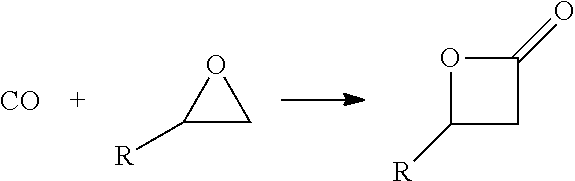Patents
Literature
853results about "Oxygen compounds preparation by hydrocarbon oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthesis of liquid fuels from biomass
ActiveUS20100076233A1Hydrocarbon by metathesis reactionLiquid hydrocarbon mixture productionFuranAlkane
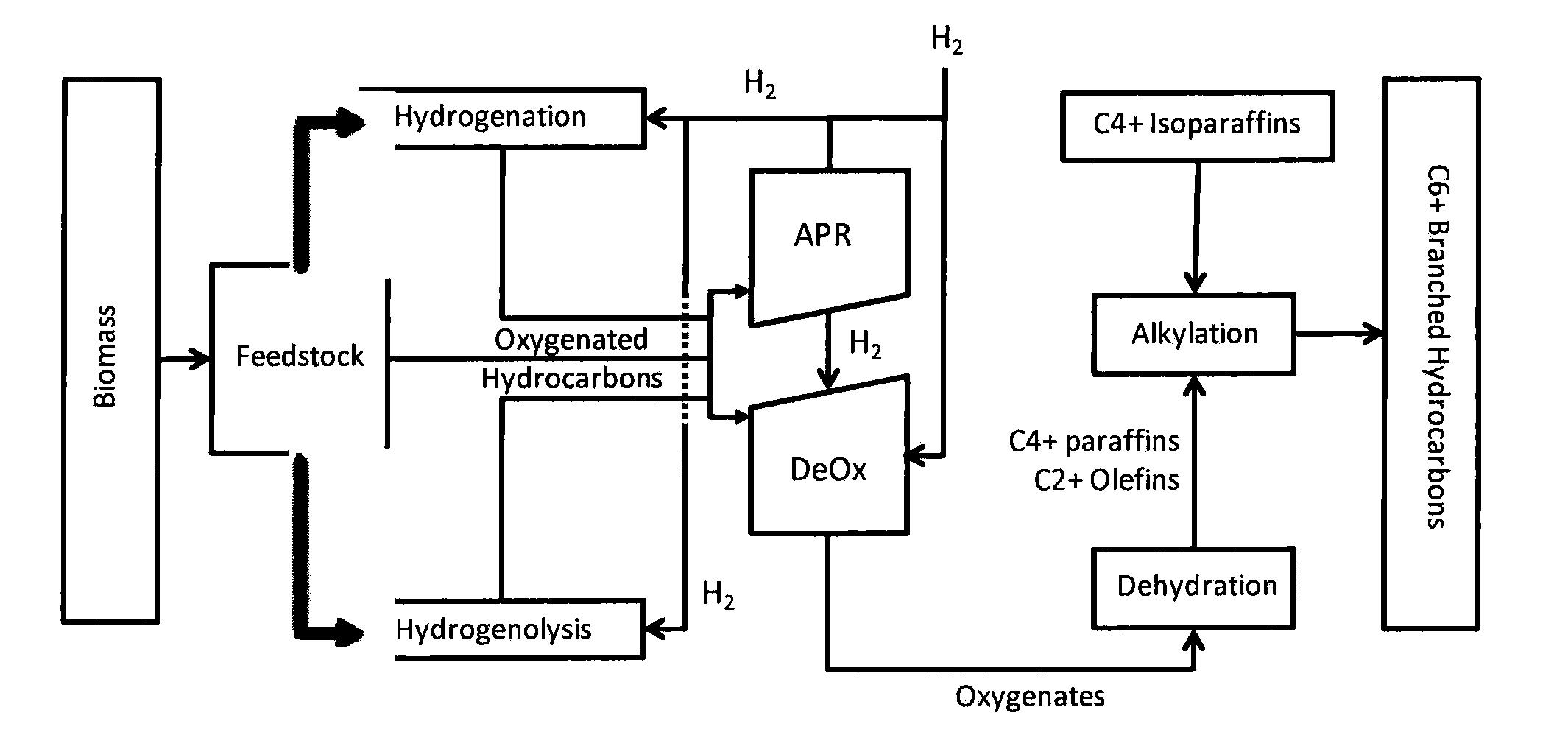
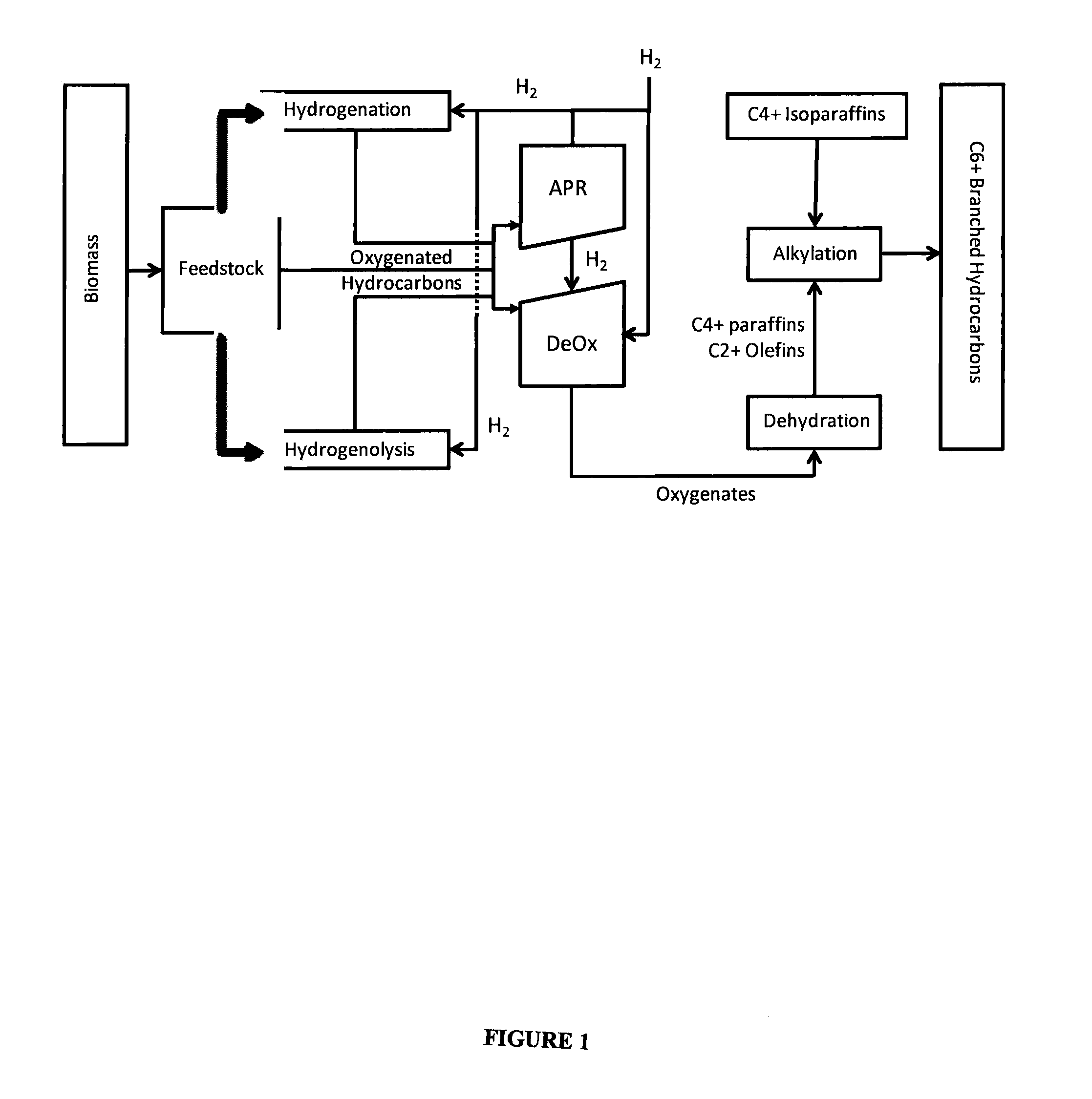
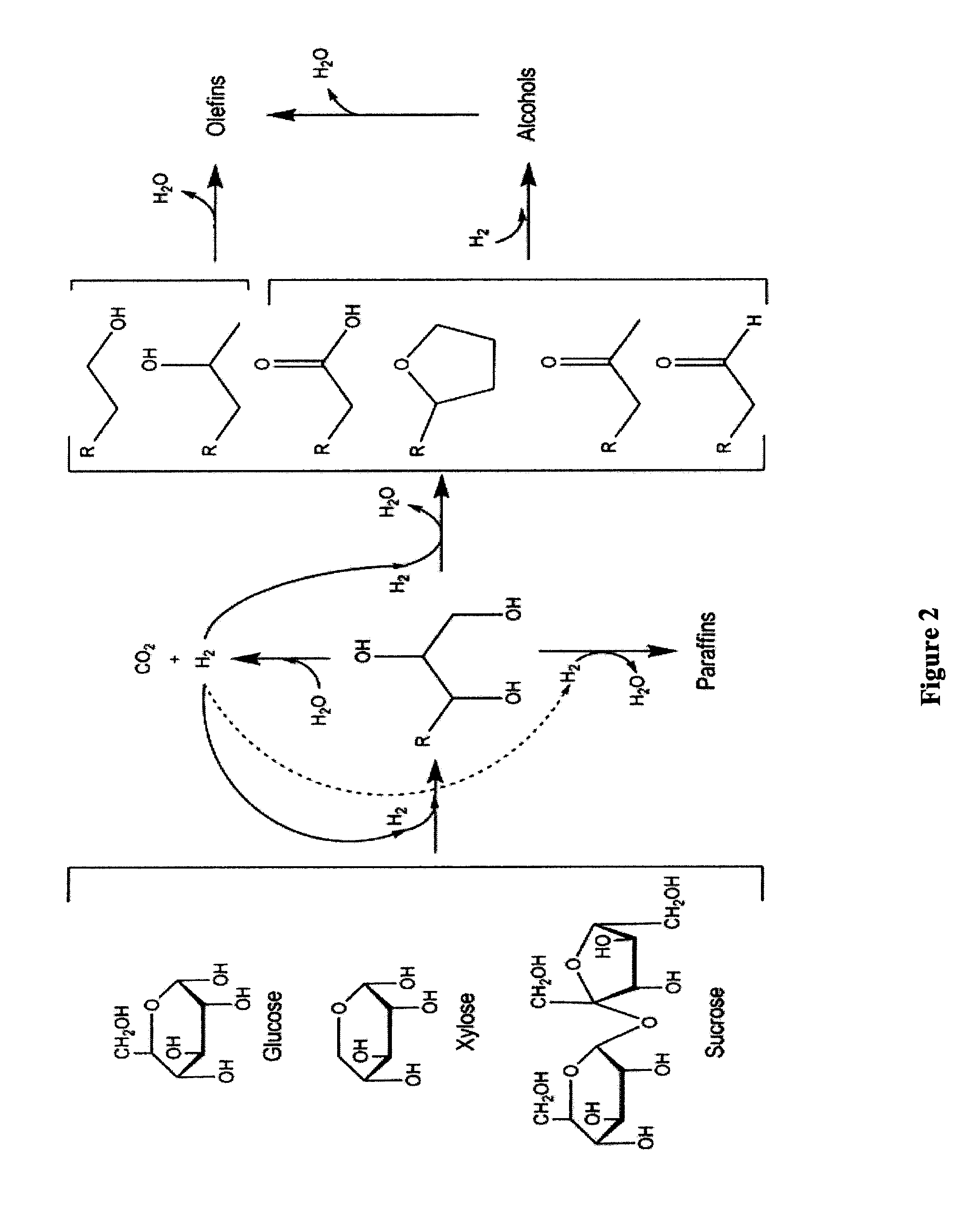
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to paraffins useful as liquid fuels. The process involves the conversion of water soluble oxygenated hydrocarbons to oxygenates, such as alcohols, furans, ketones, aldehydes, carboxylic acids, diols, triols, and / or other polyols, followed by the subsequent conversion of the oxygenates to paraffins by dehydration and alkylation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
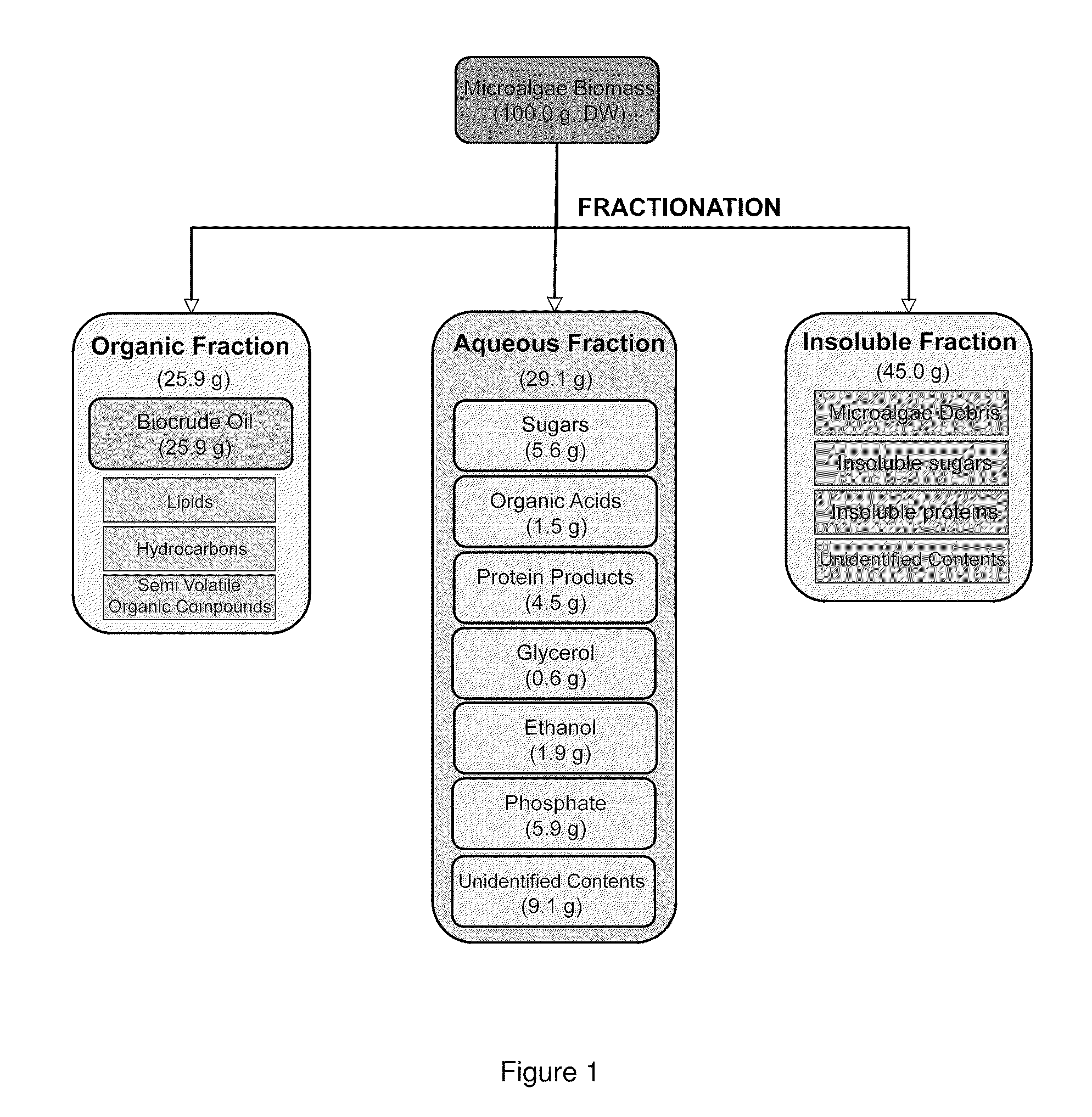
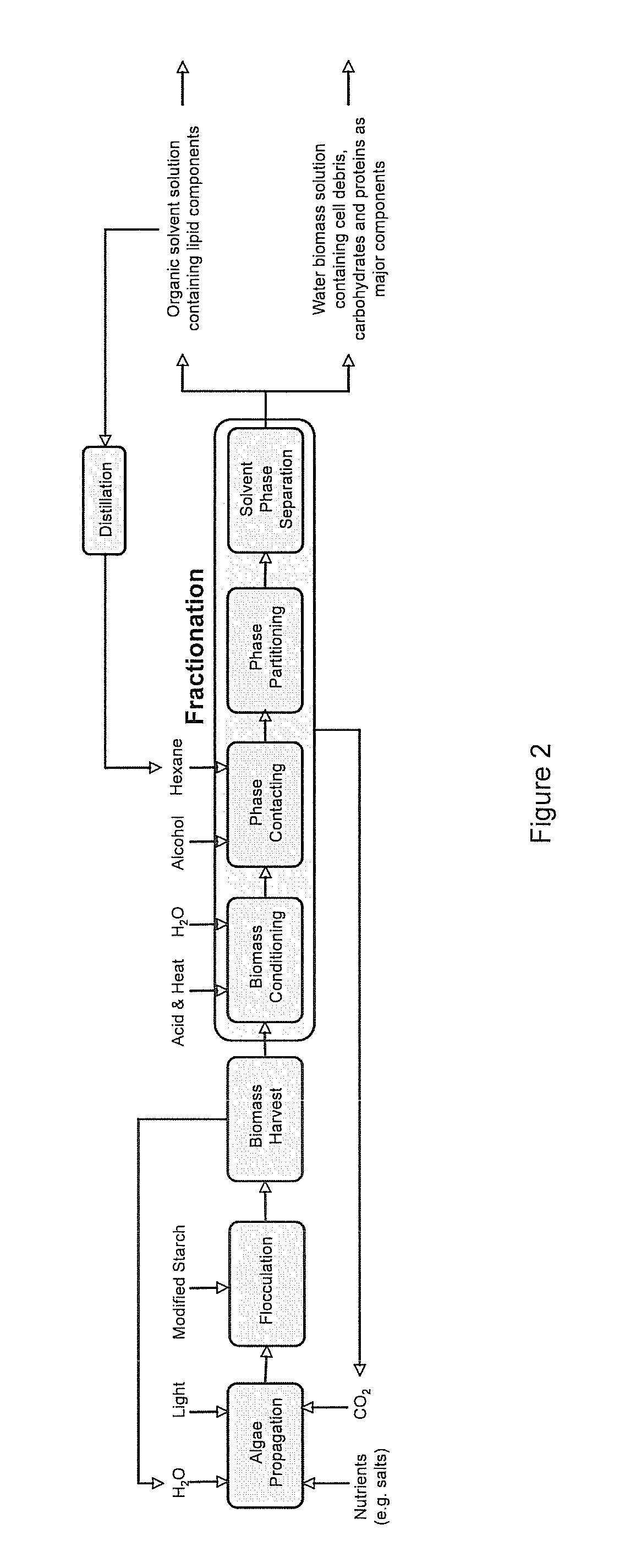
Algae biomass fractionation
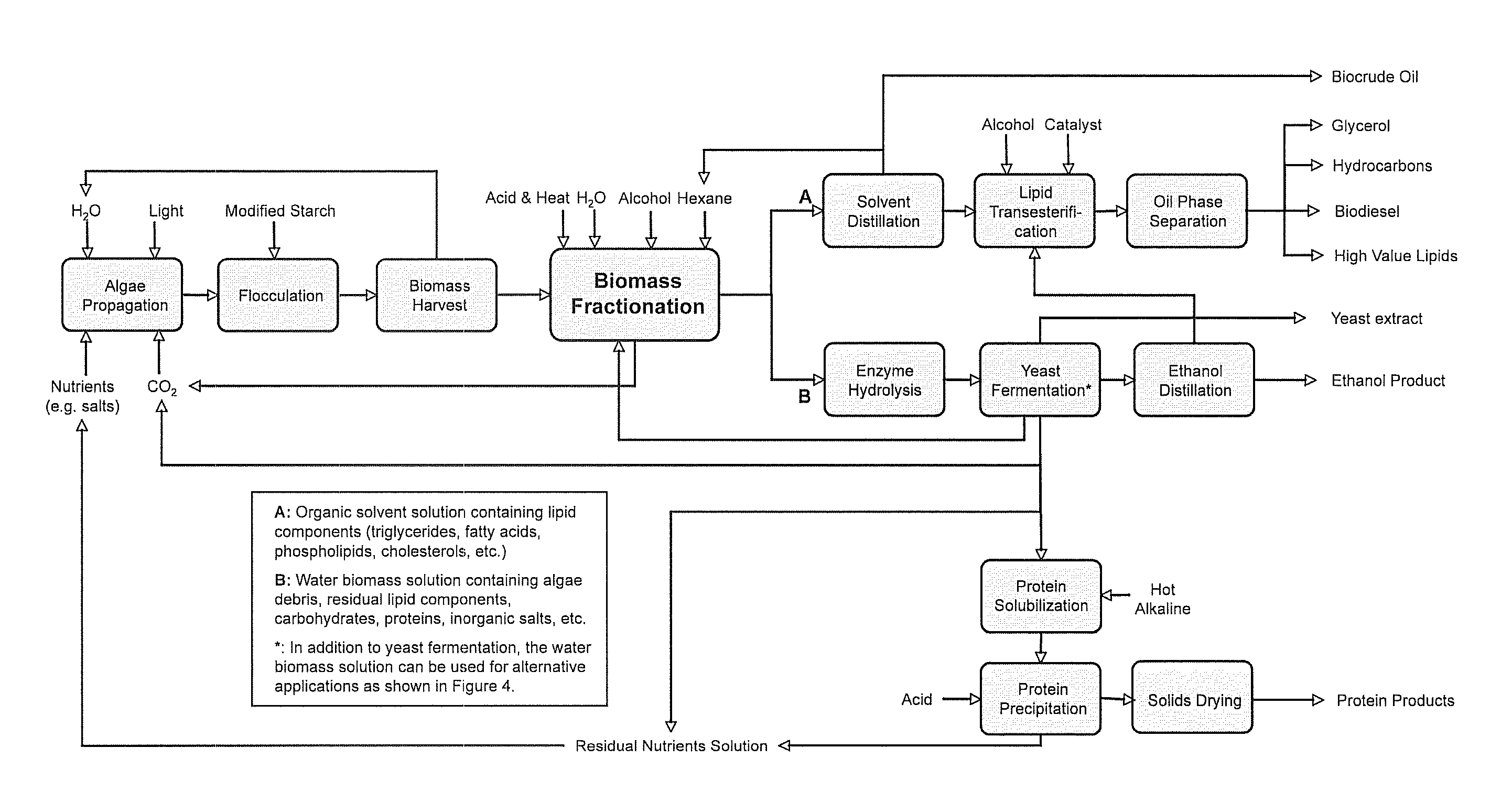
A method of fractionating biomass, by permeability conditioning biomass suspended in a pH adjusted solution of at least one water-based polar solvent to form a conditioned biomass, intimately contacting the pH adjusted solution with at least one non-polar solvent, partitioning to obtain an non-polar solvent solution and a polar biomass solution, and recovering cell and cell derived products from the non-polar solvent solution and polar biomass solution. Products recovered from the above method. A method of operating a renewable and sustainable plant for growing and processing algae.
