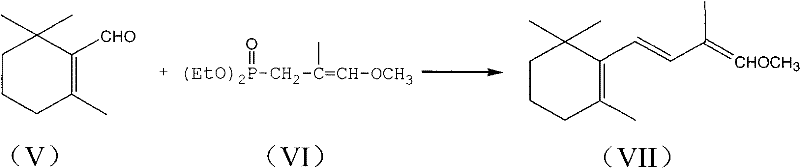
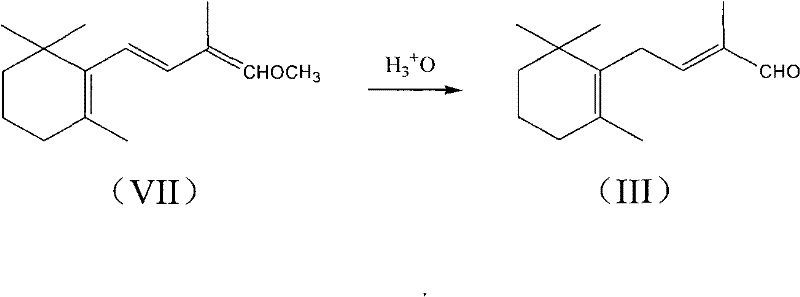
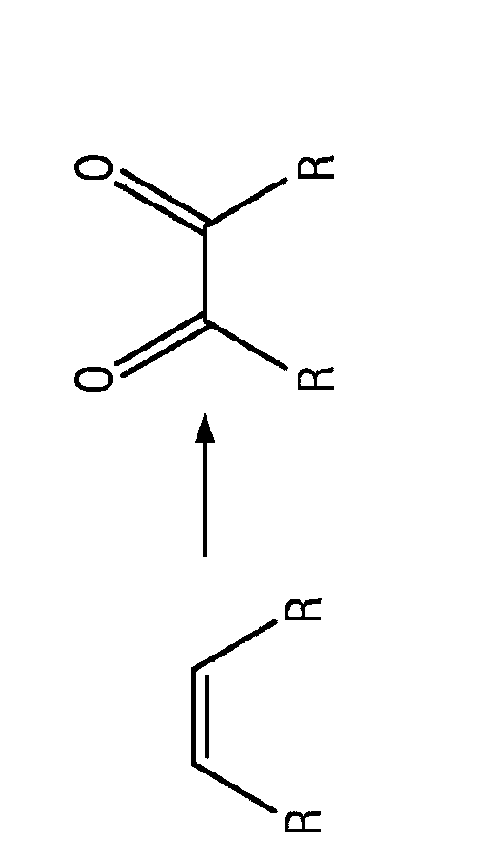
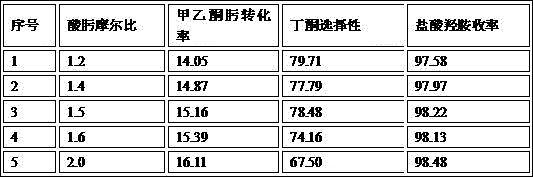
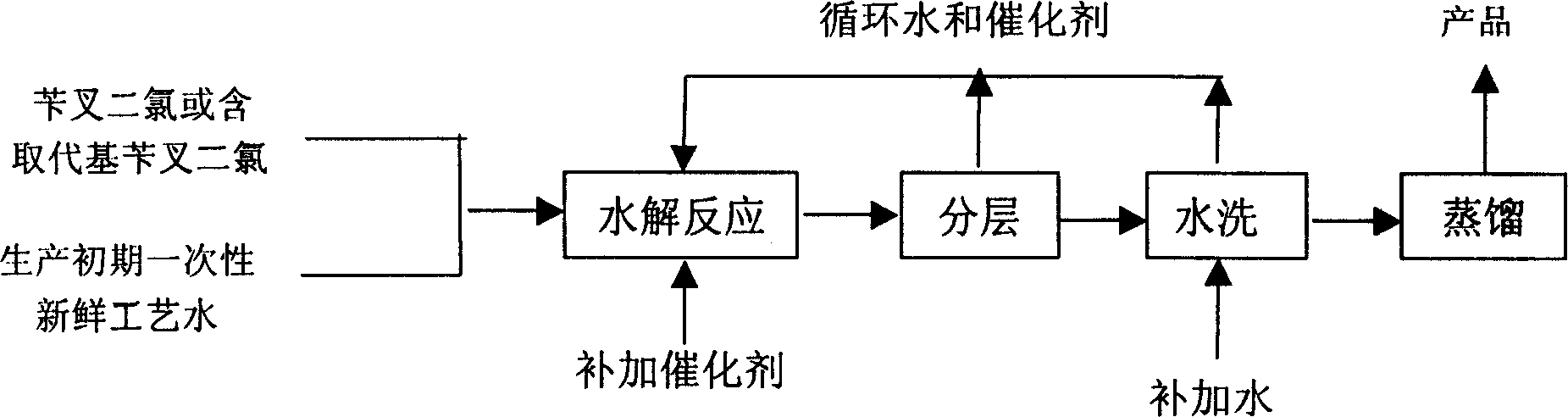
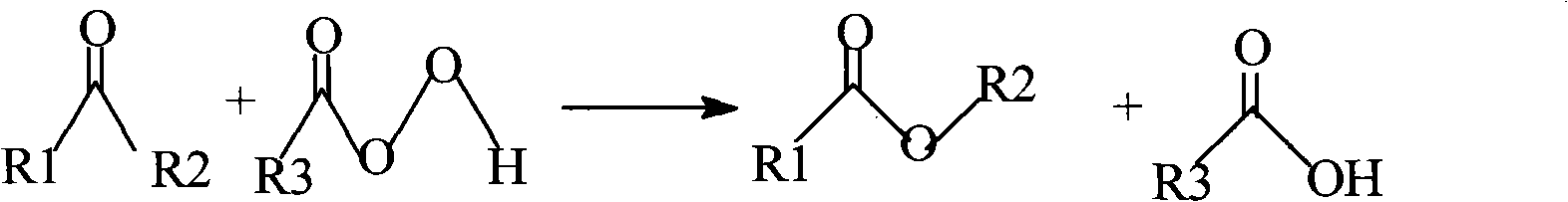
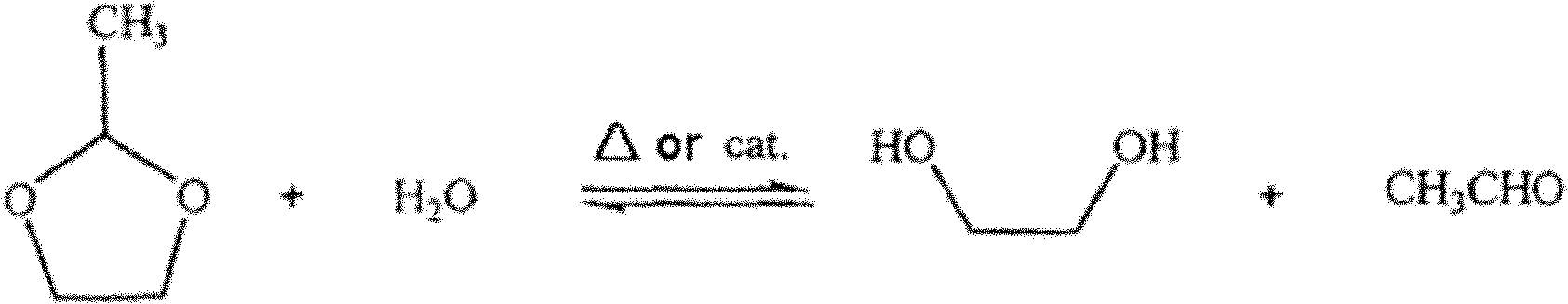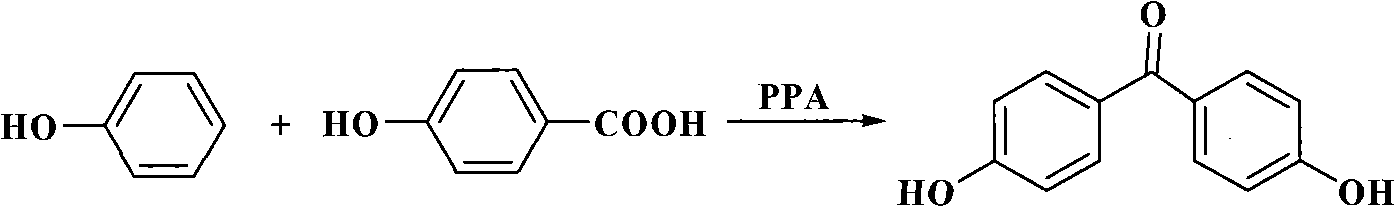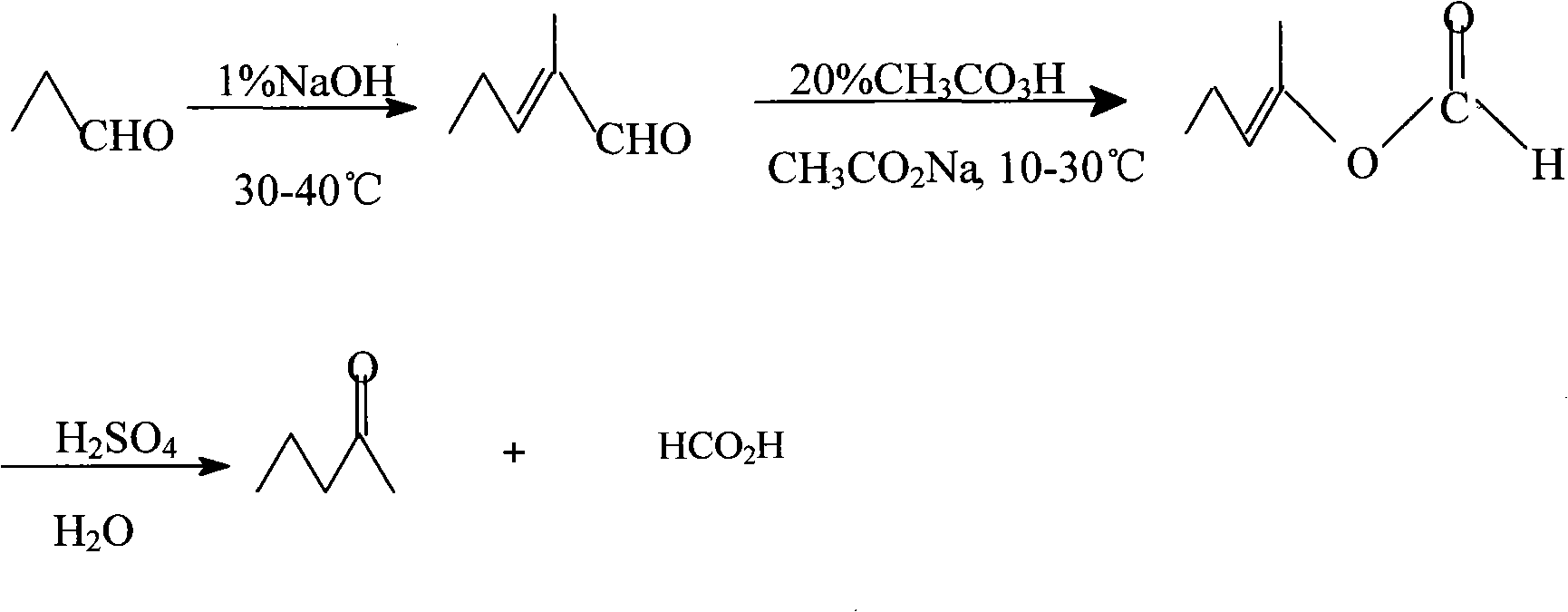Patents
Literature
435results about "Carbonyl compound preparation by hydrolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Depolymerization of lignin using solid acid catalysts
ActiveUS20120302796A1Organic compound preparationCarbonyl compound preparation by hydrolysisDepolymerizationSolid acid
Owner:COUNCIL OF SCI & IND RES
Method for preparing intermediate of vitamin A, namely tetradecanal
InactiveCN102190565AOrganic compound preparationCarbonyl compound preparation by hydrolysisEnol etherDissociation reaction
The invention provides a method for preparing an intermediate of vitamin A, namely C14-aldehyde, and an intermediate of the C14-aldehyde, namely C14 enol ether, which comprises the following steps of: (1) under the protection of inert gas, performing rearrangement dissociation reaction on C4 phosphonate at the temperature of between -40 and 30DEG C in the presence of alkali in an ether solvent ora dipolar aprotic solvent; (2) adding beta-cyclocitral, and performing Wittig-Homer condensation reaction at the temperature of between -40 and 30DEG C in the presence of alkali in the ether solvent or the dipolar aprotic solvent to obtain the C14 enol ether; and (3) under the protection of inert gas, mixing the C14 enol ether, an acid catalyst, water and a homogenous phase solvent, and performing hydrolysis reaction at the temperature of between 10 and 35DEG C with stirring to obtain the C14-aldehyde. The method has the advantages of simple process, readily available raw materials and low cost, and has great industrial value.
Owner:SHAOXING UNIVERSITY +1
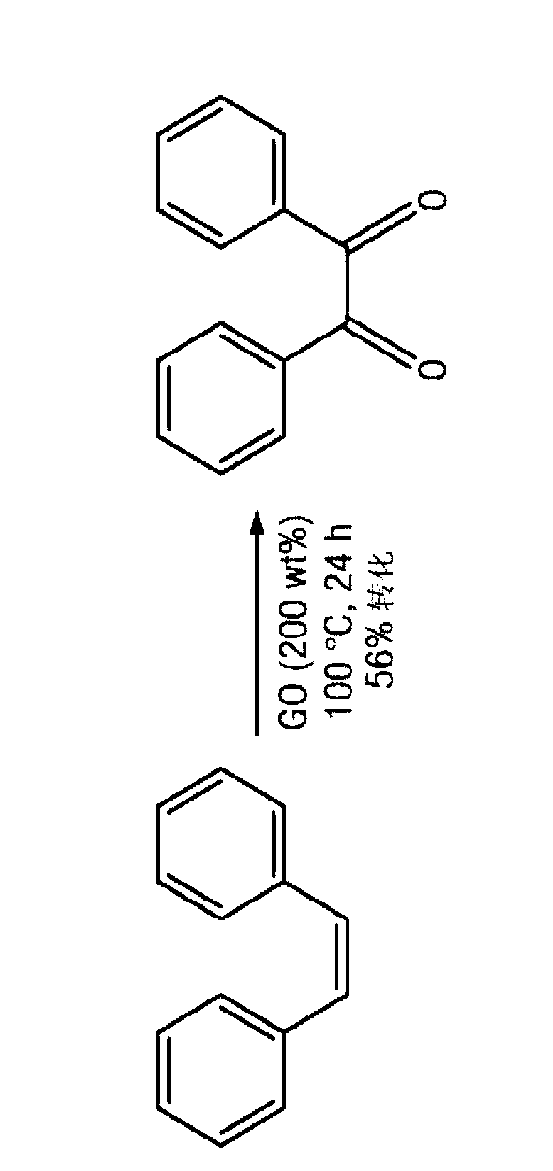
Carbocatalysts for chemical transformations
InactiveCN103025685AMaterial nanotechnologyOrganic compound preparationHydration reactionChemical reaction
The disclosure relates to catalytically active carbocatalysts, e.g., a graphene oxide or graphite oxide catalyst suitable for use in a variety of chemical transformations. In one embodiment, it relates to a method of catalyzing a chemical reaction of an organic molecule by reacting the organic molecule in the presence of a sufficient amount of graphene oxide or graphite oxide for a time and at a temperature sufficient to allow catalysis of a chemical reaction. According to other embodiments, the reaction may be an oxidation reaction, a hydration reaction, a dehydrogenation reaction, a condensation reaction, or a polymerization reaction. Some reactions may include auto-tandem reactions. The disclosure further provides reaction mixtures containing an organic molecule and graphene oxide or graphite oxide in an amount sufficient to catalyze a reaction of the organic molecule.
Owner:GRAPHEA
Methods of saponifying xanthophyll esters and isolating xanthophylls
ActiveUS20120107380A1High yieldAvoid insufficient purityOrganic active ingredientsCosmetic preparationsWaxSaponification
This invention relates to a practical and effective process for converting esterified xanthophylls, including zeaxanthin, to non-esterified xanthophylls through saponification. In addition, the invention provides a process for obtaining esterified zeaxanthin in high yields and purities, isolating the xanthophylls from interfering substances such as waxes, oils, and fats. A product of this process is a zeaxanthin rich substrate that is suitable for use in foods, nutritional supplements, cosmetics, pharmaceuticals and related products.
