Packaging shape-stabilizing method of inorganic hydrated salt phase-change heat storage material
A technology of phase-change heat storage materials and inorganic hydrated salts, which is applied in the field of material science and can solve problems such as incomplete adsorption, long time consumption, and unevenness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
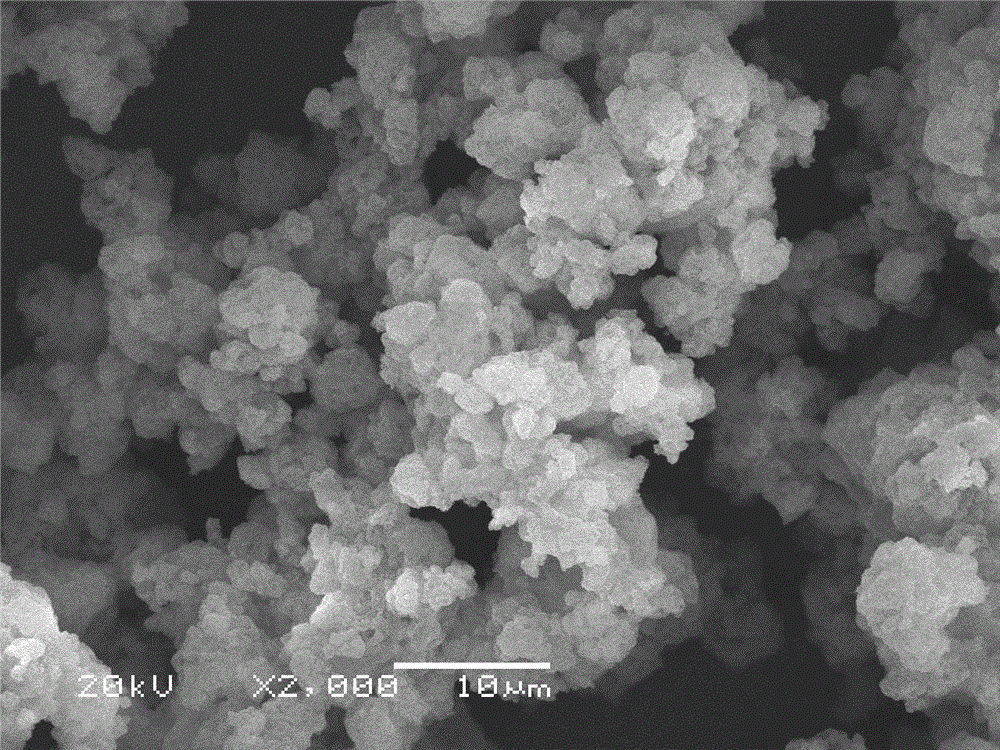
[0014] (1) Take 2g CaCl 2 ·6H 2 O, put it in a beaker, add 0.06 borax, mix evenly, add a small amount of deionized water, seal it with plastic wrap, put it in a constant temperature drying oven at 50 ℃, and heat it for 5 minutes to make it reach a molten state; (2) take 1.0 Porous Al 2 o 3 Added to the molten CaCl 2 ·6H 2 O in a beaker, and stir evenly, put the beaker of the above sample in a vacuum drying oven, vacuumize for 2h, and then put it in the refrigerator for 5h for recrystallization, that is, a new type of CaCl with uniform adsorption is obtained. 2 ·6H 2 O Porous Al 2 o 3 Composite phase change material; (3) The phase change temperature of the obtained composite shape-setting phase change heat storage material is 30.71°C, and the latent heat value of phase change is 99.81 J / g; the SEM photos and DSC test results of this example are as follows Figure 1a , Figure 2a shown.