Owner:VALICOR
Processes for making ethanol from acetic acid
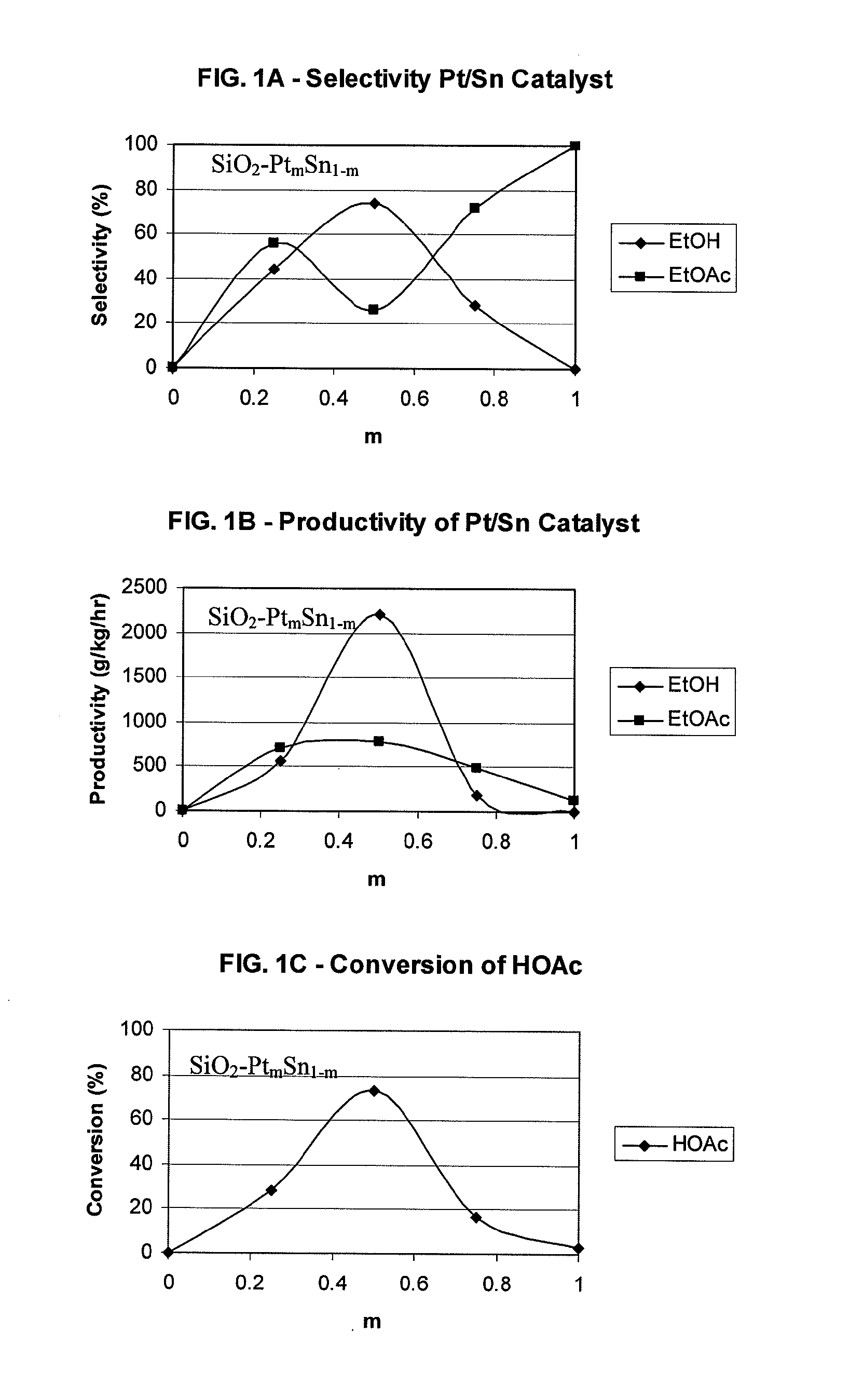
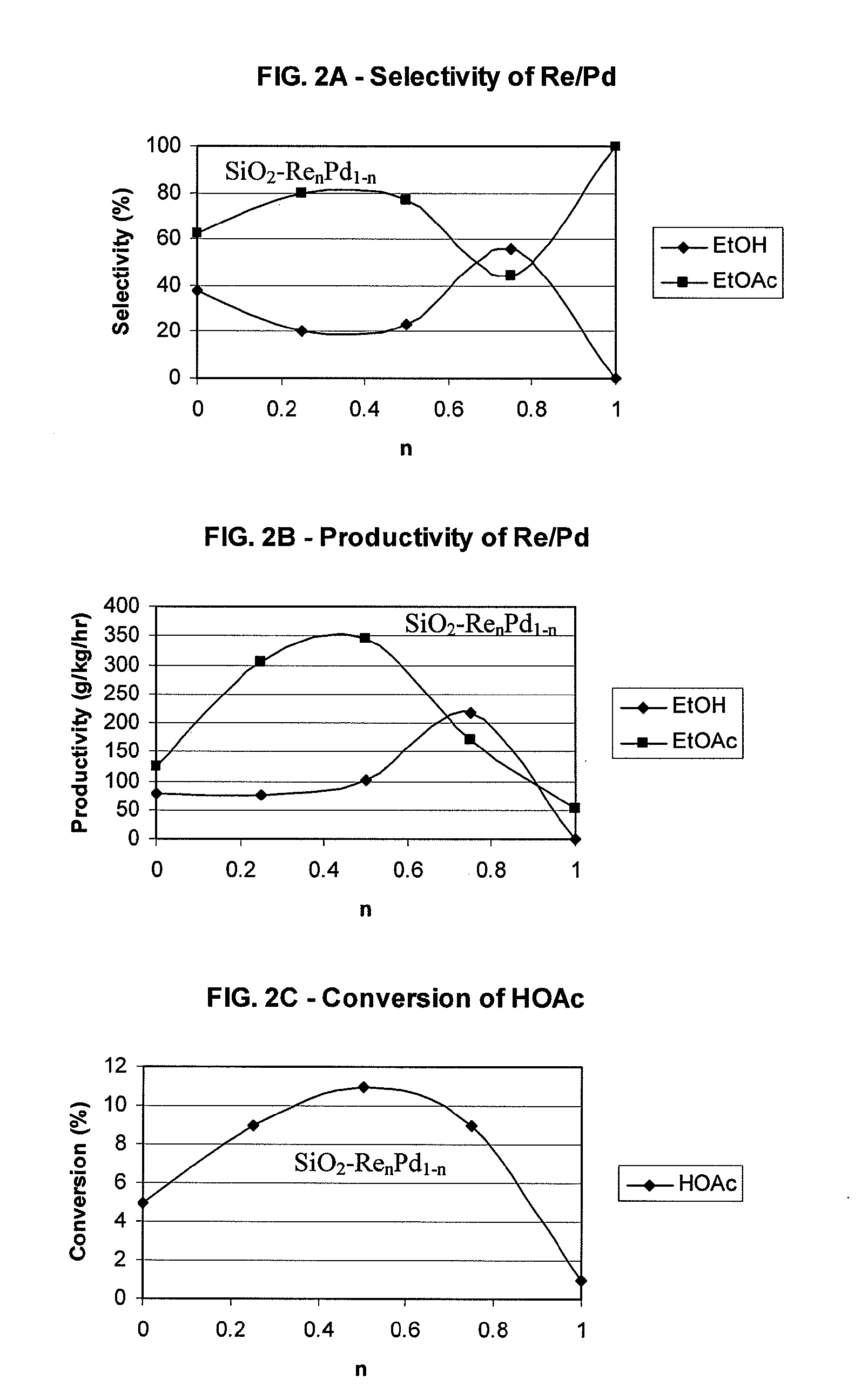
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
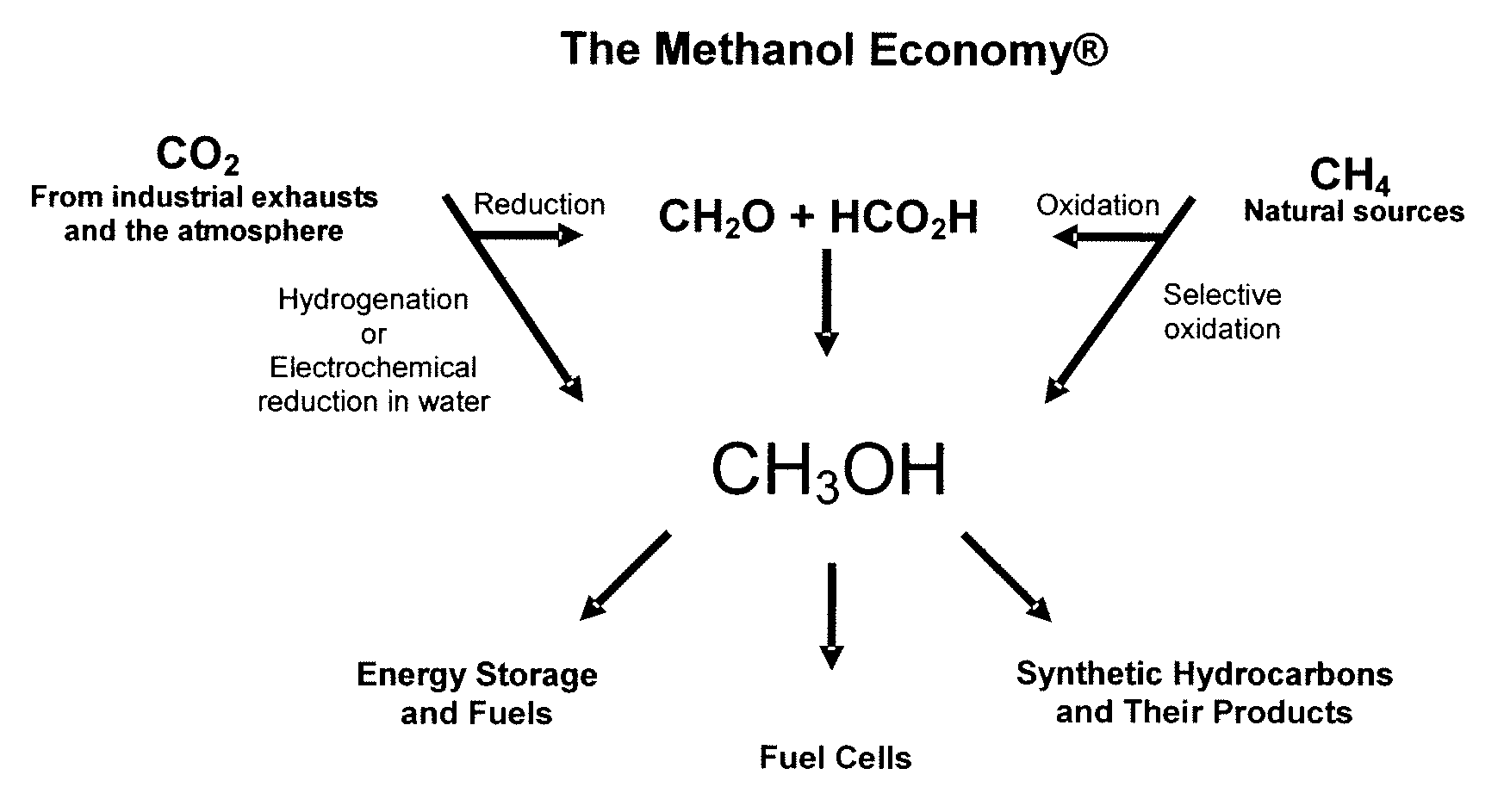
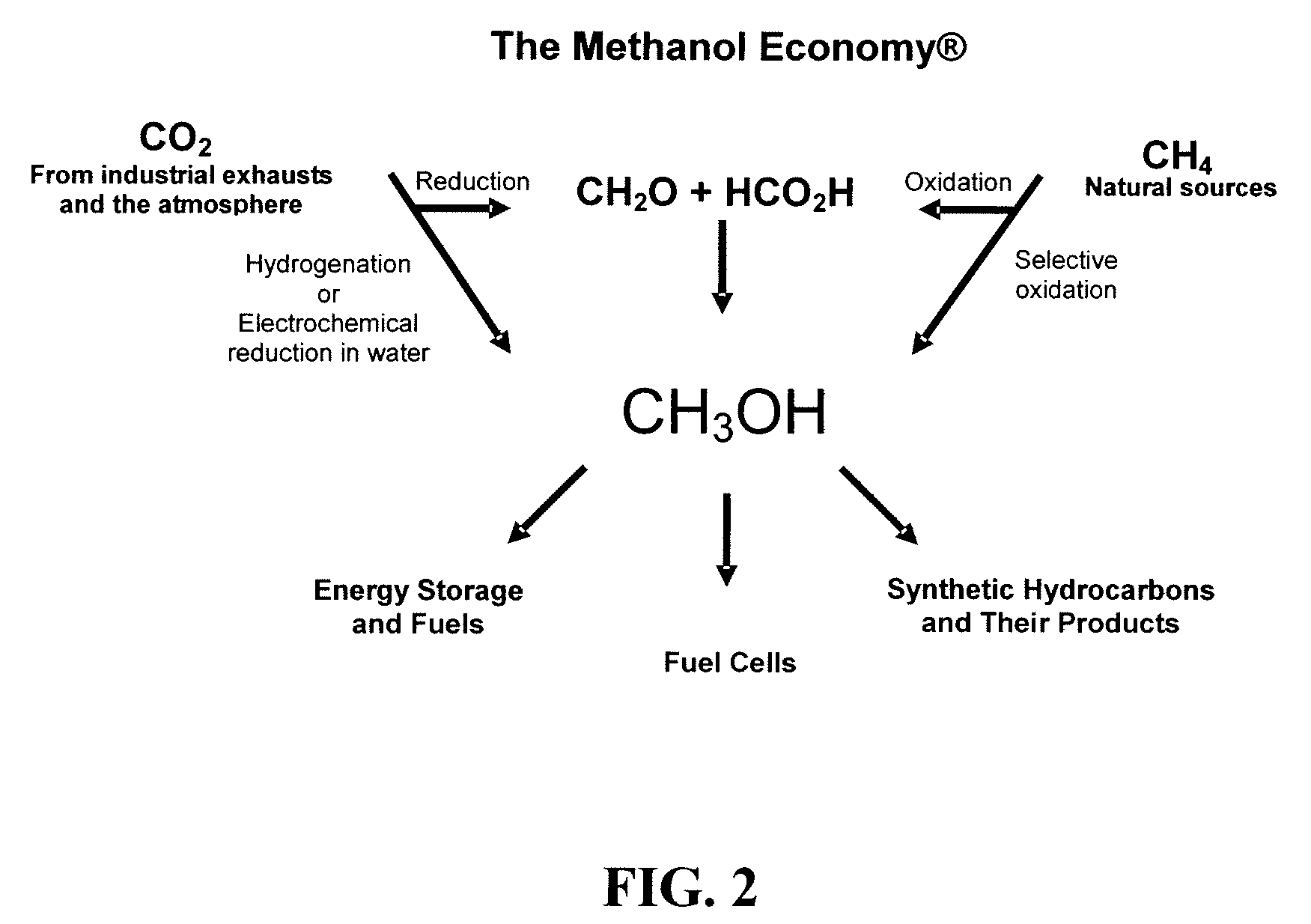
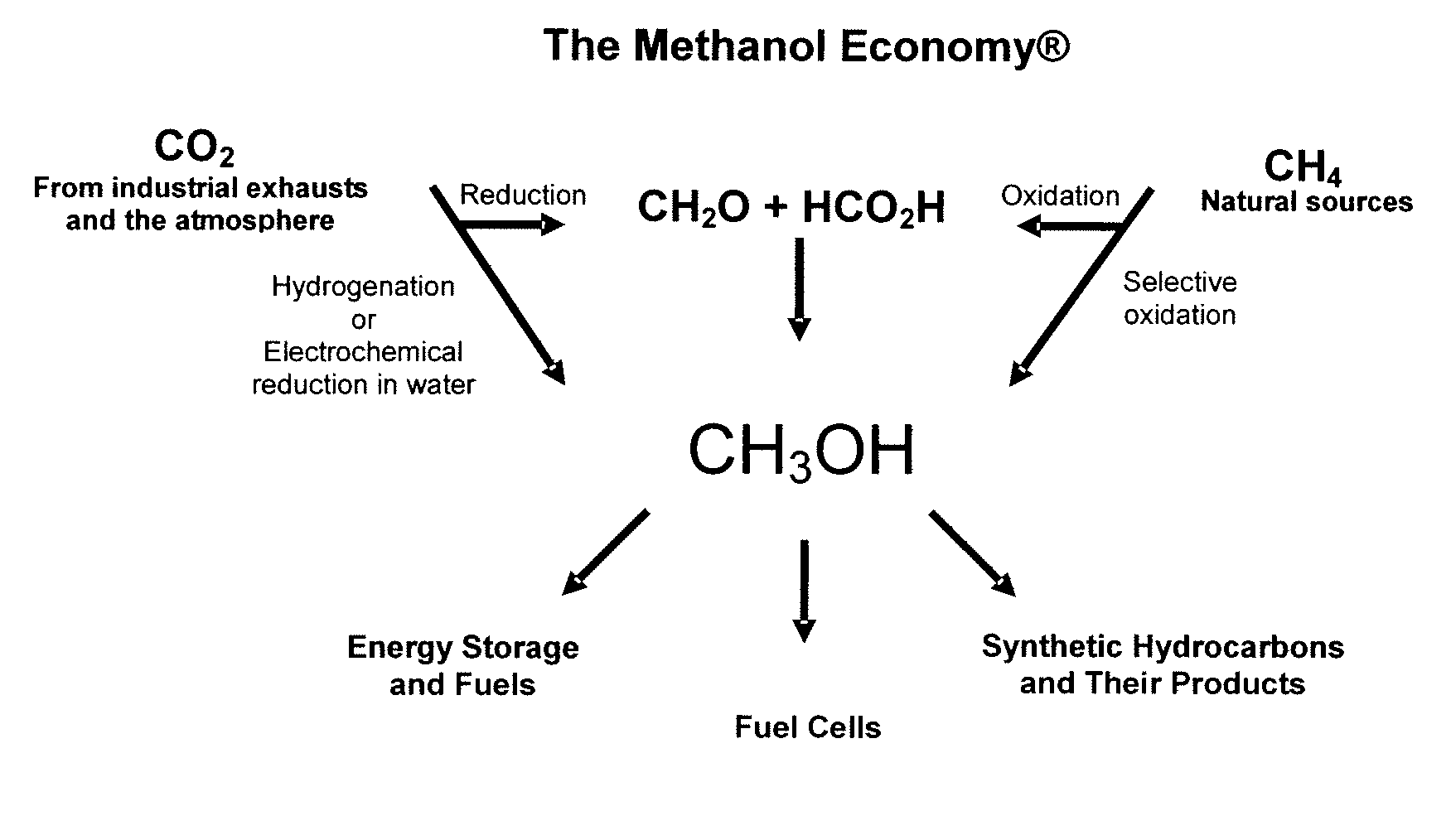
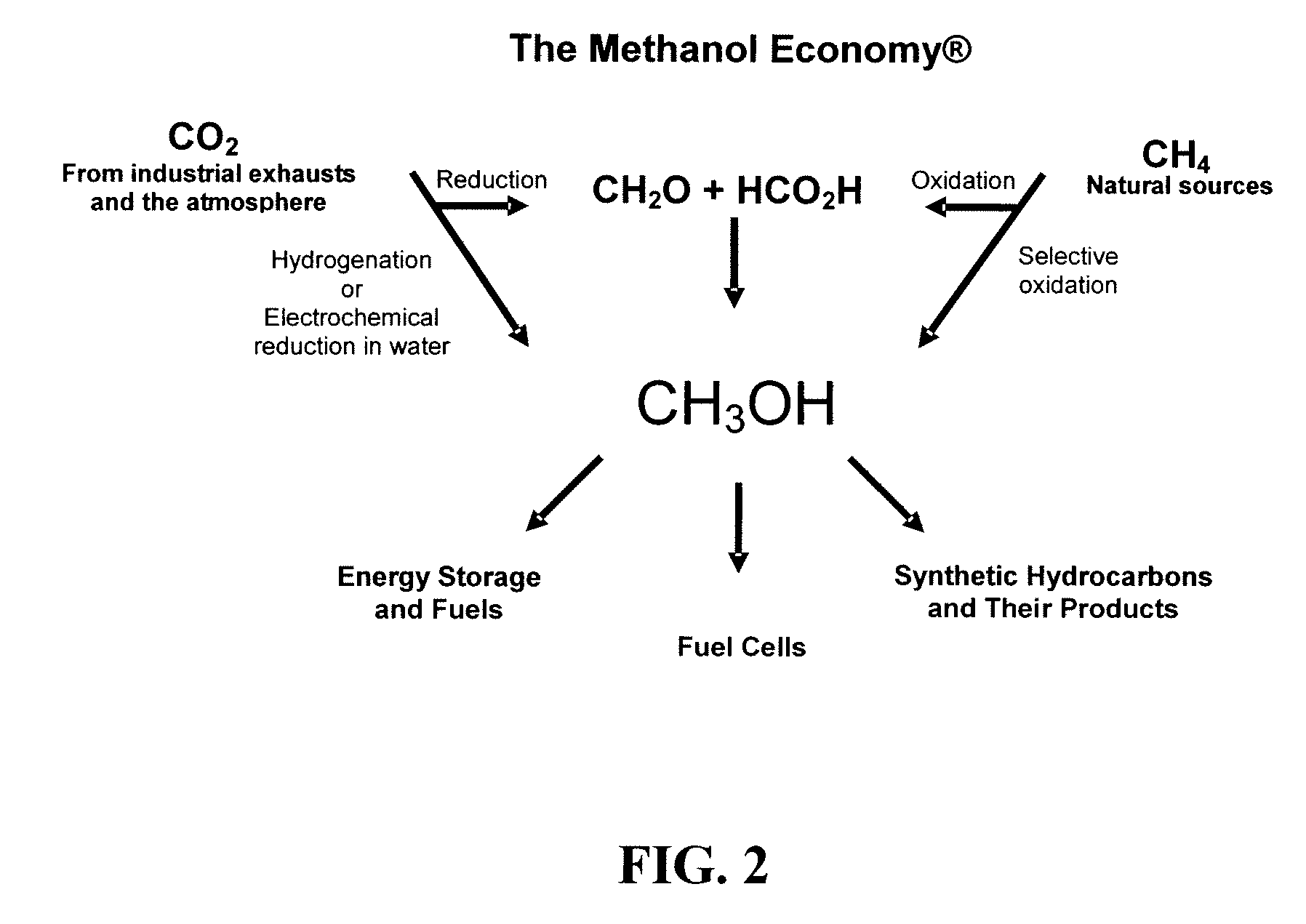
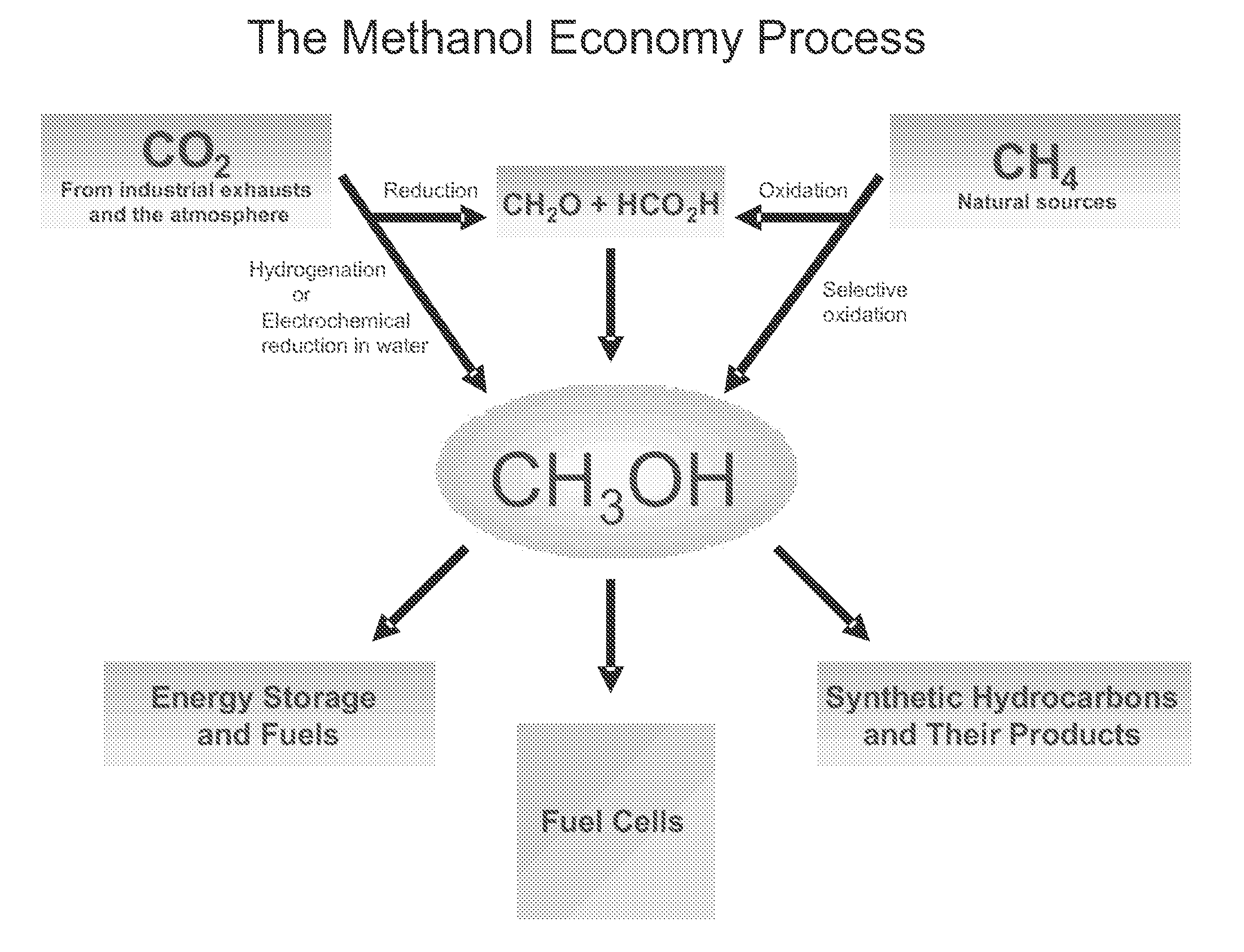
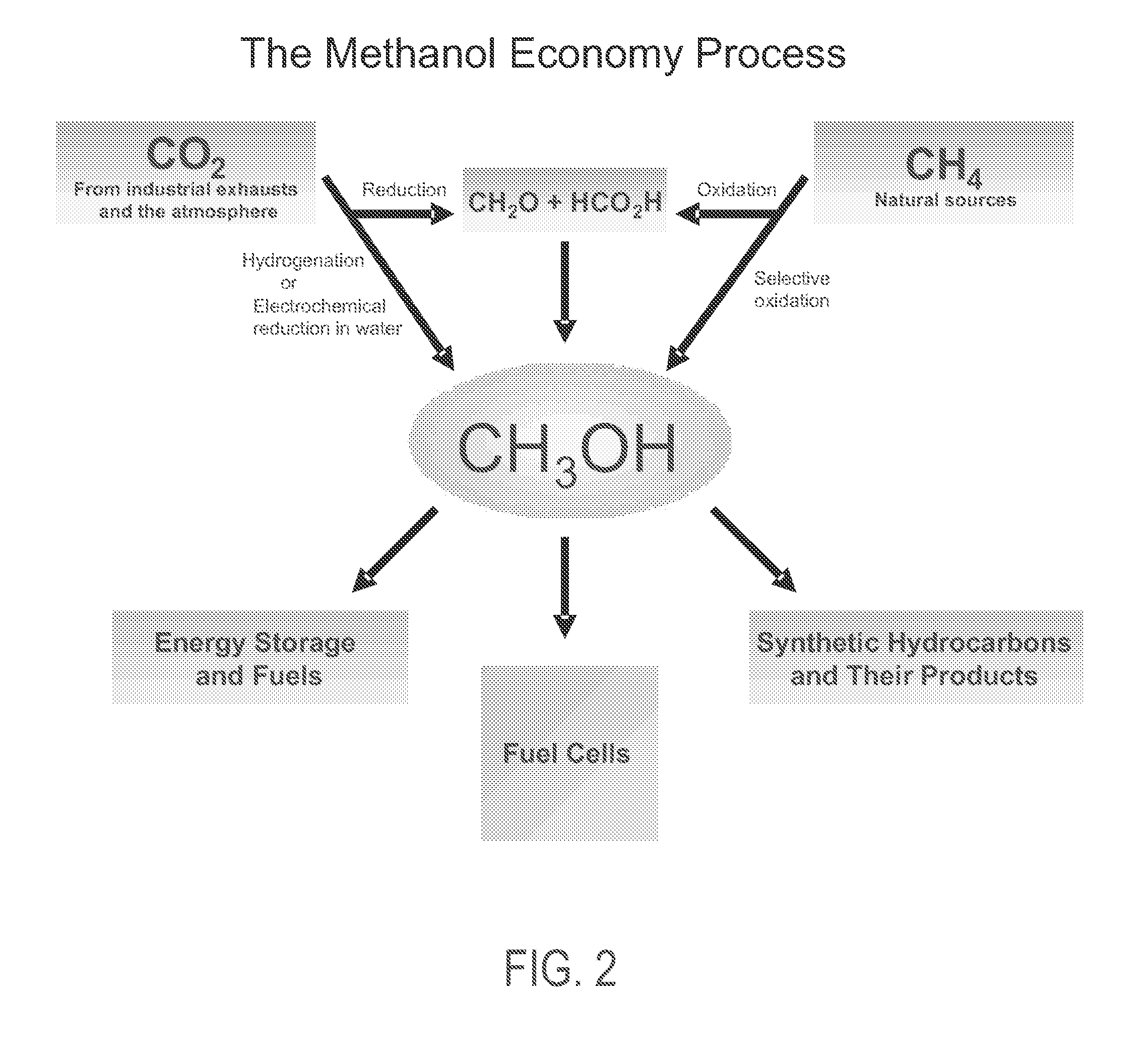
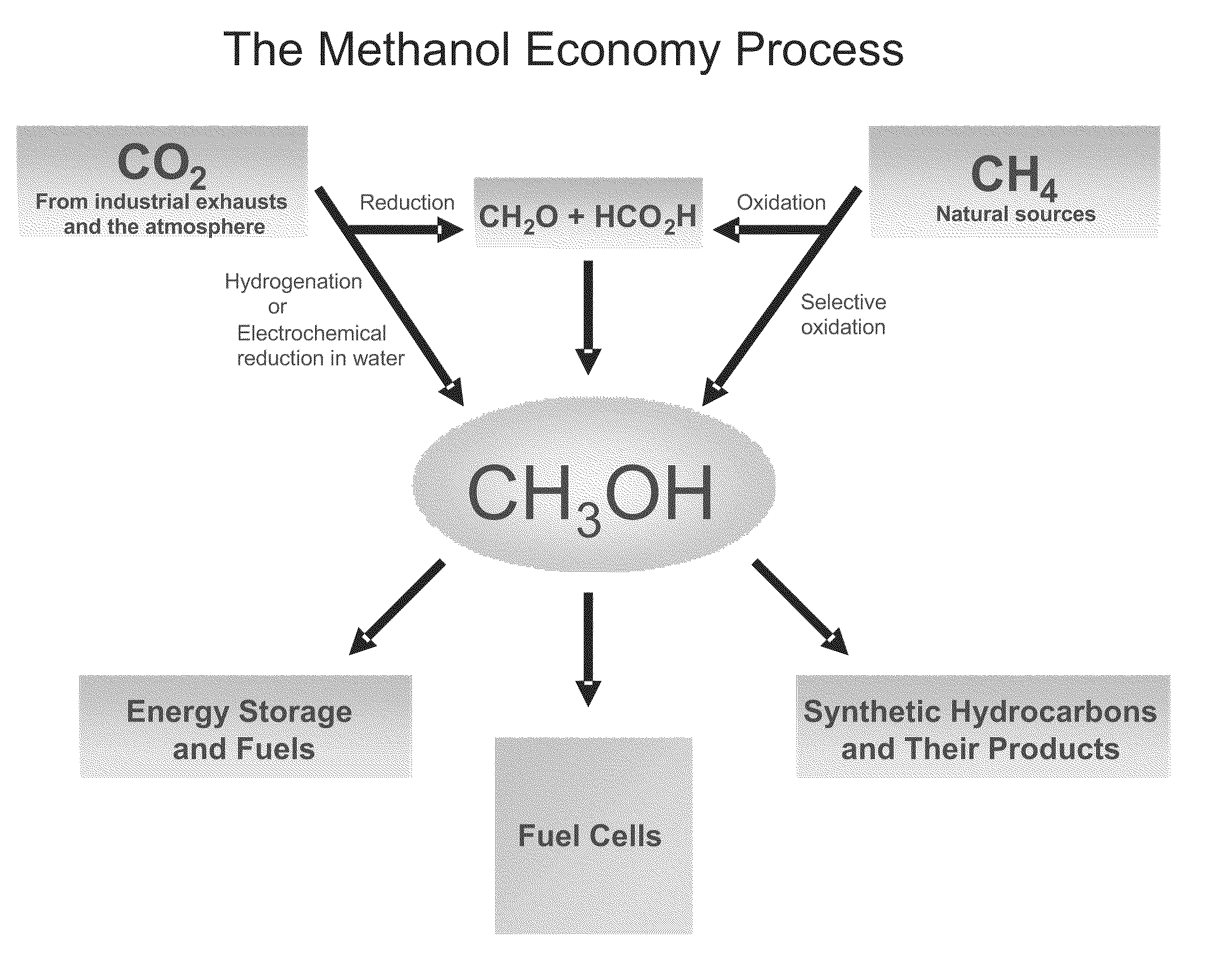