Owner:KALAMAZOO HLDG INC
Electrochemically active agents for ph modulation in biological buffers
ActiveUS20170008825A1ReactivityReduced responseOrganic compound preparationQuinone preparation by oxidationAnalyteActive agent
Device and methods for use in a biosensor comprising a multisite array of test sites, the device and methods being useful for modulating the binding interactions between a (biomolecular) probe or detection agent and an analyte of interest by modulating the pH or ionic gradient near the electrodes in such biosensor. An electrochemically active agent that is suitable for use in biological buffers for changing the pH of the biological buffers. Method for changing the pH of biological buffers using the electrochemically active agents. The methods of modulating the binding interactions provided in a biosensor, analytic methods for more accurately controlling and measuring the pH or ionic gradient near the electrodes in such biosensor, and analytic methods for more accurately measuring an analyte of interest in a biological sample.
Owner:ROBERT BOSCH GMBH
Process for directly producing natural benzaldehyde using cassia twig leaf and cassia bark as raw material
InactiveCN1749231AReduce investmentShort process routeCarbonyl compound preparation by hydrolysisBenzaldehydeGas phase
The process of producing natural benzaldehyde directly with cassia twig, leaf and bark as raw material includes the steps of: crushing cassia twig, leaf and bark, and distilling in distiller; hydrolysis of the mixed gas of steam and cassia oil from the distiller, inside hydrolysis reactor with inlet connected to the outlet of the distiller and through contact with alkaline catalyst to produce benzaldehyde in the steam mixture; and condensing the mixture to obtain coarse benzaldehyde product. The present invention can produce natural benzaldehyde by increasing one hydrolysis reactor to available cassia oil producing apparatus, and has short technological path, low power consumption, low cost and coarse benzaldehyde yield of 0.5 %.
Owner:GUANGXI UNIV
Process for producing acrolein and glycerin-containing composition
InactiveUS7951978B2Good effectReduce accumulationOrganic compound preparationCarbonyl compound preparation by hydrolysisAcroleinSolid acid
Owner:NIPPON SHOKUBAI CO LTD
Method for recycling ethylene glycol and acetaldehyde from polyester waste water
ActiveCN102126926AReduce COD valueFix security issuesMultistage water/sewage treatmentWater/sewage treatment by degassingPolyesterDistillation
The invention discloses a method for recycling ethylene glycol and acetaldehyde from polyester waste water. The method is implemented by the following steps of: (1) introducing esterification waste water into a stripping tower for treating and directly introducing organic matter gas and water vapor collected from a tower top into an acetaldehyde rectifying tower; (2) treating in the acetaldehyde rectifying tower, collecting high-purity acetaldehyde from the tower top and introducing waste water collected from a tower bottom into an ethylene glycol distillation tower; and (3) treating in the ethylene glycol distillation tower, collecting a high-purity ethylene glycol solution from the tower bottom and introducing waste gas and water vapor produced on the tower top into the stripping tower for cyclic treatment. In the method, acetaldehyde and ethylene glycol are continuously collected, so that hydrolysis of byproduct of 2-methyl-1,3-dioxolane in the esterification waste water is continuously performed positively and negatively, acetaldehyde with the concentration of over 95 percent and ethylene glycol with the concentration of over 70 percent are obtained at the end of treatment, and the 2-methyl-1,3-dioxolane is fully hydrolyzed.
