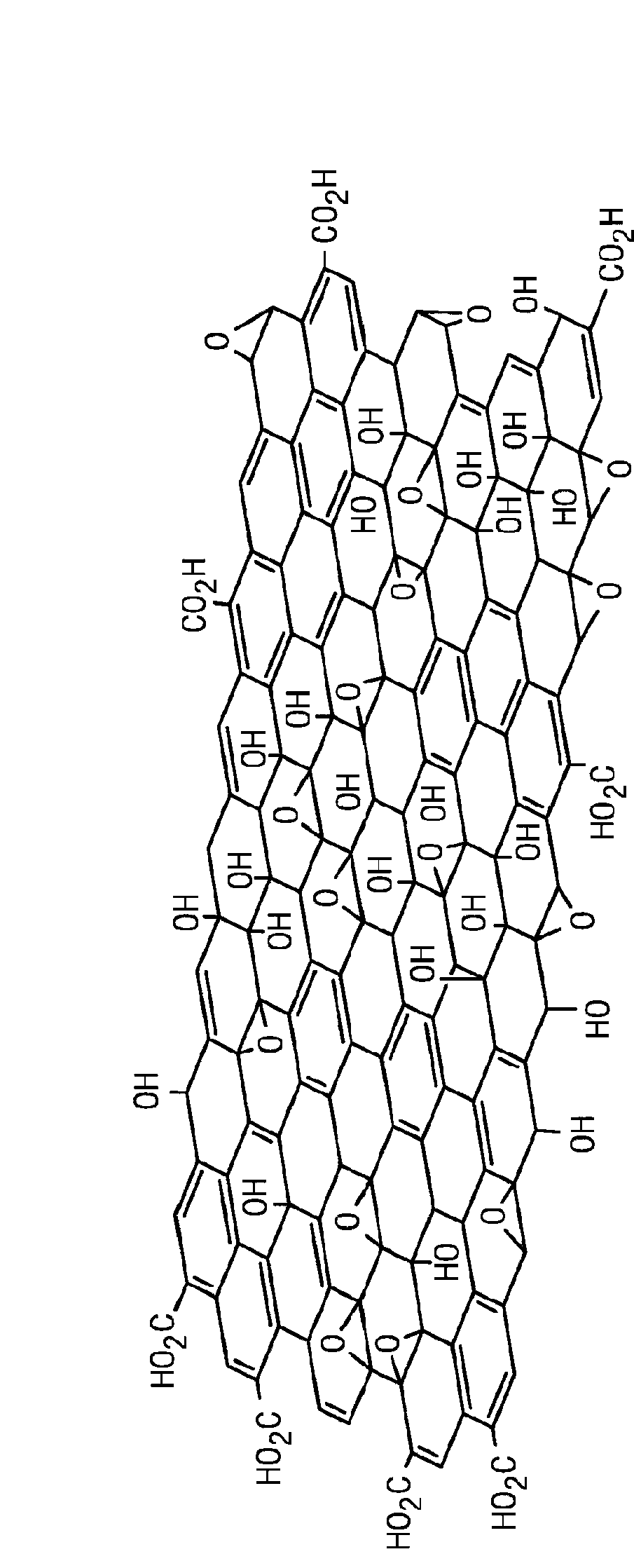
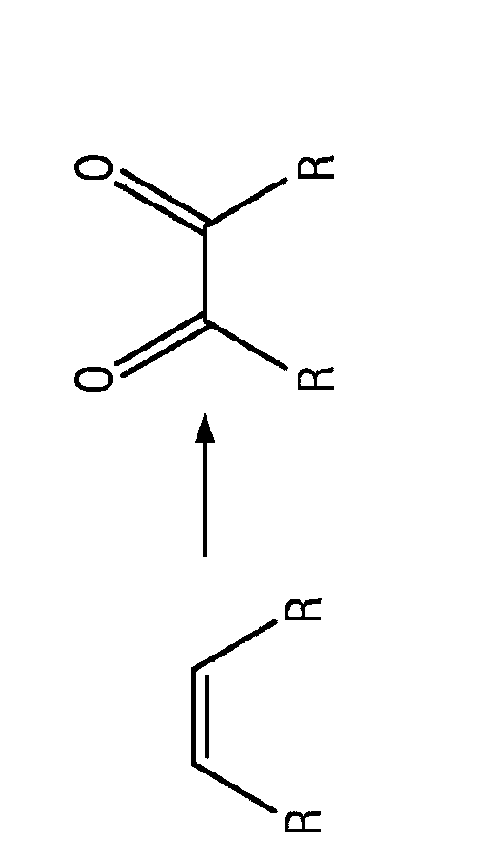
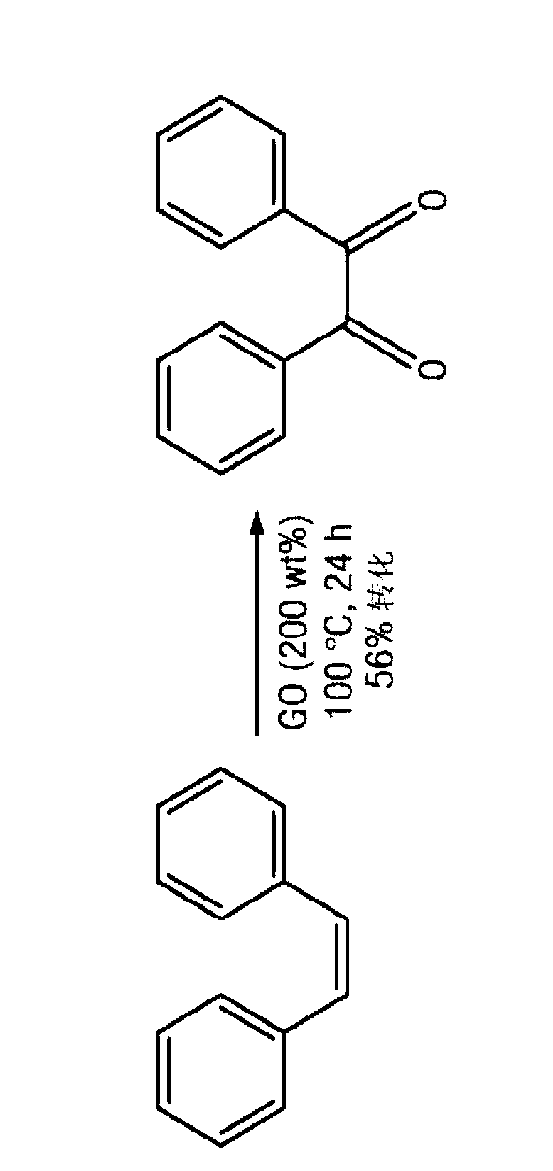
Carbocatalysts for chemical transformations
A technology of carbon catalyst and chemical transformation, applied in the direction of physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, nanotechnology for materials and surface science, etc. issues of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0258] The preparation of embodiment 1-graphene oxide or graphite oxide catalyst
[0259] Graphene oxide or graphite oxide used in some of the experiments contained in these examples was prepared according to the following method. Others were prepared using the Staudenmaier method. Both methods yield suitable catalysts.
[0260] Graphite oxide was prepared using a modified Hummers method. A 100 mL reaction flask was charged with natural flake graphite (3.0 g; SP-1, Bay Carbon Inc. or Alfa Aesar [99%; 7–10 μm]), concentrated sulfuric acid (75 mL) and a stir bar, then cooled on an ice bath . Then slowly add KMnO to the flask within 2h 4(9.0 g), resulting in a dark mixture. The rate of addition was carefully controlled to prevent the temperature of the suspension from exceeding 20 °C. After stirring for 1 h at 0 °C, the mixture was heated at 35 °C for 0.5 h. The flask was then allowed to cool to room temperature and the reaction was quenched by pouring the mixture into 1...
Embodiment 2
[0261] Embodiment 2: the preparation of graphite oxide
