Cloning and application of a new hydroxylase (cytochrome P450) gene of amycolatopsis sp.CGMCC1149
A technology of hydroxylase and amino acid, which is applied in application, genetic engineering, oxidoreductase, etc., can solve the problems of bacteria poisoning, low expression level, limiting the production of statins in Wuxi, etc., and achieve the effect of overcoming the toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: Construction of expression plasmid
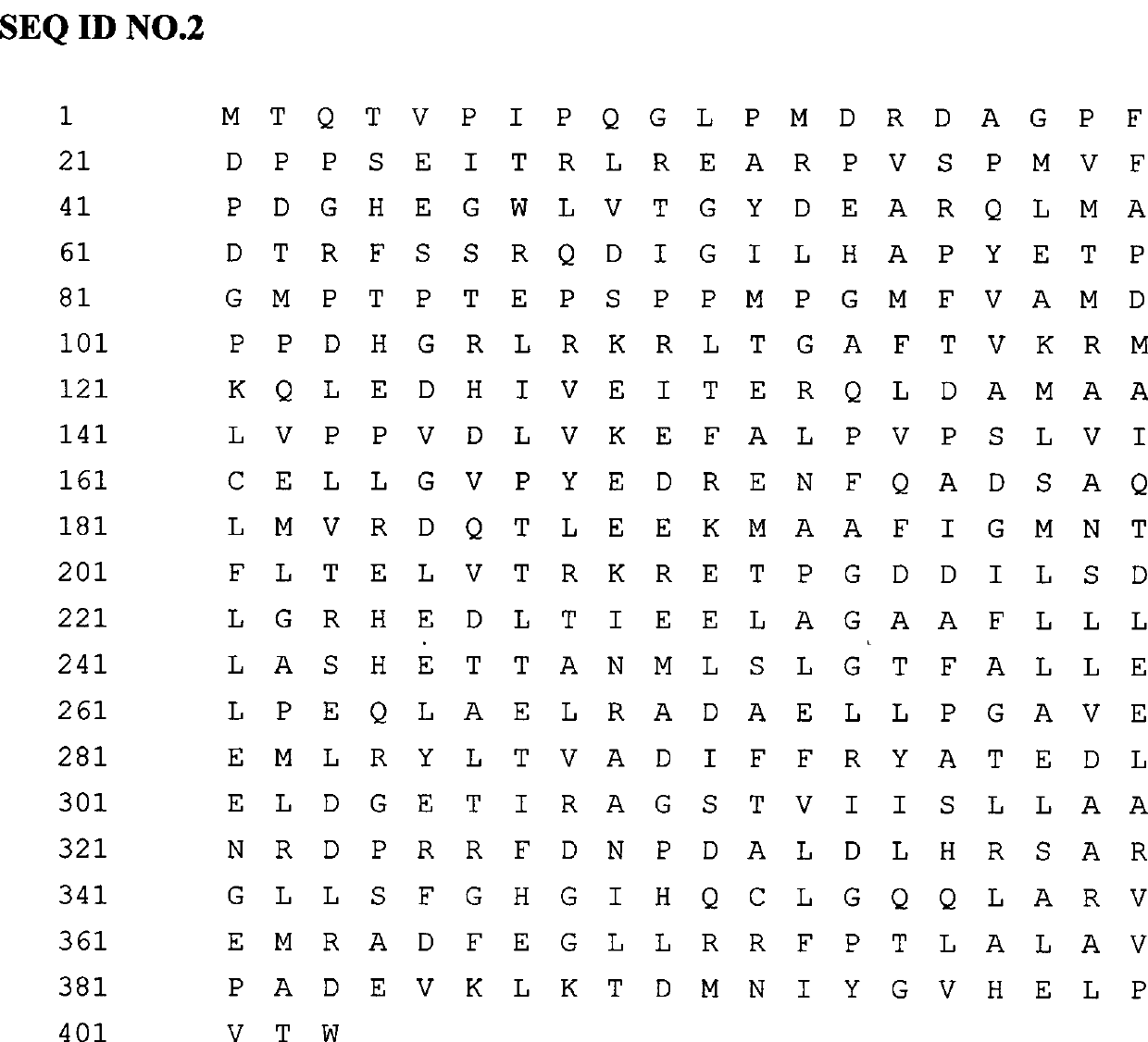
[0015] Design a pair of primers according to the nucleotide sequence of SEQIDNO.1
[0016] P-F: 5′-GAC GAATTC ATGACCCAGACGGTTCC-3';
[0017] P-R: 5′-TAG AAGCTT TTACCAGGTGACCGGCAG-3';
[0018] In order to facilitate the connection of the PCR fragment to the plasmid pEtac, EcoRI and HindIII restriction sites were introduced at the 5' ends of the two primers, respectively, and the underline "_" indicated the introduced restriction sites.
[0019] The PCR program was: 94°C pre-denaturation for 5 min; 94°C denaturation for 1 min, 65°C annealing for 1 min, 72°C extension for 2 min, 30 cycles; 72°C extension for 10 min. After the product was recovered, it was connected to the T vector, transformed into E.coliJM109, and positive transformants were screened using LB resistance plates (with a final concentration of 50ug / mL kanamycin), the plasmid was extracted, and the correctness of the plasmid was verified. The plasmid an...
Embodiment 2
[0020] Example 2: Induced expression of cytochrome P450
[0021] Insert the E.coliDH5α(pEtac-p450) transformant into LB (containing a final concentration of 50ug / mL kanamycin) medium, and culture at 37°C until OD 600 About 0.4-0.6, add IPTG to a final concentration of 0.1 mM, and continue to induce culture at 25°C for 8 hours. Collect the cells by centrifugation, wash the cells twice with 1×TE (pH8.0) buffer, wash the cells with an appropriate amount of wall-breaking buffer (50mM Tris-HCl buffer (pH7.4), containing 20% glycerol, 2mMDTT, 0.1mM EDTA) Suspend the bacteria and perform ultrasonic crushing. The crushing conditions are: power 300W, working for 1s, interval of 3s, and total time of 10min. Centrifuge at 10,000 r / min at 4°C for 15 minutes, collect the supernatant as crude cytochrome P450 enzyme solution, and obtain pure cytochrome P450 after purification.
Embodiment 3
[0022] Example 3: Establishment of lovastatin in vitro hydroxylation system
[0023] In order to overcome the unfavorable factors such as the relatively low expression level of CYP in Amycolatopsissp. and the toxic effect of lovastatin on the bacteria and to efficiently apply CYP, an in vitro hydroxylation system of lovastatin was established for the in vitro hydroxylation product I of lovastatin synthesis. The reaction system is: a total volume of 200 μL, pure CYP, 0.26 mM NADH, ferredoxin reductase (0.04 U), 320 μg ferredoxin, 0.23 mM lovastatin, 100 mMPBS buffer (pH 7.4) to make up 200 μL. React in a water bath at 30°C and 150r / min for 6h, terminate the reaction at 105°C for 5min, and detect the hydroxylated product I of lovastatin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com