Owner:SHANGHAI JUYOU CHEM ENG +1
Process for preparing hydrofluoroethers
InactiveUS6023002AFurther reactionAchieve efficiencyOrganic compound preparationEther preparation by compound additionEtherHydrofluoroether
Owner:3M INNOVATIVE PROPERTIES CO
Method for producing isophorone
InactiveCN102531866AOrganic compound preparationCarbonyl compound preparation by hydrolysisOrganic fractionIsophorone
The invention relates to a method for producing isophorone by catalyzed aldol condensation of acetone as an educt, reprocessing the reaction product, hydrolyzing the product stream, and separating into an organic and an aqueous fraction, obtaining isophorone from the organic fraction, distillatively reprocessing the aqueous fraction, and feeding the vapors from the head of the distillative reprocessing apparatus into the hydrolysis apparatus.
Owner:EVONIK DEGUSSA GMBH
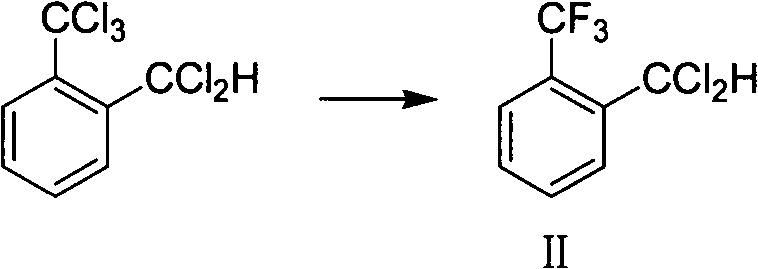
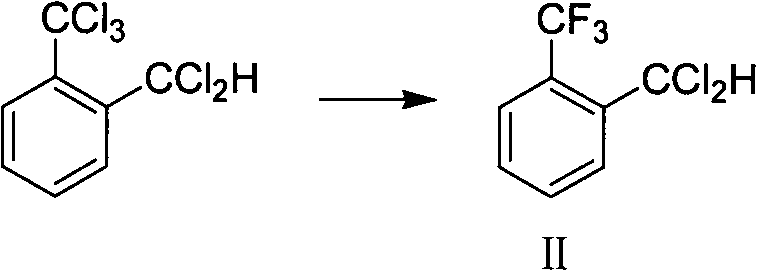
Method for preparing o-trifluoromethyl benzaldehyde
ActiveCN104016840AEase of industrial productionLow costCarbonyl compound preparation by hydrolysisBenzaldehydeWastewater
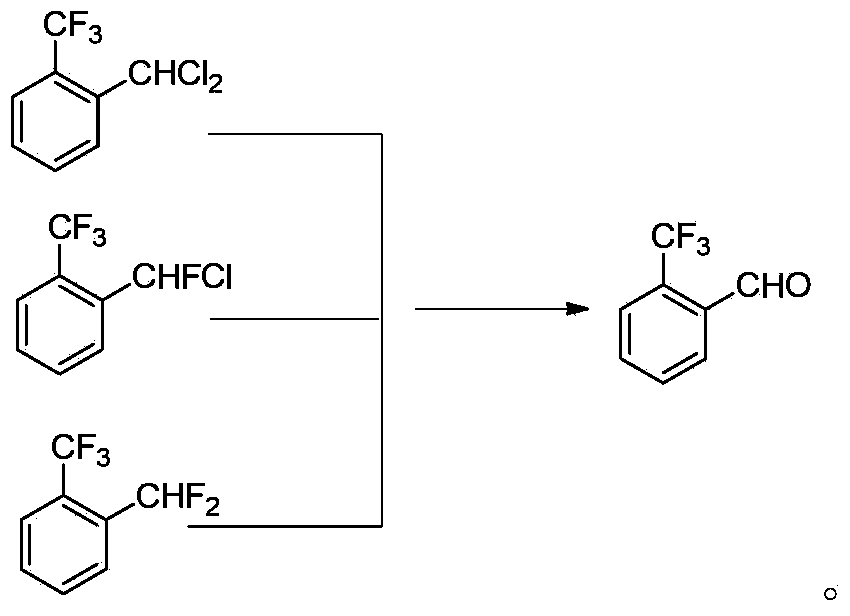
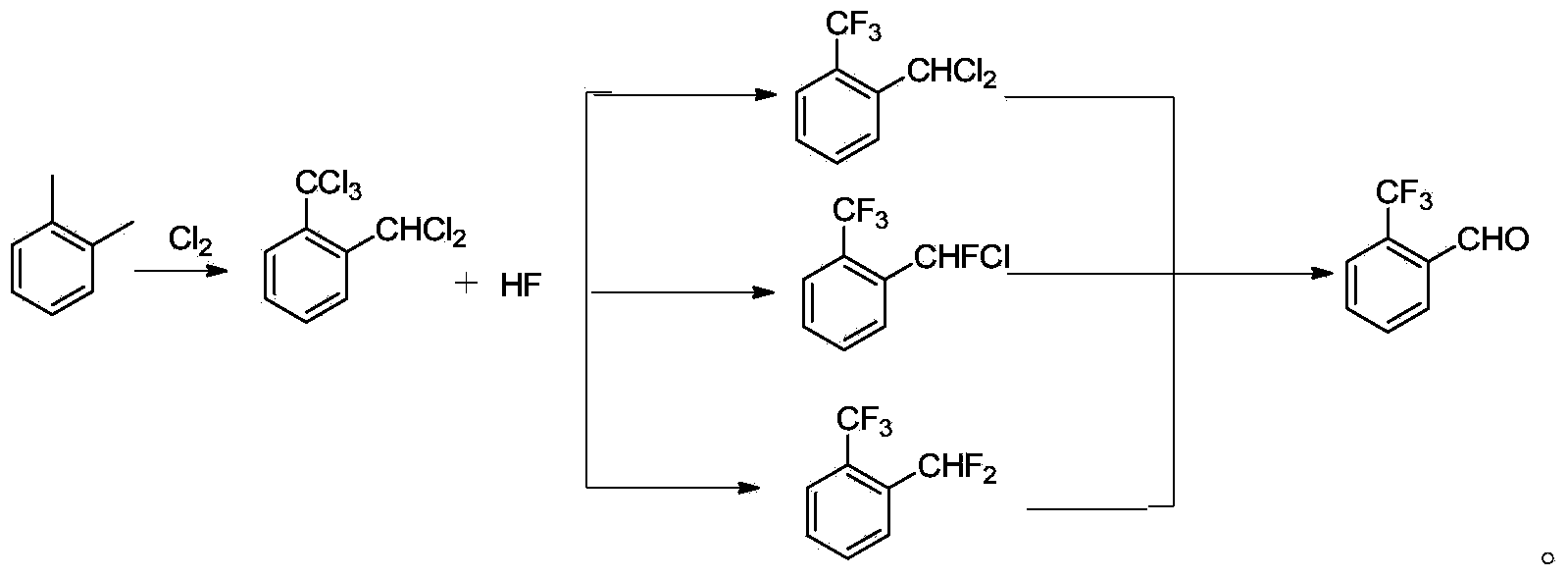
The invention discloses a method for preparing o-trifluoromethyl benzaldehyde. The method for preparing the o-trifluoromethyl benzaldehyde, disclosed by the invention, comprises the following steps: in the presence of a catalyst, carrying out hydrolysis reaction on a mixture of o-trifluoromethyl methylbenzene bichloride, o-trifluoromethyl chloro-fluoro-methylbenzene and o-trifluoromethyl methylbenzene difluoride and water at the temperature of 80-150 DEG C to obtain, wherein the mass of the catalyst accounts for 0.01-10% of the mass of the mixture. The method disclosed by the invention is cheap and easily available in raw materials, low in cost, little in wastewater, low in energy consumption and simple in operation and can be suitable for industrial production. The structural formula of the o-trifluoromethyl benzaldehyde is shown in the specification.
Owner:LIAONING TIANYU CHEM +2
Green method of synthessing benzaldehyde from near critical water
InactiveCN1597653ASolve the pollution problemImprove responseCarbonyl compound preparation by hydrolysisBulk chemical productionBenzaldehydeHigh pressure
A process for synthesizing benzaldehyde in near-supercritical water without environmental pollution nicludes such steps as adding deionized water to high-pressure agitated reactor, deoxidizing, heating, adding laurel oil and solubilizer, reaction and vacuum distilling.
Owner:ZHEJIANG UNIV
Process for regenerating catalyst
InactiveUS7612007B2Good choiceLong durationOrganic compound preparationOther chemical processesGas phaseCatalytic oxidation
A process for regenerating a catalyst consisting of a mixed oxide having molybdenum, bismuth and iron used for preparing an unsaturated aldehyde and / or an unsaturated carboxylic acid by catalytically oxidizing propylene, isobutylene and / or tert.-butanol with molecular oxygen in a gas phase, in which the catalyst is regenerated by thermally treating the deteriorated catalyst in an atmosphere of a gas containing molecular oxygen at a temperature of 200 to 500° C., and then thermally treating the catalyst in the presence of a reducing compound at a temperature of 200 to 500° C.
