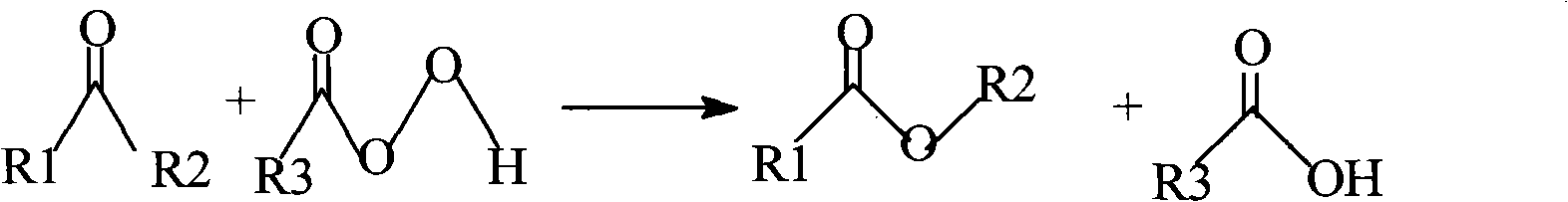
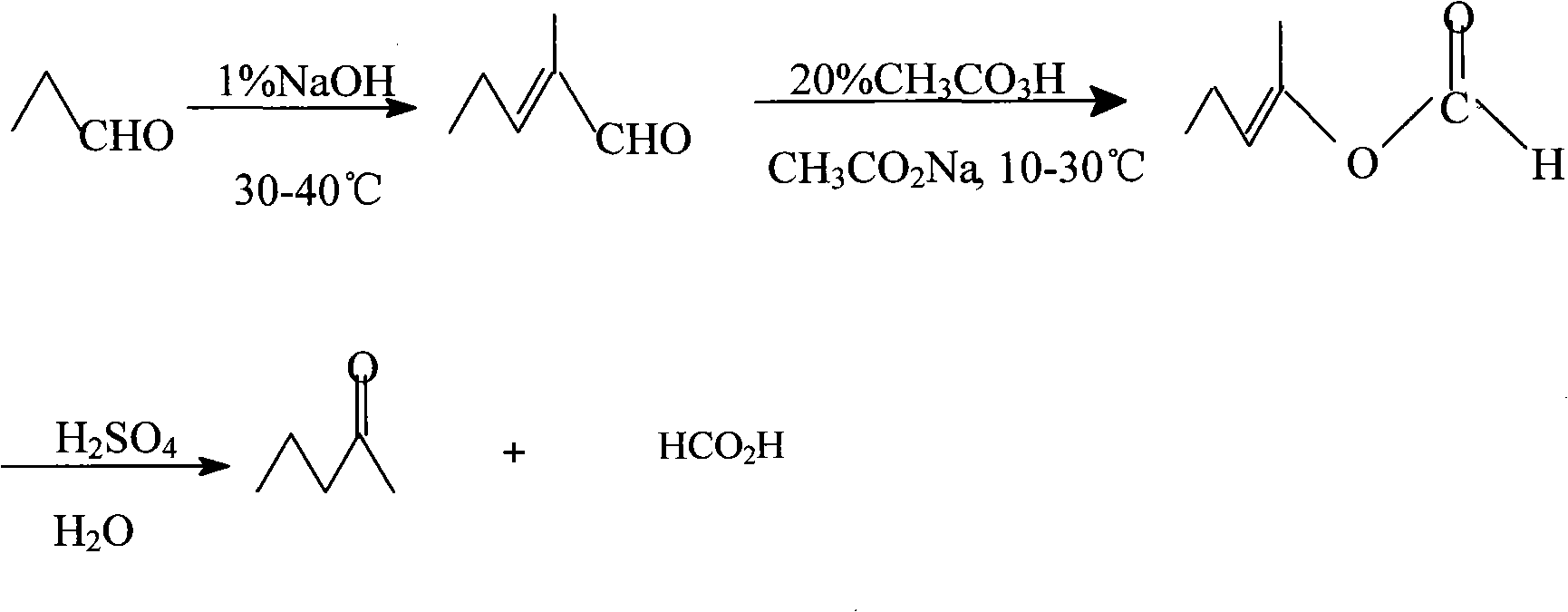Method for synthesizing pentanone-2 through Bayer-Vlieger oxidation reaction
A technology of oxidation reaction and pentanone, which is applied in the direction of hydrolysis to prepare carbonyl compounds, etc., can solve the problem of low yield of formate, and achieve the effect of single product composition, easy refining, and good product quality
Inactive Publication Date: 2010-08-25
WUTONG AROMA CHEM CO LTD
View PDF1 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Ketones can smoothly generate esters or lactones when B-V reactions occur. When aldehydes undergo B-V reactions, due to the easy oxidation of aldehyde groups, the main product is that the aldehyde group is oxidized to carboxylic acid, which is oxidized to form formate. very low rate
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a method for synthesizing pentanone-2 through a Bayer-Vlieger oxidation reaction. The pentanone-2 is prepared by using propionaldehyde as a raw material, performing alkaline condensation and the Bayer-Vlieger oxidation reaction on the propionaldehyde, and finally performing hydrolysis under an acid condition. The method comprises the following process steps of: (1) condensing the propionaldehyde at the temperature of 30 to 40 DEG C under the action of solution of NaOH to obtain 2-methyl-2-pentenal; (2) preparing buffer solution of acetic acid and sodium acetate from the product obtained in the step (1) by taking the acetic acid as solvent in the presence of the sodium acetate, controlling the pH of a reaction system to be between 3.7 and 6, and at the temperature of 10 to 30 DEG C, using peroxyacetic acid to perform B-V oxidation so as to obtain formic acid-2-amylene-2-alcohol ester; and (3) using H2SO4 aqueous solution to reflux and hydrolyze the product obtained in the step (2) under an acidic condition to prepare the pentanone-2. The quality of the pentanone-2 meets the requirements of preparing a flavoring essence and an essence for household and personal care chemicals. The method has the advantages of readily available raw materials, simple process, high yield, single product component, easy refining, high product quality, low cost and suitability for industrial production.
Description
technical field The invention belongs to the technical field of spice organic synthesis, and relates to a method for synthesizing pentanone-2, in particular to a Bayer-Villiger reaction (Baeyer-villiger, B-V reaction) for synthesizing pentanone-2 using a peroxygen reagent as an oxidant. 2 new methods. Background technique Pentanone-2 is an important aroma raw material, which has a fresh and elegant ether aroma, sweet aroma, fermented cheese and banana fruit-like aroma. It can be widely used in cheddar cheese, milk, banana, jackfruit and other fruity food flavors, and can also be used in various fruity daily chemical flavors. The preparation of pentanone-2 is reported in the literature by dry distillation of calcium acetate and calcium butyrate (Annalen, 108, 124, 1858), or by oxidation of sodium n-hexanoate or n-hexanoic acid with hydrogen peroxide, but these The composition of the finished product prepared by the method is complex, and the product is difficult to purify....
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C49/04C07C45/42
Inventor 舒宏福卫洁丁涛
Owner WUTONG AROMA CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com