Metal-organic framework supported heteropoly acid catalyst for synthesizing glutaraldehyde and production method of metal-organic framework supported heteropoly acid catalyst
A technology of immobilized heteropolyacids and organic frameworks, applied in the field of catalysts for the heterogeneous catalytic oxidation of cyclopentene to synthesize glutaraldehyde, can solve the problem that the total production capacity is less than 500 tons, the yield of glutaraldehyde is not high, and there is no industrial value and other problems to achieve excellent catalytic performance, facilitate production control, and prevent loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: A kind of metal-organic framework immobilized heteropolyacid catalyst for the synthesis of glutaraldehyde, its production method comprises the following steps:
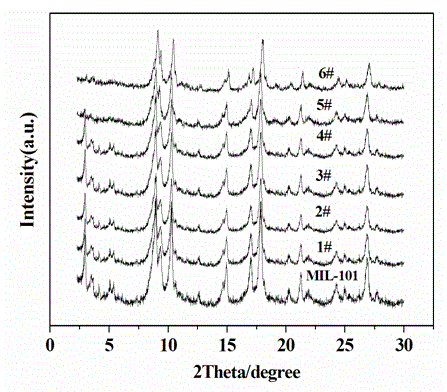
[0023] Place the reactor in a water bath at 35°C, add 80 ml of DMF (N,N-dimethylformamide) to the reactor, and dissolve 2.0 g of terephthalic acid H 2 (BDC) and 2.8 g ZrCl 4 Dissolve in DMF, stir to dissolve, then add 1.5 ml of concentrated hydrochloric acid (concentration: 1.179g / ml), stir for 3 h, then add 0.497 g of phosphotungstic heteropoly acid, continue stirring for 2 h, mix the above reaction The raw material was transferred into a stainless steel reactor, crystallized at 120°C for 30 h, taken out, filtered, washed and dried, and the product was activated at a constant temperature of 180°C for 10 h in an air atmosphere to obtain 1# catalyst.
Embodiment 2
[0024] Example 2: A kind of metal-organic framework immobilized heteropolyacid catalyst for the synthesis of glutaraldehyde, its production method comprises the following steps:
[0025] Place the reactor in a water bath at 35°C, add 80 ml of DMF (N,N-dimethylformamide) to the reactor, and dissolve 2.0 g of terephthalic acid H 2 (BDC) and 2.8 g ZrCl 4 Dissolve in DMF, stir to dissolve, then add 1.5 ml concentrated hydrochloric acid to it, stir for 3 h, then add 0.745 g phosphotungstic heteropoly acid, continue stirring for 2 h, after mixing evenly, transfer the above reaction materials into a stainless steel reaction kettle , crystallized at 120°C for 30h, took it out, filtered, washed, dried, and activated at a constant temperature of 180°C in an air atmosphere for 10h to obtain 2# catalyst.
Embodiment 3
[0026] Example 3: A kind of metal-organic framework immobilized heteropolyacid catalyst for the synthesis of glutaraldehyde, its production method comprises the following steps:
[0027] Place the reactor in a water bath at 35°C, add 80 ml of DMF (N,N-dimethylformamide) to the reactor, and dissolve 2.0 g of terephthalic acid H 2 (BDC) and 2.8 g ZrCl 4 Dissolve in DMF, stir to dissolve, then add 1.5 ml concentrated hydrochloric acid to it, stir for 3 h, then add 0.994 g phosphotungstic heteropoly acid, continue stirring for 2 h, after mixing evenly, transfer the above reaction materials into a stainless steel reaction kettle , crystallized at 120°C for 30 h. Take it out, filter, wash and dry it, and activate it at 180°C for 10 h at a constant temperature in an air atmosphere to obtain 3# catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com