Owner:SUMITOMO CHEM CO LTD
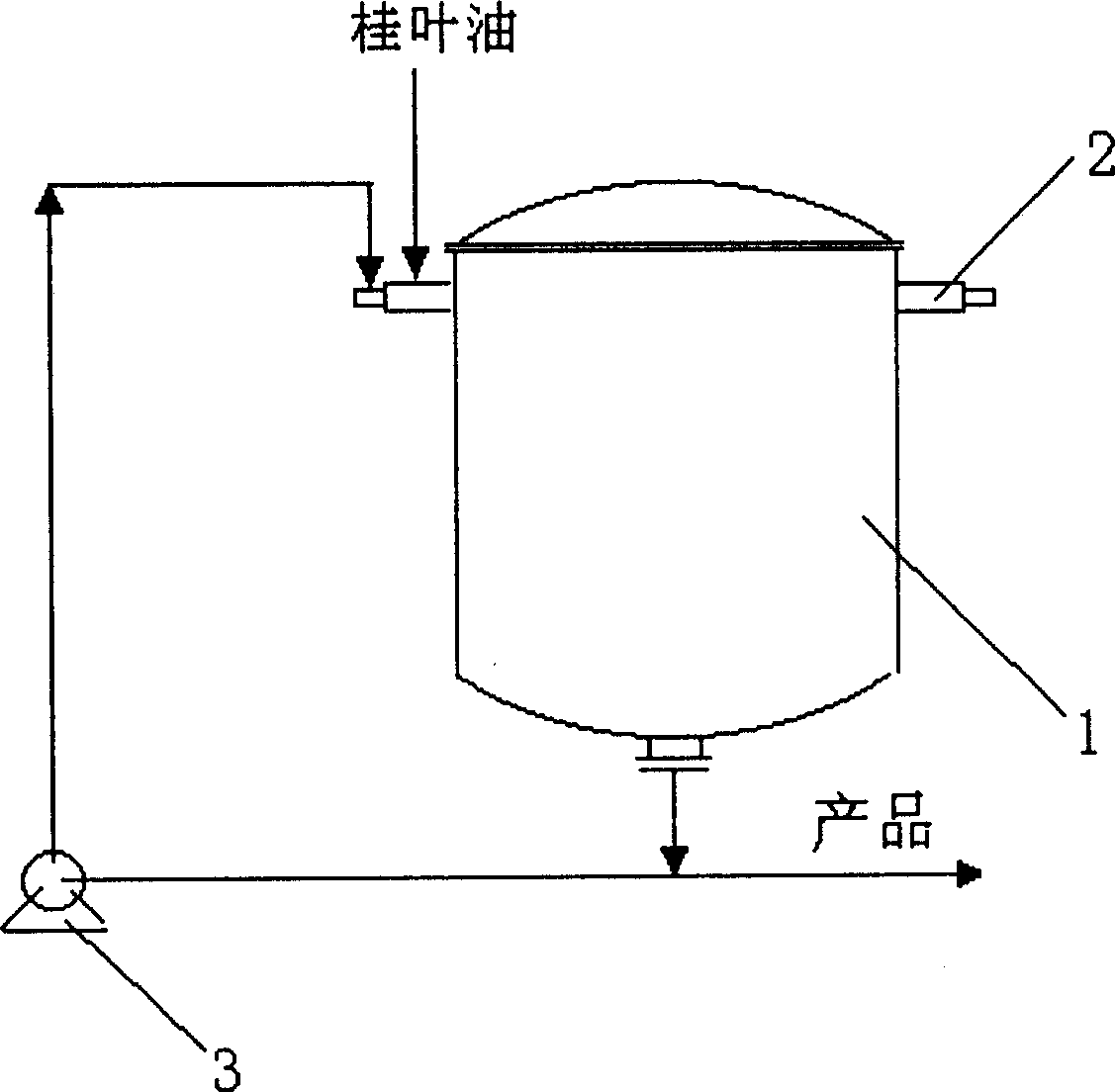
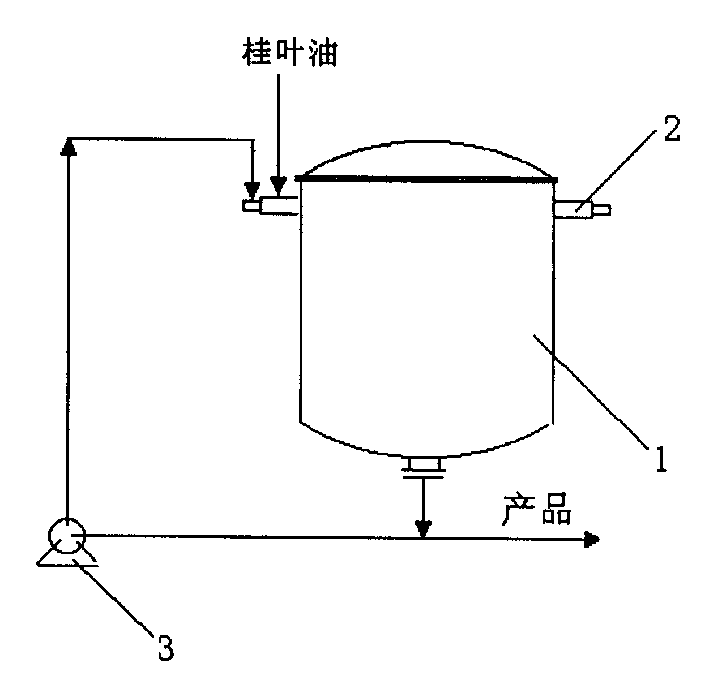
New method for preparing natural benzaldehyde by hydrolyzing bay leaves oil
InactiveCN1446789AHigh yieldFacilitate large-scale industrial productionCarbonyl compound preparation by hydrolysisBenzaldehydeAqueous solution
A process for preparing natural benzaldehyde by hydrolyzing cinnamon leaf oil includes respectively atomizing the cinnamon leaf oil and the aqueous solution of alkaline substance while spraying them in reactor, reacting to generate benzaldehyde, and collecting benzaldehyde from the resultant. Its advantage is high output rate.
Owner:SHANGHAI HUASHENG FLAVORING
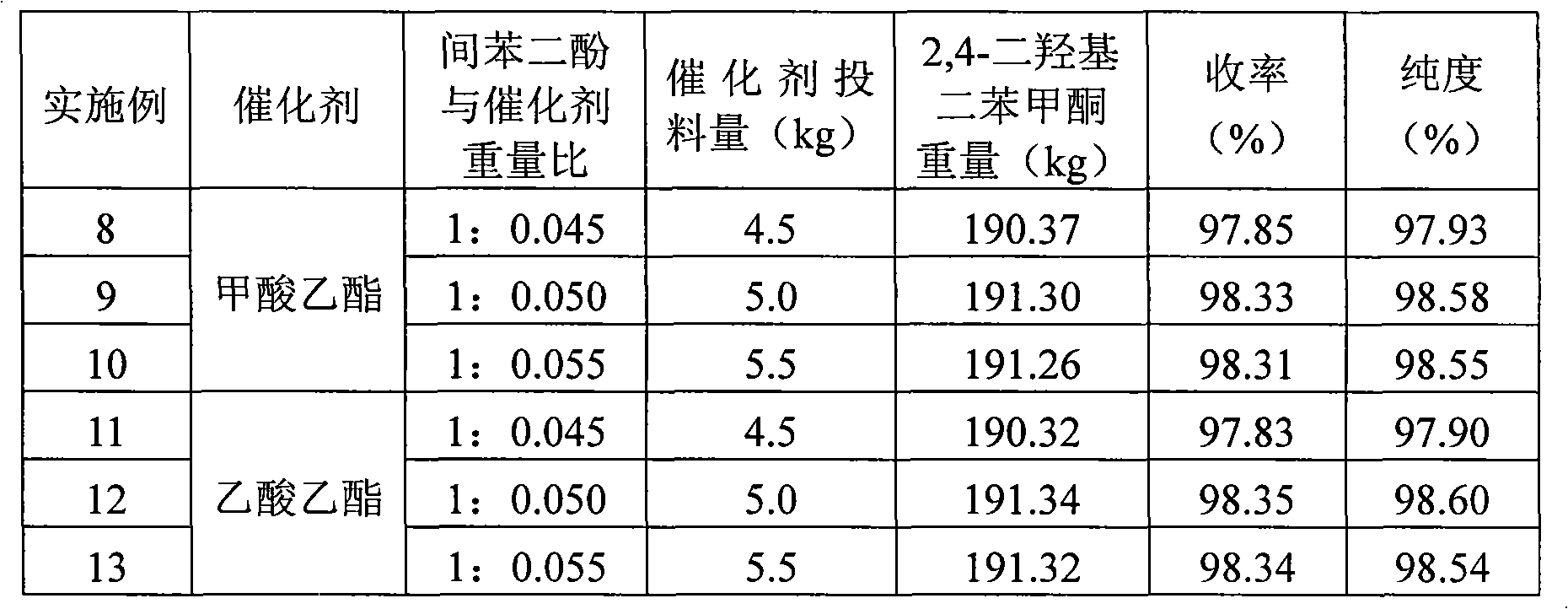
2,4-dihydroxyldiphenylketone preparation method
InactiveCN101628865ACarbonyl compound preparation by hydrolysisReaction temperatureWater temperature
A 2,4-dihydroxyldiphenylketone preparation method which adopts resorcinol, trichlorotoluene, ethanol, methanol and water as main raw materials comprises the following steps: adding water in a reaction tank, heating water with steam to 35-38 DEG C, then adding resorcinol in the reaction tank, starting stirring to dissolve resorcinol completely, then adding ethanol and methanol in the reaction tank, controlling the quantity of steam to heat the materials to 50-60 DEG C, adding trichlorotoluene to react for 3-4h while controlling the material reaction temperature to 45-55 DEG C and keeping the temperature, cooling feed liquid to reduce the material temperature to 20-30 DEG C and finally obtaining 2,4-dihydroxyldiphenylketone by centrifugal separation. The method realizes that the yield is 99% and the product purity can reach more than 98%.