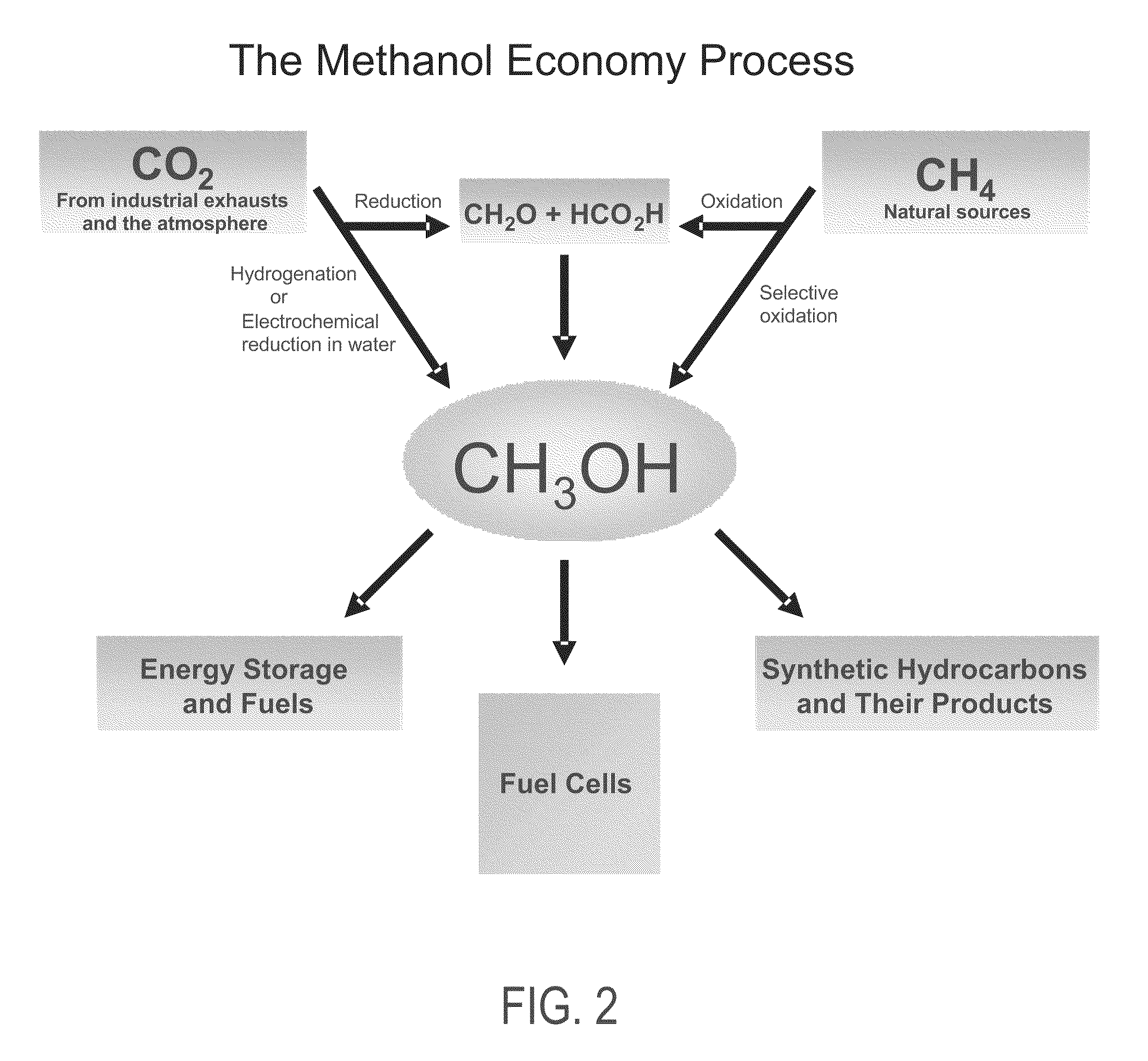
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one cell compartment and a metal cathode electrode in another cell compartment that also contains an aqueous solution comprising methanol and an electrolyte of one or more alkyl ammonium halides, alkali carbonates or combinations thereof to produce therein a reaction mixture containing carbon monoxide and hydrogen which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode.
