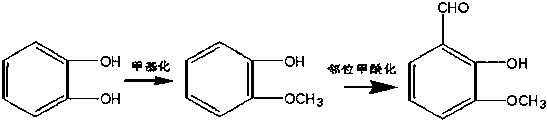
Method for in situ preparing o-vanillin by taking pyrocatechol as raw material
A technology of ortho-vanillin and catechol, which is applied in the field of preparation of pharmaceutical raw materials, can solve the problems of restricting the large-scale production of berberine drugs, the difficulty of separating isomer mixtures, and the lack of product quality assurance, and achieve economic benefits and Significant social benefits, overcoming production constraints, and cheap prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0026] (1) According to the mass ratio of 1:4, weigh catechol and sodium hydroxide, put them into a 500ml three-necked flask, add catalysts (alcohols), use methanol as a solvent, heat up and stir until the solid dissolves, and at 65°C, Add 2-chloromethane dropwise, keep warm for 3 hours, filter, concentrate under reduced pressure to recover the solvent, extract with cyclohexane, and distill off methanol to obtain the product o-methoxyphenol.
[0027] (2) Using acetonitrile as solvent, weigh 18g o-methoxyphenol, pour it into a 500ml three-neck flask, add catalyst (Lewis acid), heat up and stir to 65°C, add triethylamine dropwise, add paraformaldehyde, slowly Raise the temperature to reflux, react for 1 h, drop to room temperature and add hydrochloric acid to adjust the pH value to obtain the ortho-vanillin reaction solution.
[0028] (3) Sodium chloride was added to the reaction solution, the solution was layered, and chromatographically separated to obtain a mixture of acetoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com