Owner:宜都市华阳化工有限责任公司
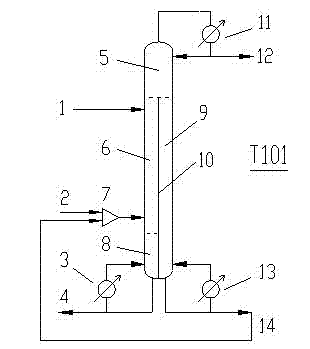
Continuous reactive distillation equipment for synthesising methoxylamine hydrochloride and process thereof
InactiveCN103113257AReduce production energy consumptionReduce material consumptionChemical industryCarbonyl compound preparation by hydrolysisGas phaseHydrolysis
The invention provides continuous reactive distillation equipment for synthesising methoxylamine hydrochloride by taking oxime methyl ether and hydrochloric acid as raw materials, and a process thereof, wherein a vertical clapboard is arranged in a reactive distillation dividing-wall column in the continuous reactive distillation equipment provided by the invention, and used for dividing the column into four areas, namely, a common distillation section, a reaction section, a stripping section and a lateral stripping section; and oxime methyl ether and hydrochloric acid solution enter from the upper part and the lower part of the reactive distillation section respectively, hydrolysis reaction for oxime methyl ether is performed in the reaction section, the generated methoxylamine hydrochloride is discharged from the bottom of the reactive distillation section, the generated by-product, namely, the mixture of acetone and oxime methyl ether, enters in the common distillation section in the form of a gaseous phase to be separated, more than 95% acetone is obtained on the top of the column, and the separated oxime methyl ether is recycled. According to the continuous reactive distillation equipment and the process provided by the invention, the dividing-wall column is combined with reactive distillation, and hydrolysis reaction and product separation are intensively performed in one column, thus effectively reducing energy consumption and equipment investment cost.
Owner:FUZHOU UNIV
Method for preparing 3,3-dimethyl butyraldehyde
InactiveCN104130115AReduce manufacturing costShort production processOrganic compound preparationCarboxylic acid esters preparationDistillationTert-Butyl chloride
The invention relates to a method for preparing 3,3-dimethyl butyraldehyde. The method comprises the following steps: (1) vacuumizing a closed reaction kettle, adding a dichloromethane solvent, dripping vinyl acetate, introducing a catalyst after dripping is ended, and introducing hydrogen chloride gas, so that the pressure reaches 0-1MPa, slowly dripping tert butyl chloride for reacting, removing excessive hydrogen chloride gas after the reaction is ended to obtain a distillation product, raising the temperature and distilling to obtain 1-chloro-3,3-dimethyl butyl acetate; and (2) adding the 1-chloro-3,3-dimethyl butyl acetate and water into the reaction kettle, controlling the temperature to be 120 DEG C, performing hydrolysis disproportionation on the 1-chloro-3,3-dimethyl butyl acetate to obtain a hydrolysis disproportionation reaction mixture containing 3,3-dimethyl butyraldehyde, and distilling and purifying to obtain the 3,3-dimethyl butyraldehyde. According to the method disclosed by the invention, the production cost is low, the production process is shortened, the reaction conditions are mild, the production safety is high, and the preparation method is simple in preparation process, easy to control, high in yield and safe.
Owner:SHANDONG BENYUE BIOTECH
Synthetic method of p-chlorobenzaldehyde
InactiveCN104447251AGood choiceHigh yieldCarbonyl compound preparation by hydrolysisHalogenated hydrocarbon preparationChlorobenzeneP-chlorobenzaldehyde
The invention discloses a synthetic method of p-chlorobenzaldehyde. The synthetic method comprises the following steps: performing illumination and chlorine reaction on p-chlorotoluene in the presence of a phosphorus trichloride initiator to generate p-chlorobenzal chloride, refining, and then performing catalytic hydrolysis for 6-12 hours to obtain p-chlorobenzaldehyde. The synthetic method of p-chlorobenzaldehyde, disclosed by the invention, is good in selectivity and high in yield.
Owner:CHANGSHU XINHUA CHEM
Preparation method of 2,4-dichloro-5-fluorobenzoyl chloride
ActiveCN107118096AEmission reductionBreak through the technical bottleneck of conversionOrganic compound preparationCarbonyl compound preparation by hydrolysisBiopolymerOrganic synthesis
The invention discloses a preparation method of 2,4-dichloro-5-fluorobenzoyl chloride and belongs to the field of organic synthesis. The preparation method comprises the following steps: with 2,4-dichlorofluorbenzene as a raw material, performing friedel-crafts reaction and hydrolyzation to generate an intermediate, 2,4-dichloro-5-fluorobenzoyl chloride; and hydrolyzing, oxidizing and acylating a byproduct, biopolymer (III), generated by the reaction to obtain a final compound (II), wherein the total yield is 88% or above. According to the invention, the raw material conversation ratio is 80% or above, the defects that existing raw materials are hardly available, and the utilization ratio is low are overcome, resources are saved, the production cost is lowered, and the preparation method is simple to operate and easy for amplified production.
Owner:HEADING NANJING PHARMTECH CO LTD
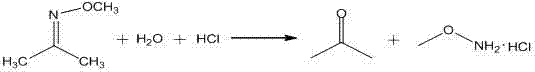
Process for preparing hydroxylamine hydrochloride by adopting oxime acidolysis method
InactiveCN107522181AIncrease profitReduce manufacturing costOrganic compound preparationHydroxy compound preparationAlcoholHydroxylamine Hydrochloride
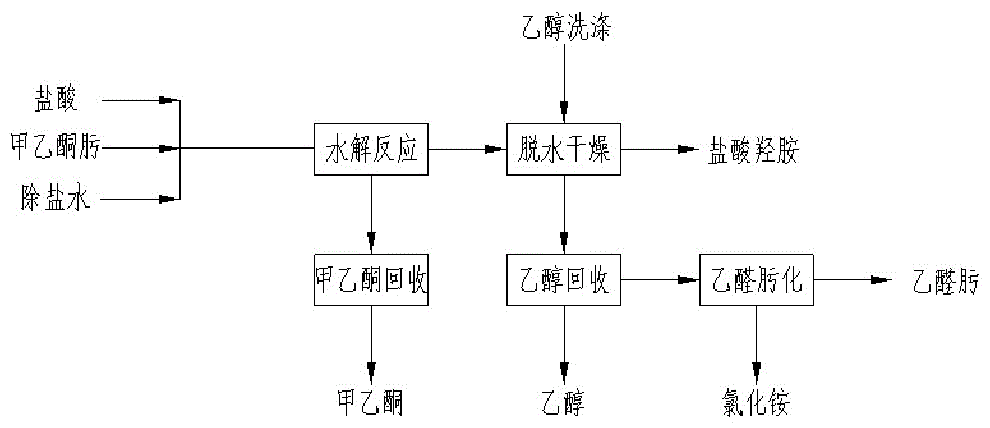
The invention belongs to the technical field of hydroxylamine hydrochloride, and discloses a process for preparing hydroxylamine hydrochloride by adopting an oxime acidolysis method. The method comprises the following steps: a) a raw material cache configuration process; b) an acidolysis reaction process; c) a ketoxime rectification process; d) a dehydration and drying process; e) an alcohol recycling process; and f) an acetaldehyde oximation process. By adopting the process, the process route and technical parameters of hydroxylamine hydrochloride production by methyl ethyl ketone acidolysis are optimized, a pervaporation coupling technology is applied, the product yield and quality can be improved, further improvement of the industrial technology is promoted, related industries can be driven to develop, and resource reasonable utilization can be realized.
Owner:临沭县华盛化工有限公司
Catalytic hydrolysis method of circulation phase transition for preparing benzaldehyde and benzaldehyde containing substituent
A process for preparing benzaldehyde or substituent benzaldehyde (C6H(5-n)XnCHO) by cyclic hydrolysis method features the hydrolyzing reaction between benzyl bichloride or substituent benzyl bichloride, water and phase-transfer catalyst at 60-110 deg.C. The water phase after reaction can be cyclically used.
Owner:NANTONG TIANSHI CHEM
Preparation method of 2, 6-dichlorobenzaldehyde
ActiveCN103396301AReduce consumptionReduce generationCarbonyl compound preparation by hydrolysisChemical synthesisCatalytic effect
The invention provides a preparation method of 2, 6-dichlorobenzaldehyde and relates to the technical field of chemical synthesis production. The preparation method of the invention comprises the following steps of: performing a chlorination reaction on 2,6-dichlorotoluene and chlorine under a reaction condition of 50-250 DEG C in the catalytic effect of phosphorus pentachloride and light, and performing rectification to prepare 2,6-dichloro dchlorobenzyl; adding the 2,6-dichloro dchlorobenzyl, an acidic solvent and zinc chloride into a hydrolysis nitrilation kettle, and performing a hydrolysis reaction under a heating reflux condition to prepare the 2, 6-dichlorobenzaldehyde. The preparation method provided by the invention has the advantages of ensuring easy production control, little material consumption and low production cost, reducing the generation of solid waste and reducing the emission of nitrogen-containing nitroso group waste, thereby being an energy-saving, emission-reducing, environment-friendly, industrial, practical and clean production process.
