid, 2-methylimidazole or terephthalic acid is used as a synthesis ligand and a suitable solvent is selected to synthesize the double-metal MOF catalyst. A double-metal MOF material is used as a catalyst and p-diethylbenzene is used as a raw material; the p-diethylbenzene is catalytically oxidized in a fixed bed to prepare p-ethylacetophenone. The method provided by the invention has the advantages of mild reaction conditions, simplicity in operation, high p-diethylbenzene conversion rate and high p-ethylacetophenone selectivity; a product and the catalyst are easy to separate and the catalyst is stable in performance; reaction is applied to fixed bed reaction to realize continuous production, so that the method has a wide industrial application prospect.
Owner:NANJING UNIV OF TECH
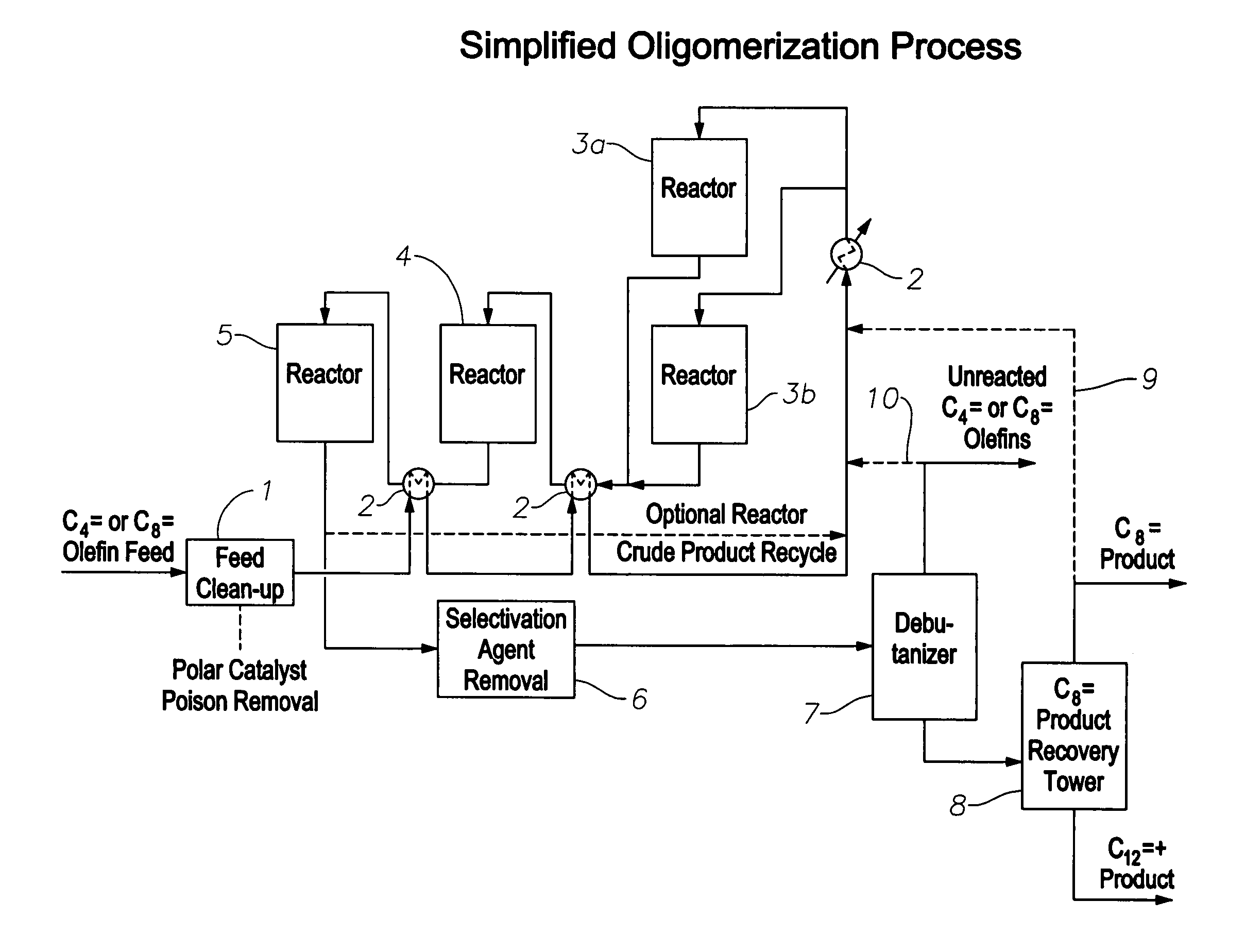
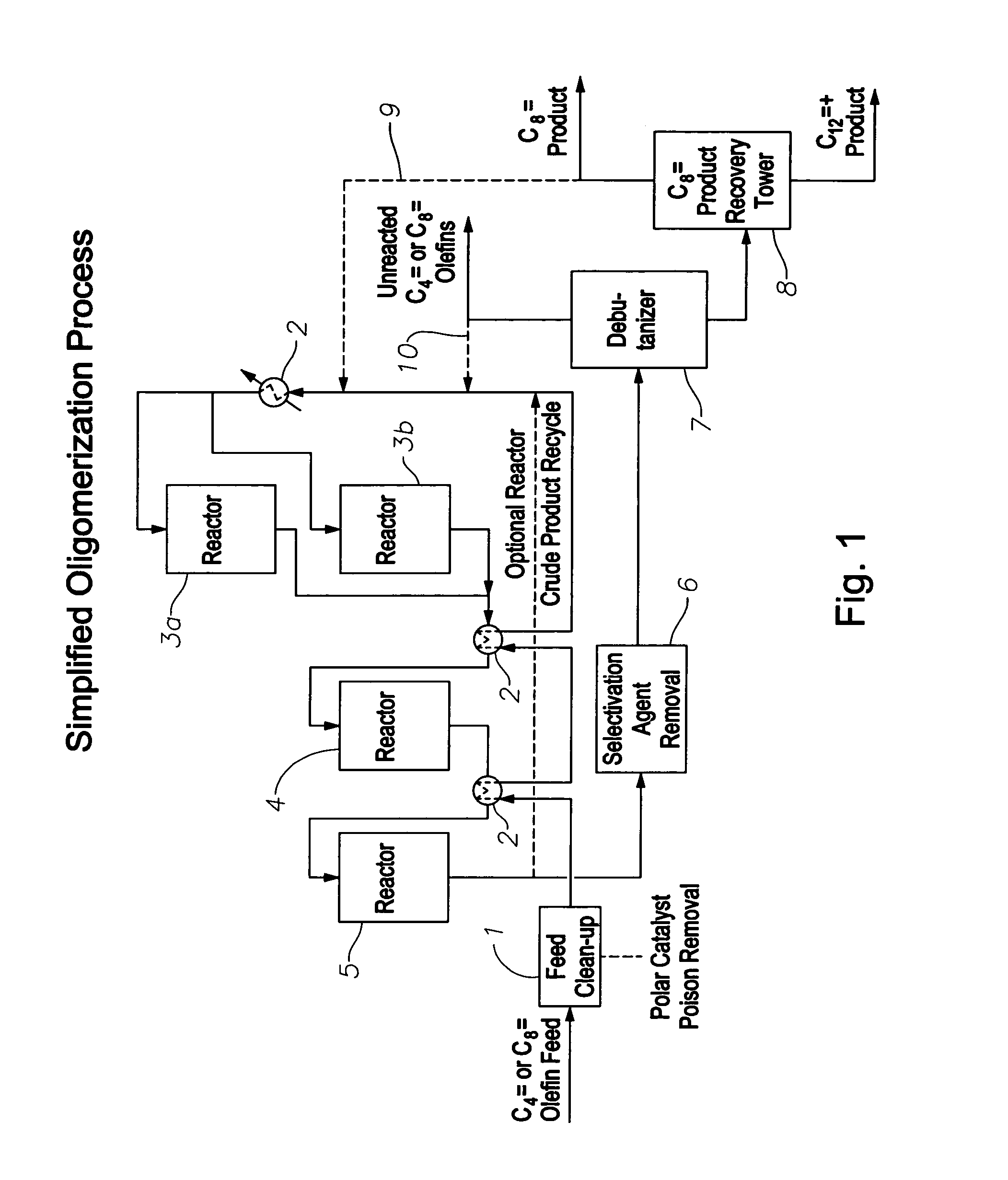