Embodiment 2
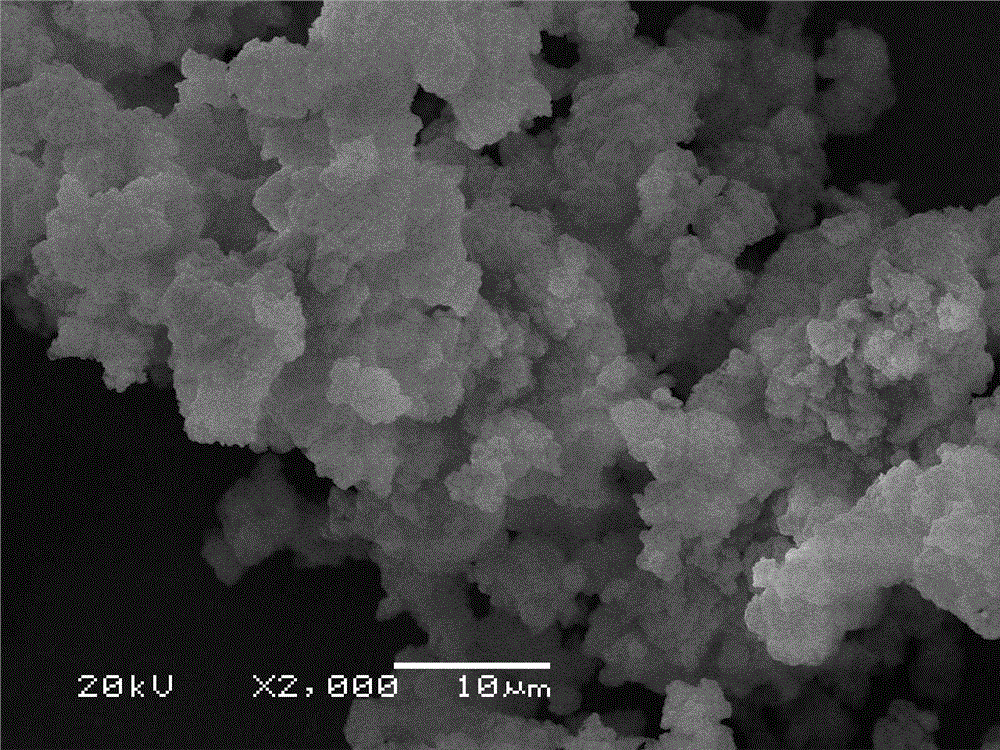
[0016] (1) Take 2g CaCl 2 ·6H 2 O, put it in a beaker, add 0.06 borax, mix evenly, add a small amount of deionized water, seal it with plastic wrap, put it in a constant temperature drying oven at 50 ℃, and heat it for 5 minutes to make it reach a molten state; (2) take 1.2 Porous Al 2 o 3 Added to the molten CaCl 2 ·6H 2 O in a beaker, and stir evenly, put the beaker of the above sample in a vacuum drying oven, vacuumize for 2h, and then put it in the refrigerator for 5h for recrystallization, that is, a new type of CaCl with uniform adsorption is obtained. 2 ·6H 2 O Porous Al 2 o 3 Composite phase change material; (3) The phase change temperature of the obtained composite shape-setting phase change heat storage material is 29.95°C, and the latent heat value of phase change is 86.42 J / g; the SEM photos and DSC test results of this example are as follows Figure 1b , Figure 2b shown.
Embodiment 3
[0018] (1) Take 2g CaCl 2 ·6H 2 O, put it in a beaker, add 0.06 borax, mix well, add a small amount of deionized water, seal it with plastic wrap, put it in a constant temperature drying oven at 50 ℃, and heat it for 5 minutes to make it reach a molten state; (2) Take 1.4 Porous Al 2 o 3 Added to the molten CaCl 2 ·6H 2 O in a beaker, and stir evenly, put the beaker of the above sample in a vacuum drying oven, vacuumize for 2h, and then put it in the refrigerator for 5h for recrystallization, that is, a new type of CaCl with uniform adsorption is obtained. 2 ·6H 2 O Porous Al 2 o 3 Composite phase change material; (3) The phase change temperature of the obtained composite shape-setting phase change heat storage material is 28.34°C, and the latent heat value of phase change is 70.61J / ; the SEM photos and DSC test results of this embodiment are as follows Figure 1c , Figure 2c shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com