Owner:永椿化工新材料有限公司
Method for extracting isoliquirtigenin from licorice
InactiveCN101328115ACarbonyl compound preparation by hydrolysisCarbonyl compound separation/purificationAcid hydrolysisGLYCYRRHIZA EXTRACT
The invention relates to a method for extracting isoliquiritigenin from iquorice. The method uses a property of flavonone which can be transformed into chalcone in the presence of alkali, an acid hydrolysis product of liquidritin is transformed into the isoliquiritigenin, thereby obviously improving the yield of extracting the isoliquiritigenin. With the method, the yield of extracting the isoliquiritigenin is 32 times of that of ethanol ultrasonic extraction, and 1.7 times of that of acid hydrolysis extraction.
Owner:SHIHEZI UNIVERSITY
Preparation method of 2,4-dyhydroxyl benzophenone
ActiveCN101830791AReduce the risk of contaminationReduce dosageCarbonyl compound preparation by hydrolysisBenzophenoneResorcinol
The invention relates to a preparation method of 2,4-dyhydroxyl benzophenone, which comprises the steps of: adding resorcinol, water and a catalyst in a reaction container, wherein the catalyst is an ester compound; dripping benzotrichloride under the stirring condition at the temperature of 38-45 DEG C according to a certain proportion at a certain dropping speed, and fully reacting by preserving the temperature at 42-45 DEG C; washing a reactant until the pH value is 6.0-7.0; and centrifuging and drying to obtain the 2,4-dyhydroxyl benzophenone. The method has simple process, safe and convenient operation, less environment pollution, low cost and high product yield and purity.
Owner:HUBEI MEIKAI CHEM
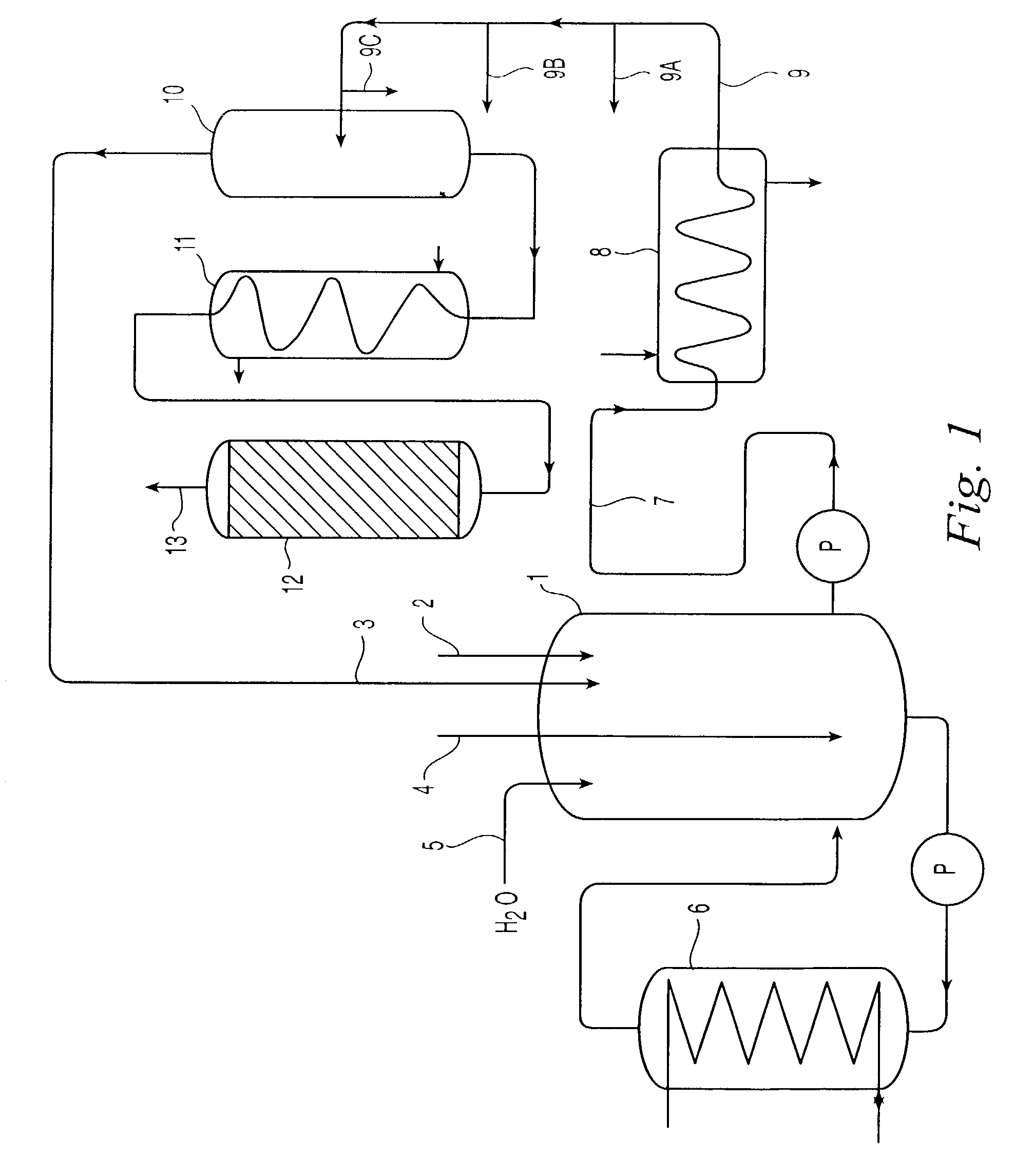
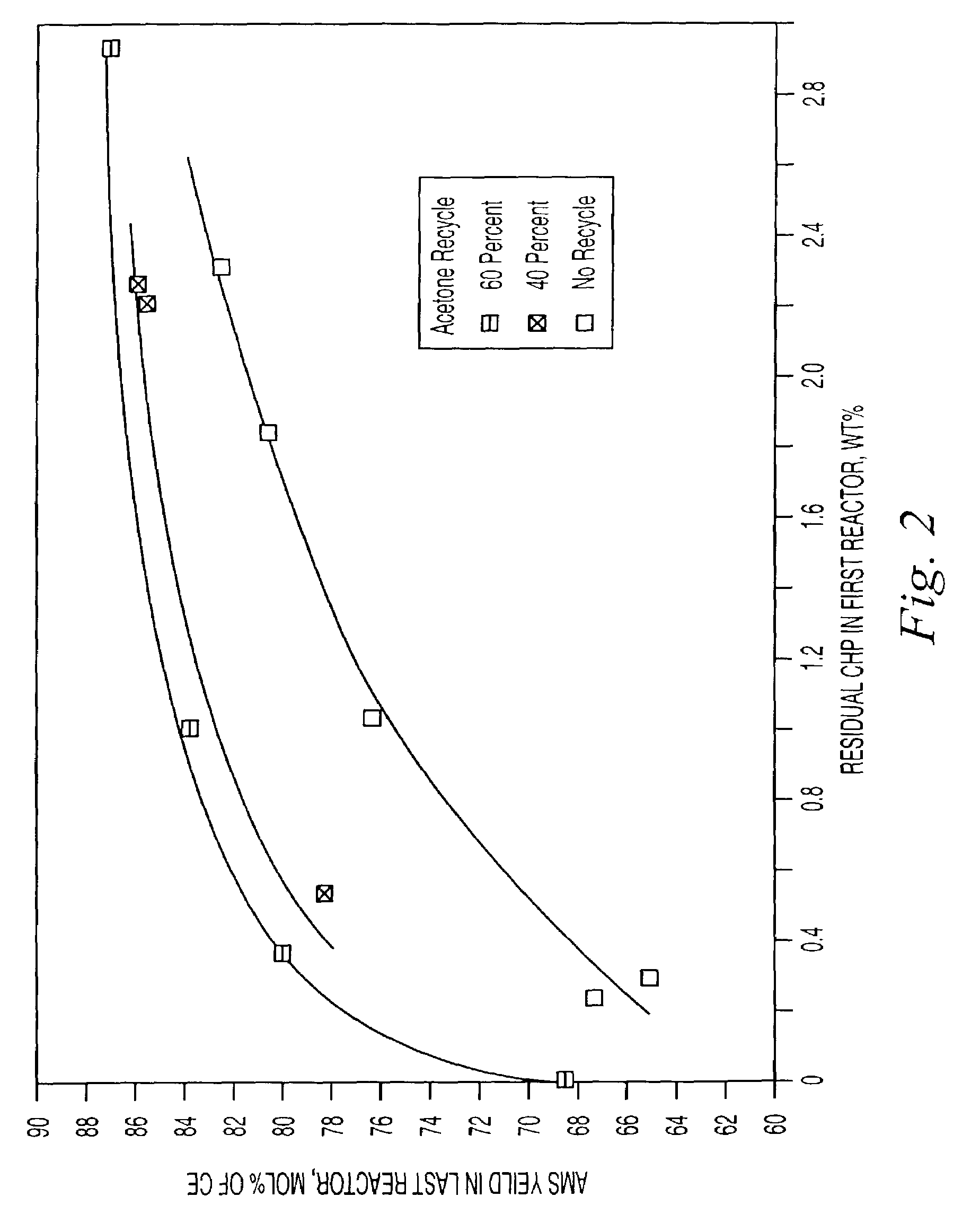
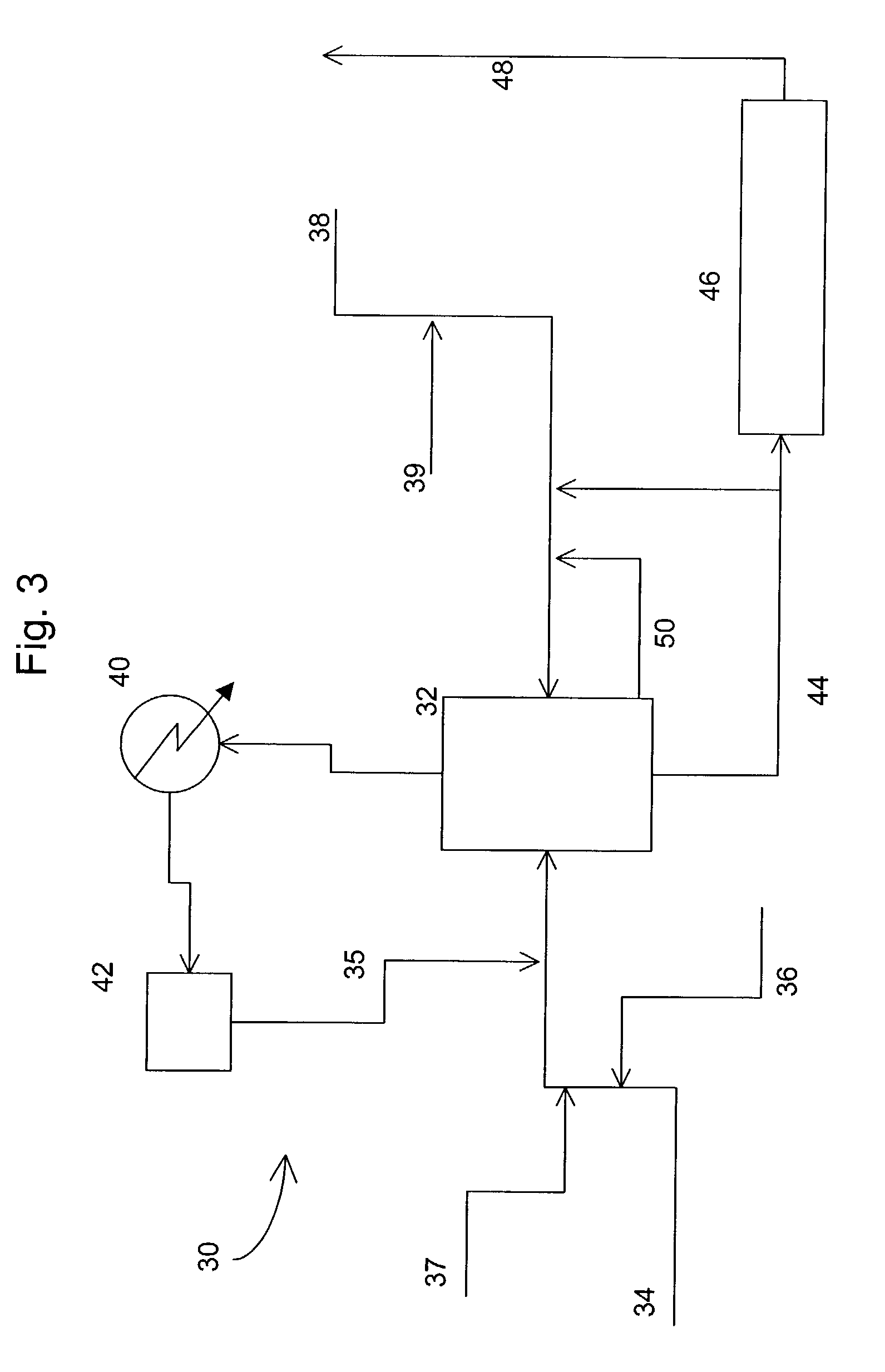
Decomposition of cumene oxidation product
InactiveUS7166752B2Stable and economical mannerHigh yieldOrganic compound preparationHydrocarbon from oxygen organic compoundsPhenolCumene
A process for decomposing a cumene oxidation product mixture containing cumene hydroperoxide (CHP) and dimethylphenyl carbinol (DMPC) to produce phenol, acetone and alpha-methyl styrene (AMS) with enhanced safety of operation and reduced by-product formation which comprises the steps: mixing the cumene oxidation product in a stirred or back-mixed reactor with an acid catalyst, with 10 to 100 percent acetone relative to the amount of acetone produced during the decomposition reaction, and with up to 4 weight percent additional amounts of water relative to the reaction mixture, at an average temperature between about 50° C. and about 90° C. for a time sufficient to lower the average CHP concentration of the reactor to between about 0.2 and about 3.0 weight percent, and wherein a portion of DMPC is converted to dicumyl peroxide (DCP); then reacting the reaction mixture from step (a) at a temperature between about 120° C. and 150° C. under plug-flow conditions for a time sufficient to decompose substantially all residual CHP and at least 90 percent of the DCP formed in step (a).