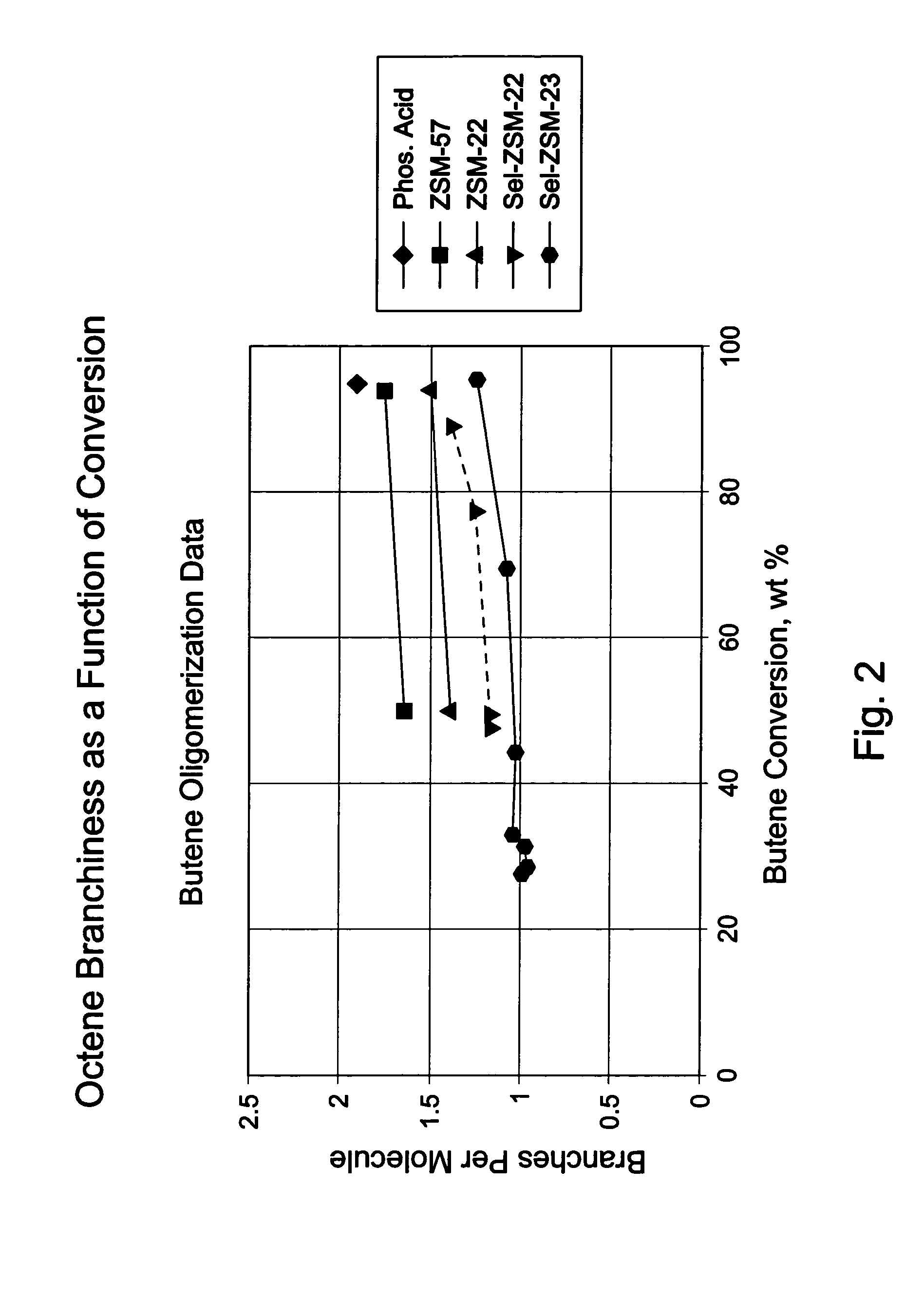
Oligomerization of olefins
ActiveUS7425662B2Increased branchingImprove linearityHydrocarbon by dehydrogenationCarboxylic acid esters preparationButeneMolecular sieve
A selectivated molecular sieve, e.g., ZSM-22 or ZSM-23, is used as olefin oligomerization catalyst to provide product, e.g., octenes and dodecenes from butene, having a low degree of branching and hindered double bonds.
