Method for preparing intermediate of vitamin A, namely tetradecanal
A technology of cyclic citral and C-14, which is applied in the field of preparation of vitamin A intermediate tetradecanal, can solve the problems of difficult industrial production, expensive methyl iodide, etc., and achieves low cost, easy to obtain raw materials, and simple process route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of β-cyclic citral (V)
[0038] Dilute 3g KOH with 600ml methanol and add it to a 2500ml three-necked flask. Under mechanical stirring at room temperature, 304g (2mol) α-cyclic citral is added dropwise. After the water bath is kept for about 2 hours, the dripping is completed, and the stirring is continued for about 1 hour. The gas chromatography tracks the completion of the reaction, and then 12 g of concentrated hydrochloric acid is dripped to terminate the reaction. The solvent is recovered, the residue is distilled under reduced pressure, and 255 g of the 60-63°C / 1mmHg fraction is collected, which is a colorless and transparent liquid with a gas content of 98.5% and a yield of 83.9%.
[0039] Product structure verification:
[0040]
[0041] GC-MS (m / e): 152, 137 (100%), 123, 109, 95, 91, 81, 67, 55, 43, 41;
[0042] IR(ν / cm -1 ): 1672(-CHO), 1612;
[0043] 1 HNMR(δ, ppm, 400MHz, CDCl 3 ): 1.19 (s, 6H, C8-H, C9-H), 1.43-1.46 (m, 2H, C6-H), 1.60-1.66 (...
Embodiment 2
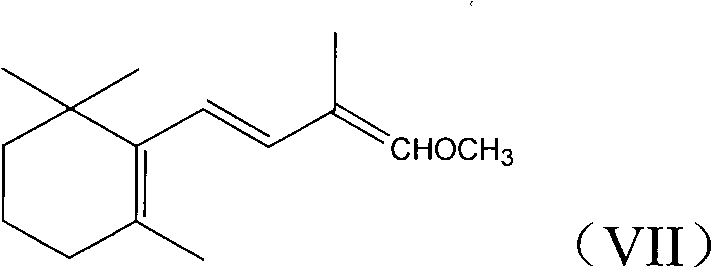
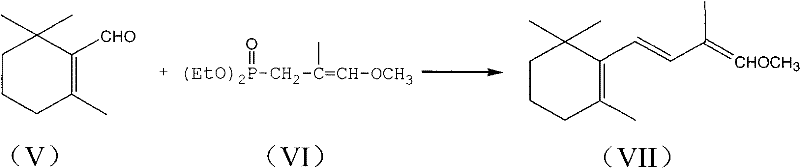
[0045] Example 2: Preparation of C-14 enol ether (VII)
[0046] Add 12.3 g of potassium tert-butoxide (0.11 mole) and 50 ml of a mixture of 8:1 (volume ratio) of tetrahydrofuran and dimethyl sulfoxide into a 250ml three-necked flask protected by nitrogen. Add 22.2 g of C-4 phosphonate (VI) (0.1 mol) dropwise, keep the temperature at -30~-25℃ for about half an hour and finish the dripping, continue to keep warm and stir for about 1 hour to make the carbanion dissociation reaction sufficient, and then keep- Add 15.2 g of β-cyclic citral (V) (0.1 mol, prepared in Example 1) dropwise at 30~-25°C. The addition is completed in about 1 hour, and the heat preservation and stirring are continued for about half an hour. The gas chromatography traces the end of the reaction and adds 50 Stir 100 ml of water and 100 ml of ether for 10 minutes, separate the layers, wash the ether layer with 5% sodium chloride aqueous solution 3 times (25 ml each time), dry the organic layer with magnesium sulf...
Embodiment 3
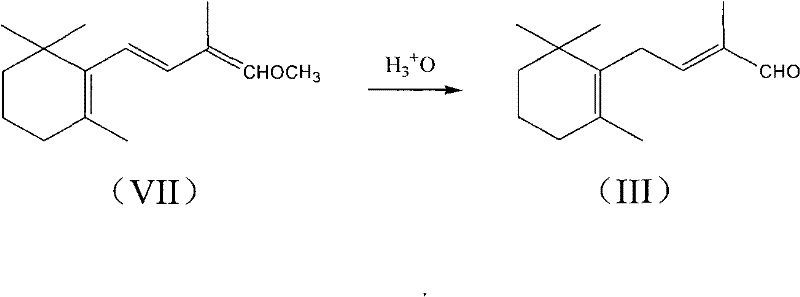
[0053] Example 3: Preparation of C-14 aldehyde (III) by hydrolysis of C-14 enol ether (VII)
[0054] Add 11.0 g (0.05 mol) of C-14 enol ether (VII) prepared in Example 2, 100 g of tetrahydrofuran and 1.1 g of p-toluenesulfonic acid into a 250 ml three-necked flask under nitrogen protection, stir well and add dropwise 22g of water, stirred for one day at 20~25℃, gas chromatography tracking the reaction is basically completed, add 2g of sodium bicarbonate and 20ml of water to make a solution to neutralize, water pump depressurize tetrahydrofuran, then add 100ml of cyclohexane The organic layer was washed with 30 ml of water, dried over anhydrous magnesium sulfate, and the solvent was recovered under reduced pressure to obtain 10.2 g of crude C-14 aldehyde (III) with a gas content of 77%. Add 10.5 g of sodium bisulfite to dissolve in 80 ml of water solution, protected by nitrogen, stir under magnetic stirring at 10-20°C for 20 minutes, the organic matter basically disappeared; add 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com