Owner:UNIV OF SOUTHERN CALIFORNIA
Fullerene nanotube compositions
InactiveUS20080063585A1High yieldModerate temperatureMaterial nanotechnologyFullerenesFullereneNanotube
This invention relates generally to a fullerene nanotube composition. The fullerene nanotubes may be in the form of a felt, such as a bucky paper. Optionally, the fullerene nanotubes may be derivatized with one or more functional groups. Devices employing the fullerene nanotubes of this invention are also disclosed.
Owner:RICE UNIV
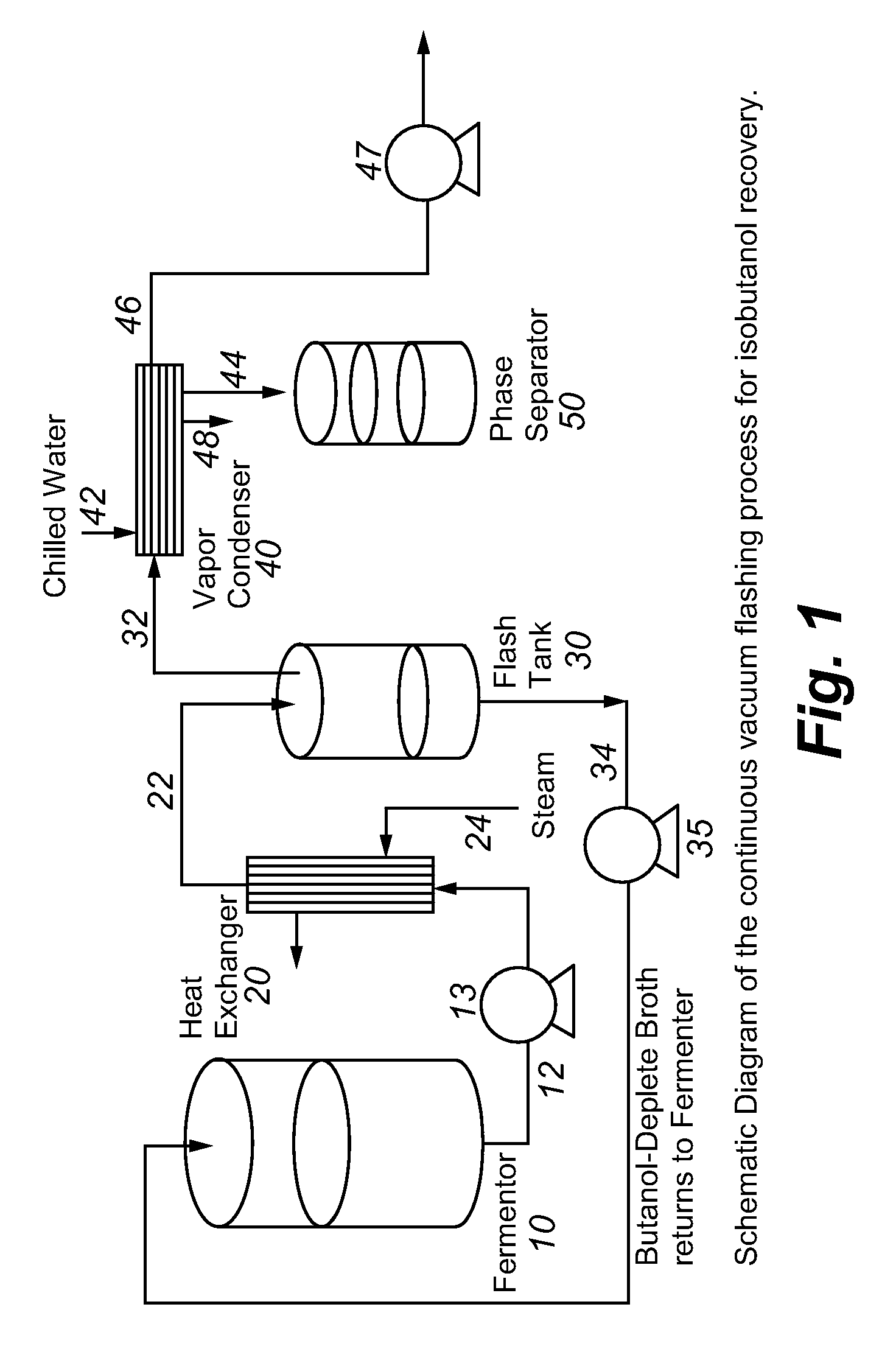
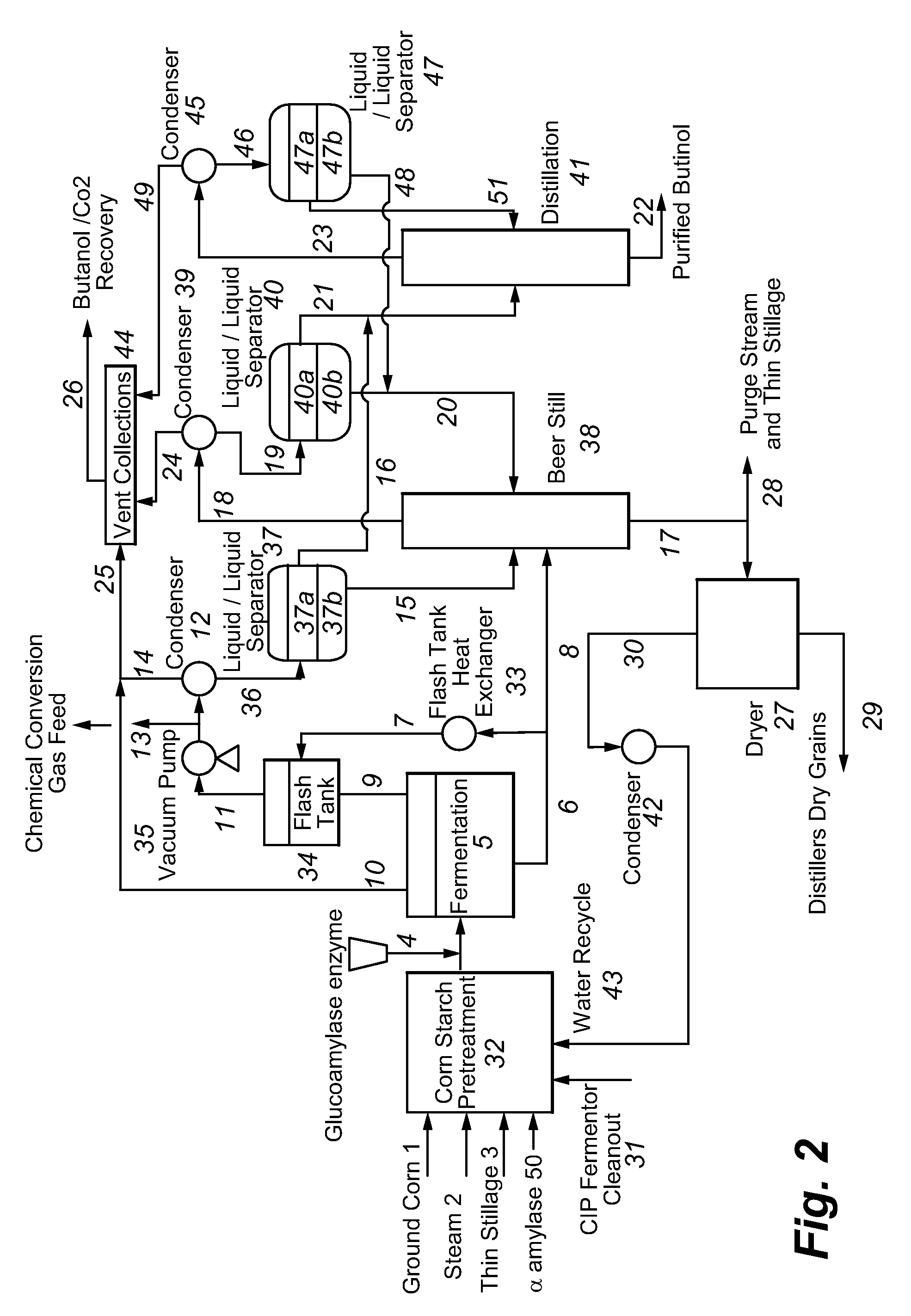
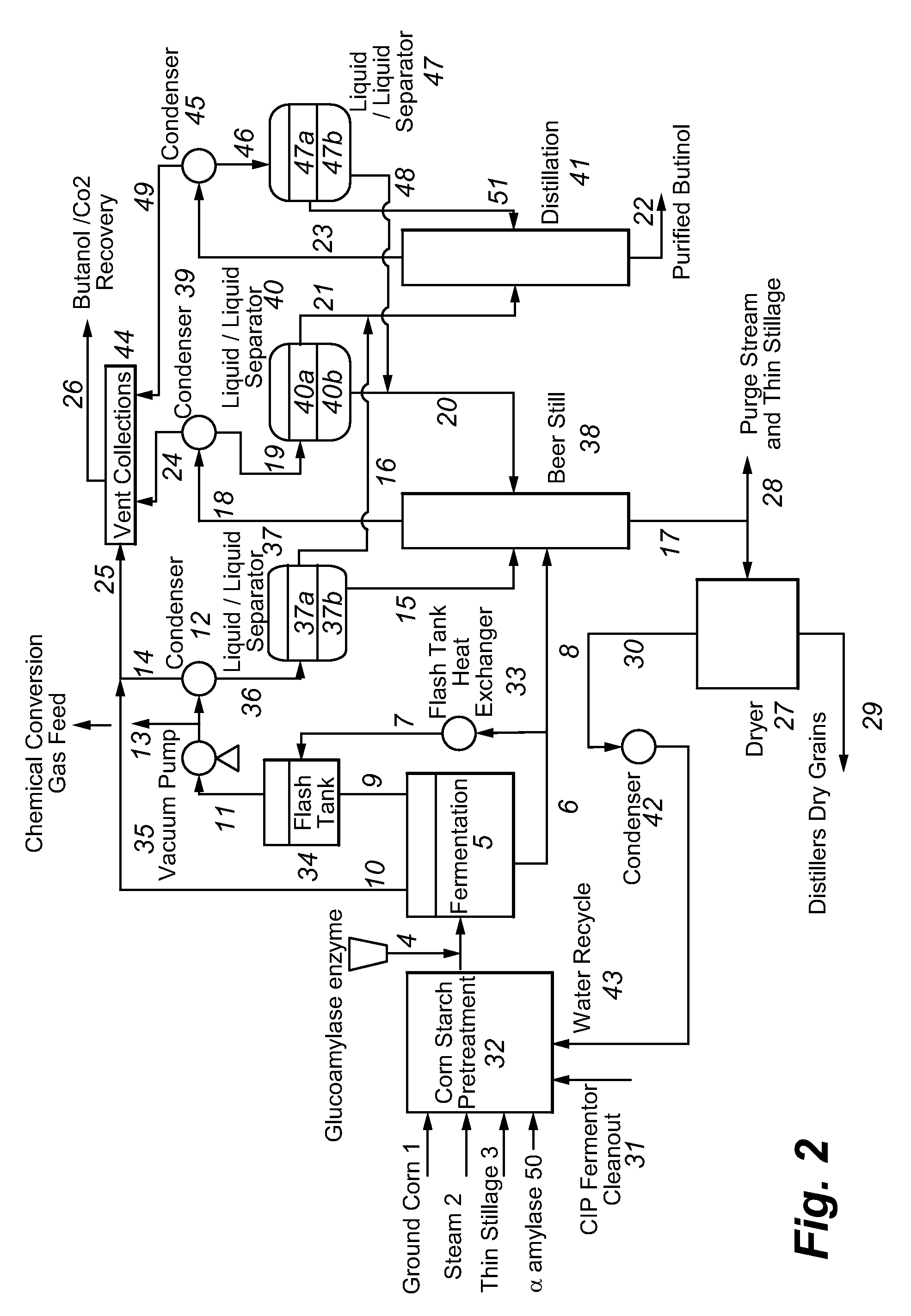
Recovery of higher alcohols from dilute aqueous solutions
ActiveUS20090171129A1High activityBioreactor/fermenter combinationsBiological substance pretreatmentsProduction rateAqueous solution
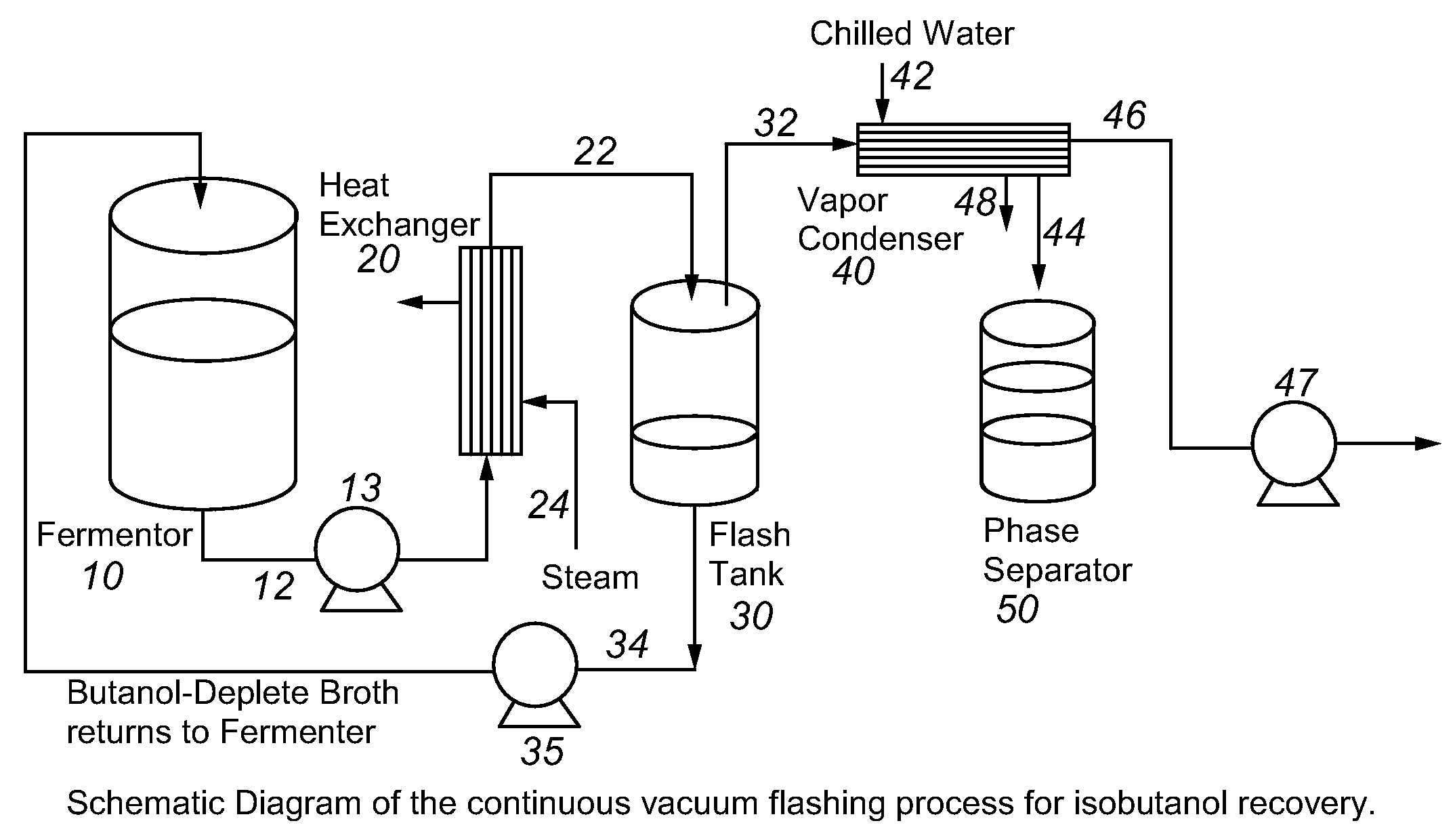
This invention is directed to methods for recovery of C3-C6 alcohols from dilute aqueous solutions, such as fermentation broths. Such methods provide improved volumetric productivity for the fermentation and allows recovery of the alcohol. Such methods also allow for reduced energy use in the production and drying of spent fermentation broth due to increased effective concentration of the alcohol product by the simultaneous fermentation and recovery process which increases the quantity of alcohol produced and recovered per quantity of fermentation broth dried. Thus, the invention allows for production and recovery of C3-C6 alcohols at low capital and reduced operating costs.