[0262] A 100 mL reaction flask was charged with natural flake graphite (6.0 g; SP-1, BayCarbon Inc. or Alfa Aesar [99%; 7-10 μm]), concentrated sulfuric acid (25 mL), K 2 S 2 o 8 (5g), P 2 o 5 (5 g) and a stir bar, the mixture was then heated at 80° C. for 4.5 h. The mixture was then allowed to cool to room temperature. Next, the mixture was diluted with water (1 L) and left to stand for about 8-10 hours. The pretreated graphite was collected by filtration and washed with water (0.5 L). The pellet was air-dried for 1 day and transferred to concentrated H 2 SO 4 (230mL). Then slowly add KMnO to the mixture within 2h 4 (30 g), resulting in a dark mixture. Carefully control the rate of addition to prevent the temperature of the suspension from exceeding 10 °C. The mixture was stirred at 0 °C for 1 h. The mixture was then heated at 35 °C for 2 h. The flask was then cooled to room temperature and the reaction ...
Embodiment 3
[0263] Embodiment 3: the preparation of graphite oxide
[0264] Fill a 250mL reaction flask with natural flake graphite (1.56g; SP-1, BayCarbon Inc. or Alfa Aesar [99%; 7-10μm]), 50mL of concentrated sulfuric acid, 25mL of fuming nitric acid and a stirring bar, and then Cool in medium. NaClO was then added to the flask with stirring 3 (3.25g; Note: In some cases, due to the possible formation of KClO 4 Water insoluble, NaClO 3 better than KClO 3 ). Additional NaClO every hour for 11 consecutive hours 3 (3.25g) was added. This procedure was repeated for 3 days. The resulting mixture was poured into 2 L of deionized water. The heterogeneous dispersion was then filtered through a coarse sintered funnel or nylon membrane filter (0.2 μm, Whatman) and the isolated material was washed with additional deionized water (3 L) and 6N HCl (1 L). The filtered solid was collected and dried under high vacuum to give the product as a dark brown powder (3.61 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com