Owner:EXXONMOBIL CHEM PAT INC
Carbocatalysts for chemical transformations
InactiveCN103025685AMaterial nanotechnologyOrganic compound preparationHydration reactionChemical reaction
The disclosure relates to catalytically active carbocatalysts, e.g., a graphene oxide or graphite oxide catalyst suitable for use in a variety of chemical transformations. In one embodiment, it relates to a method of catalyzing a chemical reaction of an organic molecule by reacting the organic molecule in the presence of a sufficient amount of graphene oxide or graphite oxide for a time and at a temperature sufficient to allow catalysis of a chemical reaction. According to other embodiments, the reaction may be an oxidation reaction, a hydration reaction, a dehydrogenation reaction, a condensation reaction, or a polymerization reaction. Some reactions may include auto-tandem reactions. The disclosure further provides reaction mixtures containing an organic molecule and graphene oxide or graphite oxide in an amount sufficient to catalyze a reaction of the organic molecule.
Owner:GRAPHEA
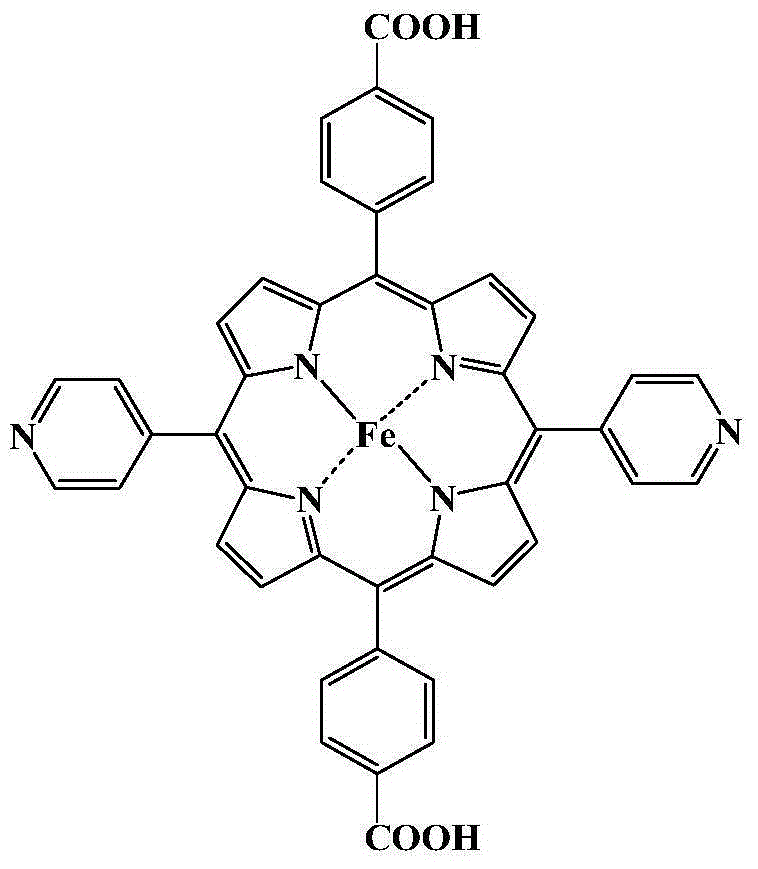
Metal organic framework material of Fe porphyrin ligand, preparation method therefor and application thereof
InactiveCN105061776ANovel structureImprove catalytic performancePreparation by oxidation reactionsOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidN dimethylformamide
The invention discloses a metal organic framework material of a Fe porphyrin ligand, a preparation method therefor and an application thereof, and belongs to the technical field of crystalline materials. A chemical formula of the Fe porphyrin ligand is FeL, wherein L is am organic ligand, namely 5, 15-bipyridine-10 and 20-dicarboxyphenyl ironporphyrin. In a closing condition, a crystal of the metal organic framework material is obtained by the organic ligand, namely 5, 