Owner:GEVO INC
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one cell compartment and a metal cathode electrode in another cell compartment that also contains an aqueous solution comprising methanol and an electrolyte of one or more alkyl ammonium halides, alkali carbonates or combinations thereof to produce therein a reaction mixture containing carbon monoxide and hydrogen which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode.
Owner:UNIV OF SOUTHERN CALIFORNIA
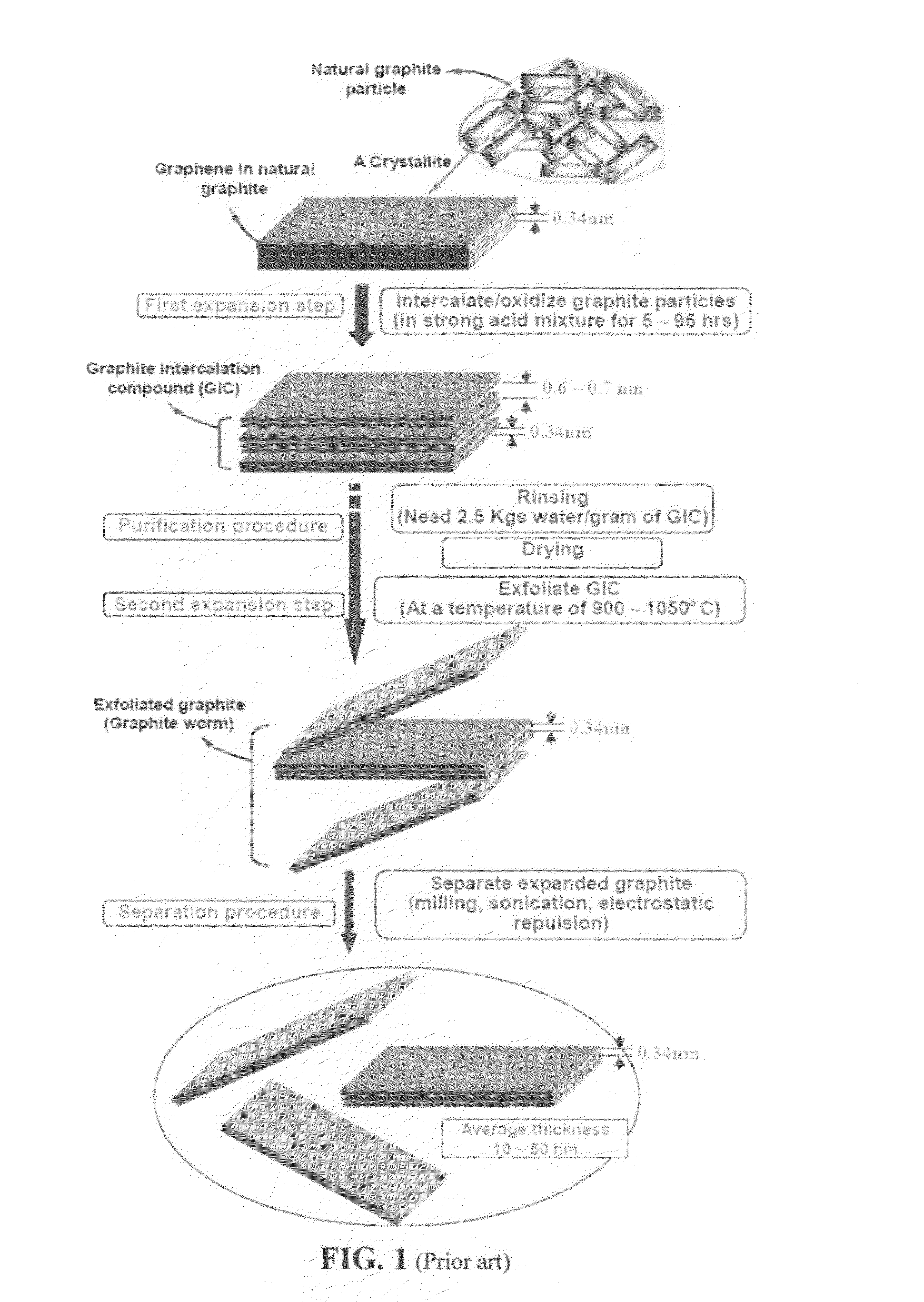
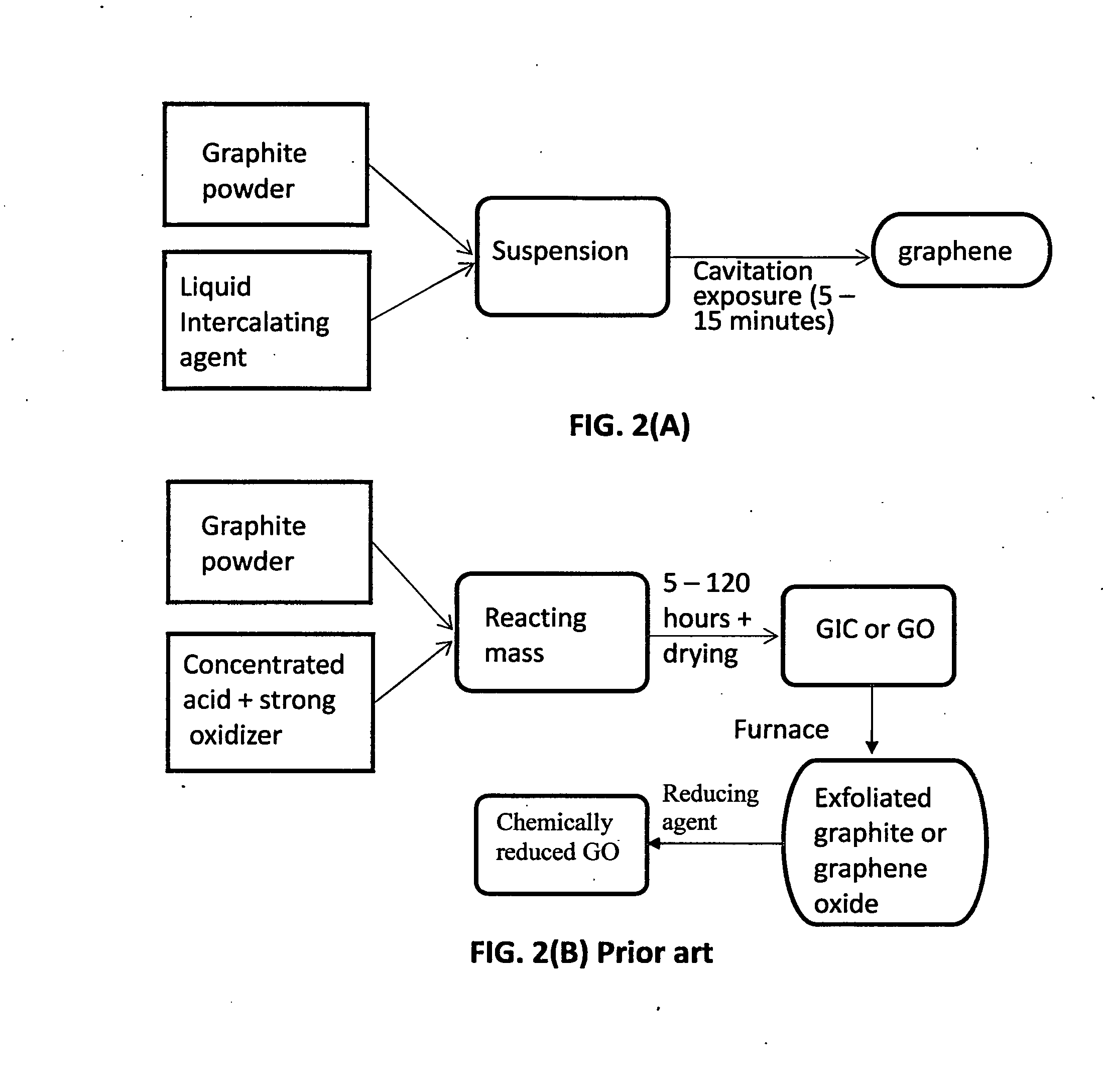
Production of graphene materials in a cavitating fluid
ActiveUS20150239741A1Shorten the timeReduce lossesCarboxylic acid nitrile preparationGraphiteCavitationLiquid medium
The invention provides a method of producing a graphene material from a starting graphitic material. In an embodiment, the method comprises: (a) dispersing the starting graphitic material in a liquid medium to form a graphite suspension; and (b) introducing the graphite suspension into a hydrodynamic cavitation reactor that generates and collapses cavitation or bubbles in the liquid medium to exfoliate and separate graphene planes from the starting graphitic material for producing the graphene material. The process is fast (minutes as opposed to hours or days of conventional processes), environmentally benign, and highly scalable. The reactor can concurrently perform the functions of graphene production, chemical functionalization, dispersion, and mixing with a polymer to make a composite.
Owner:GLOBAL GRAPHENE GRP INC
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
ActiveUS20100193370A1Efficient combustionElectrolysis componentsPhotography auxillary processesElectrolysisAtmospheric air
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one compartment and a metal cathode electrode in a compartment that also contains an aqueous solution comprising methanol and an electrolyte. An anion-conducting membrane can be provided between the anode and cathode to produce at the cathode therein a reaction mixture containing carbon monoxide and hydrogen, which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode. The oxygen produced at the anode can be recycled for efficient combustion of fossil fuels in power plants to exclusively produce CO2 exhausts for capture and recycling as the source of CO2 for the cell.
Owner:UNIV OF SOUTHERN CALIFORNIA
Recovery of higher alcohols from dilute aqueous solutions
ActiveUS8101808B2High activityBioreactor/fermenter combinationsBiological substance pretreatmentsAqueous solutionAlcohol products
This invention is directed to methods for recovery of C3-C6 alcohols from dilute aqueous solutions, such as fermentation broths. Such methods provide improved volumetric productivity for the fermentation and allows recovery of the alcohol. Such methods also allow for reduced energy use in the production and drying of spent fermentation broth due to increased effective concentration of the alcohol product by the simultaneous fermentation and recovery process which increases the quantity of alcohol produced and recovered per quantity of fermentation broth dried. Thus, the invention allows for production and recovery of C3-C6 alcohols at low capital and reduced operating costs.
Owner:GEVO INC
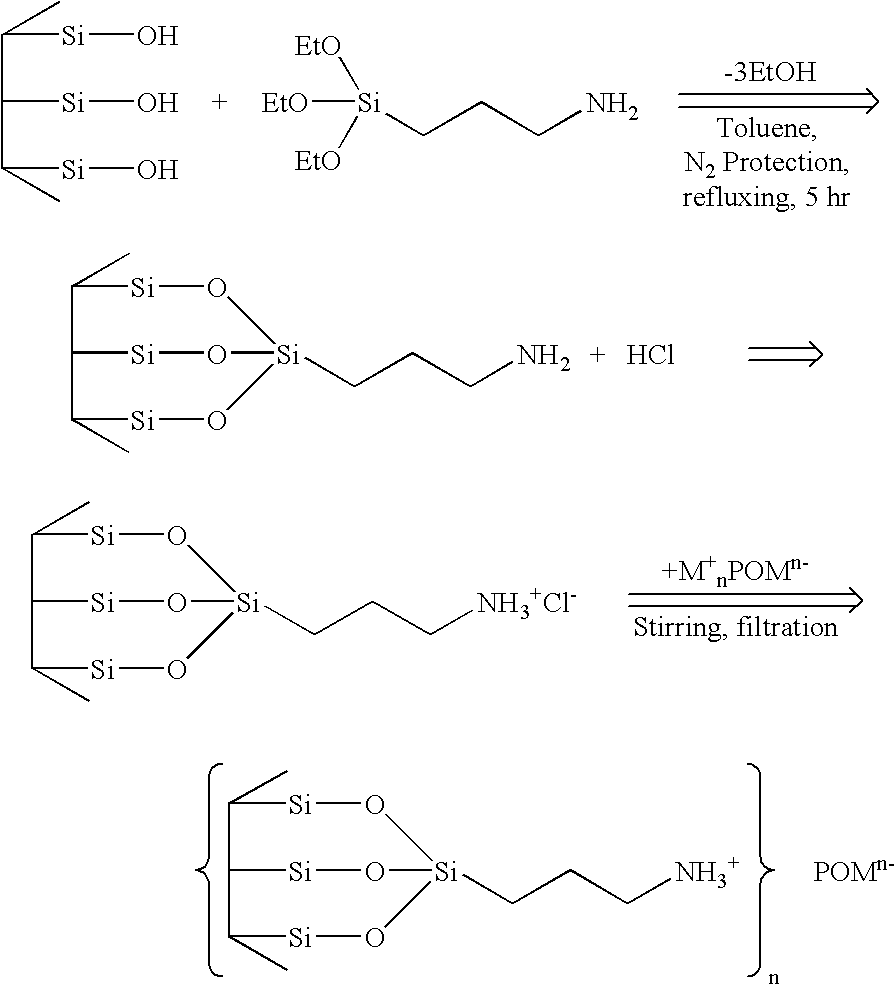
Supported polyoxometalates and process for their preparation
InactiveUS7417008B2Group 4/14 element organic compoundsOxygen/ozone/oxide/hydroxideCatalytic oxidationMesoporous silica
Owner:EXXONMOBIL CHEM PAT INC
Manufacture of alcohols
Owner:EXXONMOBIL CHEM PAT INC
Method of functionalizing a carbon material
InactiveUS20110053050A1Material nanotechnologyGroup 8/9/10/18 element organic compoundsCarboxylic acidMaterials science
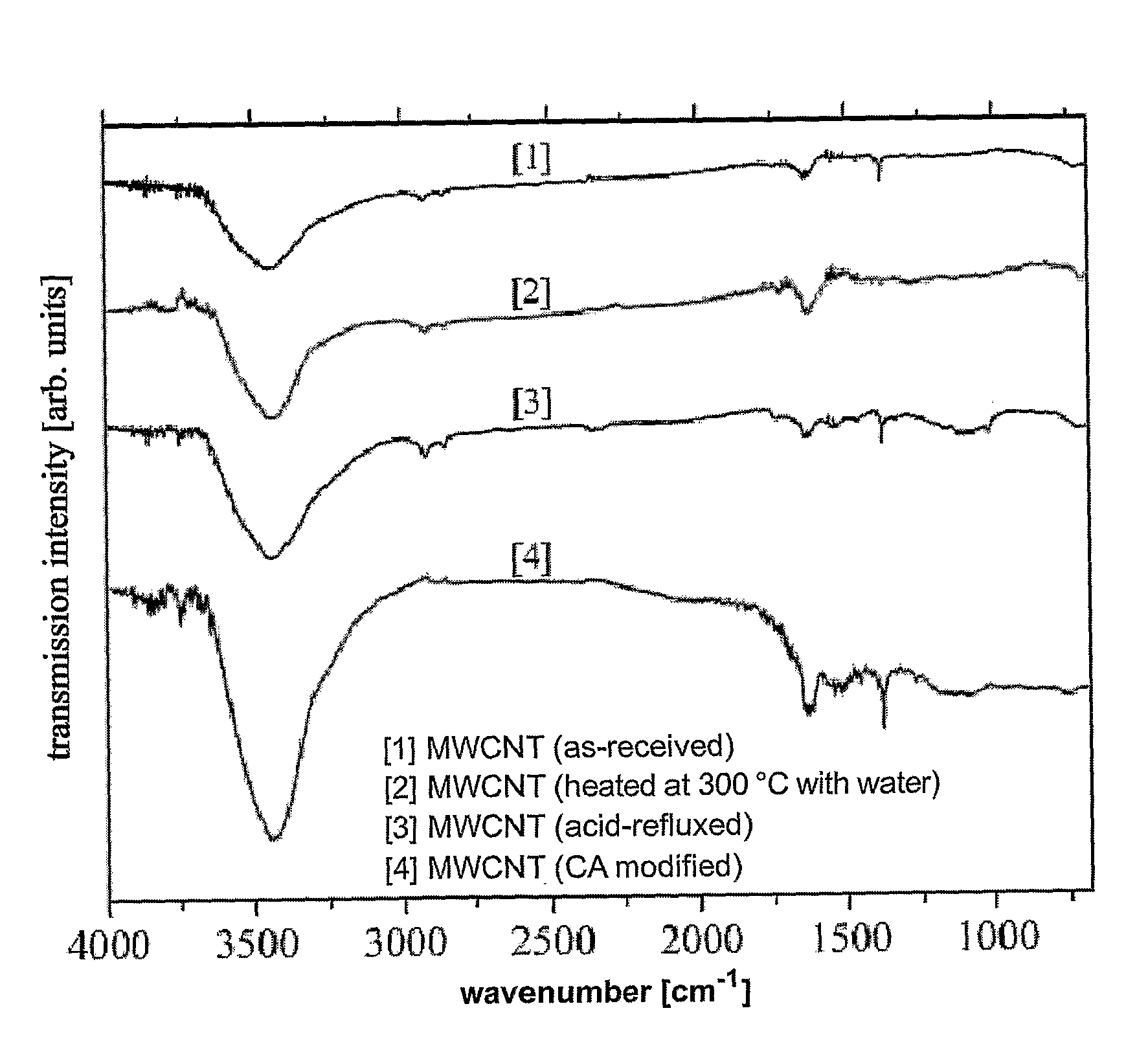
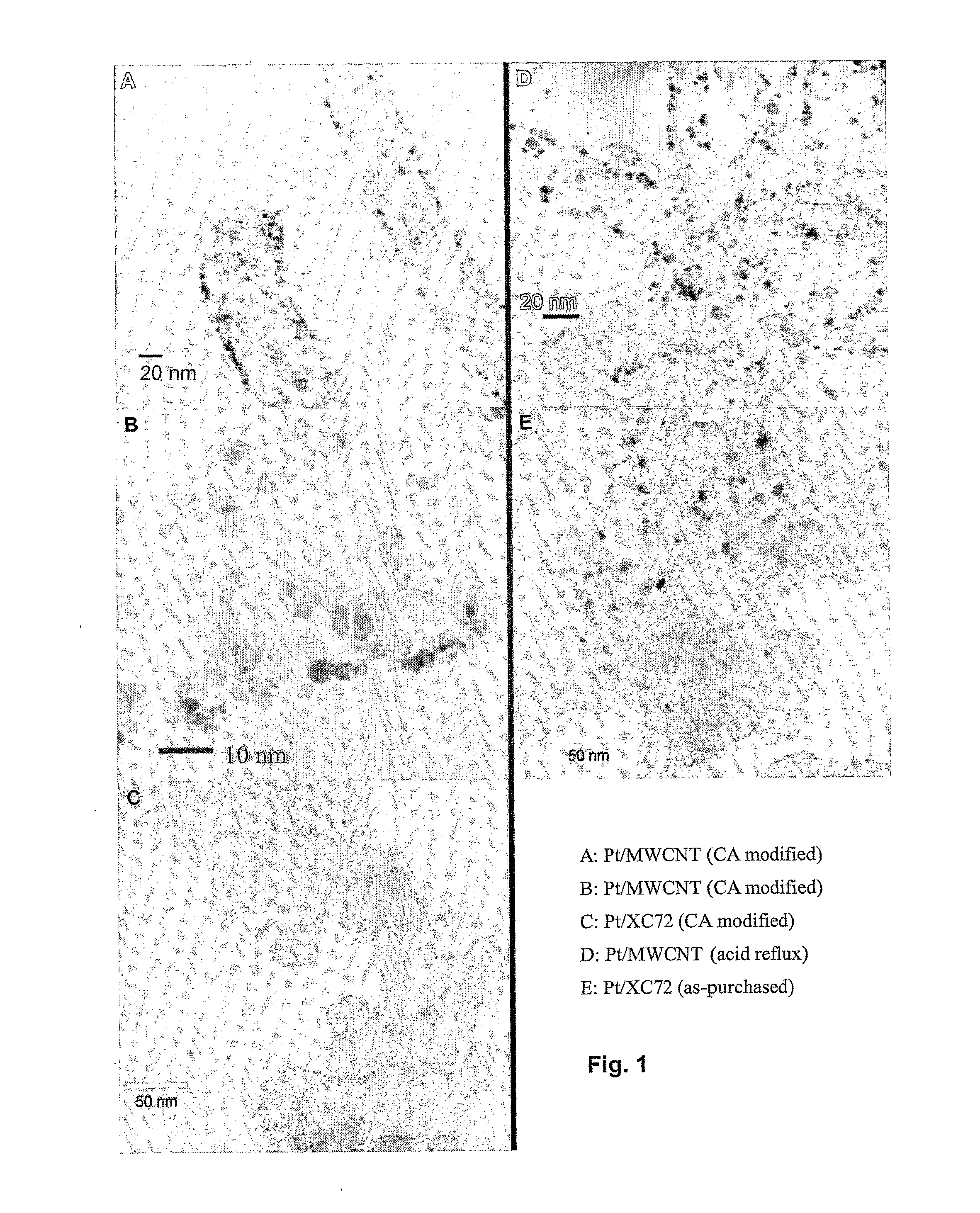
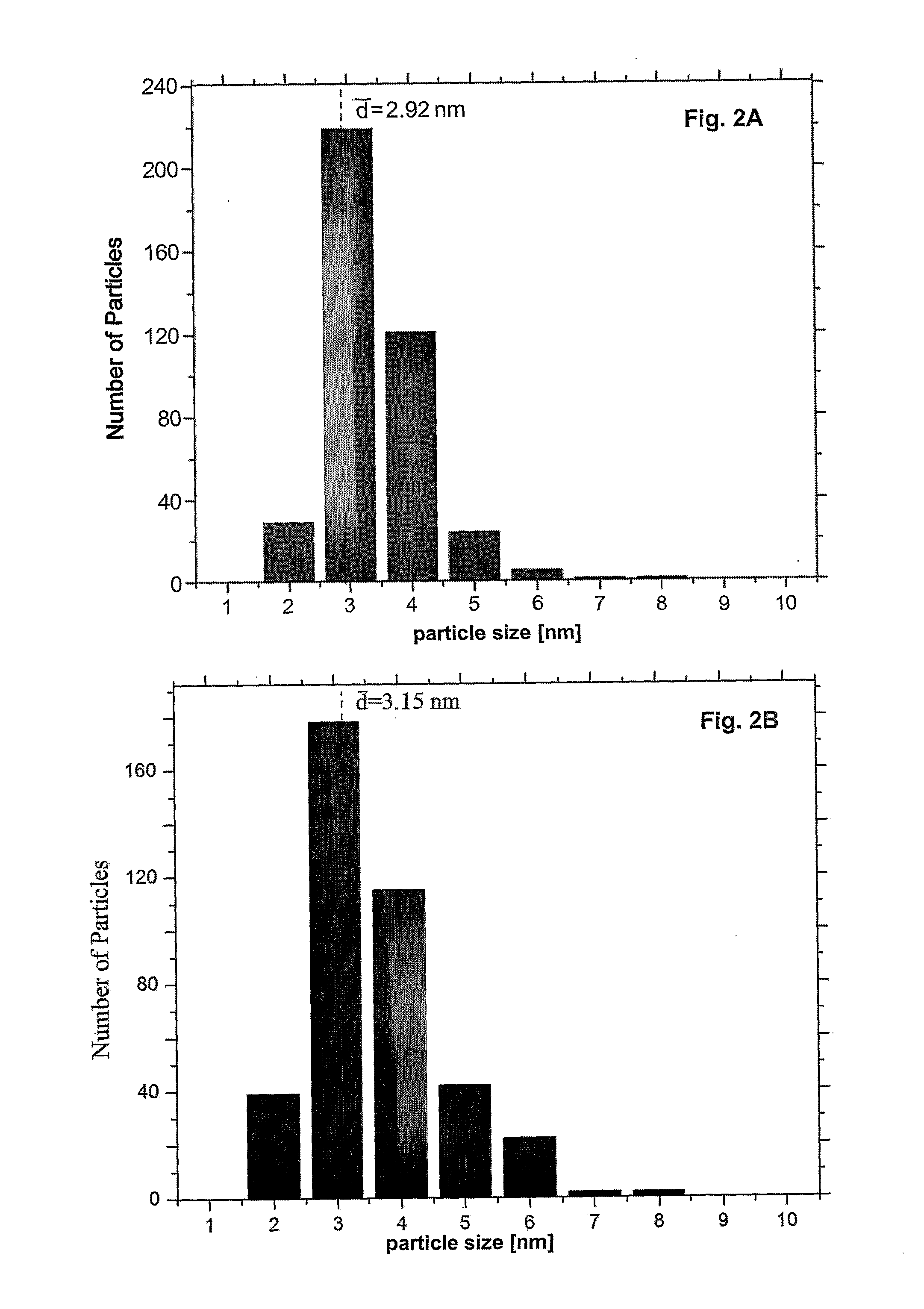
The present invention relates to a method of functionalizing a carbon material. A carbon material is contacted with a carboxylic acid, whereby a mixture is formed. The mixture is heated for a suitable period of time at a temperature below the thermal decomposition temperature of the carbon material.
Owner:AGENCY FOR SCI TECH & RES
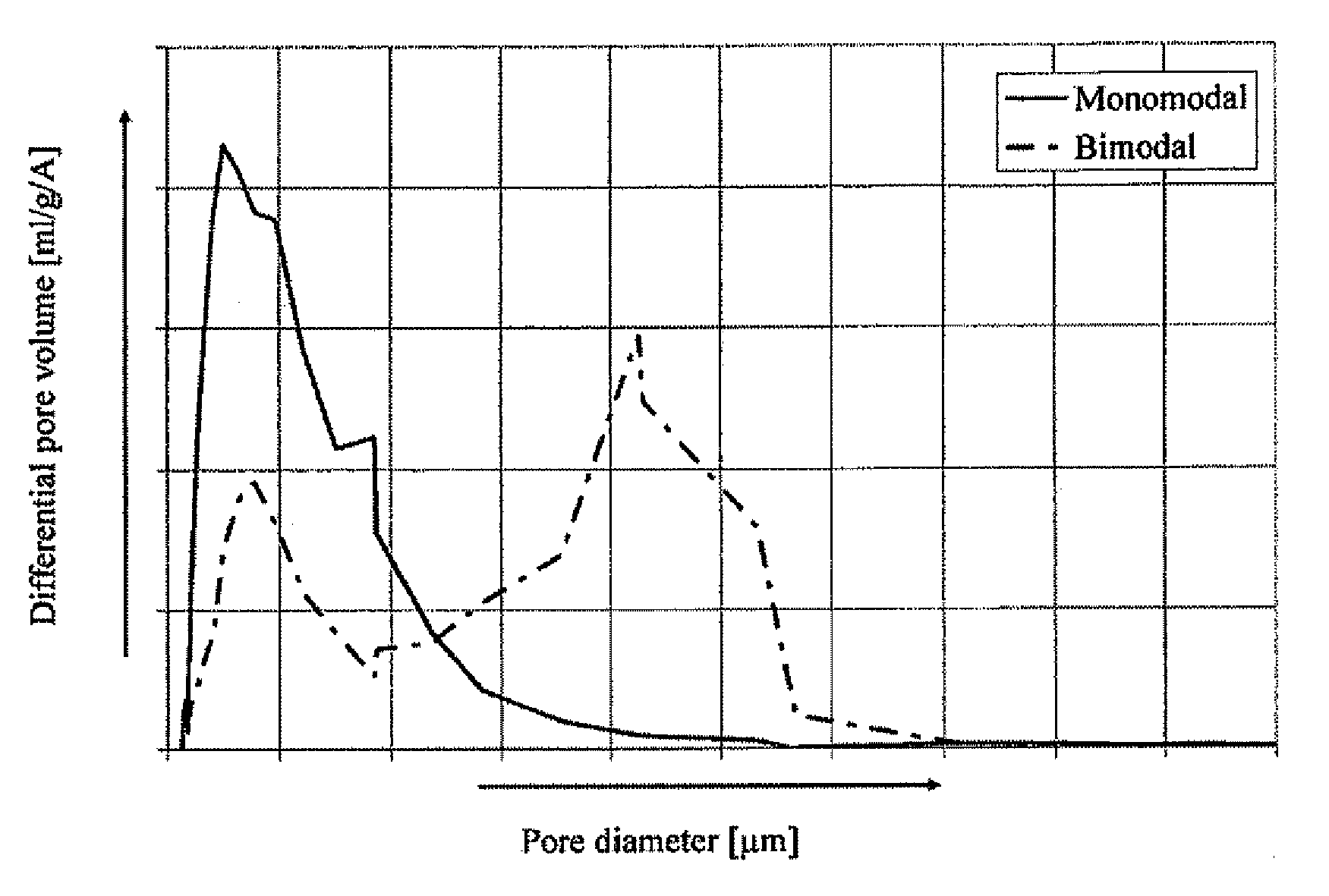
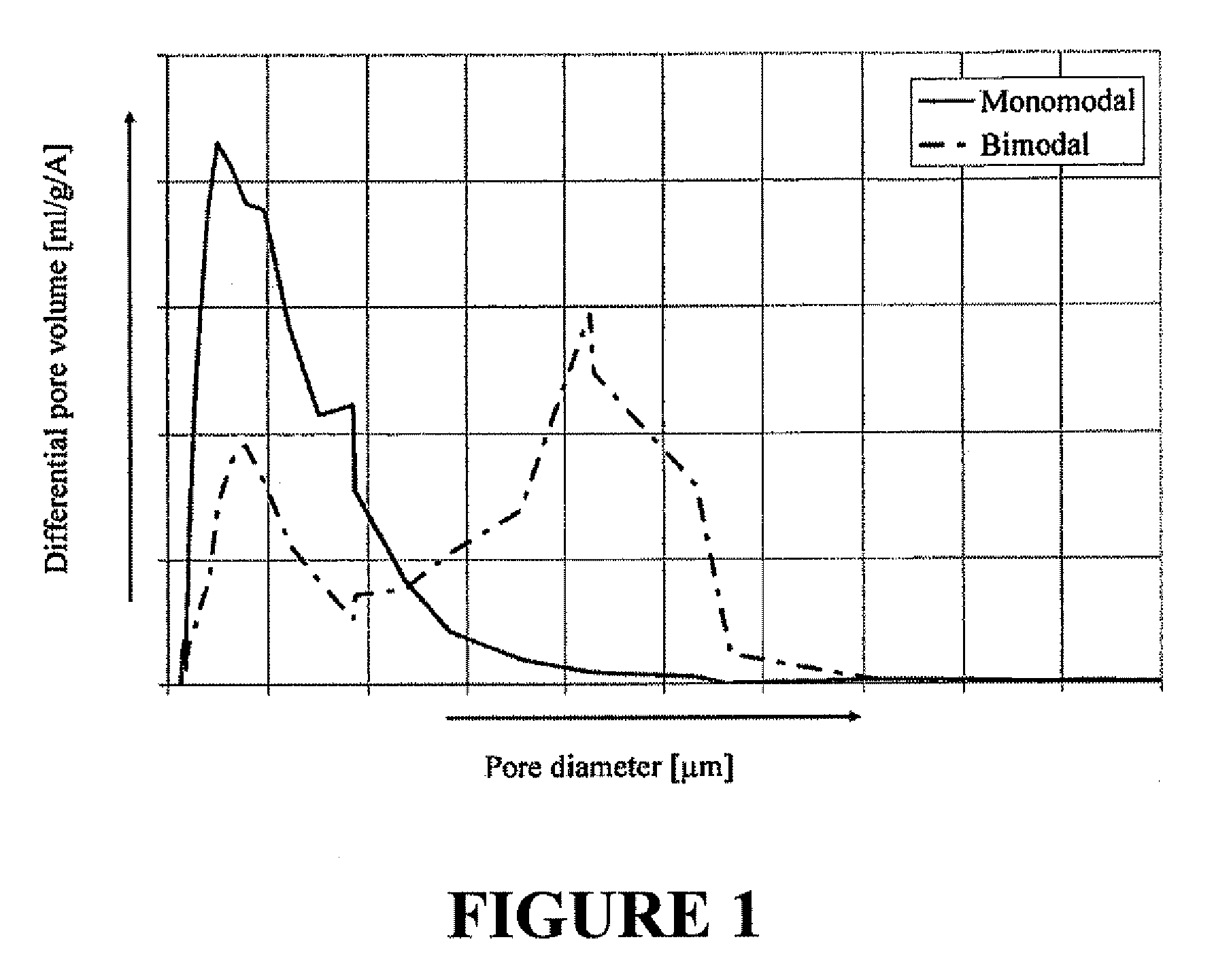
Catalyst with bimodal pore size distribution and the use thereof
ActiveUS20090198076A1Promoting amountExcessive amountMolecular sieve catalystsOther chemical processesRheniumPore diameter
The invention is directed to a catalyst for the epoxidation of an olefin to an olefin oxide, the catalyst comprising a support having at least two pore size distributions, each pore size distribution possessing a different mean pore size and a different pore size of maximum concentration, the catalyst further comprising a catalytically effective amount of silver, a promoting amount of rhenium, and a promoting amount of one or more alkali metals, wherein the at least two pore size distributions are within a pore size range of about 0.01 μm to about 50 μm. The invention is also directed to a process for the oxidation of an olefin to an olefin oxide using the above-described catalyst.