Owner:ADVANSIX RESINS & CHEM LLC
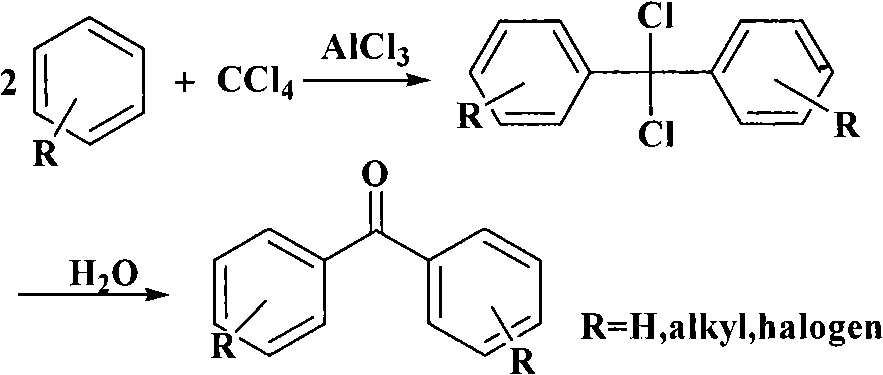
Process for synthesizing 4,4'-dihydroxy diphenylketone
InactiveCN101270038ARealize resource utilizationReduce manufacturing costCarbonyl compound preparation by hydrolysisChemical recyclingIce waterResource utilization
The present invention provides a synthetic method of 4, 4'-dihydroxy benzophenone (I). In the method, carbon tetrachloride and phenol are used as raw materials and react for 3 to 20 hours at the temperature of 80 to 200 DEG C, with solid superacid catalytic, then the mixture is cooled to be at the room temperature, an appropriate amount of ice water is added for mixing and hydrolysis for 20 to 60 minutes; after the hydrolysis reaction, the reaction solution is separated and purified to prepare the 4, 4'-dihydroxy benzophenone; the formula of the solid super acidic catalyst can be one of the following: SO4<2-> / Fe2O3, SO4<2-> / Al2O3, SO4<2-> / SnO2, and SO4<2-> / Sb2O3. The method has the following beneficial effects: the carbon tetrachloride is used as the raw material to synthesize the 4, 4'-dihydroxy benzophenone, which realizes the resource utilization of the carbon tetrachloride and reduces the production cost; the solid super acidic catalyst can be easily treated and stored and has no corrosion to the equipment; the catalyst can be separated from the reaction mixture after the reaction is completed; and the catalyst can be recycled and has little pollution.
Owner:ZHEJIANG UNIV OF TECH
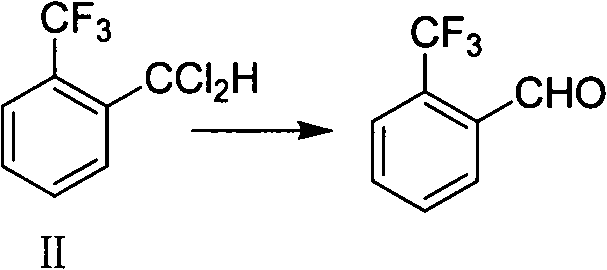
Preparation methods of 2-(trifluoromethyl)benzaldehyde and intermediate thereof
ActiveCN102516047ASimple processRaw materials are easy to getPreparation by halogen replacementCarbonyl compound preparation by hydrolysisHydrogen fluorideBenzaldehyde
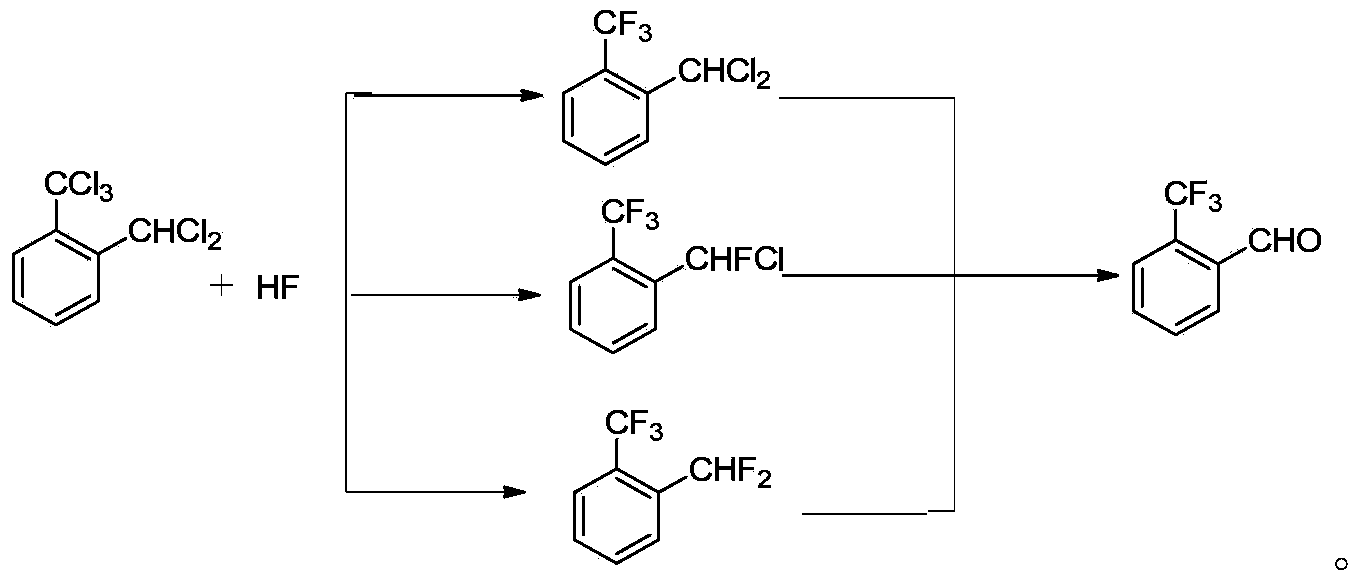
The invention discloses a preparation method of 2-(trifluoromethyl)benzaldehyde, which comprises the following step: under the action of C1-C4 linear chain saturated monobasic fatty acid and C1-C4 linear chain saturated monobasic fatty acid salt of alkali metal, hydrolyzing 2-(trifluoromethyl)dichlorotoluene with water at a temperature of 150-190 DEG C and under a pressure of 0.3-0.78MPa. The invention also discloses a preparation method of an intermediate of 2-(trifluoromethyl)benzaldehyde, which comprises the following steps: performing selective fluoridation of 2-(trifluoromethyl)dichlorotoluene and hydrogen fluoride under the catalysis of antimony halidesantimony halides, wherein the molar concentration is 2-6 times of that of 2-(trifluoromethyl)dichlorotoluene, and the temperature of selective fluoridation is 60-110 DEG C. The preparation methods disclosed by the invention is simple in operation, low in cost, environment-friendly, and high in yield and purity, and can be better applicable for industrial production.