15-bipyridine-10, 20-dicarboxyphenyl ironporphyrin and iron nitrate nonahydrate by virtue of thermal reaction in a solution of N,N-dimethylformamide and acetic acid. The metal organic framework material has an iron porphyrin catalytic property, and can be used for selective catalysis of cycloalkane.
Owner:BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
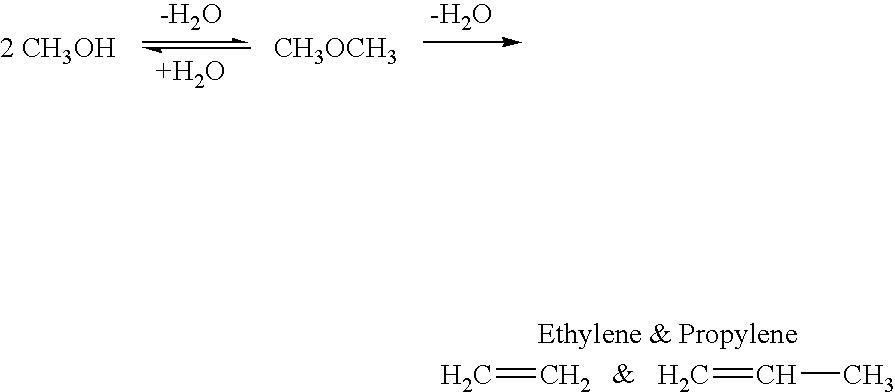
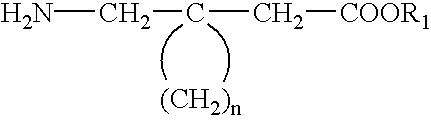
![Novel fragrance and methods for production of 5-epi-beta-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one, and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one Novel fragrance and methods for production of 5-epi-beta-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one, and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one](https://images-eureka.patsnap.com/patent_img/8b1305f6-4bff-4016-a8f5-365a23d58585/US20100129306A1-20100527-D00000.png)
![Novel fragrance and methods for production of 5-epi-beta-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one, and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one Novel fragrance and methods for production of 5-epi-beta-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one, and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one](https://images-eureka.patsnap.com/patent_img/8b1305f6-4bff-4016-a8f5-365a23d58585/US20100129306A1-20100527-D00001.png)
![Novel fragrance and methods for production of 5-epi-beta-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one, and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one Novel fragrance and methods for production of 5-epi-beta-vetivone, 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-2,6-dien-8-one, and 2-isopropyl-6,10-dimethyl-spiro[4.5]deca-1,6-dien-8-one](https://images-eureka.patsnap.com/patent_img/8b1305f6-4bff-4016-a8f5-365a23d58585/US20100129306A1-20100527-D00002.png)