Owner:SCI DESIGN
Process for producing composite oxide catalyst
InactiveCN1697701AImprove conversion rateGood choiceOrganic compound preparationOrganic chemistry methodsAdditive ingredientSolvent
A catalyst for producing from an olefin the corresponding unsaturated aldehyde and unsaturated carboxylic acid in high yield; and a process for producing the catalyst. The process is for producing a composite oxide catalyst which is for use in catalytically oxidizing an olefin in a vapor phase with a gas containing molecular oxygen to produce the corresponding unsaturated aldehyde and corresponding unsaturated carboxylic acid, and which comprises (A) molybdenum, (B) bismuth, (C) cobalt and / or nickel, and (D) iron. It is characterized in that catalyst precursor particles produced through a preceding step in which an aqueous dispersion containing a raw material for the ingredient (A), raw material for the ingredient (C), and raw material for the ingredient (D) which have been united with one another and having a nitric acid radical content satisfying the relationship 1.2<=NO3 / (3xFe+2x(Co+Ni)) is dried and then heated are united with a raw material for the ingredient (B) in a water-miscible solvent and the resultant united matter is dried and burned. In the relationship, NO3, Fe, Co, and Ni indicate the molar contents of nitric acid radicals, iron, cobalt, and nickel, respectively.
Owner:MITSUBISHI CHEM CORP
Mesoporous material with active metals
A process for treating organic compounds includes providing a composition which includes a substantially mesoporous structure of silica containing at least 97% by volume of pores having a pore size ranging from about 15 Å to about 30 Å and having a micropore volume of at least about 0.01 cc / g, wherein the mesoporous structure has incorporated therewith at least about 0.02% by weight of at least one catalytically and / or chemically active heteroatom selected from the group consisting of Al, Ti, V, Cr, Zn, Fe, Sn, Mo, Ga, Ni, Co, In, Zr, Mn, Cu, Mg, Pd, Pt and W, and the catalyst has an X-ray diffraction pattern with one peak at 0.3° to about 3.5° at 2θ. The catalyst is contacted with an organic feed under reaction conditions wherein the treating process is selected from alkylation, acylation, oligomerization, selective oxidation, hydrotreating, isomerization, demetalation, catalytic dewaxing, hydroxylation, hydrogenation, ammoximation, isomerization, dehydrogenation, cracking and adsorption.
Owner:ABB LUMMUS GLOBAL INC
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
ActiveUS8138380B2Efficient combustionPhotography auxillary processesElectrolysis componentsElectrolysisOxygen
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one compartment and a metal cathode electrode in a compartment that also contains an aqueous solution comprising methanol and an electrolyte. An anion-conducting membrane can be provided between the anode and cathode to produce at the cathode therein a reaction mixture containing carbon monoxide and hydrogen, which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode. The oxygen produced at the anode can be recycled for efficient combustion of fossil fuels in power plants to exclusively produce CO2 exhausts for capture and recycling as the source of CO2 for the cell.
Owner:UNIV OF SOUTHERN CALIFORNIA
Process for production of acrylates from epoxides
ActiveUS20140309399A1Low costAvoiding isolation and storageOrganic compound preparationPreparation by ester-hydroxy reactionReaction zoneMetal carbonyl
The methods of the present invention comprise the steps of: providing a feedstock stream comprising an epoxide and carbon monoxide; contacting the feedstock stream with a metal carbonyl in a first reaction zone to effect conversion of at least a portion of the provided epoxide to a beta lactone; directing the effluent from the first reaction zone to a second reaction zone where the beta lactone is subjected to conditions that convert it to a compound selected from the group consisting of: an alpha beta unsaturated acid, an alpha beta unsaturated ester, an alpha beta unsaturated amide, and an optionally substituted polypropiolactone polymer; and isolating a final product comprising the alpha-beta unsaturated carboxylic acid, the alpha-beta unsaturated ester, the alpha-beta unsaturated amide or the polypropiolactone.
Owner:NOVOMER INC
Catalysts for catalytic oxidation of propane to acrylic acid, methods of making and using the same
InactiveUS6114278AIncrease productivityHigh yieldOrganic compound preparationOrganic chemistry methodsPartial oxidationCatalytic oxidation
A mixed metal oxide Mo-V-Ga-Pd-Nb-X (where X=La, Te, Ge, Zn, Si, In or W) catalytic system providing a higher selectivity to acrylic acid in the low temperature partial oxidation of propane with a molecular oxygen-containing gas.