Owner:LIANHE CHEM TECH +2
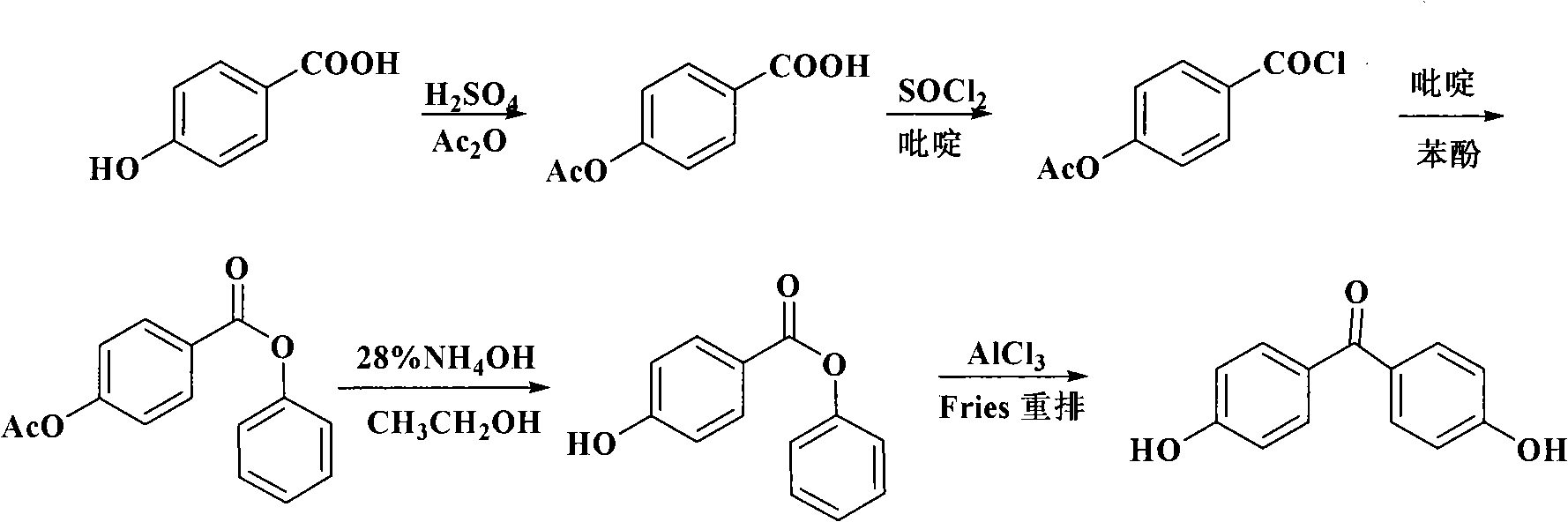
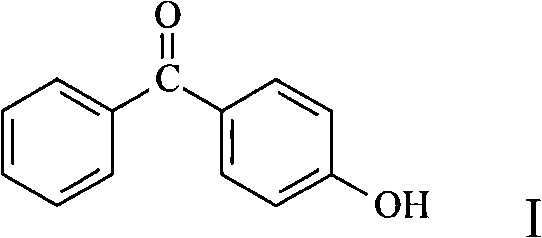
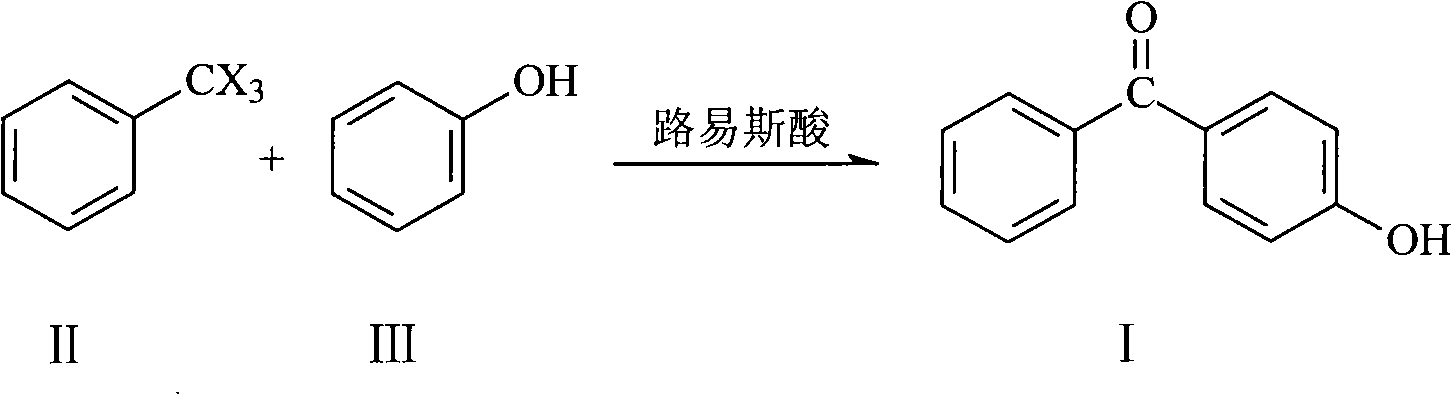
Preparation of 4-hydroxy benzophenone
InactiveCN101298414ASimple stepsOrganic compound preparationCarbonyl compound preparation by hydrolysisMethyl benzeneBenzophenone
The invention relates to a preparation method of 4-hydroxybenzophenone. The preparation method mainly includes the step of: preparing a target compound by using trihalo-methyl benzene to react with phenol by Friedel-Crafts. The product yield by adopting the preparation method of the 4-hydroxybenzophenone of the invention can reach 90 percent; besides, the preparation method of the 4-hydroxybenzophenone also has the advantages of simple steps and low operation cost, etc; the invention is a method which is easy for scale commercial preparation.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Process for regenerating catalyst for preparing unsaturated aldehyde and/or unsaturated carboxylic acid and process for preparing unsaturated aldehyde and/or unsaturated carboxylic acid
InactiveUS20080004173A1Good choiceLong durationOther chemical processesOrganic compound preparationGas phaseCatalytic oxidation
A process for regenerating a catalyst consisting of a mixed oxide having molybdenum, bismuth and iron used for preparing an unsaturated aldehyde and / or an unsaturated carboxylic acid by catalytically oxidizing propylene, isobutylene and / or tert.-butanol with molecular oxygen in a gas phase, in which the catalyst is regenerated by thermally treating the deteriorated catalyst in an atmosphere of a gas containing molecular oxygen at a temperature of 200 to 500° C., and then thermally treating the catalyst in the presence of a reducing compound at a temperature of 200 to 500° C.
Owner:SUMITOMO CHEM CO LTD
Method for synthesizing pentanone-2 through Bayer-Vlieger oxidation reaction
InactiveCN101811948AThe process is easy to getSimple processCarbonyl compound preparation by hydrolysisSodium acetatePersonal care
The invention relates to a method for synthesizing pentanone-2 through a Bayer-Vlieger oxidation reaction. The pentanone-2 is prepared by using propionaldehyde as a raw material, performing alkaline condensation and the Bayer-Vlieger oxidation reaction on the propionaldehyde, and finally performing hydrolysis under an acid condition. The method comprises the following process steps of: (1) condensing the propionaldehyde at the temperature of 30 to 40 DEG C under the action of solution of NaOH to obtain 2-methyl-2-pentenal; (2) preparing buffer solution of acetic acid and sodium acetate from the product obtained in the step (1) by taking the acetic acid as solvent in the presence of the sodium acetate, controlling the pH of a reaction system to be between 3.7 and 6, and at the temperature of 10 to 30 DEG C, using peroxyacetic acid to perform B-V oxidation so as to obtain formic acid-2-amylene-2-alcohol ester; and (3) using H2SO4 aqueous solution to reflux and hydrolyze the product obtained in the step (2) under an acidic condition to prepare the pentanone-2. The quality of the pentanone-2 meets the requirements of preparing a flavoring essence and an essence for household and personal care chemicals. The method has the advantages of readily available raw materials, simple process, high yield, single product component, easy refining, high product quality, low cost and suitability for industrial production.
Owner:WUTONG AROMA CHEM CO LTD
Popular searches
Carboxylic compound preparation Ketenes preparation Ether preparation Graphite Organic-compounds/hydrides/coordination-complexes catalysts Catalyst activation/preparation Carbonyl compound preparation by oxidation Chemical/physical/physico-chemical stationary reactors Functional group formation/introduction Hydropoly/poly sulfide preparation
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com