Owner:SAUDI BASIC IND CORP SA
Process for converting a hydrocarbon to an oxygenate or a nitrile
InactiveUS7294734B2Maximize contactOrganic compound preparationCarboxylic acid esters preparationOxygenateOxygen
This invention relates to a process for converting a hydrocarbon reactant to a product comprising an oxygenate or a nitrile, the process comprising: (A) flowing a reactant composition comprising the hydrocarbon reactant, and oxygen or a source of oxygen, and optionally ammonia, through a microchannel reactor in contact with a catalyst to convert the hydrocarbon reactant to the product, the hydrocarbon reactant undergoing an exothermic reaction in the microchannel reactor; (B) transferring heat from the microchannel reactor to a heat exchanger during step (A); and (C) quenching the product from step (A).
Owner:VELOCYS CORPORATION
Methods, compositions, and apparatuses for forming macrocyclic compounds
ActiveUS20070217965A1Improve production efficiencyHigh yieldHydrocarbon by dehydrogenationCyclic peptide ingredientsOligomerFiltration
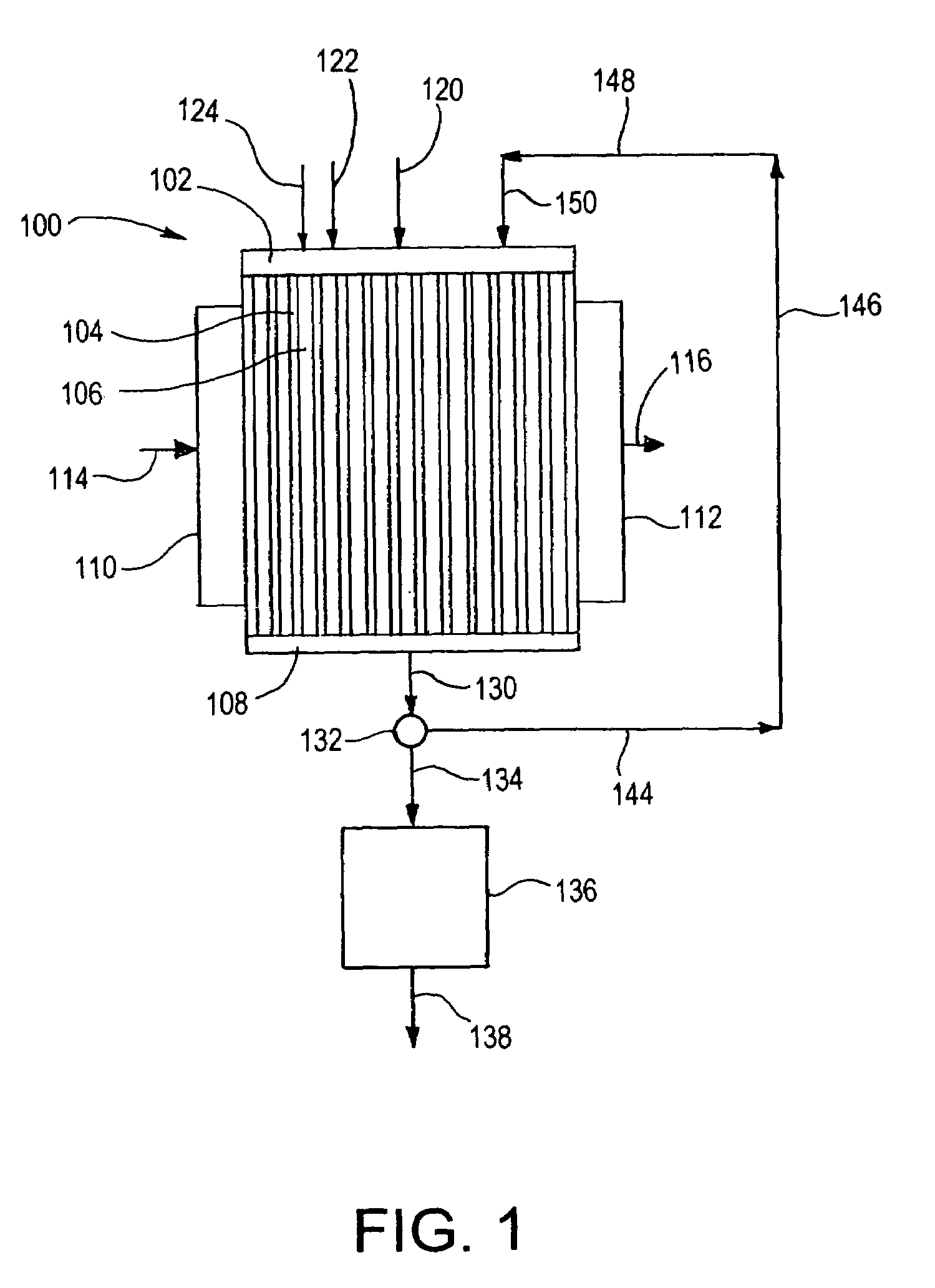
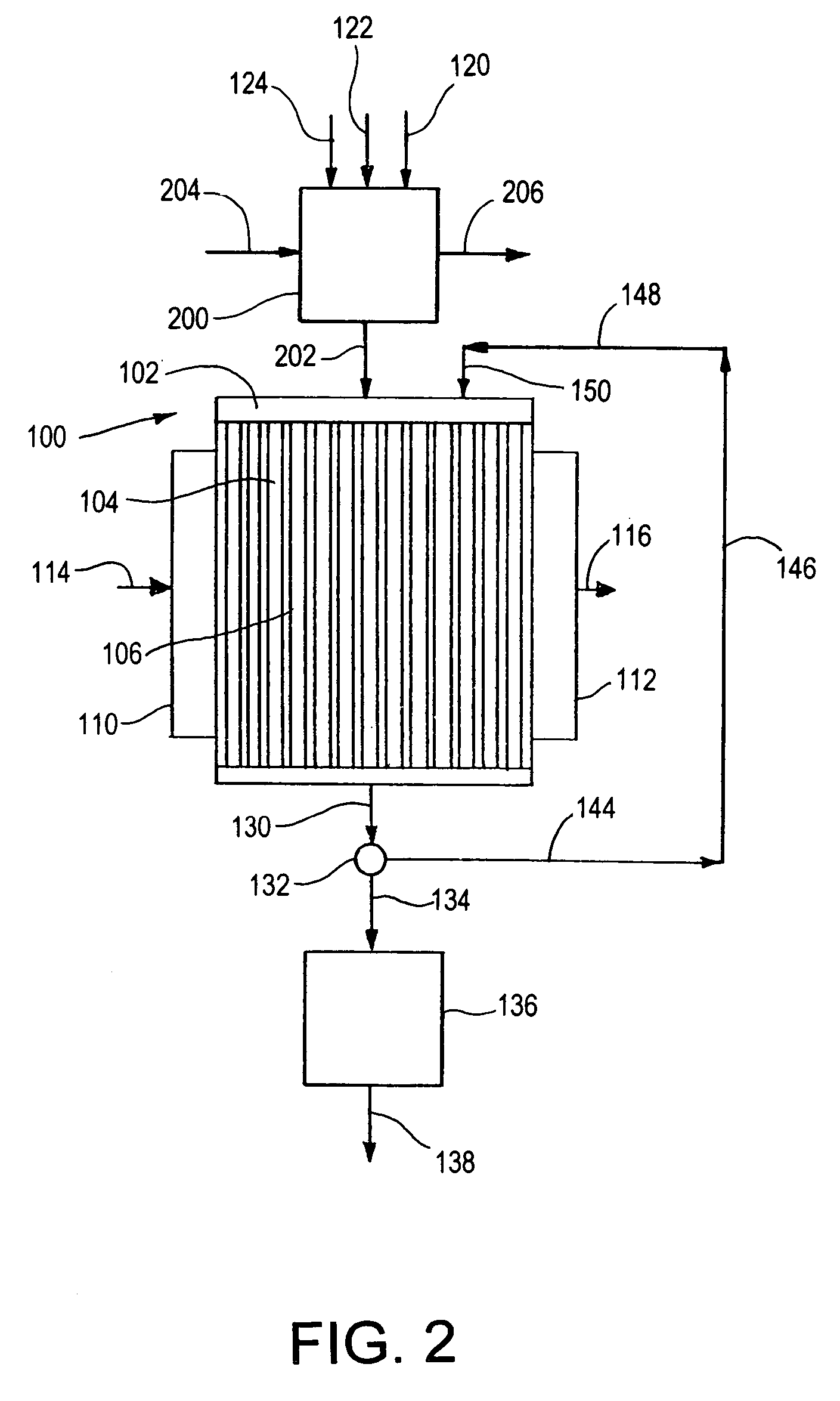
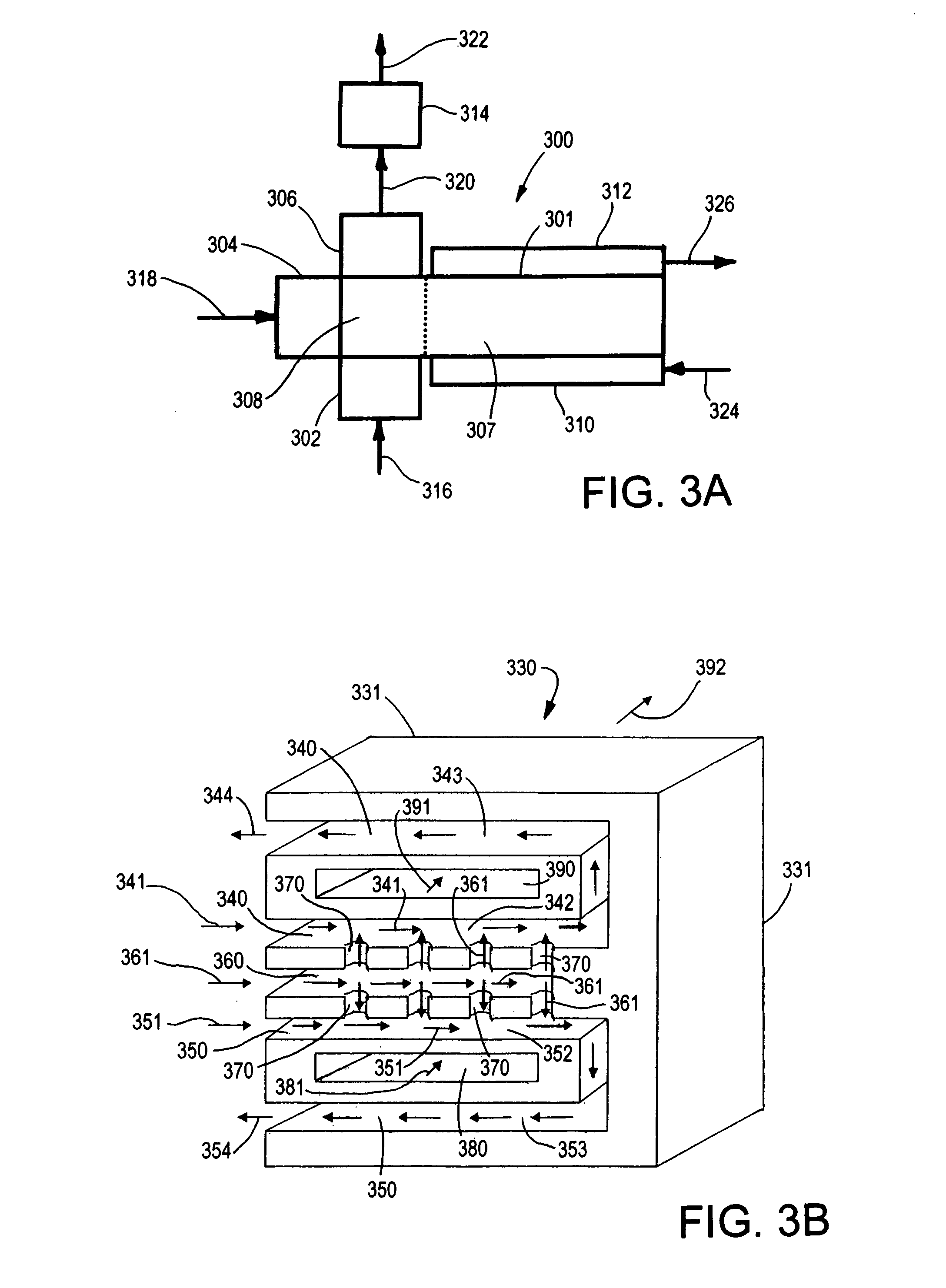
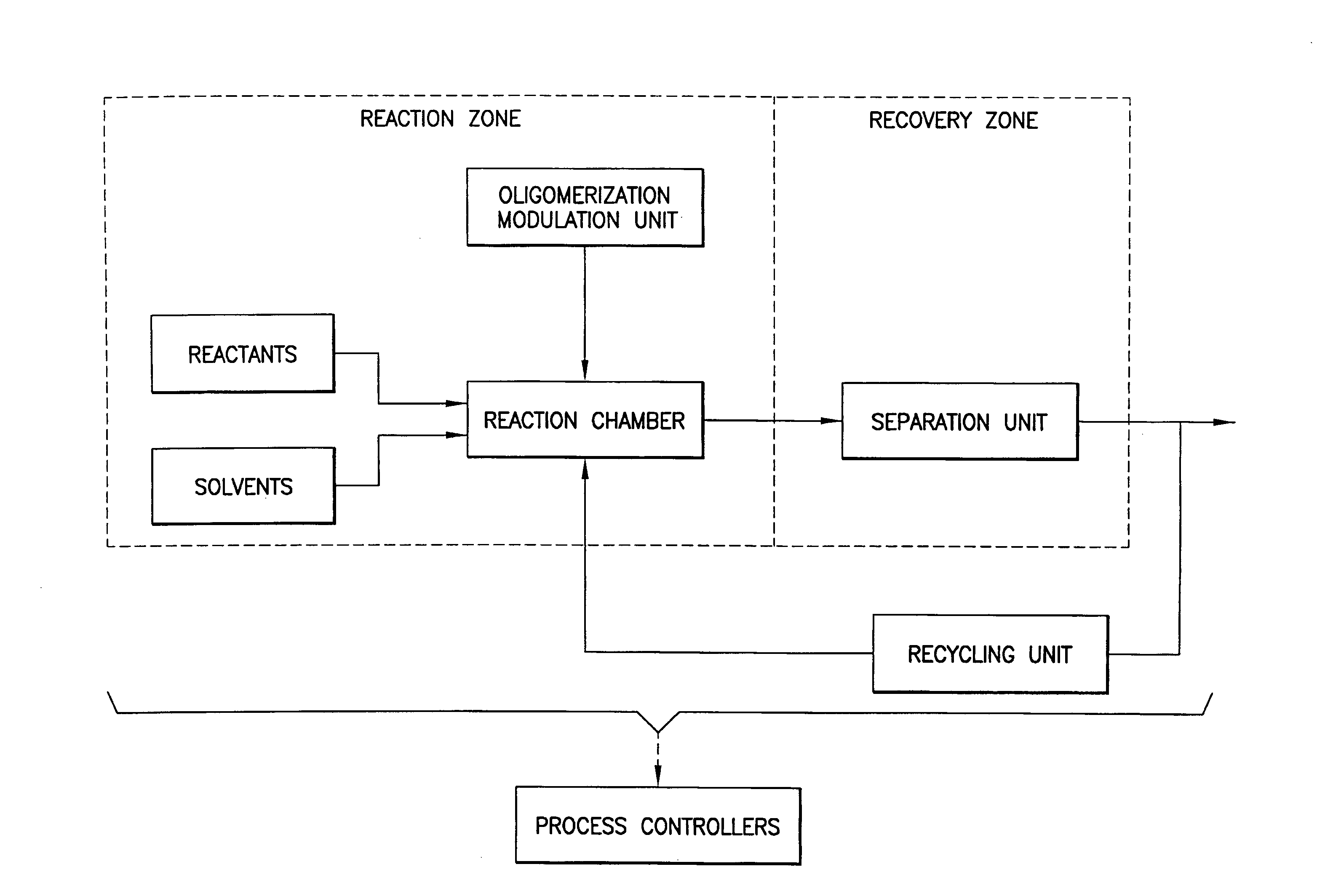
This invention relates to methods, compositions, and apparatuses for producing macrocyclic compounds. First, one or more reactants are provided in a reaction medium, which are capable of forming the macrocyclic compound through a desired reaction pathway that includes at least cyclization, and which are further capable of forming undesired oligomers through a undesired reaction pathway that includes undesirable oligomerization. Oligomerization of such reactions in the reaction medium is modulated to reduce formation of undesired oligomers and / or to reduce separation of the undesired oligomers from the reaction medium, relative to a corresponding unmodulated oligomerization reaction, thereby maximizing yields of the macrocyclic compound. The macrocyclic compound so formed is then recovered from the reaction medium. Preferably, the macrocyclic compound spontaneously separates from the reaction medium via phase separation. More preferably, the macrocyclic compound spontaneous precipitates from the reaction medium and therefore can be easily recovered by simple filtration.
Owner:JOHNSON THOMAS E +1
Method for producing water-absorbent resins
InactiveUS7179875B2Use directlyOrganic compound preparationUsing liquid separation agentScavengerGas phase
In a process for preparing water-absorbent resins based on acrylic acid, crude acrylic acid is firstly isolated from the reaction gases from the catalytic gas-phase oxidation of propane, propylene and / or acrolein. This is treated with an aldehyde scavenger and pure acrylic acid is separated by distillation from the treated crude acrylic acid, and this pure acrylic acid can be subjected directly to a free-radical polymerization.
Owner:BASF AG
Process for the start-up of an epoxidation process, a process for the production of ethylene oxide, a 1,2-diol, a 1,2-diol ether, a 1,2-carbonate, or an alkanolamine
InactiveUS20090281339A1Improve catalytic selectivityHigh selectivityProductsOrganic compound preparationOrganic chloride compoundOxygen
A process is provided for the start-up of an ethylene epoxidation process comprising: (a) contacting a catalyst bed comprising a high selectivity epoxidation catalyst with a feed comprising ethylene, oxygen and an organic chloride for a period of time until an increase of at least 1×10−5 mole-% of vinyl chloride (calculated as the moles of vinyl chloride relative to the total gas mixture), preferably 2×10−5 mole-% of vinyl chloride is detected in a reactor outlet gas or a recycle gas loop; and (b) subsequently adjusting the quantity of organic chloride in the feed to a value sufficient to produce ethylene oxide at a substantially optimum selectivity.
Owner:SHELL OIL CO
Method for atmospheric catalytic oxidation of cyclohexane by metalloporphyrin
InactiveCN1405131APreparation by oxidation reactionsOxygen compounds preparation by hydrocarbon oxidationCyclohexanoneReaction temperature
The invention relates to a new process in which under the catalytic action of metal porphyrin the cyclohexane can be selectively oxdated by air and made into cyclohexanol and cyclohexanone. Under the condition of 2-10 atm air, reaction temp. is 125-150 deg.C, it selects and uses micro-oxo double metal porphyrin and single metal porphyrin or their solid carrier as main catalyst, and uses transition metal salt or oxide as co-catalyst, the metal porphyrin can high-effectively and high-selectively catalyze oxidation of cyclohexane by using air in the biological concentratino as biological enzyme. The dose of metal porphyrin is small and said metal porphyrin can be repeatedly used, and said catalyst dose also is small, its catalystic effect is good, said catalyst can be used for making homogeneous catalysis, and after it is solid-carried, it also can be used for making heterogeneous catalysis.
Owner:HUNAN UNIV
Preparation method of monatomic dispersion catalyst with high catalytic performance
InactiveCN103566935AImprove catalytic performanceImprove stabilityPreparation by oxidation reactionsOrganic compound preparationAlkaneMicrosphere
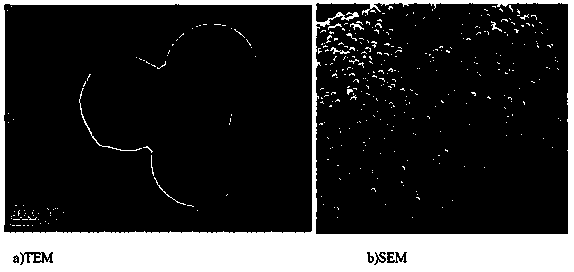
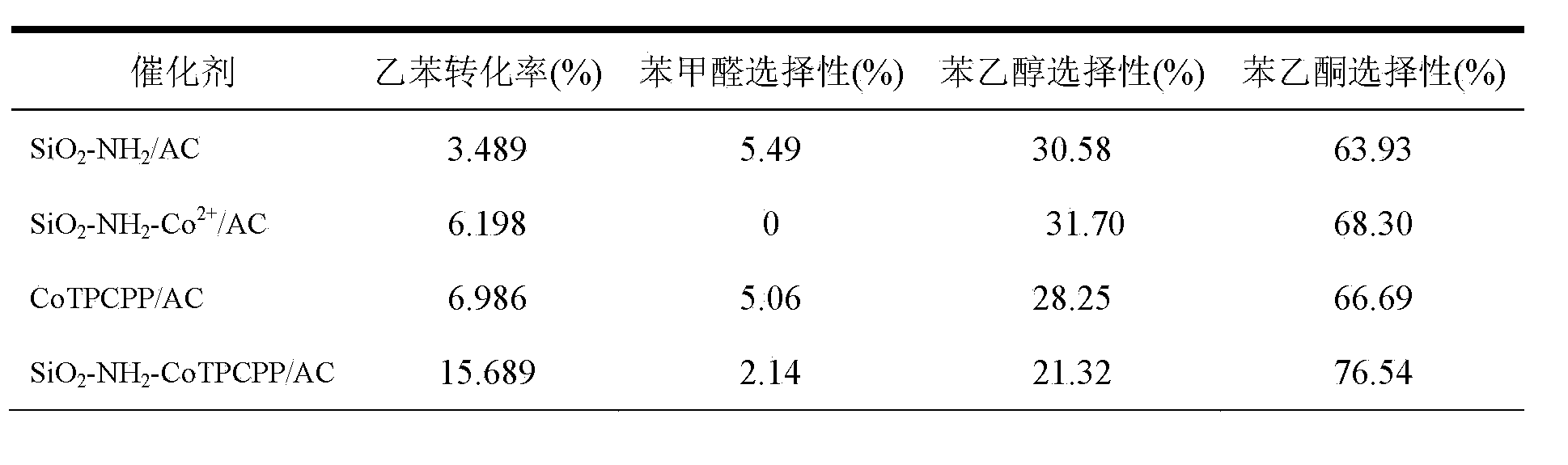
The invention belongs to the technical field of catalysis and relates to a method for preparing a monatomic cobalt catalyst by roasting silicon dioxide pellets and immobilizing metalloporphyrin. The method is realized by adopting the following technical scheme: namely stirring and dispersing nano silicon dioxide particles, dropwise adding 3-aminopropyl triethoxy silane, so as to obtain amino modified SiO2 pellets, then linking monocarboxyl metalloporphyrin (CoTPCPP) onto an amino modified SiO2 carrier through organic complexing, carbonizing porphyrin through high-temperature calcination in an inert atmosphere, and loading highly dispersed cobalt atoms onto the amino modified SiO2 pellets, thus an amino modified silicon dioxide pellet loaded monatomic cobalt catalyst is obtained. The monatomic dispersion catalyst has high catalytic activity and stability on a molecular oxygen selective ethylbenzene catalytic oxidation reaction in absence of solvent; meanwhile, a preparation method is simple, and product quality can be easily controlled, so that the monatomic dispersion catalyst is applicable to the fields of alkane selective oxidation reactions and synthesis of medical intermediates.
Owner:HUNAN UNIV
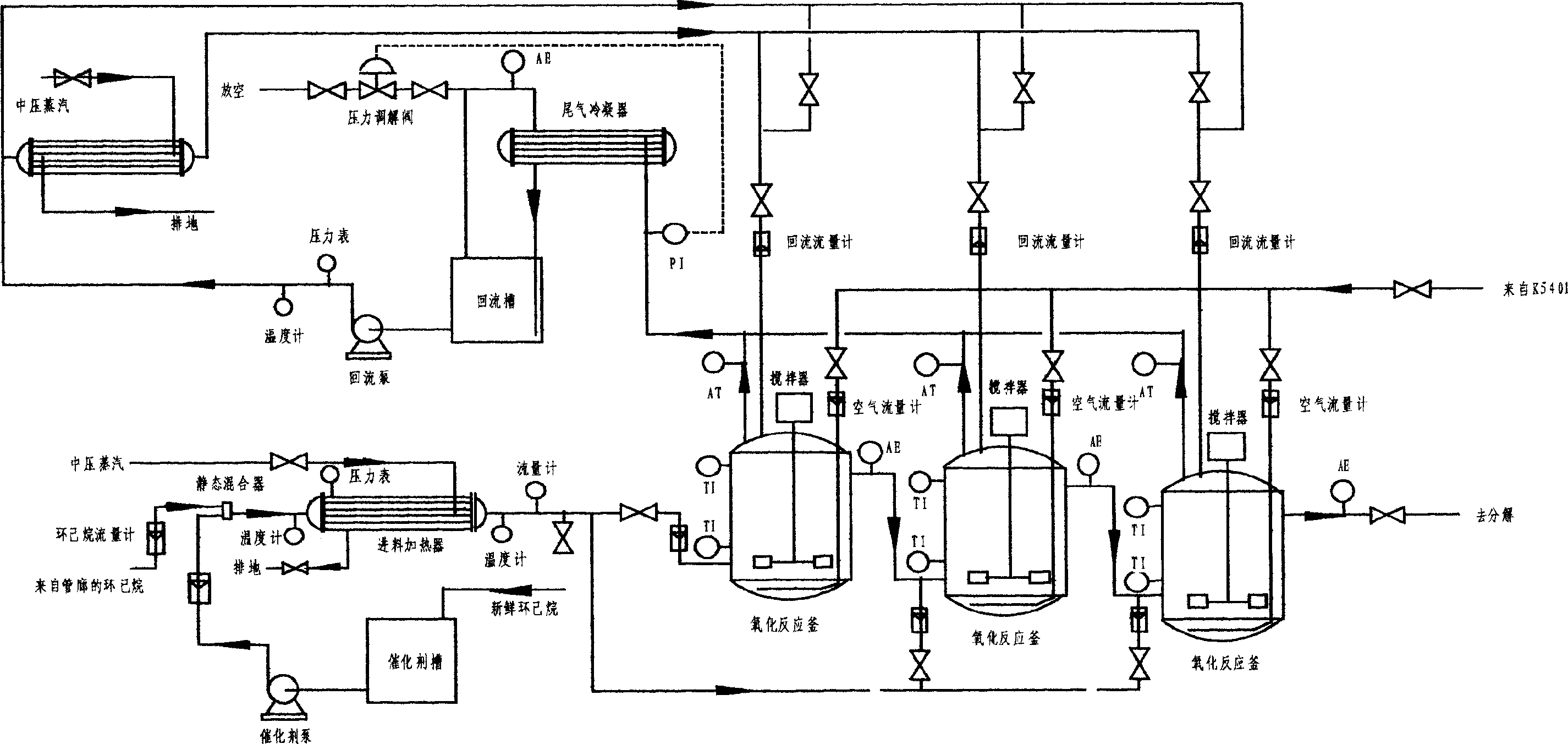
Catalyst oxdie cyclohexane process
ActiveCN1530358AIncrease conversion rate per passImprove one-way yieldOxygen compounds preparation by hydrocarbon oxidationCyclohexanonePorphyrin
A process for preparing cyclohexanol, cyclohexanone and adipic acid by catalytic oxidization of cyclohexane under existance of metallic porphyrin catalyst and a certain temp and pressure through combination of different reactors is disclosed. Its advantages are high single-pass conversion rate of cyclohexane and total output rate, low energy consumption and cost, and no need of cyclohexanol or acetone solvent and cocatalyst.
Owner:CHINA PETROLEUM & CHEM CORP
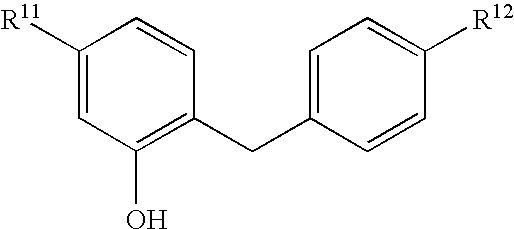
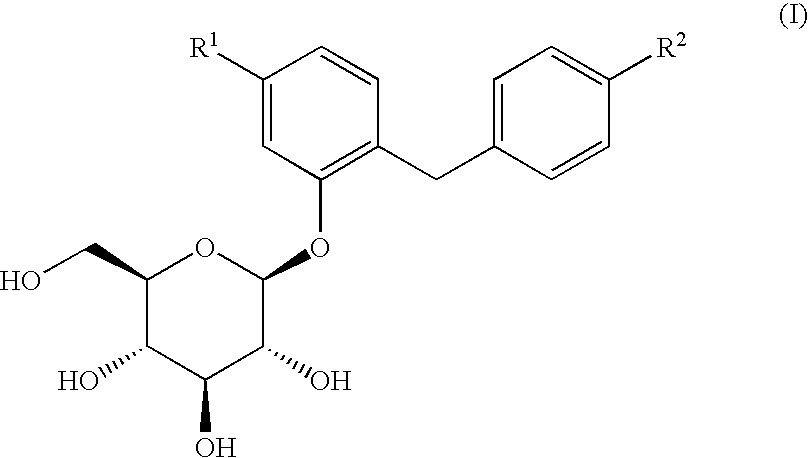
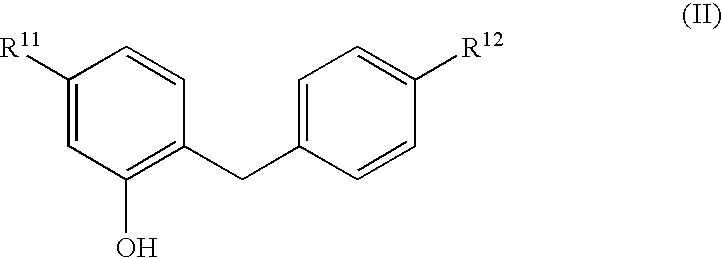
Glucopyranosyloxybenzylbenzene derivatives, medicinal compositions containing the same and intermediates for the preparation of the derivatives
InactiveUS7045665B2Strong inhibitory activityInhibitory activityBiocideSugar derivativesAcute hyperglycaemiaAlkoxy group
The present invention relates to benzylphenol derivatives represented by the general formula:wherein R11 represents a hydrogen atom or a protected hydroxy(lower alkyl) group; and R12 represents a lower alkyl group, a lower alkoxy group, a lower alkylthio group, a protected hydroxy(lower alkyl) group, a protected hydroxy(lower alkoxy) group, a protected hydroxy(lower alkylthio) group, a lower alkoxy-substituted (lower alkyl) group, a lower alkoxy-substituted (lower alkoxy) group or a lower alkoxy-substituted (lower alkylthio) group; with the proviso that R12 is not a methyl group, an ethyl group, an isopropyl group, a tert-butyl group or a methoxy group when R11 is a hydrogen atom, or a salt thereof, which are used as intermediates for making glucopyranosyloxybenzylbenzene compounds having inhibitory activity in human SGLT2 and are useful in treating diseases associated with hyperglycemia, such as diabetes.
Owner:KISSEI PHARMA
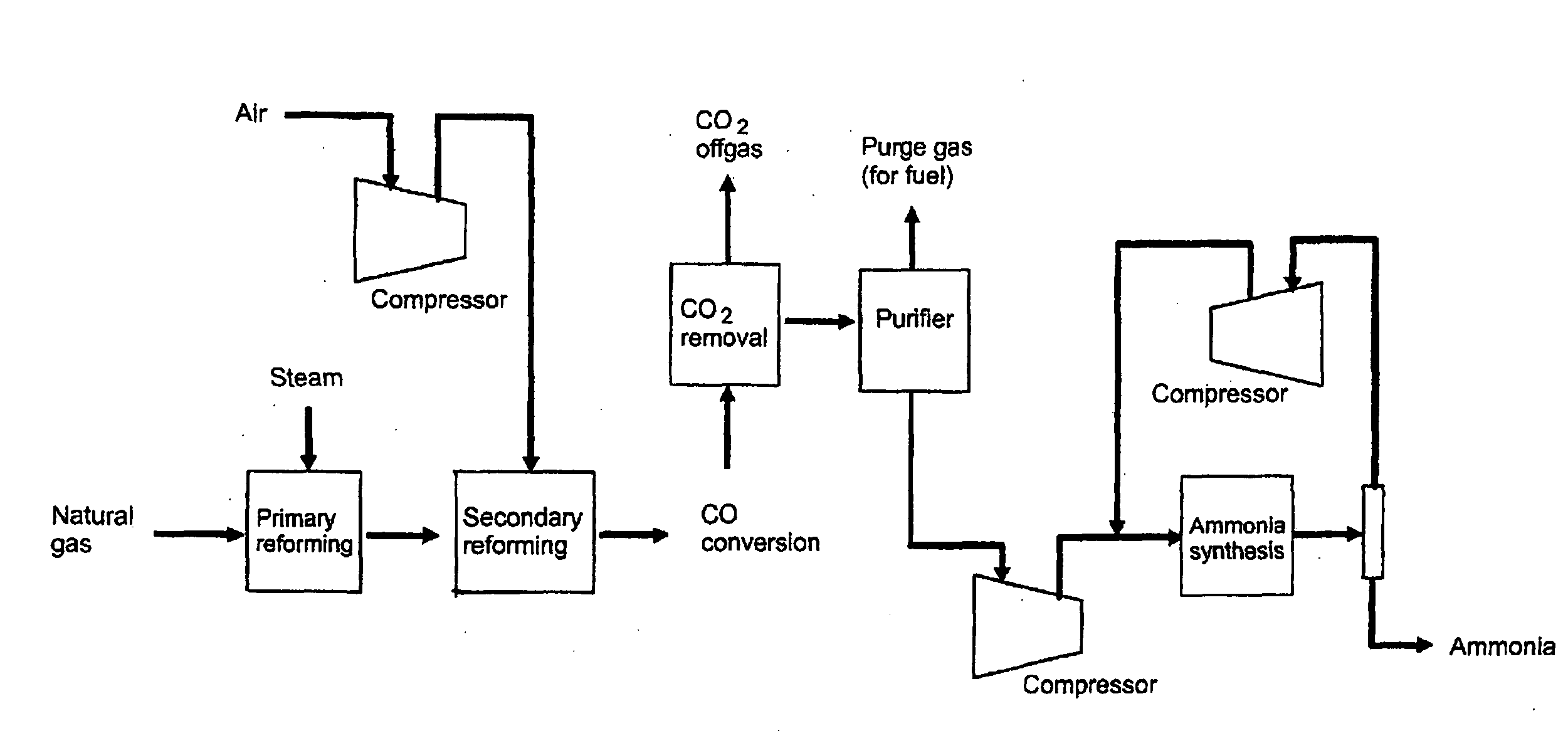
Method of coproducing methanol and ammonia
ActiveUS20100150810A1Efficiently coproduceIncreasing and decreasing specific raw material consumptionCyanogen compoundsHydroxy compound preparationSteam reformingSyngas
The present invention enables the coproduction of methanol and ammonia by using a remarkably smaller amount of raw material, natural gas, as compared with the case of independently producing either of methanol or ammonia. Specifically, the present invention provides a process of coproducing methanol and ammonia by using natural gas, LPG, butane, or naphtha as raw material, having a methanol production process (A) composed of specific steps and an ammonia production process (B) also composed of specific steps, in which (1) directing a gas rich in hydrogen recovered in a hydrogen recoverying step in the methanol production process (A) to a certain step after an air reforming step and before a ammonia synthesis step in the ammonia production process (B) to prepare additional feedstock gas for ammonia synthesis; and also (2) cooling a synthesis gas, formed in a steam reforming step in the methanol production process (A) and separating and removing condensed water from the cooled synthesis gas, mixing the water removed synthesis gas and carbon dioxide obtained by pre-compressing carbon dioxide obtained in a CO2 removing step in the ammonia production process (B), compressing up to the methanol synthesis pressure to be supplied as reactant gas for methanol synthesis to the methanol synthesis step (A-2).
Owner:TOYO ENG CORP
Supported nano Au catalyst and method for preparing the same
InactiveCN1827213AEvenly dispersedEasy to adjust particle sizeCatalyst carriersOxygen compounds preparation by hydrocarbon oxidationActive componentGold particles
The invention discloses a carrier nanometer gold catalyst gold catalyst and relative preparing method. Said invention is formed by Au, Al, and Ti / Si, Ag, Cu, Ce, Fe, or Zn. Wherein, Au is the main active component of catalyst, whose mass percentage is 0.1-4.0%; Al is the carrier of catalyst, whose mass percentage is larger than 90%; Ti or Si is the agent of catalyst carrier, whose mass percentage is 0.01-1.0%; Ag, Cu, Ce, Fe, or Zn is used as the auxiliary active component of catalyst, whose mass percentage is 0.1-5.0%. The invention has the advantages that: the gold is distributed uniformly, and the graininess of attained gold particle can be adjusted easily; the catalyst has high stability; and it can apply the oxide carrier for carrying gold whose isoelectric point pH is less than 6 and made by coprecipitation method. The invention has be used to apply the cyclohexane to prepare the nadone and cyclohexanol, with better activity and selectivity, less used amount and recycle support.
Owner:ZHEJIANG UNIV
Catalytic oxidation of alkanes to corresponding acids
A mixed metal oxide Mo-V-Ga-Pd-Nb-X catalytic system, where X is selected from La, Te, and Zn, provides the oxidation of C2-C8 hydrocarbons to corresponding acids with a molecular oxygen-containing gas.
Owner:SAUDI BASIC IND CORP SA
Popular searches
Treatment with hydrotreatment processes Hydrogen/synthetic gas production Hydrocarbon from saturated and unsaturated hydrocarbon addition Bulk chemical production Hydrocarbon oils treatment products Refining to eliminate hetero atoms Hydrogen Hydrocarbon from oxygen organic compounds Hydrocarbons Metal/metal-oxides/metal-hydroxide catalysts
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com